Abstract
This study reports an integrated microfluidic system which utilizes virus-bound magnetic bead complexes for rapid serological analysis of antibodies associated with an infection by the dengue virus. This new microfluidic system integrates one-way micropumps, a four-membrane-type micromixer, two-way micropumps and an on-chip microcoil array in order to simultaneously perform the rapid detection of immunoglobulin G (IgG) and immunoglobulin M (IgM). An IgM/IgG titer in serum is used to confirm the presence of dengue virus infection. By utilizing microfluidic technologies and virus-bound magnetic beads, IgG and IgM in the serum samples are captured. This is followed by purification and isolation of these beads utilizing a magnetic field generated from the on-chip array of microcoils. Any interfering substances in the biological fluids are washed away automatically by the flow generated by the integrated pneumatic pumps. The fluorescence-labelled secondary antibodies are bound to the surface of the IgG/IgM complex attached onto the magnetic beads. Finally, the entire magnetic complex sandwich is transported automatically into a sample detection chamber. The optical signals are then measured and analyzed by a real-time optical detection module. The entire process is performed automatically on a single chip within 30 min, which is only 1/8th of the time required for a traditional method. More importantly, the detection limit has been improved to 21 pg, which is about 38 times better when compared to traditional methods. This integrated system may provide a powerful platform for the rapid diagnosis of dengue virus infection and other types of infectious diseases.
Keywords: Dengue virus, Magnetic beads, Immunoglobulin G, Immunoglobulin M, Fluorescent immunoassay, Microfluidics
Nomenclature
- a.u.
arbitrary unit
- BP
band pass
- CCD
charge-coupled device
- CDC
Centers for Disease Control
- DI
deionized
- DV
dengue virus
- DC
direct current
- ELISA
enzyme-linked immunosorbent assay
- EMV
electromagnetic valve
- EV
enterovirus
- FIA
fluorescent immunoassay
- FITC
fluorescein isothiocyanate
- HAI
hemagglutination inhibition
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- LP
long pass
- MEMS
micro-electromechanical-systems
- PBS
phosphate buffer saline
- PDMS
polydimethylsiloxane
- PFU
plaque-forming unit
- PMT
photo-multiplier tube
- R-PE
R-phycoerythrin
- RT-PCR
reverse-transcription polymerase chain reaction
- SARS
severe acute respiratory syndrome
- SEM
scanning electron microscope
- S/N
signal-to-noise
- UV
ultraviolet
Greek letter
- μ-TAS
micro-total-analysis-systems
1. Introduction
In recent years, emerging, extremely contagious, infectious diseases such as severe acute respiratory syndrome (SARS) and avian influenza have attracted considerable concern from public health organizations. Consequently, tools that enable the fast, accurate and sensitive diagnosis of these infections becomes crucial for the Centers for Disease Control (CDC) in every country. During the acute phase of these types of infections, rapid diagnosis is essential in order to provide immediate and necessary clinical treatment and infection control. More importantly, it may limit the spread of further infection.
Dengue fever is one of these acutely infectious diseases. Traditionally, a hemagglutination inhibition (HAI) assay is commonly used for the diagnosis of dengue virus (DV) infection (Groen et al., 2000). It is an easy protocol with reasonable accuracy. Recently, a reverse-transcription polymerase chain reaction (RT-PCR) assay, a molecular diagnostic method for the detection of dengue fever, provides a highly sensitive and accurate diagnosis protocol (Shu et al., 2003a). However, it requires a complicated protocol and is relatively costly. Alternatively, enzyme-linked immunosorbent assay (ELISA) is another well-recognized serological diagnostic method for the detection of a DV infection (Shu et al., 2003b, Andrew et al., 2006). It provides for a rapid diagnosis at a relatively low cost. The principle behind this serological diagnosis is based on the detection of immunoglobulin G (IgG) and immunoglobulin M (IgM), which are specific to pathogens. The presence of IgM indicates an acute infection whereas IgG is a marker for a previous infection. Therefore, the simultaneous detection of IgM and IgG would provide a more accurate diagnosis. The measurements of IgG or IgM associated with a DV infection have been reported in the literature (Hapugoda et al., 2007, Chanama et al., 2004). However, a conventional ELISA is usually performed on 96-well plates, which involves a series of tedious processes, including incubation and washing steps. Not only is it a time-consuming (over 4 h) and labor-intensive process, but it also requires well-trained personnel to precisely perform the entire protocol. In addition, it cannot be used to detect IgM and IgG simultaneously. More importantly, the microorganism-induced antibody response of the host can be early diagnosed during the infection by a more sensitive immunological detection technique. The detection limit of the developed method is the detectable signals of immunoglobulins in the clinical bio-samples that can be more specifically and accurately analyzed. The sensitivity of the conventional ELISA is not adequate because the antigen is coated onto a two-dimensional 96-well plate, which limits binding capacity of antibody. Using the virus-holding magnetic complexes can increase the binding capacity of the specific antibody with their 3D structures, and therefore increase the detection sensitivity. Therefore, there exists a critical need to develop a platform for the rapid, automatic, and simultaneous detection of IgM and IgG associated with a DV infection with a higher sensitivity.
