Abstract
Lymphoid chemokines play an essential role in the establishment and maintenance of lymphoid tissue microarchitecture and have been implicated in the formation of tertiary (or ectopic) lymphoid tissue in chronic inflammatory conditions. Here, we review recent advances in lymphoid chemokine research in central nervous system inflammation, focusing on multiple sclerosis and the animal model experimental autoimmune encephalomyelitis. We also highlight how the study of lymphoid chemokines, particularly CXCL13, has led to the identification of intrameningeal B-cell follicles in the multiple sclerosis brain paving the way to the discovery that these abnormal structures are highly enriched in Epstein–Barr virus-infected B cells and plasma cells.
Keywords: Chemokines, Inflammation, Multiple sclerosis, Lymphoid neogenesis, Epstein–Barr virus
1. Introduction
Lymphoid or homeostatic chemokines, including CCL19, CCL21, CXCL12 and CXCL13, are constitutively expressed in lymphoid organs and regulate the migration and compartmentalization of lymphocytes and antigen presenting cells within lymphoid tissues (Yoshie et al., 1997, Zlotnik et al., 1999). CCL19 and CCL21 are ligands for CCR7 and are produced by stromal cells and dendritic cells in the T cell zone (Cyster, 1999). CCL21 is also expressed in endothelial cells in high endothelial venules (HEV) and lymphatic vessels (Cyster, 1999). Genetic and functional studies have established an important role for these chemokines in dendritic cell and T cell trafficking to T cell areas of secondary lymphoid organs (Yoshie et al., 1997, Müller et al., 2003). CXCL13 is produced by stromal cells in B cell areas (follicles), binds to CXCR5 and regulates homing of B cells and subsets of T cells (follicular B helper T cells) to lymphoid follicles (Förster et al., 1996, Legler et al., 1998, Ansel et al., 2000, Moser et al., 2002, Müller et al., 2003). CXCL12 is expressed broadly, acts as a potent chemoattractant for different types of immune cells by binding CXCR4, regulates hematopoiesis and, together with CXCL13, mediates germinal center organization in secondary lymphoid organs (Campbell et al., 2003, Allen et al., 2004). Chemokine expression in T and B cell areas of lymphoid organs is dependent on lymphotoxin (LT) α1β2 and tumor necrosis factor (TNF) signaling through the LTβR and TNFR1, respectively (Mackay and Browning, 1998, Ngo et al., 1999, Fu and Chaplin, 1999).
Over the past several years it has become evident that, in addition to their role in lymphoid tissue development and maintenance, lymphoid chemokines are also implicated in inflammation. Since the early 70's histopathologists have observed that in chronic inflammatory conditions, like autoimmunity and infections, the immune cells infiltrating the target organs may organize themselves in structures that resemble the T and B cell areas of secondary lymphoid organs, the so called tertiary or ectopic lymphoid organs (Hjelmström, 2001). The discovery that lymphoid chemokines are involved in the formation of ectopic lymphoid tissue has led to the recognition that this process (also termed lymphoid neogenesis) shares similar mechanisms with lymphoid organogenesis (Cupedo and Mebius, 2003). Following the initial observation that CXCL13 is expressed in Helicobacter pylori-induced gastric mucosa-associated lymphoid structures (Mazzucchelli et al., 1999) and the findings of elegant studies showing that transgenic expression of LT (Kratz et al., 1996, Drayton et al., 2003) or individual lymphoid chemokines (Fan et al., 2000, Luther et al., 2000, Luther et al., 2002, Chen et al., 2002b, Martin et al., 2004) in non-lymphoid tissues (pancreas, thyroid) leads to formation of lymphoid-like structures, the role of lymphoid chemokines in regulating immune cell trafficking and organization of ectopic lymphoid tissue in inflammatory conditions has received increasing attention (Aloisi and Pujol-Borrell, 2006, Drayton et al., 2006). Formation of ectopic lymphoid tissue is viewed as part of an adaptive response against infection but may also have the potential to support autoimmunity through expansion and activation of autoreactive B and T lymphocytes (reviewed in Aloisi and Pujol-Borrell, 2006). In this article, we summarize current knowledge of lymphoid chemokines in neuroinflammation, particularly in multiple sclerosis, the most common inflammatory disease of the central nervous system (CNS), and the animal model experimental autoimmune encephalomyelitis (EAE), as these are the disease conditions in which lymphoid chemokines have been mostly investigated. We also review how the study of this particular set of molecules has led us to achieve novel and unexpected insights into the elusive aetiopathogenesis of MS.
