Abstract
Background
Severe community-acquired pneumonia (SCAP) represents a frequent and potentially life-threatening condition. About 10% of all hospitalized patients with CAP require admission to the intensive care unit (ICU), and the mortality of these patients reaches 20–50%.
Objective
To evaluate the clinical presentation, bacteriological profile and outcome of severe community-acquired pneumonia (SCAP).
Patients and methods
54 patients presented by symptoms and sign of severe community acquired pneumonia who were admitted to respiratory care unit of Alhussein, Al-Azhar University Hospital from August 2015 to March 2016 were subjected to full clinical examination, chest X ray, complete blood picture, sputum and blood culture, PCR for suspected cases of Influenza H1N1 and MERS-COV, treatment, follow up, data collections and statistical analysis.
Results
The present study included 54 patients 26 males and 28 females with SCAP who were admitted to respiratory care unit of Alhussein, Al-Azhar University Hospital. The most common comorbidities were diabetes mellitus and hypertension. The most common presentations were fever, cough, dyspnea and hypoxemia. Two patients developed renal failure and 4 patients developed septic shock. The most common isolated organism was Streptococcus pneumoniae, Influenza H1N1, and Staphylococcus aureus. Mortality was 24% and it was common in patients with comorbidity than in patients without comorbidities.
Conclusion
SCAP occurs more frequently in those with comorbidities. The most frequent isolated causative organism of SCAP is S. pneumoniae, Influenza H1N1 and S. aureus. SCAP is associated with significant mortality, early recognition and prompt treatment may improve outcome.
Keywords: Presentation, Outcome, Severe community acquired pneumonia
Introduction
Community-acquired pneumonia (CAP) is defined as an infection of the lung parenchyma that is not acquired in a hospital, long-term care facility, or other contact with the health care system [1]. CAP continues to be a major cause of morbidity and mortality. Despite the availability of adequate anti biological agents to treat this illness, it has remained the fourth most common cause of death in Japan since 1975 [2]. Common causative agents of pneumonia in ambulatory patients are Streptococcus pneumoniae, Mycoplasma pneumoniae, Haemophilus influenzae, Chlamydia pneumoniae and respiratory viruses (influenza A and B, adenovirus, respiratory syncytial virus and parainfluenza) [3], [4]. Mortality was the highest for Staphylococcus aureus (50%), and the lowest for Chlamydia pneumoniae (4.5%). Mortality was not seen with M. pneumoniae. Pneumonia due to aerobic Gram negative organisms was uncommon, even though empirical therapy with a combination of broad-spectrum antibiotics was often used in this subgroup [5]. Severe community-acquired pneumonia (SCAP) represents a frequent and potentially life-threatening condition. About 10% of all hospitalized patients with CAP require admission to the intensive care unit (ICU), and the mortality of these patients reaches 20–50% [6]. Severe CAP has been defined as those cases that require admission to the ICU. Direct admission to an ICU is required for patients with septic shock or acute respiratory failure requiring invasive mechanical ventilation, which are defined as major severity criteria in the modified score of the American Thoracic Society (ATS) guidelines that are used to define severe CAP [7].
Aim of the work
To evaluate the clinical presentation, bacteriological profile and outcome of severe community acquired pneumonia.
Study design
Prospective study.
Patients and methods
After the approval of local ethics committee 54 patients (26 males and 28 females) presented by symptoms and sign of severe community acquired pneumonia who were admitted to respiratory care unit of Alhussein, Al-Azhar University Hospital from August 2015 to March 2016 were subjected to the following:
-
(1)
History taking: Fever, cough, pleuritic chest pain, dyspnea, mental confusion and comorbidities.
-
(2)
Clinical examination: Both general and local examination of the chest.
-
(3)
Plain chest X-ray (CXR): A chest radiograph was done for all patients who were likely to have pneumonia to establish the diagnosis, and follow up when needed.
-
(4)
Chest computed tomography (CT) was done when indicated.
-
(5)Laboratory Investigations: It was done in clinical pathology department of Alhussien University Hospital, Central laboratories of ministry of health and private laboratories.
-
–Complete blood picture: Total leukocyte count, (TLC), hemoglobin concentration. Blood film to demonstrate differential white blood cell count (WBCs) and morphology of red blood cells (RBCs), erythrocyte sedimentation rate (ESR) using Western Green method. Liver function test (alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP)-total and direct bilirubin-total proteins, and albumin. Kidney function test (creatinine and urea) Fasting blood sugar, and Post prandial (2 h) blood sugar.The diagnosis of severe community acquired pneumonia (SCAP) was done according to the Infectious Diseases Society of America/American Thoracic Society.
-
–
-
(6)
Microbiological evaluation: Sputum, blood culture (2 samples from 2 different sites after complete aseptic condition to avoid contamination), pleural fluid, transthoracic needle aspiration, tracheobronchial aspirate, and bronchoalveolar lavage (BAL) fluid in selected cases (BALF). Samples were plated on the following media: blood agar, MacConkey agar, chocolate agar and Sabouraud agar. Staining of selected samples was done by gram stain. Urine was tested for the presence of S. pneumoniae and Legionella antigen. Identification of microorganisms and susceptibility testing was performed according to standard methods [8].
