Abstract
Glycyrrhizin is one of the main bioactive components in liquorice (Glycyrrhiza uralensis Fisch) which has recently been found to be highly active in inhibiting replication of the severe acute respiratory syndrome (SARS)-associated virus. The separation and purification of glycyrrhizin from a methanol–water (70:30 (v/v)) extract of liquorice roots was achieved using high-speed counter-current chromatography. The separation was performed at a preparative scale in a one-step separation with a two-phase solvent system composed of ethyl acetate–methanol–water (5:2:5 (v/v)). The lower phase was used as the mobile phase in the head-to-tail elution mode. The present method yielded 42.2 mg glycyrrhizin at 96.8% purity from 130 mg of the crude exact with 95.2% recovery as determined by HPLC analysis.
Keywords: Preparative chromatography, Counter-current chromatography, Pharmaceutical analysis, Plant materials, Glycyrrhiza uralensis, Glycyrrhizin
1. Introduction
Glycyrrhizin is one of the main bioactive components in the plant liquorice (Glycyrrhiza uralensis Fisch), a common ingredient of herbal medicines. Liquorice is native to Turkey, Iraq, Spain, Greece, and northern China. The plant has been used as sweetening and flavoring agent and for the treatment of a variety of health problems for thousands of years. Recently, glycyrrhizin has been found to be highly active in inhibiting replication of the severe acute respiratory syndrome (SARS)-associated virus as well as a potential therapeutic agent for chronic hepatitis and acquired immunodeficiency syndrome (AIDS) [1], [2], [3]. Moreover, glycyrrhizin has many other pharmacological uses that include anti-inflammatory, anti-ulcerous, anti-hepatotoxic, anti-oxidative and anti-allergic activities [1], [2], [3]. The chemical structure of glycyrrhizin is shown in Fig. 1 . At present, glycyrrhizin is commercially purified from liquorice by several steps such as crystallization and chromatography. However, its recovery is only between 20 and 30% and the cost is high [4]. Existing HPLC methods are not suitable for large-scale isolation of glycyrrhizin. Hence alternative methods are being sought. The support-free techniques such as high-speed counter-current chromatography (HSCCC) have gained growing importance in the separation of naturally occurring compounds [5]. HSCCC as an all-liquid chromatographic technique operates under gentle conditions and allows non-destructive isolation even of labile natural compounds [5]. Due to the absence of solid stationary phase, adsorption losses are minimized and hence excellent sample recovery is guaranteed. Furthermore, it permits introduction of the crude sample directly into the hallow column. As a result, the application of HSCCC in purification of natural products is steadily increasing, because of its superior separation abilities and excellent recovery rates [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. The aim of the present study was to investigate the preparative separation and purification of glycyrrhizin from the root of liquorice by HSCCC.
Fig. 1.
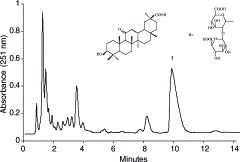
HPLC chromatogram of the crude sample extracted from the root of liquorice: 1, glycyrrhizin. HPLC conditions: reversed-phase Symmetry C18 column (150 mm×3.9 mm i.d., 5 μm); column temperature, 30 °C; mobile phase, methanol–water–acetic acid (65:34:1 (v/v)); flow rate, 1.0 ml min−1; diode array detection at 251 nm; injection volume, 20 μl.
2. Experimental
2.1. Apparatus
Preparative HSCCC instrument employed in the present study is a Model CCC-1000 high-speed counter-current chromatograph (Pharma-Tech Research, Baltimore, MD, USA). It holds a set of three multilayer coil separation columns, connected in series (diameter of tube, 1.6 mm; total volume, 342 ml). The β value of the preparative column varied from 0.47 at the internal layer to 0.73 at the external layer (β=r/R, where r is the distance from the coil to the holder shaft, and R (=7.5 cm) is the revolution radius or the distance between the holder axis and central axis of the centrifuge). The two-phase solvent system was pumped into the column using a Model Series II HPLC pump (Parma-Tech Research). A Model SPD-10 Avp UV-Vis detector (Shimadzu, Japan) was used to continuously monitor the effluent, while a Model L 120 E flat-bed recorder (Linseis, Germany) was used to record the chromatogram.