2. Expression of lymphoid chemokines in the inflamed CNS
2.1. CCL19 and CCL21
In an early study addressing the role of lymphoid chemokines in the CNS, Sergio Lira's group showed that contrary to what had been observed in the pancreas, transgenic expression of CCL21 in the brain parenchyma was not associated with lymphocyte recruitment or lymphoid neogenesis (Chen et al., 2002a), suggesting the existence of tissue-specific requirements for the biological activity of CCL21. Concomitantly, Alt et al. (2002) described functional expression of CCL19 and CCL21 in inflamed venules in the brain and spinal cord of mice with EAE and suggested that these chemokines besides regulating lymphocyte trafficking in lymphoid organs were also involved in the migration of encephalitogenic T cells in the CNS. We and others also found upregulation of CCL19, CCL21 and their common receptor CCR7 in CNS inflammatory lesions of mice with EAE, particularly during the chronic phase (Columba-Cabezas et al., 2003, Bagaeva et al., 2006, Bielecki et al., 2007).
Further support to the idea that CCR7-binding chemokines could play a role in neuroinflammation was provided by analyses of brain tissue and cerebrospinal fluid (CSF) from patients with inflammatory neurological diseases. Several studies showed increased amounts of CCL19 in the CSF of patients with MS and with infectious diseases of the CNS as compared to patients with non-inflammatory neurological disease, whereas CCL21 was less consistently detected (Pashenkov et al., 2003, Giunti et al., 2003, Krumbholz et al., 2007). Krumbholz et al. (2007) also described constitutive gene expression of CCL19 in normal brain tissue and elevation of CCL19 transcripts in MS lesions, suggesting a role for CCL19 both in normal immune surveillance of the CNS and in inflammatory conditions. In the MS CSF, CCL19 levels were found to correlate with intrathecal Ig production, but only weakly with CSF cell counts (Pashenkov et al., 2003, Krumbholz et al., 2007). The observation that CCL21 mRNA and protein are undetectable in MS inflammatory lesions and meninges (Kivisäkk et al., 2004, Serafini et al., 2004, Krumbholz et al., 2007) is consistent with the absence of markers of HEV and lymphatic vessels in the MS brain (Serafini et al., 2006; Serafini and Aloisi, unpublished data). These findings are in contrast with data obtained in the EAE model (Cannella et al., 1990, Alt et al., 2002, Columba-Cabezas et al., 2003), suggesting species differences and/or existence of disease-specific mechanisms in the acquisition of HEV-like features by CNS blood vessels. However, Kivisäkk et al. (2004) reported expression of CCL21 in the human choroid plexus in both non-inflammatory and inflammatory CNS conditions.
Interestingly, abundant expression of CCR7 was found in activated, resident microglia and myeloid dendritic cells but not in lymphocytes infiltrating MS lesions (Kivisäkk et al., 2004, Serafini et al., 2006), whereas CCR7 was consistently found on most CD4+ memory T cells obtained from the CSF of individuals without CNS inflammation and of patients with MS (Giunti et al., 2003, Kivisäkk et al., 2003). About 30% of the dendritic cells present in the CSF of MS patients were also found to express CCR7 (Kivisäkk et al., 2004). The latter findings suggest that CCL19 could be involved in the trafficking of CCR7+ T lymphocytes and antigen presenting cells through the ventricular compartment and perhaps from this compartment to CNS-draining lymph nodes. Upregulation of CCR7 on activated microglia is intriguing as it suggests regulation of microglia migratory properties or function by lymphoid chemokines (Dijkstra et al., 2006).
2.2. CXCL12
CXCL12 is a chemokine that is constitutively expressed in the CNS (Lazarini et al., 2003) and is upregulated during inflammation. Increased levels of CXCL12 have been detected in the CSF of patients with MS and other inflammatory neurological diseases (Pashenkov et al., 2003; Giunti et al., 2003, Corcione et al., 2004 Krumbholz et al., 2006). Cerebrovascular endothelia and astrocytes have been identified as major sources of CXCL12 in inflamed MS lesions (Ambrosini et al., 2005, Krumbholz et al., 2006). Because CXCR4 is expressed on immature myeloid dendritic cells, plasmacytoid dendritic cells, T cells, B cells and plasma cells (Campbell et al., 2003), it is likely that CXCL12 production in the CNS regulates intracerebral homing of different immune cell populations. Using in vitro assays, we demonstrated that human astrocytes release biologically relevant amounts of B-, T- and dendritic cell-attracting chemokines, including CXCL12 (Ambrosini et al., 2003, Ambrosini et al., 2005). It is interesting to note that some plasma cells infiltrate the parenchyma in chronic MS lesions (Serafini et al., 2004), where abundant expression of CXCL12 in activated astrocytes is also noted (Ambrosini et al., 2005). Because plasma blasts can use CXCL12 as a chemoattractant, it is likely that the CXCL12–CXCR4 axis plays a role in creating, together with B-cell survival factors like BAFF (Krumbholz et al., 2005), a survival niche for antibody-producing cells inside the brain parenchyma.
