Abstract
The biopharmaceutical industry is slowly absorbing the idea of collaborative patent licensing models. Recently, two patent pools for developing countries have been launched: the Pool for Open Innovation against Neglected Tropical Diseases initiated by GlaxoSmithKline (GSK), which is referred to as the BIO Ventures for Global Health (BVGH) pool, and the Medicines Patent Pool (MPP) initiated by UNITAID. Various organizations have recommended using pools or clearinghouses beyond the humanitarian dimension where many patents are owned by many different actors. As a first attempt, MPEG LA, which administers patent pools in various technology fields, is now setting up a clearinghouse for patents related to molecular diagnostics. These examples as well as the results from an empirical study provide useful insights for the design and administration of future pools and clearinghouses in the life sciences.
A valuable tool in the life sciences
Increasingly, patent pools and clearinghouses are being considered as a tool to facilitate access to large numbers of inventions in the biomedical sector. The biopharmaceutical industry is slowly absorbing the idea that collaborative patent licensing models, such as pools and clearinghouses, could function as an interesting alternative to exclusive single-firm production, simple bilateral licensing or cross-licensing (Box 1, Box 2 ). These models could be particularly useful in situations where many related inventions are patented by many different organizations and where access to these inventions is essential for the development and commercialization of a (new) product. Such situations are commonly referred to as ‘patent thickets’ [1] in cases where it is cumbersome to safeguard one's freedom to operate (FTO) because the commercial production, marketing and use of a new product, process or service is likely to infringe many existing patent rights owned by many third parties (third party patent rights). To gain access to those third party patent rights, one will need to enter into a multitude of licensing negotiations often leading to an accumulation of royalties (royalty stacking). For some companies or researchers this might be a reason to redesign or completely stop a research project 2, 3, 4. However, others are taking up the challenge to experiment with licensing models that could overcome the negotiation hurdle.
Box 1. Licensing.
Patent owners (licensors) can grant licenses giving recipients (licensees) permission to exploit the patent and promising not to bring suit against the licensees for infringing the patent. A person who uses the technology protected by a patent must, provided this activity is not covered by a research exception or other type of exception, request a license. Licenses between two negotiating partners are often called bilateral licenses. It is common to distinguish between in- and out-licensing. In-licensing refers to situations where the organization concerned is the user of the patented technology, i.e. the licensee. Out-licensing refers to situations where the organization concerned is the owner of the patented technology, i.e. the licensor.
A cross-license is a special form of bilateral license. In general, it is an agreement between two patent owners where the patent owners grant one another a license for exploitation of the subject matter claimed in the patents concerned. Both patent owners act as licensor and licensee. It can be regarded as a mutual pact not to sue for patent infringement. Cross-licenses are widely used in many technology fields in order to grant reciprocal access to patented inventions. Cross-licenses are often used to settle ongoing disputes, to prevent litigation or to ensure FTO. However, cross-licenses are only proper tools for transferring technology if both parties have ‘licensable’ objects to offer in return.
Box 2. Collaborative licensing models.
Patent pools and clearinghouses are often referred to as collaborative licensing models. The effective execution of these models requires the collaboration of many different players, including the licensing entity itself, its staff, patent owners and technology users. In general, bilateral licenses and cross-licenses only concern two contract partners and do not involve a separate ‘hub’ (the pool or clearinghouse).
A patent pool consists of a set of agreements. First, patent owners license their technology to one another, often by way of a multiparty agreement between two or more patent owners. As a result, the pool is established. Second, licenses are bundled into one package license and licensed out by the pool to third party licensees on FRAND terms. The package license can be granted either by one of the patent owners representing the pool or through an independent licensing authority. Patent pools allow interested parties to gain access to all patents to use an invention with one single package license, sometimes called a ‘one-stop-license’, rather than obtaining licenses from each patent owner individually.
Pools also include a ‘gatekeeper’ function that vets the patents that are pooled. This is needed to ensure that the patents are likely to be valid and enforceable. If there is no vetting procedure, then pool members could run the risk of expensive litigation procedures with an uncertain outcome.
Clearinghouses can be depicted as platforms or intermediaries bringing together owners and users of goods, services and information to lower transaction costs. There are many types of clearinghouses ranging from mere databases of information to technology exchange platforms and royalty-collecting organizations performing many functions 25, 26, 27. The clearinghouse operates as a neutral intermediary or platform for a wide variety of licensable technologies (a type of ‘supermarket’ for licensable technologies) with substantial expertise in licensing. It matches patent owners and licensees by delivering standard or one-stop-licenses. Licensors benefit from the visibility and expertise of the clearinghouse, save negotiation costs and maximize dissemination of their inventions. Licensees gain from clearinghouses through potential economies of scale in search costs and negotiation cost savings. Because licensees are making choices of what to license on a case-by-case basis, there might be lesser demand on a vetting process than in the case of a pool because the infringement liability risk falls on the licensee and not on the clearinghouse. Clearinghouses can also offer additional services related to monitoring, enforcement, royalty collection, royalty distribution and mediation or arbitration in the case of disputes.
Patent pools and clearinghouses could act as facilitators. Their use of one-stop-licenses will normally reduce royalties and transaction costs, increase legal certainty and reduce enforcement litigation. In this way, the costs and risks of R&D might be mitigated, and the costs for end-users might be reduced significantly.
