Abstract
Aquatic organisms, such as microalgae (Chlorella, Arthrospira (Spirulina), Tetrasselmis, Dunalliela etc.) and duckweed (Lemna spp., Wolffia spp. etc.) are a potential source for the production of protein-rich biomass and for numerous other high-value compounds (fatty acids, pigments, vitamins etc.). Their cultivation using agro-industrial wastes and wastewater (WaW) is of particular interest in the context of a circular economy, not only for recycling valuable nutrients but also for reducing the requirements for fresh water for the production of biomass. Recovery and recycling of nutrients is an unavoidable long-term approach for securing future food and feed production. Agro-industrial WaW are rich in nutrients and have been widely considered as a potential nutrient source for the cultivation of microalgae/duckweed. However, they commonly contain various hazardous contaminants, which could potentially taint the produced biomass, raising various concerns about the safety of their consumption. Herein, an overview of the most important contaminants, including heavy metals and metalloids, pathogens (bacteria, viruses, parasites etc.), and xenobiotics (hormones, antibiotics, parasiticides etc.) is given. It is concluded that pretreatment and processing of WaW is a requisite step for the removal of several contaminants. Among the various technologies, anaerobic digestion (AD) is widely used in practice and offers a technologically mature approach for WaW treatment. During AD, various organic and biological contaminants are significantly removed. Further removal of contaminants could be achieved by post-treatment and processing of digestates (solid/liquid separation, dilution etc.) to further decrease the concentration of contaminants. Moreover, during cultivation an additional removal may occur through various mechanisms, such as precipitation, degradation, and biotransformation. Since many jurisdictions regulate the presence of various contaminants in feed or food setting strict safety monitoring processes, it would be of particular interest to initiate a multi-disciplinary discussion whether agro-industrial WaW ought to be used to cultivate microalgae/duckweed for feed or food production and identify most feasible options for doing this safely. Based on the current body of knowledge it is estimated that AD and post-treatment of WaW can lower significantly the risks associated with heavy metals and pathogens, but it is yet unclear to what extent this is the case for certain persistent xenobiotics.
Keywords: Agro-industrial waste and wastewater, Contamination, Duckweeds, Microalgae, Feed, Food, Safety
1. Introduction
More than 15% of the people today do not have access to sufficient protein and energy in their diets, and even more suffer from micronutrient malnutrition (Godfray et al., 2010). The increasing trend of population growth (United Nations, 2017),the intensification of industrialization and depletion of resources, the inefficient utilization of nutrients for food and feed production, all in the context of the observed and projected climate changes, will expectedly accentuate the impacts on the environment. Furthermore, the decline of available irrigation water and arable land, along with rising global temperatures that destabilize farming systems, possess a threat for feed and food security (Godfray et al., 2010; Vermeulen et al., 2012; Wheeler and von Braun, 2013). All these pressures make the searching for alternative ways for producing feed and food, unavoidable.
Today feed and food production is based mainly on the cultivation of terrestrial plants. It is expected that protein consumption will increase, resulting in a higher demand for protein rich feed. In the EU most vegetable feed for animal consumption is based on soybean (35–40% proteins) which, given that its growth requirements are met most easily in sub- and tropical climates and require large land areas, is imported from third countries mainly Brazil and USA. However, the sustainability of soybean production on lands converted from forest or pasture use (Arima et al., 2011; Fehlenberg et al., 2017) and of its long-distance importation is questionable, making the long-term stability of any locally or globally feasible food-security programme dependent on finding alternative ways of producing protein-rich biomass for feed or food production (Taelman et al., 2015). Although improvements in crop yields through improved cultivation and production practices can be projected (Balafoutis et al., 2017; Vermeulen et al., 2012), alternative feed and food production systems must be considered for true food security (Rumpold and Schlüter, 2013; Walsh et al., 2015).
Microalgae (including cyanobacteria) and aquatic plants such as duckweed (for example Lemna spp. or Wolfia spp.) are a very promising renewable source of nutrients and high-value compounds that could be used as feedstock for the production of a wide range of products (Appenroth et al., 2017; Borowitzka, 2013; Christaki et al., 2011; Laurens et al., 2017; Spolaore et al., 2006). Besides proteins, they also contain fatty acids, pigments, anti-oxidants etc., that are a very interesting source to be used as food and feed supplements (Christaki et al., 2011; Leng, 1999; Markou and Nerantzis, 2013; Pulz and Gross, 2004). Some microalgal species, such as Arthrospira (Spirulina), Chlorella, and Dunaliella are rich in proteins (>50%dw), display good productivities (20–30 tDM ha−1 year−1 (Walsh et al., 2015)) and have a favorable amino acid profile compared to that of reference and food proteins (Becker, 2007). Given that cultivation of microalgae and duckweed offers some interesting options, such as a year-round biomass production and continuous harvesting, use of non-arable land, and use of brackish- or sea-water as the basis of the cultivation medium, they have been long suggested as a potential protein source to cope with increasing global food demand (Rusoff et al., 1980; Spoehr, 1953).
For a sustainable food and feed production, recovery and recycling of nutrients that are lost with waste and wastewater is of particular importance. Nutrients recovery and recycling is an unavoidable long-term approach for securing food and feed production (Schoumans et al., 2015; Zacharof and Lovitt, 2015). Microalgae and duckweed have been proven to integrate highly efficient nutrient recovery from wastewaters while producing highly versatile biomass (Cheng and Stomp, 2009; Olguín, 2012; Solovchenko et al., 2016). Potential waste and wastewater streams that have been investigated for nutrient recovery include municipal wastewater, agro-industrial WaW, such as poultry, piggery, cattle, food wastes and food processing by-products (cheese whey, olive-oil mill wastes etc.), as well gaseous waste streams such as flue gases. The aptitude of microalgae and duckweed to grow on a range of waste streams has been extensively reviewed (Abinandan and Shanthakumar, 2015; Cai et al., 2013; de la Noüe and de Pauw, 1988; Iqbal, 1999; Journey et al., 1991; Markou and Georgakakis, 2011; Markou et al., 2014; Van Den Hende et al., 2012, 2016; Whitton et al., 2015).
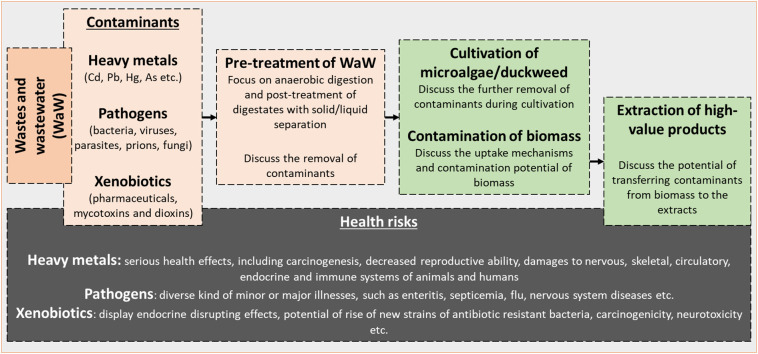
Nevertheless, waste streams, besides their content in valuable nutrients, may contain also various other organic and/or inorganic compounds that could contaminate the biomass, rendering it unsuitable for feed or food production. Such contaminants include inorganic pollutants like heavy metals & metalloids (HMs), pathogens, and various organic pollutants. The lack of a comprehensive review on this topic prompted us to try filling this gap. Due to the high heterogeneity in the physico-chemical characteristics of WaW, the paper will focus only on the nutrient rich agro-industrial waste streams, especially on manures and livestock wastewater. A particular focus is put on waste streams from AD technology, which is widely used to treat the agro-industrial WaW; the digestion effluents (digestates) preserve most of the inorganic nutrients from the feedstock and therefore they have been suggested as a promising source of nutrient source for microalgae and duckweed cultivation (Markou, 2015; Wang et al., 2010). We discuss the presence of pollutants that have potential health risks, the mechanism of their uptake by the cells, and pre-treatment methods that might possibly decrease the contamination of the biomass. In the following sections the most important potential contaminants from the agro-industrial WaW streams that could influence the quality of the produced biomass are discussed. The main categories of contaminants that are taken into consideration are: HMs, pathogens, and xenobiotics. An overview of the content of our review is given in Fig. 1 .
Fig. 1.
Schematic overview of the main discussion points of cultivation of microalgae/duckweed using agro-industrial wastes and wastewater.
2. Heavy metals
One of the greatest concerns about using WaW for the production of aquatic organisms for feed and food is the high potential of bioaccumulation and contamination of biomass with HMs. HMs are those chemical elements that have molecular densities higher than 5 g cm−3. They are highly toxic and their excessive intake could have diverse serious health effects, including carcinogenesis, decreased reproductive ability, damages to nervous, skeletal, circulatory, endocrine and immune systems of animals and humans (Abarikwu, 2013; Li et al., 2014; Morais et al., 2012). Among the various HMs, Cd, Pb, Hg and inorganic As are potentially the most toxic and are included among the ten chemicals of major public health concern (WHO, 2017). As far it is known, these HMs have no beneficial effects in humans (Abarikwu, 2013; Morais et al., 2012). In contrast, other elements regarded as heavy metals, such as Cu, Cr, Co, Mn, Ni, Se, Mo and Zn support essential physiological functions in animals and humans and are required in very small quantities (i.e. micronutrients) (Mertz, 2012). However, their intake in high quantities can likely cause health problems. Since HMs cannot be metabolized, if an organism takes them up at a rate faster than that lost by excretion, they accumulate into the animal or human body. Therefore, the maximum levels for certain HMs and other contaminants in foodstuffs and feed are regulated and legislated worldwide (see Section 6).
2.1. Heavy metal content in agro-industrial wastes and wastewater
Agro-industrial WaW frequently contain HMs. The species of HMs as well their concentrations range widely depending on the WaW type, the production/processing practices and the origin of the feedstock (Markou and Georgakakis, 2011; Napan et al., 2015; Paranychianakis et al., 2015; Zhang et al., 2012). Some HMs concentrations in selected agro-industrial WaW are listed in Table 1 . Most livestock manures (poultry, pig, and cattle) contain relative high amounts of HMs especially for the ones (e.g. Zn, Cu and As) that are commonly supplemented in animal feed, for growth promotion or for treatment of illnesses. HMs are usually added as highly soluble metal salts, frequently above physiological requirements and their excess amounts are excreted (Zhang et al., 2012). HMs content on manures reflects their content in feedstock, their uptake efficiency by animals, and their excretion in faeces and urine (Zhang et al., 2012).
Table 1.
Heavy metal content of some selected agro-industrial wastes and wastewaters.
| Heavy metal |
References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd (ppb) | Cr (ppb) | Co (ppb) | Cu (ppb) | Mn (ppb) | Pb (ppb) | Zn (ppb) | As (ppb) | Ni (ppb) | ||
| Poultry manure/litter | 0.08–38 | 5–2403 | 1.5–487 | 990 | 0.7–22 | 15–1063 | 0.5–10.4 | 32.6 | (Markou et al., 2015; Zhang et al., 2012) | |
| Swine manure | 9 | 2100 | 2700 | 3.6 | 8800 | (Kebede-Westhead et al., 2006) | ||||
| Cattle manure | <3.6 | <3.6 | 14–113 | 0.5–5.5 | 17–377 | 0.5–19 | (Zhang et al., 2012) | |||
| Pig manure | 0.05–203 | 1–43 | 78–1521 | 1.2–5.1 | 63–1622 | (Zhang et al., 2012) | ||||
| Olive oil mill wastewater | 9 | 1 | 1 | 1 | 1 | 12 | (Paredes et al., 2005) | |||
| Molasses | 8.7 | 11 | 20 | 0.24 | (Teclu et al., 2009) | |||||
| Winery wastewater | 50–80 | 200–720 | 110–300 | 200–3260 | 200–1740 | 550–1340 | 90–1400 | 200–650 | (Bustamante et al., 2005) | |
| Vinase | 20–160 | 10–950 | 90–610 | 50–8570 | 730–3520 | 320–1740 | 410–2670 | 60–810 | (Bustamante et al., 2005) | |
| Digestates (AD) | 7.1–19 | 120–500 | 16–74 | 650–1800 | 140–13,000 | 70–240 | 2800–10,000 | 74–210 | (Valeur, 2011) | |
Regarding the food processing industry, some WaW types contain relative high HMs concentrations, primarily as a consequence of the use of industrial products (fertilizers, plant protection chemicals, processing agents etc.) that contain HMs in the agri-food production chain. The species and concentrations of HMs in AD effluents are also highly depended on the feedstock (typically a mixture of animal manures and other organic wastes) and the specific ratio of the different WaW used. While stricter regulations are expected to minimize HM's in waste and wastewaters this is not always possible for every contaminant (Mattsson et al., 2017).
2.2. HMs uptake capacity
Microalgae and duckweed have been frequently considered as means for HMs removal from aqueous solutions due to their relative high affinities and sorption capacities (Chan et al., 2013; Dixit and Singh, 2014; Markou et al., 2015; Napan et al., 2015). As several reviews have adequately covered this topic (Anastopoulos and Kyzas, 2015; Kaplan, 2013; Perales-Vela et al., 2006; Rezania et al., 2016; Suresh Kumar et al., 2015; Ziegler et al., 2016) this overview only provides a brief description. Sorption capacities of microalgae and duckweed range between 1 and 100 mg g−1; however significantly lower or higher values are also reported (Table 2 ). The great variation in sorption capacities is due to the different affinities of the microalgae/duckweed species for given HMs species. Sorption is governed by the electrostatic parameters of the cell walls and the organic matrices that often encapsulate algae (Rossi and de Philippis, 2016) or by the biofilms that often cover aquatic plants (Xu and Shen, 2011); the extent and composition of these, mainly polysaccharide, matrices are dependent on the organism's physiologic status (Boney, 1981; El-Sheekh et al., 2012). Therefore, it is expected that different experimental conditions that impose different stresses on the organisms will affect effective uptake and sorption of HMs. In any case, given the high affinity of microalgae and duckweed for HMs sorption and their high accumulation into the biomass, the use of agro-industrial WaW streams presents a potential for the contamination of the produced biomass with toxic HMs. However, it should be pointed out that most research on this topic has been conducted in synthetic and mono-metallic aqueous HMs solutions containing HMs concentration of one to three orders of magnitude higher than what is eventually contained in the real WaW types (Anastopoulos and Kyzas, 2015; Rezania et al., 2016; Suresh Kumar et al., 2015), and references therein), resulting in possibly exaggerated bioaccumulation of HMs. It is expected that for HM's at the considerably lower concentrations found in real WaW, the kinetics of the HMs adsorption and uptake rates by microalgae/duckweed would be significantly distinct, with maximum accumulation values possibly lower than the ones reported for laboratory conditions. This statement is based on the HMs concentration dependent sorption isotherms where the lower the concentration of HMs in the aqueous phase the lower the equilibrium sorption. However, active uptake and intracellular bioaccumulation of HMs might not be dosage dependent as organisms can effectively remove most of the aqueous HMs from the solution (Basile et al., 2012).
Table 2.
