Abstract
Madin Darby canine kidney (MDCK) cells were adapted to serum-free RPMI 1640 medium and used for cultivation of canine viruses. RPMI 1640 medium was supplemented with a soybean peptone, l-glutamine and antibiotics, so that the protein concentration was less than 5 μg/ml (RPMI/SP medium). The resulting adapted MDCK-SP cells showed steady growth after the twenty-eighth passage in RPMI/SP medium (MDCK-SP cell culture). Canine distemper virus, canine parvovirus, canine adenoviruses and canine parainfluenza virus, which are the principal components of canine combined virus vaccines, grew in the MDCK-SP cell culture as efficiently as the parental MDCK cells cultured in the conventional Eagle's MEM containing fetal bovine serum. Consequently, the use of MDCK-SP cell culture can make current canine vaccine products much safer, of higher quality and at lower cost.
Keywords: Canine viruses, MDCK cell, Serum-free medium
1. Introduction
There are at least 48 species of virus that infect dogs, but only about half are considered to cause clinical problems, and fewer than 10 of these are regarded as important pathogens of dogs. These are canine herpesvirus (CHV), infectious canine hepatitis virus (canine adenovirus type 1: CAV-1), infectious canine laryngotracheitis virus (canine adenovirus type 2: CAV-2), canine parvovirus type 2 (CPV-2), canine parainfluenza virus (CPIV), canine distemper virus (CDV), rabies virus, and canine coronavirus (CCoV). Of these, CAV-1, CPV-2 and CDV cause serious infections with high mortality, especially in non-immune puppies, and therefore vaccines against these infections are regarded as ‘core’ vaccines for dogs. Vaccination against these pathogens is widely practiced throughout the world. Non-core vaccines include those for CHV, CPIV and CCoV, and are essentially optional.
In recent years, vaccines containing CAV-2, CPV-2, CDV and CPIV have routinely been combined modified live virus vaccines. CAV-2 vaccination provides immunity against infectious canine hepatitis caused by CAV-1 because of their shared immunogens. In practice, canine vaccination starts in pups as young as 4 weeks of age, and puppies have to receive several injections until 3 months old, both because these pathogens are ubiquitous, and residual high levels of maternally derived antibodies may inhibit immunization. It is recommended that thereafter dogs should have a booster vaccination with an appropriate interval. For reviews of canine vaccination, see ref. [1].
A general concern regarding multiple vaccine injections in a short period is that they may cause local and/or systemic adverse reactions. Fetal bovine serum (FBS) has traditionally been used in cell cultures for vaccine production, and it is now widely recognized that FBS, serum components such as albumin, and other proteins of animal origin are not only the cause of such adverse events but may also be the source of adventitious microbial contaminations. Nuisance contaminants are generally fungi, bacteria, viruses, microbial nucleic acids, and more specially the prion of bovine spongiform encephalopathy [2]. Consequently, there is a requirement for a method of growing viruses without the use of animal proteins.
In the present study, a new cell culturing system was established from Madin Darby canine kidney (MDCK) cells by consecutively culturing MDCK cells in several kinds of serum-free media. The growth of canine viruses in the adapted culture was examined and compared to the growth of the viruses in the original parental MDCK cell culture.
