Graphical abstract
Keywords: Drug shortage, Drug manufacturing, Drug distribution, Drug unavailability, Shortage impact, Mitigation strategy
Abbreviations: AIFA, Agenzia Italiana del Farmaco (Italian Medicines Agency); ATC, Anatomical Therapeutic Chemical Classification Systems; EEA, European Economic Area; EMA, European Medicines Agency; EU, European Union; GMP, Good Manufacturing Practice; HMA, Heads of Medicines Agencies; MAH, Marketing Authorization Holder; NDMA, N-nitrosodimethylamine; SPOC, Single National points of contact; TFEU, Treaty on the Functioning of the European Union; VEN, Vital-Essential-Non essential
Abstract
Medicine shortages have been spreading in European countries. In many cases, the unavailability of medicinal products has a substantial impact on the capability of National Healthcare Systems in ensuring the continuity of care. Shortages originate from multifactorial causes. In particular, they can be due to supply-related factors (e.g., manufacturing issues, regulatory issues, logistics, distribution) and demand-related ones (e.g., fluctuating drug demand, parallel market, tendering, price and reimbursement policies). However, some extraordinary geopolitical events (e.g., Brexit) may also affect medicines’ availability. The capability of European Regulatory Authorities and other stakeholders, which are involved in the pharmaceutical distribution chain and the healthcare assistance services, to define suitable problem-solving strategies has been limited for years by the fragmentation of the European regulatory framework, starting from the lack of a univocal definition of a medicine shortage. Only in 2019, the EMA and HMA joint task force released the first harmonized “shortage” definition in the European Economic Area (EEA) and guidance on public communication. This manuscript aims to review the current European regulatory framework on medicine shortages. To support the activities of regulators, manufacturers and other healthcare professionals, an algorithm was also proposed to be used as a harmonized procedure to determine the shortage/unavailability impact on public health and to rationalize the problem-solving strategies adopted in all different settings.
1. Introduction
Medicine shortages have risen worldwide, straining the capability of National Healthcare Systems in ensuring patients’ access to pharmacological therapies (Beck et al., 2019, Jia and Zhao, 2017, Schwartzberg et al., 2017). The resilience of the pharmaceutical distribution chain and other stakeholders varies based on different factors, such as product characteristics and the involved countries (Birhli, 2013). The unavailability of medicinal products to patients can be due to supply-related factors (e.g., manufacturing issues, regulatory issues, logistics, distribution) and demand-related ones (e.g., fluctuating drug demand, parallel market, tendering, price and reimbursement policies). Moreover, extraordinary geopolitical events [e.g., Brexit, Coronavirus disease (COVID-19) outbreak in China] can also have a strong influence on the resilience of the pharmaceutical chain and the patients’ access to therapies. Even if globalized players control the research and production of medicinal products by now, the activities of Regulatory Authorities are still based on national or supranational [i.e. European Union (EU)] frameworks. In Europe, the capability of the Regulatory Authorities and other subjects involved in the pharmaceutical distribution chain and the healthcare assistance services in defining suitable problem-solving strategies has been limited by the fragmentation of the regulatory framework (Bochenek et al., 2018, De Weerdt et al., 2015a). Only in 2019, the EMA and HMA released the first harmonized “shortage” definition in the European Economic Area (EEA) (EMA and HMA, 2019b). Although the proposed definition was relevant only for Marketing Authorization Holders (MAHs) and Regulatory Authorities, it is the first step to promote the communication and the coordination among European pharmaceutical stakeholders, regulators and professionals working in the different National Healthcare Systems for improving their resilience to shortages. However, the kaleidoscopic regulatory framework on shortage is not the only critical factor, but the heterogenicity of the National legislation, reimbursement and price policies, and medicines’ market around Europe limited the establishment of a solid European emergency plan to solve the shortage problem.
Considering that numerous factors cause shortages, a univocal problem-solving strategy cannot be established at the European level. The needs of big-pharmaceutical markets, such as Germany (Said et al., 2018) or Italy (Di Giorgio et al., 2019), are far from those of small ones like as Finland (Heiskanen et al., 2017). Moreover, excessive fragmentation of the National regulatory frameworks may be itself a cause of drug shortage. The relationship between the price policies and availability of medicinal products is known (Dave et al., 2018, De Weerdt et al., 2015b). Considering the different reimbursement policies of Member States (WHO, 2018), the price of the same medicinal product may vary significantly around Europe. In this context, the exportation of drug products to more profitable markets (e.g., parallel trade) is frequently cited as one of the demand-related causes of drug shortages (De Weerdt et al., 2015b). Noteworthy, such a cause-effect relationship is still debated (Aguiar and Ernest, 2020). The importation of medicinal products from other EU or extra-EU countries is also the most common strategy to mitigate the adverse effects of severe shortages (Said et al., 2018). Notably, small European countries might not be profitable enough for MAHs to justify building new manufacturing sites and marketing medicinal products. In this context, for justified public health reasons, the National Regulatory Authorities can adopt regulatory pathways to speed up the release of the marketing authorization (e.g., Art. 126a of Directive 2001/83/EC) and, therefore, to make medicines available on the National market. Otherwise, parallel trade can be a possible solution to ensure the patients’ access to treatments. For example, besides the granting of National marketing authorizations, a small English-speaking country like Malta ensures its medicines' supply mainly by Art. 126a authorizations, while parallel import licences contribute only to the residual needs (less than 10%) (Farrugia, 2018). Indeed, the application of Art. 126a permits the MAHs of products already authorized in other English-speaking EU countries (e.g., UK, Ireland) to have easy access to the Maltese market. However, Brexit (EMA, 2019a) and some concurrent updates of the European regulatory framework might worsen such a delicate balance. For example, the new medicine traceability system introduced in the EU by the Regulation (EU) 2016/161 increased the costs of both the production and the distribution of medicinal products, affecting the economic sustainability of the all actors involved in the pharmaceutical supply chain (e.g., MAHs, parallel distributors). The potential repercussions on patients’ access to treatments in the more fragile EU countries are foreseeable, especially in the case of low-price medicinal products (e.g., generics).
In the current situation, it is evident that the same strategy may have different efficacy in shortage mitigation according to the features of European countries involved. As a consequence, rational and practical shortage emergency plans should originate from stronger cooperation among European countries and based on the assessment of pros/cons balance at a supranational level.
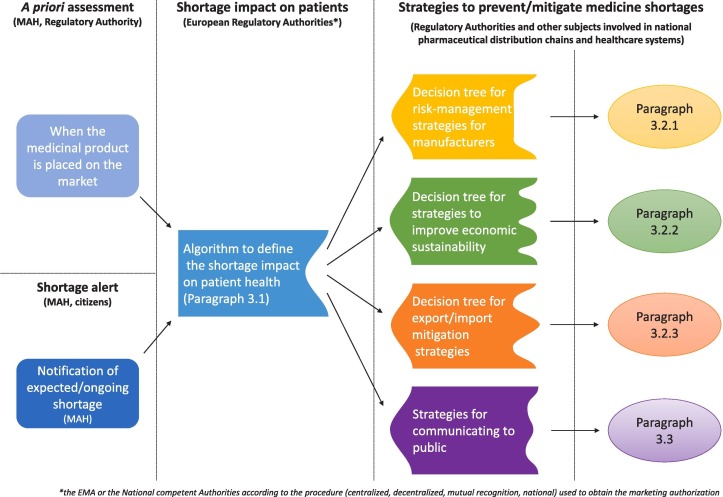
This manuscript aims to review the current upgrade of the European regulatory framework on medicine shortages and to propose a theoretical approach for 1) determining the shortage/unavailability impact on the capability of the National Healthcare Systems and 2) rationalizing the problem-solving strategies to be adopted in function of the leading cause of shortage (e.g., production, price and importation/exportation). Such an approach would be useful to the European Regulatory Authorities in the harmonization of the metrics around the Union. Moreover, it would also be helpful to other subjects involved in the National pharmaceutical distribution chains and healthcare systems (e.g., manufacturers, wholesalers, hospitals, pharmacies, insurance companies, regional healthcare Authorities). In this light, it should be flexible enough to rationalize the risk-assessment and risk-management strategies for the mitigation of the shortage effects in different industrial and healthcare settings.
