Abstract
Background
A seasonal peak in asthma exacerbations in the fall has previously been reported. The association between fall exacerbations and viral respiratory tract infections (RTI) remains uncertain.
Objective
To investigate the number of fall exacerbations and the incidence of RTIs in a pediatric asthmatic population using an at-home mucus collection methodology.
Methods
This was a 16-week, multicenter, randomized, double-blind, parallel-group exploratory study. Children, 4–11 years of age with a clinical diagnosis of asthma requiring use of an inhaled corticosteroid, a morning peak expiratory flow ≥70% predicted and a history of ≥1 asthma exacerbation during the previous respiratory viral season were eligible for enrollment. Subjects were randomized (1:1) to receive fluticasone propionate/salmeterol (FP/SAL) 100/50 mcg or FP 100 mcg prior to starting school. Subjects collected mucus samples using an at-home kit when they experienced respiratory symptoms. Mucus samples obtained during symptomatic periods were analyzed for common respiratory viruses by multiplex polymerase chain reaction. The number of exacerbations requiring systemic corticosteroids was recorded.
Results
In total, 339 (FP/SAL, n = 171; FP, n = 168) subjects were randomized and included in the intent-to-treat population; 292 (86%) completed the study. Of the 537 mucus samples collected, 64% tested positive for viruses, but only 6% of positive samples were associated with an asthma exacerbation. Exacerbations were infrequent, with only 41 subjects reporting 49 exacerbations in total. Adverse events were reported in 66% of subjects.
Conclusions
In a susceptible population, the fall asthma exacerbation rates in children were low despite frequent detection of viral RTIs.
NCT01192178; GSK ID: ADA113872.
Keywords: Fluticasone propionate/salmeterol combination, Fluticasone propionate, Asthma exacerbation, Human rhinovirus, Upper respiratory tract infection
Abbreviations: AE, adverse event; C-ACT, Childhood Asthma Control Test; ER, emergency room; FP/SAL, fluticasone propionate/salmeterol combination; FP, fluticasone propionate; HRV, human rhinovirus; ICS, inhaled corticosteroids; ITT, intent-to-treat; PEF, peak expiratory flow; RT-PCR, real-time polymerase chain reaction; RSV, Respiratory Syncytial Virus; RTI, respiratory tract infection; SAE, serious adverse event; SCS, systemic corticosteroids
Highlights
-
•
Upper respiratory viral infections determined by at-home mucus collection at the time of symptoms.
-
•
The majority of subjects reported ≥1 period of upper respiratory symptoms.
-
•
Asthma exacerbations were infrequent, despite treatment and it being the fall season.
-
•
The majority of upper respiratory viral infections were not associated with exacerbations.
-
•
Additional virus, environment or host factors may influence exacerbation risk.
1. Introduction
In primary school-aged children, a frequent presentation of asthma is a sudden deterioration in health status, associated with a viral respiratory tract infection (RTI), resulting in signs of airway obstruction, cough, and wheeze [1]. A fall seasonal peak in asthma exacerbations has been reported in many studies and is widely associated with human rhinovirus (HRV) infections [2], [3], [4], [5], [6], [7], [8]. Severe asthma exacerbations, requiring the use of systemic corticosteroids (SCS), are a major cause of disease suffering and morbidity, account for significant asthma-related healthcare costs in the USA [9], [10], [11], and are the main risk factor and predictor for future exacerbations [12], [13], [14].
The pediatric asthma population, with a past history of fall exacerbations, is particularly vulnerable to respiratory infection upon return to school. This study set out to identify a pediatric population (4–11 years of age) at least 2 weeks prior to returning to their school, who were considered at risk for seasonal exacerbation based on recent prior history of exacerbation requiring treatment with SCS, emergency room (ER) visits or hospitalization. To investigate the viral etiology of exacerbations as they occurred during the fall season, this study utilized an at-home, symptom-driven, method for collection of mucus samples which were subsequently analyzed for viral content. As exacerbations are rare events, this study was not designed to demonstrate a statistically significant difference in exacerbation rates between treatment groups but rather to explore the relationships between treatment, respiratory symptoms, exacerbations, and viral etiology. Mucus sample collection was triggered by the presence of worsening asthma and/or cold symptoms. Samples were analyzed by real-time polymerase chain reaction (RT-PCR) for the presence and identification of virus. The date of mucus collection, the asthma and/or cold symptom pattern, viral etiology, and date of exacerbation treatment facilitated the investigation of the association between viral presence and asthma exacerbation.
This is the first study in an at-risk pediatric population to prospectively explore viral etiology and association with fall asthma exacerbations through symptom-driven collection of mucus samples. It differs from many previous studies in that nasal mucus samples were collected in real time from children following a report of moderate-to-severe respiratory symptoms, rather than at the time of or shortly after asthma exacerbation. As the study design allowed for more frequent mucus collection, above and beyond times of exacerbation, this study allowed for the observation of viral infection patterns throughout the fall period. The results presented here demonstrate the feasibility of at-home mucus collection in a randomized, clinical trial setting, and provide evidence that the majority of viral infections in children being treated with control medications for persistent asthma do not cause exacerbations of asthma.