Quantitative sputum culture (QSC)
Sputum obtained from adults having clinically and/or radiologically diagnosed CAP. All of the samples contained >=25 polymorphonuclear leukocytes (PNL), and <10 epithelial cells per low-power field (LPF) under light microscopy. Quantitative cultures were performed. Blood and chocolate and MacConkey agar plates were used for culture and standard microbiological methods were used for bacterial identification. 24 and 48 h later both direct plates and quantitative cultures were observed. Cut off point >=105 CFU/ml was determined to be positive for the QSC [9].
Quantitative cultures of bronchoalveolar lavage (BAL) fluid, a colony count of ⩾104 CFU/ml represents a bacterial load which is indicative of bacterial pneumonia. A BAL fluid colony count below the 104 CFU/ml threshold points to oropharyngeal contamination. Quantitative cultures of transthoracic needle aspiration, tracheobronchial aspirate, a colony count of ⩾103 CFU/ml represents a bacterial load which is indicative of bacterial pneumonia. A BAL fluid colony count below the 103 CFU/ml threshold points to contamination [10].
Quantitative culture of the blood
A single bacterial count >100 CFU/ml in the quantitative culture of the blood specimen in the presence of a positive qualitative peripheral blood culture of the same organism is an indication of sepsis [11].
Ziehl Neelsen stain: Direct smear stained with Gram stain and Ziehl Neelsen stain (to detect acid fast bacilli).
Polymerase chain reaction: PCR to detect nucleic acids of Influenza H1N1 and Corona virus.
| Table [A]: Definition of severe community-acquired pneumonia according to the 2007 Infectious Disease Society of America/American Thoracic Society community-acquired pneumonia guidelines [8]. |
| Minor criteria: three or more of |
| Respiratory rate >30 breaths/min. |
| PaO2/FiO2 ratio <250. |
| Multilobar infiltrates. |
| Confusion/disorientation. |
| Uremia (BUN level >20 mg/dL). |
| Leukopenia (WBC count <4000 cells/mm3). |
| Thrombocytopenia (platelet count <100,000 cells/mm3). |
| Hypothermia (core temperature <36 °C). |
| Hypotension requiring aggressive fluid resuscitation. |
| Major criteria: one or more of |
| Invasive mechanical ventilation. |
| Septic shock with the need for vasopressors |
| Abbreviations: PaO2 = arterial oxygen tension; FiO2 = inspiratory oxygen fraction; BUN = blood urea nitrogen; WBC = white blood cell. |
Inpatient, ICU treatment [8]
-
(1)
A beta-lactam (cefotaxime, ceftriaxone, or ampicillin–sulbactam) plus either azithromycin (level II evidence) or a fluoroquinolone (level I evidence) (strong recommendation) (For penicillin-allergic patients, respiratory fluoroquinolone and aztreonam are recommended.)
-
(2)
For Pseudomonas infection, use an antipneumococcal, antipseudomonal beta-lactam (piperacillin–tazobactam, cefepime, imipenem, or meropenem) plus either ciprofloxacin or levofloxacin (750-mg dose) or the above beta-lactam plus an aminoglycoside and azithromycin or the above beta-lactam plus an aminoglycoside and an antipneumococcal fluoroquinolone (for penicillin-allergic patients, substitute aztreonam for the above beta-lactam). (Moderate recommendation; level III evidence.)
-
(3)
For community-acquired methicillin-resistant S. aureus infection, add vancomycin or linezolid. (Moderate recommendation; level III evidence).
Statistical analysis of data
Statistical analysis was carried out using the SPSS computer package version 17.0 (SPSS Inc., Chicago, IL, USA). The collected data were statistically managed as follows:
-
–
For descriptive statistics: mean ± SD were used for quantitative variables while the number and percentage were used for qualitative variables.
-
–
For analytic statistics: chi-square test was used to assess the differences in frequency of qualitative variables, while Fischer’s exact test (FET) was applied if any expected cell values in a 2́2 table was <5.
-
–
In order to assess the differences in means of quantitative variables between both groups, independent sample t-test was applied.
-
–
The statistical methods were verified, assuming a significant level of P < 0.05 and a highly significant level of P < 0.001.
Results
The present study included 54 patients 26 males and 28 females with SCAP. From 54 there were 28 patients 15 males and 13 females with comorbidities and 26 patients 11 males and 15 females without comorbidities. The most common comorbidities was diabetes mellitus and hypertension (13%), chronic bronchitis (11.1%) and bronchial asthma (7.4%) (Table 1 ).
Table 1.