2. Current regulatory framework and problems till on the ground
According to Article 81 of the Directive 2001/83/EC, MAHs and their distributors should ensure appropriate and continued supplies to pharmacies and authorized persons to meet the needs of patients in the different European countries. If a temporary or permanent disruption of the supply occurs, the MAH has to notify the competent Authorities, also providing information about the causes (Art. 23a of the Directive 2001/83/EC). The notification should be made no less than two months before the interruption of the supply is in place. In some cases, such deadline was extended, e.g. to 4 months in Italy (Italian Republic, 2019). Moreover, Art. 123 of the Directive 2001/83/EC leaves plenty of room to the Member States in establishing appropriate measures to ensure the prompt communication among stakeholders about authorizing marketing, supplying and withdrawing a medicinal product, together with the reasons on which such decisions are based.
In the absence of specific, harmonized and detailed guidance on the detection and notification of supply interruption at the European level, the regulation fragmentation occurred at the National level. The term “shortage” has taken on different meanings around Europe (De Weerdt et al., 2015a, De Weerdt et al., 2018). The French legislation defined shortages the inability for a community or hospital pharmacy to deliver a drug to a patient within 72 hours (Bocquet et al., 2017). In Germany, different words were used to identify the drug shortages at a delivery-level (i.e. lie-ferengpass) and those that compromise the patient therapies in the absence of available therapeutic alternatives (i.e. versorgungsengpass) (Said et al., 2018). In Italy, the Italian Medicines Agency (AIFA) distinguishes between carenze, which identifies all manufacturing-related shortages including also those caused by MAH voluntary withdrawal (temporary or permanent), and indisponibilità, which identifies the unavailability of a medicinal product in a specific geographical area due to inefficiencies of the pharmaceutical distribution chain (Di Giorgio et al., 2019). Such fragmentation has also been evident in the communication strategies adopted by the different EU Member States (Bochenek et al., 2018).
According to a survey carried out by the EMA and HMA in 2019, about 87% of the EU competent Authorities published shortages alerts to healthcare professionals and patients, whereas industries and other regulators were the alerts’ recipients only in the 60% of the cases (EMA and HMA, 2019b). In general, the information on ongoing/expected shortages have been publicly available in web-accessible catalogues periodically upgraded, but other communication tools have also been used (e.g., relevant organizations’ channel, press releases, professional/medical journal, TV, newsletter, social media). In some cases, shortage alerts have been shared in the National electronic patient health systems or the electronic prescribing systems used by physicians and pharmacists (7%). However, in the majority of cases (69%), no specific criteria have been set by the National Authorities for the publication. Moreover, the published data vary significantly case-by-case and country-by-country, improving the confusion for professionals and patients at National and European levels.
In this light, significant efforts to implement the regulatory framework were undertaken by the European Authorities. Single National points of contact (SPOC) have been created to facilitate sharing of the information about nationwide medicine shortages and the coordination of emergency plans among the competent National Authorities and the EMA. For multi-country shortages, the EMA’s scientific committees should also be involved to write harmonized recommendations to healthcare professionals.
In 2019, the EMA and HMA released two joint guidelines on shortages, which provided recommendations to improve the collaboration among Regulatory Authorities and stakeholders (EMA and HMA, 2019a, EMA and HMA, 2019b). The “Guidance on detection and notification of shortage of medicinal products for Marketing Authorization Holders (MAHs) in the Union (EEA)” contained the first harmonized definition of shortage valid for all the EU (EMA and HMA, 2019a). “A shortage of a medicinal product for human and veterinary use occurs when supply does not meet demand at a national level”. According to such definition, unavailability of a medicinal product can be classified as a shortage only if the MAH supply is insufficient to fulfil the demand of a specific country overall, at least. Consequently, delivery-related issues due to regional supply disruption or inefficient National redistribution of stock are explicitly out of the definition, leaving to National Authorities plenty of room in establishing their approaches to prevent the unavailability issues. According to the new regulatory provisions, the MAH should notify any shortages, including impending/anticipated supply interruptions, to the Regulatory Authorities of the affected European countries (EMA for medicinal products authorised by a centralised procedure). Moreover, MAHs are asked to provide detailed information about the product, the nature and time of the shortage, the availability of alternative medicinal products, the population affected by shortage and the risk for patient safety or a reduction in treatment access (Annex to the guideline). Such information is then used by the competent Authority to triage and assess the shortage of risks.
The second HMA/EMA guideline, “Good practice guidance for communication to the public on medicines’ availability issues”, aimed to enhance and align the European communication on a shortage to improve the awareness of healthcare professionals and patients and the cooperation among European stakeholders (EMA and HMA, 2019b). Several key recommendations are provided to National Authorities about what should be publicly available on shortages or unavailability due to revocation/cessations of the marketing authorization. The guideline states, for example, that all nationwide shortages should be published in systematic lists, which should be easily accessible and searchable by the public in all the EU countries. The shortage information should also be publicly communicated as quickly as possible to ensure the continuity of care, and it should remain accessible, at least, after six months from shortage resolution. For each ongoing/expected shortage, the systematic list should report details about the affected medicinal product, the shortage duration and cause, the adopted mitigation strategies, the advice for healthcare professionals, including potential alternative medicinal products and recommendation to change in clinical practice/in use of medicine. The best communication tool should be selected based on the shortage gravity; the high-profile tools (e.g., press release) should be preferred for severe shortages with a high impact on public health.
Following the publication of HMA/EMA guidelines, several European stakeholder associations have released position papers confirming their significant concerns on the shortages in the EU and proposing solutions to overcome criticalities still on the ground regardless of the HMA/EMA task force interventions. In May 2019 more than 30 organisations representing patients, consumers, healthcare professionals and public health advocates stimulated the European Commission to do more in identifying the factors leading to medicines shortages and providing clear and transparent information on the root causes (Various Associations, 2019a). In December 2019, the European associations representing manufacturers, parallel distributors, pharmaceutical full-line wholesalers and industrial pharmacists issued a position paper in which they stated their view on the root causes’ classification and provided suggestions to improve the European cooperation in preventing shortage impact on public health (Various Associations, 2019b). In particular, it has been highlighted the need to find collaborative solutions for medicine shortages to coordinate EU and National policies and communication campaigns. The cooperation should start from the adoption of a harmonized shortage definition and the rational definition of a “risk of shortage” for essential products in the EU. Moreover, they also underlined the importance of improving the monitoring of shortages and cooperation in the EU by the establishment of a priority ranking of shortages to address “high-risk” medicinal products efficiently without introducing disproportionate initiatives that could have opposite effects to the ones intended.