Potential range for heavy metal uptake for selected living microalgae and duckweeds.
| Heavy metal | Specific HMs uptake (mg g−1 dw) | Species | Reference |
|---|---|---|---|
| Cd | 44.5 | Arthorspira platensis (M) | (Murugesan et al., 2008) |
| 13.5 | Tetraselmis chii (M) | (Sjahrul, 2012) | |
| 0.02–1055 | Various species (M) | (Suresh Kumar et al., 2014) | |
| 4.7–7.7 | Lemna minor and Spirodela polyrhiza (D) | (Chaudhuri et al., 2014) | |
| 0.28–1.56 | Lemna gibba (D) | (Verma and Suthar, 2015) | |
| 2.5–3 | Lemna trisulca (D) | (Huebert and Shay, 1992) | |
| Cr | <0.25 | Arthorspira (M) | (Belokobylsky et al., 2004) |
| 226–333 | Various species (M) | (Suresh Kumar et al., 2014) | |
| 0.6–1.2 | Lemna minor (D) | (Wahaab et al., 1995) | |
| 0.1–1.1 | Spirodela polyrrhiza (D) | (Tripathi and Chandra, 1991) | |
| 0.5–3 | Spirodela polyrhiza (D) | (Liu et al., 2017) | |
| Co | 0.89–1.3 | Chlamydomonas reinhardtii (M) | (Macfie and Welbourn, 2000) |
| Up to 21 | Lemna minor (D) | (Sree et al., 2015) | |
| Cu | 6.42–7.54 | Chlamydomonas reinhardtii (M) | (Macfie and Welbourn, 2000) |
| 0.5–3.25 | Scenedesmus obliquus, Chlorella pyrenoidosa and Closterium lunula (M) | (Yan and Pan, 2002) | |
| 1–1.8 | Lemna minor (D) | (Wahaab et al., 1995) | |
| Up to 5.5 | Lemna trisulca (D) | (Prasad et al., 2001) | |
| Pb | 4.49–5.11 | Pseudochlorococcum typicum (M) | (Shanab et al., 2012) |
| 188 | Arthrospira platensis (M) | (Arunakumara et al., 2008) | |
| 0.28–1.60 | Lemna gibba (D) | (Verma and Suthar, 2015) | |
| 10 | Lemna polyrrhiza (D) | (Sharma and Gaur, 1995) | |
| Zn | 72.1 | Scenedesmus subspicatus (M) | (Schmitt et al., 2001) |
| 0.8–4.3 | Lemna minor (D) | (Dirilgen and Inel, 1994) | |
| 8–20 | Lemna trisulca (D) | (Huebert and Shay, 1992) | |
| As | 0.3–1.4 | Dunaliella sp. (M) | (Yamaoka et al., 1990) |
| >1 | Wolffia globosa (D) | (Zhang et al., 2009) | |
| 0.5–2.2 | Lemna gibba (D) | (Mkandawire and Dudel, 2005) | |
| Hg | 9.2 | Scenedesmus subspicatus (M) | (Schmitt et al., 2001) |
| 15.1 | Pseudochlorococcum typicum (M) | (Shanab et al., 2012) | |
| >2 | Not specified (D) | (Mo et al., 1989) | |
| Ni | 0.4–0.63 | Chlamydomonas reinhardtii (M) | (Macfie and Welbourn, 2000) |
| 15.4 | Chlorella vulgaris (M) | (Al-Rub et al., 2004) | |
| 90.9 | Arthrospira platensis (M) | (Markou et al., 2015) | |
| 7.1–12.9 | Lemna minor (D) | (Goswami and Majumder, 2015) | |
| 5.5 | Lemna polyrrhiza (D) | (Sharma and Gaur, 1995) |
The uptake capacity of microalgal/duckweed for HMs is influenced by various parameters such as, microalgae or duckweed species, HMs chemical species and concentration, co-existing ions, pH or salinity and nutrient status of the solution (Suresh Kumar et al., 2015). WaW co-existing ions or compounds (organic or inorganic), might decrease or increase HMs sorption degree through antagonistic or synergistic effects, respectively. However, the typical, and therefore more frequent observation, is that co-existing ions or other charged compounds counteract or compete with HMs decreasing the available sorption sites on the surface of biomass resulting in lower HMs uptake by the cells (Deng et al., 2006; Malik, 2004; Tsezos et al., 1996). This is of particular significance as WaW are usually rich in various ions that could interact with HMs inhibiting their uptake by cells and eventual decreasing the contamination degree of the biomass.
Moreover, especially when culturing microalgae without continuous supplementation of CO2 for pH control, the pH of the growth medium tends to increase due to photosynthesis, as OH– ions are released during uptake of CO2 from bicarbonate. Under such conditions pH reaches values ≫9 (Markou et al., 2014). It is expected that alkalization enhances sorption of cationic HMs (Verma and Suthar, 2015) as the functional groups on the biomass surface become deprotonated, with increased negative charges that increasingly favour the binding of metal cations (Monteiro et al., 2012). However, at high pH, the complex ionic structure of WaW creates condition favorable for ionic complexation of HMs and co-precipitation (Monteiro et al., 2012; Toumi et al., 2000), decreasing thus the availability of HMs for uptake by microalgae. More research is required to develop applied strategies for inhibiting HMs uptake by the cells. Regarding duckweed, they typically tend to equilibrate the pH at 8–8.5 (McLay, 1976; Xu et al., 2012), where HMs precipitation potential still exists, however lower than that for microalgal cultivation systems.
Studies targeting HMs sorption by microalgae and duckweed using real agro-industrial WaW are scarce (Kebede-Westhead et al., 2006; Onalo et al., 2014; Ziegler et al., 2016). More research that considers true agro-industrial WaW and realistic HMs concentrations, in the typical range of WaW, is needed in order to understand and monitor the degree of biomass contamination with HMs and to offer tools that can be deployed in the context of a food and feed safety regulatory and legislative environment.
2.3. Uptake mechanisms
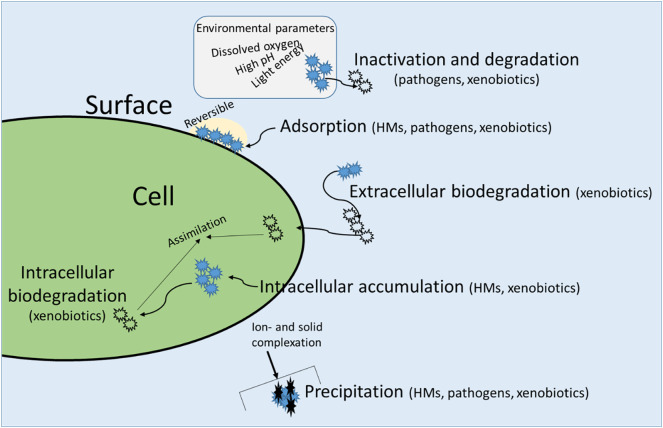
Uptake of HMs by microalgae and duckweed follows several pathways, which could be generally categorized into: (i) cell surface sorption and (ii) intracellular accumulation (or bioaccumulation) (Fig. 2 ) (Basile et al., 2012; Perales-Vela et al., 2006; Suresh Kumar et al., 2015). The uptake kinetics of HMs by microalgae or duckweed could be linear or biphasic, depended on the species of HMs or the aquatic organism. In the biphasic process a first rapid removal from the solution occurs through surface sorption/precipitation followed by a second, slow phase of intracellular accumulation, i.e. the diffusion/transportat of HMs into the interior of the cell (Megateli et al., 2009; Suresh Kumar et al., 2015).
Fig. 2.
Schematic representation of removal and uptake mechanisms of contaminants by microalgae or duckweed.
2.3.1. Surface sorption
The term sorption refers to the adhesion of atoms, ions or molecules to a surface. Surface sorption is an electrostatic phenomenon that is governed by the totality of charged loci on the surface of cells, associated with compounds such as polysaccharides and mucilage (Boney, 1981) or cell walls components. The latter consist mainly of carbohydrates, proteins and lipids, which offer several surface functional groups such as —COOH, —OH, —PO3, —NH2, —SH that adsorb HMs through counter-ion interactions (Kaplan, 2013; Suresh Kumar et al., 2015). Surface sorption could be further divided into (i) ion-exchange, (ii) physical adsorption, (iii) complexation, (iv) precipitation, and (v) entrapment (Perales-Vela et al., 2006; Suresh Kumar et al., 2014). Surface sorption is a non-metabolic mechanism, i.e. no active metabolic process takes place for HMs uptake and therefore it is rapid, and occurs in both living and non-living cells (Kaplan, 2013). Surface sorption is a reversible process (Suresh Kumar et al., 2014) and therefore provides the opportunity of de-contamination of biomass by desorbing HMs, with salts/acids/bases or chelators.
2.3.2. Intracellular accumulation
Intracellular accumulation occurs also in both living and non-living cells and occurs by either passive diffusion or active transmembrane transport (Argüello et al., 2012). Active transport is a metabolically-driven process by which HMs cross the cell wall membrane through energy expending mechanisms, and thus only occurs in living cells. Intracellularly the HMs are finally bound in various organelles (in case of eukaryotic microalgae) and cell compartments (in case of cyanobacteria), such as mitochondria and chloroplasts, polyphosphate bodies, vacuoles, endodermal cells, or also on surface of tissues (in case of duckweed) (Basile et al., 2012; Perales-Vela et al., 2006). Even while the cells have the ability to discriminate between non-essential and essential HMs for their metabolism (Perales-Vela et al., 2006), when the extracellular concentration of HMs is considerably higher than its intracellular concentration, then they may actively transport HMs across their cell membranes into the cytoplasm in order to store them and neutralize their potential toxicity (Monteiro et al., 2009; Perales-Vela et al., 2006). However, for both microalgae and duckweeds, if the HMs concentration exceeds a certain threshold (a function of both the HMs and the organism), it might result in inhibition of growth and lowered uptake as toxicity manifests itself in lowered metabolic activity. Intracellular accumulation is slow and irreversible, restricting the potential for de-contaminating the biomass by desorption. The overall degree of intracellular accumulation of HMs is species and strain dependent (Matsunaga et al., 1999; Suresh Kumar et al., 2015) and can reach 15–65% of the total bioaccumulated HMs. Surface adsorption or intracellular accumulation of HMs in duckweeds is insufficiently studied.
2.4. Removal of heavy metals during pretreatment and biomass contamination potential
To produce biomass free of HMs it is important to identify strategies and devise methods to either reduce the concentration of HMs in the growth media, e.g. by pre-treating of WaW streams, or to manipulate the cultivation conditions in order to render HMs (bio-)unavailable and thus decreasing or nullifying contamination.
Several methods may be employed for the removal of HMs from media prior to cultivation, such as chemical precipitation, ion-exchange, adsorption, membrane filtration, coagulation and flocculation, flotation, and electrochemical treatment. However, each one of these method has advantages and limitations (Fu and Wang, 2011). Chemical precipitation is effective only at high HMs concentrations and consumes a lot of chemicals. Ion-exchange is expensive especially when treating large volumes of wastewater containing HMs at low concentrations. Adsorption using activated carbon is also expensive, while adsorption by low cost adsorbents, such as certain bio-residues, is a relatively new process and yet technically undeveloped. Membrane filtration technology while very efficient for HMs removal, it is, however, uneconomical for agro-industrial wastewaters that contain high organic loads and suspended solids which leads to membrane fouling and low permeate flux. Coagulation and flocculation are efficient but consume a lot of chemicals. Flotation and electrochemical treatments have high initial capital, maintenance and operation costs. In any case, the treatment method used for HMs removal prior cultivation should not remove essential nutrients (such as P and Mg), limit growth, or produce by-products that might inhibit growth or be themselves contaminants.
For AD effluents, a typical pre-treatment step that is applied in biogas plants to facilitate handling, transportation or storing of digestates, is solid/liquid separation (Möller and Müller, 2012); HMs are generally retained in the solid fraction (Tijero et al., 1990) but the degree of retention depends on the separation method and particularly on the particle size of the solids. Marcato et al. (2008) found that in anaerobically digested pig slurry most of Cu and Zn was retained in particle with sizes between 3 and 25 μm, with only 3% bound on >250 μm particles. While, duckweed could be cultivated on unseparated digestates, microalgae may be cultured only in the liquid fraction of digestates, preferably filtered, as any residual solids decrease light penetration or may lead to cells clumping (Uggetti et al., 2014; Xia and Murphy, 2016). Nevertheless, solid/liquid separation may be effective for decreasing HMs in the liquid fraction of an AD effluents used for microalgae or duckweed growth. Moreover, given that usually the AD liquids are diluted at least 10 times, this may lead to a significant decrease in the concentration of HMs in the cultivation media. However, calibration work is needed to ensure that biomass is not contaminated with HMs.
Several studies demonstrated that employing chelators, such as ethylenediaminetetraacetic acid (EDTA), decreases or even eliminates the uptake of HMs by microalgae or duckweed (Huebert and Shay, 1992; Srivastava and Appenroth, 1995). Microalgae and duckweed can synthetize polypeptides with the amino acid sequence (gGlu-Cys)n-Gly (n = 2–11), known as phytochelatins (Le Faucheur et al., 2005), as a protective response to the stress induced by the presence of HMs. Chelating agents bond with metal ions, creating metal-chelate coordination complexes, either inside or outside of the cells, which render metal ions inactive (Miazek et al., 2015). However, where HMs are adsorbed onto the suspended particles, as might be the case for WaW, the addition or synthesis of chelators could create an unbalance in the concentration of HMs between the adsorbed and soluble compartments, which can lead to desorption and possibly an increase in HMs uptake by microalgae or duckweed. Such a scenario has been confirmed for duckweed cultured in the presence of EDTA (Dipu et al., 2012).
As already mentioned in Section 2.2, a high pH could possibly decrease biomass contamination by facilitating the complexation and precipitation of HMs with other co-existing ions. However, a change of pH would also affect growth parameters and thus at this point this is only a hypothesis that requires more investigation. On the other hand, certain micronutrient HMs (e.g. Cu, Zn, Se), are essential for plant and animal metabolism. Saeid et al. (2016) demonstrated that Spirulina biomass enriched in Cu and Fe, through biosorption, enhanced the growth of laying hens and egg characteristics, above the results obtained by direct addition of these micronutrients to feed in a salt form. Thus, biosorption of essential HMs from WaW could be of interest for the production of feed biomass enriched in recycled micronutrients.
3. Pathogens
Pathogens is a broad term that covers all microorganisms that can cause disease. This includes bacteria, fungi, protozoa, worms, viruses, and infectious proteins called prions. There is an enormous heterogeneity of disease types, symptoms, transmission pathways, virulence (pathogenicity), persistence, and eco-physiology, among pathogens. Livestock manures and wastewaters commonly carry pathogens that can be transferred to biomass obtained on them. The abundance and diversity of pathogens varies significantly among WaW types and can vary among farms and geographical regions due to different production practices. Animal pathogens of concern are habitually excreted in faeces, and urine. Many pathogens are zoonotic, i.e. they may cause infections in both animals and humans, and can survive under various environmental conditions. Numerous extensive reviews have addressed livestock pathogens and their impact on animal and human health (Bicudo and Goyal, 2003; Kumar et al., 2013; Manyi-Loh et al., 2016; Mor-Mur and Yuste, 2009; Sahlström, 2003; Sobsey et al., 2006; Spencer and Guan, 2004; Turner and Burton, 1997; Ziemer et al., 2010).
3.1. Pathogen content in agro-industrial wastes and wastewater
The ubiquity of pathogens in farming settings leads to the common working assumption that livestock WaW do carry pathogens (Buchanan et al., 2013; Gerardi and Zimmerman, 2004). A selected list of pathogens that can be harboured in agro-industrial WaW are presented in Table 3 .
Table 3.
Selected pathogens found in livestock wastes and wastewater.
| Pathogen | Waste and wastewater type present | Disease/symptoms | Notes |
|---|---|---|---|
| Bacteria | |||
| Campylobacter spp. | Poultry, cattle | Gastro-enteritis, fever, headache, nausea, and vomiting | Sensitive to heat and anaerobic digestion. Not regarded as high risk. Has a low infective dose (100–800 cells can cause disease). It does not survive at a pH within the range of 1–4 or at temperatures >47 °C |
| Clostridium sp. | Poultry, swine, cattle | Tetanus, botulism, blackleg (clostridial myositis)/respiratory and muscular paralysis, muscle spasms | Spores remain viable in the soil for years and are claimed to be a source of infection. Very resistant. |
| Escherichia coli | Cattle, swine poultry (less) | Bloody diarrhea, vomiting, hemorrhagic colitis, haemolytic uremic syndrome | Facultative anaerobic. A strain of major concern is E. coli O157:H7. Grows on adverse conditions and can survive at low pH and temperatures. Can survive for long periods in soil and water. E. coli does not grow pH <3.6 or in high saline conditions. Infective dose about 10 cells. |
| Listeria monocytogenes | Cattle, poultry | Listeriosis/meningitis, meningoencephalitis, brain abscess, cerebritis | Facultative anaerobic. Grows under adverse conditions and is resistant to heat and freezing. |
| Salmonella sp. | Poultry, swine, cattle | Salmonellosis/food borne enteritis, diarrhea, fever, vomiting | Facultative anaerobic. Grows at pH of 4–8, and between 8 and 45 °C. Can survive for long periods in soil and water. |
| Yersinia enterocolitica | Swine | Yersiniosis/Acute enteritis, lymphadenitis, nosodum ethema, septicemia, poliartitis and maybe death | Non-sporulated, non-capsulated; infrequent. Grows at pH 4–10 and at 4–43 °C. |
| Viruses | |||
| Porcine circovirus | Swine | Porcine dermatitis and nephropathy syndrome, porcine respiratory disease complex, postweaning multisystemic wasting syndrome | It is heat (70 °C) and chemical resistant. Can survive for long periods. Anaerobic digestion reduces infectivity. |
| Coronavirus | Many animals | Sensitive to stresses. Does not survive for long periods. | |
| Rotavirus | Many animals | Acute viral gastroenteritis/diarrhea | Potential zoonotic. Resistant to detergent and many antiseptics. Anaerobic digestion and UV reduces infectivity. |
| Hepatitis E virus | Swine, sheep, poultry | Liver disease/anorexia, nausea and vomiting, hepatomegaly | Zoonotic. Persistence characteristics are not known. |
| Influenza | Many animals | Flu | Zoonotic. Sensitive to heat, irradiation, detergents and oxidizing agents. |
| Parasites | |||
| Ascaris suum | Many animals | Ascariasis | Parasitic nematode, zoonotic; eggs survive under anaerobic stabilization (>80% viability after 20 days). |
| Giardia sp. | Many animals | Giardiasis/diarrhea, cramps, flatulence. | Flagellate protozoan parasite, zoonotic. Very low infection dose. Cysts survive for long periods. In water oocysts survive <14 days at 25 °C. |
| Cryptosporidium parvum | Many animals | Cryptosporidiosis/diarrhea, dehydration, nausea, vomiting | Very low infection dose (132 oocysts) Oocysts are resistant to disinfectants Relative sensitive to heat. |
| Prions | Nervous system disease such as Creutzfeldt–Jakob disease | Resistant to high temperature, in general difficult to be disinfected. | |
Data summarized from (Bicudo and Goyal, 2003; Costantini et al., 2007; Kumar et al., 2013; Manyi-Loh et al., 2016; Sahlström, 2003; Spencer and Guan, 2004; Ziemer et al., 2010).