Demographic data & distribution of comorbidity among the studied sample.
| Characteristics | No (%) (total no = 54) |
|---|---|
| Age in years (Mean ± SD) | 44.4 ± 5.8 |
| Gender | |
| Male | 26 (48.1%) |
| Female | 28 (51.9%) |
| Comorbidities | 28 (51.9%) |
| Male | 15 (27.8%) |
| Female | 13 (24.1%) |
| No comorbidities | 26 (48.1%) |
| Males | 11 (20.3%) |
| Females | 15 (27.8%) |
| Comorbidities | |
| 1-Bronchial asthma | 4 (7.4%) |
| 2-Chronic bronchitis | 6 (11.1%) |
| 3-Hypertension + DM | 7 (13.0%) |
| 4-Cardiomyopathy | 1 (1.9%) |
| 5-Mitral stenosis | 2 (3.7%) |
| 6-Systemic lupus erythematosus | 2 (3.7%) |
| 7-Liver cirrhosis | 1 (1.9%) |
| 8-Pulmonary fibrosis | 1 (1.9%) |
| 9-Hypogammaglobulinemia | 1 (1.9%) |
| 10-Wagner,s granulomatosis | 1 (1.9%) |
| 11-Septic emboli | 1 (1.9%) |
| 12-Hodgkin’s lymphoma | 1 (1.9%) |
As regards clinical presentation, fever was present in 85.7% of patients with comorbidities compared to 100% in patients without comorbidities, cough 100%, shortness of breath 92.5%, hypoxemia was 82.1% in patients with comorbidities compared to 76.9% in patients without comorbidities, expectoration was 78.6% in patients with comorbidities compared to 73.1% in patient without comorbidities, confusion was 25% in patients with comorbidities compared to 7.7% in patients without comorbidities. Low systolic blood pressure (BP) was 39.3% in patients with comorbidities compared to 11.5% in patients without comorbidities, low diastolic BP was 35.7% in patients with comorbidities compared to 7.7% in patients without comorbidities, differences were statistically significant. Complication in form of renal failure occurred in 7.1% and septic shock in 14.3% in patients with comorbidities compared to septic shock in 7.7% in patients without comorbidities, the difference was statistically non significant (see Table 2 ).
Table 2.
Comparison between patients with and without comorbidities regarding clinical presentation.
| Clinical presentation | Patients with comorbidities no = 28 (%) | Patients without comorbidities no = 26 (%) | P-value |
|---|---|---|---|
| Fever > 38.5 | 24 (85.7) | 26 (100.0) | 0.112 |
| Cough | 28 (100.0) | 26 (100.0) | – |
| Shortness of breath | 26 (92.9) | 24 (92.3) | 1.000 |
| Expectoration | 22 (78.6) | 19 (73.1) | 0.754 |
| Confusion | 7 (25.0) | 2 (7.7) | 0.144 |
| PaO2/FiO2 <250 | 23 (82.1) | 20 (76.9) | 0.741 |
| Systolic blood pressure <90 mmHg | 11 (39.3) | 3 (11.5) | 0.030⁎ |
| Diastolic blood pressure <60 mmHg | 10 (35.7) | 2 (7.7) | 0.021⁎ |
| Complications | |||
| Renal failure | 2 (7.1) | 0 (0.0) | 0.2611 |
| Septic shock | 4 (14.3) | 2 (7.7) | |
| No complications | 22 (78.6) | 24 (92.3) | |
Values analyzed by Fisher’s exact test.
Values analyzed by Chi-square test.
Significant.
Table 3 showed initial laboratory investigation, complete blood picture, blood urea nitrogen, serum creatinine, serum sodium, serum potassium and blood gas analyses. The difference between both groups was statistically significant as regards hemoglobin which was low in patients with comorbidities, high neutrophils count, high blood urea and serum creatinine in patients with comorbidities, compared to patients without comorbidities. Low PH and PaO2 with high PaCo2 in patients with comorbidities compared to patients without comorbidities the differences was statistically significant.
Table 3.
Comparison between patients with and without comorbidities regarding initial laboratory investigations.
| Laboratory investigations | Patients with comorbidity no = 28 | Patients without comorbidity no = 26 | P-value |
|---|---|---|---|
| Hemoglobin (g/dL) | 10.5 ± 2.3 | 14.8 ± 2.1 | <0.001⁎ |
| Total leukocyte count (×109/L) | 16.3 ± 5.1 | 15.4 ± 5.0 | 0.529 |
| Neutrophils (%) | 84.4 ± 8.3 | 78.3 ± 7.9 | 0.007⁎ |
| Lymphocytes (%) | 28.2 ± 7.6 | 31.1 ± 6.2 | 0.142 |
| Platelets (×109/L) | 223.0 ± 55.0 | 243.0 ± 68.4 | 0.242 |
| Serum BUN (mg/dL) | 45.6 ± 6.1 | 35.6 ± 4.8 | <0.001⁎ |
| Serum creatinine (mg/dL) | 2.2 ± 0.8 | 1.1 ± 0.6 | <0.001⁎ |
| Serum sodium (mmol/L) | 139.1 ± 5.0 | 135.0 ± 3.4 | 0.001⁎ |
| Serum potassium (mmol/L) | 3.6 ± 1.4 | 4.2 ± 0.7 | 0.056 |
| ABG | |||
| PH | 7.26 ± 0.09 | 7.36 ± 0.08 | <0.001⁎ |
| PaCo2 (mmHg) | 48.1 ± 8.7 | 33.0 ± 4.6 | <0.001⁎ |
| PaO2 (mmHg) | 41.1 ± 7.5 | 49.1 ± 7.9 | <0.001⁎ |
Values present as mean ± SD and analyzed by independent samples t-test.
Significant.
