Abstract
The common cold has intrigued physicians and the general public for centuries. It has been defined as an acute epidemic respiratory disease characterized by mild coryzal symptoms of rhinorrhea, nasal obstruction, and sneezing. The nasal discharge is usually copious and thin during the first 2 days of illness, then it generally becomes more viscous and purulent.22 The disease is self-limited. Symptoms may persist for 2 days to more than 14 days; however, the cold may abort after only 1 day. Fever, cough, sore throat, or lacrimation may or may not be present. The common cold is of itself harmless, but bacterial invasion frequently follows the initial infection. It is these secondary invaders that may produce disorders of serious consequence.
The common cold is the most frequent acute illness in the United States and throughout the industrialized world. About half the population gets at least one cold every year.5 Colds account for 40% of all time lost from jobs among employed people (23 million days of work per year) and about 30% of absenteeism from schools (26 million school days per year). Estimates vary, but the average preschool child has somewhere between 4 and 10 colds per year, and the average adult has about two to four colds per year. The actual cost of caring for patients with colds in US physicians' offices is estimated to be $1.5 billion annually.64
Seasonal patterns of infection can be identified for some of the various types of viruses that are responsible for outbreaks of the common cold. For example, available epidemiologic data suggest early fall and late spring are the most common times to find more outbreaks of rhinovirus. Respiratory syncytial virus (RSV) tends to follow winter and spring incidence, with a peak number of cases found mainly in January. Parainfluenza types 1 and 2 seem to peak during the autumn, whereas parainfluenza type 3 has an increased incidence during the late spring. Adenoviruses and coronaviruses tend to produce epidemics during the winter and spring (Fig. 1)
This article presents data concerning the cause, pathogenesis, and treatment of the common cold, as well as discussion of the available diagnostic tests and their use in formulating differential diagnoses.
HISTORICAL PERSPECTIVES
In 1904 Bishop4 described the state-of-the-art approach to colds:
Figure 1.
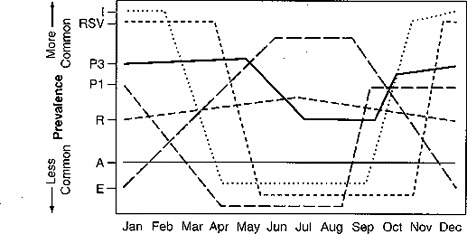
Seasonal prevalence of common cold viruses. I = influenza; RSV = respiratory syncytial virus; P3 = parainfluenza type 3; P1 = parainfluenza type 1; R = rhinovirus; A = adenovirus; E = enterovirus.
The early manifestation of cold in the head is a sensation of dryness or irritation in the nose, prompting one to snuff the air as if to dislodge some foreign substance. This gives place to itching, tickling, or stinging sensations, followed by paroxysms of sneezing, copious flow of serum and mucus from the nostrils, suffusion of the eyes, lacrimation, flushed countenance, and possibly sensations of constriction over the eyes in the frontal sinuses and headache. The patient is put to bed and the bowels relaxed if necessary. When the temperature is high it is reduced with anti-pyrin or one of its efficient substitutes, and the pain and other distressing symptoms are relieved by the coryza tablets containing a combination of morphia, atropia, and caffeine in the proportion of one-twelfth grain of morphia with one-six hundredth grain of atropia and one-sixth grain of caffeine. The morphia relieves the pain and nervous irritability, surpresses the excessive secretions and stimulates the circulation; the atropia elevates the tone of the blood vessels, quickens the pulse, decreases all the secretions except the urine, stimulates the respiratory center and counteracts the constipating effect of the morphia; and the caffeine stimulates the nervous centers and the kidneys and diminishes the tendency of the morphia to produce nausea. The sneezing and nasal discharge cease, the nostrils open up, and the pain disappears.4
By 1930 an infectious cause for the common cold was being considered. In his article “The Common Cold” that appeared in the Archives of Otolaryngology in August of 1930, Hilding31 says “colds seem definitely to fall into two groups: (1) those due to exposure and (2) those due to infection.”
In another article written in 1944, Hilding32 makes the comment “it is now well established that the etiological factor is a filterable virus. Krause determined this in 1914. It was collaborated by Foster in 1916 and again by the masterly work of Dochez and his group in the 1920's.”
Although he did not mention a viral cause in his 1930 article, the concept apparently was well established if not widely accepted. The idea that a virus was an organism that could not be seen under the microscope was deduced by passing solutions of nasal secretions through filters fine enough to stop the passage of the bacteria that were culturable and still being able to induce infection with the product of the filtration.
With the introduction of sulfa drugs in the late 1930s some clinicians claimed the drugs were effective against colds. Numerous articles6, 13, 35 44 59 about sulfa drugs in the literature of that time made such claims. The excitement about using antibiotics may have resulted from their effectiveness against pneumonia, a common disease of the time associated with high mortality.
ORGANISMS CAUSING UPPER RESPIRATORY INFECTIONS
The variety of viruses that cause upper respiratory infections, including the common cold, are shown in Table 1 . The types of common cold virus vary with population, age, and time of year. The most frequent etiologic agent is rhinovirus, which rarely causes anything more serious than the common cold. Rhinoviruses are variously estimated to cause illness 10% to 40% of the time.14 Rhinoviruses are the smallest single-stranded, RNA picornaviruses. The Rhinoviruses include more than 100 serotypes. In some studies a relatively small number of serotypes were detected in certain populations. In a study3 of nursery school children, 14 serotypes were detected in a 10-month period, but only 7 of the 14 spread to another child and only 3 spread extensively in the same 10-month period. In another study,51 there were only 8 serotypes recovered from military and university students.
Table 1.
VIRUSES REPORTED TO CAUSE COMMON COLDS
| Virus | Notes |
|
|
|
|
|---|---|---|---|---|---|
| Rhinoviruses | 52 | 27–35 | 10 | 16 | |
| Type 21 |
|
|
Study of children only | ||
| 25 | 14 |
|
|||
| 39 | |||||
| HH |
|
||||
| S | |||||
| 22 | |||||
| 51 |
|
||||
| 43 | |||||
| 55 | |||||
| 80 types |
|
||||
| Coronaviruses | 21 | ||||
| Type oc43 |
|
||||
| 229E | |||||
| Echoviruses | |||||
| Type 28 | — | 2 | 26-35 | — | 2 |
| 22 | |||||
| 20 | |||||
| Coxsackievirus | |||||
| Type A21 |
|
2 | 15 | — | 4 |
| RSV Type B |
|
8 | 10 | 10 | 8 |
| Parainfluenza | |||||
| Type 3 | — | 10 | 17 | 24 | 10 |
| 1 | 12 | ||||
| 8 | |||||
| 4 | |||||
| Adenoviruses | |||||
| Types 1, 4, & 7 |
|
5 | — | 22 | 10 |
| 2 |
|
4 | |||
| 5 | 8 | ||||
| 3 | 9 | ||||
| HH and S = older classification systems for rhinovirus subtypes; RSV = respiratory syncytial virus. | |||||
There is a subpopulation of rhinoviruses that are fastidious and can be cultured only in living organisms; they cannot be cultured reliably in tissue culture. In addition, the only animal type other than human beings that reliably can be infected with rhinoviruses is the chimpanzee. A trivial infection in humans can be lethal to chimpanzees, however.