Bacteria: Animals and humans host an enormous population of different bacteria. Bacteria are single-cell prokaryotic microorganisms with a vast number of species, with a variety of lifestyles and morphologies (cocci/spherical, bacilli/rod-shaped, spirilla/spiral, spirochaetes/tight coils etc.). Most bacteria are not intrinsically pathogenic, however there are numerous species and strains that contain specific virulence genes. Bacteria are frequently responsible for disease outbreaks in humans and animals that originate in livestock production. The extensive use of drugs (antibiotics) in the animal production poses an additional threat to humans related to the selection and transmission of drug resistance genes and thus resistant bacteria (Bicudo and Goyal, 2003; Ray et al., 2013).
3.1.1. Viruses
Particularly important are the enteric and respiratory viruses, including animal enteroviruses, rotaviruses, adenoviruses, hepatitis E viruses, caliciviruses, reoviruses, parvoviruses and other non-enveloped viruses. They are primarily of concern to animal health because they are responsible for high morbidity and mortality and reduced production. Caliciviruses, rotaviruses, myxoviruses and hepatitis E viruses, are or may be capable of infecting humans as well (Costantini et al., 2007; Sobsey et al., 2006).
3.1.2. Parasites
By definition, parasites are those organisms which live in, with or on another organism (called host) and benefits by taking up nutrients at the hosts expense. This include protozoa, helminths, and arthropods, but of significant concern are only the former two. Animal parasites that can potentially pose risks to human health include: Acaris suum, Balantidium coli, Cryptosporidium parvum, Giardia lamblia, Microsporidia spp., and Toxoplasma gondii (Sobsey et al., 2006). However, many of the parasites are of low risk for humans and others are not found in important agricultural animals (Sobsey et al., 2006).
3.1.3. Prions
Prions are infectious proteinaceous agents that cause diseases of the nervous system. Prions reproduce themselves by recruiting the normal cellular isoform of the prion protein and stimulating its conversion into a disease-causing isoform (Colby and Prusiner, 2011). A significant characteristic of prions is their resistance to inactivation by ultraviolet (UV), ionizing irradiation and degradation by proteases (Aguzzi and Calella, 2009). Prion infections are typically restricted to the central nervous and lymphatic systems of infected hosts and it is probably that they are excreted with urine. However excretion of prions has been observed only in cases of chronic inflammation (Seeger et al., 2005).
3.1.4. Fungi
Animal diseases caused by fungi (mycoses) are not a major concern for human health because animals are not typically a reservoir for human infections. However, some animals manure can harbour mycotic agents that could be transmitted to humans (Sobsey et al., 2006). On the other hand, agro-industrial WaW and especially of the food/feed sector, could be contaminated by fungi that produce mycotoxins (e.g. aflatoxins, zearalenone, ochratoxin) which are toxic to humans causing a wide range of effects including carcinogenicity, neurotoxicity, and developmental toxicity (Kolpin et al., 2014). Some fungi, such as Aspergillus sp. Penicillium sp., Rhizomucor etc. are pathogenic as well (Schnürer and Schnürer, 2006).
3.2. Removal of pathogens during pretreatment
A major challenge when utilizing agro-industrial WaW for production of food and feed based on photosynthetic organisms is to reduce the pathogen content and eliminate the possibility of transmitting pathogens to humans and animals. Pathogens could be either inactivated (losing their virulence) or thoroughly removed. Due to the biological origin, there are several environmental factors that affect pathogens' survival. The most important factors include: pH, temperature, humidity, ionic and osmotic strength, competition with other flora, light (ultra-violet – UV). The mechanisms of pathogen inactivation include cell disruption, proteolysis, protein denaturation, antibiosis, antagonism and nutrient deficiencies. Many WaW treatment technologies are based on the effect of these factors on the survival of pathogens. There are three main categories of treatment methods: (i) physical, (ii) chemical, and (iii) biological. Extensive research on this matter can be found in numerous extensive reviews (Amin et al., 2013; Asghar et al., 2015; Bicudo and Goyal, 2003; Franke-Whittle and Insam, 2013; Manyi-Loh et al., 2016; Matilainen and Sillanpää, 2010; Oller et al., 2011; Särkkä et al., 2015; Sobsey et al., 2006; Verbyla and Mihelcic, 2015). We therefore offer here only a brief description of the various treatment options, with a focus, however, on AD. In practice, it is not feasible to detect every pathogen in WaW and therefore indicator pathogens are frequently used as risk indicators for putative presence of pathogens.
3.2.1. Physical methods
This category includes pasteurization, UV irradiation and filtration. Pasteurization entails increasing the temperature, typically to 70 °C, for several minutes (30–60 min). It is effective and eliminates most pathogens (e.g. bacterial indicators, Ascaris suum eggs, swine vesicular disease virus), however it does not eliminate spore-forming bacteria, like Clostridium spores or viruses such as porcine parvovirus (Bagge et al., 2010; Sahlström et al., 2008). Increasing the pasteurization temperature at 90 °C could result in a more reliable inactivation of bacteria, heat-resistant viruses and parasites (Martens and Böhm, 2009). It is however an energy intensive process that could be economically feasible when waste thermal energy is available, such as in biogas production plants. UV irradiation can achieve high reduction of enteric bacterial and protozoan pathogens (2–5 log10), however some viruses are relatively resistant to UV irradiation (Bilotta and Kunz, 2013; Sobsey et al., 2006). UV irradiation does not generate any unwanted residuals and does not alter the nutritional composition of WaW. Although filtration (ultra- and nano-filtration) is effective to remove pathogens (even prions) (Yunoki et al., 2008), its application for large volumes of WaW, at an industrial scale as required for effective biomass production, is hindered by the presence of the various suspended and dissolved solids.
3.2.2. Chemical methods
This category includes alkaline treatment, chlorination, ozonation, and advanced oxidation processes. Alkaline treatment involves the addition of alkali, like ΚΟΗ, CaO or Ca(OH)2, and mixes with other materials like ash, to reach a pH > 12. The most frequent alkaline treatment is lime stabilization (CaO or Ca(OH)2). This is effective for most pathogens, with reduction of fecal coliforms, Salmonella sp., helminth eggs and protozoan oocysts up to 98–99.99% that can be achieved after a few hours of stabilization (Sobsey et al., 2006; Viancelli et al., 2015; Wong and Selvam, 2009). However, alkaline stabilization can also precipitate nutrients such as phosphorus. Combining alkaline treatment with heat favours alkaline hydrolysis, a confirmed reliable method for inactivation of pathogens (Kaye et al., 1998). Hydrolysates thus obtained might contain amino acids and sugars that may be directly assimilated by microalgae or duckweeds (Markou et al., 2014) offering an attractive source of organics for mixotrophic or heterotrophic growth. Chlorination is the most cost-effective process for wastewater disinfection (Amin et al., 2013; Rodríguez-Chueca et al., 2015); however the main drawback is the formation of toxic and carcinogenic by-products. Moreover, although chlorination is very effective for inactivation of bacteria it is less effective for reduction of viruses and protozoa (Sobsey et al., 2006). Ozonation is effective for inactivation of bacteria, viruses and protozoa; however organic matter in WaW can inactivate ozone which limits the efficacy of the method leading to requirements for high ozone doses (1–2 g L−1) (Sobsey et al., 2006; Watkins et al., 1997; Wu et al., 1998). Ozonation does not alter the nitrogen and phosphorus content of the wastewater (Watkins et al., 1997; Wu et al., 1998). The use of advanced oxidation processes for agro-industrial WaW could be more appropriate since organic matter is degraded during the process and does not interfere with the disinfection ability. Advanced oxidation processes are effective for disinfection giving bacterial reduction higher than 3 log10 (Rodríguez-Chueca et al., 2015) and have been widely studied for agro-industrial WaW (Deng and Zhao, 2015; Matilainen and Sillanpää, 2010; Oller et al., 2011; Wagner et al., 2002). However, more research is needed around the formation of potentially toxic intermediate products (Oller et al., 2011; Särkkä et al., 2015).
3.2.3. Biological methods
Agro-industrial WaW are treated mainly by biological methods, such as stabilization lagoons and AD. Other biological methods such as aerobic digestion, biofilters, activated sludge or constructed wetlands are not commonly employed for agro-industrial WaW. Stabilization lagoons (aerated, anaerobic, facultative or multiple configurations) offer low-cost options for WaW treatment. Especially multiple stabilization ponds are more effective in pathogens reduction (2–6 log10) than single ponds (1–3 log10) (Sobsey et al., 2006; Viancelli et al., 2013). However, they require long retention times (>3 months), their performance is not consistent, and depend significantly on environmental parameters (Sobsey et al., 2006). Moreover, due to the long retention times required high loss of nutrients could occur; over 80% of N could be lost through ammonia volatilization (Rockne and Brezonik, 2006). Among the biological methods for agro-industrial WaW treatment, AD is increasingly gaining interest because of the simultaneous treatment of the WaW and the production of biogas, which is an energy carrier that can be used to produce electricity/thermal energy or transportation fuels (biomethane) (Weiland, 2010). AD may be performed either in the psychrophilic (temperature of the environment), mesophilic (30–40 °C) or thermophilic (45–65 °C) temperature ranges. Most frequently AD is carried out in the mesophilic range, as it is more robust compared to the thermophilic range digestion. The latter exhibits uncertainty in methanogenesis, especially when the ammoniacal nitrogen concentration of the waste liquor is relative high (Moset et al., 2015; Yenigün and Demirel, 2013). Thermophilic AD is nonetheless more effective for pathogen reduction (Table 4 ). Nevertheless, thermophilic digestion does not inactivate spore-forming pathogens of Clostridium or Bacillus (Bagge et al., 2010; Sahlström, 2003). An increase in organic acids (volatile fatty acids, such as acetate) in the digestion liquor may substantially reduce spore-forming pathogens (Salsali et al., 2008; Xu et al., 2015) and thus a two-phase thermophilic/mesophilic configuration could be applied to address such pathogens (Huyard et al., 2000). Such an approach might also overcome the instabilities of the thermophilic reactors. In practice, a pasteurization stage (70 °C for 1 h) either before or after the mesophilic AD is included to ensure the hygiene of the effluents (Sahlström, 2003). Still, pasteurization does not fully inactivates all spore-forming microorganisms (bacteria or fungi) (Bagge et al., 2010; Sahlström, 2003; Schnürer and Schnürer, 2006) and it was proposed to combine it with alkaline hydrolysis for increasing the reliability of disinfection (Kaye et al., 1998). In general, the wide diversity of pathogens renders WaW difficult to disinfection via a single technology, and often a combination of technologies is required (Viancelli et al., 2013). Wastewater treatment research shows that pathogens partition between the solid and the liquid phase at variable proportions is a function of the treatment option and the status of the abiotic parameters in the treatment system (van der Drift et al., 1977; Zhang and Farahbakhsh, 2007). Nevertheless, studies directly focusing on the distribution of pathogens between the solid and liquid fractions after separation are scarce. It was found however, that most indicator pathogens were retained in the liquid fraction (Liu et al., 2017). Additionally, improved animal management and housing techniques could also contribute in reducing pathogen load in animal WaW. Such techniques include vaccination and antibiotic therapy, adjustments of animal diets, on-farm hygienic and sanitation actions, and livestock housing management (Sobsey et al., 2006).
Table 4.
Inactivation of selected pathogens during anaerobic digestion.
| Pathogens | Disinfection method | Reduction (log10) | Time of complete inactivation | References |
|---|---|---|---|---|
| Campylobacter spp | Thermophilic anaerobic digestion | >24 h@53 °C | (Wagner et al., 2008) | |
| Listeria monocytogenes | Thermophilic anaerobic digestion | >24 h@53 °C | (Wagner et al., 2008) | |
| Listeria monocytogenes | Mesophilic anaerobic digestion | 2.23@35 °C > 14 d | (Horan et al., 2004) | |
| Clostridium sp. | Thermophilic anaerobic digestion | Inactivation rate 0.188–2.681 CFU/d @ 55 °C | (Xu et al., 2015) | |
| Escherichia coli | Mesophilic anaerobic digestion | 1.66@35 °C > 14 d | (Horan et al., 2004) | |
| Escherichia coli | Thermophilic anaerobic digestion | Decimal reduction 10 min at 55 °C | (Aitken et al., 2007) | |
| Escherichia coli | Thermophilic anaerobic digestion | <4 d@52.5 °C | (Pandey and Soupir, 2011) | |
| Fecal enterococci | Anaerobic digestion | 4 log10 after 300 h@35 °C or 1–2 h@55 °C | (Shilton et al., 2008) | |
| Salmonella sp. | Mesophilic anaerobic digestion | 2.23@35 °C > 14 d | (Horan et al., 2004) | |
| Ascaris suum | Thermophilic anaerobic digestion | >6 h@49 °C <0.5 h @ 55 °C |
(Aitken et al., 2005) | |
| Giardia muris | Anaerobic digestion | 3 log10 after 20.5 h@37 °C or 11 min@55 °C | (Van Praagh et al., 1993) | |
| Cryptosporidium parvum | 2 log10 after 10 d@37 °C or 2 d@55 °C | (Kato et al., 2003) | ||
| Porcine parvovirus | Anaerobic digestion | MGRT 11–12 h@55 °C | (Lund et al., 1996) | |
| Bovine enterovirus | Anaerobic digestion | MGRT 23 h@35 °C, or <0.5 h@55 °C | (Lund et al., 1996) |
MGRT: minimum guaranteed retention time.
1 log10 corresponds to 90% inactivation, 2 log10 to 99%, 3 log10 to 99.99% etc.
3.3. Contamination potential of produced biomass with pathogens mechanisms of contamination
A question of particular interest regarding the growth of aquatic organisms in agro-industrial WaW is whether the various pathogens harboured by WaW may contaminate biomass and what are the mechanisms of contamination. There are numerous studies investigating the interaction of microalgae or duckweeds with bacteria (Fuentes et al., 2016; Underwood and Baker, 1991), viruses (Short, 2012) or protozoan grazers (Tillmann, 2004) and their symbiotic, antagonistic or parasitic relationships. However, there is insufficient direct evidence on whether microalgae or duckweeds might host human or animal pathogens. Considering the known capacity of viruses or bacteria to interact with particles in suspension, it can be hypothesized that potential mechanisms for contamination of biomass with pathogens would involve adsorption of pathogens on microalgal/duckweed biomass surface (Fig. 2) through the variably distributed hydrophilic and hydrophobic loci on both the surface of bacteria and the algae or duckweeds (Marshall, 1985; Verbyla and Mihelcic, 2015) and the role of the ionic composition, i.e. pH and ionic strength, in modifying the net expression of these charges. The geometry of the interacting surfaces is also critical, and as this varies among microorganisms it affects the effective range of the electrostatic interactions, and the thus strength of adsorption. Production of charged compounds, such as polysaccharide capsules, or certain proteins (e.g. adhesins) will enhance the likelihood and strength of attachment. Often such compounds are produced under non-ideal conditions and thus management of the growth conditions might modify the attachment kinetics.
Bacterial pathogens (such as Salmonella and E. coli) can also invade plants via roots or shoots (Cooley et al., 2003; Zhang et al., 2010) and establish themselves within intercellular spaces, in plant tissues (Ávila-Quezada et al., 2010). As in the case of HMs, it can be speculated that the inter/intracellular contamination with pathogens would be more difficult to addressed, whereas surface bound pathogens could be desorbed or treated for their inactivation.
On the other hand, it has been frequently reported that during microalgae or duckweeds cultivation on wastewater certain indicator bacteria were significantly reduced (Heubeck et al., 2007; Iqbal, 1999; Posadas et al., 2015; Schumacher et al., 2003). For example, Staphylococcus aureus, Escherichia coli, and Enterococcus faecalis were reduced by 3–4 log in cultures of the microalga Scenedesmus sp. (Al-Gheethi et al., 2017). It is unclear, however, if such reduction was attained through pathogen death, inactivation, or is an artefact of the testing procedure. In general, potential mechanisms for the removal/inactivation of pathogen in microalgal cultures systems include (i) inactivation through solar irradiation (UV), (ii) drastic shift in pH, either due to the photosynthesis linked pH increase (>9–9.5), or due to rapid acidification to pH of 1–2, as obtained when extremophiles such as Galdieria sp. are cultured in wastewaters (Henkanatte-Gedera et al., 2017), (iii) photosynthesis driven increases in dissolved oxygen concentrations in the cultivation media, and (iv) the production and excretion of antibacterial substances by algae (Heubeck et al., 2007; Posadas et al., 2015). In case of duckweeds, many of these pathogen removals methods are expected to be unpractical or less effective as the floating duckweed plants shade the cultivation media minimizing solar irradiation and, given that photosynthesis occurs above the water-air interface, also minimizes any drastic effect on the pH or dissolved oxygen of the media (pH 8–8.5) (Dewedar and Bahgat, 1995; McLay, 1976; Smith and Moelyowati, 2001; Xu et al., 2012 ). There are some indications however, that duckweeds cultivated in municipal (Moyo et al., 2003) or even hospital-based wastewater (Islam et al., 2004) might be safe to use as feed because it was found that they were not contaminated with pathogens. More insight into such observations is needed to support any decision making.