Table 4 showed chest X-ray finding. Bilateral patchy infiltrate in 35.1%, bilateral patchy infiltrate with cavitations in 3.7%, bilateral lower lobes infiltrate in 16.6%, bilateral lower lobes consolidation with pleural effusion in 3.7%, right lower lobe consolidation in 14.8%, left lower lobe consolidation in 11.1%, right upper lobe consolidation with cavitations in 3.7% and right lower lobe consolidation with cavitations in 3.7% and right lower lobe consolidation and pleural effusion in 7.4%.
Table 4.
Chest X-ray finding.
| Chest X-ray finding | Causative organism | No | No = 54 (%) |
|---|---|---|---|
| 1-Bilateral patchy infiltrate | – Influenza H1N1 | 7 | 19 (35.1%) |
| – Staphylococcus aureus | 5 | ||
| – Hemophilus influenza | 4 | ||
| – Streptococcus pneumonia | 3 | ||
| 2-Bilateral patchy infiltrate with cavitations | – Pseudomonas aeruginosa | 1 | 2 (3.7%) |
| – Staphylococcus aureus | 1 | ||
| 3-Bilateral lower lobes infiltrates | – Streptococcus pneumonia | 5 | 9 (16.6%) |
| – Moraxella catarrhalis | 3 | ||
| – Klebsiella pneumonia | 1 | ||
| 4-Bilateral lower lobes consolidation with pleural effusion | – Acinetobacter baumannii | 1 | 2 (3.7%) |
| – Streptococcus pneumonia | 1 | ||
| 5-Right lower lobe consolidation | – E. coli | 4 | 8 (14.8%) |
| – Pseudomonas aeruginosa | 3 | ||
| – Streptococcus pneumonia | 1 | ||
| 6-Left lower lobe consolidation | – Streptococcus pneumonia | 2 | 6 (11.1%) |
| – Klebsiella pneumonia | 2 | ||
| – Hemophilus influenza | 1 | ||
| – Acinetobacter baumannii | 1 | ||
| 7-Right upper lobe consolidation and cavitations | – Mycobacterium tuberculosis | 1 | 2 (3.7%) |
| – Klebsiella pneumonia | 1 | ||
| 8-Right lower lobe consolidation and cavitations | – Acinetobacter baumannii | 1 | 2 (3.7%) |
| – Klebsiella pneumonia | 1 | ||
| 9-Right lower lobe consolidation and pleural effusion | – Acinetobacter baumannii | 1 | 4 (7.4%) |
| – Klebsiella pneumonia | 1 | ||
| – Legionella species | 1 | ||
| – Hemophilus influenza | 1 |
Table 5 showed sputum culture results, S. pneumoniae was the most common organism, it occurred in 18.5%, S. aureus in 11.1%, Influenza H1N1 in 12.9%, Klebsiella pneumonia in 7.4%, H. influenzae in 9.2%, Moraxella catarrhalis in 3.7%, Legionella species in 1.8%, Pseudomonas aeruginosa in 5.5%, Acinetobacter baumannii in 3.7%, and Mycobacterial tuberculosis in 1.8%. No bacterial growth occurred in 24%.
Table 5.
Comparison between patients with and without comorbidities regarding sputum culture.
| Sputum culture | Patients with comorbidities no = 28 (%) | Patients without comorbidities no = 26 (%) | Total | P-value |
|---|---|---|---|---|
| 1-Streptococcus pneumonia | 5 (17.9) | 5 (19.2) | 10 (18.5%) | 1.000 |
| 2-Hemophilus influenza | 3 (10.7) | 2 (7.7) | 5 (9.2%) | 1.000 |
| 3-Moraxella catarrhalis | 1 (3.6) | 1 (3.8) | 2 (3.7%) | 1.000 |
| 4-Staphylococcus aureus | 3 (10.7) | 3 (11.5) | 6 (11.1%) | 1.000 |
| 5-Legionella species | 1 (3.6) | 0 (0.0) | 1 (1.8%) | 1.000 |
| 6-Klebsiella pneumonia | 4 (14.3) | 0 (0.0) | 4 (7.4%) | 0.112 |
| 7-Pseudomonas aeruginosa | 3 (10.7) | 0 (0.0) | 3 (5.5%) | 0.237 |
| 8-Acinetobacter baumannii | 2 (7.1) | 0 (0.0) | 2 (3.7%) | 0.491 |
| 9-Influenza H1N1 | 4 (14.3) | 3 (11.5) | 7 (12.9%) | 1.000 |
| 10-Mycobacterium tuberculosis | 1 (3.6) | 0 (0.0) | 1 (1.8%) | 1.000 |
| 11-Negative | 0 (0.0) | 13 (50.0) | 13 (24%) | <0.001⁎ |
Values analyzed by Fisher’s exact test.
Significant.
Table 6 showed blood culture result of both groups, organism isolated only in (31.5%), the organisms isolated were Escherichia coli in 7.4%, S. pneumoniae in 5.5%, S. aureus in 5.5%, K. pneumonia in 5.5%, P. aeruginosa in 1.8%, and A. baumannii in 3.7%.
Table 6.
