Abstract
Respiratory infections such as SARS-CoV in humans are often accompanied by mild and self-limiting hepatitis. As a respiratory disease, influenza A virus (IAV) infection can lead to hepatitis, but the mechanism remains unclear. This study aimed to investigate the occurrence of hepatitis by establishing a model for infected mice for three different subtypes of respiratory IAVs (H1N1, H5N1, and H7N2). Histological analysis was performed, and results showed increase serum aminotransferase (ALT and AST) levels and evident liver injury on days 3 and 7, especially on day 5 post infection. Immunohistochemistry (IHC) results indicated a wide distribution of IAV's positive signals in the liver of infected mice. Real-time PCR results further revealed a similar viral titer to IHC that presented a remarkedly positive correlation with histology injury. All these data showed that the mouse model suitably contributed valuable information about the mechanism underlying the occurrence of hepatitis induced by respiratory influenza virus.
Keywords: Influenza A virus, Liver, Injury, Distribution
Highlights
-
•
Respiratory influenza A virus elevated aminotransferase levels in infected mice.
-
•
IAVs could replicate in liver and lead mice model liver injury.
-
•
IAVs induced injury in liver is a short-term.
1. Introduction
Influenza A virus (IAV) is a typical enveloped negative-strand RNA virus that belongs to the Orthomyxovirida family. Its genome consists of eight RNA fragments, two of which encode two important surface glycoproteins called hemagglutinin (HA) and neuraminidase (NA). According to its antigenicity, this virus can be divided into 18 HA subtypes (H1~H18) and 11 NA subtypes (N1~N11). At present, some of the combination between different HA subtypes and NA subtypes have been detected [1]. Depending on the virus strain, different IAVs have different pathogenesis for the infection. Low pathogenic avian influenza viruses (LPAIV) cause mild respiratory symptoms and are sometimes accompanied with a gastrointestinal infection. Highly pathogenic avian influenza viruses (HPAIV), including H5N1 and H7N9, can cause multi-organ systemic infection and even fatal diseases [2]. The main clinical feature of these cases is the different degrees of pneumonia manifesting as fever, cough, and difficulty in breathing.
Humans infected with SARS-CoV usually demonstrate virus-associated hepatitis, which is characterized by a viral signal distributed in the liver [3,4]. However, studies on this issue during the influenza virus infection are limited. A case reported that a 3-year-old boy infected with the H5N1 avian influenza virus died of Reye's syndrome and respiratory distress syndrome [5]. A novel pandemic 2009 influenza A ⁄ H1N1 different from the seasonal influenza infection induced hepatocellular injury with increased serum aminotransaminase levels [6,7]. When humans are infected with H7N9, hypoxic and fatty changes in the liver tissue have been observed [8,9]. Moreover, in advanced chronic liver disease, diseases caused by the common bacteria Streptococcus pneumoniae or influenza virus increased the risk for serious health complications and death [10]. These data are interesting because liver damage occurs after influenza infection, but no viral pathogen is detected.
In this study, a model was built for three different subtypes of respiratory IAV, namely, H1N1 (A/WSN/33), H5N1 (A/Chicken/Henan/1/04), and H7N2 (A/Chicken/Hebei/2/02), which was used to infect BALB/c mice. We detected and examined the viral expression profiles and histological distributions in the mice liver during infection to generate basic data regarding the influenza virus infection in liver and further understand the role of the liver in the virus induced systemic symptoms in mammalian hosts. To date, this study first investigates whether the respiratory influenza virus infection can induce liver injury in mice and the correlation between them.
2. Materials and methods
2.1. Virus
Three influenza viruses, H1N1 (A/WSN/33), H5N1 (A/chicken/Henan/1/2004), and H7N2 A/Chicken/Hebei/2/02) were used in this study. These viruses were propagated in the allantoic cavities of 10-day-old embryonated chicken eggs at 37 °C for 48–96 h. The allantoic fluid was harvested, aliquoted, and stored at −80 °C. Serial dilution of the stock was carried out to measure the lethal dose (LD50) and tissue culture infective dose (TCID50) in mice and cells.