Although the HMA/EMA guidelines released in 2019 represent an essential first step to provide a harmonized solution for the shortages in Europe, the proposed approach seems perfectible. For example, the cooperation process among the concerned National Authorities is not detailed, since no systematic procedure on the information sharing among National SPOC has been provided yet (EMA and HMA, 2019a, EMA and HMA, 2019b). At the moment, only EMA’s decision tree specific for shortages due to GMP non-compliance/quality defects is publicly available (EMA, 2013). Moreover, no procedures to triage the shortage impact on patient’s health were detailed in the guidelines, leaving plenty of room to the Member States in defining the measures to assess the information notified by the MAH and mitigate the risk of shortages. However, there is a strong need for proper and effective risk-assessment and problem-solving strategies to reduce nationwide shortages without wasting money and time. In the absence of a harmonized triage procedure, there is the risk of increasing the workload for MAHs and Regulatory Authorities to manage shortages with a low impact on public health, even though they are already mitigated and managed effectively at dispensing level. As reported by the Royal Dutch Pharmacists Association Farmanco® platform, the continuity of treatments is ensured in about 90% of the medicine shortage cases by the medicine substitution with therapeutic alternatives (PGEU, 2019). When more than one country is involved in a shortage, the situation is even more critical. For example, the same medicinal product may be triaged in different matters by competent National Authorities and different mitigation plan may be required to the MAHs. This hypothesis is particularly relevant for co-marketing products produced by the same contractor or medicinal products authorized by decentralized or mutual recognition procedures. The involved SPOC may not be able to assess the shortage crisis quickly and to coordinate the mitigation strategies in a useful matter. Consequently, the efforts of MAH/contractor in restoring the National supply may be slowed down by different National obligations that contrast each other. For example, in the presence of reduced manufacturing capacity, the obligation for MAH to maintain a preventive stockpiling in some countries (e.g., Switzerland) may worsen the product availability in the other involved markets.
Another critical issue, which has not been adequately tackled by the guidelines yet, is the management of product shortages induced by raw materials unavailability. Indeed, the ongoing/expected shortages of raw material may cause, for a ripple effect, shortages of medicinal products in several countries without MAHs can predict or notify them in advance. Economic globalization promotes the delocalization and gathering of manufacturing sites of pharmaceutical raw materials, impacting on the resilience of the pharmaceutical supply chain of many medicinal products to the shortage. For example, between 2008 and 2009, the so-called “Great Acetonitrile Shortage” caused shortages of several drug substances, for which synthesis the solvent was used, and drug products (PDA, 2014). Sartans and immunoglobulins give other examples. In the former case, after the discovery of cancerogenic impurities in sartans (i.e. valsartan, losartan irbesartan) produced by some manufacturers, the blockage of the drug substances’ production and multiple medicines’ recalls caused severe shortages in most of the European countries (EMA, 2019b). In the latter case, shortages are due to a low-manufacturing capacity in comparison to the growing demand of immunoglobulins. Although the immunoglobulins use has been tripled worldwide in the past 20 years, their production is limited by the availability of human plasma, from which the immunoglobulins are extracted and purified (Carrock Sewell et al., 2014). In this context, several National Authorities (e.g., France, Germany, UK) have published priority rankings and guidelines for the use of immunoglobulins in clinical practice to reduce the shortage risks and rationalize the patients’ access to therapies. In all these cases, the notification process proposed by the EMA/HMA guideline seems not sufficient to detect the risk of shortage since the MAHs are not aware of the resilience of its raw material suppliers to shortage and, therefore, they are not able to monitor effectively raw materials suppliers and predict failures in their manufacturing chain. For these products, more centralized coordination of the risk control strategies by the EMA and, consequently, a more efficient sharing of information among National Regulatory Authorities are needed to better mitigation of shortage impact at a national level and to fulfil the needs of all the patients.
3. “Antibodies to medicine shortages”
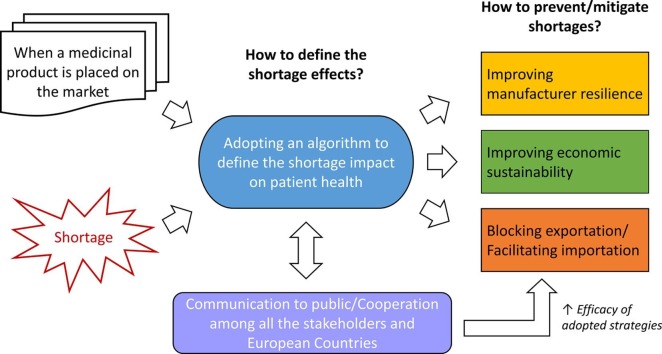
Considering such critical issues and the latest position papers of stakeholder associations (Various Various Associations, 2019a, Various Associations, 2019b), the establishment of harmonized metrics to classify the impact of medicine shortage on the National Healthcare Systems is a mandatory step to enhance the cooperation among competent National Authorities of the European countries and to rationalize the efforts to mitigate them. Regulators and professional associations have proposed several systematic measures to assess shortage problems (Beck et al., 2019, Bochenek et al., 2018, EMA, 2019c, Jia and Zhao, 2017, Panzitta et al., 2017, PDA, 2014). However, the proposed strategies are generally designed to face a specific shortage’s root cause (e.g., manufacturing failures, low price, distribution-problem). Such strategies cannot be applied to solve all shortage types, and the communications/cooperation among stakeholders may be limited. However, in the end, the existing problem-solving strategies aimed to face the same issue: the medicine unavailability for the patients and the increased risks for their health (Phuong et al., 2019). Consequently, in the estimation of the shortage impact on the patient needs, this aspect should be primary for harmonizing the existing metrics and rationalizing the problem-solving strategies to be adopted. Based on this assumption, it is proposed a procedure to determine the impact of shortage/unavailability on the healthcare assistance and, therefore, the patient accessibility to therapies, other than the communication strategies to adopt (Fig. 1 ).
Fig. 1.
Scheme of the harmonized procedure to tackle drug shortage in the EU.
A shortage can be imagined as a disease for a National healthcare system. Consequently, the procedure can ideally be considered as a shortage-against antibody. Two parts form an antibody: one specific to the antigen (variable region) it has to bind, the other for the recognition by the immune system (constant part). Similarly, an ideal regulatory procedure should be composed by a “constant part”, which should be recognized by all EU countries and stakeholders, and a “variable part”, which should be more focused on the shortage cause to tackle or the peculiarities of the National regulatory framework. In this way, the resulting metrics allow promoting, on the one hand, the harmonization of the risk assessment of the shortages at EU level and, on the other hand, the rationalization of the preventing management strategies in a cause-specific matter.
Such a procedure may be fully integrable with other existing and adopted strategies and, therefore, it may be an added value for both European Regulatory Authorities and other subjects involved in the National pharmaceutical distribution chains and healthcare systems (e.g., manufacturers, wholesalers, hospitals, pharmacies, insurance companies, regional healthcare Authorities). Indeed, a unique triage procedure for shortage impacts can improve the stakeholders’ cooperation in managing the emergencies. At the same time, it can be useful for European Regulatory Authorities to assess the potential risks of shortage for a medicinal product since the obtaining of the marketing authorization, allowing them to establish preventive actions to improve MAH resilience and minimize risks for patients’ health. Moreover, the procedure can also be adapted and applied in a hospital setting to allow pharmacists to identify those medicinal products in their stocks which are more likely to be exposed to the risk of shortages, and to set up most appropriate strategies to prevent inefficiencies in healthcare assistance caused by the shortage.
3.1. Definition of shortage impact on patients’ health
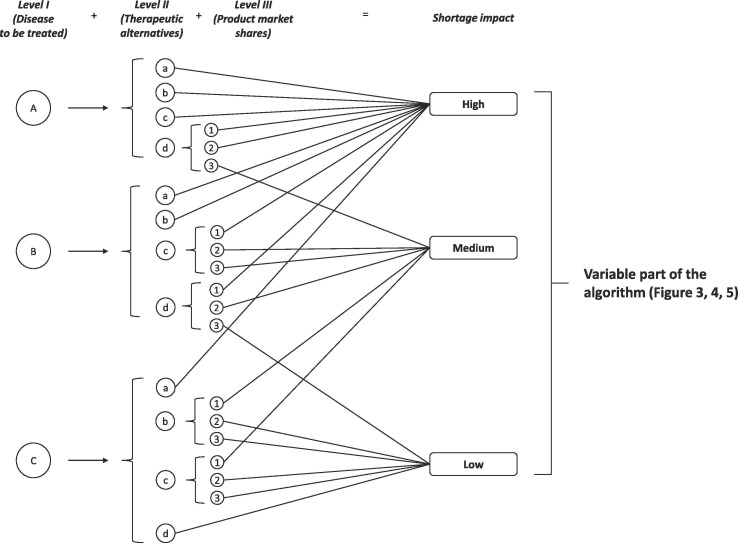
The constant part of the algorithm may be applied by the EMA in consensus with HMA members as part of the activities of the HMA/EMA task force (HMA, 2019). The constant part of the algorithm aims to provide a harmonized tool for evaluating the impact of drug shortage on clinical demand in each European country. Considering that the negative effects of a medicine shortage strongly varies according to the type of treated disease and the real-world patient assess to therapies, a procedure to assign the overall score for shortage impact (i.e. high, medium, low) may be designed on three levels: I) type of disease to be treated, II) the availability of therapeutic alternatives and III) the market shares of the product in a specific country in comparison to the available alternatives. Table 1 reportes the score for each level. The data needed for each level are either available to competent National Authorities or included in the notification the MAHs have to send them for any expected/ongoing shortages according to the guideline for MAHs (EMA and HMA, 2019a).