Comparison between patients with and without comorbidity regarding blood culture.
| Blood culture | Patients with comorbidity no = 28 (%) | Patients without comorbidity no = 26 (%) | Total no = 54(%) | P-value |
|---|---|---|---|---|
| 1-Streptococcus pneumonia | 2 (7.1) | 1 (3.8) | 3 (5.5) | 1.000 |
| 2-Hemophilus influenza | 1 (3.6) | 0 (0.0) | 1 (1.8) | 1.000 |
| 3-Staphylococcus aureus | 2 (7.1) | 1 (3.8) | 3 (5.5) | 1.000 |
| 6-Klebsiella pneumoniae | 3 (10.7) | 0 (0.0) | 3 (5.5) | 0.237 |
| 7-Pseudomonas aeruginosa | 1 (3.6) | 0 (0.0) | 1 (1.8) | 1.000 |
| 8-Acinetobacter baumannii | 2 (7.1) | 0 (0.0) | 2 (3.7) | 0.491 |
| 7-E. coli | 3 (10.7) | 1 (3.8) | 4 (7.4) | 0.612 |
| 8-No growth | 14 (50.0) | 23 (88.5) | 37 (68.5) | 0.003⁎ |
Values analyzed by Fisher’s exact test.
Significant.
Table 7 showed that 42.9% from patients with comorbidities needed invasive mechanical ventilation compared to 23.1% for patient without comorbidities, and 21.4% from patients with comorbidities needed non invasive mechanical ventilation compared to 19.2% for patients without comorbidity. Inotropic support needed in 21.4% of patients with comorbidities compared to 7.7% in patients without comorbidities. ICU stay was 7 ± 1.2 days for patients with comorbidities compared to 4 ± 0.9 days for patients without comorbidities. Hospital stay was 13 ± 1.5 days for patients with comorbidities compared to 8 ± 1.6 days for patients without comorbidities. Mortality was 32.1% for patients with comorbidities compared to 15.3% for patients without comorbidities, the total mortality was 24%.
Table 7.
Comparison between patients with and without comorbidities regarding the need for mechanical ventilation (MV), hospital stay & mortality.
| Patients with comorbidities no = 28 (%) | Patients without comorbidities no = 26 (%) | Total no = 54 (%) | P-value | |
|---|---|---|---|---|
| Needed invasive MV | 12 (42.9) | 6 (23.1) | 18 (33.3) | 0.155 |
| Needed non invasive MV | 6 (21.4) | 5 (19.2) | 11 (20.4) | 1.000 |
| Inotropic support | 6 (21.4) | 2 (7.7) | 8 (14.8) | 0.253 |
| ICU stay in days1 | 7.0 ± 1.2 | 4.1 ± 0.9 | 5.6 ± 1.8 | <0.001⁎ |
| Hospital stay in days1 | 13.0 ± 1.5 | 8.0 ± 1.6 | 10.6 ± 2.9 | <0.001⁎ |
| Mortality | 9 (32.1) | 4 (15.3) | 13 (24) | 0.207 |
Values analyzed by Fisher’s exact test.
Values present as mean ± SD & analyzed by independent samples t-test.
Significant.
Mortality was 100% in patients with A. baumannii multidrug resistant (MDR) pneumonia, (66.6%) in patients with P. aeruginosa pneumonia, 50% in patients with K. pneumonia, 33.3% in patients with S. aureus pneumonia, 28.5% in patients with Influenza H1N1 pneumonia, and 33% in patients with S. pneumoniae (see Table 8 , Figure 1, Figure 2, Figure 3, Figure 4 ).
Table 8.
Percentage of mortality according to causative organism.
| Causative organism | Percentage of mortality no = percentage (%) |
|---|---|
| Influenza H1N1 | 2/7 (28.57%) |
| Staphylococcus aureus | 2/6 (33.3%) |
| Acinetobacter baumannii MDR | 2/2 (100%) |
| Klebsiella pneumoniae | 2/4 (50%) |
| Pseudomonas aeruginosa | 2/3 (66.6%) |
| Streptococcus pneumonia | 3/10 (33%) |
Figure 1.
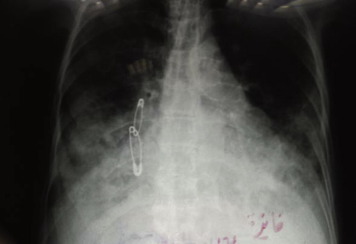
Chest X ray of 40 year old female with severe CAP.
Figure 2.
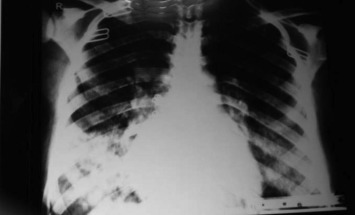
Chest X ray of 35 year old male with severe CAP.
Figure 3.
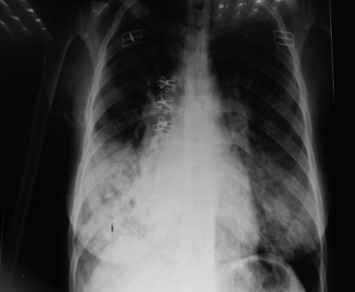
Chest X ray of 38 year female with severe CAP.
Figure 4.