Recent working examples
For many years, numerous proposals have been made for collaborative licensing models in the life sciences, but most of these have not led to concrete steps. Recently, we have observed a number of endeavors in the life sciences to set up patent pools and clearinghouses. It is still uncertain whether all these projects will succeed because they are still in an early stage, but at least the organizations that drive these initiatives have taken a strong lead and companies seem more supportive of this type of endeavor than in the past. This might be related to a growing interest in open innovation (see Box 3 ) and a stronger push for corporate social responsibility with respect to biopharmaceutical companies, which is also reflected in calls for socially responsible patenting and licensing strategies (E. van Zimmeren, PhD thesis, University of Leuven, 2011) 5, 6.
Box 3. Collaborative licensing models for facilitating open innovation.
A trend towards open collaborative innovation appears to be emerging. Although some claim that open innovation is ‘old wine in new bottles’ [53], others regard open innovation as the new paradigm for fostering innovation in the life sciences [54]. Open innovation is defined as the use of inflows and outflows of knowledge to stimulate and accelerate innovation within firms and organizations, and to expand the markets for the exploitation of innovation by others 55, 56, 57. The central principle of open innovation is that firms can and should use external ideas as well as internal ideas and employ internal and external paths to market as they advance their technology 55, 56, 57.
In the life sciences, organizational modes of open innovation are blossoming. Public–private partnerships exemplify such open innovation organizational modes. Public–private partnerships include the Innovative Medicines Initiative (IMI) (http://www.imi-europe.org/), the Centre for Translational Molecular Medicine (CTMM) (http://www.ctmm.nl/) and the Diabetes Genetics Initiative (DGI) (http://www.broad.mit.edu/diabetes/). These examples indicate a growing willingness and openness of the pharmaceutical and biotechnology industries to set up new forms of public–private partnerships in order to make significant advances in the life sciences. Open innovation principles might catalyze commercialization and accelerate innovation in global health. These open collaborative initiatives require active involvement and knowledge spill-overs between companies, customers, suppliers, universities, research institutes, consortia and start-ups. Hence, there is a need for well-tailored IP strategies that support the open spirit of the collaboration, enable knowledge flows, allow for value appropriation and facilitate commercialization. In this respect, collaborative licensing models, operating as one-stop-licensing shops, might be a useful alternative for (one-by-one) bilateral agreements and might further facilitate open innovation.
The subject, nature, initiators, profile of the patent owners, main incentives and governance schemes of these pools and clearinghouses vary significantly (Table 1 ). The first (known) project for a patent pool in the life sciences was the Severe Acute Respiratory Syndrome (SARS) pool [7]. The World Health Organization (WHO) set up a network of laboratories to control the disease. As various patent applications were filed by public and private organizations, a patent pool was proposed to prevent disputes, to enhance R&D and to advance the development of vaccines. The relevant (public and private) patent owners had been identified, and principal agreement on the pool had been gained between them. However, the SARS pool is no longer actively pursued because, with no further outbreaks, the economic drive for the formation of the pool has disappeared (J. Simon, personal communication, 21 January 2009). When the initiative was called off, the detailed arrangements for the pool's operation were not yet fully determined and the pool never worked in practice (Table 1).
Table 1.
Features of patent pools and clearinghouses
| Electronics and telecommunications pools | SARS pool | MPP | BVGH pool | SNP nutrigenomics clearinghouse | Librassay™ | |
|---|---|---|---|---|---|---|
| Subject | Standard-related technology in consumer electronics and telecommunications | Genomic sequences SARS | Medicines against HIV/AIDS | R&D regarding NTDs | SNPs related to nutrigenomics | Diagnostic testing |
| Nature | Profit | Non-profit | Non-profit | Hybrid | Profit | Profit |
| Initiative | Patent owners | WHO/patent owners | WHO/UNITAID | GSK and other patent owners | DSM | MPEG LA |
| Profile patent owners (public/private) | Mainly private partners | Mixed | Mixed | Mixed | Mixed | Mixed |
| Main incentives | Downstream, standards compatibility, opening up a market | Upstream, humanitarian, quick and broad availability of vaccines | Downstream, humanitarian, lower prices, combinations for specific populations | Upstream, humanitarian, opening up a market, risk sharing | Upstream, opening up a market, risk sharing | Downstream, broad availability of tests, enabling personalized medicines, lower prices |
| Administration pool | One of the patent owners/independent third party, e.g. MPEG LA, VIA Licensing, SISVEL | Unknown | MPP | BVGH/patent owners | Independent third party | MPEG LA |
| Licensing practices | Package license, essentiality standard, FRAND | Package license | Individual licenses | Individual licenses, royalty-free, qualified participants | Customized package license | Customized package license |
In 2008, the Executive Board of UNITAID, an international facility for the purchase of drugs against HIV/AIDS, malaria and tuberculosis for the populations of developing countries hosted by the WHO, approved the establishment of the Medicines Patent Pool (MPP). This pool would be set up as a separate legal entity to facilitate the manufacture of antiretroviral pharmaceutical products for the treatment of HIV/AIDS, in particular fixed-dose combinations and child-specific formulations, and to provide cheaper second-line treatments for patients who developed resistance to conventional therapies 8, 9, 10. The MPP is now operational. It is strongly supported by the WHO. In September 2010, the US National Institutes of Health (NIH) set an important precedent by announcing that it was licensing patents and patent applications related to the protease inhibitor class of HIV medicines to the MPP [11]. Several commercial partners have also expressed their support for the pool. Hoffman-La Roche, Gilead, Sequoia Pharmaceuticals and ViiV Healthcare [a joint venture of GlaxoSmithKline (GSK) and Pfizer] have taken the lead and have entered into negotiations with the MPP. These negotiations are still ongoing. By contrast, several other key patent owners have informed the MPP that, for now, they prefer to focus on their own longstanding efforts to improve access to HIV/AIDS medicines (http://www.medicinespatentpool.org/LICENSING/Company-Engagement); it might be difficult to convince these patent owners to participate.