Table 1.
Levels and relative scores of the procedure to determine the shortage impact on patient health.
| Level | Score |
|---|---|
| I (the type of disease to be treated) |
|
| II (therapeutic alternatives) |
|
| III (product market shares in a specific country) |
|
3.1.1. Level I – Type of disease to be treated
As shown in Fig. 2 , the first step is to classify the medicinal products for which a shortage is expected/ongoing based on the therapeutic indications (Level I), namely: A) products for life-supporting, life-sustaining or rare diseases, B) products for severe or debilitating diseases (acute or chronic), C) products for other diseases. A shortage of a life-supporting medicinal product resulted in a higher impact on patient health than one used to treat a nonserious illness, such as allergic rhinitis. The score assignment for a medicinal product can be carried out adopting the principles of the VEN (Vital-Essential-Non-essential) analysis (WHO, 2003) or other approaches. Alternatively, an indication of how a competent National Authority considers much essential medicine may be indirectly obtained from the official documentation provided to support its inclusion/exclusion from the list of reimbursed medicines. Indeed, in many European countries (e.g., Italy), the seriousness of the disease is one of the most relevant criteria on which the competent National Authority performs the Health Technology Assessment (HTA) to determine the eligibility of the medicinal product to be reimbursed. For example, only medicinal products considered “essential” and those for chronic medicinal products get access to the reimbursement after the AIFA assessment (Italian Republic, 1993). If the same medicinal product is indicated for the treatment of several diseases, the most serious and with the low prevalence should be considered to guarantee the continuity of care to the most fragile patient population.
Fig. 2.
Determination of the shortage impact on patient health.
3.1.2. Level II - The availability of therapeutic alternatives
The seriousness of the shortage impact on public health is also influenced by the existence of therapeutic alternatives that can ensure the continuity of the care (PGEU, 2019). Therefore, each class should be analysed assessing the availability of other therapeutic options (Level II) (Fig. 2, Table 1). A different score is assigned, in decreasing order of importance, based on the negative impact on public health (Table 1). The lower the number of therapeutic alternatives on the market, the higher the negative effect of the shortage on public health. The scores for the availability of treatments are: a) not more than two medicinal products containing drug substances in the same ATC level III (same therapeutic/pharmacological subgroup) or IV (same chemical/therapeutic/pharmacological subgroup); b) more than two medicinal products for the same ATC level III, but not for the same ATC level IV; c) more than two medicinal products containing drug substances in the same ATC level IV, but no generic products are available for the same ATC level V (same chemical substance or therapeutic moiety); d) more than two generic products for the same ATC level V.
It is noteworthy that available treatments are those that are marketed in a European country. Medicinal products should not be considered in the assessment if they have obtained the marketing authorization but have not been placed in the market by the MAH yet. Moreover, medicines with negligible market share (lower than 2% of the total demand of reference for the product in shortage) should not be taken into account as well, since their negligible impact in preserving patient access to therapies. The differences among scores are based on the criticality associated with the medicine interchangeability. Indeed, it is expectable that a shortage can be easily mitigated by the availability of therapeutically equivalent products, which can be substituted in a pharmacy setting in most of the European countries (Wouters et al., 2017). Two medicinal products are therapeutically equivalent if they contain the same active substance or therapeutic moiety (ATC Level V) and, clinically, show the same efficacy and safety (EMEA, 2000). Generic products fall in this class.
On the contrary, the unavailability of generic products implies that, in the best scenario, the benefit/risk balance of the therapeutic interchangeability should be evaluated by a physician. It is the case of medicinal products for which there are alternatives with the same ATC-level III (e.g., penicillins vs tetracyclines) or IV alternatives (e.g., amoxicillin vs ampicillin). In both cases, the efficacy/safety patterns of two alternatives may not be the same and, therefore, the substitution cannot be automatically carried out by the community pharmacists. Consequently, the shortage impact is potentially higher because the patient is exposed to a higher risk of cure discontinuity if the physician is not promptly accessible.
3.1.3. Level III – The market shares of the product
The existence of therapeutic alternatives may not be enough to mitigate the negative effect of a medicine shortage. Indeed, if the therapeutic alternative has low market shares, the MAHs supply cannot be enough to fulfil the patients’ needs during the crisis. The risks of shortage for the low-market-share alternatives is also increased for a ripple effect. The more monopolistic is the market of a medicinal product, the higher the probability that competitors are not able to sustain the patient demands during a shortage. Indeed, Parsons and colleagues suggested the existence of an inverse correlation between the number of suppliers for antineoplastic drugs in the US and shortages’ occurrence (Parsons et al., 2016). Regardless of the presence of therapeutic alternatives on the market, the lower the supplier number, the greater the number of drug shortages. Therefore, for algorithm level III, the higher the market shares of a medicine (expressed as annual volumes) in comparison to the existing therapeutic alternatives, the higher the potential risks for the public health in case of shortage.
Consequently, the following scores were empirically defined (Table 1): 1) a market share higher than 50% of the entire National market; 2) a market share between 25 and 50%; 3) a market share lower than 25%. For scores b) and c) of the Level II, the product market shares should be estimated concerning the ATC level III or IV in which the product is included. For score d) of Level II, the Level-III score should be determined in comparison to all the products included in the same ATC level V.
3.2. Risk-management strategies
Based on the scores obtained in the constant part of the algorithm, decision trees for risk-management strategies (i.e. variable part of algorithm) can be built up according to the features of different settings (e.g., manufacturers, wholesalers, hospitals, pharmacies) or National regulatory frameworks. Some examples of the possible applications to the variable part of the algorithm are reported below.
