Abstract
A new deterministic model for the spread of a communicable disease that is controllable using mass quarantine is designed. Unlike in the case of the vast majority of prior quarantine models in the literature, the new model includes a quarantine-adjusted incidence function for the infection rate and the quarantine of susceptible individuals suspected of being exposed to the disease (thereby making it more realistic epidemiologically). The earlier quarantine models tend to only explicitly consider individuals who are already infected, but show no clinical symptoms of the disease (i.e., those latently-infected), in the quarantine class (while ignoring the quarantine of susceptible individuals). In reality, however, the vast majority of people in quarantine (during a disease outbreak) are susceptible. Rigorous analysis of the model shows that the assumed imperfect nature of quarantine (in preventing the infection of quarantined susceptible individuals) induces the phenomenon of backward bifurcation when the associated reproduction threshold is less than unity (thereby making effective disease control difficult). For the case when the efficacy of quarantine to prevent infection during quarantine is perfect, the disease-free equilibrium is globally-asymptotically stable when the reproduction threshold is less than unity. Furthermore, the model has a unique endemic equilibrium when the reproduction threshold exceeds unity (and the disease persists in the population in this case).
Keywords: Quarantine, Equilibrium, Reproduction number, Stability
1. Introduction
Quarantine (of individuals feared exposed to a communicable disease) is one of the oldest public health control measures for the spread of communicable diseases in given populations. More recently, this measure was successfully used to combat the spread of some emerging and re-emerging human and animal diseases, such as the severe acute respiratory syndrome (SARS) [1], [2], [3], [4], [5], [6], [7], [8], foot-and-mouth disease [9] and the 2009 swine influenza pandemic [10]. Mathematical models have been designed and used to gain qualitative insights into the spread of diseases in the presence of quarantine of individuals suspected of being exposed to a disease, and the subsequent isolation or hospitalization of those with clinical symptoms of the disease (see, for instance, [1], [2], [3], [4], [5], [6], [7], [8], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]). In the aforementioned models, with the exception of the models in [2], [5], the dynamics of the quarantined susceptible individuals is not explicitly incorporated into the model (the models only count quarantined individuals who are actually latently-infected, and essentially ignore those who remain susceptible at the end of the quarantine period).
In other words, in the aforementioned models (with the exception of the models in [2], [5]) the class (or compartment) of quarantined individuals contain only those who are latently-infected (and the temporary removal of susceptible individuals from the susceptible pool is not accounted for). The justification given for this assumption (of ignoring the back-and-forth dynamics of quarantined susceptible individuals: from susceptible to quarantine and back to susceptible class if they show no disease symptoms) is that, in general, the fraction of infected contacts that can be traced and quarantined at the time of infection is very small; and that the total population is large in comparison to the size of the infected individuals [13]. In practice, however, quarantine involves the removal of all individuals suspected of being exposed to a given disease (regardless of infection status) from the rest of the population (this often involves a lot of people; the term mass quarantine is often used to describe this process). Those who show disease symptoms during quarantine are hospitalized (or isolated), and those who remain susceptible at the end of the quarantine period are returned to the susceptible class. It is, therefore, instructive to study the impact of the aforementioned assumption (of not explicitly accounting for the quarantine of susceptible individuals) on the transmission dynamics of a disease that is controllable using quarantine.
The purpose of this study is to investigate the qualitative impact of explicitly including the dynamics of quarantined susceptible individuals on the spread of a disease that is controllable by quarantine and isolation. To achieve this objective, a new deterministic model, which includes the dynamics of quarantined susceptible individuals and quarantine-adjusted incidence function, is designed and analyzed. It should be mentioned that quarantined-adjusted incidence has also been used in a number of quarantine models, such as those in [5], [14], [15], [20].