The second most common cause of colds are the coronaviruses. Coronaviruses can be identified from about 20% of colds.66 Coronaviruses are single-stranded, RNA viruses that produce a clinical picture similar to rhinoviruses infections. In Monto and Sullivan's52 study of Tecumseh, Michigan, coronaviruses were second only to rhinoviruses and actually were related to approximately 18% of illnesses. Tecumseh, a small town in southeastern Michigan, was the site of an 11-year longitudinal study of acute respiratory infections. At any one time approximately 1000 persons were under weekly surveillance to discover the onset of symptoms and to follow with serial laboratory studies. The problem with coronaviruses is that no suitable tissue culture method has been devised for culturing; therefore, the only method of following coronavirus infection is in a volunteer population.
In the Tecumseh study influenza type A was responsible for the third largest group of respiratory infections. Influenza is not discussed in detail in this article, however. Influenza produces a pattern of respiratory illness that usually is distinctly different from the common cold.
Fourth in Monto's study was the parainfluenza virus. Parainfluenza viruses accounted for approximately 12% of the isolates in the younger population and 2% to 7.5% in the older population. Parainfluenza viruses are single-stranded, RNA paramyxoviruses in the same family with measles and mumps. There is much greater seasonality seen with parainfluenza viruses. Parainfluenza viruses are associated much more commonly with a croup or bronchiolitis pattern in very young children and are found less commonly in the mild infections in older individuals.
RSV is a medium-sized, membrane-coated, single-stranded, RNA pneumovirus with two antigenically distinct groups (types A and B). RSV, particularly type A, is associated more commonly with severe infections, including bronchiolitis and croup, especially in children. Some of each type is associated with more mild, common cold-type symptoms, however, especially in older individuals. RSV generally is found during the winter and early spring, with the peak incidence in January. RSV causes outbreaks of disease every winter. In the Tecumseh, Michigan study, RSV was found in 29% of the age group that included children younger than 4 years, but the majority of these children had lower respiratory disease. In the population older than age 5, RSV was found in approximately 4% of patients with cold symptoms. These were indistinguishable from the symptoms produced by the other viruses.52
A large proportion of the subtypes of adenovirus can be found responsible for the common cold, with the pattern of illness being mainly winter and spring seasons. Adenoviruses are double-stranded, DNA viruses with 41 recognized serotypes and more than 100 subtypes. The genetic heterogeneity of adenoviruses makes population studies difficult.
To a much lesser extent some echoviruses and coxsackieviruses have been associated with common cold symptoms that classically occur in summer.29 The coxsackieviruses are well known for causing epidemic cervical myalgia, pleurisy, hand-foot-mouth disease, and herpangina, along with respiratory infections suggestive of the common cold.
PATHOGENESIS AND ENVIRONMENTAL FACTORS
In 1933 Paul and Freese56 reported on Spitsbergen, a mountainous archipelago lying about midway between Norway and the North Pole. The population was isolated for all but 4 months of the year. During the 4 months of the summer when the oceans were not frozen numerous boats picked up coal mined in Spitsbergen and brought new workers to the area. After this brief contact with the outside world, the community became totally isolated for the remaining 8 months of the year. The incidence of common colds in the city of Longyear was one attack per person per year. Local history proved that epidemics of the common cold would never break out before the ships started to arrive each summer. In 1931 the first boat arriving in May had only one seaman who had symptoms suggestive of the incubating stage of a fresh common cold. Two men on the ship's crew had recovered from a recent attack of a cold in the week before arriving. Forty-eight hours after the arrival of the boat three cases of common cold developed in the town. There had been no common colds in the 3 months preceding the arrival of the first boat. By the fourth day after arrival there were 12 new cases of common colds seen in the dispensary and that same pattern continued through the tenth day, at which the number of cases peaked at 26 and then tapered off during the following 3 weeks.
In 1954 Gohd22 reported that the incubation period from the time of contact with infectious material until the onset of symptoms was 24 to 72 hours and that colds would persist for 3 to 7 days. He reported that the virus in some experimental colds was present not only in the nasal discharge of the patient with a fully developed cold but also in nasal washings obtained during the incubation period. The most successful means of viral spread is transmission of infectious mucous secretions to the fingers and hands and subsequently to the nose or eyes of a susceptible recipient. This finding has been reported in numerous studies during the past 20 years.63 Most respiratory viruses produce reinfection after re-exposure. Subsequent infections with the same or similar agents are generally more mild and last shorter periods.56 Risks factors for increased rates of infections and increased severity of disease include young age, low birth weight, prematurity, chronic disease, congenital immunodeficiency disorders, malnutrition, crowding, the presence of large numbers of susceptible people in the community, and exposure of the child to other infected persons. Rhinoviral infections are spread from person to person by virus-contaminated respiratory secretions. Possible routes for spread are inhalation of small airborne particles, inhalation or impaction of large particles transmitted over a short distance, and contact directly or indirectly via contaminated environmental objects. Although all three routes probably are involved to some extent, the most likely is direct contact.24 In a series of studies with experimental infections Gwaltney and Hendley26 showed the feasibility of contact transmission. Persons with high concentration of virus in their respiratory secretions exhibited a propensity for contamination of their hands and environmental objects with the virus. Rhinovirus was recovered from 40% to 90% of hands of persons with colds and from 6% to 15% of environmental objects such as doorknobs and coffee cups. Rhinoviruses were shown to exhibit good survival on many environmental surfaces for hours, and infections were transmitted readily by finger contact with a contaminated object and then back to the eye or nasal mucosa.25
The quality and quantity of the inoculation and the duration of the exposure are equally important. The family is the major site for spread of rhinoviruses in society. The pool of infected donors often is the school population. The virus then is brought into the home and spread through the family.33
Efforts to transmit the virus include experimentally infecting donors by way of inanimate objects such as coffee cups, pens, playing cards, poker chips, and plain porcelain wall tile. In one study of transmitting cold virus by kissing, only one person of 16 susceptible recipients became infected by a 1.5-minute kiss from an infected donor. It has been found that many thousand times as much virus is needed for infection than is found in the saliva or outer nares. Symptomatic donors have no detectable virus in their saliva 90% of the time.