2.2. Animals and infection
Female BALB/c mice (18–20 g) were purchased from Vital River Laboratories (Beijing, China). The mice were randomly divided into four groups and housed under specific pathogen-free conditions. To investigate the virus infection, the mice were anesthetized by intramuscular administration of Zoletil (Virbac, Carros, France) and infected intranasally with 3 LD50 of three different subtypes virus in phosphate-buffered saline (PBS) (50 μL/mouse).The mice infected with H1N1 IAV were randomly divided into two groups, one group (7 mice) was used for measuring survival, and the other group (15 mice) was utilized for sample collection. The mice infected with H5 and H7 were treated in the same manner. Animal care and experimental procedures were followed according to the Regulations of Experimental Animals of Beijing Authority and approved by the Animal Ethics Committee of China Agricultural University (approval number 201206078).
2.3. Analysis of liver function
The serum from infected or control mice were collected and stored at −80 °C until analysis. Liver function was determined by measuring the serum ALT (alanine aminotransferase) and AST (aspartate aminotransferase) levels with alanine aminotransferase (C009-2-1) and aspartate aminotransferase (C010-2-1) assay Kit in accordance with the manufacturer's instructions (Jiancheng, Nanjing, China).
2.4. Histopathological and immunohistochemical analysis
The tissues were removed and fixed with 4% neutral formalin at room temperature for 48 h. Serial tissue sections were cut into 4-μm thickness after embedding in paraffin. Each slide was stained with hematoxylin and eosin (H & E) and then to determine histological changes by light microscopy (Olympus BX41, Olympus Optical Co., Tokyo, Japan). The pathological changes were evaluated by a veterinary pathologist and given a score from 0 to 4 in a blinded study. The indications for the scores were as follows: 0 = no microscopic lesions; 1 = extremely mild, characterized by slight congestion and hemorrhage; 2 = mild, characterized by congestion, hemorrhage, vacuolization and acidophilic degeneration, 3 = moderate, characterized by congestion, hemorrhage, serious vacuolization and acidophilic degeneration, and necrosis with dissolved nucleus in the liver cells; 4 = severe, characterized by congestion, hemorrhage, serious vacuolization and acidophilic degeneration, serious necrosis with dissolved nucleus in liver cells as well as inflammatory cell infiltration.
The examination of influenza viral antigen in the tissue samples was performed by immunohistochemical analysis. Sections were blocked in 10% normal goat serum in PBS for 30 min then incubated in anti-influenza nucleoprotein (NP) mAb (AA5H, Abcam, Cambridge, MA, USA) at 1:1000 dilution for 2 h. The slides were further incubated with goat anti-mouse immunoglobin G (IgG) conjugated with avidin (Chemicon, Temecula, CA, USA) for 1 h, followed by incubation with biotinylated peroxidase (Victoria, BC, Canada) for an additional 1 h. Staining was visualized by the addition of 3, 3-diaminobenzidin (DAB, Sigma, St. Louis, MO, USA) for 15 min and counterstained with hematoxylin mounted with neutral balsam. The detection of the IAV antigen was scored from 0 to 4 according to the number of positive cells per section. The indications for the scores were as follows: 0 = no positive cells, 1 = 1–10 positive cells, 2 = 11–50 positive cells, 3 = 51–150 positive cells and 4 ≥ 150 positive cells.