The model to be considered is that for the transmission dynamics of a communicable disease that can be controlled using quarantine and isolation, such as ebola, measles, pandemic influenza and SARS [1], [2], [3], [4], [5], [7], [8], [11], [12], [14], [19], [21]. It is based on splitting the total population at time , denoted by , into the sub-populations of non-quarantined susceptible (), quarantined susceptible (), non-quarantined latently-infected (i.e., infected but show no clinical symptoms of the disease) , quarantined latently-infected (), non-quarantined symptomatic (), quarantined symptomatic (), hospitalized () and recovered () individuals, so that
The model is given by the following system of non-linear differential equations (a flow diagram of the model is depicted in Fig. 1 ):
| (1) |
where is the infection rate given by Safi et al. [20]:
| (2) |
and is the total actively-mixing population given by (see also [20])
| (3) |
In (2), is the effective contact rate, and the modification parameters accounts for the assumed reduction of infectiousness of exposed and hospitalized individuals, respectively, in relation to the symptomatically-infected (infectious) individuals in the class. Similarly, accounts for the assumed reduction of infectiousness of infected quarantined individuals in relation to individuals in the class (the parameter represents the assumed reduction of infectiousness of exposed quarantined individuals, in the class, in relation to infectious quarantined individuals in the class).
Fig. 1.
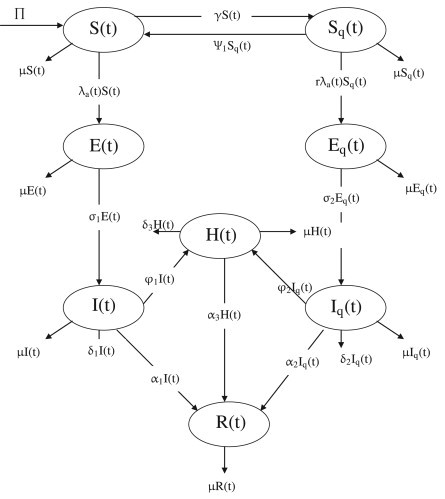
Flow diagram of the model.
In (3), is a modification parameter that measures the efficacy of quarantine in preventing individuals in quarantine from having contact with members of the general public. If , then quarantine is perfectly-implemented (so that individuals in the quarantine classes are not part of the actively-mixing population, and do not transmit infection).
The non-quarantined susceptible population () is increased by the recruitment of individuals into the community, at a rate . This population is reduced by infection (at the rate ), quarantine (at a rate ) and natural death (at a rate ; populations in all epidemiological compartments are assumed to suffer natural death at this rate). This population is increased by the return of quarantined susceptible individuals at the end of the quarantine period (at a rate ) and the loss of natural immunity by recovered individuals (at a rate ). The population of quarantined susceptible individuals () is generated by the quarantine of non-quarantined susceptible individuals (at the rate ). It is decreased by infection (at a reduced rate , with accounting for the efficacy of quarantine in preventing infection of quarantined susceptible individuals) and by the reversion to the non-quarantined susceptible class at the end of the quarantine period (at the rate ).
Non-quarantined latently-infected individuals () are generated at the rate and decreased by the development of clinical symptoms of the disease (at a rate ). Similarly, the population of quarantined latently-infected individuals () is generated at the rate and decreased by the development of clinical symptoms (at a rate ). Non-quarantined symptomatic individuals () are generated at the rate . This population is decreased by hospitalization (at a rate ), natural recovery (at a rate ) and disease-induced mortality (at a rate ). The population of quarantined symptomatic individuals () is generated at the rate and decreased by hospitalization (at a rate ), recovery (at a rate ) and disease-induced death (at a rate ).
The population of hospitalized individuals () is generated by the hospitalization of non-quarantined and quarantined symptomatic individuals (at the rate and , respectively). It is diminished by recovery (at a rate ) and disease-induced death (at a rate ). The recovered population () is generated at the rates and diminished by loss of natural immunity (at the rate ).