2. Methods
2.1. Subject selection
Boys or girls, 4–11 years old, attending daycare or school, with a clinical diagnosis of asthma that required the use of an inhaled corticosteroid (ICS) as mono- or combination controller medication were eligible for inclusion in this study. Subjects were required to have a morning peak expiratory flow (PEF) of ≥70% of predicted at baseline and a history of ≥1 exacerbation of asthma during the previous respiratory viral season (September 2009–May 2010) that required the use of SCS, or an asthma-related urgent care visit, ER visit, or hospitalization during that period. Exclusion criteria included a history of life-threatening asthma in the previous 12 months, evidence of unstable asthma in the week prior to randomization and worsening asthma in the 4 weeks prior to screening, including use of SCS.
2.2. Study design
This was a 16-week, multicenter, randomized, double-blind, parallel-group study designed to explore the relationship between the number of exacerbations and viral RTIs during the fall viral season (September 2010–mid-December 2010; NCT01192178; GSK study identifier: ADA113872). Enrollment was planned for 316 subjects across 40 sites. Subjects were randomized 1:1 to receive fluticasone propionate/salmeterol (FP/SAL) 100/50 mcg or FP 100 mcg, both delivered twice daily via the DISKUS® device for 16 weeks. Subjects were pre-screened for eligibility and were randomized between August 2nd, 2010 and August 13th, 2010. This ensured study treatment was administered for at least 2 weeks prior to the subjects' return to school. To allow the inclusion of subjects who may have taken a “summer holiday” from their controller medications, subjects were not required to be compliant with the use of their controller medication at the time of randomization. The type of school attended (e.g. traditional calendar, year-round, etc.) and whether it was in session at the time of randomization was collected. Non-study ICS mono- or combination medication was suspended for the duration of the study. Short-acting β2-agonist medication was replaced with albuterol. Subjects were required to withhold albuterol ≥6 h pre-study visit. A placebo group was not included in this study as the population was not required to be on therapy at the time of study entry.
The study protocol, informed consent, and assent documentation were approved by either the central institutional review board or independent ethics committees at each participating site, and written informed consent was obtained from the parents of all subjects prior to any study-specific procedures. In addition, an age-appropriate assent form was used to inform study participants. The study was carried out in accordance with the Declaration of Helsinki, and in compliance with International Conference on Harmonisation Good Clinical Practice guidelines. Funding for this study was provided by GlaxoSmithKline (ADA113872).
2.3. Randomization and blinding
Subjects were assigned to treatment in accordance with a computer-generated randomization schedule through a telephone call to the Interactive Voice Response System. At subsequent visits, a telephone call was made for treatment pack numbers. Both investigators and subjects were blinded to treatment assignment.
2.4. Assessments
Caregivers of subjects kept a daily electronic diary (eDiary) to record asthma measures including morning PEF, asthma symptoms (using a 6-point scale), nighttime awakenings due to asthma, rescue medication use, missed school due to asthma, and upper respiratory symptoms (i.e. cold symptoms, using a 4-point scale). eDiary responses triggered mucus sample collection throughout the study, as described below. Information regarding allergy status (presence/absence and type) was collected by parent report only. The baseline for each of the diary measures was defined as the mean of the diary scores collected in the 7 days immediately following the Randomization Visit.
2.5. Mucus collection
To assess whether asthma exacerbations were associated with respiratory virus infection, subjects were prompted, on a symptom-driven basis, to collect mucus samples. The subjects' eDiary alerted the subjects when criteria for at-home nasal mucus sample collection were met. Subjects and parents were trained in the at-home collection and freezer storage of mucus samples using the methodology previously reported [15].
The protocol defined the symptom triggers for mucus collection which included the presence of moderate-to-severe upper respiratory symptoms (i.e. cold symptoms) or the worsening of asthma. Worsening asthma symptom triggers were as follows: asthma symptom score of ≥3 on any 2 consecutive days, increased rescue medication use from baseline, morning PEF below the determined stability limit on 2 consecutive days, nighttime awakening due to asthma on 2 consecutive nights. The morning PEF stability limit was defined as a 20% decrease from the mean morning PEF, which itself was determined from data collected during the first week in the study. The eDiary alerted the subject to collect a mucus sample only once within any given 7-day period.
The presence of moderate-to-severe upper respiratory cold-like symptoms were also collected in the daily eDiary (0 = not present, 1 = mild, clearly present; 2 = moderately severe, uncomfortable; 3 = severe, interfering with sleep or activity). The onset date of a cold was defined retrospectively by the first day of report of moderate-to-severe upper respiratory symptoms as reported in the eDiary (i.e., score of 2 or 3 respectively). A “cold period” was defined as the cold onset day and the following 6 days. Cold onset days were separated by at least 7 days and cold periods did not overlap.
2.6. Viral analysis
At the end of the study, samples were shipped to the University of Wisconsin for viral analysis. Viral analysis was conducted as previously reported using multiplex RT-PCR [15]. A panel of 19 commonly reported respiratory viruses was assessed (Adenovirus B, C and E, Bocavirus, Coronavirus 229E, NL63 and OC43; Enterovirus; Influenzavirus A and B, Metapneumovirus, Parainfluenzavirus 1, 2, 3, 4a, and 4b; Rhinovirus; Respiratory Syncytial Virus [RSV] A and B).
2.7. Efficacy assessments
The primary measure of efficacy was the number of asthma exacerbations that occurred during the 2010 fall viral study period (August 30th, 2010–December 16th, 2010). An exacerbation was defined per the protocol as a deterioration of asthma that required the use of outpatient SCS (tablets, suspensions, or injection) or an urgent care visit, hospitalization, or ER visit. Secondary efficacy measures included assessments of symptom severity and duration, and incidence of exacerbations associated with moderate-to-severe upper respiratory infections.