2.3. CXCL13
Because abnormal humoral immune responses characterized by intrathecal accumulation of B cells and plasma cells, B-cell clonal expansions and Ig synthesis are a prominent feature of MS and several infectious diseases of the CNS (reviewed in Uccelli et al., 2005), several recent studies have addressed the role of the B-cell attracting chemokine CXCL13 in CNS inflammation. In 2004, our group reported induction of CXCL13 mRNA in the CNS of mice with EAE and by using immunohistochemical techniques showed that CXCL13-expressing cells were present inside large B-cell aggregates in the inflamed brain meninges (Magliozzi et al., 2004). We also provided evidence that such abnormal B-cell aggregates exhibited some features of germinal center organization as they comprised proliferating B cells and a reticulum of stromal/follicular dendritic cells, which constitutively produce CXCL13 in B-cell follicles of secondary lymphoid organs. These observations led us to propose that CNS inflammation, through local production of LTα1β2 and TNF by tissue-infiltrating immune cells (Columba-Cabezas et al., 2006), induces expression of CXCL13 which in turn attracts B cells and promotes their compartmentalization with formation of germinal centers in the inflamed CNS meninges. In agreement with our findings, Bagaeva et al. (2006) found upregulation of CXCL13 mRNA and protein and infiltration of CXCR5+ cells in the spinal cord of EAE-affected mice.
The subsequent step was to find out whether CXCL13 and germinal center-like structures could be detected in the MS brain. In a first immunohistochemical analysis of post-mortem brain tissue from 6 MS cases with relapsing-remitting and progressive clinical courses, we observed the presence of B-cell aggregates containing a well developed network of CXCL13-expressing cells, numerous proliferating B cells, plasma cells and T cells in the subarachnoid space (the space lying between the pial and arachnoid membranes and filled with CSF) of 2 of 3 cases with secondary progressive MS (Serafini et al., 2004). Notably, despite prominent, perivascular accumulation of B cells and plasma cells in chronic white matter lesions, neither CXCL13 nor proliferating B cells were detected at these sites, suggesting that the meninges, but not the Virchow–Robin space surrounding CNS intraparenchymal blood vessels, provide a permissive milieu for lymphoid neogenesis. Because chemokine-producing stromal cells in lymphoid organs are of mesenchymal origin (Parsonage et al., 2005), we hypothesized that stromal cells involved in the organization of MS ectopic follicles could differentiate from precursors residing in the meninges or migrating there from the blood circulation (Serafini et al., 2004). The above findings were confirmed in a larger set of brain specimens from cases with primary and secondary progressive MS (n = 36) (Magliozzi et al., 2007). Variable numbers of intrameningeal B cell follicles, all containing CXCL13-producing stromal cells, were detected only in the brain of a proportion of cases that had developed secondary progressive MS (12 out of 29 examined), but in none of the 7 cases with primary progressive MS analyzed. In Fig. 1 , an intrameningeal B-cell follicle stained for the pan B-cell marker CD20 and for CXCL13 is shown (panels A and B, respectively).
Fig. 1.
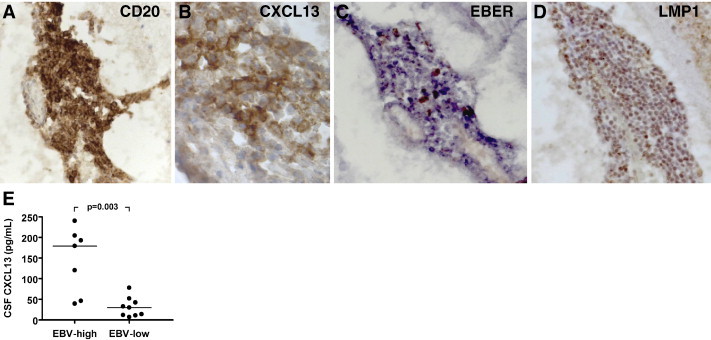
CXCL13 in MS ectopic B-cell follicles and CSF. Immunostainings for the pan B-cell marker CD20 (A) and for the lymphoid chemokine CXCL13 (B) were performed on serial brain sections from a case with secondary progressive MS, as previously described (Serafini et al., 2007). An intrameningeal B-cell follicle comprising numerous CD20+ B cells (A) aggregated around a network of CXCL13+ stromal cells/follicular dendritic cells (B) is shown. In situ hybridization for EBER (C) and immunostaining for the EBV latent protein LMP1 (D) (see Serafini et al., 2007 for staining protocols) reveal enrichment in EBV-infected cells inside the same B-cell follicle shown in A, B. Original magnifications: 250× in A, C and D, 1000× in B. Panel E shows the levels of CXCL13 protein in post-mortem CSF of MS cases that were classified as EBV-high (n = 7) and EBV-low (n = 9) based on the frequency of EBV-infected cells accumulating in the meninges and in white matter lesions (Serafini et al., 2007). The levels of CXCL13 are significantly higher in the CSF of MS cases characterized by more prominent deposits of EBV infection in brain tissue. CXCL13 levels were determined with a quantitative sandwich ELISA, in accordance with manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). Bars indicate median values. Between-group comparison was performed by Mann–Whitney test.