PATHOLOGY
In 1930, Hilding31 took 125 biopsies and scrapings of the inferior turbinates of cold sufferers during various stages of their illness. Pathologic changes during the development of a common cold begin with edema of the submucosal layers. The first change to appear in the epithelium is separation of the cells by edema fluid. The surface cells loosen and begin to slough off. A necrotic zone then develops next to the surface cells. This may extend deeper into the submucosal layer. The entire epithelium finally disintegrates. Later in the disease the cells begin to regenerate, starting with those nearest the basement membrane and then gradually replacing cells layer by layer.31 Bardin et al2 reported much later on several studies of the histologic appearance of nasal mucosa infected by rhinoviruses and other respiratory viruses. They found considerably less evidence of serious epithelial disruption caused by the rhinoviruses. Influenza viruses, adenoviruses, and parainfluenza viruses tended to have the more severe cytopathic effects on the respiratory epithelium.
Epithelial cells of the nasopharynx have intercellular adhesion molecule-1 receptors by which the rhinovirus enters human cells. Nasal epithelial cells do not have these receptors.67 Rhinoviruses cause minimal destruction of nasal mucosa but more consistent damage to the higher nasopharyngeal structures, such as the mucosa of the turbinates and in the paranasal sinuses. This difference may explain partly the variation in histologic appearance of mucosa from one study group to another.
CHEMICAL MEDIATORS
The pathologic changes found after viral infection of the upper respiratory tract bear many similarities to those found with allergic rhinitis and some chemical irritations of the upper airway. One mechanism by which viral infections cause pathologic changes is release of tissue-damaging substances from leukocytes and other cells. Respiratory viruses may produce epithelial cell slough, cell death, and the resulting release of tissue-damaging enzymes (such as lysosomal enzymes). Recent evidence indicates that viruses may have the capacity to induce directly the release of proinflammatory mediators from macrophages, neutrophils, eosinophils, and mast cells and may augment superoxide production.
Histamine
Several studies have shown that the release of histamine from leukocytes (including basophils and mast cells) following immunologic and nonimmunologic stimuli is increased by infection or incubation with respiratory viruses, including influenza A, rhinovirus, parainfluenza virus, and RSV.7, 12 Recent work11 with parainfluenza viruses in brown Norway rats has shown increased release of histamine coupled to a viral-induced increase in mast cells.
Interferon
Interferon is involved in the pathogenesis of and recovery from viral infections. Interferon is produced when a host cell such as a macrophage is stimulated by viral infection or other chemical mediator. Some viruses are good interferon stimulators, such as the influenza and parainfluenza groups. Other viruses are poor stimulators of interferon, such as adenoviruses, herpes viruses, and enteroviruses. The antiviral action of interferon is mediated by time-related contact with the host cells to increase the cells' resistance to viruses. This period of interaction is necessary for the synthesis of the antiviral proteins. Those viruses that are better stimulators of interferon are generally more sensitive to its antiviral action. Studies by Ida and Hooks38 and Chonmaitree12 related interferon to T-cell availability and histamine production.
Bradykinin
Bradykinin and lysyl bradykinin are potent inflammatory mediators causing vasodilation, increased vascular permeability, and glandular secretion. They stimulate the production of pain by way of neuronal reflexes. Using rhinovirus 39 and rhinovirus HH, Naclerio54 has shown there is close association between symptoms of a cold and rises in the levels of bradykinin and lysyl bradykinin in nasal secretions. In Naclerio's study, levels of histamine and prostaglandins in nasal secretions were normal, suggesting that degranulation of mast cells had not occurred. Kinins increased tenfold over baseline in nasal secretions from rhinovirus-infected volunteers.54 These findings suggest that even though histamine and kinins produce similar nasopharyngeal symptoms, the kinins more likely are related to viral infection and histamine is not a mediator of cold symptoms.
IMMUNE RESPONSES
Antibody Reaction
Respiratory viral infections generally do not lead to marked immunologic responses. Although humoral and cellular immunity are activated, it is doubtful that humoral response is necessary for recovery from viral illnesses. There is evidence to suggest that with some viral infections the severity of the infection may be increased by low levels of antibodies, a phenomenon called antibody-dependent enhancement. It is postulated that low levels of antibodies insufficient to neutralize the virus will bind and facilitate viral entry into cells. Those infected cells then can promote spread of the viral infection to other parts of the respiratory tract in addition to allowing viral replication to take place.57
Cellular Immune Response
The role of systemic cellular immune responses in viral infections continues to be somewhat confusing. Levandowski45 showed decreases in numbers of circulating T lymphocytes in peripheral blood after rhinoviral infection and proposed that this finding might be related to recruitment of these T lymphocytes to the nasal mucosa where they performed local immunologic functions. He also showed increased numbers of lymphocytes in nasal secretions during rhinoviral infections. It is possible that the decrease in circulating T lymphocytes may be related to increased levels of interferon. Lymphocytes release cytokines that are involved intimately in the inflammatory process. The production of cytokines by viruses and T lymphocytes may be one of the major influences on airway inflammation. In elegant studies by Hsia, human peripheral blood cells were incubated with rhinovirus. The cellular immune response was evaluated by measuring the production of interleukin-2. Interleukin-2 increased by a factor of four and was related inversely to the duration of virus shedding but was unrelated to total symptom scores and to convalescent antibody titers. Interleukin-2 may play a major role in the duration of viral shedding, and further studies may implicate cytokines produced by lymphocytic activation.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of the common cold sometimes can be difficult. It is caused by a diverse group of viruses, each of which has its own collection of syndromes. Except for rhinoviruses, the common cold is only one of the patterns of illness that a particular virus can cause. The symptoms most prevalent in patients with the common cold, in order of prevalence, are throat clearing, nasal obstruction, nasal discharge, cough, and postnasal drip.15 These symptoms often are found in association with sneezing, sore throat, drowsiness, weakness, dry mouth, and dizziness. In a study by Curley et al,15 sore throat, sneezing, and drowsiness were found to have decreased significantly by day 2 or 3 of the illness. Cough, nasal discharge, postnasal drip, and throat clearing frequently still were present on day 14 after onset. In young children, RSV and parainfluenza viruses cause cough by direct invasion of the lower respiratory tract. These same viruses cause cough in older children and adults indirectly by stimulation of the upper respiratory tract. Coronaviruses produce a syndrome that is typical of the common cold but are more likely to produce myalgias, headaches, and general malaise than rhinoviruses or RSVs. Like the parainfluenza viruses, coronaviruses commonly will produce a bit more fever than the rhinoviruses and the RSVs. Adenoviruses are well known to produce a syndrome similar to the common cold but much more commonly produce conjunctivitis and laryngitis as part of the syndrome. Adenovirus types 3 and 7 cause pharyngoconjunctival fever distinguished by severe conjunctivitis and pharyngitis with minor contribution of other cold symptoms. Pharyngoconjunctival fever most often is found in summer when people are swimming in lakes and other nonchlorinated pools. The echoviruses and coxsackieviruses can produce coryza alone, an acute episode of rhinitis with minimal other symptoms. This is a summer disease also. Table 2 presents the major symptoms of common cold by the frequency with which they are associated with each virus type.