2.5. Quantitative polymerase chain reaction (qPCR)
Total RNA was prepared from 10 mg of tissue homogenized in Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The DNaseI-treated RNA (0.2 μg) was reverse-transcribed into cDNA using universal primers for IAV (Uni 12) with an EasyScript First-Strand cDNA Synthesis Super Mix (TransGen Biotech, Beijing, China). Quantification of influenza virus (nonstructural protein, NS) gene expression was conducted using a Power SYBR® Green PCR Master Mix kit (Applied Biosystems, Foster City, CA, USA). The following primers were used in the qPCR: forward primer, 5′-GCA ATT GGA ATC CTC ATC GG-3′, and reverse primer, 5′-CAA CTC GTT TCG CCA TGT AGC-3′. The reaction was run on a 7500 thermal cycler (Applied Biosystems) with an initial denaturation step at 95 °C for 10 min, followed by 35 cycles of 95 °C for 15 s, 56 °C for 30 s, and 72 °C for 40 s. Data analysis was performed using 7500 software v2.0 (Applied Biosystems). The copy number of the NS gene was calculated using an NS-containing plasmid of known concentration as a standard.
2.6. Statistical analysis
Data are expressed as means ± standard error (SE). The variability among the different groups were determined by the two-way tests of variance using the GraphPad Prism software (version 5.0), and the statistical significance was set at p < 0.05.
3. Results
3.1. Respiratory IAV infection model displayed increased ALT and AST
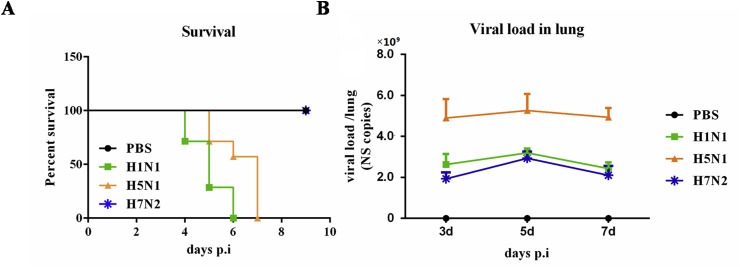
We investigated the IAV infection by using three of its subtype IAVs (H1N1, H5N1, and H7N2) in an infected mouse model. After infection, the animals in the infected groups demonstrated respiratory illness symptoms, such as fever, shortness of breath, and gradual decrease in body weight from days 2–8 compared with those under PBS treated controls, which appeared normal over the entire period (data not shown). H7N2 is a low pathogenic virus that can cause mild respiratory symptoms in mice instead of death. The survival of mice in the four groups is shown in Fig. 1 A. At the same time, qPCR assays were further performed on viral RNA extracted from the lungs of these animals to detect the viral copies of the NS gene. Results showed that viral RNA was detectable on day 1 and then increased. H5N1 infected mice presented the highest vial load among the three virus subtype infected groups. No viral RNA was detected in any of the control mice (Fig. 1B). These data clearly indicated that three subtypes of the respiratory influenza A virus infection model were successfully built.
Fig. 1.
Three different subtypes respiratory influenza A virus infection model was build in BALB/c mice. BALB/c mice were i.n. infected with PBS or H1N1, H5N1, and H7N2 respectively. (A) Survival was monitored after infection (N = 7). (B) Viral load in the lungs was measured by real-time PCR (N = 3).
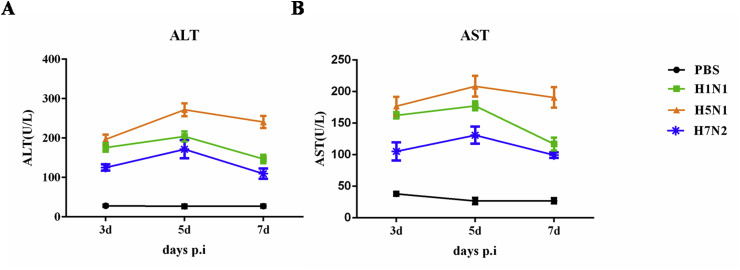
The elevation of liver aminotransferase levels is common during viral infections. However, the incidence of liver involvement has not been established in influenza infection because clinical attention is on the acute infection in the respiratory tract. To test the involvement of liver in influenza infection, the ALT and AST levels in serum were analyzed. Three infected group animals experienced a significant increase in ALT and AST levels after infection especially in H5N1 infected individuals (Fig. 2 ). The high ALT and AST values probably reflect the liver damage suffered by the infected mice.