At variance with our findings (Serafini et al., 2004, Magliozzi et al., 2007), Krumbholz et al. (2006) reported increased CXCL13 immunoreactivity in active MS lesions, both in the perivascular cuffs and inside the parenchyma, and confirmed CXCL13 expression in MS brain tissue by using real time PCR. Since it has been reported that macrophages and dendritic cells can produce CXCL13 under inflammatory conditions (Perrier et al., 2004, Carlsen et al., 2004), discrepant immunohistochemical findings in the MS brain could be due to technical issues or differences in the inflammatory activity of the lesions analyzed. Krumbholz et al. (2006) also demonstrated increased levels of CXCL13 in the CSF of patients with MS and other inflammatory neurological diseases and found a correlation between CXCL13 concentration and presence of B cells, plasma blasts, T cells and intrathecally produced Ig in the CSF. In addition, the same group detected expression of the CXCL13 receptor CXCR5 on nearly all B cells and on a proportion of T cells isolated from the CSF of MS patients.
Taken together, the above studies link CXCL13 expression in the CNS with the degree of inflammation and the development of ectopic lymphoid structures and point to CXCL13 as an attractive target for therapeutic interventions aimed at blocking B-cell recruitment and/or lymphoid neogenesis in the inflamed CNS. In a study performed in the EAE model we found that blocking LTα1β2 signaling with the fusion protein LTβR-Ig, which acts as a decoy receptor (Browning et al., 1997), reduced EAE severity and prevented induction of CXCL13 and formation of organized B-cell follicles in the meninges, but failed to inhibit intrameningeal B-cell migration and aggregation (Columba-Cabezas et al., 2006). We interpreted these findings as evidence that CXCL13 may be more important for the development of ectopic lymphoid tissue than for B-cell recruitment in the CNS. Along the same lines, Bagaeva et al. (2006) showed that both CXCL13-deficiency and treatment with anti-CXCL13 neutralizing antibodies reduced clinical severity in EAE and proposed that CXCL13 may coordinate the localization and interactions of immune cells within the CNS rather than attracting CXCR5+ cells from the periphery. Interestingly, CXCL13 expression was not induced in the CNS of mice developing encephalomyelitis after infection with neurotropic coronavirus despite prominent accumulation of virus-specific antibody-secreting cells in the target tissue, a finding that may suggest lack of ectopic B-cell follicle formation (Tschen et al., 2006). In this model, CXCL9 and CXCL10, both of which act on plasma blasts through CXCR3, have been implicated in the recruitment of antibody-producing cells in the infected CNS (Tschen et al., 2006).
Increased expression of CXCL13 was also found in the brain and CSF of patients with Lyme neuroborreliosis, a chronic infection in which B-cell activation, plasma cell infiltration and enhanced Ig production in the CNS are a prominent feature (Narayan et al., 2005, Rupprecht et al., 2006). Moreover, CXCL13 has been detected in primary CNS lymphoma where it is produced by malignant B cells and transported on the luminal surface of cerebrovascular endothelial cells (Smith et al., 2003, Brunn et al., 2007), suggesting that this chemokine may influence tumor development and localization in the CNS.
3. Pathological relevance of ectopic lymphoid tissue in MS
Because ectopic lymphoid tissue forming in an inflamed tissue could sustain a local immune response against microbial or self antigens, the discovery of B-cell follicles in the MS brain raised a number of questions concerning the possible contribution of these structures to MS immunopathology, particularly to intrathecal Ig synthesis (Serafini et al., 2004, Aloisi and Pujol-Borrell, 2006).
Careful analysis of the cerebral cortex adjacent to ectopic B-cell follicles revealed the presence of demyelinated lesions whose extension and degree of neuronal loss and microglia activation were greater than those observed in the cerebral cortex of MS cases with lower meningeal inflammation (Magliozzi et al., 2007). Of importance, such a dramatic pattern of cortical pathology correlated with a more severe disease course in MS cases with follicles compared to those without (Magliozzi et al., 2007). These findings suggested the possibility that ectopic B-cell follicles are a source of diffusible factors, such as (auto)antibodies, pro-inflammatory cytokines and/or proteolytic enzymes, that cause injury to neural cells directly or through induction of microglia activation.