4. Xenobiotics
Besides HMs and pathogens, agro-industrial WaW contain also several other hazardous compounds that could contaminate the produced biomass of microalgae/duckweed. The most important of these, that will be briefly discussed herein, are pharmaceuticals (steroidal hormones, antibiotics and parasiticides), mycotoxins and dioxins.
4.1. Pharmaceuticals
4.1.1. Steroidal hormones
Hormonal growth promoters are often added to livestock feed to increase feed efficiency and accelerate weight gain. These are mainly estrogens, testosterone and progesterone, and various synthetic hormones that regulate growth and development of animals (Ray et al., 2013). Once excreted by livestock into the environment these compounds display endocrine disrupting effects (Combalbert and Hernandez-Raquet, 2010; Ray et al., 2013). Among the estrogens, 17α-estradiol (E2α), 17β-estradiol (E2β), and estrone (E1) account for >90% of the excreted estrogens by cattle, while E2β is the most prevalent in poultry (Table 5 ) (Ray et al., 2013). In general, estrogens concentrations vary significantly (Table 5) with age, diet, and health status of the animals, as well by the manure/urine collection and handling practices (Combalbert and Hernandez-Raquet, 2010; Ray et al., 2013).
Table 5.
Estrogen excretions by selected animals and estrogen content in their manures. Adapted from (Combalbert and Hernandez-Raquet, 2010; Ray et al., 2013).
| Species | Fecal excretion (μg d−1 per animal) | Urine excretion (μg d−1 per animal) | Droppings (μg d−1 per animal) | Daily excretion (μg d−1 per animal) | Estrogen content in manure (μg kg−1) |
|---|---|---|---|---|---|
| Cattle | >30–360 | 15–180 | 45–540 | E2α; <1.1–1113 | |
| E2β; <1.9–485 | |||||
| E1; <5–865 | |||||
| Swine | 14–270 | 100–2000 | 120–2300 | E2α; 9 | |
| E2β; 115 | |||||
| E1; 243 | |||||
| Poultry | 2.5–6 | E2α; 93 | |||
| E2β; 150 | |||||
| E1; 44 |
4.1.2. Antibiotics
Besides their therapeutic use antibiotics are also used as growth promoters (Hughes and Heritage, 2004; Van Boeckel et al., 2017). Most antibiotics though are poorly absorbed by the gastrointestinal tract and therefore are largely (17–90%) excreted in faeces and urine (Van Boeckel et al., 2017) either unmodified or as active metabolites (epimers or isomers) of the parent compounds (Massé et al., 2014; Sarmah et al., 2006). In some cases, such in N-acetyl-sulfonamides, the metabolized fraction contained in manures can be transformed again into parent compound (Mohring et al., 2009). The most serious threat associated with the use and environmental contamination with antibiotics is the rise of new strains of antibiotic resistant bacteria (Sarmah et al., 2006). The content of antibiotics in urine, that enters manure, (fresh or stored) varies widely (Table 6 ) but typically ranges between 1 and 10 mg L−1 or mg kg−1 (Massé et al., 2014).
Table 6.
Antibiotics content in fresh or stored animal manure/urine and their removal during anaerobic digestion (adapted from (Massé et al., 2014)).
| Antibiotic | Chemical structure | mg L−1 or mg kg−1 | Removal (%) | References |
|---|---|---|---|---|
| Oxytetracycline | 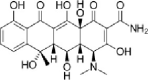 |
0.4–354 | 59–85% | (Álvarez et al., 2010; Massé et al., 2014) |
| Chlortetracycline | 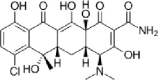 |
1–139 | 27–90 | (Álvarez et al., 2010; Gartiser et al., 2007; Massé et al., 2014) |
| Tetracycline | 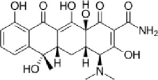 |
30–98 | – | (Massé et al., 2014) |
| Doxycycline | 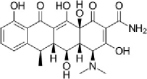 |
37 | – | (Massé et al., 2014) |
| Sulfadiazine | 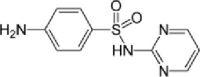 |
7.1 | 70–100 | (Massé et al., 2014; Mohring et al., 2009) |
| Tylosin | 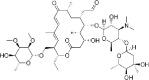 |
0.11–8.1 | 100 | (Massé et al., 2014; Mitchell et al., 2013) |
| Monensin | 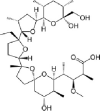 |
120 | 60 | (Gartiser et al., 2007; Massé et al., 2014) |
4.1.3. Parasiticides
Parasiticides refer to the application of chemicals to treat and control endo- or ecto-parasites (organisms that live on the inside or outside of its host, respectively), such as flies, lice, acari, mosquitoes, worms, protozoa and coccidia. There is a wide range of chemical compounds employed as antiparasiticides, such as chlorinated hydrocarbons, organophosphates and carbamates, synthetic pyrethroids, amides, macrocyclic lactones and benzylphenyl ureas (Bártíková et al., 2016; Khan et al., 2008). Some parasiticides, such as synthetic pyrethroids are of concern for human health as they are known disruptors of the endocrine system (Coleman et al., 2013; Khan et al., 2008). Given their diversity in chemistry they vary considerably in their excretion rates by animals. Some organophosphate and carbamate compounds might be efficiently metabolized, while other compounds, such as synthetic pyrethroids, benzylphenyl ureas, macrocyclic lactones and fluazuron, are excreted without much change in mass (Khan et al., 2008). Their discontinuous use in livestock, seasonal or as needed, leads to large variations in their concentrations in manures. For example, Coleman et al. (2013) found that the concentration of abamectin, ivemectin and doramectin in fresh or stored manure range between <1 and 36 μg kg−1.
4.2. Mycotoxins and dioxins
Mycotoxins or dioxins are also potential contaminants but there is little known about their presence in manure/urine. Mycotoxins could be a concern if contaminated agro-food wastes and by-products are used as nutrient source for growing microalgae/duckweed. Fungi can easily contaminate agro-food products generating mycotoxins, such as ochratoxin, zearalone, deoxynivalenol, which can have a wide range of effects including carcinogenicity, neurotoxicity, and developmental toxicity (Ajila et al., 2012; Kolpin et al., 2014). Dioxins are halogenated organic compounds generated in industrial processes. Many dioxins are persistent and have become abundant in the environment; livestock is exposed to dioxins mainly by consuming feed contaminated by airborne dioxins (Khan et al., 2008). It is unclear if these are of great significance in liquid growth systems, but they can easily contaminate biomass post-growth.
4.3. Cyanotoxins and microcystins
An additional category of xenobiotics that possess a health risk, not carried in WaW, is the presence of cyanotoxins and microcystins produced by cyanobacterial species that invade and contaminate microalgal and duckweed cultures. Open pond production systems are susceptible to such contaminations. Cyanotoxins and microcystins are highly soluble and have numerous toxic effects causing gastroenteritis, allergic and inflamatory reactions, liver injury and even death (Bláha et al., 2009; Dawson, 1998). There have been reports about contaminated commercial algae dietary supplements with various toxins at levels above the tolerable daily intake values (Roy-Lachapelle et al., 2017).
4.4. Removal of chemical compounds during pretreatment
Many technologies employed for pathogen removal could be also used for the removal of contaminants. Briefly, some physico-chemical technologies that have been investigated for the removal of pharmaceuticals and other chemical compounds are: adsorption, chemical advanced oxidation processes (ozonation, Fenton oxidation, UV treatment and ionizing irradiation) (Wang and Wang, 2016). The removal efficiencies range significantly depending on the chemical compound, the type of the WaW and the operation parameters (Wang and Wang, 2016). However, each of these technologies has various limitations (see Section 2.4) and are not easily implementable in practice. Efficiency of AD for removal of chemical compounds has been shown to vary widely, from negligible to almost complete removal, and is mainly a function of the chemical characteristics of the compound in question (Stasinakis, 2012). Nevertheless, few studies focused on the value of AD for the removal of chemical compounds from agro-industrial wastes (des Mes et al., 2008; Zhao, 2008).
4.4.1. Steroid estrogens
Under anaerobic conditions (anaerobic digesters, anaerobic storage or anoxic lagoons) E1 is reduced to E2 (α and β) and further to E3. The total estrogen content is generally removed only to a small degree (<22%) (Combalbert et al., 2012; des Mes et al., 2008; Zhang et al., 2014b; Zheng et al., 2012), although, removals higher than 50% have also been reported (Paterakis et al., 2012). Hormones have low Henry's constants (in the order of magnitude of 10−11 (atm L−1 mol−1) and have hydrophobic properties (octanol/water partition coefficient, Kow, of about 2.41–4.01) and consequently they display a great affinity for adsorbtion onto (bio)solids (Chawla et al., 2014). It is expected therefore that after solid/liquid separation of digestates estrogens will be largely retained within the solid fraction, thus reducing the amount in the liquid phase that would be used for cultivation of algae and duckweeds. Moreover, it might be possible that they form sulfate-conjugated forms in manures, that are more recalcitrant to biodegradation than the free estrogen forms (Combalbert et al., 2012; des Mes et al., 2008). It seems that aerobic conditions, either as aerated reactors or by aerobic composting, are necessary for a significant (>70%) removal of estrogens (Zhang et al., 2014b; Zhao, 2008).
4.4.2. Antibiotics
The fate of veterinary antibiotics during AD of manures have been studied by various researchers (Álvarez et al., 2010; Arikan, 2008; Arikan et al., 2006; Gartiser et al., 2007; Mitchell et al., 2013; Mohring et al., 2009). Table 6 lists the impact of AD on the removal rates of antibiotics. The degree of degradation varies significantly and depends on the antibiotic compound and the AD parameters. A general trend observed in studies dealing with oxytetracycline, chlortetracycline, sulfonamides and tylosin is that they are relatively quickly transformed into intermediate products, which are then either further degraded or persist. Persistence of antibiotics is probably related to their capacity to be adsorbed onto (bio)solids increasing their stability (Álvarez et al., 2010); e.g. the water soluble fraction of oxytetracycline was degraded significantly more (up to 85%) than the solid bound fraction (Álvarez et al., 2010). Tylosin was completely removed after 4 days; however the two degradation products formed persisted at least 40 days with 20–50% adsorbed onto solids and the rest remaining in the liquid phase (Mitchell et al., 2013). In some cases, like for chlortetracycline, the concentrations of water-soluble degradation products increased about 2-fold during digestion (Arikan, 2008). Any concern regarding these degradation by-products is directly related to whether the transformation products display the same or different antibiotic activities.
4.4.3. Parasiticides, mycotoxins and dioxins
Investigations into the degradation of parasiticides, mycotoxins and dioxins during AD of agro-industrial WaW are scarce. Kupper et al. (2008) found that about 50% of parasiticides originating in feedstock were 96% to100% degraded, about 35% were degraded at a proportion of 51–95%, with the rest degraded at proportions of <50%. After the solid/liquid separation of the digestates it was found that parasiticides end up preferentially in the liquid fraction. In the study of Salati et al. (2014) 69% to 87% of the mycotoxin aflatoxin B1 was degraded under batch mode of AD while at semi-continuous mode the degradation was 42% in average. Dioxins are considered to be highly resistant to biodegradation probably due to their low water solubility and high Kow coefficients (Kulkarni et al., 2008). After 6 months of AD the concentration of some dioxins (polychlorinated dibenzo-p-dioxins and dibenzofurans) has been shown to remain unchanged while some other dioxins (3-chlorophenol and 3,4-dichlorophenol) were newly generated; however, pentachlorophenol concentration decreased 57% (Najmanová et al., 2014).
4.5. Uptake mechanisms and biomass contamination potentials
During cultivation of microalgae/duckweed in media that contain xenobiotics, various removal mechanisms take place such as adsorption onto (bio)solids, cell uptake, volatilization, photodegradation and biological degradation and transformation (Wang et al., 2017; Zhang et al., 2014a). However, the type of removal mechanism depends on the physico-chemical characteristics of the compounds resulting in a significant variation in the degree and type of removal or uptake (Fig. 2) (Matamoros et al., 2015; Wang et al., 2017; Zhang et al., 2014a).
4.5.1. Steroid estrogens
During microalgae/duckweed cultivation estrogens are converted from one form into another; E1, E2 and EE2 can be interconverted rapidly, during a few hours of cultivation, while estrone (E1) is transformed into estriol and after an extended period (>7 days) is further degraded into unknown lipophilic products (Lai et al., 2002; Shi et al., 2010; Zhang et al., 2014c). Among the removal mechanisms, it was shown that photodegradation and direct oxidation accounted for a small fraction of the total estrogen removal (Maes et al., 2014; Shi et al., 2010; Zhang et al., 2014c). On the other hand, even as the final removal of total estrogen can reach over than 85%, it was shown that the estrogenic activity of the medium was not reduced suggesting that the degradation products displayed the same or higher estrogenic activity compared to the parent compounds (Zhang et al., 2014c). Estrogen sorption onto biomass can range between 4 and 58% and might depend on the species of the organism, the estrogen concentration, or other cultivation parameters (Hom-Diaz et al., 2015; Zhang et al., 2014c). The degree of intracellular concentration of estrogen has not yet been extensively investigated; however, it was shown that the microalga Desmodesmus subspicatus incorporated around 23% EE2 from its surrounding medium after 24 h, with no further uptake. After re-incubation of contaminated cells in clean medium a portion of EE2 was desorbed, but 30% of the initially incorporated estrogen remained in the microalgal biomass (Maes et al., 2014), suggesting, at least partially, a gradient driven adsorption onto cell surfaces.
4.5.2. Antibiotics
Investigations into growing microalgae/duckweed in the presence of veterinary antibiotics are very scarce. When studied, microalgae and duckweed have been shown to be successfully employed for the removal of various antibiotics (Wang et al., 2017; Zhang et al., 2014a). The microalgal uptake, along with photodegradation (contributing around 21%) eliminated >88% of the compound (Santaeufemia et al., 2016). However, living cells of the microalga Phaeodactylum tricornutum have been shown to have a sorption capacity for oxytetracycline of about 29 mg g−1 (Santaeufemia et al., 2016).
4.5.3. Parasiticides, mycotoxins and dioxins
These categories include numerous compounds with diverse physico-chemical characteristics and persistence. There is a vast literature about their toxic effects on phytoplankton and plants, however fewer reports exist on their removal using microalgae or duckweed, and research about the contamination of microalgal or duckweed biomass is scarce (Dosnon-Olette et al., 2010; Olette et al., 2008; Weiner et al., 2004). Microalgae and duckweed might degrade some parasiticides like deltamethrin (Muir et al., 1985) or diazinon (Kurade et al., 2016) forming several other degradation by-products. There are no available data regarding mycotoxins, only one report that indicates that algae (unspecified) were not able to degrade aflatoxin (Ciegler et al., 1966); there are only a few reports on the ability of microalgae to degrade dioxins and various other persistent organic pollutants (Cepoi et al., 2016). Concerning the biomass contamination, it was shown that the pyrethroid deltamethrin parasiticide was rapidly accumulated by Lemna sp. during the initial 24 h reaching 253 and 308 ng g−1 dry weight, and that this dropped to <50% after 3 days due to degradation (Muir et al., 1985). Weiner et al. (2004) found that a correlation between atrazine uptake and microalgal species-sensitivity to the compound exists. The more sensitive species accumulated more atrazine than less sensitive species. It was hypothesized that less sensitive species may actively pump atrazine out of the cell or may degrade it intracellularly.
5. Contamination potentials of extracted products
As was mentioned before, a variety of high-value metabolites e.g. pigments, fatty acids, proteins, antioxidants etc. could be extracted from microalgae and duckweed. Ruiz et al. (2016) conducted a techno-economical evaluation of microalgae feasibility and concluded that nowadays the production of high-value metabolites could be profitable. Thus, it is of interest to identify if the production of high-value metabolites could be coupled with the use of WaW in the context of circular economy, while on the other hand might reduce biomass production costs. However, the potential of transferring contaminants, e.g. HMs, pathogens or xenobiotics that originate in WaW, to the extracted products is possible and thus a concern. There is however a paucity of studies demonstrating this potential, indicating that there are research opportunities on this topic.