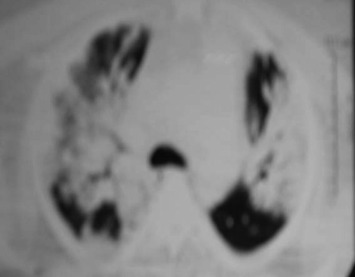
CT chest of 53 year male patient with severe CAP.
Discussion
Severe community acquired pneumonia (SCAP) occurs in approximately 18–36% [12] of all CAP. The mortality rate for CAP is <5% for outpatient cases, it rises to 10% in admitted ward patients and can exceed 30% in patients admitted to intensive care unit (ICU) [13]. In our study 28 out of 54 patients (51.85%) admitted with SCAP had comorbidities. The most common comorbidities were diabetes mellitus and hypertension (13%), chronic bronchitis (11.1%) and bronchial asthma (7.4%). There were 2 patients diagnosed as Systemic lupus erythematosus, 1 patient diagnosed as Hodgkin’s lymphoma, 2 patients diagnosed as severe mitral stenosis, 1 patient diagnosed as Wagner’s granulomatosis, 1 patient diagnosed as Hypogammaglobulinemia and 1 patient diagnosed as Septic pulmonary emboli, and all these 8 patients were diagnosed for the first time.
In this study the most common clinical presentations were fever, cough, dyspnea and hypoxemia. 2 patients developed acute renal failure and 6 patients developed septic shock. In study done by Aya et al. [14], they found that (72.2%) was complaining of fever, (89.9%) was complaining of cough, sputum production was present in (66.66%), dyspnea was present in (66.6%), pleuritic chest pain was present in (35.11%), and non respiratory complaints in the form of nausea, vomiting, myalgia and headache were found in (38.69%).
In our study there was a low hemoglobin level, high neutrophil count, elevated blood urea and serum creatinine with low PH, high PaCo2 and low PaO2 in patients with comorbidities compared to patients without comorbidities, the differences were statistically significant. This indicates more severe diseases and multiple organ dysfunction in patients with comorbidities.
In this study chest X-ray showed Bilateral patchy infiltrate in 35.1% which is common with Influenza A H1N1, S. aureus, Haemophilus influenza, and S. pneumoniae; bilateral patchy infiltrate with cavitations in 3.7%, and it was common with S. aureus, and P. aeruginosa; bilateral lower lobes infiltrate in 16.6% which occurred with S. pneumoniae, M. catarrhalis, and Klebsiella pneumonia; bilateral lower lobe consolidation with pleural effusion in 3.7%; right lower lobe consolidation in 14.8%; left lower lobe consolidation in 11.1%; right upper lobe consolidation in 3.7% and right lower lobe consolidation with cavitations in 3.7% and right lower lobe consolidation and pleural effusion in 7.4%. In the study done by Kantor [15] he found the patterns of lobar pneumonia and bronchopneumonia were equally frequent in pneumococcal pneumonia. The radiographic findings of Legionella consist of segmental peripheral consolidations, there may also be lobar involvement, and bilateral disease is seen in more than half of the patients [16]. Viral pneumonia has a radiologic pattern consisting of poorly defined air-space nodules of 4–10 mm, patchy areas of peribronchial ground glass opacity, and air-space consolidation. Hyperinflation is also commonly present because of the associated bronchiolitis. Pneumonia could progress as seen by the rapid confluence of consolidation leading to diffuse alveolar damage, which consists of homogenous or patchy unilateral or bilateral air-space consolidation and ground-glass opacity or poorly defined centrilobular nodules [17]. The predominant radiographic patterns in H1N1 pneumonia are bilateral ground glass opacity and alveolar consolidation [18]. Striking characteristic of S. pneumonia is its tendency to involve the pleura, parapneumonic effusions are common in Pneumococcal pneumonia [19]. In the study conducted by Ali Khawaja et al. [20] they found that the most common chest X ray finding in patient with CAP was lobar consolidation in (63%) followed by pleural effusion in (37%) and interstitial infiltrates in (28%). In another study by Seksan et al. [21] they found that the most common pulmonary infiltration pattern of X-ray in patients with CAP was patchy infiltrates in (69.3%), followed by interstitial infiltrations in (22.2%), diffuse alveolar infiltrations in (5.0%) and multilobar infiltrations in (3.5%).
In this study, sputum culture showed that S. pneumoniae was the most common isolated organism, it occurred in 18.5%, S. aureus in 11.1%, Influenza H1N1 in 12.9%, K. pneumonia in 7.4%, H. influenzae in 9.2%, M. catarrhalis in 3.7%, Legionella species in 1.8%, P. aeruginosa in 5.5%, A. baumannii multidrug resistant (MDR) in 3.7%, and M. tuberculosis in 1.8%. No bacterial growth occurred in 24%. P. aeruginosa, K. pneumonia, E. coli and A. baumannii were predominantly present in patients with comorbidities, which can be explained by airway colonization by these organisms, also Influenza H1N1 is considered high because our study was done mainly during winter season. In the study done by Boixeda et al. [22], they found that the most frequently isolated microorganism in patients with comorbidities and COPD was P. aeruginosa in 27 (30.7%), followed by S. pneumoniae in 23 (26.1%), Enterobacteriaceae in 18 (20.4%), H. influenza in 14 cases (15.9%), and finally M. catarrhalis in 6 (6.8%). In another study done File [4] he found that the most common causes of severe pneumonia are S. pneumoniae, S. aureus, Legionella spp. Gram-negative bacilli and H. influenza.