Further immunohistochemical analysis revealed that ectopic B-cell follicles in MS meninges also contain numerous CD8+ cytotoxic T cells and plasmacytoid dendritic cells, both of which play an essential role in antiviral responses. This observation prompted us to search for the presence of a viral agent inside these abnormal structures (Serafini et al., 2007). The most likely candidate appeared to be Epstein–Barr virus (EBV) for two main reasons. The first one stems from the bulk of seroepidemiological data and immunological findings supporting an association between EBV infection and MS (reviewed in Ascherio and Munger, 2007, Lünemann et al., 2007). The second one was related to the unique ability of EBV to establish a latent infection in B cells and, through expression of viral latency proteins, to drive B-cell proliferation and activation (Thorley-Lawson, 2001), these features being compatible with the presence of expanded B cell clones and Ig-producing plasma cells in the MS brain (Uccelli et al., 2005). The prediction turned out to be correct as in situ hybridization for EBV-encoded small RNAs (EBER) and immunohistochemical stainings for two EBV latency proteins (EBNA-2, LMP1) revealed that ectopic B-cell follicles were highly enriched in EBV-infected cells (Serafini et al., 2007) (see Fig. 1C, D for EBER and LMP1 stainings, respectively). EBER+ cells were identified essentially as B cells and plasma cells. Variable numbers of EBV-infected cells were also detected in the meninges and perivascular immune infiltrates of active white matter lesions in nearly 100% of the MS cases analyzed (21 out of 22 with different clinical courses), their frequency being correlated with the degree of inflammation. Expression of markers of the EBV lytic cycle appeared restricted to acute inflammatory lesions in the white matter of MS cases with progressive disease and was abundant in the highly inflamed brain of cases with very severe, fulminant MS. Of note, EBV lytic proteins were consistently found in ectopic B-cell follicles indicating that these structures represent major occult sites of viral reactivation in the MS brain (Serafini et al., 2007).
We also provided evidence that CD8+ T cells accumulating in ectopic follicles and active MS lesions show signs of activation, such as proliferation and IFN-γ production, and display cytotoxic activity toward EBV-infected B cells/plasma cells (Serafini et al., 2007). These findings led us to propose that a CNS compartmentalized and dysregulated infection with EBV drives MS-associated immunopathology through expansion and activation of latently infected B cells, periodic viral reactivation, and induction of an EBV-specific cytotoxic immune response. Most probably, the latter plays a major role in mediating CNS tissue damage.
Interestingly, analysis of post-mortem CSF revealed the presence of significantly higher levels of CXCL13 in the MS cases with ectopic follicles and a higher frequency of CNS-infiltrating EBV-infected cells as compared to the MS cases with less brain inflammation and a lower frequency of EBV-infected cells (Fig. 1E). This finding suggests that CXCL13 could play a key role in the compartmentalization of EBV-infected B cells within intrameningeal niches, allowing their expansion and organization in germinal center-like structures. Of interest, a recent study has shown that lymphoid chemokines (CCL19, CCL21, CXCL12 and CXCL13), in addition to their chemoattractant properties, are able to enhance the survival of chronic lymphocytic leukemia B cells, but not of normal B cells, in vitro (Ticchioni et al., 2007). The hypothesis that lymphoid chemokines might play a role in promoting the survival of EBV-infected B cells should therefore be tested.
4. Concluding remarks
The work summarized in this article provides a remarkable example of how the study of lymphoid chemokines as markers of chronic inflammation in a complex neurological disease like MS has helped to direct (or redirect) the focus of neuropathological analyses on a compartment, the meninges, that has received little attention during the past decades (Guseo and Jellinger, 1975, Prineas and Wright, 1978, Lassmann et al., 2007). Immune surveillance of the CNS routinely occurs across the meningeal-ventricular compartment (Engelhardt and Ransohoff, 2005) and it is therefore plausible that events taking place therein play an important role in MS pathogenesis. Because the analysis of CXCL13 in the MS brain has been the starting point for the identification of intrameningeal B-cell follicles, their characterization as foci of EBV infection, and the recognition of a link between dysregulated EBV infection and inflammatory activity in MS lesions, it is fair to state that research on lymphoid chemokines has played a key role in advancing our comprehension of this complex disease. The challenge for the future is to understand how this knowledge can be exploited for therapeutic purposes.
Acknowledgments
The authors' studies summarized in this article were supported by grants of Italian Ministry of Health and Italian Ministry of University and Research, 6th Framework Program of the European Union NeuroproMiSe LSHM-CT-2005-01863, and Italian Multiple Sclerosis Foundation. Most tissue samples were supplied by the UK Multiple Sclerosis Tissue Bank (www.ukmstissuebank.imperial.ac.uk), funded by the Multiple Sclerosis Society of Great Britain and Northern Ireland (registered charity 207495).