In 2009, GSK proposed a pool for medicines for neglected tropical diseases for Least Developed Countries (LDCs). This proposal is part of GSK's Open Innovation Agenda, which is aimed at tackling challenges for improving global public health 12, 13, 14. The agenda favors a more flexible approach regarding intellectual property (IP) to overcome the lack of R&D in neglected tropical diseases 12, 13. A pool could boost R&D by enabling access to relevant patented inventions and related know-how. The pool would be restricted to neglected tropical diseases, which by definition do not appear in developed countries. Thus, the market for medicines for these diseases is quite small and not so competitive. This is a major difference with the MPP, which concerns patents related to HIV/AIDS medicines with all the associated risks of parallel importation of medicines produced under a pool license into the developed world. Given this fundamental difference, these pools will probably develop in very different ways.
GSK agreed to put 800 small molecule compound or process patents (or patent applications) and relevant know-how in the pool. The administration of the Pool for Open Innovation against Neglected Tropical Diseases (NTDs) has now been taken over by BIO Ventures for Global Health (BVGH). Hereinafter, the pool is referred to as the BVGH pool (http://ntdpool.org/). BVGH acts as the non-profit independent administrator of the pool offering a database with the available patents and know-how and conducting outreach to potential contributors and licensees. Pool contributors grant worldwide, royalty-free, non-exclusive licenses to (i) qualified participants with a concrete proposal, and (ii) in accordance with the ‘de minimis’ standards set by the pool for research, development, manufacture and export of therapeutics for the 16 major NTDs as identified by the US FDA* for sales into LDCs† as defined by the United Nations [15]. According to BVGH, these two standards are not intended to be exclusive but aim at safeguarding the quality of the research enabled by the pool. The exact criteria for qualification (e.g. potential licensee's scope of work, the nature of its resources and capabilities) are not public. It is important to reveal these criteria because a lack of transparency might be regarded by some as a way to discriminate certain actors, whereas the whole purpose of the pool is to broaden access and lower costs of access.
In the meantime, in addition to GSK, Alnylam Pharmaceuticals, Massachusetts Institute of Technology, the product development partnership Medicines for Malaria Venture, the University of California Berkeley, the California Institute of Technology, the Sandler Center for Drug Discovery at the University of California and Stanford University have joined the BVGH pool as contributors. Emory Institute for Drug Discovery, iThemba Pharmaceuticals, the University of Cape Town and the South Africa's Technology Innovation Agency have signed up as users of the pool.
These three examples, the SARS pool, the MPP and the BVGH pool, differ considerably from patent pools in other technology sectors (i.e. consumer electronics and telecommunications) (Table 1). In these sectors, pools are more common and they (i) are usually for profit, (ii) are initiated by industry and (iii) are generally developed in parallel with standard-setting, which requires interoperability between different products. By contrast, the pools described above (i) are non-profit and/or have a humanitarian objective, (ii) are initiated or strongly supported by international organizations, such as the WHO and (iii) are generally not supported by standard-setting and interoperability. With respect to the latter, we note, however, that several authors have pointed to the role standards could play in pools in the life sciences 16, 17, 18.
Moreover, the MPP and BVGH pool models do not include a multiparty agreement between the patent owners and, therefore, seem closer to the clearinghouse concept than to many classical patent pools in this respect. In addition, the direct negotiations and grant of licenses by contributors to licensees of the BVGH pool is different from most pools in other technology fields and from the MPP. Normally, patent owners license their patents to the pool and the pool issues sub-licenses on pre-agreed terms. In this sense, the BVGH pool also seems to operate more as a clearinghouse than as a patent pool.
Some have suggested applying pool and clearinghouse models in the life sciences beyond humanitarian uses, i.e. in a for-profit context 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 and believe that pools and clearinghouses are particularly apt to deal with problems of highly fragmented patent rights in the area of genetic testing 16, 17, 18, 19, 20, 22, 23, 26, 27. Embracing this line of reasoning, MPEG LA, which administers patent pools in various fields (http://www.mpegla.com/), announced a molecular diagnostics licensing clearinghouse (‘licensing supermarket’) in April 2010, now known as Librassay™. Its aim is to aggregate patent rights for existing and emerging tests that could lead to personalized treatment (e.g. in relation to cancer, cardiovascular disease, diabetes, neurological disorders and various hereditary conditions such as hearing loss), and to license those patents non-exclusively for diagnostic use. According to Larry Horn, President and CEO of MPEG LA, MPEG LA has received support for its diagnostic licensing supermarket from different sectors (L. Horn, personal communication, 13 January 2011). Agreements have been concluded with several initial anchor patent owners, and discussions are under way with others. MPEG LA offers three different packages to patent owners. Under the most advanced package, patent owners enter into a commitment to provide their patents or patents applications in the diagnostic field to Librassay™ for a minimum of 5 years. MPEG LA assumes the cost for any enforcement actions that might be necessary based on the interests of the patent owners and licensees enrolled in the supermarket. Patent owners have the option of delegating to MPEG LA the responsibility and costs associated with patent maintenance fees and with continuing patent prosecution. All packages provide patent owners with the opportunity to retain rights for research, education and drug development carried out by, or on behalf, of the patent owner. MPEG LA aims at having the supermarket up and running in the first quarter of 2012 (L. Horn, personal communication, 22 May 2011 and http://www.mpegla.com/main/pid/mds/default.aspx).