3.2.1. Manufacturers’ strategies to reduce shortages
A medicine shortage may be caused by a blockage of the production for different causes (e.g., the unavailability of raw materials, quality-failures, GMP no-compliance, quality defects). In this context, considerable efforts have been made from Regulatory Authorities and professional associations to improve the quality in the pharmaceutical processes to minimize the risk for the patients and improve the effectiveness of the manufacturing processes (ISPE, 2014, PDA, 2014, EMA, 2019c; Various Associations, 2019b). For example, a nine-fold increase in the EMA quality guidelines was observed between 2005 and 2015. To mitigate the risk of quality-failures, the EMA promoted the application of the quality-by-design and quality risk assessment principles to the manufacturing of medicinal products (EMA, 2015a, EMA, 2015b, EMA, 2017). The rationalization of pharmaceutical manufacturing and the control strategies can be derived from a better knowledge of the product and process. The traditional quality control process has been generally based on the testing of most critical parameters and manufacturing steps can impact on the quality of the intermediates or the final medicinal product. The control setpoints and operating ranges are, therefore, fixed based on the experiments carried out in the pharmaceutical development, and their change should be notified/authorized by a Regulatory Authority as post-approval change. The application of a modern pharmaceutical quality system promotes a systematic and continuous evaluation, understanding and refining of the formulation and the manufacturing process by the identification of the critical material attributes and process parameters and by the determination of the existing functional relationship between such factors and the responses (i.e. quality profile of the products). However, effective pharmaceutical quality systems require close collaboration between the MAH and their suppliers of raw materials to promote a more rational definition of the critical quality attributes of the drug product and to improve the production resilience. The implementation of the quality risk management plan, including shortage-specific prevention and management plan, was proposed to enhance the resilience of manufacturers to shortages, (ISPE, 2014, PDA, 2014, Panzitta et al., 2017). For example, Panzitta and co-workers proposed a qualitative risk assessment based on a classification of different risk factors, causing a shortage in the manufacturing process (Panzitta et al., 2017). Based on the resulting risk ranking, the manufacturer can define proper strategies to prevent shortage (e.g., increase of the raw material suppliers, expansion of the material stocks). However, some measures may not apply to all the situation since their high impact on economic sustainability. For example, the maintenance of large material stockpiles or the expansion of the suppliers’ number, with the relative increase of inspections and audits, might be not sustainable for manufacturers of the low-price drug products. For these considerations, the adoption of the preventing tools proposed by the stakeholders’ associations (PDA, 2014, ISPE, 2014) could be carried out gradually by the MAHs based on both the shortage impact of a product on the treated patients and the economic sustainability for the MAHs production.
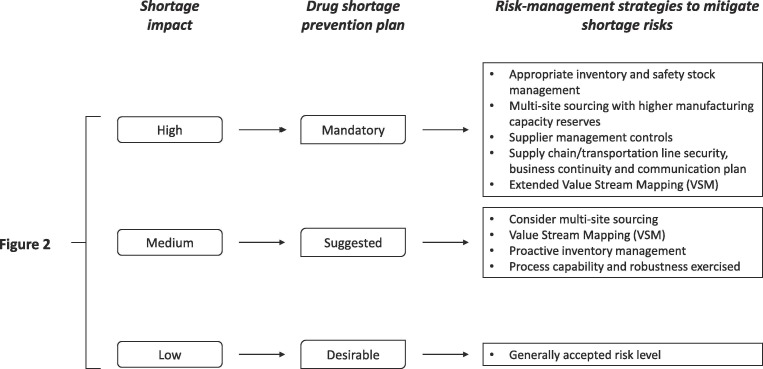
In this context, the proposed algorithm can be applied to enforce the gradual implementation of the pharmaceutical quality systems for MAHs most vulnerable to shortage risks. As shown in Fig. 3 , the obligations for MAHs are determined based on the shortage impact score. For products with a high shortage impact, the establishment of a shortage prevention plan seemed essential to ensure an appropriate and continued supply to fulfil patients’ needs. For such products, manufacturer’s resilience should be improved by more onerous provisions, such as the obligation for MAHs to qualify at least two suppliers of drug substance, the stockpiles’ expansion and the implementation of the manufacturing technologies and risk assessment tools. In this context, the establishment of ad hoc agreements between European countries and MAH to conduct exceptional manufacturing activities in specific National sites can also be pursued. As an example, in Italy, National military manufacturing site has been involved in such activities to mitigate the impact of severe shortage, especially in the case of old and low-cost medicinal products (Di Giorgio et al., 2019). On the other side, if the shortage impact of medicinal products on the public health is low, it is not reasonable to charge the MAHs with additional costs and, therefore, affect the economic sustainability for the implementation of quality assurance systems that may have a limited positive impact on the continuity of care.
Fig. 3.
Decision tree for risk control strategies for manufacturers. For drug shortage prevention plan please refers to the ISPE document (ISPE, 2014), while control strategies are extrapolated in agreement with the priority level proposed in the PDA Technical report no. 68 (PDA, 2014).
3.2.2. Strategies to improve economic sustainability
Medicine prices are cited among the causes of shortages worldwide (Jia and Zhao, 2017, Bochenek et al., 2018, Heiskanen et al., 2017, De Weerdt et al., 2015b, Dave et al., 2018). Indeed, especially for low-price medicinal products, the market competition may affect the economic sustainability of the MAHs significantly, decreasing their manufacturing capacity and resilience to demand fluctuation. In this context, MAHs may not be able to fulfil appropriately the guidance reported in the ISPE drug shortage prevention plan as suggested by the last HMA/EMA guideline (EMA and HMA, 2019a). Indeed, especially for old medicinal products, the costs of the upgrade to the required high-quality standards and risk assessment plan may be too high to maintain the MAH economic sustainability. In this context, European associations representing manufacturers, parallel distributors, pharmaceutical full-line wholesalers and industrial pharmacists recently stressed the need of establishing proper reward actions by the competent Authorities for supporting their activities to comply with regulation upgrades (e.g., Falsified Medicines Directive) (Various Associations, 2019b).
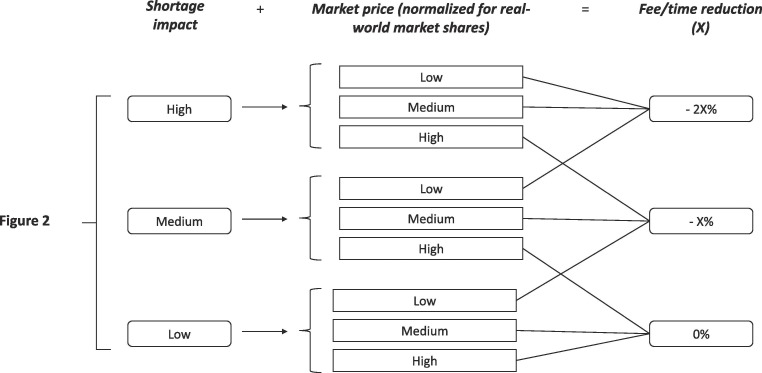
The application of economic incentives to MAHs has been proposed as a solution to prevent shortages. For example, Jia and Zhao demonstrated that a price increase of 30% results in a significant reduction of risk of shortage in the US market (Jia and Zhao, 2017). However, a similar approach cannot be fully applied in the EU, considering the fragmentation of the European price and reimbursement policies (WHO, 2018). Economic incentives to MAHs producing medicinal products can be provided as cut-off of the regulatory costs/times to implement the quality in their manufacturing process and to improve their resilience to production blockage. It is not feasible to introduce economic incentives for all medicinal products since it can affect the budgets of the Regulatory Authorities or, more in general, those of the National Healthcare Systems. Therefore, proper harmonized decision trees should be designed to apply regulatory incentives only to the most vulnerable medicinal products to shortages. Since fees and times for dossier variations vary significantly among the European Regulatory Authorities (EGA, 2015), the extent of the regulatory incentives cannot be defined a priori at EU-level, but they should be calculated as percentages of current fee and regulatory time to be more adaptable to the different National settings (Fig. 4 ).
Fig. 4.
Decision tree for regulatory incentives.
Fig. 4 showes a hypothetical decision tree for regulatory incentives that merge the shortage impact score obtained by the constant part of the algorithm (Fig. 2) and the product price. The latter was normalized by the real-world market share. Indeed, at the same market price, the incentive impact is more relevant for low-volume medicinal products than high-volume ones.
However, as Jia and Zhao demonstrated (Jia and Zhao, 2017), the efficacy of the regulatory incentives in preventing shortages is also linked to the creation or the strengthened of failures-to-supply clauses for MAHs in the reimbursement agreements. These strategies permit to improve MAH sustainability and to ensure a proper supply for the patient demand. Noteworthy, it is not expectable that such provisions can solve the economic sustainability of a product in a long-term perspective, but there is no doubt that they can mitigate the MAH situations in short and medium-term, allowing the adoption of proper strategies to rationalize the production and to improve MAH sustainability.