Advances in micromachining and microfluidic technologies have enabled the miniaturization of many chemical and biomedical instruments. A micro-total-analysis-system (μ-TAS) can be realized using Micro-electromechanical-systems (MEMS) technology, which integrates several functional modules on a single chip for sample preparation, reaction, transportation and detection (Auroux et al., 2002, Bini et al., 2008). Microfluidic techniques have shown its great potential for automatic processing (Lien et al., 2008). Recently, several magnetic bead-based microfluidic systems have been demonstrated for biomedical applications (Huang et al., 2002, Gijs, 2004, Choi et al., 2000). For example, a magnetic bead-based immunoassay utilizing immobilization surfaces and bio-molecule carriers for protein analysis has been reported (Choi et al., 2000). Recently, magnetic beads coated with antibodies specific to target pathogens have been commonly used to purify and to enrich bio-samples (Fuentes et al., 2006, Lien et al., 2007). These microfluidic systems usually provide advantages over their large-scale counterparts including a shorter diagnosis time, less sample/reagent consumption, a lower operating cost and even higher sensitivity (Auroux et al., 2002, Reyes et al., 2002). More importantly, a variety of integrated devices for sample preparation, reaction and detection have been successfully demonstrated, the entire diagnostic process can be performed automatically using integrated micropumps and microvalves on the same chip (Lien et al., 2008). Consequently, various bead-based immunoassays implemented via microfluidic systems have been reported. Generally speaking, bead-based immunoassays have several advantages when compared with substrate-based assays (Vignali, 2000). In addition to their capability for sample purification, the micro-beads have a three-dimensional structure which increases the surface-to-volume ratio, thus providing more binding sites for target bio-samples. Furthermore, surface modification of a microfluidic device was also reported to enhance the sensitivity and selectivity of an immunoassay (Bai et al., 2006). For example, it has been demonstrated that rabbit IgG and human IgG can be detected with the incorporation of these nano-particles into microfluidic systems (Matsunaga et al., 2007, Holmes et al., 2007). However, there are still several lengthy off-chip sample pre-treatment processes necessary to purify the samples prior to usage and numerous bulky apparatus are normally required for the entire procedure.
Immunoassay-based systems using MEMS technology to perform on-chip detection of infectious diseases have been explored (Henry, 1997). For example, a microsystem has been successfully demonstrated that can detect sheep IgM with a detection limit of 17 nM (Eteshola and Balberg, 2004). Similarly, the detection of DV can be performed using a microfluidic-based fluorescent biosensor (Zaytseva et al., 2005). A microfluidic device capable of rapid analysis of fluorescence-labelled beads could be used to count and sort micron-sized polymer beads that are used as a solid support for a fluorescence immunoassay (Gijs, 2004, Aytur et al., 2006). However, no attempts have been made for the simultaneous detection of IgG and IgM in a microfluidic immunoassay system.
Consequently, this study presents an innovative fluorescent immunoassay (FIA)-based microfluidic system for the rapid serological analysis of IgM and IgG associated with the detection of DV infection by utilizing virus-bound magnetic beads. The system can perform transportation, mixing and separation of the sandwich-like magnetic bead complexes automatically. Furthermore, the total experimental time is decreased to within 30 min. By using virus-bound magnetic beads, the developed system can be used to purify the virus-specific IgM and IgG from serum samples such that the subsequent detection can be performed with a higher sensitivity and specificity. Most importantly, this microfluidic chip can detect IgG and IgM simultaneously. By analyzing the antibody titer of patients with suspected infections, it can be used to quickly and to automatically diagnose infectious diseases.
2. Materials and methods
2.1. Experimental procedure
A microfluidic system is designed and fabricated for rapid diagnosis of DV infection by using virus-bound magnetic beads in a sandwich-based immunoassay. To utilize the affinity between the antibodies and the antigens (Haukanes and Kvam, 1993), the immunoglobulins in the sample serum are captured by the virus-bound magnetic beads with a high selectivity. Fig. 1(a) is an illustration of a sandwich-based immunoassay with magnetic beads. Using the DV–bead complex ensures that any captured IgM and IgG is specific to the DV. Therefore, this enhances the selectivity of the virus detection. The virus is treated with ultraviolet (UV) rays prior to use to inactivate its infectivity, but not its structure. Consequently, infection and contamination issues are eliminated. The surface of the superparamagnetic beads is coated with an epoxy and is conjugated with the capture antibodies. The DV is bound to the antibody-conjugated beads by the affinity between the antibody and the antigen. The DV–bead complex can then detect the target IgM and IgG in serum samples during the incubation process. Finally, the target IgM and IgG are recognized by the specific attached antibodies (anti-human IgG antibody labelled with fluorescein isothiocyanate (FITC) and anti-human IgM antibody labelled with R-phycoerythrin (R-PE)). Fig. 1(b) illustrates the protocol for the magnetic bead-based microfluidic system. First, the DV-antibody-conjugated magnetic beads (5 μl) and the serum samples (100 μl) are loaded in a “mixing chamber.” A four-membrane-type micromixer is used to incubate these virus-bound magnetic beads and the serum samples for 5 min, so that the virus-specific IgM and IgG in the serum samples can be recognized and can be attached onto the virus-bound magnetic beads (Fig. 1(b-1)). This is followed by purifying and isolating these beads utilizing a magnetic field generated from the on-chip microcoils. Any interfering material in the biological fluids would be washed away into a waste chamber by flowing a wash buffer (1× phosphate buffer saline (PBS) with 0.5% Tween 20) (Fig. 1(b-2)). After washing, the purified beads with IgM and IgG are re-suspended into a smaller volume, and the purified beads are then transported into the mixing chamber using a two-way micropump. This is followed by adding the developed antibody fluorescent conjugates (anti-human IgM-R-PE and anti-human IgG-FITC) into the mixing chamber (Fig. 1(b-3)). After incubating for 2 min, anti-human IgM-R-PE antibodies and anti-human IgG-FITC should be individually bound onto the surface of the IgM and IgG that are attached to the magnetic bead complex. Then, a washing step is repeated to flush out other impurities and any un-bound developed antibodies (Fig. 1(b-4)). Finally, the sandwich-like magnetic bead complexes are transported to a “detection chamber” where the fluorescent signal is excited and is analyzed by an optical detection module consisting of a photo-multiplier tube (PMT) and a mercury lamp (Fig. 1(b-5)). Detailed information about the experimental procedure including the reaction volume of the samples and the on-chip operation conditions of the miniature platform can be found in Table 1 .