The Dutch multinational DSM, active in life and material sciences, has also shown interest in the clearinghouse model to overcome the emerging patent thicket problem on single nucleotide polymorphisms (SNPs) in the area of nutrigenomics to unlock the market for personalized nutrition. A model has been designed for an SNP Nutrigenomics Clearinghouse, which would offer standard licenses enabling companies to develop genetic tests based on SNPs with a specific predisposition profile (see case study in E. van Zimmeren, PhD thesis, University of Leuven, 2011). As long as these tests are not available for a reasonable price, the market for personalized nutrition will remain blocked. DSM is seeking partners for the formation of a consortium to finance the start-up of the clearinghouse but has encountered serious difficulties in doing so. Most industry partners are highly risk-averse and seem reluctant to engage in a model they do not know for a market (nutrigenomics) where any commercial success is still quite uncertain (E. van Zimmeren, PhD thesis, University of Leuven, 2011).
Prior experience of MPEG LA and other pool administrators in other industries has shown that voluntary for-profit collaborative licensing models set up by patent owners or an administering entity can work [28]. But to what extent are patent owners in the life sciences eager to apply such models beyond the non-profit sphere? To what extent are these models economically viable alternatives compared to single-firm production, bilateral licensing or cross-licensing (Box 1) for the biomedical sector?
The pool model is promising in terms of its operational efficiency and feasibility 28, 29, as well as its potential impact on health care in developing countries [29]. However, it is doubtful whether voluntary participation in a patent pool will generate a critical mass of high quality patents, particularly outside a very specific area, such as neglected tropical diseases or HIV/AIDS [29]. These apprehensions also arise with respect to clearinghouses and they are even more prominent in a for-profit context. Concerns about the lack of a critical mass of high quality patents have also been confirmed as a major point of concern by the respondents in the survey, which will be described below [30]. These issues point to the need to offer additional incentives to patent holders to supply patents to pools in the life sciences. Most pools in the life sciences are initiated by the beneficiaries (the demand side) of the pool (licensees, non-governmental organizations, international organizations, patient groups, etc.). This situation differs from pools in, for example, consumer electronics and telecommunications, where most patent owners have a clear interest in initiating or collaborating with the establishment of a pool because they need licenses for the inventions of the other patent owners in the pool to legally pursue their own product development.
Although patent pools and clearinghouses have repeatedly been recommended as a remedy against patent fragmentation in the life sciences, such models have not emerged widely to date in a for-profit context. One explanation could be that the perceived risk of patent fragmentation and blockage from thickets has not yet occurred in practice.
Fragmentation of patent rights?
The literature on the emergence of patent thickets and anticommons 2, 3, 4 in genetics has been heavily criticized 31, 32. In the USA, the fear of deleterious consequences caused by patent thickets in genetics has largely disappeared, although doubts concerning genetic diagnostic testing remain [33]. In Europe, the more stringent approach of European patent examiners has been argued to largely allay the concerns about the effects of patenting in genetics [34]. Moreover, empirical evidence appears to indicate that, apart from some exceptional cases, the initial apprehensions about wide patent thickets have not materialized in the USA [22] or Europe [35].
Although this empirical evidence calls into question the widespread existence of patent thickets, the same studies have expressed fears that fragmented ownership is likely to interfere in the new era of personalized medicine and complicate the use of multiplex genotyping, multiplex sequencing and whole genome sequencing 22, 35, 36. Patent pools and clearinghouses are seen as an interesting option for solving future problems in this field 22, 37.
Knowledge and experience with collaborative licensing models
Another reason for the limited uptake of collaborative licensing models could be that stakeholders simply do not know such models or have a negative perception of them. Little empirical research has been carried out regarding collaborative licensing models as a remedy for patent fragmentation. In the USA 38, 39, Australia [40] and Switzerland [41], scholars have conducted surveys on the impact of patenting and licensing policies, particularly with regard to access to biomedical research. These surveys primarily reviewed conventional patenting and licensing practices (e.g. bilateral and cross-licenses). Recently, empirical studies focusing on collaborative licensing in medical biotechnology in Europe [30] and Australia [42] have been carried out. The results of the Australian study are discussed in detail in [43]. Here, we summarize the findings of the European study [30].
The European survey was fully completed by 177 respondents: 19 from pharmaceutical companies, 53 from biotechnology companies, 43 from universities and public and private research institutes, 20 from hospitals, 26 from law and patent firms and 16 from other types of organizations (e.g. private or publicly owned technology transfer offices, investors, technology parks and incubators) (Figure 1 ).
Figure 1.
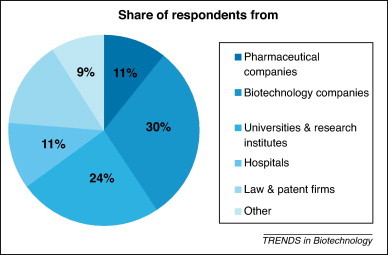
The pie chart indicates the share of respondents from the different types of organizations in an empirical study focusing on collaborative licensing in medical biotechnology in Europe.
Most of the 177 respondents (67.8%) work for organizations that are not at all or not heavily engaged in in-licensing (universities, hospitals, most biotechnology companies and some pharmaceutical companies). For universities and hospitals, this seems rather obvious, but this is not the case for biopharmaceutical companies. This might be explained by the fact that many European biopharmaceutical companies are still relatively young and have not yet developed a licensing policy or because they are focusing on upstream research. With regard to the out-licensing profile, a relatively large part of the sample does not know to what extent their organization's patent portfolio is licensed out, 19.8% of the respondents indicate that their organization is not licensing out at all and 24.9% are licensing out no more than 10% of the patent portfolio. It is interesting to compare these results with the findings of Pressman et al. [38]. In their sample, approximately 70% of the patents had been licensed. Yet, their sample was limited to only 19 universities and only covered DNA patents. In this light, we emphasize that in the current survey 18.7% of the sample is indeed licensing out more than 30% of their patent portfolio, which can be considered a significant fraction (16.7% pharmaceutical companies, 30.4% universities and research institutes and 20.3% biotechnology companies).