The model (1) is an extension of many of the models for quarantine published in the literature (including those in [1], [3], [4], [6], [11], [12], [13], [14], [15], [16], [17], [18]), by considering the dynamics of quarantined susceptible individuals (this entails adding the epidemiological compartments and ), in line with the models in [2], [5]. Furthermore, the model (1) extends the model in [5] by:
-
(i)
including a compartment for hospitalized individuals ();
-
(ii)
allowing for the infection of quarantined susceptible individuals (at the rate ; that is, unlike in [5], it is assumed that the efficacy of quarantine to prevent infection of quarantined susceptible individuals is not 100%);
-
(iii)
allowing for disease transmission by infected quarantined and hospitalized individuals (i.e., it is assumed, unlike in [5], that quarantine and hospitalization are not 100% effective in preventing transmission by infected quarantined or hospitalized individuals).
The model (1) also extends the model in [2] by:
-
(a)
incorporating demographic parameters (i.e., using an endemic model as against the epidemic model used in [2]);
-
(b)
using a standard incidence function to model the infection rate (the mass action incidence function was used in [2] by assuming constant total population);
-
(c)
allowing for the infection of quarantined susceptible individuals (quarantine was assumed to be perfect against infection in [2]);
-
(d)
allowing for disease transmission by non-quarantined latently-infected individuals.
Furthermore, detailed qualitative analysis of the model will be carried out in this study (this is not done in [2]). The model (1) extends the quarantine models with quarantined-adjusted incidence in [15], [20], by including the dynamics of quarantined susceptible individuals () as well as adding classes for quarantined exposed () and symptomatic () individuals.
The model (1) will now be analyzed to gain insight into its dynamical features.
2. Analysis of the model
2.1. Basic properties
Since the model (1) monitors human populations, all its associated parameters are non-negative. The following basic results can be established using the method in Appendix A of [22].
Theorem 1
The variables of the model (1) are non-negative for all time. In other words, solutions of the model system (1) with positive initial data will remain positive for all time .
Lemma 1
The closed set
is positively-invariant for the model (1) .
Proof
Adding all the equations of the model (1) gives,
Since , it follows that if . Thus, a standard comparison theorem [23] can be used to show that . In particular, if . Thus, the region is positively-invariant. Further, if , then either the solution enters in finite time, or approaches asymptotically. Hence, the region attracts all solutions in . □
2.2. Local stability of disease-free equilibrium (DFE)
The DFE of the model (1) is given by
| (4) |
The local stability of will be explored using the next generation operator method [24], [25]. Using the notation in [25], the non-negative matrix, , of the new infection terms, and the -matrix, , of the transition terms associated with the model (1), are given, respectively, by
and,
where,
It follows that the control reproduction number [26], [27], denoted by , where is the spectral radius, is given by
Using Theorem 2 in [25], the following result is established.
Lemma 2
The DFE of the model (1) , given by (4) , is locally-asymptotically stable (LAS) if , and unstable if .
The threshold quantity measures the average number of new infections generated by a single infectious individual in a population where a certain fraction of the susceptible population is vaccinated. Lemma 2 implies that the disease can be eliminated from the community (when ) if the initial sizes of the sub-populations of the model are in the basin of attraction of the DFE ().
2.3. Backward bifurcation analysis
In this section, the existence of endemic equilibria (that is, equilibria where the infected compartments of the model are non-zero) of the model (1) is established. Let,
represents any arbitrary endemic equilibrium point (EEP) of the model (1). Further, define
| (5) |
(the force of infection of the model (1) at steady-state). It follows, by solving the equations in (1) at steady-state, that
| (6) |
Substituting the expressions in (6) into (5) shows that the non-zero equilibria of the model satisfy the following quadratic equation (in terms of ):
| (7) |
where,
The endemic equilibria of the model (1) can then be obtained by solving for from (7), and substituting the positive values of into the expressions in (6). The quadratic equation (7) can be analyzed for the possibility of multiple endemic equilibria when . It should be noted that the coefficient, , of the quadratic (7) is always positive and is positive (negative) if is less (greater) than unity. Hence, the following result is established.