Fig. 1.
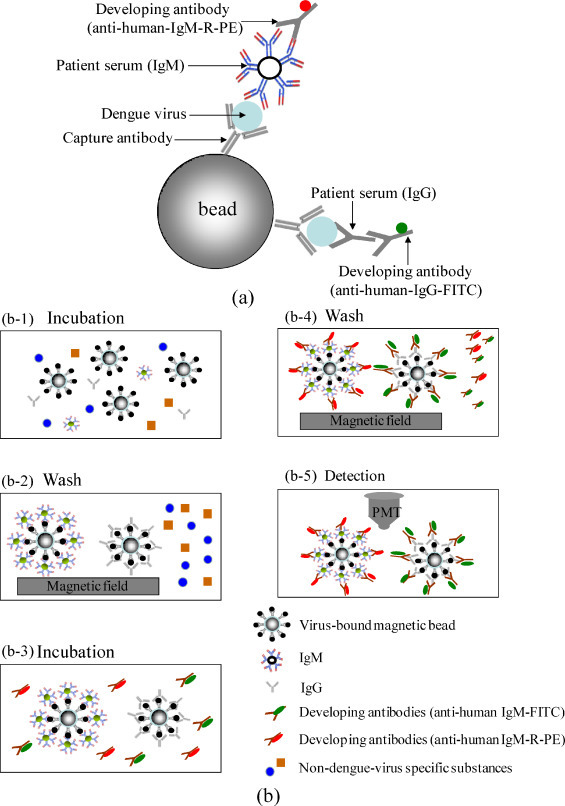
(a) Schematic illustration of a sandwich-based immunoassay with magnetic beads; (b) schematic illustration of the protocol for purification and detection of IgG/IgM by using the virus-bound magnetic beads in the microfluidic system.
Table 1.
Detailed information about the experimental protocol on the magnetic bead-based microfluidic platform.
| Step | Procedure | Sample volume | On-chip operation condition |
|---|---|---|---|
| 1 | Load virus-bound magnetic beads in the sample loading/mixing chamber. | 5 μl | |
| Load serum sample in the sample loading/mixing chamber. | 100 μl | ||
| Deflect the membranes of the micropump to block the sample inside the sample purification chamber. | 20 psi for the micropump | ||
| 2 | Mix the bio-sample with the magnetic beads for 5 min in the incubation process by using the membrane-type micromixer. | 20 psi and 1.37 Hz for the micromixer | |
| 3 | Pump the well-mixed sample into the sample purification chamber at a flow rate of 620 μl/min. | 20 psi and 35 Hz for the micropump | |
| 4 | Turn on the magnetic field to collect the IgM/IgG-bound magnetic beads onto the surface of the sample purification chamber for 1 min by using the microcoils array. | 150 mA for microcoils | |
| 5 | Load the washing buffer into the sample loading chamber. | 200 μl | |
| Pump the washing buffer through the purification chamber at a flow rate of 620 μl/min for washing. | 20 psi and 35 Hz for the micropump | ||
| Re-suspend the purified samples into a volume of 100 μl and transport the sample into the sample loading/mixing chamber. | 100 μl | 20 psi and 35 Hz for the micropump | |
| 6 | Pump half of the purified sample to the sample chamber at a flow rate of 620 μl/min. | 50 μl | 20 psi and 35 Hz for the micropump |
| 7 | Load developing antibody (anti-human IgG-FITC) in the sample loading/mixing chamber. | 100 μl | |
| Mix the developing antibody with the purified samples for 2 min in the incubation process by using the membrane-type micromixer. | 20 psi and 1.37 Hz for the micromixer | ||
| 8 | Pump the well-mixed sample into the sample purification chamber at a flow rate of 620 μl/min. | 20 psi and 35 Hz for the micropump | |
| 9 | Apply the DC current into the microcoils array to trap the magnetic complexes within the sample purification chamber for 1 min. | 150 mA for microcoils | |
| Load the washing buffer in the sample loading chamber and then flow the buffer through the purification chamber at a flow rate of 620 μl/min. | 300 μl | 20 psi and 35 Hz for the micropump | |
| Re-suspend the purified magnetic complexes into a volume of 5 μl and transport the complexes to the sample detection chamber at a flow rate of 182.5 μl/min. | 5 μl | 20 psi and 35 Hz for the micropump | |
| 10 | Pump the purified sample in the sample chamber to sample loading/mixing chamber at a flow rate of 620 μl/min. | 50 μl | 20 psi and 35 Hz for the micropump |
| Load developing antibody (anti-human IgM-R-PE) in the sample loading/mixing chamber. | 100 μl | ||
| Mix the developing antibody with the purified samples for 2 min in the incubation process by using the membrane-type micromixer. | 20 psi and 1.37 Hz for the micromixer | ||
| 11 | Pump the well-mixed sample into the sample purification chamber at a flow rate of 620 μl/min | 20 psi and 35 Hz for the micropump | |
| 12 | Apply the DC current into the microcoils array to trap the sandwich-like magnetic bead complexes within the sample purification chamber for 1 min. | 150 mA for microcoils | |
| Load the washing buffer in the sample loading chamber and then flow the buffer through the purification chamber at a flow rate of 620 μl/min. | 300 μl | 20 psi and 35 Hz for the micropump | |
| Re-suspend the purified sandwich-like magnetic bead complexes into a volume of 5 μl and transport the complexes to the sample detection chamber at a flow rate of 182.5 μl/min. | 5 μl | 20 psi and 35 Hz for the micropump | |
| 13 | The fluorescent signal is excited and is analyzed by an optical detection module. | ||