On the basis of these data, approximately four categories of players can be identified when it comes to licensing: (i) organizations that do not pursue any licensing activity; (ii) organizations that own patents and license them out, but are not licensing in (‘net IP sellers’); (iii) organizations that license in but are not licensing out (‘net IP buyers); (iv) organizations that license in and out. Depending on the category, one's viewpoint towards collaborative licensing will differ. However, a strict classification of the different types of organizations in one of the four categories is impossible. The study shows significant variety within the different types of organizations. For example, a considerable number of biotechnology companies belong to the first category but some fall in one of the other categories.
When respondents were asked whether they perceive the existing number of third party patent rights in the biomedical sector as an ‘undue burden’, defined as ‘the number of third party patent rights is a substantial obstacle on the organization's path to research, product development and/or the provision of (clinical testing) services’, for their organization's FTO, one in four answered yes. More than 50% of this group works for biotechnology companies. Problems especially arise for drug discovery, pharmaceuticals and diagnostics and in particular for gene sequences, processes and methods. For biotechnology companies, particularly, this is an important finding, which points to the need to further elaborate on efficient models that could reduce this undue burden.
In most cases, organizations overcome patent fragmentation by negotiating a license or inventing around the patents concerned. However, some organizations (in particular biotechnology companies) decide to abandon research projects altogether. This finding seems to confirm that it is essential to start thinking about alternative models to deal with patent fragmentation. Furthermore, respondents from European universities and research institutes and even biotechnology companies tend to presume that their work is covered by a research exception. However, this might not be the ‘safe harbor’ they think it is: in Europe, the text of the research exception differs substantially from country to country, and, in most countries, there is no case law that clearly defines the scope of the exception [25]. In the USA, there is no general research exception at all 25, 44. Nonetheless, ‘rational forbearance’ by patent owners who want to maintain goodwill and ensure access to future inventions, in particular if the damages are likely to be very small, might result in a de facto research exception [38]. We doubt, however, whether this is a stable and sustainable basis for R&D in the life sciences, in particular in view of the fact that an increasing number of lawsuits are based on inventions resulting from academic research licensed out exclusively [45]. Therefore, patent owners might not remain so tolerant in the future towards academic researchers who ignore patents [46].
Pools and clearinghouses could be an alternative for bilateral and cross-licensing, inventing around and reliance on the research exception, which appear unsatisfactory for 25% of the respondents to the survey. However, when the survey was conducted (2008), only 50% of the respondents had heard about pools and only 40% about clearinghouses. This number might be somewhat higher if the questionnaire were sent out now following the press coverage of the MPP and BVGH pool. Among respondents who were familiar with the models, 22.7% had experience with pools and 21.6% had experience with clearinghouses. The survey does not show any significant difference among the types of organizations involved in pools. However, universities and research institutes appear to have significantly more experience with clearinghouses than biotechnology and pharmaceutical companies. It is likely that universities, which primarily are net IP sellers, use clearinghouses to market their technology more effectively than they could do themselves. Universities that try to maximize the use of their patented technology by drawing on the services of intermediaries seem to act in line with the concept of open innovation (Box 3).
Overall, experience with pools is rated positively. In addition to being useful for gaining FTO and for facilitating licensing out, pools are regarded as an appropriate way to reduce the risks of refusals to license. However, the complexity of patent pools, the loss of secrecy, exclusivity and control in the bargaining process, and the time-consuming negotiations needed to establish pools partially thwart these advantages.
The results for clearinghouses are more diverse: respondents from biotech and pharma are more positive than respondents from universities and research institutes, but these non-profit institutions have more experience with clearinghouses. Although clearinghouses have mainly been recommended as an instrument to safeguard FTO in the scholarly literature, they are not perceived in this way in practice. Clearinghouses are rather thought to facilitate licensing out, to generate licensing revenue or to enable organizations to focus on their core activities. The relatively negative perception among part of the respondents largely stems from fears about the loss of control in the bargaining process in setting licensing conditions and the loss of exclusivity. These issues are also mentioned for patent pools, but for clearinghouses some additional weaknesses are stated. First, respondents point to the risk that clearinghouses would not contain valuable, key technologies (‘markets for lemons’). It is claimed that patent owners would prefer to manage their valuable patented technologies themselves and leave the ‘lemons’ (e.g. low quality patents, unmarketable technologies) to clearinghouses. Second, it seems unlikely that clearinghouses will be able to guarantee full FTO and, hence, they would fail to solve patent thicket problems.
The survey also shows that for most respondents competition law is not a key consideration in choosing a particular licensing model. In most survey questions, competition law was included as a choice option but only a few respondents indicated that competition law had been an important factor in designing a specific licensing strategy.
Patent pools and clearinghouses are considered especially promising for drug discovery, pharmaceuticals, diagnostics and genetic testing and, in particular, as tools to administer patents related to gene sequences, processes and methods.
Concluding remarks
Recent examples of patent pools and clearinghouses in the life sciences have elicited a growing interest among international governmental organizations and industry in collaborative licensing models. For now, four important sets of observations and recommendations can be inferred, which might be helpful in setting up such models.