3.2.3. Importation/exportation mitigation strategies
If a medicinal product is affected by an expected/ongoing shortage in a specific country, the competent National Authorities can authorize its importation from other countries (EU or extra-EU) to ensure the patients’ needs. A recent survey on German Pharmacists demonstrated that 38% of community and about 90% of hospital pharmacies have imported medicinal products in the last three months (Said et al., 2018). German pharmacists are allowed to import medicinal products only in presence of an order for a specific individual patient and in a small quantity when no products, which are identical in terms of drug substances and comparable in strength, are available in Germany for the same therapeutic indications [Section 73 (3) of the Medicinal Products Act (Arzneimittelgesetz – AMG)]. In other European countries, the importation of medicinal products is also permitted if the importer obtained a formal authorization of the competent National Authorities (e.g., Italy). For example, the AIFA periodically releases on its portal a list of medicinal products in shortage for which hospitals are authorized to import them (234 products are included in the list at 9th August 2019) (AIFA, 2019a). Unlike the AIFA list of medicinal products in shortage (more than 2000 items) (AIFA, 2019a), the import-authorization list includes only medicinal products for with no therapeutic alternatives are available on the Italian market. Regardless of the procedure required at the National level, the medicine importation is, in most of the cases, the unique possibility for healthcare professionals to ensure the continuity of care to patients if a National therapeutic alternative is not available. However, an excessive recourse to parallel imports from other European countries may harm medicine unavailability in other ones for a ripple effect. This effect may be worsened by uncontrolled medicine movements for economic reasons by parallel trade, under the Art. 34 of the Treaty on the Functioning of the European Union (TFEU) (Di Giorgio et al., 2019). However, Art. 36 of TFEU enabled competent National Authorities to establish prohibitions or restrictions on parallel imports, exports on the grounds of the protection of health and life of humans (European Commission, 2018). Consequently, several European countries included specific restrictions of the product exportation to preserve the National stockpiles during shortages in their regulatory frameworks (Bochenek et al., 2018). However, restriction criteria for medicines affected by shortage are not harmonized at the EU level. In France and Poland, the exportation ban applies to all medicinal products of high therapeutic value that are in shortages. In Greece, the ban was first applied to vaccines and then extended to all medicinal products in shortages. In Spain, the competent Authority can restrict exportation only to medicinal products with not therapeutically equivalents. In Italy, the competent Authorities adopted a combined approach to prevent that exportations could worsen the products shortages/unavailabilities. On the one side, the AIFA has started a preventive verification program on the exporting wholesalers and distributors to detect potential violations of good distribution practice and traceability rules, causing abnormal exportation of medicinal products. As reported by Di Giorgio et al., a pilot verification project conducted showed effectiveness in reducing the distribution-related unavailability on specific tracing products (Di Giorgio et al., 2019). In particular, the adopted counteracting measures seemed more efficient in preventing unavailabilities that affected community pharmacies than hospital ones. On the other side, the Italian regulatory framework has been updated to permit AIFA to impose temporary blockage of exportations to prevent or mitigate drug shortages or unavailability of a medicinal product authorized in Italy (Art. 13, Decree-Law n. 35 of 30th April 2019, as converted by Law n. 60 of 25 June 2019). At the moment, seven medicinal products [i.e. four Sinemet® strengths (levodopa/carbidopa), Questran® (cholestyramine), Famotidina EG® (famotidine), Ongentys® (opicapone)] are included in the AIFA list for exportation ban (AIFA, 2019b, AIFA, 2019). In most of the cases, the list includes products for which the shortage has recently ended and for which there are distribution-related unavailabilities in Italy (i.e. products containing levodopa/carbidopa and cholestyramine). Indeed, the provision aims to prevent that parallel exportation may delay the wholesalers’ capability in restoring the supply to the Italian community and hospital pharmacies during the post-emergency period. The medicinal products containing opicapone (Ongentys® 50 mg, 30 capsules) and famotidine (Famotidina EG® 40 mg, 10 tablets) have been included for preventive purpose. Ongentys® is the only medicinal product containing opicapone, which is used to treat adults with Parkinson’s disease. Since the MAH had communicated the medicine shortage from 6th November 2019 to 15th January 2020 for manufacturing issues, the exportation ban was activated by the AIFA to prevent any medicine depletion of the National stock that could affect the patient’s access to therapy. Famotidine is the only drug substance with the same ATC level IV of ranitidine available on the Italian market. After the detection of a possible cancerogenic impurity (i.e., NDMA) in several batches of drug substance, EMA required the massive withdrawal of ranitidine products around the EU (EMA, 2019d). Consequently, the famotidine demand has exceeded the MAH production capability, running out the available stocks. In this context, the exportation ban established by AIFA aimed to preserve the National product stocks for removing as long as possible the onset of famotidine shortage.
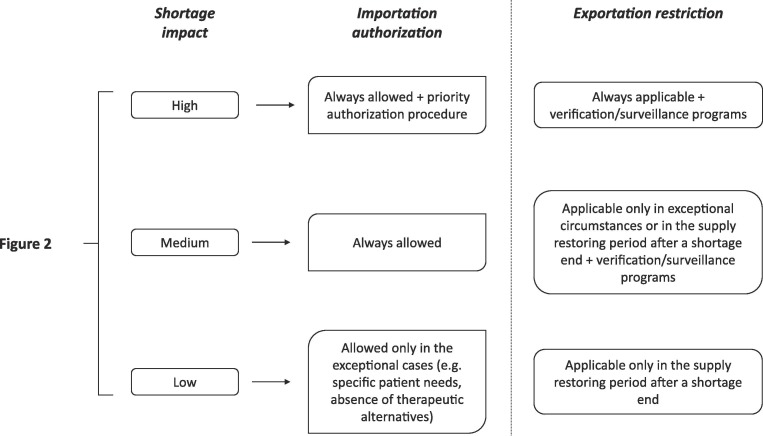
Here again, the establishing a unique decision tree to support National decision about medicine importation/exportation could be useful to reduce the fragmentation of shortage-mitigating strategies among European countries and to improve the effectiveness of the National strategic plan to satisfy the patients’ needs, without altering the National regulatory framework.
As shown in Fig. 5 , a hypothetical decision tree may link the possibility to import or block the exportation to the shortage impact score established with the constant part of the algorithm (Fig. 2). For high/medium impact medicinal products, the importation should be allowed during an ongoing shortage. In the case of high-impact products, fast authorization procedures should also be adopted to speed up the importation from other EU (parallel imports) or extra-EU countries with similar pharmaceutical quality standards to the EU, from which the medicine can be exported without any ripple effect. On the other side, it may not be convenient in the case of low-shortage-impact products, since therapeutic alternatives are available at the National level, and they may worsen the shortage situation in other European countries. However, the importation of medicinal products should always be allowed in exceptional cases, such as a substantial reduction of the National supply of all therapeutically equivalents or specific patients’ needs justified by their clinical conditions (e.g., allergies to excipients, rare metabolic diseases). Moreover, a similar decision tree can also be used to support National Competent Authorities in the adoption of regulatory pathways such as Art. 126a of Directive 2001/83/EC to ensure the availability of medicines in a specific market, particularly for those with a high shortage impact on public health.
Fig. 5.
Decision tree for importation/exportation mitigation strategies.
The blockage/restriction of exportations can be feasible as preventing/mitigating strategies in specific circumstances. It is the case of all medicinal products for which the shortage has recently ended, and the supply is lower than a warming level and cannot adequately fulfil the National demand. Here again, the establishment of an exportation ban can be useful for high-impact products since the maintenance of National stocks directly influence the capability of healthcare systems to ensure patient access to therapy. For low-shortage-impact products, such strategies may be a too restrictive provision because of the negligible effects on the continuity of the care. Indeed, therapeutically equivalent medicinal products can be substituted with therapeutic alternatives without compromising the safety of patients. For medium-shortage-impact products, the adoption of exportation restrictions should be evaluated case-by-case based on the availability of other therapeutic options.