2. Materials and methods
2.1. Cells and media
The parental MDCK cells were obtained from the Department of Veterinary Microbiology of Tokyo University. They were cultured in Eagle's MEM (Nissui) supplemented with 7.5% FBS (Invitrogen), 10% Tryptose Phosphate Broth (Difco), and an antibiotic mixture (penicillin 100 U/ml, streptomycin 100 μg/ml, Amphotericin B 0.25 μg/ml) (7.5% FBS/MEM) were used for adaptation to media. In the first step of adaptation to serum-free cell culture, these cells were subcultured twice in 25 cm2 plastic T-Flasks containing “Opti-Pro SFM” (Invitrogen). This medium contained 10 ng/ml recombinant epidermal growth factor. When the cells made a monolayer, they were trypsinized by using a mixture of 0.25% Trypsin (Difco Trypsin 250, swine pancreas origin) and 0.02% EDTA solution, and were re-suspended in fresh medium. The cells were subcultured a further 33 times in the “Opti-MEM I Reduced-Serum medium” (Invitrogen) without a supplement of FBS. In the third step they were adapted to Roswell Park Memorial Institute (RPMI) 1640 medium (GIBCO) supplemented with 750 μg/ml soybean peptone (Fluka, Product No. 87972), l-glutamine 300 μg/ml and the antibiotic mixture (RPMI/SP medium). After a further 28 subcultures in RPMI/SP medium, the cells (MDCK-SP cells) showed steady growth, and a regular fourfold expansion every 5–7 days was possible. The MDCK-SP cells between 40 and 50 passages in the RPMI/SP medium were used for the experiments described here.
Protein concentration of the RPMI/SP medium was determined by the Bradford method (Bio-Rad Laboratories).
2.2. Cell cryopreservation
The MDCK-SP cells in log phase growth, usually a 24–72-h culture, were collected and suspended at 106 or more cells/ml in the RPMI/SP medium containing 10% dimethyl sulfoxide (Wako). The ampoules were placed in a freezing container (BIOCELL™, Nihon Freezer), which was then placed in a −80 °C freezer overnight. The next day the frozen ampoules were transferred into a liquid nitrogen refrigerator.
2.3. Mycoplasma examination of the MDCK-SP cells
A 9-day culture of the MDCK-SP cells at the 50th passage in the RPMI/SP medium was examined for mycoplasma contamination using the method described in the Japanese Manufacturing Standards of Veterinary Biologicals. This is a general culturing method using a combination of liquid and agar media for Mycoplasma gallisepticum, Mycoplasma synoviae, Mycoplasma hypopneumoniae and Mycoplasma orale. One milliliter of the MDCK-SP cell suspension was added into 100 ml of the Mycoplasma-liquid medium and incubated at 35–37 °C for 14 days. On the 3rd, 7th, 10th and 14th days of the liquid-culturing period, an aliquot of the medium was inoculated into a Mycoplasma-agar plate and the plate was incubated at 35–37 °C for 10 days in a 5% CO2 incubator. Mycoplasma synoviae was used as a positive control in all experiments.
2.4. Viruses
The Snyder-Hill strain of CDV, PR109 strain of CAV-1, Manhattan strain of CAV-2, MD97-037 strain (2b antigenic type) of CPV-2, and Tsukuba strain of CPIV were used. All viruses except CPV-2 were inoculated into the monolayer cells. CPV-2 was inoculated into the cell suspension before establishing a stationary culture.
Since each virus stock had been produced in the parental MDCK cells in 7.5% FBS/MEM, it was necessary to eliminate FBS components from the virus stock. Therefore, fresh stocks of all of the viruses were made by using the MDCK-SP cells grown in RPMI-SP medium (MDCK-SP cell culture). All viruses were cultivated in the serum-free MDCK-SP cell culture three times before use. For CPV-2 the parvovirus culturing method described previously [3] was applied.
2.5. Virus growth determination
Parental MDCK and the MDCK-SP cell cultures were prepared in 25 cm2 T-flasks for each virus. For CDV, CAV-1, CAV-2 and CPIV the following protocol was applied. The stock viruses were inoculated at multiplicity of infection (m.o.i.) 0.01, allowed to adsorb for 1 h at 37 °C, and then the monolayer was washed once to remove unadsorbed viruses.
For CPV-2, the virus was inoculated into the cells suspended in the flask at a m.o.i. 0.05, and the flask was then cultured stationary at 37 °C for 24 h. Then the medium was replaced to remove unadsorbed viruses, and the incubation was continued.
At regular incubation intervals virus-infected cell culture were frozen at −80 °C and thawed, and the supernatant fluid was obtained after low-speed centrifugation.