2.2. Chip design and experimental setup
In this study, the entire protocol assay can be performed automatically in a microfluidic system. A photograph of this magnetic bead-based microfluidic system is shown in Fig. 2(a). The dimensions of the chip are measured to be 53 mm × 37 mm. The microfluidic chip integrates several modules into a single chip, including a sample chamber, a four-membrane-type micromixer, microchannels, two-way micropumps, a circular array of microcoils, a sample loading/mixing chamber, a sample purification chamber, two sample detection chambers and a waste collection chamber. The details about design parameters of the microfluidic system can be found in the supplemental Fig. 2. This chip is composed of three polydimethylsiloxane (PDMS, Sylgad 184A/B, Dow Corning Corp., USA) layers and one glass layer. In order to perform the simultaneous detection of IgG and IgM, microcoils are first used in a bio-separator and micropumps and microvalves are used for sample transportation. Scanning electron microscope (SEM) images of the components and the SU-8 molds can be found in the supplemental Fig. 1(a). Details of the fabrication process for the circular microcoil array, micropumps and microvalves can be found in our previous work (Lien et al., 2007, Lien et al., 2008). The circular array of microcoils is used as a bio-separator when the washing buffer flows through the sample purification chamber. The array is supplied with a direct current (DC) to generate the required magnetic field so that the magnetic beads with IgM and IgG are attracted onto the surface of the microcoils. In this study, a two-way micropump is used to transport fluids forward and backwards. There are two inlets for the compressed air in the two-way micropump which is regulated by electromagnetic valves (EMV, SMC Inc., S070 M-5BG-32, Japan). The liquid in the microchannels can be delivered forward or backwards by the sequential activation of the peristaltic micropumps which are driven by the air pressure. Besides, the two-way micropump can be also used as a microvalve, if the PDMS membranes are deformed completely to block the fluid in the microchannel.
Fig. 2.
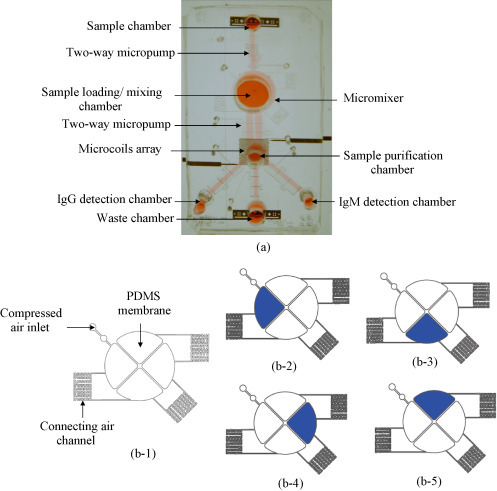
(a) A photograph of the magnetic bead-based microfluidic chip. The dimensions of this chip are measured to be 53 mm × 37 mm. Note that the detailed design parameters of the microfluidic can be found in the Supplemental Information. (b-1) A schematic diagram of the four-membrane-type micromixer; (b-2)–(b-5) show the motion of the fluids in the micromixer.
The four-membrane-type micromixer contains four thin-film PDMS membranes as air chambers and three connecting air channels, as shown in Fig. 2(b-1). The working principle of the micromixer is illustrated in Fig. 2(b-2)–(b-5). Air tubing is connected to the “compressed air inlet” to convey the compressed air to the air chamber of the four-membrane-type micromixer. When the compressed air passes through the connecting air channels, the air cavities under the four thin-film PDMS membranes are filled up in sequence and a time-phased deflection of the membranes is then generated. Therefore, the sample fluid can be mixed efficiently. A gentle mixing effect can be induced as the compressed air is released.
In addition, an optical detection module is used to detect the fluorescent signals for serological diagnosis and the entire experimental setup is schematically shown in the supplementary Fig. 1(b).
2.3. Materials
2.3.1. Virus strain and serum sample
Dengue virus serotype 2/PL046 and enterovirus (EV) 71/4643 are used to verify the performance of the developed microfluidic system and the initial titers of the virus pool are 1 × 108 plaque-forming unit/ml (PFU/ml) and 1 × 107 PFU/ml, respectively. Serum samples have been obtained from the Department of Pediatrics, National Cheng Kung University, Tainan, Taiwan. All serum samples have been verified by the CDC, Taipei, Taiwan. Detailed information regarding the virus strains can be found in a previous study (Lee et al., 2008).