Moreover, the promising results obtained by some National competent Authorities (e.g., AIFA) suggested that setting up cooperation schemes between administration and other stakeholders, fostering a responsible approach in managing crisis in medicines supply, also allowing, for instance, surveillance/verification programs that may represent rational and effective preventive strategies to monitor the activities of exporting wholesalers and distributors for reducing the risks of product unavailability. If such tools are strongly necessary for high-shortage-impact products, their adoption as a preventive action to monitor medium- and low-impact products is also desirable. However, for low- and medium-impact products, the development of protocols to rationalize the clinical medicine substitution or to activate importation procedures may be more effective. In this context, education programs should be performed by competent Authorities to ensure update of healthcare professionals on these topics.
3.3. Communication to the public
The shortage impact scores calculated with the constant part of the algorithm can be used to improve and rationalize how to communicate a shortage to the public and patients. Recommendations how to better communicate shortages to patients were introduced by the last HMA/EMA guideline (EMA and HMA, 2019b). In this context, it is worth underlining that communication strategies should be carefully planned based on the level of shortage criticality for public health (high, medium, low). Without any doubt, the patients’ awareness about shortage is an important aspect that the competent Authorities have to consider in every mitigation action they can adopt. However, it is essential to avoid alarmism in patients, especially when therapeutic alternatives are promptly available. High-profile communication tools (e.g., press release, social network, television, radio) should be preferred to communicate the shortages of medicinal products with high impact score, whereas drug alerts to healthcare professionals and patient’s associations can be sufficient for products with medium and low impact scores. Moreover, the shortage-impact scores can be used as a basis for harmonizing the graphical formats of the communication campaigns in all the EU countries. For examples, red, yellow, and green colours can be used in the systematic National shortage lists, but also in the paper communications, posts on social networks, alerts in the software used by physicians and pharmacists to better identify the medicinal products with high, medium and low impact scores, respectively.
4. Conclusion
Economic globalization has promoted the delocalization and gathering of manufacturing sites of drug substances and medicinal products, stressing the resilience of the pharmaceutical supply chain of many medicinal products to the shortages. The European countries cannot face the different shortage crisis alone, but the cooperation at EU-level is necessary to provide practical and rational solutions to the problem. For mitigating shortages that affect the Union, the stronger coordination at EU-level and the harmonization of communication, risk-assessment and risk-management strategies are desirable and urgent. Although further studies in real-world settings are needed to complete the validation of the procedure and decision trees, the approach proposed in this article may contribute to improving the information sharing and cooperation among European countries. Indeed, risk-assessment and risk-management strategies adopted by professionals (e.g., pharmacists) and other stakeholders can be rationalized and harmonized based on the medicines shortage impact scores calculated by a competent National Authority.
Moreover, the structure of procedure and decision trees may be adapted and used as a model to build up risk-management strategies for local needs of a specific healthcare assistance setting (e.g., single hospital). However, considering that the shortage-impact assessment is linked to the authorized therapeutic indications of medicinal products in the procedure here proposed, a limitation of the latter, which is also valid for other shortage-mitigation strategies, is that the off-label use of medicinal products in specific patient populations (e.g., paediatrics) is not tracked and, therefore, the shortage impact may not be assessed adequately in this field. In this context, following the example of the provision adopted by several National Authorities to ensure patient access to immunoglobulins treatment (e.g., France, Germany, UK), the establishment of treatment priority rankings, clinical practice guidelines and clinical records may be possible solutions. For these activities, the involvement of EMA scientific committees can be considered an added value for supporting the efforts of competent National Authorities. In parallel, the cooperation among Regulatory Authorities and stakeholders of the pharmaceutical distribution chain should also be improved to set up shared strategies to mitigate distribution-related unavailability in specific geographic regions and to prevent shortages. In this context, the establishment of permanent consultation or task force groups is desirable both at National and European levels for speeding up the resolution of medicine shortage emergency.
CRediT authorship contribution statement
Umberto M. Musazzi: Conceptualization, Methodology, Investigation, Writing - original draft. Domenico Di Giorgio: Methodology, Writing - review & editing. Paola Minghetti: Visualization, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Paola Minghetti and Umberto M. Musazzi have no conflict of interest. Domenico Di Giorgio states that this scientific publication does not imply any current or potential conflict of interest with the Administration of affiliation; the view and opinions expressed are those of co-author and should not be attributed to AIFA.
Acknowledgement
Umberto M. Musazzi would like to express his gratitude to the COST Action CA15105 “European Medicines Shortages Research Network – addressing supply problems to patients (Medicines Shortages)” for supporting the research work and promoting collaboration among European experts on shortages.
References
- Aguiar E., Ernest K. Letter to the editor - Factual inaccuracies of the article Causes of drug shortages in the legal pharmaceutical framework. Regul. Toxicol. Pharm. 2020;111 doi: 10.1016/j.yrtph.2019.104551. [DOI] [PubMed] [Google Scholar]
- AIFA, 2019a. Farmaci carenti. https://www.aifa.gov.it/web/guest/farmaci-carenti (accessed: 21st August 2019).
- AIFA, 2019b. Determination n. 1635/2019 of 31st October 2019 “Elenco dei medicinali che non possono essere sottratti alla distribuzione e alla vendita per il territorio nazionale al fine di prevenire o limitare stati di carenza o indisponibilità” [List of medicinal products that cannot be removed from distribution and dispensation in the national territory to prevent or limit states of shortage or unavailability].
- Beck M., Buckley J., O’Reilly S. Managing pharmaceutical shortages: an overview and classification of policy responses in Europe and the USA. Int. Rev. Adm. Sci. 2019 [Google Scholar]
- Birgli . Birgli® on behalf of EAEPC, Brussels. 2013. An evaluation of medicines shortages in Europe with a more in-depth review of these in France, Greece, Poland, Spain and the United Kingdom. [Google Scholar]
- Bochenek T., Abilova V., Alkan A., Asanin B., de Miguel Beriain I., Besovic Z., Vella Bonanno P., Bucsics A., Davidescu M., De Weerdt E., Duborija-Kovacevic N., Fürst J., Gaga M., Gailīte E., Gulbinovič J., Gürpınar E.U., Hankó B., Hargaden V., Hotvedt T.A., Hoxha I., Huys I., Inotai A., Jakupi A., Jenzer H., Joppi R., Laius O., Lenormand M.C., Makridaki D., Malaj A., Margus K., Marković-Peković V., Miljković N., de Miranda J.L., Primožič S., Rajinac D., Schwartz D.G., Šebesta R., Simoens S., Slaby J., Sović-Brkičić L., Tesar T., Tzimis L., Warmińska E., Godman B. Systematic measures and legislative and organizational frameworks aimed at preventing or mitigating drug shortages in 28 European and Western Asian countries. Front. Pharmacol. 2018;8:942. doi: 10.3389/fphar.2017.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet F., Degrassat-Theas A., Peigné J., Paubel P. The new regulatory tools of the 2016 Health Law to fight drug shortage in France. Health Policy. 2017;121:471–476. doi: 10.1016/j.healthpol.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Carrock Sewell W.A., Kerr J., Behr-Gross M.-E., Peter H.-H., on behalf of the Kreuth Ig Working Group European consensus proposal for immunoglobulin therapies. Eur. J. Immunol. 2014;44:2207–2214. doi: 10.1002/eji.201444700. [DOI] [PubMed] [Google Scholar]
- Dave C.V., Pawar A., Fox E.R., Brill G., Kesselheim A.R. Predictors of drug shortages and association with generic drug price: a retrospective cohort study. Value in health. 2018;21:1286–1290. doi: 10.1016/j.jval.2018.04.1826. [DOI] [PubMed] [Google Scholar]
- De Weerdt E., Simoens S., Casteels M., Huys I. The necessity for a European definition of drug shortages. Int. J. Pharm. Pract. 2018;26:289–290. doi: 10.1111/ijpp.12459. [DOI] [PubMed] [Google Scholar]
- De Weerdt E., Simoens S., Casteels M., Huys I. Toward a European definition for a drug shortage: a qualitative study. Front. Pharmacol. 2015;6:253. doi: 10.3389/fphar.2015.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weerdt E., Simoens S., Hombroeckx L., Casteels M., Huys I. Causes of drug shortages in the legal pharmaceutical framework. Regul. Toxicol. Pharm. 2015;71:251–258. doi: 10.1016/j.yrtph.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Di Giorgio, D., Scrofina, G., Scognamiglio, B., Di Carluccio, N., Tulimero, R., Pietrosanto, A., Petrone, P., De Iure, M., Chimenti, M.G., Pozzetti, E., Giacomazzi, M., Berno, R., Lupo, M., Giaccone, M., Pani, M., Cesta, E., Cruciani, O., Maione, C., Gramazio, M., Derossi, G., 2019. Tackling distribution-related shortages of medicines: an Italian case of study evaluated in the European Union framework. MA@PoC. https://doi.org/10.1177/2399202619856859.