Fig. 2.
Liver aminotransferase levels in serum were determined after infection. Changes in (A)ALT and (B)AST; (N = 3).
3.2. Influenza A virus infection causes liver histopathologic injury
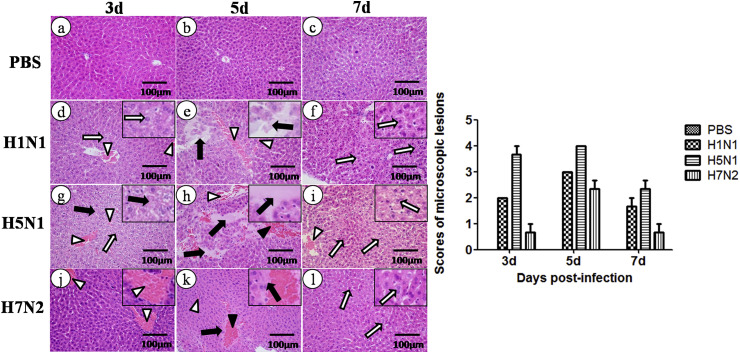
To test whether liver injury also occurred after influenza virus infection, the histological changes in the liver of infected mice at days 3, 5, and 7 post-infection (p.i.) were evaluated. On day 3 p.i., the liver cell appeared have slight vacuolization and focal dilation, and congestion was observed in the hepatic sinusoidal and central vein. Moreover, the structural disorder of hepatic lobules and necrosis with dissolved nucleus in the liver cells was seen in H5N1 infected mice. On day 5 p.i., a more noticeable hemorrhaging was seen in the hepatic sinusoidal and central veins and a large amount of red blood cells was observed between the cells, in which certain cells dissolved. Inflammatory cell infiltration, including lymphocyte and neutrophils in the hepatic sinusoida veinl was noted in the H5N1 infected group. On day 7 p.i., the liver appeared to have vacuolization and acidophilic degeneration accompanied with abundant eosinophilic cytoplasm. H5N1 infected animals displayed more severe pathological damage among the three IAV infected groups. The pathological damage in these infected mice was most serious on day 5 p.i but mild on day 3 and 7. No clear histopathological change was seen in PBS treated mice (Fig. 3 ). The scores in the pathological changes were also shown. These results suggested that the three IAV subtypes can induce liver injury and H5N1causes the most serious pathological lesions among them.
Fig. 3.
Pathology tests on the liver performed by using hematoxylin and eosin (H & E) staining. The hollow arrows indicate vacuolation or acidophilic degeneration; black arrows indicate necrosis or degeneration; hollow triangles indicate congestion; black triangles indicate hemorrhage. Pathological changes were evaluated by a veterinary pathologist and scored 0 to 4 in a blinded study (N = 3).