A first key step for the extraction of the metabolites is the disruption of cells to facilitate access on the intracellular ingredients. Microalgae and duckweed have relatively rigid cell walls (compared to bacteria or yeast) requiring harsher techniques for the disruption of cell walls or tissues. Cell disruption methods may be grouped into two categories: (1) mechanical and (2) non-mechanical (Günerken et al., 2015): mechanical methods include (i) bead milling, (ii) high-pressure homogenization, (iii) high-speed homogenization, (iv) ultrasonication, (v) microwave, and (vi) pulsed electric field treatments; non-mechanical methods include (i) enzymatic cell lysis and (ii) chemical cell disruption (Günerken et al., 2015). Some cell disruption technologies (like the chemical ones) can also simultaneously extract some target metabolites. The core stage of metabolite extraction is conducted mainly by chemical methods (organic solvents, alkali or acid salts, polymer -salts, super- or subcritical fluids extraction etc.) that might be assisted by mechanical methods (such as microwave) followed by product purification using chromatographic technologies (ion-exchange, gel filtration, expanded bed absorption etc.), membrane separation (micro-, ultra-, and nano-filtration or reverse osmosis) or chemical (caustic refining etc.) (Cuellar-Bermudez et al., 2015; Günerken et al., 2015; Gerardo et al., 2014). The method applied for cell disruption, metabolite extraction and purification depends on the physico-chemical characteristics of tissues and the cell membranes and the target compound(s), the latter determined mainly by their hydrophilic (phycobilins, some proteins, sugars etc.) or hydrophobic (lipid-based pigments, fatty acids etc.) properties. Some cell disruption methods such as high-speed homogenization (Dong et al., 2015) or bead milling (Doucha and Lívanský, 2008) could further contribute to cell lysis, however it is expected to have little to no effect on HMs or xenobiotics. Given that contaminants have also hydrophilic or hydrophobic properties they could be extracted along with the target compound(s). It seems that the purification step might be the most critical because here highly efficient separation technologies could be applied in order to obtain a pure and safe product for consumption. More research to elucidate these points is critical.
6. Regulations regarding feed and food safety and the treatment of agro-industrial wastes and wastewater
Food and feed can become contaminated by various causes and processes during their production and this may pose a risk to human or animal health. Therefore, most jurisdictions regulate the presence of contaminants or unwanted compounds in feed and food, setting various recommendations, regulations, and standards to ensure their quality and safety. While there are major commonalities, the particularities of the local regulatory systems offer a wide range of variability in the regulations and their implementation strategies. The Food and Agriculture Organization (FAO) of the United Nations has set a series of standards and recommendations in the Codex Alimentarius (Codex Stan 193-1995) to address the presence of contaminants, such as HMs and toxins in food and feed and listing their maximum levels (ML) (Table 7 lists the ML of most significant contaminants). FAO Codex Alimentarius points out the significance of the Good Agricultural Practice (GAP) and Good Manufacturing Practice (GMP) in order to achieve a low as reasonably presence of contaminants in feed and food. GAP and GMP includes the following activities to prevent or to reduce contamination of feed and food (CAC, 1995): “(i) preventing food and feed contamination at the source, e.g. by reducing environmental pollution, (ii) applying appropriate technology control measure(s) in food and feed production, manufacture, processing, preparation, treatment, packing, packaging, transport or holding, and (iii) applying measures aimed at decontamination of contaminated feed or food and measures to prevent contaminated feed or food to be marketed for consumption”. Regarding biological contamination, most regulations demand the absence of pathogens in feed and food, setting standards for the sampling and analytical procedures.
Table 7.
Maximum levels of the most significant contaminants in feed and food set by Codex Alimentarius, FAO (CAC, 1995).
| Ochratoxin A (μg kg−1) | 5 |
| As (mg kg−1) | 0.01–0.5 |
| Cd (mg kg−1) | 0.05–2 |
| Pb (mg kg−1) | 0.01–1 |
| Hg (methylmercury) (mg kg−1) | 0.5–1 |
| Sn | 50–250 |
Likewise, most countries lay down rules for agro-industrial wastes and wastewater management, in order to ensure an adequate level of safety and protection of public health. Typically, WaW are categorized based on their risk potential. Some WaW are not allowed to be applied to crops intended for human consumptions, e.g. municipal source WaW, while some highly hazardous WaW, e.g. hospital waste, are not permitted to be used for agriculture and must be disposed after appropriate treatment, including incineration. Other categories (including agro-industrial wastes and wastewater) might be used for the production of various commodities (such as organic fertilizers etc.) after appropriate treatment, such as composting or AD (see for example the EU REGULATION (EC) No 1069/2009). For most terrestrial plants transfer of contaminants from land applied waste to plant occurs via roots which act to mitigate the movement of contaminants into the above ground edible parts of the plant, and while many contaminants may accumulate in plant tissues they are often below most relevant risk thresholds (Chiou, 2003). Inadvertent transfer, e.g. via splashing during rain of irrigation events, is less likely to occur in massive doses (Heaton and Jones, 2008).
However, microalgae and duckweed are aquatic organisms, and unlike terrestrial plantsthey come in direct contact with any suspended or dissolved contaminants and therefore are more likely to be contaminated. Although there is an increasing interest to use agro-industrial WaW for biotechnological applications, like microalgal or duckweed cultivation, there are no known regulations or standards addressing the contamination risk of using WaW in such systems.
7. Conclusions
In many countries, agro-industrial WaW are legislatively categorized according to their origin (animal by-products, food waste etc.) which is then linked to their level of health risk and which in turn informs the regulatory approaches to their further usage or disposal. Human waste and animal by-products are more strictly regulated due to their more obvious health risks. Thus, many countries have developed their own regulations regarding the presence of various contaminants in feed or food, by setting Acceptable Daily Intakes, Acute Reference Doses, or recommendations for Maximum Residue Levels, and often impose strict safety monitoring processes. These regulations are of particular relevance for the initiation of a discussion on whether processed agro-industrial WaW might and ought to be used for cultivating microalgae or duckweed for feed or food production. In Table 8 the various potential contaminants, their removal during AD and cultivation along with an estimation of contamination risks are summarized. Contamination of biomass could occur either due to the adsorption of contaminants on surface or due to the intracellular accumulation. Surface adsorbed contaminants could be desorbed thus decontaminating the biomass, while the intracellularly accumulated contaminants cannot be removed, unless they are biodegraded into harmless byproducts. It is important to identify strategies and devise methods to either reduce the concentration of contaminants in the growth media, e.g. by pre-treating of WaW streams, or to manipulate the cultivation conditions in order to inhibit uptake of contaminants by microalgae and duckweed, thus mitigating biomass contamination. Nevertheless, it is rather obvious that the topic of biomass contamination deserves further investigation and offers a wide scope for research.
Table 8.
Summary of the potential for contamination of microalgae and duckweed cultivated with anaerobic digestates.
| Contaminant | Comments |
|---|---|
| HMs | HMs are not removed during anaerobic digestion. However, most HMs are attached to the solid fraction after solid/liquid separation thus decreasing their amount available to microalgae and duckweed cultivated in the liquid fraction. The necessary dilution of digestates to prepare the cultivation medium, further decreases HMs concentration. There is a high potential of complexation and precipitation when pH of the medium reaches relative alkaline pH (>8.5). The contamination risk with HMs might be low. |
| Pathogens | Most pathogens are removed during anaerobic digestion and especially under thermophilic conditions. However, there are some spore forming bacteria (Clostridium or Bacillus) that are resistant to thermophilic anaerobic digestion. A combination of different technologies might have higher removal efficiencies. Solid/liquid separation do not remove pathogens from the liquid fraction. The necessary dilution of digestates to prepare the cultivation medium, further decreases the abundance of pathogens and there is a high potential that pathogens are further inactivated during cultivation. The contamination risk with pathogens might be mainly low, but variable, and not always negligible. |
| Xenobiotics |
Hormones: Low degradability under anaerobic digestion; after solid/liquid separation most of hormones are retained in the solid fraction. The necessary dilution of digestates to prepare the cultivation medium, further decreases the concentration of hormones. During cultivation of microalgae and duckweed estrogens are transformed, but the estrogenic activity of the degradation products might not be significantly decreased, or might even increase. A fraction of hormones is adsorbed on cell surfaces; thus the contamination potential risk is variable, but estimated as low to moderate. Antibiotics: degradation of antibiotics under anaerobic digestion varies significantly. Solid bound antibiotics are more persistent than the soluble fractions. The necessary dilution of digestates to prepare the cultivation medium, further decreases concentration of antibiotics and there is a high potential that antibiotics are further inactivated during the cultivation. Microalgae and duckweed might adsorb a fraction of the antibiotics. The contamination potential risk is low to moderate. Parasiticides, mycotoxins and dioxins: Their degradation under anaerobic digestion varies significantly. Some are complete, while other are negligibly degraded. During anaerobic digestion some dioxins might be newly formed. During cultivation some of the compounds might be degraded. Little data exists to assess the potential contamination risk. |
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Abarikwu S.O. Springer; 2013. Lead, Arsenic, Cadmium, Mercury: Occurrence, Toxicity and Diseases, Pollutant Diseases, Remediation and Recycling; pp. 351–386. [Google Scholar]
- Abinandan S., Shanthakumar S. Challenges and opportunities in application of microalgae (Chlorophyta) for wastewater treatment: a review. Renew. Sust. Energ. Rev. 2015;52:123–132. [Google Scholar]
- Aguzzi A., Calella A.M. Prions: protein aggregation and infectious diseases. Physiol. Rev. 2009;89(4):1105–1152. doi: 10.1152/physrev.00006.2009. [DOI] [PubMed] [Google Scholar]
- Aitken M.D., Sobsey M.D., Blauth K.E., Shehee M., Crunk P.L., Walters G.W. Inactivation of Ascaris suum and poliovirus in biosolids under thermophilic anaerobic digestion conditions. Environ. Sci. Technol. 2005;39(15):5804–5809. doi: 10.1021/es048004h. [DOI] [PubMed] [Google Scholar]
- Aitken M.D., Sobsey M.D., Van Abel N.A., Blauth K.E., Singleton D.R., Crunk P.L., Nichols C., Walters G.W., Schneider M. Inactivation of Escherichia coli O157:H7 during thermophilic anaerobic digestion of manure from dairy cattle. Water Res. 2007;41(8):1659–1666. doi: 10.1016/j.watres.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Ajila C., Brar S., Verma M., Tyagi R., Godbout S., Valéro J. Bio-processing of agro-byproducts to animal feed. Crit. Rev. Biotechnol. 2012;32(4):382–400. doi: 10.3109/07388551.2012.659172. [DOI] [PubMed] [Google Scholar]
- Al-Gheethi A.A., Mohamed R.M., Jais N.M., Efaq A.N., Abd Halid A., Wurochekke A.A., Amir-Hashim M.K. Influence of pathogenic bacterial activity on growth of Scenedesmus sp. and removal of nutrients from public market wastewater. J. Water Health. 2017;15(5):741–756. doi: 10.2166/wh.2017.080. [DOI] [PubMed] [Google Scholar]
- Al-Rub F.A., El-Naas M., Benyahia F., Ashour I. Biosorption of nickel on blank alginate beads, free and immobilized algal cells. Process Biochem. 2004;39(11):1767–1773. [Google Scholar]
- Álvarez J.A., Otero L., Lema J., Omil F. The effect and fate of antibiotics during the anaerobic digestion of pig manure. Bioresour. Technol. 2010;101(22):8581–8586. doi: 10.1016/j.biortech.2010.06.075. [DOI] [PubMed] [Google Scholar]
- Amin M.M., Hashemi H., Bovini A.M., Hung Y.T. A review on wastewater disinfection. Int. J. Environ. Health Eng. 2013;2(1):22. [Google Scholar]
- Anastopoulos I., Kyzas G.Z. Progress in batch biosorption of heavy metals onto algae. J. Mol. Liq. 2015;209:77–86. [Google Scholar]
- Appenroth K.-J., Sree K.S., Böhm V., Hammann S., Vetter W., Leiterer M., Jahreis G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017;217:266–273. doi: 10.1016/j.foodchem.2016.08.116. [DOI] [PubMed] [Google Scholar]
- Argüello J.M., Raimunda D., González-Guerrero M. Metal transport across biomembranes: emerging models for a distinct chemistry. J. Biol. Chem. 2012;287(17):13510–13517. doi: 10.1074/jbc.R111.319343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikan O.A. Degradation and metabolization of chlortetracycline during the anaerobic digestion of manure from medicated calves. J. Hazard. Mater. 2008;158(2):485–490. doi: 10.1016/j.jhazmat.2008.01.096. [DOI] [PubMed] [Google Scholar]
- Arikan O.A., Sikora L.J., Mulbry W., Khan S.U., Rice C., Foster G.D. The fate and effect of oxytetracycline during the anaerobic digestion of manure from therapeutically treated calves. Process Biochem. 2006;41(7):1637–1643. [Google Scholar]
- Arima E.Y., Peter R., Robert W., Marcellus M.C. Statistical confirmation of indirect land use change in the Brazilian Amazon. Environ. Res. Lett. 2011;6(2) [Google Scholar]
- Arunakumara K.K.I.U., Zhang X., Song X. Bioaccumulation of Pb2+ and its effects on growth, morphology and pigment contents of Spirulina (Arthrospira) platensis. J. Ocean Univ. China. 2008;7(4):397–403. [Google Scholar]
- Asghar A., Raman A.A.A., Daud W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: a review. J. Clean. Prod. 2015;87:826–838. [Google Scholar]
- Ávila-Quezada G., Sánchez E., Gardea-Béjar A.A., Acedo-Félix E. Salmonella spp. and Escherichia coli: survival and growth in plant tissue. N. Z. J. Crop. Hortic. Sci. 2010;38(2):47–55. [Google Scholar]
- Bagge E., Persson M., Johansson K.E. Diversity of spore-forming bacteria in cattle manure, slaughterhouse waste and samples from biogas plants. J. Appl. Microbiol. 2010;109(5):1549–1565. doi: 10.1111/j.1365-2672.2010.04790.x. [DOI] [PubMed] [Google Scholar]
- Balafoutis A., Beck B., Fountas S., Vangeyte J., Wal T.v.d., Soto I., Gómez-Barbero M., Barnes A., Eory V. Precision agriculture technologies positively contributing to GHG emissions mitigation, farm productivity and economics. Sustain. For. 2017;9(8):1339. [Google Scholar]
- Bártíková H., Podlipná R., Skálová L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere. 2016;144:2290–2301. doi: 10.1016/j.chemosphere.2015.10.137. [DOI] [PubMed] [Google Scholar]
- Basile A., Sorbo S., Conte B., Cobianchi R.C., Trinchella F., Capasso C., Carginale V. Toxicity, accumulation, and removal of heavy metals by three aquatic macrophytes. Int. J. Phytoremediat. 2012;14(4):374–387. doi: 10.1080/15226514.2011.620653. [DOI] [PubMed] [Google Scholar]
- Becker E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007;25(2):207–210. doi: 10.1016/j.biotechadv.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Belokobylsky A., Ginturi E., Kuchava N., Kirkesali E., Mosulishvili L., Frontasyeva M., Pavlov S., Aksenova N. Accumulation of selenium and chromium in the growth dynamics of Spirulina platensis. J. Radioanal. Nucl. Chem. 2004;259(1):65–68. [Google Scholar]