2.8. Health outcomes
Controller status was assessed using the Childhood Asthma Control Test (C-ACT) during clinical visits at baseline and Weeks 4, 8, 12, and 16. C-ACT is a 7-item validated questionnaire completed by subjects (questions 1–4) and the subject's parent/legal guardian (questions 5–7) [16]. The investigator calculated the total score and transcribed it into the Clinical Trial Report. Scores of <20 indicate that asthma may not be well controlled.
2.9. Safety
Safety was assessed by adverse event (AE) and serious AE (SAE) monitoring throughout the study and 7 days after the last clinical visit by a follow-up phone call.
2.10. Data analysis and statistical methods
The study was designed to be exploratory; therefore, hypothesis tests were considered to be descriptive rather that inferential. Consequently, no multiplicity adjustments to the results of the hypothesis tests were made. Descriptive statistical analysis used SAS version 9.1 software in a UNIX reporting environment. While there were no formal power or sample size calculations, a sample size of 150 subjects per treatment was chosen to support the nature of asthma exacerbations as infrequently occurring events. The analysis population was the intent-to-treat (ITT) population, which included all subjects randomized to study drug.
Secondary measures of efficacy were summarized by treatment group and included severity of asthma symptoms associated with positive viral sample and exacerbations associated with positive viral sample. Exacerbations associated with positive viral sample were analyzed in the same manner as the primary efficacy measure.
3. Results
3.1. Study subjects
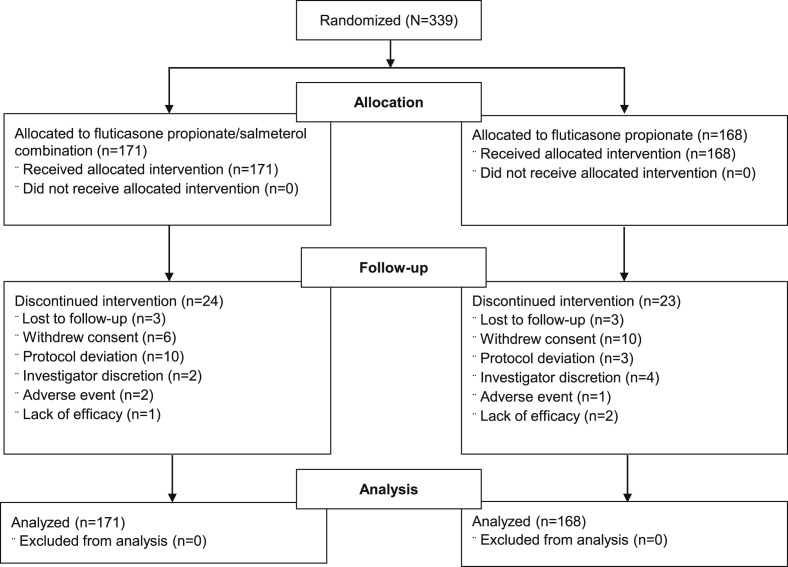
A total of 339 subjects (FP/SAL, n = 171; FP, n = 168) from 39 centers in the United States were randomized and included in the ITT population. Of these, 292 (86%) completed the full 16-week study (FP/SAL, n = 147; FP, n = 145). Patient disposition and the reasons for subject withdrawal are shown in Fig. 1 . The mean age of the study population was 7.4 years and mean duration of asthma was 4.6 years. The majority of the study population attended traditional calendar school and had not yet returned back to school at the time of randomization (Table 1 ).
Fig. 1.
Summary of subject disposition and flow (ITT population).
Table 1.
Demographics and other baseline characteristics (ITT population).
| Total (N = 339) | |
|---|---|
| Mean age, years (SD) | 7.4 (2.09) |
| Sex, male, (%) | 220 (65) |
| Race, % | |
| Caucasian | 256 (76) |
| African American | 60 (18) |
| Asian | 14 (4) |
| Other | 9 (3) |
| Mean duration of asthma, years (SD) | 4.6 (2.52) |
| Allergy history | |
| No allergies, n (%) | 135 (40) |
| One or more allergies | 203 (60) |
| Number of subjects reporting exacerbations in the prior year, n (%)a | |
| Requiring hospitalization | 25 (7) |
| Requiring oral corticosteriods | 331 (98) |
| Type of school | |
| Traditional, not started at randomization | 309 (91) |
| Traditional, started when randomized | 14 (4) |
| Daycare | 11 (3) |
| Year-round, not started at randomization | 1 (<1) |
| Year-round, started when randomized | 4 (1) |
An exacerbation was defined per the protocol as a deterioration of asthma that required the use of outpatient SCS (tablets, suspensions, or injection) or an urgent care visit, hospitalization, or emergency room visit; ITT, intent-to-treat; SCS, systemic corticosteroid; SD, standard deviation.
3.2. Collection of mucus samples and identification of respiratory viruses
In total, 429 cold period events, defined by moderate-to-severe asthma symptoms for 6 days, were reported and 75 subjects reported having at least one cold period event. The most common reason for eDiary alert for mucus sample collection was a decrease in PEF below the stability limit (523 events) and rescue medication use (112 events) (Table 2 ).
Table 2.