First, knowledge of patent pools and clearinghouses is sparse and experience with pools and clearinghouses is also limited. Informing stakeholders about the features of such models and their benefits and shortcomings is essential to take well-informed decisions in setting up such collaborative licensing models. In this respect, further research on the compatibility of different institutional designs and licensing schemes with competition law and on their economic viability is desirable. In particular, research on the compatibility of exclusive licenses for very specific fields of use, customized licensing terms and qualification criteria with the obligation in competition law for pools to adopt non-exclusive, fair, reasonable and non-discriminatory terms (FRAND terms) would be worthwhile. Another interesting topic for research would be the proportionality of the costs of establishing and administering a pool or clearinghouse in relation to the perceived benefits of such models for the life sciences. In other words, to what extent should the patent landscape be fragmented in order to justify the cost and labor intensive establishment of a pool or clearinghouse? What type of additional incentives can and should be used to attract patent owners to participate in a pool or clearinghouse if they believe they can make more money by staying outside those models? In this respect, more detailed case studies of the newly developed pools and clearinghouses once they are more mature will be particularly interesting.
Second, pools and clearinghouses are considered promising tools in the sectors of drug discovery, pharmaceuticals, diagnostics and genetic testing, especially for patents on gene sequences, processes and methods. Industry players who are active in these particular sectors might reflect on setting up pools or clearinghouses, or get involved in ongoing initiatives such as the MPEG LA licensing supermarket. In the same vein, policymakers and funding agencies (e.g. European Commission, NIH) involved in public–private partnerships in these particular sectors could encourage their partners to apply these models in case of fragmented ownership of patent rights flowing from such partnerships, similar to what the WHO has done in several instances.
Third, patent owners are concerned about the loss of control of the bargaining process and licensing conditions, the loss of exclusivity and the loss of secrecy when they participate in a collaborative licensing scheme. Moreover, potential licensees doubt whether clearinghouses will license out valuable, key technologies or low quality ‘lemons’ and whether pools and clearinghouses would be able to secure a portfolio with a critical mass of essential patented technologies. Redesigning some conventional features of pools and clearinghouses might help. For example, the models could be reorganized in such a way that they give more freedom to patent owners to negotiate with licensees within the scope of the respective model, such as the BVGH pool.
However, increasing the freedom of patent owners to negotiate directly with would-be licensees also encompasses some risks. If one stretches the boundaries of the models too far, one might eliminate the significant pro-competitive transaction cost savings and undermine the purpose of the model in the first place. Moreover, one might encounter limitations imposed by competition law. One important issue is that typical one-stop pool licenses should comply with FRAND terms 47, 48, 49. In a pool model where patent owners have extensive freedom to impose qualification criteria and freely decide on the licensing conditions, the prohibition to discriminate in competition law could be particularly problematic. Instead of offering no standard terms at all, it might be better to propose customized menus of licensing terms tailored to the needs of different licensee profiles [50]. This would increase transparency and public trust in the patent pool.
Another cure for fears about loss of control might be pools or clearinghouses for a specific ‘niche’, e.g. a specific application or field of use outside the core activities of most patent owners. For such a ‘niche’, patent owners might be less worried about exclusivity and control. The MPEG LA and DSM initiatives are presented as niche models for patent owners in the pharmaceutical sector for diagnostic and genetic assessment technologies that are not their main line of business.
A further option would be to provide stronger incentives for patent owners to contribute essential and valuable technologies to the pool or clearinghouse. One set of incentives to contribute would be services that add value for patent owners. For example, start-up companies and universities might be interested in legal or financial assistance in filing and maintaining patents or in formulating a standard/customized set of licensing conditions. Alternatives could be partial tax exemptions for royalties obtained from the pool or prize schemes linked to the patent pool for new inventions that result from collaborative research that uses patents in the pool.
Fourth, the role of competition law is often largely ignored. However, competition law contains a lot of rules on how patent licenses should be drafted and how market players should behave. This intersection between patent and competition law is notoriously complex 51, 52. For patent pools, competition agencies have issued guidelines that can be used as a checklist during negotiation processes 16, 18, 28, 47, 48, 49. However, particular circumstances in the life sciences might require specific features, as we noticed with respect to licensing terms, qualification criteria and incentive schemes for the MPP and BVGH pool. Therefore, it is always important to contact competition law experts to check innovative pooling or clearinghouse arrangements on their compatibility with competition law.
None of these recommendations requires legislative changes. However, they entail further research on how licensing mechanisms could be tailored to respond to challenges in the life sciences, an open mind in thinking about IP management and a strong commitment and leadership among policymakers and stakeholders to foster a collaborative attitude. In this way, patent fragmentation might well transform from problematic patent thickets into opportunities for further collaboration and (open) innovation.
Acknowledgments
The authors gratefully acknowledge the financial support of the Flemish Research Fund (FWO) and EuroGentest in addition to the assistance of the Licensing Executives Society International, the European Commission, EuroGentest, ProTon Europe and EuropaBio with the survey. We also thank the experts on our test panel for their helpful suggestions on the survey design, M. Suckers and J. Van den Broeck for their research assistance and D. Nicol for the collaboration on the Australian counterpart of the survey. E.v.Z. and G.V.O. appreciate the opportunity of working with DSM on a model for the SNP Nutrigenomics Clearinghouse. Finally, we would also like to thank the two anonymous reviewers for their detailed and helpful comments.
Footnotes
The 16 major neglected tropical diseases identified by the US FDA are: tuberculosis, malaria, blinding trachoma, buruli ulcer, cholera, dengue/dengue hemorrhagic fever, dracunculiasis (guinea-worm disease), fascioliasis, human African trypanosomiasis, leishmaniasis, leprosy, lymphatic filariasis, onchocerciasis, schistosomiasis, soil transmitted helminthiasis and yaws.