References
- Allen C.D., Ansel K.M., Low C., Lesley R., Tamamura H., Fujii N., Cyster J.G. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- Aloisi F., Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- Alt C., Laschinger M., Engelhardt B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2002;32:2133–2144. doi: 10.1002/1521-4141(200208)32:8<2133::AID-IMMU2133>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Ambrosini E., Columba-Cabezas S., Serafini B., Muscella A., Aloisi F. Astrocytes are the major intracerebral source of macrophage inflammatory protein-3α/CCL20 in relapsing experimental autoimmune encephalomyelitis and in vitro. Glia. 2003;41:290–300. doi: 10.1002/glia.10193. [DOI] [PubMed] [Google Scholar]
- Ambrosini E., Remoli M.E., Giacomini E., Rosicarelli B., Serafini B., Lande R., Aloisi F., Coccia E.M. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 2005;64:706–715. doi: 10.1097/01.jnen.0000173893.01929.fc. [DOI] [PubMed] [Google Scholar]
- Ansel K.M., Ngo V.N., Hyman P.L., Luther S.A., Förster R., Sedgwick J.D., Browining J.L., Lipp M., Cyster J.G. A chemokine driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Ascherio A., Munger K.L. Environmental risk factors for multiple sclerosi. Part I: the role of infection. Ann. Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- Bagaeva L.V., Rao P., Powers J.M., Segal B.M. CXC chemokine ligand 13 plays a role in experimental autoimmune encephalomyelitis. J. Immunol. 2006;176:7676–7685. doi: 10.4049/jimmunol.176.12.7676. [DOI] [PubMed] [Google Scholar]
- Bielecki B., Mazurek A., Wolinski P., Glabinski A. Expression of chemokine receptors CCR7 and CCR8 in the CNS during ChREAE. Scand. J. Immunol. 2007;66:383–392. doi: 10.1111/j.1365-3083.2007.01954.x. [DOI] [PubMed] [Google Scholar]
- Browning J.L., Sizing I.D., Lawton P., Bourdon P.R., Rennert P.D., Majeau G.R., Ambrose C.M., Hession C., Miatkowski K., Griffiths D.A., Ngam-ek A., Meier W., Benjamin C.D., Hochman P.S. Characterization of lymphotoxin-alpha beta complexes on the surface of mouse lymphocytes. J. Immunol. 1997;159:3288–3298. [PubMed] [Google Scholar]
- Brunn A., Montesinos-Rongen M., Strack A., Reifenberger G., Mawrin C., Schaller C., Deckert M. Expression pattern and cellular sources of chemokines in primary central nervous system lymphoma. Acta Neuropathol. 2007;114:271–276. doi: 10.1007/s00401-007-0258-x. [DOI] [PubMed] [Google Scholar]
- Campbell D.J., Kim C.H., Butcher E.C. Chemokines in the systemic organization of immunity. Immunol. Rev. 2003;195:58–71. doi: 10.1034/j.1600-065x.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- Cannella B., Cross A.H., Raine C.S. Upregulation and coexpression of adhesion molecules correlate with relapsing autoimmune demyelination in the central nervous system. J. Exp. Med. 1990;172:1521–1524. doi: 10.1084/jem.172.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen H.S., Baekkevold E.S., Morton H.C., Haraldsen G., Brandtzaeg P. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood. 2004;104:3021–3027. doi: 10.1182/blood-2004-02-0701. [DOI] [PubMed] [Google Scholar]
- Chen S.C., Leach M.W., Chen Y., Cai X.Y., Sullivan L., Wiekowski M., Dovey-Hartman B.J., Zlotnik A., Lira S.A. Central nervous system inflammation and neurological disease in transgenic mice expressing the CC chemokine CCL21 in oligodendrocytes. J. Immunol. 2002;168:1009–1017. doi: 10.4049/jimmunol.168.3.1009. [DOI] [PubMed] [Google Scholar]
- Chen S.C., Vassileva G., Kinsley D., Holzmann S., Manfra D., Wiekowski M.T., Romani N., Lira S.A. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J. Immunol. 2002;168:1001–1008. doi: 10.4049/jimmunol.168.3.1001. [DOI] [PubMed] [Google Scholar]
- Columba-Cabezas S., Serafini B., Ambrosini E., Aloisi F. Lymphoid chemokines CCL19 and CCL21 are expressed in the central nervous system during experimental autoimmune encephalomyelitis: implications for the maintenance of chronic neuroinflammation. Brain Pathol. 2003;13:38–51. doi: 10.1111/j.1750-3639.2003.tb00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columba-Cabezas S., Griguoli M., Rosicarelli B., Magliozzi R., Ria F., Serafini B., Aloisi F. Suppression of established experimental autoimmune encephalomyelitis and formation of meningeal lymphoid follicles by lymphotoxin β receptor-Ig fusion protein. J. Neuroimmunol. 2006;179:76–86. doi: 10.1016/j.jneuroim.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Corcione A., Casazza S., Ferretti E., Giunti D., Zappia E., Pistorio A., Gambini C., Mancardi G.L., Uccelli A., Pistoia V. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11064–11069. doi: 10.1073/pnas.0402455101. Electroni publication 2004 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupedo T., Mebius R.E. Role of chemokines in the development of secondary and tertiary lymphoid tissues. Semin. Immunol. 2003;15:243–248. doi: 10.1016/j.smim.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Cyster J.G. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- Dijkstra I.M., de Haas A.H., Brouwer N., Boddeke H.W., Biber K. Challenge with innate and protein antigens induces CCR7 expression by microglia in vitro and in vivo. Glia. 2006;54:861–872. doi: 10.1002/glia.20426. [DOI] [PubMed] [Google Scholar]