2.6. Virus titration assays
Infectivity titers of all viruses were obtained by a micro-titration method performed in 96-wells, flat-bottomed polystyrene plates (Iwaki). Serial 10-fold dilutions of the virus fluid were prepared with 7.5% FBS/MEM, and a volume of 100 μl of each was transferred to four wells per dilution. Then 100 μl of cell suspension at 105/ml in 7.5% FBS/MEM was added to all wells. The plate was mixed by gently tapped and then incubated at 37 °C for 7 days in the 5% CO2 chamber.
The parental MDCK cell culture was used for virus titration of CAV-1, CAV-2, CPV-2 and CPIV, and Vero cells expressing the CDV-receptor, canine CD150 [4], were used for CDV titration. The Vero cells were cultured by using a 7.5% FBS/MEM supplemented with 0.5 mg/ml of Geneticin (Invitrogen).
The end point of the infectivity titer of CDV, CAV-1 and CAV-2 was determined by the presence of cytopathic effect, and for CPV-2 and CPIV by the presence of hemagglutinin (HA) production in the culture supernatant. A 50-μl volume of supernatant of each well was mixed with the same volume of phosphate buffered saline (PBS), and a further volume of 0.75% of rhesus monkey red blood cell suspension in PBS. The HA reaction was performed at pH 6.8 in PBS containing 0.01% bovine serum albumin at 4 °C and the pH 7.0 PBS at room temperature for CPV-2 and CPIV, respectively.
3. Results
3.1. Characteristics of the MDCK-SP cell culture
A growth curve of MDCK-SP cells at passage 41 is shown in Fig. 1 . The MDCK-SP cells increased approximately 4 times and the parental MDCK cell culture approximately 5.5 times growth by the seventh day.
Fig. 1.
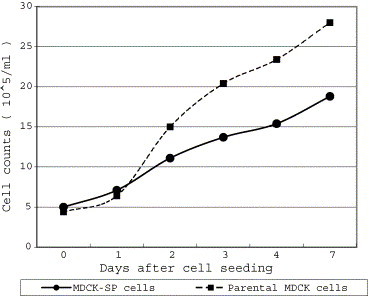
Cell growth curves of the 41st culture of the MDCK-SP cells and the parental MDCK cells.
No mycoplasma was detected from the MDCK-SP cell culture. The protein concentration of the RPMI/SP medium was calculated as less than 5 μg/ml.
No obvious morphological change in the cell culture was detected after the adaptation to the RPMI/SP medium; however, their anchoring ability to the glass surface was reduced. However, they were able to grow and form a monolayer on the surface of plastic culture vessels.
The MDCK-SP cells recovered from cryopreservation as described above were identical to the cells before freezing in respect of cell morphology and growth ability.
3.2. Stock seed virus
Infectivity titers of the stock seed viruses, grown in the MDCK-SP and the parental MDCK cell cultures, are shown in Table 1 . Similar titers were obtained for the viruses grown in either cell culture.
Table 1.
Stock seed viruses
| Virus | Strain | Infectivity titer (TCID50/0.1 ml) |
|
|---|---|---|---|
| Parental MDCK cell culture | MDCK-SP cell culture | ||
| Canine distemper virus | Snyder-Hill | 104.7 | 105.5 |
| Canine adenovirus type1 | PR109 | 108.5 | 109 |
| Canine adenovirus type2 | Manhattan | 106.7 | 105.3 |
| Canine parvovirus type 2 | MD97-037 | 105.5 | 104.5 |
| Canine parainfluenza virus | Tsukuba | 107.7 | 108.3 |
3.3. Comparison of growth characteristics of canine viruses grown in MDCK-SP or parental MDCK cell cultures
The Snyder Hill strain of CDV grew continuously in both MDCK-SP and parental MDCK cell cultures (Fig. 2 ). The infectivity of the viruses in the MDCK-SP cell culture were 32–100 times greater than those of the viruses grown in the parental MDCK cells at all harvest time points.
Fig. 2.