Cold and worsening asthma triggers (ITT population).
| Total (N = 339) | |
|---|---|
| Total number of cold period events | 429 |
| Subjects with ≥1 reported cold perioda | 75 |
| Subjects with 2 reported cold periods | 61 |
| Subjects with ≥3 or more reported cold periods | 56 |
| Number of asthma triggersb | |
| Asthma Symptoms | 30 |
| PEF below stability limit | 523 |
| Nighttime awakenings | 41 |
| Rescue medication use | 112 |
| Multiple asthma triggers | 46 |
A “cold period” was defined as the cold onset day (day of report of moderate or severe symptoms) and the following 6 days.
Number of times eDiary alerted subject to collect mucus sample because of increased asthma symptoms; ITT, intent-to-treat.
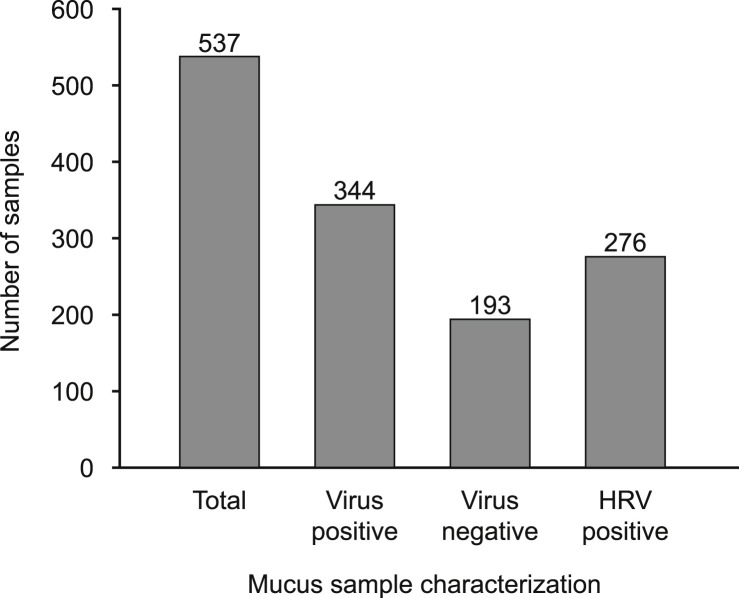
Of the 339 study subjects, 227 (67%) collected at least one mucus sample during the study. A total of 537 mucus samples were collected and analyzed. The number of cold period events, eDiary alerts and mucus samples collected were similar between treatment groups (data not shown). In total, 64% (344) of all mucus samples tested positive for at least one respiratory virus. Of the total number of virus positive samples, 80% (276) were HRV positive (Fig. 2 ). Additional HRV typing was not conducted. The next most frequently detected viruses were Parainfluenzavirus 2 and Coronavirus (NL63 and OC43). The majority (93%) of the virus-positive samples tested positive for the presence of only 1 virus; 24 samples (7%) tested positive for more than 1 virus (Table 3 ).
Fig. 2.
Summary of mucus sample characterization (ITT population): Mucus samples were collected as described in methods and analyzed by PCR for virus content. The cumulative total number of samples collected depicted by the number collected per treatment group and further characterized by the number of virus positive samples and HRV positive samples collected per treatment group. HRV, human rhinovirus; ITT, intent-to-treat.
Table 3.
Virology from symptom-drivena mucus sample collection (ITT population).
| Total (n = 339) | |
|---|---|
| Subjects with ≥1 sample, n | 227 |
| Samples collected, n | 537 |
| Samples containing virus, n | 344 |
| Virus type, n (%)b | |
| Rhinovirus | 276 (80) |
| Parainfluenzavirus 2 | 29 (8) |
| Coronavirus NL63 | 14 (4) |
| Coronavirus OC43 | 15 (4) |
| Otherc | 35 (10) |
| Viruses per sample n (%) | |
| Positive for 1 virus | 320 (93) |
| Positive for 2 viruses | 23 (7) |
| Positive for 3 viruses | 1 (<1) |
Samples can contain ≥1 virus.
Upon presence of either moderate to severe cold symptoms or worsening asthma symptoms.
Percentage of the total number of virus positive samples.
Enterovirus, Bocavirus, Adenovirus C, Parainfluenza 4b, Parainfluenza 1, Influenza B; ITT, intent-to-treat.
3.3. Exacerbations: virus and non-virus associated
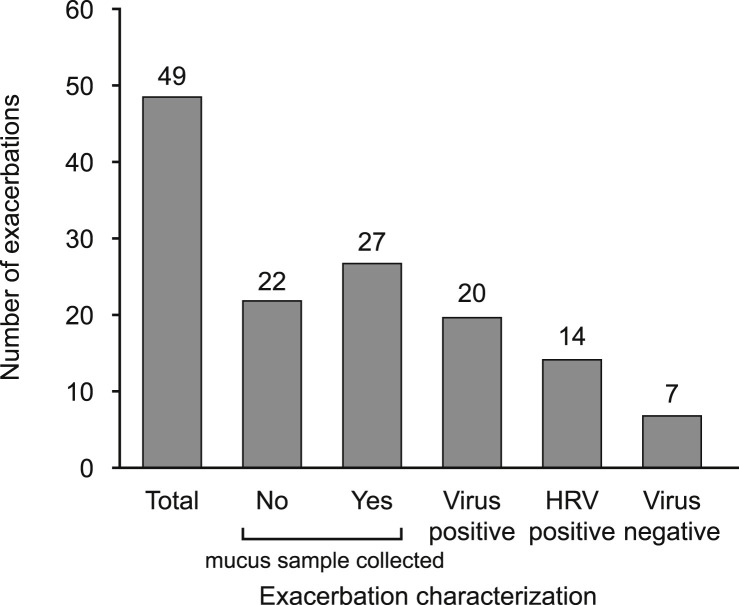
During the study, 41 (12%) subjects experienced at least one exacerbation and a total of 49 exacerbations were reported. Mucus samples were collected within 7 days of the exacerbation (i.e. 5 days prior to exacerbation onset and 2 days post) for 27 exacerbations. Of these samples, 20 (74%) were virus positive and 14 (70%) of these were rhinovirus positive. Therefore, of the 344 virus positive mucus samples collected, only 6% were associated with an asthma exacerbation. There were 22 exacerbations for which no mucus sample was collected within the 7-day period surrounding the exacerbation (Fig. 3 ).