- Drayton D.L., Ying X., Lee J., Lesslauer W., Ruddle N.H. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J. Exp. Med. 2003;197:1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayton D.L., Liao S., Mounzer R.H., Ruddle N.H. Lymphoid organ development: from ontogeny to neogenesis. Nat. Immunol. 2006;7:344–453. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- Engelhardt B., Ransohoff R.M. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Fan L., Reilly C.R., Luo Y., Dorf M.E., Lo D. Cutting edge: ectopic expression of the chemokine TCA4/SLC is sufficient to trigger lymphoid neogenesis. J. Immunol. 2000;164:3955–3959. doi: 10.4049/jimmunol.164.8.3955. [DOI] [PubMed] [Google Scholar]
- Förster R., Mattis A.E., Kremmer E., Wolf E., Brem G., Lipp M. A putative, chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Fu Y.-X., Chaplin D.D. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- Giunti D., Borsellino G., Benelli R., Marchese M., Capello E., Valle M.T., Pedemonte E., Noonan D., Albini A., Bernardi G., Mancardi G.L., Battistini L., Uccelli A. Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS. J. Leukoc. Biol. 2003;73:584–590. doi: 10.1189/jlb.1202598. [DOI] [PubMed] [Google Scholar]
- Guseo A., Jellinger K. The significance of perivascular infiltrations in multiple sclerosis. J Neurol. 1975;211:51–60. doi: 10.1007/BF00312463. [DOI] [PubMed] [Google Scholar]
- Hjelmström P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J. Leukoc. Biol. 2001;69:331–339. [PubMed] [Google Scholar]
- Kivisäkk P., Mahad D.J., Callahan M.K., Sikora K., Trebst C., Tucky B., Wujek J., Ravid R., Staugaitis S.M., Lassmann H., Ransohoff R.M. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann. Neurol. 2004;55:627–638. doi: 10.1002/ana.20049. [DOI] [PubMed] [Google Scholar]
- Kivisäkk P., Mahad D.J., Callahan M.K., Trebst C., Tucky B., Wei T., Wu L., Baekkevold E.S., Lassmann H., Staugaitis S.M., Campbell J.J., Ransohoff R.M. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz A., Campos-Neto A., Hanson M.S., Ruddle N.H. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J. Exp. Med. 1996;183:1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz M., Theil D., Derfuss T., Rosenwald A., Schrader, Monorau C.-A., Kalled, Hess D.M., Serafini B., Aloisi F., Wekerle H., Hohllfeld R., Meinl E. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J. Exp. Med. 2005;201:195–200. doi: 10.1084/jem.20041674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz M., Theil D., Cepok S., Hemmer B., Kivisäkk P., Ransohoff R.M., Hofbauer M., Farina C., Derfuss T., Hartle C., Newcombe J., Hohlfeld R., Meinl E. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129:200–211. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- Krumbholz M., Theil D., Steinmeyer F., Cepok S., Hemmer B., Hofbauer M., Farina C., Derfuss T., Junker A., Arzberger T., Sinicina I., Hartle C., Newcombe J., Hohlfeld R., Meinl E. CCL19 is constitutively expressed in the CNS, up-regulated in neuroinflammation, active and also inactive multiple sclerosis lesions. J. Neuroimmunol. 2007;190:72–79. doi: 10.1016/j.jneuroim.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Lassmann H., Brück W., Lucchinetti C.F. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F., Tham T.N., Casanova P., Arenzana-Seisdedos F., Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- Legler D.F., Loetscher M., Roos R.S., Clark-Lewis I., Baggiolini M., Moser B. B-cell attracting chemokine 1, a human CXC chemokine expressed inlymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther S.A., Lopez T., Bai W., Hanahan D., Cyster J.G. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- Luther S.A., Bidgol A., Hargreaves D.C., Schmidt A., Xu Y., Paniyadi J., Matloubian M., Cyster J.G. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- Lünemann J.D., Kamradt T., Martin R., Münz C. Epstein Barr virus: environmental trigger for multiple sclerosis? J. Virol. 2007;81:6777–6784. doi: 10.1128/JVI.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F., Browning J.L. Turning off follicular dendritic cells. Nature. 1998;395:26–27. doi: 10.1038/25630. [DOI] [PubMed] [Google Scholar]
- Magliozzi R., Columba-Cabezas S., Serafini B., Aloisi F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2004;148:11–23. doi: 10.1016/j.jneuroim.2003.10.056. [DOI] [PubMed] [Google Scholar]