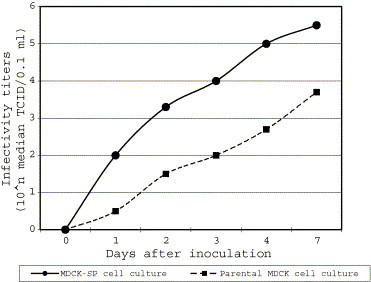
Growth curves of CDV in the MDCK-SP and the parental MDCK cell cultures.
Fig. 3 shows the growth curves of the CAVs. There were no obvious differences in the titers of CAV-1 and CAV-2 grown in either culture.
Fig. 3.
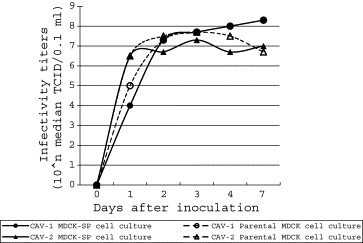
Growth curves of CAVs in the MDCK-SP and the parental MDCK cell cultures.
The infectivity titer of CPIV in the parental MDCK cell culture reached a plateau 24 h earlier than in the MDCK-SP cells, but there was no difference between the maximum titers obtained (Fig. 4 ).
Fig. 4.
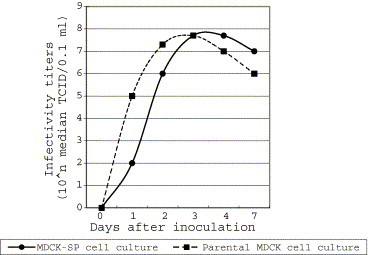
Growth curves of CPIV in the MDCK-SP and the parental MDCK cell cultures.
CPV-2b type MD97-037 virus could grow in both cell cultures (Fig. 5 ); however, the maximum infectivity titer obtained in the MDCK-SP cell culture was approximately 100 times lower than the parental MDCK cell culture.
Fig. 5.
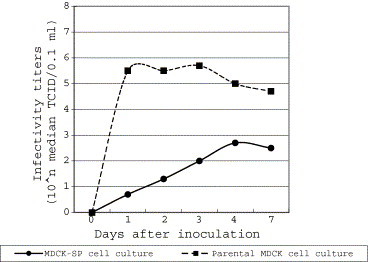
Growth curves of CPV-2 in the MDCK-SP and the parental MDCK cell cultures.
4. Discussion
In addition to primary and diploid cells, continuous cell lines such as MDCK and Vero cells have been accepted for use in the production of viral vaccines for human use, and there have been a few industrial-scale productions of viruses entirely using a serum-free process [2]. For example, influenza viruses were produced in serum-free MDCK cell cultures [5], [6], [7] in which original serum-free media were designed: Ultra-MDCK medium (BioWHITTAKER) [5], MDSS2 medium (Axcell Biotechnologies) [6], [8], MDSS2N medium (AXCEVIR-Vero™) [9], [10], and EpiSerf medium (Gibco-BRL) [7]. However, it is difficult to know the ingredients of these media since they have not been published in full.
The most prominent characteristic of the MDCK-SP cell culture system described here is its simplicity. The RPMI/SP medium was prepared by using only a few materials which are readily obtained, and contains no animal protein. The protein concentration of the RPMI/SP medium was lower than the serum-free media previously used for MDCK cell cultures [5], [6], [8].
A few serum-free media combined with substances of plant origin in place of the animal proteins such as insulin, transferrin, albumin, or peptones have been published. For example, a “plant extract” was used instead of casein peptone in the MDSS2N [9], [10], and “soja-hydrolysate” was added as a further additive to the serum-free CHO-T1 medium that contains several animal proteins [11]. It is obvious that the soybean peptone used in the RPMI/SP medium may act as a substitute for such animal proteins, though its precise ingredients are unknown.
The primary purpose of this study was to establish the susceptibility of the newly established MDCK-SP cells to several canine viruses. Although the data are not shown here, CHV D004 strain (ATCC VT-552) and canine parvovirus type-1 HM-6 strain [12] also grew in the MDCK-SP cell culture. Together with the data presented in the present study, it was considered that the MDCK-SP cells possess comparable virus susceptibility to the parental MDCK cells.