3.3. Viral replication probably induced liver injury
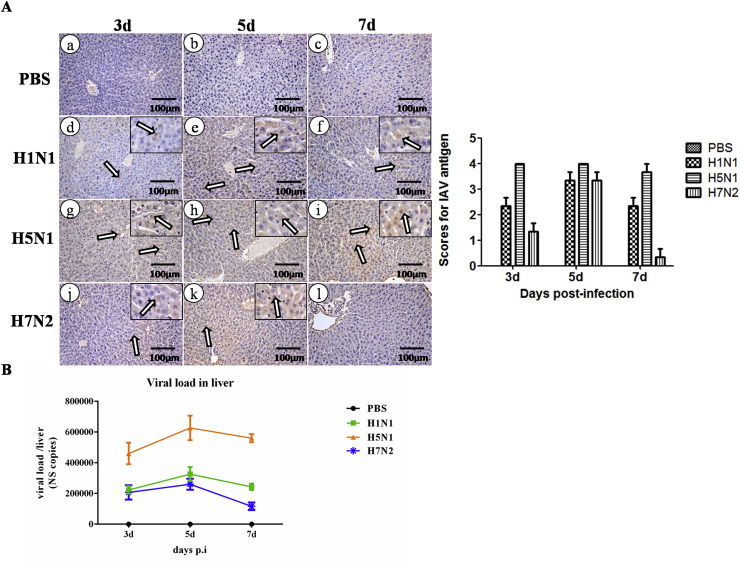
To investigate whether viral replication led to tissue damage, the antigens of IAV in liver of the three subtype-infected mice were examined by immunohistochemistry(IHC), which used an NP specific mouse monoclonal antibody. It was demonstrated that antigens of IAV were detected on day 3 p.i in the liver infected with H1N1,H5N1, and H7N2, whereas a significantly higher amount of positive signals were observed on day 5 p.i than that on day 3 and 7 p.i. However, no positive signals of NP were found in the liver of H7N2-infected mice on day 7.In the H5N1 infected mice, an extensively distributed positive signal can be detected in the liver during entire infection period (Fig. 4 A). The scores of IAV antigen were also shown.
Fig. 4.
Viral load in the liver detected after infection. (A) Viral distribution in the liver detected through immunohistochemistry (IHC) a the specific NP antibody. The brown cells are positive cells and indicated by hollow arrows. The detection of the IAV antigens were evaluated by a veterinary pathologist and scored 0 to 4 in a blinded study(N = 3). (B) Viral load in the liver etected by qRT-PCR through NS gene (N = 3).
An absolute quantification of PCR was applied to rule out the possibility that the error from the semi-quantitative detection method of IHC and further quantify the viral load in the liver. The findings showed that the virus can be detected in all of the three infected groups during the entire infection period. Furthermore, an increased number of viral load was observed at the early stage, wherein their peak values at day 5, and then decreased in H1N1- and H7N2- infected mice. By contrast, in the H5N1- infected group, a continuously high level of viral load was observed during the entire infection stage (Fig. 4B). Similar to the IHC data, these findings demonstrated a positive correlation with the degree of histological injury in liver. These data suggested that the viral replication in liver may contribute to its injury.
4. Discussion
As a respiratory disease, the liver injury does not typically occur during influenza A virus infection. That respiratory, gastrointestinal, and other mucosal tissues have experienced viral replication or immune damage, but do not influence non-mucosal organs, such as the liver or kidney [[11], [12], [13]]. However, some contradicting findings were obtained, such that a pandemic A ⁄ H1N1 (2009) influenza A virus, which is different from seasonal influenza, can lead to liver damage [14]. An abrupt and massive increase in aminotransferase levels is the first direct evidence of liver injury [[15], [16], [17]]. Unexpectedly, we observed that the three subtypes of respiratory influenza infection in mice caused not only damage in the lungs but also in the liver accompanied with clear upregulation of AST in serum. Given that these symptoms resembles those exhibited by humans after influenza infection, our influenza virus infected mice provided a good model for studying the underlying mechanisms of how respiratory influenza infection causes liver damage.
Influenza productively infects the upper respiratory tract to host a trypsin-like enzyme and activate the viral hemagglutin. Liver injury associated with influenza virus infection is uncommon. In case of influenza with accompanying liver damage, no direct evidence is available for viral replication in liver. Therefore, the means for associating influenza infection with damage to tissues or organs is still unclear when these injuries are not induced by direct viral infection. After respiratory A/Kawasaki/86 (H1N1) virus infection, the C57BL/6 (B6) mice displayed “collateral damage” in liver without viral replication. Further study found that Kupffer cells and apoptotic hepatocytes mediated inflammatory injury [18]. Hypoxic hepatitis was a clinical characteristic in the H7N9 infection [9]. Recent data further confirmed that hypoxic hepatitis can be an important causative factor for acute liver failure associated with influenza virus infection instead of virus related hepatitis [19]. We know that highly pathogenic influenza virus leads to respiratory failure, which reduces the oxygen supply to the liver, and hypoxic hepatitis is a likely cause of severe liver impairment in our study. In our study, viral antigens were widely distributed in the liver of the different influenza virus subtype-infected mice during the infection stage, especially the H5N1- infected group. As expected, a certain relationship exists between the degree of pathological injury in liver (revealed by HE) and the viral load in liver (detected by qRT-PCR). In this work, we conclude that the virus existing in the liver probably induced pathological injury. However, the underlying mechanisms need further investigation.