- Bicudo J., Goyal S. Pathogens and manure management systems: a review. Environ. Technol. 2003;24(1):115–130. doi: 10.1080/09593330309385542. [DOI] [PubMed] [Google Scholar]
- Bilotta P., Kunz A. Swine manure post-treatment technologies for pathogenic organism inactivation. Engenharia Agríc. 2013;33(2):422–431. [Google Scholar]
- Bláha L., Babica P., Maršálek B. Toxins produced in cyanobacterial water blooms-toxicity and risks. Interdiscip. Toxicol. 2009;2(2):36–41. doi: 10.2478/v10102-009-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boney A.D. Mucilage: the ubiquitous algal attribute. Br. Phycol. J. 1981;16(2):115–132. [Google Scholar]
- Borowitzka M.A. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013;25(3):743–756. [Google Scholar]
- Buchanan A., Bolton N., Moheimani N., Svoboda I., Grant T., Batten D., Cheng N., Borowitzka M.A., Fallowfield H. Co-operative Research Centre for High Integrity Australian Pork; 2013. Algae for Energy and Feed: A Wastewater Solution. A review. [Google Scholar]
- Bustamante M.A., Paredes C., Moral R., Moreno-Caselles J., Pérez-Espinosa A., Pérez-Murcia M. Uses of winery and distillery effluents in agriculture: characterisation of nutrient and hazardous components. Water Sci. Technol. 2005;51(1):145–151. [PubMed] [Google Scholar]
- CAC . Codex Stan 193-1995. 1995. Codex alimentarius commission, codex general standard for contaminants and toxins in food and feed. [Google Scholar]
- Cai T., Park S.Y., Li Y. Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew. Sust. Energ. Rev. 2013;19:360–369. [Google Scholar]
- Cepoi L., Donţu N., Şalaru V., Şalaru V. Removal of organic pollutants from wastewater by Cyanobacteria. In: Zinicovscaia I., Cepoi L., editors. Cyanobacteria for Bioremediation of Wastewaters. Springer International Publishing; Cham: 2016. pp. 27–43. [Google Scholar]
- Chan A., Salsali H., McBean E. Heavy metal removal (copper and zinc) in secondary effluent from wastewater treatment plants by microalgae. ACS Sustain. Chem. Eng. 2013;2(2):130–137. [Google Scholar]
- Chaudhuri D., Majumder A., Misra A.K., Bandyopadhyay K. Cadmium removal by Lemna minor and Spirodela polyrhiza. Int. J. Phytoremediat. 2014;16(11):1119–1132. doi: 10.1080/15226514.2013.821446. [DOI] [PubMed] [Google Scholar]
- Chawla C., Sarkar S., Ali S., Rehmann L., Nakhla G., Ray M.B. Anaerobic digestibility of estrogens in wastewater sludge: effect of ultrasonic pretreatment. J. Environ. Manag. 2014;145:307–313. doi: 10.1016/j.jenvman.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Cheng J.J., Stomp A.M. Growing duckweed to recover nutrients from wastewaters and for production of fuel ethanol and animal feed. Clean–Soil, Air, Water. 2009;37(1):17–26. [Google Scholar]
- Chiou C.T. Contaminant uptake by plants from soil and water. In: Chiou C.T., editor. Partition and Adsorption of Organic Contaminants in Environmental Systems. 2003. pp. 214–233. [Google Scholar]
- Christaki E., Florou-Paneri P., Bonos E. Microalgae: a novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011;62(8):794–799. doi: 10.3109/09637486.2011.582460. [DOI] [PubMed] [Google Scholar]
- Ciegler A., Lillehoj E., Peterson R., Hall H. Microbial detoxification of aflatoxin. Appl. Microbiol. 1966;14(6):934–939. doi: 10.1128/am.14.6.934-939.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby D.W., Prusiner S.B. Prions. Cold Spring Harb. Perspect. Biol. 2011;3(1) doi: 10.1101/cshperspect.a006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman H.M., Trinh T., Le-Minh N., Klein M., Roser D.J., Tucker R.W., Stuetz R.M., Peters G., Khan S.J. Occurrence of ectoparasiticides in Australian beef cattle feedlot wastes. Environ. Pollut. 2013;174:265–272. doi: 10.1016/j.envpol.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Combalbert S., Hernandez-Raquet G. Occurrence, fate, and biodegradation of estrogens in sewage and manure. Appl. Microbiol. Biotechnol. 2010;86(6):1671–1692. doi: 10.1007/s00253-010-2547-x. [DOI] [PubMed] [Google Scholar]
- Combalbert S., Bellet V., Dabert P., Bernet N., Balaguer P., Hernandez-Raquet G. Fate of steroid hormones and endocrine activities in swine manure disposal and treatment facilities. Water Res. 2012;46(3):895–906. doi: 10.1016/j.watres.2011.11.074. [DOI] [PubMed] [Google Scholar]
- Cooley M.B., Miller W.G., Mandrell R.E. Colonization of Arabidopsis thaliana with Salmonella enterica and enterohemorrhagic Escherichia coli O157: H7 and competition by Enterobacter asburiae. Appl. Environ. Microbiol. 2003;69(8):4915–4926. doi: 10.1128/AEM.69.8.4915-4926.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini V.P., Azevedo A.C., Li X., Williams M.C., Michel F.C., Saif L.J. Effects of different animal waste treatment technologies on detection and viability of porcine enteric viruses. Appl. Environ. Microbiol. 2007;73(16):5284–5291. doi: 10.1128/AEM.00553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar-Bermudez S.P., Aguilar-Hernandez I., Cardenas-Chavez D.L., Ornelas-Soto N., Romero-Ogawa M.A., Parra-Saldivar R. Extraction and purification of high-value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015;8(2):190–209. doi: 10.1111/1751-7915.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. The toxicology of microcystins. Toxicon. 1998;36(7):953–962. doi: 10.1016/s0041-0101(97)00102-5. [DOI] [PubMed] [Google Scholar]
- Deng Y., Zhao R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015;1(3):167–176. [Google Scholar]
- Deng L., Su Y., Su H., Wang X., Zhu X. Biosorption of copper (II) and lead (II) from aqueous solutions by nonliving green algae Cladophora fascicularis: equilibrium, kinetics and environmental effects. Adsorption. 2006;12(4):267–277. [Google Scholar]
- van der Drift C., van Seggelen E., Stumm C., Hol W., Tuinte J. Removal of Escherichia coli in wastewater by activated sludge. Appl. Environ. Microbiol. 1977;34(3):315–319. doi: 10.1128/aem.34.3.315-319.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewedar A., Bahgat M. Fate of faecal coliform bacteria in a waste water retention reservoir containing Lemna gibba L. Water Res. 1995;29(11):2598–2600. [Google Scholar]
- Dipu S., Kumar A.A., Thanga S.G. Effect of chelating agents in phytoremediation of heavy metals. Remediat. J. 2012;22(2):133–146. [Google Scholar]
- Dirilgen N., Inel Y. Effects of zinc and copper on growth and metal accumulation in duckweed, Lemna minor. Bull. Environ. Contam. Toxicol. 1994;53(3):442–449. doi: 10.1007/BF00197238. [DOI] [PubMed] [Google Scholar]
- Dixit S., Singh D. An evaluation of phycoremediation potential of cyanobacterium Nostoc muscorum: characterization of heavy metal removal efficiency. J. Appl. Phycol. 2014;26(3):1331–1342. [Google Scholar]
- Dong P., Georget E.S., Aganovic K., Heinz V., Mathys A. Ultra high pressure homogenization (UHPH) inactivation of Bacillus amyloliquefaciens spores in phosphate buffered saline (PBS) and milk. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosnon-Olette R., Trotel-Aziz P., Couderchet M., Eullaffroy P. Fungicides and herbicide removal in Scenedesmus cell suspensions. Chemosphere. 2010;79(2):117–123. doi: 10.1016/j.chemosphere.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Doucha J., Lívanský K. Influence of processing parameters on disintegration of Chlorella cells in various types of homogenizers. Appl. Microbiol. Biotechnol. 2008;81(3):431. doi: 10.1007/s00253-008-1660-6. [DOI] [PubMed] [Google Scholar]
- El-Sheekh M.M., Khairy H.M., El-Shenody R. Algal production of extra and intra-cellular polysaccharides as an adaptive response to the toxin crude extract of Microcystis aeruginosa. Iran. J. Environ. Health Sci. Eng. 2012;9(1):10. doi: 10.1186/1735-2746-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlenberg V., Baumann M., Gasparri N.I., Piquer-Rodriguez M., Gavier-Pizarro G., Kuemmerle T. The role of soybean production as an underlying driver of deforestation in the South American Chaco. Glob. Environ. Chang. 2017;45(Supplement C):24–34. [Google Scholar]
- Franke-Whittle I.H., Insam H. Treatment alternatives of slaughterhouse wastes, and their effect on the inactivation of different pathogens: a review. Crit. Rev. Microbiol. 2013;39(2):139–151. doi: 10.3109/1040841X.2012.694410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu F., Wang Q. Removal of heavy metal ions from wastewaters: a review. J. Environ. Manag. 2011;92(3):407–418. doi: 10.1016/j.jenvman.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Fuentes J.L., Garbayo I., Cuaresma M., Montero Z., González-del-Valle M., Vílchez C. Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs. 2016;14(5):100. doi: 10.3390/md14050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartiser S., Urich E., Alexy R., Kümmerer K. Anaerobic inhibition and biodegradation of antibiotics in ISO test schemes. Chemosphere. 2007;66(10):1839–1848. doi: 10.1016/j.chemosphere.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Gerardi M.H., Zimmerman M.C. John Wiley & Sons; 2004. Wastewater Pathogens. [Google Scholar]
- Gerardo M.L., Oatley-Radcliffe D.L., Lovitt R.W. Integration of membrane technology in microalgae biorefineries. J. Membr. Sci. 2014;464:86–99. [Google Scholar]
- Godfray H.C.J., Beddington J.R., Crute I.R., Haddad L., Lawrence D., Muir J.F., Pretty J., Robinson S., Thomas S.M., Toulmin C. Food security: the challenge of feeding 9 billion people. Science. 2010;327(5967):812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- Goswami C., Majumder A. Potential of Lemna minor in Ni and Cr removal from aqueous solution. Pollution. 2015;1(4):373–385. [Google Scholar]
- Günerken E., D'Hondt E., Eppink M.H.M., Garcia-Gonzalez L., Elst K., Wijffels R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015;33(2):243–260. doi: 10.1016/j.biotechadv.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Heaton J.C., Jones K. Microbial contamination of fruit and vegetables and the behaviour of enteropathogens in the phyllosphere: a review. J. Appl. Microbiol. 2008;104(3):613–626. doi: 10.1111/j.1365-2672.2007.03587.x. [DOI] [PubMed] [Google Scholar]
- Henkanatte-Gedera S.M., Selvaratnam T., Karbakhshravari M., Myint M., Nirmalakhandan N., van Voorhies W., Lammers P.J. Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: Laboratory to field scale demonstration. Algal Res. 2017;24(Part B):450–456. [Google Scholar]
- Heubeck S., Craggs R., Shilton A. Influence of CO2 scrubbing from biogas on the treatment performance of a high rate algal pond. Water Sci. Technol. 2007;55(11):193–200. doi: 10.2166/wst.2007.358. [DOI] [PubMed] [Google Scholar]
- Hom-Diaz A., Llorca M., Rodríguez-Mozaz S., Vicent T., Barceló D., Blánquez P. Microalgae cultivation on wastewater digestate: β-estradiol and 17α-ethynylestradiol degradation and transformation products identification. J. Environ. Manag. 2015;155:106–113. doi: 10.1016/j.jenvman.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Horan N., Fletcher L., Betmal S., Wilks S., Keevil C. Die-off of enteric bacterial pathogens during mesophilic anaerobic digestion. Water Res. 2004;38(5):1113–1120. doi: 10.1016/j.watres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Huebert D.B., Shay J.M. The effect of EDTA on cadmium and zinc uptake and toxicity in Lemna trisulca L. Arch. Environ. Contam. Toxicol. 1992;22(3):313–318. [Google Scholar]
- Hughes P., Heritage J. FAO; Rome, Italy: 2004. Antibiotic Growth-Promoters in Food Animals, Assessing Quality and Safety of Animal Feeds; pp. 129–151. [Google Scholar]
- Huyard A., Ferran B., Audic J.-M. The two phase anaerobic digestion process: sludge stabilization and pathogens reduction. Water Sci. Technol. 2000;42(9):41–47. [Google Scholar]
- Iqbal S. SAn-DEC Report(6/99) 1999. Duckweed aquaculture. Potentials, possibilities and limitations for combined wastewater treatment and animal feed production in developing countries. [Google Scholar]
- Islam M., Kabir M., Khan S., Ekramullah M., Nair G., Sack R., Sack D. Wastewater-grown duckweed may be safely used as fish feed. Can. J. Microbiol. 2004;50(1):51–56. doi: 10.1139/w03-102. [DOI] [PubMed] [Google Scholar]
- Journey W.K., Skillicorn P., Spira W. The World Bank; 1991. Duckweed Aquaculture: A New Aquatic Farming System for Developing Countries. [Google Scholar]
- Kaplan D. Absorption and adsorption of heavy metals by microalgae. In: Richmond A., Hu Q., editors. Handbook of Microalgal Culture: Applied Phycology and Biotechnology. Second Edition. John Wiley & Sons, Ltd; 2013. [Google Scholar]
- Kato S., Fogarty E., Bowman D. Effect of aerobic and anaerobic digestion on the viability of Cryptosporidium parvum oocysts and Ascaris suum eggs. Int. J. Environ. Health Res. 2003;13(2):169–179. doi: 10.1080/0960312031000098071. [DOI] [PubMed] [Google Scholar]
- Kaye G.I., Weber P.B., Evans A., Venezia R.A. Efficacy of alkaline hydrolysis as an alternative method for treatment and disposal of infectious animal waste. J. Am. Assoc. Lab. Anim. Sci. 1998;37(3):43–46. [PubMed] [Google Scholar]
- Kebede-Westhead E., Pizarro C., Mulbry W.W. Treatment of swine manure effluent using freshwater algae: production, nutrient recovery, and elemental composition of algal biomass at four effluent loading rates. J. Appl. Phycol. 2006;18(1):41–46. [Google Scholar]
- Khan S., Roser D., Davies C., Peters G., Stuetz R., Tucker R., Ashbolt N. Chemical contaminants in feedlot wastes: concentrations, effects and attenuation. Environ. Int. 2008;34(6):839–859. doi: 10.1016/j.envint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Kolpin D.W., Schenzel J., Meyer M.T., Phillips P.J., Hubbard L.E., Scott T.-M., Bucheli T.D. Mycotoxins: diffuse and point source contributions of natural contaminants of emerging concern to streams. Sci. Total Environ. 2014;470:669–676. doi: 10.1016/j.scitotenv.2013.09.062. [DOI] [PubMed] [Google Scholar]
- Kulkarni P.S., Crespo J.G., Afonso C.A. Dioxins sources and current remediation technologies—a review. Environ. Int. 2008;34(1):139–153. doi: 10.1016/j.envint.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Kumar R.R., Park B.J., Cho J.Y. Application and environmental risks of livestock manure. J. Korean Soc. Appl. Biol. Chem. 2013;56(5):497–503. [Google Scholar]
- Kupper T., Bucheli T.D., Brändli R.C., Ortelli D., Edder P. Dissipation of pesticides during composting and anaerobic digestion of source-separated organic waste at full-scale plants. Bioresour. Technol. 2008;99(17):7988–7994. doi: 10.1016/j.biortech.2008.03.052. [DOI] [PubMed] [Google Scholar]
- Kurade M.B., Kim J.R., Govindwar S.P., Jeon B.-H. Insights into microalgae mediated biodegradation of diazinon by Chlorella vulgaris: microalgal tolerance to xenobiotic pollutants and metabolism. Algal Res. 2016;20(Supplement C):126–134. [Google Scholar]
- de la Noüe J., de Pauw N. The potential of microalgal biotechnology: a review of production and uses of microalgae. Biotechnol. Adv. 1988;6(4):725–770. doi: 10.1016/0734-9750(88)91921-0. [DOI] [PubMed] [Google Scholar]
- Lai K., Scrimshaw M., Lester J. Biotransformation and bioconcentration of steroid estrogens by Chlorella vulgaris. Appl. Environ. Microbiol. 2002;68(2):859–864. doi: 10.1128/AEM.68.2.859-864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens L.M., Markham J., Templeton D.W., Christensen E.D., Van Wychen S., Vadelius E.W., Chen-Glasser M., Dong T., Davis R., Pienkos P.T. Development of algae biorefinery concepts for biofuels and bioproducts; a perspective on process-compatible products and their impact on cost-reduction. Energy Environ. Sci. 2017;10(8):1716–1738. [Google Scholar]
- Le Faucheur S., Behra R., Sigg L. Phytochelatin induction, cadmium accumulation, and algal sensitivity to free cadmium ion in Scenedesmus vacuolatus. Environ. Toxicol. Chem. 2005;24(7):1731–1737. doi: 10.1897/04-394r.1. [DOI] [PubMed] [Google Scholar]
- Leng R. 1999. Duckweed: a tiny aquatic plant with enormous potential for agriculture and environment. [Google Scholar]
- Li Z., Ma Z., van der Kuijp T.J., Yuan Z., Huang L. A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci. Total Environ. 2014;468:843–853. doi: 10.1016/j.scitotenv.2013.08.090. [DOI] [PubMed] [Google Scholar]