Besides virus susceptibility, virus yield is also an important issue for virus vaccine production. In the present study, as shown in Table 1 and Fig. 2, Fig. 3, Fig. 4, the maximal titers of CDV, CAV-1, CAV-2 and CPIV in the MDCK-SP cell cultures were constantly equal to or rather higher than those obtained in the parental MDCK cell cultures. Similar results have been described, for instance, in the cases of rabies virus and poliovirus titers obtained in serum-free BHK-21 or Vero cell cultures [8], [13], [14].
On the other hand, the virus titers of CPV-2 obtained in the MDCK-SP cell culture were lower than those in the parental MDCK cell culture, as shown in Fig. 5. The difference may relate to the host cell activity shown in Fig. 1. The parental MDCK cells grew faster than the MDCK-SP cells under the present experimental conditions. It is well known that actively dividing cells are required for efficient replication of parvoviruses, and the same is true for CPV-2. The cellular DNA polymerase that is expressed only in mammalian cells during the S phase of the cell cycle is an essential factor for parvovirus replication [15]. Thus, it may be necessary to devise methods for obtaining the virus fluid with higher titers; for example, making use of a higher m.o.i. and of higher cell density in a smaller volume of medium, or of the parvovirus efficient culturing method [3] as applied for the preparation of the stock seed CPV-2 (Table 1).
The third point to be considered is whether a serum-free virus culturing system can be industrialized in the field of veterinary medicine. It is known that there some practical vaccines have been made by using serum-free cell culturing systems; for example, a live combined bovine virus vaccine containing attenuated bovine herpesvirus-1 and bovine parainfluenza type 3 virus [16]; or an inactivated rabies vaccine [17]. To our knowledge this is the first demonstration of an animal protein-free cell culture of canine viruses adopted for current canine vaccine products. Although further experiments are needed for development of practical vaccine, the present study clearly demonstrated that the MDCK-SP cell culture system could be used to produce much safer biologicals such as vaccines for not only dogs but also other animal species including man.
Acknowledgments
Vero cells expressing canine CD150 were generously provided by Dr. Ryoji Yamaguchi of Miyazaki University. Dr. Yasuhito Yamazaki, Tsukuba Research Laboratory of Kyoritsu Seiyaku Corporation, carried out for mycoplasma examination. The author wishes to thank Emeritus Prof. Oswald Jarrett of Glasgow University for critical reviewing of the manuscript.
References
- 1.Greene CE, Schultz RD, Ford RB. Canine vaccination. In: Ford RB, guest editor. Vaccines and vaccinations. The veterinary clinics of North America. Philadelphia: W.B. Saunders Co.; 2001. p. 473–92. [DOI] [PMC free article] [PubMed]
- 2.Merten O-W. Development of serum-free media for cell growth and production of viruses/viral vaccines – Safety issues of animal products used in serum-free media. In: Brown F, Hendriksen C, Sesardic D, Cussler K, editors. Advancing science and elimination of the use of laboratory animals for development and control of vaccines and hormones. Dev Biol Basel, Karger, 2002;111:233–57. [PubMed]
- 3.Johnson R.H., Siegl G., Gautschi M. Characteristics of feline panleukopaenia virus strains enabling definitive classification as parvoviruses. Arch Virusforsch. 1974;46:315–324. doi: 10.1007/BF01240073. [DOI] [PubMed] [Google Scholar]