- Magliozzi R., Howell O., Vora A., Serafini B., Nicholas R., Popolo M., Reynolds R., Aloisi F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- Martin A.P., Coronel E.C., Sano G., Chen S.C., Vassileva G., Canasto-Chibuque C., Sedgwick J.D., Frenette P.S., Lipp M., Furtado G.C., Lira S.A. A novel model for lymphocytic infiltration of the thyroid gland generated by transgenic expression of the CC chemokine CCL21. J. Immunol. 2004;173:4791–4798. doi: 10.4049/jimmunol.173.8.4791. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli L., Blaser A., Kappeler A., Schärli P., Laissue J.A., Baggiolini M., Uguccioni M. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J. Clin. Invest. 1999;104:49–54. doi: 10.1172/JCI7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser B., Schaerli P., Laetscher P. CXCR5(+) T cells: follicular homing takes center stage in T-helper-cell responses. Trends Immunol. 2002;23:250–254. doi: 10.1016/s1471-4906(02)02218-4. [DOI] [PubMed] [Google Scholar]
- Müller G., Höpken U.E., Lipp M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol. Rev. 2003;195:117–135. doi: 10.1034/j.1600-065x.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- Narayan K., Dail D., Li L., Cadavid D., Amrute S., Fitzgerald-Bocarsly P., Pachner A.R. The nervous system as ectopic germinal center: CXCL13 and IgG in Lyme neuroborreliosis. Ann. Neurol. 2005;57:813–823. doi: 10.1002/ana.20486. [DOI] [PubMed] [Google Scholar]
- Ngo V.N., Korner H., Gunn M.D., Schmidt K.N., Riminton D.S., Cooper M.D., Browning J.L., Sedgwick J.D., Cyster J.G. Lymphotoxin-α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonage G., Filer A.D., Haworth O., Nash G.B., Rainger G.E., Salmon M., Buckley C.D. A stromal address code defined by fibroblasts. Trends Immunol. 2005;26:150–156. doi: 10.1016/j.it.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashenkov M., Söderström M., Link H. Secondary lymphoid organ chemokines are elevated in the cerebrospinal fluid during central nervous system inflammation. J. Neuroimmunol. 2003;135:154–160. doi: 10.1016/s0165-5728(02)00441-1. [DOI] [PubMed] [Google Scholar]
- Perrier P., Martinez F.O., Locati M., Bianchi G., Nebuloni M., Vago G., Bazzoni F., Sozzani S., Allavena P., Mantovani A. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. J. Immunol. 2004;172:7031–7042. doi: 10.4049/jimmunol.172.11.7031. [DOI] [PubMed] [Google Scholar]
- Prineas J.W., Wright R.G. Macrophages, lymphocytes, and plasma cells in the perivascular compartment in chronic multiple sclerosis. Lab. Invest. 1978;38:409–421. [PubMed] [Google Scholar]
- Rupprecht T.A., Koedel U., Angele B., Fingerle V., Pfister H.W. Cytokine CXCL13 — a possible early CSF marker for neuroborreliosis. Nervenarzt. 2006;77:470–473. doi: 10.1007/s00115-005-2021-7. [DOI] [PubMed] [Google Scholar]
- Serafini B., Rosicarelli B., Magliozzi R., Stigliano E., Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini B., Rosicarelli B., Magliozzi R., Stigliano E., Capello E., Mancardi G.L., Aloisi F. Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake, and interaction with proliferating T cells. J. Neuropathol. Exp. Neurol. 2006;65:124–141. doi: 10.1097/01.jnen.0000199572.96472.1c. [DOI] [PubMed] [Google Scholar]
- Serafini B., Rosicarelli B., Franciotta D., Magliozzi R., Reynolds R., Cinque P., Androni L., Trivedi P., Salvetti M., Faggioni A., Aloisi F. Dysregulated Epstein–Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 2007;204:2899–2912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.R., Braziel R.M., Paoletti S., Lipp M., Uguccioni M., Rosenbaum J.T. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101:815–821. doi: 10.1182/blood-2002-05-1576. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D.A. Epstein–Barr virus: exploiting the immune system. Nat. Rev. Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- Ticchioni M., Essafi M., Jeandel P.Y., Davi F., Cassuto J.P., Deckert M., Bernard A. Homeostatic chemokines increase survival of B-chronic lymphocytic leukemia cells through inactivation of transcription factor FOXO3a. Oncogene. 2007;26:7081–7091. doi: 10.1038/sj.onc.1210519. [DOI] [PubMed] [Google Scholar]
- Tschen S.-I., Stohlman S.A., Ramakrishna C., Hinton D.R., Atkinson R.D., Bergmann C.C. CNS viral infection diverts homing of antibody-secreting cells from lymphoid organs to the CNS. Eur. J. Immunol. 2006;36:603–612. doi: 10.1002/eji.200535123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A., Aloisi F., Pistoia V. Unveiling the enigma of the CNS as a B-cell fostering environment. Trends Immunol. 2005;26:254–259. doi: 10.1016/j.it.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Yoshie O., Imai T., Nomiyama H. Novel lymphocyte-specific CC chemokines and their receptors. J. Leukoc. Biol. 1997;62:634–644. doi: 10.1002/jlb.62.5.634. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Morales J., Hedrick J.A. Recent advances in chemokines and chemokine receptors. Crit. Rev. Immunol. 1999;19:1–47. [PubMed] [Google Scholar]