The molecular structure and sensitivity of HA to host cell proteases directly affect the virulence of the virus. The majority of LPAIV, which contain a single arginine (R) or rarely include a lysine (K) at the HA cleavage site, usually cause a local infection. By contrast, the HPAIV of subtypes H5 and H7 possesses the consensus sequence R-X-R/K-R, which supports systemic infections [20]. In this study, among the three virus subtypes, H1N1 and H5N1 caused a high viral load in liver on the basis of the qPCR results. By contrast, H7N2 was a low pathogenic virus that caused a very small amount of virus in the liver. The results of the histopathological and immunohistochemical analyses further showed that pathological damage was present in the same data. However, only H5N1 virus possesses the R-K-K-R amino acid sequence at the cleavage site. The different levels of liver injury caused by influenza infection in this research were independent from the viral HA genes. Thus, a large scale study should be performed to determine the contribution of respiratory influenza virus inducing hepatic tropism and deduce the correlation between them.
In this study, our data demonstrated that pathological injury appeared on day 3 and aggravated to a peak on day 5, and then gradually decreased on day 7. This finding can be regarded as a short stage compared with virus induced respiratory damage during the entire infection period. Our data were similar to those of a previous research wherein respiratory infection induced mild liver damage and was self-limiting. However, the underlying mechanism needs further exploration. In summary, our present data suggested that respiratory influenza A virus infection induced liver injury and likely contributed to the viral replication in the liver. This work contributes to the research on the interaction between IAV and host and further explains the complex pathogenesis of influenza virus infection.
Acknowledgments
The authors would like to thank the Key Laboratory of Animal Epidemiology and Zoonosis of Ministry of Agriculture, China.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.micpath.2019.103736.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wu Y., Wu Y., Tefsen B., Shi Y., Gao G.F. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol. 2014;22:183–191. doi: 10.1016/j.tim.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mostafa A., Abdelwhab E.M., Mettenleiter T.C., Pleschka S. Zoonotic potential of influenza a viruses: a comprehensive overview. Viruses. 2018;10 doi: 10.3390/v10090497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chau T.N., Lee K.C., Yao H., Tsang T.Y., Chow T.C., Yeung Y.C. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams D.H., Hubscher S.G. Systemic viral infections and collateral damage in the liver. Am. J. Pathol. 2006;168:1057–1059. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ku A.S., Chan L.T. The first case of H5N1 avian influenza infection in a human with complications of adult respiratory distress syndrome and Reye's syndrome. J. Paediatr. Child Health. 1999;35:207–209. doi: 10.1046/j.1440-1754.1999.t01-1-00329.x. [DOI] [PubMed] [Google Scholar]
- 6.Cho W.H., Kim Y.S., Jeon D.S., Kim J.E., Kim K.I., Seol H.Y. Outcome of pandemic H1N1 pneumonia: clinical and radiological findings for severity assessment. Korean J. Intern. Med. 2011;26:160–167. doi: 10.3904/kjim.2011.26.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potapova O.V., Kovner A.V., Anikina A.G., Cherdantseva L.A., Sharkova T.V., Shkurupy V.A. Studies of influenza A/H1N1 A/Tomsk/13/2010 virus topology during development of infectious process in mammals. Bull. Exp. Biol. Med. 2016;160:683–686. doi: 10.1007/s10517-016-3249-x. [DOI] [PubMed] [Google Scholar]