Table 2.
PERCENTAGE OF PATIENTS SHOWING INDIVIDUAL SYMPTOMS WITH EACH COMMON COLD VIRUS
| Virus | Sore Throat (%) | Cough (%) | Rhinitis (%) |
|
Fever (%) | Malaise (%) | Conjunctivitis (%) |
|---|---|---|---|---|---|---|---|
| Adenovirus | 95 | 80 | 70 | — | 70 | 60 | 15 |
| Coxsackievirus | 65 | 60 | 75 | — | 35 | 30 | 30 |
| RSV | 90 | 65 | 80 | 95 | 20 | 65 | — |
| Echovirus | 60 | 50 | 99 | 90 | 10 | 45 | — |
| Rhinovirus | 55 | 45 | 90 | 90 | 15 | 40 | 10 |
| Coronavirus | 55 | 50 | 90 | 90 | 15 | 40 | 10 |
| Parainfluenza | 75 | 50 | 65 | — | 30 | 70 | 5 |
| All viruses as a group | 70 | 80 | 95 | 95 | No data | 60 | No data |
Early in the course of any respiratory infection, the symptoms may suggest the common cold. It is only as the symptoms progress during a period of days and other features become more prominent that one can dismiss the diagnosis of common cold and be more specific about the diagnosis. In clinical practice, one rarely gathers cultures for the viral causes of the common cold, so the suspect virus usually can be presumed only from the progression of symptoms. The most common confusion in the differential diagnosis is between the common cold and patients with respiratory allergies and vasomotor rhinitis. One first must distinguish viral causes from allergic and vasomotor causes, then secondarily decide which virus.
The five factors to consider in assembling the differential diagnosis are (1) age, (2) epidemiology, (3) physical findings, (4) progression of symptoms, and (5) laboratory tests.
Age
In the age group of 5 years to 40 years, rhinoviruses are by far the most common cause of respiratory illnesses. Most are manifested as the common cold. Symptoms in patients older than age 40 may be more severe. In very young children with presumptive symptoms of common cold, the physician must be suspicious of the symptoms progressing to croup or bronchiolitis. In the group of children older than age 4 and in the group of young adults, what begins as the common cold usually needs to be distinguished only from allergies and vasomotor rhinitis. Elderly patients may die if cold symptoms are not distinguished from influenza (Table 3)
Table 3.
VIRUSES CAUSING COMMON COLD BY AGE GROUP
| Younger than Age 4 | 5-10 Years Old | Adolescents | Young Adults | Older Adults | |
|---|---|---|---|---|---|
| Most Common | Rhinovirus | Para influenza | Rhinovirus | Rhinovirus | Rhinovirus |
| RSV | Adenovirus | Influenza | Echovirus | Influenza | |
| Parainfluenza | Enterovirus | Echovirus | Influenza | Echoviruses | |
| Adenovirus | RSV | Coronaviruses | Coxsackie-virus | Coxsackie-virus | |
| Influenza | Rhinovirus | RSV | Coronaviruses | Coronaviruses | |
| Influenza | Parainfluenza | RSV | RSV | ||
| Adenovirus | Parainfluenza | Parainfluenza | |||
| Least Common | Adenovirus | Adenovirus |
Epidemiology
Improvement in the accuracy of one's differential diagnosis can be acquired by paying close attention to patterns of upper respiratory illness in the community. Knowing the time of year and paying attention to what patterns of illness are making their way through the nearby community are helpful in distinguishing the common cold from other masqueraders.
Physical Findings
Physical findings in a case of common cold should be limited to rhinorrhea (usually clear) and swelling with erythema of the mucous membranes in the nose and nasopharynx. When there is a physical finding beyond the nasopharynx, clinicians frequently can remove the common cold from the differential diagnosis. A red throat with exudate would not suggest the common cold, but rather streptococcus,
adenovirus, or infectious mononucleosis as the cause. Mild redness without exudate is compatible with colds. Any abnormal lung findings such as wheezing suggest lower respiratory involvement and exclude the common cold (see Table 2).
Progression of Symptoms
If a physician has the advantage of seeing the patient later in the course of the illness after symptoms have had a chance to develop and progress, the differential diagnosis is much easier. On day 3 of symptoms, when the patient has symptoms of only rhinitis, congestion, nasal obstruction, and low-grade cough without any physical findings in the lungs, one can be fairly secure in diagnosing the common cold. If other symptoms such as wheezing or conjunctivitis or laryngitis or muscular aching have developed since the onset of symptoms, the common cold probably can be excluded.
Laboratory Testing
The advent of the new rapid influenza type A slide test has made confirmation of influenza much more accurate. By following patterns of influenza infection worldwide in Morbidity and Mortality Weekly Reports, published by the Massachusetts Medical Society, one can be relatively secure in suspecting the A or B influenza virus when myalgias and fever are prominent symptoms. The rapid test for RSV has improved diagnostic accuracy in the very young population and should be considered in any high-risk child younger than age 2 who has symptoms compatible with the common cold early in the course of an illness. This group has a high likelihood of progressing to bronchiolitis and croup.
If the physician is compelled to discriminate viral from bacterial cause, determination of the C-reactive protein in the serum of a patient may help differentiate them.41 Many studies have shown that using a cutoff of 40 mg per liter as a screening limit can be useful in differentiating a bacterial cause from a viral cause. There is a broad band of crossover, particularly in the range of 20 to 40 mg per liter; however, when the result is much more than 40 mg per liter, nearly all the infections are bacterial. When there is less than 20 mg per liter, bacteria rarely are involved.
Studies by Korppi et al42 in Finland were fairly conclusive in showing that high serum white blood counts and granulocyte counts are evidence for a bacterial cause of respiratory infection. Korppi's group did not find any evidence of value for lymphocyte counts in distinguishing between viral and bacterial infections. It long has been suspected in an informal, unproven way, however, that a predominance of lymphocytes associated with a low white blood count is suggestive of viral cause, at least in adults. These conclusions cannot be depended on to extend to young infants and children.
None of the five factors always will individually separate viral from allergic or vasomotor causes of rhinorrhea, but considered together, the differentiation becomes more clear. The specific viral type also may be suggested.
LABORATORY STUDIES
Two reasons why in the past more laboratory testing of patients with the common cold was not done are (1) the testing was expensive and (2) it generally took a long time to get the results back. Patients were frequently well and back at work long before the results were returned. Microbial diagnosis of the common cold traditionally has depended on the detection of the virus during the course of the illness or on a rise in antibody titer from the acute to the convalescent phase blood samples. Despite advances in antigen detection and other techniques, however, culture for viral identification remains an important gold standard.