Fig. 3.
Summary of the number of exacerbations (ITT population): Exacerbations characterized by the number with or without a mucus sample collected within the 5 days prior or 2 days following the exacerbation. The exacerbations with a mucus sample were characterized as virus-positive and virus-negative exacerbations. Of the virus-positive exacerbations the number associated with HRV infection is shown. HRV, human rhinovirus; ITT, intent-to-treat.
3.4. Asthma control associated with exacerbation
In total, 75% of subjects had well-controlled asthma at baseline with a C-ACT score of ≥20. When comparing baseline control status of those subjects who had an exacerbation during the study with those who did not, it was noted that 34% of the subjects who had an exacerbation reported being not well-controlled (C-ACT = 13–≤19) at baseline, compared with 24% of those who did not have an exacerbation during the study. At Week 16, the proportion of patients with well-controlled status (C-ACT = ≥20) increased to 88% (Table 4 ).
Table 4.
Asthma control statusa (ITT population).
| Total | |
|---|---|
| Baseline – ITT | N = 339 |
| Total Scorea, n (%) | |
| ≥20 | 254 (75) |
| 13 to ≤19 | 85 (25) |
| ≤12 | 0 |
| Baseline – Exacerbators | n = 41 |
| Total scorea, n (%) | |
| ≥20 | 27 (66) |
| 13 to ≤19 | 14 (34) |
| ≤12 | 0 |
| Baseline – Non-exacerbators | n = 298 |
| Total scorea, n (%) | |
| ≥20 | 227 (76) |
| 13 to ≤19 | 71 (24) |
| ≤12 | 0 |
| Week 16 – ITT | n = 292 |
| Total scorea, n (%) | |
| ≥20 | 256 (88) |
| 13 to ≤19 | 33 (11) |
| ≤12 | 3 (1) |
C-ACT ≥20 = well controlled status; 13 to ≤19 = not well controlled status; ≤12 = poorly controlled status; C-ACT, Childhood Asthma Control Test; ITT, intent-to-treat.
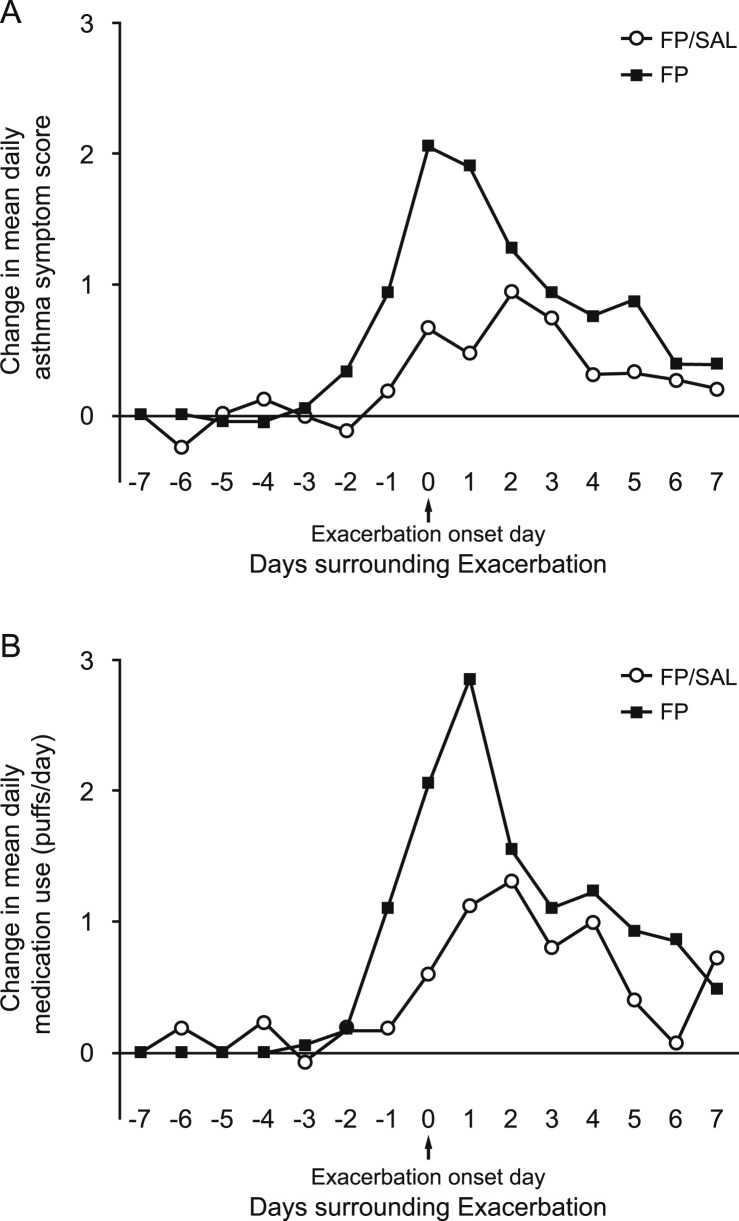
In the period surrounding an exacerbation, FP/SAL compared with FP treatment was associated with reduction in rescue medication use and asthma symptoms (Fig. 4 ). Morning PEF was similarly variable in both treatment groups in the days surrounding exacerbation. A similar ad-hoc analysis was conducted to examine these parameters associated with virus-associated exacerbations, but results were inconclusive due to the low number of virus-associated exacerbations (data not shown).