The LDCs defined by the United Nations are: Afghanistan, Angola, Bangladesh, Benin, Bhutan, Burkina Faso, Burundi, Cambodia, Central African Republic, Chad, Comoros, Democratic Republic of the Congo, Djibouti, Equatorial Guinea, Eritrea, Ethiopia, Gambia, Guinea, Guinea-Bissau, Haiti, Kiribati, Lao People's Democratic Republic, Lesotho, Liberia, Madagascar, Malawi, Maldives, Mali, Mauritania, Mozambique, Myanmar, Nepal, Niger, Rwanda, Samoa, São Tomé and Príncipe, Senegal, Sierra Leone, Solomon Islands, Somalia, Sudan, Timor-Leste, Togo, Tuvalu, Uganda, United Republic of Tanzania, Vanuatu, Yemen and Zambia.
References
- 1.Shapiro C. Navigating the patent thicket: cross licenses, patent pools, and standard-setting. In: Jaffe A., editor. Innovation Policy and the Economy. MIT Press; 2001. pp. 119–150. [Google Scholar]
- 2.Heller M.A. The tragedy of the anticommons: property in the transition from Marx to markets. Harv. Law Rev. 1998;111:621–688. [Google Scholar]
- 3.Heller M.A. Basic Books; 2008. The Gridlock Economy: How Too Much Ownership Wrecks Markets, Stops Innovation, and Costs Lives. [Google Scholar]
- 4.Heller M.A., Eisenberg R.S. Can patents deter innovation? The anticommons in biomedical research. Science. 1998;280:698–701. doi: 10.1126/science.280.5364.698. [DOI] [PubMed] [Google Scholar]
- 5.Access to Medicines Foundation . Access to Medicines Foundation; 2010. Access to Medicines Index 2010. [Google Scholar]
- 6.Hunt, P. (2008) The Right to Health: Report of the Special Rapporteur on the Right of Everyone to the Enjoyment of the Highest Attainable Standard of Physical and Mental Health, UN General Assembly
- 7.Simon J.H.M. Managing severe acute respiratory syndrome (SARS) intellectual property rights: the possible role for patent pooling. Bull. World Health Org. 2005;83:707–710. [PMC free article] [PubMed] [Google Scholar]
- 8.UNITAID (2008) UNITAID Moves Towards a Patent Pool for Medicines, Press Release, UNITAID, Geneva, 9 July 2008
- 9.UNITAID Executive Board (2010) Provisional Minutes: Resolution No. 5, Executive Board Meeting, 11th Session, Special Session on Patent Pool, 5 February 2010, WHO, Geneva
- 10.WHO and Medicines Patent Pool Foundation (2010) Memorandum of Understanding, 14 September 2010
- 11.UNITAID (2010) US National Institutes of Health (NIH) First to Share Patents with Medicines Patent Pool, Press Release, UNITAID, Geneva, 30 September 2010
- 12.Witty, A. (2009) Big Pharma as a Catalyst for Change, Speech to Harvard Medical School, Boston, 13 February 2009
- 13.Witty, A. (2010) Open Labs, Open Minds: Breaking Down Barriers to Innovation and Access to Medicines and Vaccines in the Developing World, Remarks delivered to the Council on Foreign Relations, New York, 20 January 2010
- 14.Gamo F.-J. Thousands of chemical starting points for antimalarial lead identification. Nature. 2010;465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 15.BVGH . BVGH; 2010. Pool for Open Innovation against Neglected Tropical Diseases: Core Principles. [Google Scholar]
- 16.Ebersole T.J. Patent pools and standard setting in diagnostic genetics. Nat. Biotechnol. 2005;23:937–938. doi: 10.1038/nbt0805-937. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein J.A. Biotechnology patent pools and standards setting. In: Takenaka T., editor. Patent Law and Theory: A Handbook of Contemporary Research. Edward Elgar Publishing; 2008. pp. 712–721. [Google Scholar]
- 18.Verbeure B. Patent pools and diagnostic testing. Trends Biotechnol. 2006;24:115–120. doi: 10.1016/j.tibtech.2006.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Organization for Economic Co-operation and Development (OECD) (2002) Genetic Inventions, Intellectual Property Rights and Licensing Practices: Evidence and Policies, OECD
- 20.Human Genome Organization (HUGO) Intellectual Property Committee (2003) Statement on the Scope of Gene Patents, Research Exemption and Licensing of Patented Gene Sequences for Diagnostics, HUGO [PubMed]
- 21.Australian Law Reform Commission (ALRC) (2004) Genes and Ingenuity: Gene Patenting and Human Health, ALRC, Final Report No. 99
- 22.US Secretary's Advisory Committee on Genetics, Health, and Society (SACGHS) (2010) Revised Draft Report on Gene Patents and Licensing Practices and Their Impact on Patient Access to Genetic Tests, Office of Science Policy, National Institutes of Health: Office of Biotechnology Activities
- 23.European Society of Human Genetics (ESHG) Working Party on Patenting and Licensing (2008) Patenting and licensing in genetic testing: recommendations of the European Society of Human Genetics. Eur. J. Hum. Gen. 16, S3–S9 [DOI] [PubMed]
- 24.Nicol D., Hope J. Cooperative strategies for facilitating use of patented inventions in biotechnology. Law in Context. 2006;24:85–112. [Google Scholar]
- 25.Van Overwalle G. Models for facilitating access to patents on genetic inventions. Nat. Rev. Genet. 2006;7:143–148. doi: 10.1038/nrg1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Zimmeren E. A clearing house for diagnostic testing: the solution to ensure access to and use of patented genetic inventions? Bull. World Health Org. 2006;84:352–359. doi: 10.2471/blt.06.030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Overwalle G., editor. Gene Patents and Collaborative Licensing Models: Patent Pools, Clearinghouses, Open Source Models and Liability Regimes. Cambridge University Press; 2009. [Google Scholar]