There are currently two rapid enzyme immunoassay (EIA) tests for the detection of influenza A viral antigen and RSV antigen. These tests are available in most hospital laboratories and provide 1-hour turnaround time for a test that is sensitive and specific. An EIA test detects the large quantity of viral antigens that are produced by infected epithelial cells and applies a fluorescent-tagged monoclonal antibody to identify the antigen. Monoclonal one-step time-resolved fluoroimmunoassays (TR-FIAs) have been developed for the detection of respiratory viruses and are the most sensitive and convenient solid-phase assays available. TR-FIA involves incubating the specimen for just 1 hour with the antibody that tags the viral antigen and the antibody that is labeled to tag the capture antibody.30
Nucleic acid hybridization and, more recently, sandwich hybridization with solid-phase capture are now available to identify some of the viruses that previously had been untypeable because of the many serotypes. Nucleic acid hybridization has only modest sensitivity, requiring as many as 105 molecules for identification. When polymerase chain reaction is used to increase the amount of available nucleic acid the sensitivity can be dropped to as few as 10 molecules for detection. Nucleic acid hybridization now has allowed the identification of rhinoviruses as well as other picornaviruses and enteroviruses.
There are also serologic techniques to study respiratory infections. Whereas immunoassay or hybridization can be performed only during the acute illness when nasal secretions are full of antigen, serologic assay for IgG antibodies in the serum can be carried out anytime. EIAs for IgG antibodies are the most sensitive tests and far superior to the older complement fixation test.
Standard tissue culture requires 3 to 14 days to develop cytopathic effect adequate for diagnosing a particular virus. The use of shell vial cultures59 together with sequentially applied monoclonal antibodies more recently has shortened the time period necessary for diagnosis to fewer than 2 days, sometimes as little as 1 day, and is a specific test (specificity greater than 97%; sensitivity greater than 95%). Table 4 shows the variety and utility of the available viral tests.
Table 4.
TESTS AVAILABLE BY VIRUS TYPE
| Test Type | EIA | TR-FIA |
|
Biotin EIA |
|
|
|
|---|---|---|---|---|---|---|---|
| Sensitivity | Adequate | Moderately sensitive | Very sensitive | — | Sensitivity 95% | Gold standard |
|
| Specificity | Adequate | Very specific | Very specific | — | Specificity: 97% | Very specific | |
| Time Required | 1 h | 1 h | 24-48 h | 12 h | 24-36 h | 2-4 w | 6-12 h |
|
|
|
RSV A/B |
|
|
|
|
The key to getting good results from viral testing is obtaining good, quality specimens. Nasal swabs that recover only a small quantity of viral protein or a small number of viral particles have been shown to give poor results. If nasal secretions are collected directly in sterile containers or if the nasopharynx is irrigated with a small quantity of viral culture media or even normal saline and the irrigating fluid is collected in sterile containers, however, the results are remarkably improved.
TREATMENTS
Treatment of the common cold should include rest, adequate fluid intake, and no prescriptions for antibiotics. Studies,35, 39, 43, 58 especially in the 1940s and 1950s, examined the usefulness of antibiotics as treatment for the common cold. Although antibiotics can reduce the complication rate of secondary infections and might reduce the morbidity of associated conditions, they are not appropriate for actually treating cold symptoms. The treatment and prevention of respiratory viral infections have been the focus of interest since the early 1940s when influenza vaccines were developed. In the 1960s amantadine, an anti-influenza drug, was licensed. In the intervening 55 years, however, little significant progress has been made.
Some antiviral agents have shown promise, notably WIN51711 and R61837. These two drugs probably work by binding to the receptor portion of the viral surface, thereby preventing entrance of the virus into the cells of the mucous membranes. They are administered by intranasal aerosol, beginning soon after exposure to a virus. In 1989 Al-Nakib et al1 described double-blind, placebo-controlled trials of R61837, showing it effective in suppressing colds in human volunteers. The drug does not prevent infection but binds to the capsid proteins of the rhinovirus and reduces symptoms. It must be administered six times a day by intranasal spray.
Numerous studies have shown that interferon when delivered as a nasal spray, prevents the common cold.28, 34 These interferons seem to be effective only against the rhinoviruses. Prolonged use in dosages large enough to prevent infection produced so many side effects (nasal congestion, nosebleeds) that long-term use could not be continued. Interferon inducers such as propane diamine and enviroxime showed great promise in studies of tissue culture and are apparently nontoxic but have no in vivo effect.14 One additional approach to preventing the common cold is by enhancing the individual's immunity using immunomodulators. Of these drugs, muramyl dipetide and a thioguanosine derivative have shown great promise when administered to mice and guinea pigs. Good protection against influenza was shown, but the same agents showed no effect in human volunteers.
SYMPTOMATIC TREATMENT
It long has been believed that antihistamines reduce the rhinitis related to the common cold. Many studies9, 18, 21, 37, 61 have concluded that antihistamines theoretically should not work and practically do not produce much relief. In 1988 Callow8 found very small amounts of leukotrienes, prostaglandins, and histamine in nasal secretions during colds. When Naclerio53 challenged volunteers with rhinovirus, the infected group produced large quantities of nasopharyngeal mucus with relatively low levels of histamine and albumin but very high levels of kinins, including bradykinin and lysyl bradykinin. The kinins have been shown to induce symptoms of nasal irritation and rhinorrhea, symptoms similar to those produced by histamines. Kinins are not affected by antihistamine drugs. Consequently, antikinin drugs are being developed. One such candidate, NPC567, a bradykinin antagonist, has been tested but has been unsuccessful in reducing nasal irritation and rhinorrhea.
Antihistamines and combinations of antihistamines with decongestants are the ingredients in at least 800 over-the-counter products. The majority of studies have concluded, however, that antihistamines are of marginal benefit in treating cold symptoms. There are a few notable exceptions. Curly et al15 in 1988 concluded that the cough of the common cold arose from stimuli in the upper respiratory tract and was reduced along with other symptoms by antihistamine or decongestant therapy. Smith and Feldman61 found no evidence of effectiveness of over-the-counter cold medications in preschool children but did find them useful in reducing cold symptoms in adolescents and adults. They also found that when applied by nasal spray or taken orally, most decongestants had a positive effect on reducing nasal stuffiness and some seemed to reduce rhinorrhea by way of vasoconstriction of the nasopharyngeal mucosa. Because of rebound congestion on withdrawal of decongestants (leading to addiction) and adverse effects on hypertensive patients, these drugs should be used with great caution and only for the most brief period.