- 8.Yu L., Wang Z., Chen Y., Ding W., Jia H., Chan J.F. vol. 57. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America; 2013. Clinical, Virological, and Histopathological Manifestations of Fatal Human Infections by Avian Influenza A(H7N9) Virus; pp. 1449–1457. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Liu J., Yu L., Zhou N., Ding W., Zheng S. Prevalence and characteristics of hypoxic hepatitis in the largest single-centre cohort of avian influenza A(H7N9) virus-infected patients with severe liver impairment in the intensive care unit. Emerg. Microb. Infect. 2016;5:e1. doi: 10.1038/emi.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harmala S., Parisinos C., Shallcross L., O'Brien A., Hayward A. Effectiveness of pneumococcal and influenza vaccines to prevent serious health complications in adults with chronic liver disease: a protocol for a systematic review. BMJ open. 2018;8:e018223. doi: 10.1136/bmjopen-2017-018223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huo C., Xiao K., Zhang S., Tang Y., Wang M., Qi P. H5N1 influenza a virus replicates productively in pancreatic cells and induces apoptosis and pro-inflammatory cytokine response. Front. Cell. Infect. Microbiol. 2018;8:386. doi: 10.3389/fcimb.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J., Li F., Wei H., Lian Z.X., Sun R., Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J. Exp. Med. 2014;211:2397–2410. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S., Wei T., Tianv H., Cheng J., Xiao J., Wang M. Small intestinal injury in mice infected with respiratory influenza A virus: evidence for virus induced gastroenteritis. Biotechnol. Lett. 2015;37:1585–1592. doi: 10.1007/s10529-015-1847-8. [DOI] [PubMed] [Google Scholar]
- 14.Papic N., Pangercic A., Vargovic M., Barsic B., Vince A., Kuzman I. Liver involvement during influenza infection: perspective on the 2009 influenza pandemic. Influenza Other Respir. Viruses. 2012;6:e2–5. doi: 10.1111/j.1750-2659.2011.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhut M., Thorburn K., Ahmed T. Transaminase levels in ventilated children with respiratory syncytial virus bronchiolitis. Intensive Care Med. 2004;30:931–934. doi: 10.1007/s00134-004-2236-2. [DOI] [PubMed] [Google Scholar]
- 16.Whitworth J.R., Mack C.L., O'Connor J.A., Narkewicz M.R., Mengshol S., Sokol R.J. Acute hepatitis and liver failure associated with influenza A infection in children. J. Pediatr. Gastroenterol. Nutr. 2006;43:536–538. doi: 10.1097/01.mpg.0000232332.00677.3d. [DOI] [PubMed] [Google Scholar]
- 17.Morton A. Presumed acute fatty liver of pregnancy following influenza A hepatitis. Obstet. Med. 2017;10:186–188. doi: 10.1177/1753495X17695173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polakos N.K., Cornejo J.C., Murray D.A., Wright K.O., Treanor J.J., Crispe I.N. Kupffer cell-dependent hepatitis occurs during influenza infection. Am. J. Pathol. 2006;168:1169–1178. doi: 10.2353/ajpath.2006.050875. quiz 404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nonaka K., Matsuda Y., Kakizaki M., Takakuma S., Hamamatsu A., Sakashita Y. Acute liver failure associated with influenza A virus infection: an autopsy case report. Jpn. J. Infect. Dis. 2019 doi: 10.7883/yoken.JJID.2018.494. [DOI] [PubMed] [Google Scholar]
- 20.Baron J., Tarnow C., Mayoli-Nussle D., Schilling E., Meyer D., Hammami M. Matriptase, HAT, and TMPRSS2 activate the hemagglutinin of H9N2 influenza A viruses. J. Virol. 2013;87:1811–1820. doi: 10.1128/JVI.02320-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.