Fig. 4.
Summary of the mean change in daily asthma symptoms and rescue medication use (ITT population): A) Change from day −7 in mean daily asthma symptom score and 7 days prior and 7 days following exacerbation. B) Change from day −7 in mean daily rescue medication use in the 7 days prior and 7 days following exacerbation. Mean values were determined for subjects in each treatment group on each day for a 2-week period surrounding the exacerbation. The first day of exacerbation treatment with ICS was determined as Day 0. FP, fluticasone propionate; FP/SAL, fluticasone propionate/salmeterol; ITT, intent-to-treat.
3.5. Safety assessments
In total, 66% (224) of subjects reported AEs and 7% (24) experienced drug-related AEs. A total of 5 non-fatal SAEs were reported in 3 subjects, one of which was hospitalized due to an asthma exacerbation. Reported SAEs included gastroenteritis and syncope in 1 subject each, and RSV pneumonia, respiratory distress and status asthmaticus in 1 subject. No SAEs were considered treatment related. There were no deaths in either treatment group. A total of 2 subjects experienced AEs considered non-serious leading to study withdrawal (deteriorating asthma [n = 1] and abnormal behavior [n = 1]) and SAEs leading to study withdrawal included RSV pneumonia, respiratory distress, status asthmaticus (all in the same subject). The most common AEs reported included upper RTI (18%), headache (16%), cough (14%), pyrexia (11%), and nasopharyngitis (10%). All AEs had a similar incidence between treatment groups, with the exception of sinusitis, which was more commonly reported with FP/SAL (6%) compared with the FP (2%). The most common drug-related AEs were headache (1%) and Upper RTI (1%).
4. Discussion
The most common etiology of exacerbations of asthma in children is viral RTIs [17]. The severity and risk of having a viral associated exacerbation may be influenced by factors including atopic status, previous history of exacerbations, pathogenicity of the infecting virus, exposure to allergens, medications taken and medication adherence [17], [18], [19], [20], [21]. Several in vitro studies have suggested synergistic benefits of asthma treatment including ICS combined with a long-acting beta-adrenoceptor agonist against HRV [22], [23]. This study set out to prospectively assess the frequency and etiology of exacerbations in school-aged subjects during the fall viral epidemic, on a background of FP and FP/SAL maintenance therapy. This study also addressed and confirmed the feasibility of using at-home collection of mucus samples to detect viral etiology in further studies of viral RTIs in subjects with asthma.
The majority of the study population experienced moderate-to-severe cold symptoms at least once during the fall study period. Based on the time since randomization, the first reports of moderate-to-severe cold symptoms occurred between the second and third week in September and continued throughout the treatment period with no obvious peaks. Worsening asthma symptoms were reported throughout the treatment period with no obvious peaks in reporting.
Viruses were detected in 64% of the mucus samples collected; this frequency is similar to results found in a recent study [24]. As our study employed symptom-driven mucus collection, the viral detection frequency suggests that causes other than viral infection (e.g. allergens, air pollution, etc.) also contributed to respiratory symptoms. Of the samples with positive virology, rhinovirus was the most common respiratory virus identified. This finding is consistent with other reports from fall studies of asthma [25], [26].
The number of exacerbations seen during the fall 2010 study treatment period was low; only 41 of 339 (12%) children experienced an exacerbation requiring treatment with SCS or hospitalization. This is notable considering the study was conducted during the peak viral season (September–early December) and in a population considered to be at higher risk of exacerbation based on their past exacerbation history. The frequency of viral association with exacerbation (74%) is similar to viral-associated exacerbation rates reported in other studies conducted in outpatient settings, emergency departments, and hospitals [8], [26], [27].
A strength of this study, differentiating it from most previous studies of virus-induced exacerbations is that it was a prospective interventional study in which blinded medication was provided to the participants, and adherence to asthma control therapy was monitored and nasal sampling was symptom-driven. This study design is in contrast to observational, cross-sectional, or cohort studies in which adherence to asthma control medications preceding the exacerbation is presumably low and in which nasal samples were collected at scheduled times [28]. Additional strengths of the study included a design which required the population to enter the study in a 2-week period in early August 2010; this enabled treatment of the entire study population for a minimum of 2 weeks prior to the start of most traditional school calendars. Also, the study employed “just in time” mucus sample collection that was driven by the presence of moderate-to-severe upper respiratory symptoms and/or by the presence of signs of worsening asthma. Doing so demonstrated that symptom-driven, at-home, sample collection is feasible in a randomized controlled trial setting. Even though mucus collection was dependent on the parent's and child's compliance with the procedure at-home, this feature allowed for accurate classification of exacerbations in terms of viral etiology. The study also used a stringent and generally accepted definition of asthma exacerbation that required either SCS use, an acute care visit or hospitalization in accordance with the statement from the American Thoracic Society and European Respiratory Society on asthma control and exacerbations [29].