Aspirin and acetaminophen suppress the serum antibody response but increase nasal symptoms and signs.23 Graham et al23 and Stanley et al62 showed that there was a trend toward longer duration of virus shedding in the aspirin and acetaminophen groups when compared with patients given placebo. Aspirin has been shown to inhibit antibody formation and any secondary antibody responses. Acetaminophen may have immune-enhancing and immune-inhibitory effects. Aspirin reduced the severity of burning eyes, sneezing, headache, chilliness, and malaise. Reduction of fever adds nothing to the therapeutic response, but over-zealous focus on fever occasionally leads to serious toxicity from aspirin and acetaminophen.
FOLK REMEDIES
There are entire books on the subject of folk remedies, many of which are directed at the common cold. There are numerous references to the use of heated vapors or local hyperthermia. Tyrrell et al65 concluded that nasal hyperthermia improved the course of the common cold and also gave immediate relief of symptoms. Ophir and Elad55 showed the effectiveness of steam inhalation in alleviating the symptoms. In 1990 Macknin et al46 found no beneficial effects of steam inhalation, however.
The reported positive effects of chicken soup are purportedly caused by the mucolytic effects of the vapors rising from a hot bowl of soup. Others have suggested that it is the hyperthermia that develops in the nose from breathing the warm vapors.
In 1984 Eby et al19 used zinc in an effort to stimulate T-cell lymphocyte responsiveness in a 3-year-old girl. When this young girl developed a cold she refused to swallow a 50-mg zinc tablet and it dissolved in her mouth instead. Within several hours her cold symptoms disappeared without any further treatment. Eby postulated that zinc ions inhibit replication of some viruses and would terminate an infection. Studies such as those by Weismann reported in the Danish Medical Bulletin in 1980 using 10% of the zinc dose failed to reproduce positive results. In 1987 Al-Nakib reported in Antimicrobial Chemotherapy good results using 23 mg zinc tablets.
The Nobel Prize-winning physicist Pauling recommended the use of vitamin C in high doses to prevent and treat the common cold. In 1975 Karlowski et al40 showed that vitamin C had at best only a minor influence on the duration and severity of colds.
COMPLICATIONS OF THE COMMON COLD
The meanest complication of the common cold is the extreme cost to society in dollars spent, days missed from school or work, and discomfort to the individual. More than $1 billion per year is spent on over-the-counter cold remedies in the United States of America.
In 1944 Hilding32 noted the connection between colds and the suppurative infections of the nose, sinuses, ears, and lower air passages that frequently followed. He believed that secondary bacterial invaders caused these complications. In 1987 Denny16 noted that the most common complication of the common cold was otitis media. He showed that the proportion of patients with colds who developed otitis media depended on many factors, including the age of the patient and the agent causing the cold. McBride et al49 studied adult volunteers' eustachian tube function before and after rhinovirus infection. They found that 2 days after rhinoviral infection tubal eustachian tube function was reduced to 50% of the usual potency and 20% of persons had significant pressure changes in the middle ear. In 1992 McIntosh et al50 showed that in acute otitis media middle ear fluid contained culturable bacteria in 50% to 70% of cases and, at the time of diagnosis, more than 90% of the patients with otitis media had cold symptoms that were suspected to be induced virally. Others have noted similar findings in the sinuses.
PREVENTION
Many elegant studies such as Gwaltney and Hendley's26 show that iodine or phenol/alcohol disinfectants can interrupt transmission of rhinoviral infections. Coxsackievirus type A 21 was shown to be transmittable from one end of a long room to the other, suggesting that coxsackievirus is carried in small, airborne droplets that can be inhaled onto the nasal mucosa. Numerous studies have shown that the use of virucidal impregnated nasal tissues can reduce the transmission of cold viruses significantly.20 The difficulty of consistently using tissues impregnated with a virucidal chemical is obvious, particularly with small children.
The traditional approach to preventing infection has been vaccination. Influenza vaccine has been used since the 1940s. The large number of serotypes of rhinovirus plus other respiratory viruses make vaccine development difficult. A major problem with developing a vaccine to the cold viruses is that durable immunity to the respiratory viral infections is difficult to produce. Parenteral immunization does not produce much nasal secretion of antibodies and provides little or no protection against a challenging virus. Even with influenza vaccine it is known that the immunity, although high initially, is lost after only 3 or 4 months.
Considerable effort has been expended in recent years to produce a form of interferon that is safe and effective. Interferon has been shown in several studies to produce good protection against infection. The high doses necessary to produce a prophylactic effect were associated with serious undesirable side effects, including nasal stuffiness, bloody mucus, and mucosal erosions.
Some viruses replicate well at low temperatures and poorly at higher temperatures. Mandell47 reasoned that because elevated body temperature is a nearly universal manifestation of infection, perhaps the increased body temperature affects phagocytosis of bacteria or viruses. Carmichael et al10 inoculated puppies with canine herpes virus. Most puppies survived the almost universally fatal infection when they were kept at elevated temperatures for several days after the inoculation. Reducing fever with antipyretics may be a counterproductive effort. Treatment with aspirin might be useful in preventing infection by cold viruses, but aspirin, acetaminophen, and other nonsteroidal anti-inflammatory agents actually can be shown to increase the duration of viral shedding.62
SUMMARY
This article studied the common cold from its historical roots to the present day research into cause and treatment. The author reviewed in detail the various viruses known to cause the symptom complex of colds. There is perhaps more to report on what not to prescribe for therapy than there is for new treatments. Research continues in the quest to find vaccines or antiviral agents to treat or prevent this most common acute ailment of humankind.