- Liu Z., Carroll Z.S., Long S.C., Roa-Espinosa A., Runge T. Centrifuge separation effect on bacterial indicator reduction in dairy manure. J. Environ. Manag. 2017;191:268–274. doi: 10.1016/j.jenvman.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Lund B., Jensen V.F., Have P., Ahring B. Inactivation of virus during anaerobic digestion of manure in laboratory scale biogas reactors. Antonie Leeuwenhoek. 1996;69(1):25–31. doi: 10.1007/BF00641608. [DOI] [PubMed] [Google Scholar]
- Macfie S., Welbourn P. The cell wall as a barrier to uptake of metal ions in the unicellular green alga Chlamydomonas reinhardtii (Chlorophyceae) Arch. Environ. Contam. Toxicol. 2000;39(4):413–419. doi: 10.1007/s002440010122. [DOI] [PubMed] [Google Scholar]
- Maes H.M., Maletz S.X., Ratte H.T., Hollender J., Schaeffer A. Uptake, elimination, and biotransformation of 17α-ethinylestradiol by the freshwater alga Desmodesmus subspicatus. Environ. Sci. Technol. 2014;48(20):12354–12361. doi: 10.1021/es503574z. [DOI] [PubMed] [Google Scholar]
- Malik A. Metal bioremediation through growing cells. Environ. Int. 2004;30(2):261–278. doi: 10.1016/j.envint.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Manyi-Loh C.E., Mamphweli S.N., Meyer E.L., Makaka G., Simon M., Okoh A.I. An overview of the control of bacterial pathogens in cattle manure. Int. J. Environ. Res. Public Health. 2016;13(9):843. doi: 10.3390/ijerph13090843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcato C.E., Pinelli E., Pouech P., Winterton P., Guiresse M. Particle size and metal distributions in anaerobically digested pig slurry. Bioresour. Technol. 2008;99(7):2340–2348. doi: 10.1016/j.biortech.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Markou G. Fed-batch cultivation of Arthrospira and Chlorella in ammonia-rich wastewater: optimization of nutrient removal and biomass production. Bioresour. Technol. 2015;193(0):35–41. doi: 10.1016/j.biortech.2015.06.071. [DOI] [PubMed] [Google Scholar]
- Markou G., Georgakakis D. Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewaters: a review. Appl. Energy. 2011;88(10):3389–3401. [Google Scholar]
- Markou G., Nerantzis E. Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013;31(8):1532–1542. doi: 10.1016/j.biotechadv.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Markou G., Vandamme D., Muylaert K. Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res. 2014;65:186–202. doi: 10.1016/j.watres.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Markou G., Mitrogiannis D., Çelekli A., Bozkurt H., Georgakakis D., Chrysikopoulos C.V. Biosorption of Cu2+ and Ni2+ by Arthrospira platensis with different biochemical compositions. Chem. Eng. J. 2015;259:806–813. [Google Scholar]
- Marshall K.C. Mechanisms of bacterial adhesion at solid-water interfaces. In: Savage D.C., Fletcher M., editors. Bacterial Adhesion: Mechanisms and Physiological Significance. Springer US; Boston, MA: 1985. pp. 133–161. [Google Scholar]
- Martens W., Böhm R. Overview of the ability of different treatment methods for liquid and solid manure to inactivate pathogens. Bioresour. Technol. 2009;100(22):5374–5378. doi: 10.1016/j.biortech.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Massé D.I., Saady N.M.C., Gilbert Y. Potential of biological processes to eliminate antibiotics in livestock manure: an overview. Animals. 2014;4(2):146–163. doi: 10.3390/ani4020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros V., Gutiérrez R., Ferrer I., García J., Bayona J.M. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: a pilot-scale study. J. Hazard. Mater. 2015;288:34–42. doi: 10.1016/j.jhazmat.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Matilainen A., Sillanpää M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere. 2010;80(4):351–365. doi: 10.1016/j.chemosphere.2010.04.067. [DOI] [PubMed] [Google Scholar]
- Matsunaga T., Takeyama H., Nakao T., Yamazawa A. Screening of marine microalgae for bioremediation of cadmium-polluted seawater. J. Biotechnol. 1999;70(1–3):33–38. doi: 10.1016/s0168-1656(99)00055-3. [DOI] [PubMed] [Google Scholar]
- Mattsson A., Finnson A., I'Ons D. Heavy metal content of Swedish municipal wastewater sludge – status and goals. Water Sci. Technol. 2017;76(4):869–876. doi: 10.2166/wst.2017.277. [DOI] [PubMed] [Google Scholar]
- McLay C. The effect of pH on the population growth of three species of duckweed: Spirodela oligorrhiza, Lemna minor and Wolffia arrhiza. Freshw. Biol. 1976;6(2):125–136. [Google Scholar]
- Megateli S., Semsari S., Couderchet M. Toxicity and removal of heavy metals (cadmium, copper, and zinc) by Lemna gibba. Ecotoxicol. Environ. Saf. 2009;72(6):1774–1780. doi: 10.1016/j.ecoenv.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Mertz W. Elsevier; 2012. Trace Elements in Human and Animal Nutrition. [Google Scholar]
- Miazek K., Iwanek W., Remacle C., Richel A., Goffin D. Effect of metals, metalloids and metallic nanoparticles on microalgae growth and industrial product biosynthesis: a review. Int. J. Mol. Sci. 2015;16(10):23929–23969. doi: 10.3390/ijms161023929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S.M., Ullman J.L., Teel A.L., Watts R.J., Frear C. The effects of the antibiotics ampicillin, florfenicol, sulfamethazine, and tylosin on biogas production and their degradation efficiency during anaerobic digestion. Bioresour. Technol. 2013;149:244–252. doi: 10.1016/j.biortech.2013.09.048. [DOI] [PubMed] [Google Scholar]
- Mkandawire M., Dudel E.G. Accumulation of arsenic in Lemna gibba L.(duckweed) in tailing waters of two abandoned uranium mining sites in Saxony, Germany. Sci. Total Environ. 2005;336(1):81–89. doi: 10.1016/j.scitotenv.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Mo S., Choi D., Robinson J. Uptake of mercury from aqueous solution by duckweed: the effects of pH, copper and humic acid. J. Env. Sci. Health A. 1989;24(2):135–146. [Google Scholar]
- Mohring S.A., Strzysch I., Fernandes M.R., Kiffmeyer T.K., Tuerk J., Hamscher G. Degradation and elimination of various sulfonamides during anaerobic fermentation: a promising step on the way to sustainable pharmacy? Environ. Sci. Technol. 2009;43(7):2569–2574. doi: 10.1021/es802042d. [DOI] [PubMed] [Google Scholar]
- Möller K., Müller T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: a review. Eng. Life Sci. 2012;12(3):242–257. [Google Scholar]
- Monteiro C.M., Marques A.P., Castro P.M., Malcata F.X. Characterization of Desmodesmus pleiomorphus isolated from a heavy metal-contaminated site: biosorption of zinc. Biodegradation. 2009;20(5):629–641. doi: 10.1007/s10532-009-9250-6. [DOI] [PubMed] [Google Scholar]
- Monteiro C.M., Castro P.M., Malcata F.X. Metal uptake by microalgae: underlying mechanisms and practical applications. Biotechnol. Prog. 2012;28(2):299–311. doi: 10.1002/btpr.1504. [DOI] [PubMed] [Google Scholar]
- Morais S., e Costa F.G., de Lourdes Pereira M. INTECH Open Access Publisher; 2012. Heavy Metals and Human Health. [Google Scholar]
- Mor-Mur M., Yuste J. Emerging bacterial pathogens in meat and poultry: an overview. Food Bioprocess Technol. 2009;3(1):24. [Google Scholar]
- Moset V., Poulsen M., Wahid R., Højberg O., Møller H.B. Mesophilic versus thermophilic anaerobic digestion of cattle manure: methane productivity and microbial ecology. Microb. Biotechnol. 2015;8(5):787–800. doi: 10.1111/1751-7915.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyo S., Dalu J., Ndamba J. The microbiological safety of duckweed fed chickens: a risk assessment of using duckweed reared on domestic wastewater as a protein source in broiler chickens. Phys. Chem. Earth Parts A/B/C. 2003;28(20):1125–1129. [Google Scholar]
- Muir D.C., Rawn G.P., Grift N.P. Fate of the pyrethroid insecticide deltamethrin in small ponds: a mass balance study. J. Agric. Food Chem. 1985;33(4):603–609. [Google Scholar]
- Murugesan A., Maheswari S., Bagirath G. Biosorption of Cadmium by Live and Immobilized Cells of Spirulina platensis. Int. J. Env. Res. 2008;2(3):307–312. [Google Scholar]
- Najmanová J., Matějů V., Kyclt R., Vosáhlová S., Mazalová M. Conference: Innovative Remediation Technologies: Research and Practical Applifcation VII, At Prague, Hotel Populus, Czech Republic. 2014. Anaerobic degradation of dioxins - technology in practice. [Google Scholar]
- Napan K., Teng L., Quinn J.C., Wood B.D. Impact of heavy metals from flue gas integration with microalgae production. Algal Res. 2015;8:83–88. [Google Scholar]
- Nations U. Working Paper No. ESA/P/WP/248. 2017. Department of Economic and Social Affairs, Population Division (2017). World population prospects: the 2017 revision, key findings and advance tables. [Google Scholar]
- Olette R., Couderchet M., Biagianti S., Eullaffroy P. Toxicity and removal of pesticides by selected aquatic plants. Chemosphere. 2008;70(8):1414–1421. doi: 10.1016/j.chemosphere.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Olguín E.J. Dual purpose microalgae–bacteria-based systems that treat wastewater and produce biodiesel and chemical products within a biorefinery. Biotechnol. Adv. 2012;30(5):1031–1046. doi: 10.1016/j.biotechadv.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Oller I., Malato S., Sánchez-Pérez J. Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Sci. Total Environ. 2011;409(20):4141–4166. doi: 10.1016/j.scitotenv.2010.08.061. [DOI] [PubMed] [Google Scholar]
- Onalo J.I., Peralta H.M.M., Sunar N.M. Growth of freshwater microalga, Botryococcus sp. in heavy metal contaminated industrial wastewater. J. Sci. Technol. 2014;6(2) [Google Scholar]
- Pandey P.K., Soupir M.L. Escherichia coli inactivation kinetics in anaerobic digestion of dairy manure under moderate, mesophilic and thermophilic temperatures. AMB Express. 2011;1(1):18. doi: 10.1186/2191-0855-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranychianakis N., Salgot M., Snyder S.A., Angelakis A. Water reuse in EU states: necessity for uniform criteria to mitigate human and environmental risks. Crit. Rev. Environ. Sci. Technol. 2015;45(13):1409–1468. [Google Scholar]
- Paredes C., Cegarra J., Bernal M., Roig A. Influence of olive mill wastewater in composting and impact of the compost on a Swiss chard crop and soil properties. Environ. Int. 2005;31(2):305–312. doi: 10.1016/j.envint.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Paterakis N., Chiu T., Koh Y., Lester J., McAdam E., Scrimshaw M., Soares A., Cartmell E. The effectiveness of anaerobic digestion in removing estrogens and nonylphenol ethoxylates. J. Hazard. Mater. 2012;199:88–95. doi: 10.1016/j.jhazmat.2011.10.075. [DOI] [PubMed] [Google Scholar]
- Perales-Vela H.V., Peña-Castro J.M., Canizares-Villanueva R.O. Heavy metal detoxification in eukaryotic microalgae. Chemosphere. 2006;64(1):1–10. doi: 10.1016/j.chemosphere.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Posadas E., del Mar Morales M., Gomez C., Acién F.G., Muñoz R. Influence of pH and CO2 source on the performance of microalgae-based secondary domestic wastewater treatment in outdoors pilot raceways. Chem. Eng. J. 2015;265:239–248. [Google Scholar]
- Prasad M., Malec P., Waloszek A., Bojko M., Strzałka K. Physiological responses of Lemna trisulca L.(duckweed) to cadmium and copper bioaccumulation. Plant Sci. (Amsterdam, Neth.) 2001;161(5):881–889. [Google Scholar]
- Pulz O., Gross W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004;65(6):635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- Ray P., Zhao Z., Knowlton K. Emerging contaminants in livestock manure: hormones, antibiotics and antibiotic resistance genes. Sustain. Anim. Agric. 2013:268–283. [Google Scholar]
- Rezania S., Taib S.M., Din M.F.M., Dahalan F.A., Kamyab H. Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. J. Hazard. Mater. 2016;318:587–599. doi: 10.1016/j.jhazmat.2016.07.053. [DOI] [PubMed] [Google Scholar]
- Rockne K.J., Brezonik P.L. Nutrient removal in a cold-region wastewater stabilization pond: importance of ammonia volatilization. J. Environ. Eng. 2006;132(4):451–459. [Google Scholar]
- Rodríguez-Chueca J., Ormad M., Mosteo R., Sarasa J., Ovelleiro J. Conventional and advanced oxidation processes used in disinfection of treated urban wastewater. Water Environ. Res. 2015;87(3):281–288. doi: 10.2175/106143014x13987223590362. [DOI] [PubMed] [Google Scholar]
- Rossi F., de Philippis R. Exocellular polysaccharides in microalgae and cyanobacteria: chemical features, role and enzymes and genes involved in their biosynthesis. In: Borowitzka M.A., Beardall J., Raven J.A., editors. The Physiology of Microalgae. Springer International Publishing; Cham: 2016. pp. 565–590. [Google Scholar]
- Roy-Lachapelle A., Solliec M., Bouchard M.F., Sauvé S. Detection of cyanotoxins in algae dietary supplements. Toxins. 2017;9(3):76. doi: 10.3390/toxins9030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J., Olivieri G., de Vree J., Bosma R., Willems P., Reith J.H., Eppink M.H., Kleinegris D.M., Wijffels R.H., Barbosa M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016;9(10):3036–3043. [Google Scholar]
- Rumpold B.A., Schlüter O.K. Potential and challenges of insects as an innovative source for food and feed production. Innovative Food Sci. Emerg. Technol. 2013;17:1–11. [Google Scholar]
- Rusoff L.L., Blakeney E.W., Jr., Culley D.D., Jr. Duckweeds (Lemnaceae family): a potential source of protein and amino acids. J. Agric. Food Chem. 1980;28(4):848–850. doi: 10.1021/jf60230a040. [DOI] [PubMed] [Google Scholar]
- des Mes T., Kujawa-Roeleveld K., Zeeman G., Lettinga G. Anaerobic biodegradation of estrogens—hard to digest. Water Sci. Technol. 2008;57(8):1177–1182. doi: 10.2166/wst.2008.102. [DOI] [PubMed] [Google Scholar]
- Saeid A., Chojnacka K., Opaliński S., Korczyński M. Biomass of Spirulina maxima enriched by biosorption process as a new feed supplement for laying hens. Algal Res. 2016;19:342–347. [Google Scholar]
- Sahlström L. A review of survival of pathogenic bacteria in organic waste used in biogas plants. Bioresour. Technol. 2003;87(2):161–166. doi: 10.1016/s0960-8524(02)00168-2. [DOI] [PubMed] [Google Scholar]
- Sahlström L., Bagge E., Emmoth E., Holmqvist A., Danielsson-Tham M.-L., Albihn A. A laboratory study of survival of selected microorganisms after heat treatment of biowaste used in biogas plants. Bioresour. Technol. 2008;99(16):7859–7865. doi: 10.1016/j.biortech.2007.09.071. [DOI] [PubMed] [Google Scholar]
- Salati S., D'Imporzano G., Panseri S., Pasquale E., Adani F. Degradation of aflatoxin B1 during anaerobic digestion and its effect on process stability. Int. Biodeterior. Biodegrad. 2014;94:19–23. [Google Scholar]
- Salsali H., Parker W.J., Sattar S.A. The effect of volatile fatty acids on the inactivation of Clostridium perfringens in anaerobic digestion. World J. Microbiol. Biotechnol. 2008;24(5):659–665. [Google Scholar]
- Santaeufemia S., Torres E., Mera R., Abalde J. Bioremediation of oxytetracycline in seawater by living and dead biomass of the microalga Phaeodactylum tricornutum. J. Hazard. Mater. 2016;320:315–325. doi: 10.1016/j.jhazmat.2016.08.042. [DOI] [PubMed] [Google Scholar]
- Särkkä H., Bhatnagar A., Sillanpää M. Recent developments of electro-oxidation in water treatment — a review. J. Electroanal. Chem. 2015;754:46–56. [Google Scholar]
- Sarmah A.K., Meyer M.T., Boxall A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere. 2006;65(5):725–759. doi: 10.1016/j.chemosphere.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Schmitt D., Müller A., Csögör Z., Frimmel F.H., Posten C. The adsorption kinetics of metal ions onto different microalgae and siliceous earth. Water Res. 2001;35(3):779–785. doi: 10.1016/s0043-1354(00)00317-1. [DOI] [PubMed] [Google Scholar]
- Schnürer A., Schnürer J. Fungal survival during anaerobic digestion of organic household waste. Waste Manag. 2006;26(11):1205–1211. doi: 10.1016/j.wasman.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Schoumans O.F., Bouraoui F., Kabbe C., Oenema O., van Dijk K.C. Phosphorus management in Europe in a changing world. Ambio. 2015;44(2):180–192. doi: 10.1007/s13280-014-0613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher G., Blume T., Sekoulov I. Bacteria reduction and nutrient removal in small wastewater treatment plants by an algal biofilm. Water Sci. Technol. 2003;47(11):195–202. [PubMed] [Google Scholar]