Theorem 2
The model (1) has
- (i)
a unique endemic equilibrium if ;
- (ii)
a unique endemic equilibrium if ( and ) or ;
- (iii)
two endemic equilibria if and ;
- (iv)
no endemic equilibrium otherwise.
Thus, it is clear from Case (i) of Theorem 2 that the model (1) has a unique EEP (of the form ) whenever . Furthermore, Case (iii) of Theorem 2 indicates the possibility of backward bifurcation, where a LAS DFE co-exists with a LAS endemic equilibrium when the associated reproduction number is less than unity (see, for instance, [28], [29], [30] for discussions on backward bifurcation) in the model (1). The epidemiological importance of the phenomenon of backward bifurcation is that the classical requirement of having is, although necessary, not sufficient for disease elimination. In this case, disease elimination will depend upon the initial sizes of the sub-populations of the model. Thus, the existence of backward bifurcation in the transmission dynamics of a disease makes its effective control difficult. A more rigorous proof of the backward bifurcation property of the model (1), based on using center manifold theory (see, for instance, [25], [29], [31], [32]), is given in the Appendix.
2.3.1. Non-existence of backward bifurcation
In this section, the scenario where the backward bifurcation property of the model can be lost is explored. Consider the model (1) with a perfect quarantine efficacy against infection (so that, ). In this case, the coefficients and of the quadratic equation (7) reduce to and whenever . Thus, for this case, the quadratic equation (7) has one solution (.) Therefore, the model (1) with a perfect quarantine has no positive endemic equilibrium whenever . This rules out the possibility of backward bifurcation in this case (since backward bifurcation requires the existence of at least two endemic equilibria whenever [28], [29], [30]). Furthermore, it can be shown that, for the case when , the DFE () of the model (1) is globally-asymptotically stable (GAS) under some conditions, as shown below.
Setting in the model (1) gives the following reduced model (it should be noted from (1) that, for the case when , as ; hence, these variables are omitted from the asymptotic analysis of the model for the special case with ):
| (8) |
with the associated force of infection , where
| (9) |
It can be shown that the reproduction number associated with the reduced model (8), with (9), is given by
Define,
The model (8) has a DFE, given by .
Theorem 3
The DFE ( ) of the reduced model (8) , with (9) , is GAS in whenever .
Proof
Consider the following Lyapunov function
with Lyapunov derivative (where a dot represents differentiation with respect to time) given by
Since all the parameters and variables of the model (1) are non-negative (Theorem 1), it follows that for (it should be noted that ) with if and only if . Hence, is a Lyapunov function on . Thus, it follows, by LaSalle’s Invariance Principle [33], that
(10) Since and (from (10)), it follows that, for sufficiently small , there exist constants and such that for all and for all . Hence, it follows from the last equation of the model (8) that, for ,
Thus, by comparison theorem [23],
so that, by letting ,
(11) Similarly (by using and ), it can be shown that
(12) Thus, it follows from (11), (12) that
Hence,
(13) Similarly, it can be shown that
(14) Thus, by combining Eqs. (10), (13), (14), it follows that every solution of the equations of the model (8), with initial conditions in , approaches as (for ). □
The above result shows that, for the case when the efficacy of quarantine in preventing infection is perfect (i.e., ), the disease can be eliminated from the community if the associated reproduction number of the model is less than unity. Furthermore, this result clearly shows that the backward bifurcation property of the model (1) is caused by the imperfect nature of the efficacy of quarantine to prevent infection (see the Appendix).
Conditions for the persistence of the disease in the population will be investigated below.
2.4. Persistence
The model system (1) is said to be uniformly-persistent if there exists a constant such that any solution satisfies [34], [35]:
provided that .
Theorem 4
System (1) is uniformly-persistent in if and only if .