- 28.Clark, J. et al. (2000) Patent Pools: A Solution to the Problem of Access in Biotechnology Patents, USPTO
- 29.WHO Expert Working Group on Research and Development Financing . WHO; 2010. Research and Development: Coordination and Financing. [Google Scholar]
- 30.van Zimmeren, E. et al. Patent Licensing in Medical Biotechnology in Europe: A Role for Collaborative Licensing Strategies? Acco (in press)
- 31.Caulfield T. Evidence and anecdotes: an analysis of human gene patenting controversies. Nat. Biotechnol. 2006;24:1091–1094. doi: 10.1038/nbt0906-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joly Y. Open source approaches in biotechnology: utopia revisited. Maine Law Rev. 2007;59:386–405. [Google Scholar]
- 33.US National Research Council of the National Academies: Committee on Intellectual Property Rights in Genomic and Protein Research and Innovation . National Academies Press; 2005. Reaping the Benefits of Genomic and Proteomic Research: Intellectual Property Rights, Innovation and Public Health. [PubMed] [Google Scholar]
- 34.Hopkins M.M. SPRU; 2006. The Patenting of Human DNA: Global Trends in Public and Private Sector Activity. [Google Scholar]
- 35.Huys I. Legal uncertainty in the area of genetic diagnostic testing. Nat. Biotechnol. 2010;27:903–909. doi: 10.1038/nbt1009-903. [DOI] [PubMed] [Google Scholar]
- 36.Chandrasekharan S., Cook-Deegan R. Gene patents and personalized medicine: what lies ahead? Genome Med. 2009;1:92–95. doi: 10.1186/gm92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Overwalle G. Turning patent swords into shares. Science. 2010;330:1630–1631. doi: 10.1126/science.1189592. [DOI] [PubMed] [Google Scholar]
- 38.Pressman L. The licensing of DNA patents by US academic institutions: an empirical survey. Nat. Biotechnol. 2006;24:31–39. doi: 10.1038/nbt0106-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh J.P. Where excludability matters: material versus intellectual property in academic biomedical research. Res. Policy. 2007;36:1184–1203. [Google Scholar]
- 40.Nicol, D. and Nielsen, J. (2003) Patents and Medical Biotechnology: An Empirical Analysis of Issues Facing the Australian Industry, Centre for Law and Genetics, Occasional Paper No. 6
- 41.Thumm, N. (2003) Research and Patenting in Biotechnology: A Survey in Switzerland, Swiss Federal Institute of Intellectual Property, Report No. 1
- 42.Nicol, D. (2010) Patent Licensing in Medical Biotechnology in Australia: A Role for Collaborative Licensing Strategies? Centre for Law and Genetics, Occasional Paper No. 7
- 43.Nicol D. Collaborative licensing in biotechnology: a survey of knowledge, experience, and attitudes in Australia. Biotechnol. Law Rep. 2010;29:465–483. [Google Scholar]
- 44.US Court of Appeals for the Federal Circuit (2002) Madey v. Duke University. 307 F.3d 1351
- 45.Holman C.M. Trends in human gene patent litigation. Science. 2008;322:198–199. doi: 10.1126/science.1160687. [DOI] [PubMed] [Google Scholar]
- 46.Yancey A., Stewart C.N. Are university researchers at risk for patent infringement? Nat. Biotechnol. 2007;25:1225–1228. doi: 10.1038/nbt1107-1225. [DOI] [PubMed] [Google Scholar]
- 47.US Department of Justice and US Federal Trade Commission (1995) Antitrust Guidelines for the Licensing of Intellectual Property, Washington
- 48.European Commission (2004) Guidelines on the Application of Article 81 of the EC Treaty to Technology Transfer Agreements, Brussels, OJ C101/02
- 49.Japan Fair Trade Commission (2005) Guidelines on Standardization and Patent Pool Arrangements, Tokyo
- 50.van Zimmeren E. Clearinghouse mechanisms in genetic diagnostics: conceptual framework. In: Van Overwalle G., editor. Gene Patents and Collaborative Licensing Models: Patent Pools, Clearinghouses, Open Source Models and Liability Regimes. Cambridge University Press; 2009. pp. 63–119. [Google Scholar]
- 51.Anderman S., Ezrachi A., editors. Intellectual Property and Competition Law: New Frontiers. Oxford University Press; 2011. [Google Scholar]
- 52.Carrier M.A., editor. Intellectual property and Competition. Edward Elgar Publishing; 2011. [Google Scholar]
- 53.Trott P., Hartmann D. Why open innovation is old wine in new bottles. Int. J. Innov. Manag. 2009;13:715–736. [Google Scholar]
- 54.Melese T. Open innovation networks between academia and industry: an imperative for breakthrough therapies. Nat. Med. 2009;15:502–507. doi: 10.1038/nm0509-502. [DOI] [PubMed] [Google Scholar]
- 55.Chesbrough H.W., editor. Open Innovation: Researching a New Paradigm. Oxford University Press; 2008. [Google Scholar]
- 56.Chesbrough H.W. Harvard Business School Press; 2006. Open Business Models: How to Thrive in the New Innovation Landscape. [Google Scholar]
- 57.Chesbrough H.W. Harvard Business School Press; 2003. Open Innovation: the New Imperative for Creating and Profiting from Technology. [Google Scholar]