In our study blood culture showed that organism isolated only in 50 % of patients with comorbidities compared to 11.5% in patients without comorbidities, the difference was statistically significant, the organisms isolated were E. coli in 7.4%, S. pneumoniae in 5.5%, S. aureus in 5.5%, K. pneumonia in 5.5%, P. aeruginosa in 1.8%, A. baumannii in 3.7%. Khawaja et al. [20] found that blood cultures were positive in 15 patients (13.3%) with severe CAP and in 9 patients (6.1%) with non-severe CAP, there were significantly more patients with positive blood cultures among those with severe CAP than non severe CAP.
In our study 42.9% from patients with comorbidities needed invasive mechanical ventilation compared to 23.1% for patient without comorbidities, and 21.4% from patients with comorbidities needed non invasive mechanical ventilation compared to 19.2% for patient without comorbidities. Inotropic support was needed in 21.4% of patients with comorbidities compared to 7.7% in patients without comorbidities, ICU stay was 7 ± 1.2 days for patients with comorbidities compared to 4 ± 0.9 days for patients without comorbidities. Hospital stay was 13 ± 1.5 days for patients with comorbidities compared to 8 ± 1.6 days for patients without comorbidities. Previous studies by Sreekanth A and Praveen Kumar Reddy. [23] they found that the hospital stay was longer for patients with comorbidities compared to patients without comorbidities, the most frequent complication recorded was sepsis in 38 cases (31%) in patients with comorbidity and 19% in patients without comorbidity, and sepsis was the cause of death in 57% cases. Previous studies by Yoshimoto et al. [24] admit that septic shock was associated with higher mortality and is a frequent complication.
In our study mortality was 32.1% for patients with comorbidities compared to 15.3% for patients without comorbidities, the total mortality was 24%. In our study the infection with P. aeruginosa was associated with worst outcome as 3 out of 3 patients infected with this pathogens died. Internationally the reported mortality of patients with severe CAP is between 35.8% and 39.1% at 5 years [25]. Mortality in patients infected with P. aeruginosa was 66.6%, the lowest mortality was with streptococcal pneumonia 22.2% [25]. In the study done by Yoshimoto et al. [24], the mortality rate for SCAP requiring ICU admission was 48.6%.
The major limitation of our study was the lack of availability of serological tests for the diagnosis of viral and atypical pathogens.
Conclusion
SCAP occurs more frequently in those with comorbidities. The most frequent causative organism of SCAP isolated is S. pneumoniae, Influenza H1N1and S. aureus. SCAP is associated with significant mortality, early recognition and prompt treatment may improve outcome.
Footnotes
Peer review under responsibility of The Egyptian Society of Chest Diseases and Tuberculosis.
Contributor Information
Mousa Elshamly, Email: dr-mousa_elshamly@hotmail.com.
Mohamed O. Nour, Email: drmun78@yahoo.com.
Abdelmaaboud M.M. Omar, Email: abdelmaaboudm@yahoo.com.
References
- 1.Marik P.E. Norasept II Study Investigators. The clinical features of severe community-acquired pneumonia presenting as septic shock. J. Crit. Care. 2000;15(3):85–90. doi: 10.1053/jcrc.2000.16460. [DOI] [PubMed] [Google Scholar]
- 2.Health and Welfare Statistics Association of Japan Annual report of national health and hygiene in Japan. Kouseinoshihyou. 2004;51:382–385. (in Japanese) [Google Scholar]
- 3.Lim W.S., Baudouin S.V., George R.C., Hill A.T., Jamieson J., Le Jeune, Macfarlane J.T., Read R.C., Roberts H.J., Levy M.L., Wani M., Woodhead M.A. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(III) doi: 10.1136/thx.2009.121434. iii1–iii55. 2. [DOI] [PubMed] [Google Scholar]
- 4.File T.M. Community-acquired pneumonia. Lancet. 2003;362:1991–2001. doi: 10.1016/S0140-6736(03)15021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang G.D., Fine M., Orloff J., Arisumi D., Yu V.L., Kapoor W., Grayston T., Wang S.P., Kohler R., Muder R., Ying Y.C., Rihs J.D., Vickers R.M. New and emerging etiologies for community-acquired pneumonia with implications for therapy: a prospective multicenter study of 359 cases. Medicine. 1990;69:307–316. doi: 10.1097/00005792-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Santiago E., Mauricio R.Z., Josep M., Aria A., Jose A., Francisco A., Michael S., Tet Antoni. Severe community-acquired pneumonia assessment of severity criteria. Am. J. Respir. Crit. Care Med. 1998;158:1102–1108. doi: 10.1164/ajrccm.158.4.9803114. [DOI] [PubMed] [Google Scholar]