2.2. Reagents
n-Butanol, ethyl acetate, chloroform, methanol, tert-butylmethyl ether, acetic acid and hydrochloric acid were all of chromatographic grade and obtained from BDH (Poole, UK). Glycyrrhizin was obtained from Wako (Japan). The dried roots of liquorice (Glycyrrhiza uralensis Fisch) were purchased from Beijing Tong-Ren-Tang drug retail outlet in Hong Kong.
2.3. Selection and preparation of two-phase solvent system
The chromatographic process in HSCCC is based on the partition of a solute between the two liquids that are used as the mobile and stationary phases, respectively. Successful separation requires a suitable choice of the two-phase solvent system, which provides an ideal partition coefficient (K). In order to achieve efficient separation of glycyrrhizin from the crude extract, seven kinds of solvent system at different volume ratios were tested (Table 1 ). The values of the partition coefficients were determined according to [5]. In brief, about 1 ml of each phase of the pre-equilibrated two-phase solvent system was mixed with 0.5 mg of glycyrrhizin. After shaken vigorously for 10 min, the mixture was separated by centrifugation at 4000×g for 5 min. Then, an aliquot of each phase was evaporated to dryness under nitrogen. Finally, the residue was diluted with methanol and analyzed by HPLC. The K-value was expressed as the peak area of glycyrrhizin in the upper phase divided by that in the lower phase.
Table 1.
The K-values (partition coefficient) of glycyrrhizin in different two-phase solvent systems
| Solvent system | K-value |
| Ethyl acetate–methanol–water (10:3:10) | 0.51 |
| Ethyl acetate–methanol–water (5:2:5) | 0.57 |
| Ethyl acetate–n-butanol–methanol–water (1:3:1:4) | 0.58 |
| Ethyl acetate–n-butanol–methanol–water (2:2:1:4) | 0.58 |
| tert-Butylmethyl ether–methanol–water (10:3:10) | 0.22 |
| tert-Butylmethyl ether–methanol–water (5:2:5) | 0.34 |
| tert-Butylmethyl ether–n-butanol–methanol–water (1:3:1:4) | 0.77 |
| tert-Butylmethyl ether–n-butanol–methanol–water (2:2:1:4) | 0.72 |
| Chloroform–methanol–water (5:6:4) | 4.30 |
| Chloroform–methanol–water (5:5:3) | 3.30 |
| Chloroform–methanol–water (4:3:2) | 5.40 |
| n-Butanol–water (1:1) | 1.56 |
| n-Butanol–ethyl acetate–water (4:1:5) | 2.00 |
| n-Butanol–ethyl acetate–water (3:2:5) | 3.20 |
| n-Butanol–ethyl acetate–water (2:3:5) | 3.00 |
| n-Butanol–ethyl acetate–water (1:4:5) | 2.80 |
The selected solvent system was thoroughly equilibrated in a separation funnel at room temperature, and the two phases were separated shortly before use. The upper phase was used as the stationary phase, while the lower phase was used as the mobile phase in the head-to-tail elution mode.
2.4. Sample and preparation of sample solution
The roots of liquorice were dried to constant weight and then pulverized. A 23 g amount of the pulverized sample was extracted with 400 ml of 70% aqueous methanol solution under sonication for 15 min. After filtration, the residue was extracted twice with 300 ml of 70% aqueous methanol solution. All the filtrates were combined, and concentrated to 25 ml. Then, 3 mol l−1 HCl (approximately 1.5 ml) was added until no precipitate appeared. The precipitate was filtrated and evaporated to dryness by freeze drying yielding 1.90 g of crude glycyrrhizin.
The sample solution was prepared by dissolving the crude extract in the mixture solution of the lower and upper phases (1:1 (v/v)) of the two-phase solvent system used for HSCCC separation.
2.5. Separation procedure
The multilayer-coiled column was first entirely filled with the upper phase. The lower phase as the mobile phase was then pumped into the column at a flow rate of 1.5 ml min−1 in the head-to-tail elution mode, while the apparatus was rotated at 1000 rpm. After the mobile phase front emerged and equilibrium was established in the column, 10 ml of the sample solution containing 130 mg of the crude glycyrrhizin in both phases was injected. The effluent was continuously monitored at 251 nm and the chromatogram was recorded. Each peak fraction was collected according to the chromatogram and determined by HPLC. The retention of the stationary phase relative to the total column capacity was computed from the volume of the stationary phase collected from the column after the separation was completed.