Proof
The theorem can be proved by using the approach used to prove Proposition 3.3 of [36], by applying a uniform persistence result in [34] and noting that the DFE of the model (1) is unstable whenever (Lemma 2). □
The consequence of this result is that each infected variable () of the model will persist above a certain threshold value at steady-state, so that the disease will persist (become endemic) in the population. It should be mentioned that some other models for quarantine, which did not explicitly include the dynamics of quarantined susceptible individuals, also showed the presence of a unique endemic equilibrium and disease persistence when the associated reproduction number exceeds unity (see, for instance, [13], [17], [18]).
Conclusions
A new deterministic model for the spread of a disease in a population in the presence of quarantine is designed. A major feature of the model is that it incorporate the dynamics of quarantine-adjusted incidence and the quarantine of susceptible individuals (that is, quarantine is modeled in terms of the temporarily removal of susceptible individuals from the susceptible pool as well as the removal of new infected individuals, detected via the contact tracing of known infectious individuals). Rigorous analysis of the model reveals that it undergoes the phenomenon of backward bifurcation when the associated reproduction number () is less than unity. The presence of this phenomenon, which does not arise if the quarantine is 100% effective, implies that the effective control of the spread of the disease, using an imperfect quarantine, depends on the initial sizes of the sub-populations of the model (when ). The model has a unique endemic equilibrium whenever . Furthermore, it is shown that the disease will persist in the population whenever .
Acknowledgments
ABG acknowledges, with thanks, the support in part of the Natural Science and Engineering Research Council (NSERC) and Mathematics of Information Technology and Complex Systems (MITACS) of Canada. The authors are grateful to the anonymous reviewers for their constructive comments.
Submitted by Juan J. Nieto
Appendix. Proof of backward bifurcation property of model (1)
The proof is based on using center manifold theory [31], [32]. In particular, Theorem 4.1 of [32] will be used. It is convenient to make the following change of variables:
Furthermore, let . Thus, the model (1) can be re-written in the form , with , as follows:
| (15) |
The Jacobian of the system (15), at the associated DFE (), is given by
where
Consider the case when . Furthermore, suppose that is chosen as a bifurcation parameter. Solving for from gives
where
The transformed system (15), with , has a hyperbolic equilibrium point (i.e., the linearized system has a simple eigenvalue with zero real part, and all other eigenvalues have negative real part), so that the center manifold theory [31], [32] can be used to analyze the dynamics of (15) near .
It can be shown that the right eigenvector of , denoted by , is given by , where,
Similarly, has a left eigenvector, given by , where,
Consequently, it follows that the associated bifurcation coefficients, and (defined in Theorem 4.1 of [32]), are given, respectively, by
| (16) |
where
Since the bifurcation coefficient is always positive, it follows (from Theorem 4.1 of [32]) that the system (15) will undergo backward bifurcation if the bifurcation coefficient is positive. This result is summarized below.
Theorem 5
The transformed model (15) , or equivalently (1) , exhibits backward bifurcation at whenever the bifurcation coefficient, , given by (16) , is positive.
It should be noted from (16) that the bifurcation coefficient , is positive whenever
Thus, the transformed model (15), exhibits backward bifurcation at whenever . Furthermore, it should be noted that for the case when quarantined susceptible individuals do not acquire infection during quarantine (i.e., ), the bifurcation coefficient becomes
since for (since all the model parameters are non-negative). Thus, since in this case, it follows from Theorem 4.1 of [32] that the model (1) will not exhibit backward bifurcation if . In other words, this study shows that the backward bifurcation property of the model (1) arises due to the infection of susceptible individuals in quarantine. This result is consistent with Theorem 3 (where it was shown that the DFE of the model (1) with is globally-asymptotically stable).