One limitation of this study was its inability to detect treatment differences, as a result of its exploratory design and the low number of viral RTI-associated exacerbations observed in the study population. Another limitation was the lack of a placebo arm, which would have enabled an assessment of the effect of asthma maintenance treatment on viral etiology and exacerbation rates. Previous randomized pediatric studies and retrospective cohort analyses of populations with similar exacerbation risk and impairment have demonstrated that approximately 30% of subjects experience an exacerbation during the fall [24], [30], [31], [32], 2.5-fold higher than the 12% exacerbation rate reported in the current study with FP and FP/SAL maintenance therapy. Further placebo-controlled studies are required to examine the effect of maintenance therapy on exacerbations of asthma.
In conclusion, these study results indicate that the large majority of viral RTIs are not associated with fall exacerbations of asthma in at-risk children who are on controller therapy and demonstrate the feasibility and utility of viral monitoring of exacerbations in a therapeutic trial. These findings suggest that additional factors related to virus (e.g. RV species), environment, and/or host help to determine the risk of virus-induced exacerbations. Understanding the relationships between exacerbations of asthma and viral RTIs may provide new insights to improve therapeutic strategies aimed at managing asthma exacerbations.
Disclosures
C.M. Prazma and D.A. Stempel are employees of GlaxoSmithKline, own stocks/shares in GlaxoSmithKline, and were involved in the study design, data collection and analysis. B.A. Prillaman was an employee of GlaxoSmithKline and holds stocks of GlaxoSmithKline and now is an employee of PAREXEL and was involved in the study design, data collection and analysis. J.E. Gern is a paid employee of the University of Wisconsin and was involved in the study design by advising on mucus sample data collection, process and technique. The viral data analysis was conducted at the University of Wisconsin–Madison. S.F. Weinstein participated as a study investigator in the clinical trial and his clinic was paid for participation in this study. S.F. Weinstein was not involved in study design but enrolled subjects into this clinical trial. Data collected from subjects at S.F. Weinstein's study site contributed to the overall study data.
All authors participated in the data analysis, interpretation of the data, initial development of the manuscript, and the decision to submit the final manuscript for publication. No honorarium, grant, or other form of payment was made to any of the authors for their contribution to the manuscript.
Acknowledgments
The authors would like to thank all study participants and study staff and investigators for participation in study ADA113872. Editorial assistance provided by Alex Lowe, PhD, from Fishawack Indicia Ltd was funded by GSK.
Footnotes
This study was funded by GlaxoSmithKline.
References
- 1.Bacharier L.B., Phillips B.R., Bloomberg G.R., Zeiger R.S., Paul I.M., Krawiec M. Severe intermittent wheezing in preschool children: a distinct phenotype. J. Allergy Clin. Immunol. 2007;119:604–610. doi: 10.1016/j.jaci.2006.12.607. [DOI] [PubMed] [Google Scholar]
- 2.Weiss K.B. Seasonal trends in US asthma hospitalizations and mortality. JAMA. 1990;263:2323–2328. [PubMed] [Google Scholar]
- 3.Dales R.E., Schweitzer I., Toogood J.H., Drouin M., Yang W., Dolovich J. Respiratory infections and the autumn increase in asthma morbidity. Eur. Respir. J. 1996;9:72–77. doi: 10.1183/09031936.96.09010072. [DOI] [PubMed] [Google Scholar]
- 4.Mao Y., Semenciw R., Morrison H., Wigle D.T. Seasonality in epidemics of asthma mortality and hospital admission rates, Ontario, 1979-86. Can. J. Public Health. 1990;81:226–228. [PubMed] [Google Scholar]
- 5.Kimes D., Levine E., Timmins S., Weiss S.R., Bollinger M.E., Blaisdell C. Temporal dynamics of emergency department and hospital admissions of pediatric asthmatics. Environ. Res. 2004;94:7–17. doi: 10.1016/s0013-9351(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 6.Lincoln D., Morgan G., Sheppeard V., Jalaludin B., Corbett S., Beard J. Childhood asthma and return to school in Sydney, Australia. Public Health. 2006;120:854–862. doi: 10.1016/j.puhe.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Rawlinson W.D., Waliuzzaman Z., Carter I.W., Belessis Y.C., Gilbert K.M., Morton J.R. Asthma exacerbations in children associated with rhinovirus but not human metapneumovirus infection. J. Infect. Dis. 2003;187:1314–1318. doi: 10.1086/368411. [DOI] [PubMed] [Google Scholar]
- 8.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moorman J.E., Rudd R.A., Johnson C.A., King M., Minor P., Bailey C. National surveillance for asthma–United States, 1980-2004. MMWR Surveill. Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 10.Akinbami L.J., Moorman J.E., Liu X. 2011. Asthma Prevalence, Health Care Use, and Mortality: United States, 2005-2009; pp. 1–14. Natl Health Stat Report. [PubMed] [Google Scholar]
- 11.Dilley J.A., Pizacani B.A., Macdonald S.C., Bardin J. Washington State Department of Health; Olympia, WA: 2005. The Burden of Asthma in Washington State. DOH Pub No. 345–201. [Google Scholar]