2.6. HPLC analysis
The crude extract from liquorice roots, glycyrrhizin (standard) and each HSCCC peak fraction were analyzed by HPLC at 30 °C. The HPLC system was composed of two Waters HPLC 510 pumps, a Symmetry C18 column (150 mm×3.9 mm i.d., 5 μm, Waters, USA, Milford, MA, USA), a Waters 996 photodiode array detector (DAD) and a Millennium chromatography data system (Waters). The separation was performed with an isocratic elution using methanol–water–acetic acid (65:34:1 (v/v)) at a flow rate of 1.0 ml min−1 and the effluent was monitored at 251 nm with DAD. The components were confirmed from their retention times and UV-Vis spectra from 200 to 360 nm against the standards. Routine sample calculations were made by comparison of the peak area with that of the standard.
3. Results and discussion
The crude extract from liquorice roots was first analyzed by HPLC, which indicated that it contained many compounds among which glycyrrhizin represented a major component accounting for 34.1% of the total (Fig. 1).
3.1. Selection of suitable two-phase solvent system
Glycyrrhizin is one of the glycuronides. Little is known about the use of n-butanol–water for the separation of glycuronides by HSCCC [16]. Using this two-phase solvent system, glycyrrhizin could be separated from the other compounds. But, the flow rate of the mobile phase was very low that resulted in an excessively broad peak with long elution time. Furthermore, the solubility of glycyrrhizin in this solvent system was poor. Consequently such a solvent system was not appropriate for the separation of a large amount of the sample due to the poor solubility. In this paper, a series of solvent systems with a broad range of hydrophobicities (by modifying the volume ratios), such as n-butanol, chloroform and ethyl acetate systems, were tested. The K-values of glycyrrhizin in these solvent systems are summarized in Table 1. When chloroform–methanol–water (5:6:4, 5:5:3, 4:3:2 (v/v)) or n-butanol–ethyl acetate–water (4:1:5, 3:2:5, 2:3:5, 1:4:5 (v/v)) was used as the two-phase solvent system, glycyrrhizin could be separated from the other compounds. But, its K-values in the two-phase solvent systems were big with which glycyrrhizin was eluted in an excessively broad peak with long elution time. When tert-butylmethyl ether–methanol–water (10:3:10, 5:2:5 (v/v)) was used as the two-phase solvent system, the K-values were small. It was difficult to separate glycyrrhizin from other compounds. When ethyl acetate–methanol–water (10:3:10, 5:2:5 (v/v)), ethyl acetate-n-butanol–methanol–water (1:3:1:4, 2:2:1:4 (v/v)) or tert-butylmethyl ether–n-butanol–methanol–water (1:3:1:4, 2:2:1:4 (v/v)) was used as the two-phase solvent system, the K-values were suitable. Glycyrrhizin could be well separated from the other compounds using these solvent systems. Among them, the two-phase solvent system composed of ethyl acetate–methanol–water at a ratio of 5:2:5 (v/v) was found to be the best.
3.2. Separation of glycyrrhizin by HSCCC
A 130 mg quantity of the crude extract was dissolved in 10 ml of both phases. Then, the sample solution was separated and purified by HSCCC using ethyl acetate–methanol–water (5:2:5 (v/v)) as the solvent system. Although the retention of the stationary phase was only 18.1%, glycyrrhizin was well separated and the total separation time was 350 min (Fig. 2 ). Based on the HPLC analysis and the elution curve of the preparative HSCCC, peak 1 corresponded to glycyrrhizin. A total of 42.2 mg of glycyrrhizin at 96.8% purity was yielded. The glycyrrhizin recovery was as high as 95.2%.
Fig. 2.
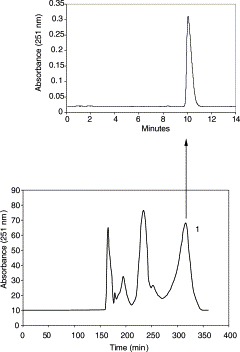
Chromatogram of the crude sample extracted from the root of liquorice by HSCCC separation, along with the HPLC chromatogram of purified glycyrrhizin from HSCCC: 1, glycyrrhizin. Conditions: column, multilayer coil of 1.6 mm i.d. PTFE tube with a total capacity of 342 ml; rotary speed, 1000 rpm; solvent system, ethyl acetate–ethanol–water (5:2:5 (v/v)); mobile phase, the lower phase; flow rate, 1.5 ml min−1; detection, 251 nm; sample size, 130 mg; injection volume, 10 ml; retention of the stationary phase, 18.1%. HPLC conditions: reversed-phase Symmetry C18 column (150 mm×3.9 mm i.d., 5 μm); column temperature, 30 °C; mobile phase, methanol–water–acetic acid (65:34:1 (v/v)); flow rate, 1.0 ml min−1; diode array detection at 251 nm; injection volume, 20 μl.