References
- 1.Gumel A.B. Modelling strategies for controlling SARS outbreaks. Proc. Roy. Soc. Ser. B. 2004;271:2223–2232. doi: 10.1098/rspb.2004.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipsitch M. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Smith J.O., Galvani A.P., Getz W.M. Curtailing transmission of severe acute respiratory syndrome within a community and its hospital. Proc. R. Soc. Lond. B. 2003;170:1979–1989. doi: 10.1098/rspb.2003.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLeod R.G., Brewster J.F., Gumel A.B., Slonowsky D.A. Sensitivity and uncertainty analyses for a SARS model with time-varying inputs and outputs. Math. Biosci. Eng. 2006;3:527–544. doi: 10.3934/mbe.2006.3.527. [DOI] [PubMed] [Google Scholar]
- 5.Mubayi A., Kribs-Zaleta C., Martcheva M., Castillo-Chavez C. A cost-based comparison of quarantine strategies for new emerging diseases. Math. Biosci. Eng. 2010;7(3):687–717. doi: 10.3934/mbe.2010.7.687. [DOI] [PubMed] [Google Scholar]
- 6.Riley S. Transmission dynamics of etiological agent of SARS in Hong Kong: the impact of public health interventions. Science. 2003;300:1961–1966. doi: 10.1126/science.1086478. [DOI] [PubMed] [Google Scholar]
- 7.Wang W., Ruan S. Simulating the SARS outbreak in Beijing with limited data. J. Theoret. Biol. 2004;227:369–379. doi: 10.1016/j.jtbi.2003.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan X., Zou Y. Optimal and sub-optimal quarantine and isolation control in SARS epidemics. Math. Comput. Modelling. 2008;47:235–245. doi: 10.1016/j.mcm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kao R.R., Roberts M.G. Quarantine-based disease control in domesticated animal herds. Appl. Math. Lett. 1998;4:115–120. [Google Scholar]
- 10.Sato H., Nakada H., Yamaguchi R., Imoto S., Miyano S., Kami M. When should we intervene to control the 2009 influenza A(H1N1) pandemic? Rapid Communications. Euro. Surveill. 2010;15(1) doi: 10.2807/ese.15.01.19455-en. 19455. [DOI] [PubMed] [Google Scholar]
- 11.Chowell G., Hengartner N.W., Castillo-Chavez C., Fenimore P.W., Hyman J.M. The basic reproductive number of ebola and the effects of public health measures: the cases of Congo and Uganda. J. Theoret. Biol. 2004;1:119–126. doi: 10.1016/j.jtbi.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Day T., Park A., Madras N., Gumel A.B., Wu J. When is quarantine a useful control strategy for emerging infectious diseases? Amer. J. Epidemiol. 2006;163:479–485. doi: 10.1093/aje/kwj056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Z., Xu D., Zhao H. Epidemiological models with non-exponentially distributed disease stages and application to disease control. Bull. Math. Biol. 2007;69:1511–1536. doi: 10.1007/s11538-006-9174-9. [DOI] [PubMed] [Google Scholar]
- 14.Hethcote H.W., Zhien M., Shengbing L. Effects of quarantine in six endemic models for infectious diseases. Math. Biosci. 2002;180:141–160. doi: 10.1016/s0025-5564(02)00111-6. [DOI] [PubMed] [Google Scholar]
- 15.Nuno M., Feng Z., Martcheva M., Castillo-Chavez C. Dynamics of two-strain influenza with isolation and partial cross-immunity. SIAM J. Appl. Math. 2005;65:964–982. [Google Scholar]
- 16.Podder C.N., Gumel A.B., Bowman C.S., McLeod R.G. Mathematical study of the impact of quarantine, isolation and vaccination in curtailing an epidemic. J. Biol. Sys. 2007;15:185–202. [Google Scholar]
- 17.Safi M.A., Gumel A.B. Global asymptotic dynamics of a model for quarantine and isolation. Discrete Contin. Dyn. Syst. Ser. B. 2010;14:209–231. [Google Scholar]