2.3.2. Immunoassay reagents
Superparamagnetic polystyrene beads (Dynabeads®M-450 Epoxy, Dynal Biotech, Norway) with a diameter of 4.5 μm are used. The magnetic microsphere is covered with surface epoxy groups and the epoxide can be used to immobilize specific antibodies onto the magnetic beads. Anti-dengue envelope (E) antibodies have been prepared in the Department of Microbiology & Immunology, National Cheng Kung University, Tainan, Taiwan. Additional information can be found in our previous work (Huang et al., 2006). The virus-bound magnetic beads have been prepared as follows: 2 μl of the anti-E antibody-conjugated magnetic beads with a concentration of 4 × 108 beads/ml and 100 μl of DV with a concentration of 108 PFU/ml are loaded into the mixing chamber, followed by incubating them for 20 min utilizing the micromixer (with an applied air pressure of 20 psi and at a driving frequency of 1.37 Hz) to form the virus-bound magnetic beads. In addition, the goat-anti-human IgM-R-PE (0.025 μg/μl with a fluorophore/protein (F/P) ratio of 4) and the goat-anti-human IgG-FITC (0.025 μg/μl with an F/P ratio of 4) are used to verify the serological detection of samples. The antibodies were purchased from Jackson Immuno Research Laboratories Inc. (West Grove, PA, USA).
3. Results and discussion
3.1. Characterization of the microfluidic system
The four-membrane-type micromixer and the micropump are used in the microfluidic system for sample incubation and transportation, respectively. The four-membrane-type micromixer is designed for gentle handling of DV-magnetic bead complexes and serum samples. A mixing efficiency index is used to quantify the mixing performance (Tseng et al., 2007). The mixing efficiency is evaluated by measuring the concentration distribution along a cross section of the mixing chamber. 1 μl of blue ink and 100 μl of deionized (DI) water are loaded into the mixing chamber to measure the efficiency of the four-membrane-type micromixer. The four-membrane-type micromixer is actuated at 1.37 Hz with an applied pressure of 20 psi. The experimental data reveal that the mixing of the DI water and the blue ink can be completed within 6 s. A normalized concentration profile across the mixing chamber is shown in Fig. 3(a), where X+ is the normalized location across the mixing chamber and C+ is the normalized concentration. Experimental data show that the mixing efficiency of the micromixer can be as high as 96% after mixing for 6 s. In order to achieve a time-phased deflection of the four membranes, a low frequency (1.37 Hz) is used to operate the four-membrane-type micromixer to generate a gradual mixing effect. The serpentine-shape air channels connecting the air chambers are used to achieve the time-phase expansion of the PDMS membranes. A high-speed, charge-coupled device (CCD, MC1311, Mikrotron, Germany) is used to verify the time-delayed expansion of each PDMS membrane. A time interval of 0.015 s is calculated between each neighboring PDMS membrane. Note that gentle mixing avoids mechanical separation of the bonding between antibodies and antigens.
Fig. 3.
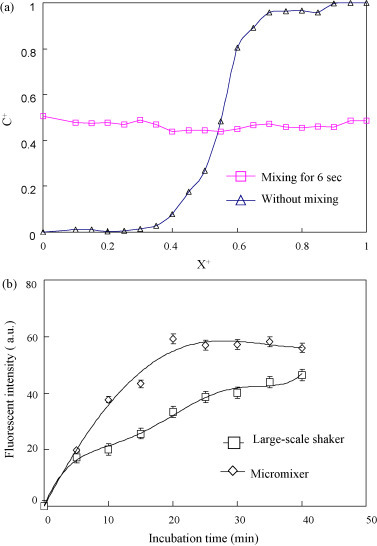
(a) The mixing efficiency of the micromixer is 96% after mixing for 6 s. (b) Comparison of the mixing effectiveness of the incubation process by utilizing the micromixer versus a large-scale shaker. A maximum fluorescent intensity can be achieved after incubation for 20 min utilizing the micromixer, while the traditional shaker may need more than 40 min. Note that X+ represents the normalized location across the mixing chamber and C+ represents the normalized concentration.