Footnotes
Address reprint requests to George L. Kirkpatrick, MD, University of South Alabama, College of Medicine, Department of Family Practice, and Community Medicine, 1504 Springhill Avenue, Mobile, AL 36604
References
- 1.Al-Nakib W., Higgins P.G., Barrow G.I. Suppression of colds in human volunteers challenged with rhinovirus by a new synthetic drug (R61837) Antimicrob Agents Chemother. 1989;33:522–525. doi: 10.1128/aac.33.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardin P.G., Johnston S.L., Pattemore P.K. Viruses as precipitants of asthma symptoms II. Physiology and mechanisms. Clin Exp Allergy. 1992;22:809–822. doi: 10.1111/j.1365-2222.1992.tb02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beem M. Rhinovirus infections in nursery school children. J Pediatr. 1969;74:818–820. doi: 10.1016/s0022-3476(69)80159-9. [DOI] [PubMed] [Google Scholar]
- 4.Bishop S.S. Diseases of the Nose, Throat and Ear and Their Accessory Cavities. FA Davis; Philadelphia: 1904. pp. 22–24. [Google Scholar]
- 5.Blueston E.C.D., Connell J.T., Doyle W.J. Symposium: Questioning the efficacy and safety of antihistamines in the treatment of upper respiratory infection. Journal of Pediatric Infectious Disease. 1988;7:215–242. [PubMed] [Google Scholar]
- 6.Bro-Kahn R.H. Sulfapyrazine: Its use in the prophylaxis of respiratory disease. Am J Med. 1946;212:170. [PubMed] [Google Scholar]
- 7.Busse W.W., Swenson C.A. Effect of influenza A virus on leukocyte histamine release. J Allergy Clin Immunol. 1983;71:382–388. doi: 10.1016/0091-6749(83)90066-0. [DOI] [PubMed] [Google Scholar]
- 8.Callow K.A. Influence of atropy on the clinical manifestations of coronavirus infections in adult volunteers. Clinical Allergy. 1988;18:119–129. doi: 10.1111/j.1365-2222.1988.tb02851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell H.J., Jr, Kantner T.R., Lilienfield L.S. Effectiveness of antihistamines in the symptomatic management of the common cold. JAMA. 1979;242:2414–2417. [PubMed] [Google Scholar]
- 10.Carmichael L.E., Barnes F.D., Percy D.H. Temperature as a factor in resistance of young puppies to canine herpesvirus. J Infect Dis. 1969;120:669–678. doi: 10.1093/infdis/120.6.669. [DOI] [PubMed] [Google Scholar]
- 11.Castleman W.I., Sorkness R.L. Virus induced airway hyper-responsiveness in brown Norway rats: Associated with increased numbers of bronchiolar mast cells;[abstract] American Review of Respiratory Disease. 1991;143:A08. [Google Scholar]
- 12.Chonmaitree T. Role of interferon in leukocyte histamine release caused by common respiratory viruses. Infect Dis Clin North Am. 1988;157:127–132. doi: 10.1093/infdis/157.1.127. [DOI] [PubMed] [Google Scholar]
- 13.Coburn A.F. Mass sulfadiazine prophylaxis of respiratory diseases in the U.S. Navy. Bul NY Acad Med. 1945;2:282. [PMC free article] [PubMed] [Google Scholar]
- 14.Couch R.B. The common cold: Control? J Infect Dis. 1984;150:167–173. doi: 10.1093/infdis/150.2.167. [DOI] [PubMed] [Google Scholar]
- 15.Curley F.J., Irwin R.S., Pratter M.R. Cough and the common cold. American Review of Respiratory Disease. 1988;138:305–311. doi: 10.1164/ajrccm/138.2.305. [DOI] [PubMed] [Google Scholar]
- 16.Denny F.W. Acute respiratory infections in children: Etiology and epidemiology. Pediatr Rev. 1987;9:135–146. doi: 10.1542/pir.9-5-135. [DOI] [PubMed] [Google Scholar]
- 17.Dowling H.F., Lefkowitz L.B. Clinical syndromes in adults caused by respiratory viruses. American Review of Respiratory Disease. 1963;88:61–72. doi: 10.1164/arrd.1963.88.3P2.61. [DOI] [PubMed] [Google Scholar]
- 18.Doyle W.J., McBride T.P., Skoner D.P. A double-blind, placebo-controlled clinical trial of the effect of chlorpheniramine on the response of the nasal airway, middle ear and eustachian tube to provocative rhinovirus challenge. Journal of Pediatric Infectious Disease. 1988;7:215–242. doi: 10.1097/00006454-198803000-00033. [DOI] [PubMed] [Google Scholar]
- 19.Eby G.A., Davis D.R., Halcomb W.W. Reduction in duration of common colds by zinc gluconate lozenges in a double-blind study. Antimicrob Agents Chemother. 1984;25:20–24. doi: 10.1128/aac.25.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farr B.M., Hendley J.O., Kaiser D.L. Two randomized controlled trials of virucidal nasal tissues in the prevention of natural upper respiratory infections. Am J Epidemiol. 1988;128:1162–1172. doi: 10.1093/oxfordjournals.aje.a115059. [DOI] [PubMed] [Google Scholar]
- 21.Gaffey M.J., Kaiser D.L., Hayden F.G. Ineffectiveness of oral terfenadine in natural colds: Evidence against histamine as a mediator of common cold symptoms. Journal of Pediatric Infectious Disease. 1988;7:215–242. doi: 10.1097/00006454-198803000-00032. [DOI] [PubMed] [Google Scholar]
- 22.Gohd R.S. The common cold. N Engl J Med. 1954;250:689–691. doi: 10.1056/NEJM195404292501704. [DOI] [PubMed] [Google Scholar]
- 23.Graham N.M., Burrell C.J., Douglas R.M. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis. 1990;162:1277–1282. doi: 10.1093/infdis/162.6.1277. [DOI] [PubMed] [Google Scholar]
- 24.Gwaltney J.M., Jr Rhinovirus. Yale J Biol Med. 1975;48:17–45. [PMC free article] [PubMed] [Google Scholar]
- 25.Gwaltney J.M., Jr Transmission of experimental rhinovirus by contaminated surfaces. Am J Epidemiol. 1982;116:828–833. doi: 10.1093/oxfordjournals.aje.a113473. [DOI] [PubMed] [Google Scholar]
- 26.Gwaltney J.M., Hendley J.O. Transmission of experimental rhinovirus infection by contaminated surfaces. Am J Epidemiol. 1982;116:828–833. doi: 10.1093/oxfordjournals.aje.a113473. [DOI] [PubMed] [Google Scholar]
- 27.Hague R.A., Burns S.E., Hargreaves F.D. Virus infections of the respiratory tract in HIV-infected children. J Infect. 1992;24:31–36. doi: 10.1016/0163-4453(92)90870-c. [DOI] [PubMed] [Google Scholar]
- 28.Hayden F.G., Kaiser D.L., Albrecht J.K. Intranasal recombinant alfa-2b interferon treatment of naturally occurring common colds. Antimicro Agents Chemother. 1988;32:224–230. doi: 10.1128/aac.32.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemming V.G. Viral respiratory diseases in children: Classification, etiology, epidemiology, and risk factors. J Pediatr. 1994;124(suppl 13):S13–S16. doi: 10.1016/S0022-3476(94)70185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hier Holzer J.C. Monoclonal Time-Resolved Fluoroimmunoassay: Sensitive Systems for the Rapid Diagnosis of Respiratory Virus Infections. Plenum Press; New York: 1990. pp. 17–45. [Google Scholar]