- 4.Seki F., Ono N., Yamaguchi R., Yanagi Y. Efficient isolation of wild strains of canine distemper virus in Vero cells expressing canine SLAM (CD150) and their adaptability to marmoset B95a cells. J Virol. 2003;77:9943–9950. doi: 10.1128/JVI.77.18.9943-9950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler N, Thomas-Roche G, Gerentes L, Aymard M. Suitability of MDCK cells grown in a serum-free medium for influenza virus production. In: Brown F, Robertson JS, Schild GC, Wood JM, editors. Inactivated influenza vaccines prepared in cell culture. Dev Biol Stand Basel, Karger, 1999;98:13–21. [PubMed]
- 6.Merten O-W, Manuguerra J-C, Hannoun C, van der Werf S. Production of influenza virus in serum-free mammalian cell cultures. In: Brown F, Robertson JS, Schild GC, Wood JM, editors. Inactivated influenza vaccines prepared in cell culture. Dev Biol Stand Basel, Karger, 1999;98:23–37. [PubMed]
- 7.Voeten J.T.M., Brands R., Palache A.M., van Scharrenburg G.J.M., Rimmelzwaan G.F., Osterhaus A.D.M.E. Characterization of high-growth reassortant influenza A viruses generated in MDCK cells cultured in serum-free medium. Vaccine. 1999;17:1942–1950. doi: 10.1016/s0264-410x(98)00464-2. [DOI] [PubMed] [Google Scholar]
- 8.Merten O.-W., Kierulff J.V., Castignolles N., Perrin P. Evaluation of the new serum-free medium (MDSS2) for the production of different biologiclas: Use of various cell lines. Cytotechnology. 1994;14:47–59. doi: 10.1007/BF00772195. [DOI] [PubMed] [Google Scholar]
- 9.Kallel H., Perrin P., Merten O.-W. Evaluation of the new medium (MDSS2N), free of serum and animal proteins, for the production of biologicals. In: Merten O.-W., Perrin P., Griffiths B., editors. New developments and new applications in animal cell technology. Kluwer Academic Press; Dordrecht: 1998. pp. 561–568. [Google Scholar]
- 10.Merten O.-W., Kallel H., Manuguerra J.-C., Tardy-Panit M., Crainic R., Delpeyroux F. The new medium MDSS2N, free of any animal protein supports cell growth and production of various viruses. Cytotechnology. 1999;30:191–201. doi: 10.1023/A:1008021317639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noe W., Schorn P., Bux R., Berthold W. Fed-batch strategies for mammalian cell cultures. In: Spier R.E., Griffiths J.B., Berthold W., editors. Animal cell technology: products of today, prospects for tomorrow. Butterworth-Heinemann; Oxford: 1994. pp. 413–418. [Google Scholar]
- 12.Mochizuki M., Hashimoto M., Hajima T., Takiguchi M., Hashimoto A., Une Y. Virologic and serologic identification of minute virus of canines (canine parvovirus type 1) from dogs in Japan. J Clin Microbiol. 2002;40:3993–3998. doi: 10.1128/JCM.40.11.3993-3998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrin P., Madhusudana S., Gontier-Jallet C., Petres S., Tordo N., Merten O.-W. An experimental rabies vaccine produced with a new BHK-21 susupension cell culture process: use of serum-free medium and perfusion-reactor system. Vaccine. 1995;13:1244–1250. doi: 10.1016/0264-410x(94)00022-f. [DOI] [PubMed] [Google Scholar]
- 14.Merten O.-W., Wu R., Couve E., Crainic R. Evaluation of the serum-free medium MDSS2 for the production of poliovirus on Vero cells in bioreactors. Cytotechnology. 1997;25:35–44. doi: 10.1023/A:1007999313566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berns K.I. Parvovirus replication. Microbiol Rev. 1990;54:316–329. doi: 10.1128/mr.54.3.316-329.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makoschey B., Patel J.R., van Gelder P.T.J.A. Serum-free produced bovine herpesvirus type 1 and bovine parainfluenza type 3 virus vaccines are efficacious and safe. Cytotechnology. 2002;39:139–145. doi: 10.1023/A:1023982003258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frazatti-Gallina N.M., Mourao-Funches R.M., Paoli R.L., Silva M.L.N., Miyaki C., Valentini HJG Vero-cell rabies vaccine produced using serum-free medium. Vaccine. 2004;23:511–517. doi: 10.1016/j.vaccine.2004.06.014. [DOI] [PubMed] [Google Scholar]