A comparison of the four-membrane-type micromixer versus a large-scale shaker (INTELLI-MIXER, ELMI Ltd., Latvia) is shown in Fig. 3(b). The mixing between the antibody-conjugated magnetic beads and the DV is the most critical process for IgM and IgG detection. Incubating 2 μl of anti-E antibody with magnetic beads (4 × 108 beads/ml) and 100 μl of DV serotype 2 (108 PFU/ml) reveals that it only takes 20 min to reach a saturated fluorescent intensity (59.2 ± 5 arbitrary unit (a.u.), n = 4) by utilizing the four-membrane-type micromixer under optimal operating conditions (an applied air pressure of 20 psi and at a driving frequency of 1.37 Hz). However, the large-scale shaker requires more than 40 min to achieve a fluorescent intensity of 46.5 a.u. Note that the sample volume of large-scale shaker and microfluidic system are the same. Since the ratio between the reaction volume of the sample and volume of the eppendorf is greater than the ratio between the reaction volume of the sample and the volume of the micromixer, the mixing efficiency for this micromixer is superior to the large-scale shaker. Therefore, each incubation process of the microfluidic system is shorter (20 min) than the large-scale shaker (40 min). Moreover, there are another two major steps in the incubation process, including the mixing of the virus-bound magnetic beads with serum samples and the mixing of the immunoglobulin-bound magnetic bead complexes with the developed antibody. The four-membrane-type micromixer provides well-mixed incubation when inflating and releasing the membranes with an air pressure of 20 psi at an operating frequency of 1.37 Hz. Under this optimal operating condition, the two other major incubation steps in the prototype microfluidic system only take 5 min and 2 min, respectively. Whereas on the second stage of detecting the human IgM or IgG to dengue virus, the anti-dengue IgM/IgG antibodies are polyclonal in nature, which can bind the dengue virus with multiple epitopes. The developing anti-human IgM or IgG R-PE or FITC conjugated antibodies are also polyclonal in nature with multiple epitope binding. The multiple binding valances with high avidity can shorten the mixing time to 5 and 2 min, respectively. Therefore, the developed magnetic bead-based microfluidic system can perform this entire protocol within 30 min. Note that the traditional method using a large-scale shaker would take more than 40 min for each incubation process. The total reaction time is only 12.5% of the time required for a traditional method using ELISA (more than 4 h).
The circular array of microcoils is used as a bio-separator and the required magnetic field is generated by a supplied DC such that the IgM/IgG-bound magnetic beads are attracted onto the surface of the microcoils. Detail information regarding the characterization of the microcoils can be found in the Supplemental Information (supplemental Fig. 3). The micropump is also characterized and the detailed experimental results can be found in supplemental Fig. 4. The maximum value of the flow rate for the one-way micropump and the two-way micropump are measured to be 214.3 μl/min and 666.7 μl/min at a driving frequency of 34.7 Hz and 40.0 Hz under an applied air pressure of 30 psi and 20 psi, respectively.
3.2. Detection of immunoglobulins
To verify the performance of the developed chip, a virus-bound magnetic bead-based sandwich immunoassay is then performed according to the protocol mentioned previously. Fig. 4 shows the experimental results for the detection of IgG and IgM by the antibody-conjugated magnetic beads. 2 μl of magnetic bead complexes (8 × 105 beads conjugated with 2 μg anti-E antibody and 107 PFU dengue virus) was employed to incubate with the IgG/IgM in the clinical serum with a volume of 100 μl, followed by mixing them with 1-μl of developing antibody (0.025 μg/μl with a F/P ratio of 4) to verify the immunological response of the infectious host. Note that data shown in Fig. 4 are the mean values calculated from five consecutive experiments (n = 5). The selectivity of the developed system is confirmed using two approaches. First, clinical patient serum samples (positive cases) and normal serum samples (negative cases) are incubated with the DV-bound beads, which can only selectively detect the anti-DV IgM and anti-DV IgG. EV71 is then used as a negative virus control and the antibody-conjugated superparamagnetic beads are incubated with the two different viruses (DV and EV71).
Fig. 4.
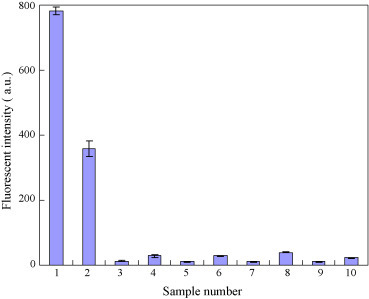
Selectivity of the virus-conjugated magnetic beads for the detection of dengue-specific IgG and IgM. Ten experiments including two positive cases (#1 and #2) and eight negative groups (from #3 to #10) are tested. Each data point is the average value from five consecutive experiments (n = 5). Note that the top/bottom values of the error bars are the maximum/minimum of the experimental data.
Ten experimental groups including two positive cases (no. 1 and no. 2) and eight negative groups (no. 3 to no. 10) are tested. The detailed conditions for each case are described as follows: (1) anti-dengue E antibody-conjugated beads incubated with DV and mixed with positive patient serum, followed by incubation with anti-human IgG-FITC (positive group for the detection of IgG); (2) anti-dengue E antibody-conjugated beads incubated with DV and mixed with positive patient serum, followed by incubation with anti-human IgM-R-PE (positive group for the detection of IgM); (3 and 4) anti-dengue E antibody-conjugated beads incubated with DV and mixed with normal serum, followed by incubation with anti-human IgG-FITC and anti-human IgM-R-PE, respectively; (5 and 6) anti-dengue E antibody-conjugated beads incubated with EV71 and mixed with positive patient serum, followed by incubation with anti-human IgG-FITC and anti-human IgM-R-PE, respectively; (7 and 8) anti-dengue E antibody-conjugated beads incubated with EV71 and mixed with normal serum, followed by incubation with anti-human IgG-FITC and anti-human IgM-R-PE, respectively; (9 and 10) anti-dengue E antibody-conjugated beads incubated with patient serum (e.g. without dengue viral particles), followed by incubation with anti-human IgG-FITC and anti-human IgM-R-PE, respectively. The signal-to-noise (S/N) ratios of the patient serum for IgG and IgM are measured to be 641.6 and 162.5, respectively. The data show the fluorescent intensity of the positive patient serum for IgG and IgM is found to be 781 a.u. and 346 a.u., respectively. In addition, the fluorescent intensity of the negative samples for IgG and IgM are found to be about 11 a.u. and 23 a.u. Therefore, the results indicate that only the serum samples have dengue IgG and IgM, which can only be specifically captured by the DV-bound magnetic beads.