Because the stationary phase was used only once, the slow eluting compounds after glycyrrhizin were removed by pumping out the stationary phase instead of eluting them with the mobile phase. After each run, methanol was used to wash the column.
4. Conclusion
Using HSCCC, we were able to purify glycyrrhizin, one of the main bioactive components from the roots of liquorice with ethyl acetate–ethanol–water (5:2:5 (v/v)) as the two-phase solvent system. The purity of glycyrrhizin was increased from 34.1 to 96.8% after only one-step separation. The overall results indicate that HSCCC is a fast and efficient technique to prepare pure bioactive compounds from natural products.
Acknowledgements
This research was supported by the FRG (Faculty Research Grant) of Hong Kong Baptist University, the Science Faculty Seed Fund Grant of the University of Hong Kong and the Outstanding Young Researcher Award of the University of Hong Kong.
References
- 1.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Lancet. 2003;361:2045. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirabayashi K., Iwata S., Matsumoto H., Mori T., Shibata S., Bata M., Ito M., Shigeta S., Yamamoto N. Chem. Pharm. Bull. 1991;39:112. doi: 10.1248/cpb.39.112. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi J., Kashiwagi S., Noguchi A., Ikematsu H., Tsuda H., Tsuji Y., Motomura M. Clin. Ther. 1989;11:161. [PubMed] [Google Scholar]
- 4.Fen F. Chin. J. Nat. Prod. 1998;10:60. [Google Scholar]
- 5.Y. Ito, W.D. Conway (Eds.), High-Speed Countercurrent Chromatography (Chemical Analysis, vol. 132), Wiley/Interscience, New York, 1996, Chapter 1, p. 36.
- 6.Lu H.T., Jiang Y., Chen F. J. Chromatogr. A. 2003;994:37. doi: 10.1016/s0021-9673(03)00454-0. [DOI] [PubMed] [Google Scholar]
- 7.Li H.B., Chen F. J. Chromatogr. A. 2001;932:91. doi: 10.1016/s0021-9673(01)01232-8. [DOI] [PubMed] [Google Scholar]
- 8.Li H.B., Lai J.P., Jiang Y., Chen F. J. Chromatogr. A. 2002;943:235. doi: 10.1016/s0021-9673(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 9.Oka H., Harada K., Suzuki M., Ito Y. J. Chromatogr. A. 2000;903:93. doi: 10.1016/s0021-9673(00)00903-1. [DOI] [PubMed] [Google Scholar]
- 10.Yang F.Q., Zhang T.Y., Ito Y. J. Chromatogr. A. 2001;919:443. doi: 10.1016/s0021-9673(01)00846-9. [DOI] [PubMed] [Google Scholar]
- 11.Jikai L., Yue H., Henkel T., Weber K. Phytochem. Anal. 2002;13:1. doi: 10.1002/pca.608. [DOI] [PubMed] [Google Scholar]
- 12.Chen L., Han Y.S., Yang F.Q., Zhang T.Y. J. Chromatogr. A. 2001;907:343. doi: 10.1016/s0021-9673(00)00960-2. [DOI] [PubMed] [Google Scholar]
- 13.Degenhardt A., Habben S., Winterhalter P. J. Chromatogr. A. 2002;943:299. doi: 10.1016/s0021-9673(01)01467-4. [DOI] [PubMed] [Google Scholar]
- 14.Han X., Zhang T.Y., Wei Y., Cao X.L., Ito Y. J. Chromatogr. A. 2002;971:237. doi: 10.1016/s0021-9673(02)01041-5. [DOI] [PubMed] [Google Scholar]
- 15.Chen L.J., Games D.E., Jones J. J. Chromatogr. A. 2003;988:95. doi: 10.1016/s0021-9673(02)01954-4. [DOI] [PubMed] [Google Scholar]
- 16.Lu H.T., Jiang Y., Chen F. J. Chromatogr. A. 2003;1017:117. doi: 10.1016/j.chroma.2003.08.012. [DOI] [PubMed] [Google Scholar]