- EGA . EMA; Brussels: 2015. Regulatory efficiency report 2015. [Google Scholar]
- EMA and HMA . EMA; Amsterdam: 2019. Guidance on detection and notification of shortage of medicinal products for Marketing Authorisation Holders (MAHs) in the Union (EEA). EMA/674304/2019. [Google Scholar]
- EMA and HMA . EMA; Amsterdam: 2019. Good practice guidance for communication to the public on medicines’ availability issues. EMA/632473/2018. [Google Scholar]
- EMA . EMA; London: 2013. Decision tree on escalation from national to European Level. EMA/314722/2013. [Google Scholar]
- EMA . EMA; London: 2015. ICH guideline Q10 on pharmaceutical quality system – step 5. EMA/CHMP/ICH/214732/2007. [Google Scholar]
- EMA . EMA; London: 2015. ICH guideline Q9 on quality risk management – step 5. EMA/CHMP/ICH/24235/2006. [Google Scholar]
- EMA . EMA; London: 2017. ICH guideline Q8 (R2) on pharmaceutical development – step 5. EMA/CHMP/ICH/167068/2004. [Google Scholar]
- EMA, 2019a. European authorities working to avoid shortages of medicines due to Brexit – questions and answers. https://www.ema.europa.eu/en/documents/other/european-Authorities-working-avoid-shortages-medicines-due-brexit-questions-answers_en.pdf (accessed: 30th January 2019).
- EMA, 2019b. Angiotensin-II-receptor antagonists (sartans) containing a tetrazole group. https://www.ema.europa.eu/en/medicines/human/referrals/angiotensin-ii-receptor-antagonists-sartans-containing-tetrazole-group (accessed: 21st August 2019).
- EMA, 2019c. Guidance for regulators on shortages due to manufacturing or quality issues. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/availability-medicines#guidance-for-regulators-on-shortages-due-to-manufacturing-or-quality-issues-section (accessed: 21st August 2019).
- Ranitidine-containing medicinal products. https://www.ema.europa.eu/en/medicines/human/referrals/ranitidine-containing-medicinal-products (accessed: 9th October 2019).
- EMEA . EMA; London: 2000. Note for guidance on the investigation of bioavailability and bioequivalence. CPMP/EWP/QWP/1401/98. [Google Scholar]
- European Commission, 2018. Paper on the obligation of continuous supply to tackle the problem of shortage of medicines. Agreed by the ad-hoc technical meeting under the Pharmaceutical Committee on shortage of medicine on 25 May 2018. European Commission, Brussels.
- Farrugia C. Malta: preparations for the impact of Brexit on the pharmaceutical supply chain in Malta – the perfect storm. Pharmaworld Magazine. 2018 Available at: https://www.pharmaworldmagazine.com/preparations-for-impact-of-brexit-pharmaceutical-supply-chain-malta/ (accessed: 17th February 2020) [Google Scholar]
- Heiskanen K., Ahonen R., Kanerva R., Karttunen P., Timonen J. The reasons behind medicine shortages from the perspective of pharmaceutical companies and pharmaceutical wholesalers in Finland. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0179479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HMA, 2019. HMA/EMA task force on availability of authorised medicines for human and veterinary use (TF AAM). https://www.hma.eu/522.html (accessed: 21st August 2019).
- ISPE . A holistic view for root cause to prevention. ISPE; Tampa: 2014. Drug shortages prevention plan. [Google Scholar]
- Italian Republic, 1993. Art. 8, Law No. 537 of 24th December 1993. Official Journal of Italian Republic No. 303 of 28th December 1993.
- Italian Republic, 2019. Art. 13, Decree-Law n. 35 of 30th April 2019, as converted by Law No. 60 of 25 June 2019. Official Journal of Italian Republic No. 101 of 2nd May 2019.
- Jia J., Zhao H. Mitigating the U.S. Drug Shortages through Pareto-improving contracts. POMS. 2017;26:1463–1480. [Google Scholar]
- Panzitta M., Ponti M., Bruno G., Cois G., D’Arpino A., Minghetti P., Mendicino F.R., Perioli L., Ricci M. The Strategic relevance of manufacturing technology: an overall quality concept to promote innovation preventing drug shortage. Int. J. Pharm. 2017;516:144–157. doi: 10.1016/j.ijpharm.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Parsons H.M., Schmidt S., Karnad A.B., Liang Y., Pugh M.J., Fox E.R. Association between the number of suppliers for critical antineoplastics and drug shortages: implications for future drug shortages and treatment. J. Oncol. Pract. 2016;12:e289–e298. doi: 10.1200/JOP.2015.007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PDA . PDA; Bethesda: 2014. PDA technical report No. 68 - Risk-Based Approach for Prevention and Management of Drug Shortages. [Google Scholar]
- PGEU . PGEU; Brussels: 2019. Position Paper on medicine Shortage. [Google Scholar]
- Phuong J.M., Penm J., Chaar B., Oldfield L.D., Moles R. The impacts of medication shortages on patient outcomes: a scoping review. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0215837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said A., Goebel R., Ganso M., Zagermann-Muncke P., Schulz M. Drug shortage may compromise patient safety: results of a survey of the reference pharmacies of the Drug Commission of German Pharmacists. Health Policy. 2018;122:1302–1309. doi: 10.1016/j.healthpol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Schwartzberg E., Ainbinder D., Vishkauzan A., Gamzu R. Drug shortage in Israel: regulatory perspectives, challenges and solutions. IJHPR. 2017;6:17. doi: 10.1186/s13584-017-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Various Associations, 2019a. Request to start an investigation into the factors leading to medicines shortages. European associations of patients, consumers, healthcare professionals and public health advocates letter to the European Commission. https://www.eahp.eu/sites/default/files/request_to_start_an_investigation_into_the_factors_leading_to_medicines_shortages.pdf (accessed: 23rd January 2020).
- Various Associations, 2019b. Addressing the root causes of medicines shortages. Supply chain stakeholder’s views on root causes and solution. https://www.efpia.eu/media/413378/addressing-the-root-causes-of-medicines-shortages-final-051219.pdf (accessed: 23rd January 2020).
- WHO, 2003. Drug and therapeutics committees - A practical guide (WHO/EDM/PAR/2004.1). Available at: https://apps.who.int/medicinedocs/en/d/Js4882e/ (accessed: 21st August 2019).
- WHO, 2018. Medicine reimbursement policies in Europe.
- Wouters O.J., Kanavos P.G., Mckee M. Comparing Generic Drug Markets in Europe and the United States: Prices, Volumes, and Spending. Milbank Q. 2017;95:554–601. doi: 10.1111/1468-0009.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]