- 18.Safi M.A., Gumel A.B. The effect of incidence functions on the dynamics of a quarantine/isolation model with time delay. Nonlinear Anal. RWA. 2011;12(1):215–235. doi: 10.1016/j.nonrwa.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webb G.F., Blaser M.J., Zhu H., Ardal S., Wu J. Critical role of nosocomial transmission in the Toronto SARS outbreak. Math. Biosci. Eng. 2004;1:1–13. doi: 10.3934/mbe.2004.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Safi M.A., Imran M., Gumel A.B. Threshold dynamics of a non-autonomous SEIRS model with quarantine and isolation. Theory Biosci. 2012;131:19–30. doi: 10.1007/s12064-011-0148-6. [DOI] [PubMed] [Google Scholar]
- 21.Chowell G., Castillo-Chavez C., Fenimore P.W., Kribs-Zaleta C.M., Arriola L., Hyman J.M. Model parameters and outbreak control for SARS. EID. 2004;10:1258–1263. doi: 10.3201/eid1007.030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thieme H.R. Princeton University Press; 2003. Mathematics in Population Biology. [Google Scholar]
- 23.Smith H.L., Waltman P. Cambridge University Press; 1995. The Theory of the Chemostat. [Google Scholar]
- 24.Diekmann O., Heesterbeek J.A.P., Metz J.A.J. On the definition and computation of the basic reproduction ratio in models for infectious disease in heterogeneous population. J. Math. Biol. 1990;28:365–382. doi: 10.1007/BF00178324. [DOI] [PubMed] [Google Scholar]
- 25.van den Driessche P., Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002;180:29–48. doi: 10.1016/s0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 26.Anderson R.M., May R.M. Springer-Verlag; Berlin, Heidelrberg, New York: 1982. Population Biology of Infectious Diseases. [Google Scholar]
- 27.Hethcote H.W. The mathematics of infectious diseases. SIAM Rev. 2000;42:599–653. [Google Scholar]
- 28.Elbasha E.H., Gumel A.B. Theoretical assessment of public health impact of imperfect prophylactic HIV-1 vaccines with therapeutic benefits. Bull. Math. Biol. 2006;68:577–614. doi: 10.1007/s11538-005-9057-5. [DOI] [PubMed] [Google Scholar]
- 29.Garba S.M., Gumel A.B., Abu Bakar M.R. Backward bifurcations in dengue transmission dynamics. Math. Biosci. 2008;1:11–25. doi: 10.1016/j.mbs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Sharomi O., Podder C.N., Gumel A.B., Elbasha E., Watmough J. Role of incidence function in vaccine-induced backward bifurcation in some HIV models. Math. Biosci. 2007;2:436–463. doi: 10.1016/j.mbs.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Carr J. Springer-Verlag; New York: 1981. Applications of Centre Manifold Theory. [Google Scholar]
- 32.Castillo-Chavez C., Song B. Dynamical models of tuberculosis and their applications. Math. Biosci. Eng. 2004;1:361–404. doi: 10.3934/mbe.2004.1.361. [DOI] [PubMed] [Google Scholar]
- 33.Hale J.K. John Wiley and Sons; New York: 1969. Ordinary Differential Equations. [Google Scholar]
- 34.H. Freedman, S. Ruan, M. Tang, Uniform persistence and flows near a closed positively invariant set, 1994.
- 35.Thieme H. Epidemic and demographic interaction in the spread of potentially fatal diseases in growing populations. Math. Biosci. 1992;1:99–130. doi: 10.1016/0025-5564(92)90081-7. [DOI] [PubMed] [Google Scholar]
- 36.Li M., Graef J., Wang L., Karsai J. Global dynamics of a SEIR model with varying total population size. Math. Biosci. 1999;160:191–213. doi: 10.1016/s0025-5564(99)00030-9. [DOI] [PubMed] [Google Scholar]