- 31.Hilding A. The common cold. Archives of Otolaryngology. 1930;12:133–150. [Google Scholar]
- 32.Hilding A.C. Summary of some known facts concerning the common cold. Ann Otol Rhinol Laryngol. 1944;53:444–460. [Google Scholar]
- 33.Hilding D.A. Literature review: The common cold. ENT Journal. 1994;73:639–647. [PubMed] [Google Scholar]
- 34.Ho M. Factors influencing the interferon response. Arch Intern Med. 1970;126:135–146. [PubMed] [Google Scholar]
- 35.Hodges C.R. The use of sulfadiazine as a prophylactic against respiratory disease. N Engl J Med. 1944;231:817–820. [Google Scholar]
- 37.Hutton N. Effectiveness of an antihistamine decongestant combination for young children with the common cold: A randomized controlled clinical trial. J Pediatr. 1991;118:125–130. doi: 10.1016/s0022-3476(05)81865-7. [DOI] [PubMed] [Google Scholar]
- 38.Ida A., Hooks J.J. Enhancement of IgE mediated histamine release from human basophils by viruses: Role of interferon. J Exp Med. 1977;145:892–906. doi: 10.1084/jem.145.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janeway C.A. The use of sulfonamides in the treatment of respiratory infections in children. N Engl J Med. 1943;229:201–207. [Google Scholar]
- 40.Karlowski T.R., Chalmers T.C., Frenkel L.D. Ascorbic acid for the common clod-A prophylactic and therapeutic trial. JAMA. 1975;231:1038–1042. [PubMed] [Google Scholar]
- 41.Korppi M., Kroger L. C-reactive protein in viral and bacterial respiratory infection in children. Scand J Infect Dis. 1992;25:207–213. doi: 10.3109/00365549309008486. [DOI] [PubMed] [Google Scholar]
- 42.Korppi M., Kroger L., Laitinen M. White blood cell and differential counts in acute respiratory viral and bacterial infections in children. Scand J Infect Dis. 1993;25:435–440. doi: 10.3109/00365549309008524. [DOI] [PubMed] [Google Scholar]
- 43.Lapin J.H. Prophylaxis of upper respiratory infections in children by oral penicillin and influenza virus vaccine inoculation. Arch Pediatr. 1947;64:121–130. [PubMed] [Google Scholar]
- 45.Levandowski R.A. Acute-phase decrease of T-lymphocyte subsets in rhinoviral infections. J Infect Dis. 1986;153:743–749. doi: 10.1093/infdis/153.4.743. [DOI] [PubMed] [Google Scholar]
- 46.Macknin M.L., Mathew S., Meldendorp S.V. Effect of inhaling heated vapor on symptoms of the common cold. JAMA. 1990;264:989–991. [PubMed] [Google Scholar]
- 47.Mandell G.L. Effect of temperature on phagocytosis by human polymorphonuclear neutrophils. Immunity. 1975:22–23. doi: 10.1128/iai.12.1.221-223.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAnerney J.M., Johnson S., Schoub B.D. Surveillance of respiratory viruses. S Afr Med J. 1994;84:473–476. [PubMed] [Google Scholar]
- 49.McBride T.P., Doyle W.J., Hayden F.G. Alterations of the eustachian tube, middle ear, and nose in rhinovirus infection. Arch Otolaryngol Head Neck Surg. 1989;115:1054–1059. doi: 10.1001/archotol.1989.01860330044014. [DOI] [PubMed] [Google Scholar]
- 50.McIntosh K., Halonen P., Ruuskanen O. Report of a workshop on respiratory viral infections: Epidemiology, diagnosis, treatment, and prevention. Clin Infect Dis. 1993;16:151–164. doi: 10.1093/clinids/16.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mogabgab W.J., Holmes B.J. Antigenic relationships of common rhinovirus types from disabling upper respiratory infections. Dev Biol Stand. 1975;28:400–411. [PubMed] [Google Scholar]
- 52.Monto A.S., Sullivan K.M. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993;110:145–160. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naclerio R.M., Proud D., Kagey-Sobotka A. Is histamine responsible for the symptoms of rhinovirus colds? A look at the inflammatory mediators following infection. Journal of Pediatric Infectious Disease. 1988;7:215–242. doi: 10.1097/00006454-198803000-00031. [DOI] [PubMed] [Google Scholar]
- 54.Naclerio R.N. Kinins are generated during experimental rhinoviral colds. J Infect Dis. 1987;157:133–142. doi: 10.1093/infdis/157.1.133. [DOI] [PubMed] [Google Scholar]
- 55.Ophir D., Elad Y. Effects of steam inhalation on nasal patency and nasal symptoms of patients with the common cold. Am J Otolaryngol. 1987;8:149–153. doi: 10.1016/s0196-0709(87)80037-6. [DOI] [PubMed] [Google Scholar]
- 56.Paul J.H., Freese H.L. An epidemiological and bacteriological study of the common cold in an isolated arctic community (Spitsbergen) The American Journal of Hygiene. 1933;17:517–535. [Google Scholar]
- 57.Porterfield J.A. Antibody dependent enhancement of viral infectivity. Adv Virus Res. 1986;31:335–355. doi: 10.1016/s0065-3527(08)60268-7. [DOI] [PubMed] [Google Scholar]
- 58.Rusk H.A., Ravenswaay A.C. Sulfadiazine in respiratory tract infections. JAMA. 1943;122:495–496. [Google Scholar]
- 59.Schirm J., Luijt D.S., Pastoor G.W. Rapid detection of respiratory viruses using mixtures of monoclonal antibodies on shell vial cultures. J Med Virology. 1992;38:147–151. doi: 10.1002/jmv.1890380214. [DOI] [PubMed] [Google Scholar]
- 60.Smillie W.G. Observations on epidemiology of common cold. N Engl J Med. 1940;223:651–654. [Google Scholar]
- 61.Smith M.B.H., Feldman W. Over-the-counter cold medications: A critical review of clinical trials between 1950–1991. JAMA. 1993;269:2258–2263. doi: 10.1001/jama.269.17.2258. [DOI] [PubMed] [Google Scholar]
- 62.Stanley E.D., Jackson G.G., Panusarn C. Increased virus shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231:1248–1251. [PubMed] [Google Scholar]
- 63.Sung R.Y.T., Murray H.G.S. Seasonal patterns of respiratory syncytial virus infection in Hong Kong: A preliminary report. J Infect Dis. 1987;156:527–528. doi: 10.1093/infdis/156.3.527. [DOI] [PubMed] [Google Scholar]
- 64.Thompkins R.K. The effectiveness and cost of acute respiratory illness: Medical care provided by physicians. Med Care. 1977;15:991–1103. doi: 10.1097/00005650-197712000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Tyrrell D., Barrow I., Arthur J. Local hyperthermia benefits natural and experimental common colds. BMJ. 1989;298:1280–1283. doi: 10.1136/bmj.298.6683.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tyrrell D.A.J. Hot news on the common cold. Ann Rev Microbiol. 1988;42:37. doi: 10.1146/annurev.mi.42.100188.000343. [DOI] [PubMed] [Google Scholar]
- 67.Winther B. Fireside conference 11: Common cold. Rhinol Suppl. 1992;14:228–232. [PubMed] [Google Scholar]