To determine the sensitivity of these virus-bound magnetic beads for detecting the target immunoglobulin, a purified monoclonal anti-dengue antibody consisting of the anti-IgG antibody labelled with FITC is used as a standard to verify the detection limit of the developed microfluidic system. Several quantified amounts of anti-dengue monoclonal antibodies samples labelled with FITC (with concentrations ranging from 45 ng to 1.09 × 10−2 ng with a 2× dilution) were incubated with a fixed amount of virus-holding magnetic complexes, followed by washing and collection processes to analyze the fluorescent signals using the optical detection module. The detailed information about the concentration of monoclonal antibodies can be found in the Supplemental Information. The sandwich-like structure of the magnetic complexes is schematically shown in Fig. 5 . At first, 5 μl of magnetic bead complexes (2 × 106 beads conjugated with 5 μg anti-E antibody and 2.5 × 107 PFU dengue virus) was used to verify the detection limit of the immunoglobulins in the clinical samples, followed by mixing them with anti-dengue monoclonal antibodies samples with different concentrations to analyze the fluorescent signals emitted from the collected magnetic complexes. From the experimental results, the detectable fluorescent intensity is measured to be 15.8 ± 2 a.u. (n = 3) in sample #7 (2.19 × 10−2 ng). Note that the fluorescent intensity of the negative control is 8.92 a.u. The S/N ratio for sample #7 is measured to be 4.02. The detection limit of this integrated microfluidic system is then found to be 21 pg based on sample #7 (Fig. 5). Therefore, the “positive” and “negative” cases in the current study can only be presented as the detectable sensitivity of the developed system due to the lack of the statistical analyses. In other words, the individual carried with the IgG/IgM with the amount higher than 21 pg, which can be detected in the proposed system, would be classified as the “positive” case. Consequently, compared to a conventional ELISA, where the same pair of capture and developed monoclonal antibodies is used to detect the DV, the sensitivity is found to be 0.8 ng. Therefore, the detection limit of this integrated microfluidic system is 38 times (0.8 ng/21 pg) better. The sensitivity is significantly increased due to the fact that the magnetic beads hold the virus particle and expose the three-dimension structure of the bound viral particles, thus increasing the surface capacity of the nano-beads to hold more antibody–virus complexes. The small amount of fluids in the microfluidic system, combined with gentle mixing assures a well-mixed reaction and enhances the interaction between the antibodies and the antigen, thus resulting in a superior affinity between antibodies and antigens, as compared to that in the large-scale system. Furthermore, the detection limit of this developed system is considerably improved by using the FIA when compared with other micro systems. For example, a polymer lab-on-a-chip for a magnetic bead-based immunoassay to detect mouse IgG was reported to have a detection limit of 16.4 ng/ml (Do and Ahn, 2008). In addition, a microfluidic immunoassay capable of detection of rabbit IgG and mouse IgG was reported (Kim and Park, 2005). The sensitivity was measured to be 244 pg and 15.6 ng, respectively. Consequently, the proposed microfluidic system integrated with FIA and a sample pre-treatment module can be used as a potential platform for more sensitive biomedical diagnosis.
Fig. 5.
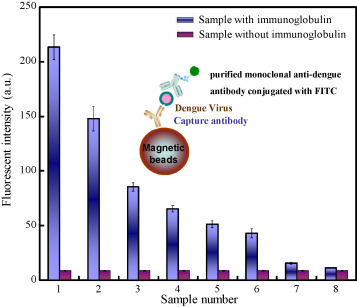
Detection limit of the microfluidic system. The detectable concentration of immunoglobulin is found to be 21 pg (sample #7) with a S/N ratio of 4.02 (n = 3). Note that the top/bottom values of the error bars are the maximum/minimum of the experimental data.
4. Conclusion
A new microfluidic system has been demonstrated for rapid, simultaneous serological analysis of IgG and IgM associated with DV infection by utilizing virus-bound magnetic beads. A microfluidic control module, a bead purification module and an optical detection module are integrated into a single chip to carry out bio-sample incubation, purification and optical analysis automatically. Experimental results indicated that the microfluidic system could reduce the total detection time to 30 min. In addition, the results also showed that IgG with a concentration as low as 21 pg can be detected successfully by this system. Therefore, this integrated system may provide a powerful platform for rapid diagnosis of DV infection. Furthermore, modification of the protocol can be also used for the rapid, automatic diagnosis of other acute infectious diseases.
Acknowledgements
The authors would like to thank the National Science Council in Taiwan for their financial support (NSC 97-2120-M-006-007). This work is also supported by the Ministry of Education, Taiwan under the NCKU Project for Promoting Academic Excellence & Developing World Class Research Centers. In addition, the authors also acknowledge assistance from Prof. Kao-Jean Huang with the biological detection methods.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bios.2009.08.020.