- Seeger H., Heikenwalder M., Zeller N., Kranich J., Schwarz P., Gaspert A., Seifert B., Miele G., Aguzzi A. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science. 2005;310(5746):324–326. doi: 10.1126/science.1118829. [DOI] [PubMed] [Google Scholar]
- Shanab S., Essa A., Shalaby E. Bioremoval capacity of three heavy metals by some microalgae species (Egyptian Isolates) Plant Signal. Behav. 2012;7(3):392–399. doi: 10.4161/psb.19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.S., Gaur J.P. Potential of Lemna polyrrhiza for removal of heavy metals. Ecol. Eng. 1995;4(1):37–43. [Google Scholar]
- Shi W., Wang L., Rousseau D.P.L., Lens P.N.L. Removal of estrone, 17α-ethinylestradiol, and 17ß-estradiol in algae and duckweed-based wastewater treatment systems. Environ. Sci. Polut. Res. 2010;17(4):824–833. doi: 10.1007/s11356-010-0301-7. [DOI] [PubMed] [Google Scholar]
- Shilton A., Mara D., Craggs R., Powell N. Solar-powered aeration and disinfection, anaerobic co-digestion, biological CO2 scrubbing and biofuel production: the energy and carbon management opportunities of waste stabilisation ponds. Water Sci. Technol. 2008;58(1):253–258. doi: 10.2166/wst.2008.666. [DOI] [PubMed] [Google Scholar]
- Short S.M. The ecology of viruses that infect eukaryotic algae. Environ. Microbiol. 2012;14(9):2253–2271. doi: 10.1111/j.1462-2920.2012.02706.x. [DOI] [PubMed] [Google Scholar]
- Sjahrul M. Phytoremediation of Cd2+ by Marine Phytoplanktons, Tetracelmis chuii and Chaetoceros calcitrans. Int. J. Chem. 2012;4(1):69. [Google Scholar]
- Smith M., Moelyowati I. Duckweed based wastewater treatment (DWWT): design guidelines for hot climates. Water Sci. Technol. 2001;43(11):291–299. [PubMed] [Google Scholar]
- Sobsey M., Khatib L., Hill V., Alocilja E., Pillai S. 2006. Pathogens in animal wastes and the impacts of waste management practices on their survival, transport and fate. [Google Scholar]
- Solovchenko A., Verschoor A.M., Jablonowski N.D., Nedbal L. Phosphorus from wastewater to crops: an alternative path involving microalgae. Biotechnol. Adv. 2016;34(5):550–564. doi: 10.1016/j.biotechadv.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Spencer J.L., Guan J. Public Health Microbiology: Methods and Protocols. 2004. Public health implications related to spread of pathogens in manure from livestock and poultry operations; pp. 503–515. [DOI] [PubMed] [Google Scholar]
- Spoehr H.A. The need for a new source of food. In: Burlew J.S., editor. Algal Culture. From Laboratory to Pilot Plant. Carnegie Institute of Washington Publication; Washington D.C.: 1953. pp. 24–30. [Google Scholar]
- Spolaore P., Joannis-Cassan C., Duran E., Isambert A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006;101(2):87–96. doi: 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- Sree K.S., Keresztes Á., Mueller-Roeber B., Brandt R., Eberius M., Fischer W., Appenroth K.-J. Phytotoxicity of cobalt ions on the duckweed Lemna minor–Morphology, ion uptake, and starch accumulation. Chemosphere. 2015;131:149–156. doi: 10.1016/j.chemosphere.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Srivastava A., Appenroth K.-J. Interaction of EDTA and iron on the accumulation of Cd2+ in duckweeds (Lemnaceae) J. Plant Physiol. 1995;146(1–2):173–176. [Google Scholar]
- Stasinakis A.S. Review on the fate of emerging contaminants during sludge anaerobic digestion. Bioresour. Technol. 2012;121:432–440. doi: 10.1016/j.biortech.2012.06.074. [DOI] [PubMed] [Google Scholar]
- Suresh Kumar K., Dahms H.-U., Lee J.-S., Kim H.C., Lee W.C., Shin K.-H. Algal photosynthetic responses to toxic metals and herbicides assessed by chlorophyll a fluorescence. Ecotoxicol. Environ. Saf. 2014;104:51–71. doi: 10.1016/j.ecoenv.2014.01.042. [DOI] [PubMed] [Google Scholar]
- Suresh Kumar K., Dahms H.-U., Won E.-J., Lee J.-S., Shin K.-H. Microalgae – a promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015;113:329–352. doi: 10.1016/j.ecoenv.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Taelman S.E., De Meester S., Van Dijk W., da Silva V., Dewulf J. Environmental sustainability analysis of a protein-rich livestock feed ingredient in the Netherlands: microalgae production versus soybean import. Resour. Conserv. Recycl. 2015;101:61–72. [Google Scholar]
- Teclu D., Tivchev G., Laing M., Wallis M. Determination of the elemental composition of molasses and its suitability as carbon source for growth of sulphate-reducing bacteria. J. Hazard. Mater. 2009;161(2):1157–1165. doi: 10.1016/j.jhazmat.2008.04.120. [DOI] [PubMed] [Google Scholar]
- Tijero J., Guardiola E., Cortijo M., Diaz-barrionuevo A. Heavy metal distribution in anaerobic sludges. Sep. Sci. Technol. 1990;25(5):653–658. [Google Scholar]
- Tillmann U. Interactions between planktonic microalgae and protozoan grazers. J. Eukaryot. Microbiol. 2004;51(2):156–168. doi: 10.1111/j.1550-7408.2004.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Toumi A., Nejmeddine A., El Hamouri B. Heavy metal removal in waste stabilisation ponds and high rate ponds. Water Sci. Technol. 2000;42(10):17–21. [Google Scholar]
- Tripathi R., Chandra P. Chromium uptake by Spirodela polyrrhiza (L.) schleiden in relation to metal chelators and pH. Bull. Environ. Contam. Toxicol. 1991;47(5):764–769. doi: 10.1007/BF01701147. [DOI] [PubMed] [Google Scholar]
- Tsezos M., Remoudaki E., Angelatou V. A study of the effects of competing ions on the biosorption of metals. Int. Biodeterior. Biodegrad. 1996;38(1):19–29. [Google Scholar]
- Turner C., Burton C.H. The inactivation of viruses in pig slurries: a review. Bioresour. Technol. 1997;61(1):9–20. [Google Scholar]
- Uggetti E., Sialve B., Latrille E., Steyer J.-P. Anaerobic digestate as substrate for microalgae culture: the role of ammonium concentration on the microalgae productivity. Bioresour. Technol. 2014;152:437–443. doi: 10.1016/j.biortech.2013.11.036. [DOI] [PubMed] [Google Scholar]
- Underwood G., Baker J. The effect of various aquatic bacteria on the growth and senescence of duckweed (Lemna minor) J. Appl. Microbiol. 1991;70(3):192–196. [Google Scholar]
- Valeur I. Norwegian University of Life Sciences; Ås: 2011. Speciation of heavy metals and nutrient elements in digestate; p. 48. (MSc Thesis) [Google Scholar]
- Van Boeckel T.P., Glennon E.E., Chen D., Gilbert M., Robinson T.P., Grenfell B.T., Levin S.A., Bonhoeffer S., Laxminarayan R. Reducing antimicrobial use in food animals. Science. 2017;357(6358):1350–1352. doi: 10.1126/science.aao1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Hende S., Vervaeren H., Boon N. Flue gas compounds and microalgae: (bio-) chemical interactions leading to biotechnological opportunities. Biotechnol. Adv. 2012;30(6):1405–1424. doi: 10.1016/j.biotechadv.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Van Den Hende S., Beyls J., De Buyck P.-J., Rousseau D.P.L. Food-industry-effluent-grown microalgal bacterial flocs as a bioresource for high-value phycochemicals and biogas. Algal Res. 2016;18:25–32. [Google Scholar]
- Van Praagh A.D., Gavaghan P.D., Sykora J.L. Giardia muris Cyst Inactivation in Anaerobic Digester Sludge. Water Sci. Technol. 1993;27(3-4):105–109. [Google Scholar]
- Verbyla M.E., Mihelcic J.R. A review of virus removal in wastewater treatment pond systems. Water Res. 2015;71:107–124. doi: 10.1016/j.watres.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Verma R., Suthar S. Lead and cadmium removal from water using duckweed–Lemna gibba L.: impact of pH and initial metal load. Alexandria Eng. J. 2015;54(4):1297–1304. [Google Scholar]
- Vermeulen S.J., Aggarwal P.K., Ainslie A., Angelone C., Campbell B.M., Challinor A., Hansen J.W., Ingram J., Jarvis A., Kristjanson P. Options for support to agriculture and food security under climate change. Environ. Sci. Pol. 2012;15(1):136–144. [Google Scholar]
- Viancelli A., Kunz A., Steinmetz R., Kich J., Souza C., Canal C., Coldebella A., Esteves P., Barardi C. Performance of two swine manure treatment systems on chemical composition and on the reduction of pathogens. Chemosphere. 2013;90(4):1539–1544. doi: 10.1016/j.chemosphere.2012.08.055. [DOI] [PubMed] [Google Scholar]
- Viancelli A., Kunz A., Fongaro G., Kich J.D., Barardi C.R.M., Suzin L. Pathogen inactivation and the chemical removal of phosphorus from swine wastewater. Water Air Soil Pollut. 2015;226(8):263. [Google Scholar]
- Wagner M., Brumelis D., Gehr R. Disinfection of wastewater by hydrogen peroxide or peracetic acid: development of procedures for measurement of residual disinfectant and application to a physicochemically treated municipal effluent. Water Environ. Res. 2002;74(1):33–50. doi: 10.2175/106143002x139730. [DOI] [PubMed] [Google Scholar]
- Wagner A.O., Gstraunthaler G., Illmer P. Survival of bacterial pathogens during the thermophilic anaerobic digestion of biowaste: Laboratory experiments and in situ validation. Anaerobe. 2008;14(3):181–183. doi: 10.1016/j.anaerobe.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Wahaab R.A., Lubberding H., Alaerts G. Copper and chromium (III) uptake by duckweed. Water Sci. Technol. 1995;32(11):105–110. [Google Scholar]
- Walsh B.J., Rydzak F., Palazzo A., Kraxner F., Herrero M., Schenk P.M., Ciais P., Janssens I.A., Peñuelas J., Niederl-Schmidinger A., Obersteiner M. New feed sources key to ambitious climate targets. Carbon Balanc. Manag. 2015;10(1):1–8. doi: 10.1186/s13021-015-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: a review. J. Environ. Manag. 2016;182:620–640. doi: 10.1016/j.jenvman.2016.07.049. [DOI] [PubMed] [Google Scholar]
- Wang L., Li Y., Chen P., Min M., Chen Y., Zhu J., Ruan R.R. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae chlorella sp. Bioresour. Technol. 2010;101(8):2623–2628. doi: 10.1016/j.biortech.2009.10.062. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu J., Kang D., Wu C., Wu Y. Removal of pharmaceuticals and personal care products from wastewater using algae-based technologies: a review. Rev. Environ. Sci. Biotechnol. 2017;16(4):717–735. [Google Scholar]
- Watkins B.D., Hengemuehle S.M., Person H.L., Yokoyama M.T., Masten S.J. Ozonation of swine manure wastes to control odors and reduce the concentrations of pathogens and toxic fermentation metabolites. Ozone Sci. Eng. 1997;19(5):425–437. [Google Scholar]
- Weiland P. Biogas production: current state and perspectives. Appl. Microbiol. Biotechnol. 2010;85(4):849–860. doi: 10.1007/s00253-009-2246-7. [DOI] [PubMed] [Google Scholar]
- Weiner J.A., DeLorenzo M.E., Fulton M.H. Relationship between uptake capacity and differential toxicity of the herbicide atrazine in selected microalgal species. Aquat. Toxicol. 2004;68(2):121–128. doi: 10.1016/j.aquatox.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Wheeler T., von Braun J. Climate change impacts on global food security. Science. 2013;341(6145):508–513. doi: 10.1126/science.1239402. [DOI] [PubMed] [Google Scholar]
- Whitton R., Ometto F., Pidou M., Jarvis P., Villa R., Jefferson B. Microalgae for municipal wastewater nutrient remediation: mechanisms, reactors and outlook for tertiary treatment. Environ. Technol. Rev. 2015;4(1):133–148. [Google Scholar]
- WHO . 2017. Ten chemicals of major public health concern.http://www.who.int/ipcs/assessment/public_health/chemicals_phc/en/ [Google Scholar]
- Wong J.W., Selvam A. Reduction of indicator and pathogenic microorganisms in pig manure through fly ash and lime addition during alkaline stabilization. J. Hazard. Mater. 2009;169(1):882–889. doi: 10.1016/j.jhazmat.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Wu J.J., Park S.-h., Hengemuehle S.M., Yokoyama M.T., Person H.L., Masten S.J. The effect of storage and ozonation on the physical, chemical, and biological characteristics of swine manure slurries. Ozone Sci. Eng. 1998;20(1):35–50. [Google Scholar]
- Xia A., Murphy J.D. Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotechnol. 2016;34(4):264–275. doi: 10.1016/j.tibtech.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Xu J., Shen G. vol. 102. 2011. Growing duckweed in swine wastewater for nutrient recovery and biomass production. [DOI] [PubMed] [Google Scholar]
- Xu J., Cheng J.J., Stomp A.M. Growing Spirodela polyrrhiza in swine wastewater for the production of animal feed and fuel ethanol: a pilot study. CLEAN–Soil, Air, Water. 2012;40(7):760–765. [Google Scholar]
- Xu C., Salsali H., Weese S., Warriner K. Inactivation of Clostridium difficile in sewage sludge by anaerobic thermophilic digestion. Can. J. Microbiol. 2015;62(1):16–23. doi: 10.1139/cjm-2015-0511. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y., Takimura O., Fuse H., Kamimura K. Accumulation of arsenic and selenium by Dunaliella sp. Appl. Organomet. Chem. 1990;4(3):261–264. [Google Scholar]
- Yan H., Pan G. Toxicity and bioaccumulation of copper in three green microalgal species. Chemosphere. 2002;49(5):471–476. doi: 10.1016/s0045-6535(02)00285-0. [DOI] [PubMed] [Google Scholar]
- Yenigün O., Demirel B. Ammonia inhibition in anaerobic digestion: a review. Process Biochem. 2013;48(5–6):901–911. [Google Scholar]
- Yunoki M., Tanaka H., Urayama T., Hattori S., Ohtani M., Ohkubo Y., Kawabata Y., Miyatake Y., Nanjo A., Iwao E. Prion removal by nanofiltration under different experimental conditions. Biologicals. 2008;36(1):27–36. doi: 10.1016/j.biologicals.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Zacharof M.-P., Lovitt R. Adding value to wastewater by resource recovery and reformulation as growth media: current prospects and potential. J. Water Reuse Desalin. 2015;5(4):473–479. [Google Scholar]
- Zhang K., Farahbakhsh K. Removal of native coliphages and coliform bacteria from municipal wastewater by various wastewater treatment processes: implications to water reuse. Water Res. 2007;41(12):2816–2824. doi: 10.1016/j.watres.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhao F.J., Huang Q., Williams P.N., Sun G.X., Zhu Y.G. Arsenic uptake and speciation in the rootless duckweed Wolffia globosa. New Phytol. 2009;182(2):421–428. doi: 10.1111/j.1469-8137.2008.02758.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Hu Y., Yang B., Ma F., Lu P., Li L., Wan C., Rayner S., Chen S. Duckweed (Lemna minor) as a model plant system for the study of human microbial pathogenesis. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Li Y., Yang M., Li W. Content of heavy metals in animal feeds and manures from farms of different scales in Northeast China. Int. J. Environ. Res. Public Health. 2012;9(8):2658–2668. doi: 10.3390/ijerph9082658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Gersberg R.M., Ng W.J., Tan S.K. Removal of pharmaceuticals and personal care products in aquatic plant-based systems: a review. Environ. Pollut. 2014;184:620–639. doi: 10.1016/j.envpol.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Zhang H., Shi J., Liu X., Zhan X., Chen Q. Occurrence and removal of free estrogens, conjugated estrogens, and bisphenol A in manure treatment facilities in East China. Water Res. 2014;58:248–257. doi: 10.1016/j.watres.2014.03.074. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Habteselassie M.Y., Resurreccion E.P., Mantripragada V., Peng S., Bauer S., Colosi L.M. Evaluating removal of steroid estrogens by a model alga as a possible sustainability benefit of hypothetical integrated algae cultivation and wastewater treatment systems. ACS Sustain. Chem. Eng. 2014;2(11):2544–2553. [Google Scholar]
- Zhao Z. Virginia Polytechnic Institute and State University; Blacksburg, Virginia: 2008. Removal of Estrogens at Full and Pilot Scale Livestock Manure Treatment Systems. (p. 104) [Google Scholar]
- Zheng W., Li X., Yates S.R., Bradford S.A. Anaerobic transformation kinetics and mechanism of steroid estrogenic hormones in dairy lagoon water. Environ. Sci. Technol. 2012;46(10):5471–5478. doi: 10.1021/es301551h. [DOI] [PubMed] [Google Scholar]
- Ziegler P., Sree K., Appenroth K.-J. Duckweeds for water remediation and toxicity testing. Toxicol. Environ. Chem. 2016;98(10):1127–1154. [Google Scholar]
- Ziemer C., Bonner J., Cole D., Vinje J., Constantini V., Goyal S., Gramer M., Mackie R., Meng X., Myers G. Fate and transport of zoonotic, bacterial, viral, and parasitic pathogens during swine manure treatment, storage, and land application. J. Anim. Sci. 2010;88(13):E84–E94. doi: 10.2527/jas.2009-2331. [DOI] [PubMed] [Google Scholar]