- 12.Zeiger R.S., Yegin A., Simons F.E., Haselkorn T., Rasouliyan L., Szefler S.J. Evaluation of the National Heart, Lung, and Blood Institute guidelines impairment domain for classifying asthma control and predicting asthma exacerbations. Ann. Allergy Asthma Immunol. 2012;108:81–87. doi: 10.1016/j.anai.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Schatz M., Zeiger R.S., Yang S.J., Chen W., Crawford W., Sajjan S. The relationship of asthma impairment determined by psychometric tools to future asthma exacerbations. Chest. 2012;141:66–72. doi: 10.1378/chest.11-0574. [DOI] [PubMed] [Google Scholar]
- 14.Covar R.A., Szefler S.J., Zeiger R.S., Sorkness C.A., Moss M., Mauger D.T. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J. Allergy Clin. Immunol. 2008;122:741–747. doi: 10.1016/j.jaci.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olenec J.P., Kim W.K., Lee W.M., Vang F., Pappas T.E., Salazar L.E. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J. Allergy Clin. Immunol. 2010;125:1001–1006. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu A.H., Zeiger R., Sorkness C., Mahr T., Ostrom N., Burgess S. Development and cross-sectional validation of the childhood asthma control test. J. Allergy Clin. Immunol. 2007;119:817–825. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- 17.Busse W.W., Lemanske R.F., Jr., Gern J.E. The role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilbert T.W., Morgan W.J., Zeiger R.S., Mauger D.T., Boehmer S.J., Szefler S.J. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N. Engl. J. Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 19.Long-term effects of budesonide or nedocromil in children with asthma. The childhood asthma management program research group. N. Engl. J. Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 20.Prazma C.M., Kral K.M., Gul N., Yancey S.W., Stempel D.A. Controller medications and their effects on asthma exacerbations temporally associated with upper respiratory infections. Respir. Med. 2010;104:780–787. doi: 10.1016/j.rmed.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Reddel H.K., Jenkins C., Quirce S., Sears M.R., Bateman E.D., O'Byrne P.M. Effect of different asthma treatments on risk of cold-related exacerbations. Eur. Respir. J. 2011;38:584–593. doi: 10.1183/09031936.00186510. [DOI] [PubMed] [Google Scholar]
- 22.Volonaki E., Psarras S., Xepapadaki P., Psomali D., Gourgiotis D., Papadopoulos N.G. Synergistic effects of fluticasone propionate and salmeterol on inhibiting rhinovirus-induced epithelial production of remodelling-associated growth factors. Clin. Exp. Allergy. 2006;36:1268–1273. doi: 10.1111/j.1365-2222.2006.02566.x. [DOI] [PubMed] [Google Scholar]
- 23.Skevaki C.L., Christodoulou I., Spyridaki I.S., Tiniakou I., Georgiou V., Xepapadaki P. Budesonide and formoterol inhibit inflammatory mediator production by bronchial epithelial cells infected with rhinovirus. Clin. Exp. Allergy. 2009;39:1700–1710. doi: 10.1111/j.1365-2222.2009.03307.x. [DOI] [PubMed] [Google Scholar]
- 24.Busse W.W., Morgan W.J., Gergen P.J., Mitchell H.E., Gern J.E., Liu A.H. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N. Engl. J. Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denlinger L.C., Sorkness R.L., Lee W.M., Evans M.D., Wolff M.J., Mathur S.K. Lower airway rhinovirus burden and the seasonal risk of asthma exacerbation. Am. J. Respir. Crit. Care Med. 2011;184:1007–1014. doi: 10.1164/rccm.201103-0585OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakes G.P., Arruda E., Ingram J.M., Hoover G.E., Zambrano J.C., Hayden F.G. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am. J. Respir. Crit. Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 27.Heymann P.W., Carper H.T., Murphy D.D., Platts-Mills T.A., Patrie J., McLaughlin A.P. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J. Allergy Clin. Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S.L., Chiu S.S., Malik P.J., Chan K.H., Wong H.S., Lau Y.L. Is respiratory viral infection really an important trigger of asthma exacerbations in children? Eur. J. Pediatr. 2011;170:1317–1324. doi: 10.1007/s00431-011-1446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddel H.K., Taylor D.R., Bateman E.D., Boulet L.P., Boushey H.A., Busse W.W. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am. J. Respir. Crit. Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 30.Martinez F.D., Chinchilli V.M., Morgan W.J., Boehmer S.J., Lemanske R.F., Jr., Mauger D.T. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:650–657. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss K.B., Gern J.E., Johnston N.W., Sears M.R., Jones C.A., Jia G. The Back to School asthma study: the effect of montelukast on asthma burden when initiated prophylactically at the start of the school year. Ann. Allergy Asthma Immunol. 2010;105:174–181. doi: 10.1016/j.anai.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Lieu T.A., Quesenberry C.P., Sorel M.E., Mendoza G.R., Leong A.B. Computer-based models to identify high-risk children with asthma. Am. J. Respir. Crit. Care Med. 1998;157:1173–1180. doi: 10.1164/ajrccm.157.4.9708124. [DOI] [PubMed] [Google Scholar]