- 7.Liapikou A., Ferrer M., Polverino E., Balasso V., Esperatti M., Piñer R., Mensa J., Luque N., Ewig S., Menendez R., Niederman M.S., Torres A. Severe community-acquired pneumonia: validation of the infectious diseases society of America/American thoracic society guidelines to predict an intensive care unit admission. Clin. Infect. Dis. 2009;48:377–385. doi: 10.1086/596307. [DOI] [PubMed] [Google Scholar]
- 8.Mandell L.A., Wunderink R.G., Anzueto A., Bartlett G., Campbell D., Dean N., Dowell S., Thomas M., Daniel Niederman M., Antonio T., Cynthia G. Infectious diseases society of America/American thoracicsociety consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007;44(Suppl. 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akan O., Ozyilmaz E., Ahmed K., Uysal S., Gulha M. 16th European Congress of Clinical Microbiology and Infectious Diseases Nice, France, Abstract number: p986. 2006. Quantitative sputum culture versus direct sputum culture. [Google Scholar]
- 10.Baselski V.S., Wunderink R.G. Bronchoscopic diagnosis of pneumonia. Clin. Microbiol. Rev. 1994;7:533–558. doi: 10.1128/cmr.7.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wing E.J., Norden C.W., Shadduck R.K., Winkelstein A. Use of quantitative bacteriologic techniques to diagnosis catheter-related sepsis. Arch. Intern. Med. 1979;139:482–483. [PubMed] [Google Scholar]
- 12.Tan Y.K., Khoo K.L., Chin S.P., Ong Y.Y. Aetiology and outcome of severe community-acquired pneumonia in Singapore. Eur. Respir. J. 1998;12:113–115. doi: 10.1183/09031936.98.12010113. [DOI] [PubMed] [Google Scholar]
- 13.Nair G.B., Niederman M.S. Community-acquired pneumonia: an unfinished battle. Med. Clin. North Am. 2011;95:1143–1161. doi: 10.1016/j.mcna.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel Dayem Aya M., Aly Alaa Ahmed, Sherif F. Pattern of community acquired pneumonia in pregnant ladies in Ain Shams University Hospitals. Egypt. J. Chest Dis. Tuberc. 2012;61:355–359. [Google Scholar]
- 15.Kantor H.G. The many radiologic faces of pneumococcal pneumonia. AJR Am. J. Roentgenol. 1981;137:1213–1220. doi: 10.2214/ajr.137.6.1213. [DOI] [PubMed] [Google Scholar]
- 16.Roig J., Sabria M., Pedro-Botet M.L. Legionella spp.: community acquired and nosocomial infections. Curr. Opin. Infect. Dis. 2003;16:145–151. doi: 10.1097/00001432-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan C.J., Jordan M.C. Diagnosis of viral pneumonia. Semin. Respir. Infect. 1988;3:148–161. [PubMed] [Google Scholar]
- 18.Abdelsalam Magdy, Samy Diab Haytham, Ragab Yasser. Radiological finding in patients with H1N1 influenza pneumonia. Egypt. J. Chest Dis. Tuberc. 2016;65:135–142. [Google Scholar]
- 19.Jay S.J., Johanson W.G., Pierce A.K. The radiographic resolution of Streptococcus pneumoniae pneumonia. N. Engl. J. Med. 1975 Oct 16;293(16):798–801. doi: 10.1056/NEJM197510162931604. [DOI] [PubMed] [Google Scholar]
- 20.Khawaja Ali, Zubairi Ali Bin Sarwar, Durrani Fahad Khan, Zafar Afia. Etiology and outcome of severe community acquired pneumonia in immunocompetent adults. BMC Infect. Dis. 2013;13:94. doi: 10.1186/1471-2334-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaisuksant Seksan, Koonsuwan Apisak, Sawanyawisuth Kittisak. Appropriateness of obtaining blood cultures in patients with community acquired. Southeast Asian J. Trop. Med. Public Health. 2013;44(2) [PubMed] [Google Scholar]
- 22.Boixeda R., Almagro P., Díez-Manglano J., Cabrera F.J., Recio J., Martin-Garrido I., Soriano J.B. Bacterial flora in the sputum and comorbidity in patients with acute exacerbations of COPD. Int. J. Chron. Obstructive Pulm. Dis. 2015;10(1):2581–2591. doi: 10.2147/COPD.S88702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sreekanth A., Praveen Kumar Reddy S. Study of clinical presentations and treatment outcome of severe community acquired pneumonia in the department of pulmonology of a tertiary care hospital. IOSR J. Dent. Med. Sci. (IOSR-JDMS) 2015;14(8 Ver. III):125–128. [Google Scholar]
- 24.Yoshimoto A., Nakamura H., Fujimura M., Nakao S. Severe community-acquired pneumonia in an intensive care unit: risk factors for mortality. Intern. Med. July 2005;44(7) doi: 10.2169/internalmedicine.44.710. [DOI] [PubMed] [Google Scholar]
- 25.Memish Z.A., Almasri M., Turkestani A., Al-Shangiti A.M., Yezli S. Etiology of severe community-acquired pneumonia during the 2013 Hajj-part of the MERS-CoV surveillance program. Int. J. Infect. Dis. 2014 Aug;25:186–190. doi: 10.1016/j.ijid.2014.06.003. Epub 2014 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]