Appendix A. Supplementary data
References
- Andrew K.I.F., Plata E.D., Vivas C.M.E.R. Clin. Vaccine Immunol. 2006;13:1044–1051. doi: 10.1128/CVI.00105-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auroux P.A., Iossifidis D., Reyes D.R., Manz A. Anal. Chem. 2002;74:2637–2652. doi: 10.1021/ac020239t. [DOI] [PubMed] [Google Scholar]
- Aytur T., Foley J., Anwar M., Boser B., Harris E., Beatty P.R. J. Immunol. Methods. 2006;314:21–29. doi: 10.1016/j.jim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Bai Y., Koh C.G., Boreman M., Juang Y.J., Tang I.C., Lee L.J., Yang S.T. Langmuir. 2006;22:9458–9467. doi: 10.1021/la061123l. [DOI] [PubMed] [Google Scholar]
- Bini A., Centi S., Tombelli S., Mascini M.M. Anal. Bioanal. Chem. 2008;390:1077–1086. doi: 10.1007/s00216-007-1736-7. [DOI] [PubMed] [Google Scholar]
- Chanama S., Anantapreecha S., Atchareeya A.N., Areerat S.G., Kurane I., Sawanpanyalert P. J. Clin. Virol. 2004;31:185–189. doi: 10.1016/j.jcv.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Choi J.W., Chong H.A., Bhansali S., Henderson H.T. Sens. Actuators B. 2000;68:34–39. [Google Scholar]
- Do J., Ahn C.H. Lab. Chip. 2008;8:542–549. doi: 10.1039/b715569g. [DOI] [PubMed] [Google Scholar]
- Eteshola E., Balberg M. Biomed. Microdev. 2004;6(1):7–9. doi: 10.1023/b:bmmd.0000013360.65653.c2. [DOI] [PubMed] [Google Scholar]
- Fuentes M., Mateo C., Rodriguez A., Casqueiro M., Tercero J.C., Riese H.H., Lafuente R.F., Guisán Jose M. Biosens. Bioelectron. 2006;21:1574–1580. doi: 10.1016/j.bios.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Gijs M.A.M. Microfluid Nanofluid. 2004;1:22–40. [Google Scholar]
- Groen J., Koraka P., Velzing J., Copra C., Osterhausa A.D.M.E. Clin. Diagn. Lab. Immun. 2000;6:867–871. doi: 10.1128/cdli.7.6.867-871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapugoda M.D., Batra G., Abeyewickreme W., Swaminathan S., Khanna N. Clin. Vaccine Immunol. 2007;14:1505–1514. doi: 10.1128/CVI.00145-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukanes B.L., Kvam C. Biotechnology. 1993;11:60–63. doi: 10.1038/nbt0193-60. [DOI] [PubMed] [Google Scholar]
- Henry C. Anal. Chem. 1997;69:359A–361A. [Google Scholar]
- Holmes D., She J.K., Roach P.L., Morgan H. Lab. Chip. 2007;7:1048–1056. doi: 10.1039/b707507n. [DOI] [PubMed] [Google Scholar]
- Huang K.J., Yang Y.C., Lin Y.S., Huang J.H., Liu H.S., Yeh T.M., Chen S.H., Liu C.C., Lei H.Y. J. Immunol. 2006;176:2825–2832. doi: 10.4049/jimmunol.176.5.2825. [DOI] [PubMed] [Google Scholar]
- Huang Y., Mather E.L., Janice L.B., Madou M. Anal. Bioanal. Chem. 2002;372:49–65. doi: 10.1007/s00216-001-1191-9. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Park J.K. Lab. Chip. 2005;5:657–664. doi: 10.1039/b502225h. [DOI] [PubMed] [Google Scholar]
- Lee W.C., Lien K.Y., Lee G.B., Lei H.Y. Diagn. Micrbiol. Infect. Dis. 2008;60:51–58. doi: 10.1016/j.diagmicrobio.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Lien K.Y., Lin J.L., Liu C.Y., Lei H.Y., Lee G.B. Lab. Chip. 2007;7:868–875. doi: 10.1039/b700516d. [DOI] [PubMed] [Google Scholar]
- Lien K.Y., Lin W.Y., Lee Y.F., Wang C.H., Lei H.Y., Lee G.B. J. Microelectromech. Syst. 2008;17:288–301. [Google Scholar]
- Matsunaga T., Maeda Y., Yoshino T., Takeyama H., Takahashi M., Ginya H., Aasahina J., Tajima H. Anal. Chim. Acta. 2007;597:331–339. doi: 10.1016/j.aca.2007.05.065. [DOI] [PubMed] [Google Scholar]
- Reyes D.R., Iossifidis D., Auroux P.A., Manz N. Anal. Chem. 2002;74:2623–2636. doi: 10.1021/ac0202435. [DOI] [PubMed] [Google Scholar]
- Shu P.Y., Chen L.K., Chang S.F., Yueh Y.Y., Chow L., Chien L.J., Chin C., Lin T.H., Huang J.H. Clin. Diagn. Lab. Immun. 2003;10:622–630. doi: 10.1128/CDLI.10.4.622-630.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu P.Y., Chang S.F., Kuo Y.C., Yueh Y.Y., Chien L.J., Sue C.L., Lin T.H., Huang J.H. J. Clin. Microbiol. 2003;41:2408–2416. doi: 10.1128/JCM.41.6.2408-2416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng H.Y., Wang C.H., Lin W.Y., Lee G.B. Biomed. Microdevices. 2007;9:545–554. doi: 10.1007/s10544-007-9062-6. [DOI] [PubMed] [Google Scholar]
- Vignali D.A.A. J. Immunol. Methods. 2000;243:243–255. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Zaytseva N.V., Montagna R.A., Baeumner A.J. Anal. Chem. 2005;77:7520–7527. doi: 10.1021/ac0509206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


