Abstract
Background
Viral respiratory infections have been associated with up to 80% of wheezing episodes and asthma exacerbations. However, studies on the role of these viruses in asthmatic patients in the interval between exacerbations are sparse. This study aimed to determine the presence of respiratory viruses, without symptoms of infection, in the airways of young asthmatics as compared to healthy controls.
Material and Methods
Patients 10–35 years of age with stable asthma and a group of healthy controls were analyzed regarding the presence of RNA from common respiratory viruses in nasopharyngeal aspirates by PCR. Self-reported asthma control and quality of life, fraction of exhaled nitric oxide (FeNO), spirometry, and bronchial responsiveness to methacholine were recorded. Blood samples were collected to assess IgE sensitisation and eosinophil cationic protein (ECP) levels.
Results
In 354 patients with asthma and 108 healthy controls, human rhinovirus (HRV) was the only virus detected (4.5% of asthmatics vs. 0.9% of controls; p = 0.08). HRV+ asthma patients had a higher degree of aeroallergen IgE sensitisation (median 37.7 vs. 10.4 kUA/L, p = 0.04), and a tendency for higher levels of serum ECP (median 17.2 vs. 12.6 μg/L, p = 0.07), as compared to their HRV− counterparts.
Conclusions
Absence of symptoms of respiratory tract infection notwithstanding, HRV seems to be more prevalent in the airways of adolescents and young adults with asthma and a high degree of aeroallergen IgE sensitisation than in controls. The presence of HRV seems also to be related to systemic eosinophilic inflammation despite ongoing treatment with inhaled corticosteroids.
Keywords: Asthma, Rhinovirus, Immunoglobulin E, Type-2 inflammation, Exhaled nitric oxide, Eosinophil cationic protein
Highlights
-
•
Cross-sectional study on adolescents and young adults with asthma and healthy controls.
-
•
Common respiratory viruses examined in nasopharyngeal aspirates by PCR.
-
•
Only rhinovirus detected in subjects without symptoms of respiratory tract infection.
-
•
Prevalence of rhinovirus tended to be higher in asthmatics compared to controls.
-
•
Presence of rhinovirus associated with high degree of aeroallergen IgE sensitisation.
1. Introduction
Viral respiratory infections have been associated with up to 80% of wheezing episodes and asthma exacerbations in both children and adults [1], [2], [3], and increased rates of hospital admissions for asthma and higher detection rates of respiratory viruses have been reported to coincide [4], [5]. However, regarding the role of respiratory viruses in asthmatic patients during stable periods between exacerbations, very little data is available. It has been speculated that human rhinovirus (HRV) has the ability to cause persistent infections in patients with asthma, but only a few small studies have suggested a preponderance of respiratory viruses in non-exacerbating asthmatic individuals compared to healthy controls [6], [7].
Viral load and clinical deterioration during acute HRV infections have been shown to be related to reduced interferon response and increased type 2-cytokine (interleukin (IL)-4, IL-5 and IL-13) response in patients with asthma compared to non-asthmatic controls [8], [9]. Further, human airway epithelial cells produce the proinflammatory molecule C-X-C motif chemokine 10 (CXCL10) in relation to HRV infection [10], and this response appears to be specific to viral-induced acute asthma [11]. Moreover, Th2 cytokines increase the release of CXCL10 in the presence of rhinovirus infection [12]. Thus, type-2 inflammation in the airways could possibly facilitate viral replication over prolonged periods or, vice versa, the infection could promote chronic airway inflammation.
In the environment of type-2 inflammation in the airways, inducible nitric oxide synthase is upregulated in the respiratory epithelium and the fraction of exhaled nitric oxide (FeNO) is consequently increased [13], [14]. Furthermore, type-2 inflammation is characterised by eosinophil recruitment and viral infections seem to cause systemic activation of eosinophils, at least in subjects with allergic asthma [15], [16].
To further elucidate the possible role of persistent virus infections, we have conducted a large cross-sectional study on young patients with asthma (n = 354) and matched controls (n = 108) (the MIDAS cohort) without symptoms of respiratory tract infection. To this end, the prevalence of HRV, respiratory syncytial virus (RSV), influenza viruses A and B, and human coronavirus (hCoV), all known to be associated with asthma exacerbations, was examined in nasopharyngeal aspirates (NPAs), and asthmatics and controls were compared. A secondary aim was to see if the presence of respiratory viruses was related to biomarkers of type-2 inflammation and/or allergic sensitisation.
2. Materials and methods
2.1. Study population
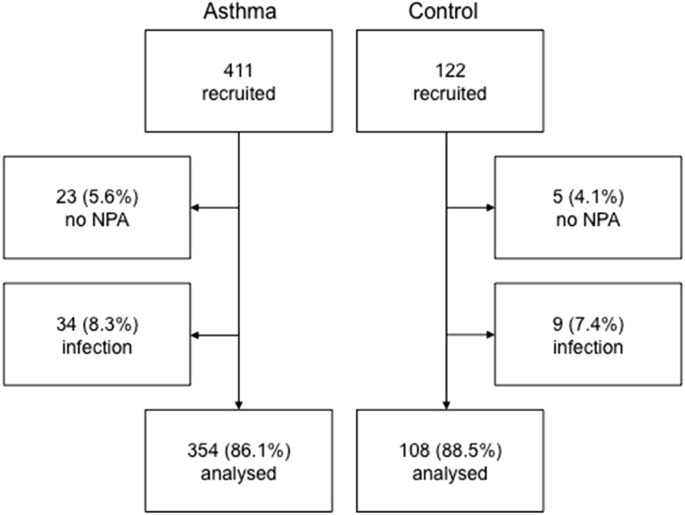
The study was based on the MIDAS cohort, and more details on the methods have been published elsewhere [17], [18], [19]. A total of 411 patients between 10 and 35 years of age with physician-diagnosed asthma as reported in their medical records, as well as daily treatment with inhaled corticosteroids (ICS) and/or oral leukotriene-receptor antagonists (LTRA) during at least three months the year before the examination were included in the study. All subjects were interviewed, examined, and sampled while in a stable state of asthma (i e no exacerbation within the past two weeks) and having refrained from taking any asthma or allergy medication for 24 h before the study. A total of 122 healthy subjects without any diagnosis of respiratory disease, randomly chosen from the Uppsala population register (Sweden), were sex- and age-matched with the asthma patients and participated as controls. All subjects were told that they should have been free from symptoms of respiratory tract infections (cough, sore throat, runny nose, sneezing, nasal congestion, pink eyes or fever) for at least two weeks on the day of examination. The subject inclusion/exclusion flow chart is shown in Fig. 1 .
Fig. 1.
Flow chart of inclusion in the study. Patients who did not volunteer to undergo sampling of nasopharyngeal aspirate (NPA) or had symptoms of respiratory tract infections during the two weeks preceding sampling were excluded from final analysis.
2.2. Asthma control and quality of life
All subjects with asthma filled in the Asthma Control Test (ACT) and the Juniper Mini Asthma-related Quality of Life Questionnaire (mAQLQ) in order to assess the degree of asthma control and asthma-related quality of life, respectively.
2.3. Respiratory measurements
Dynamic spirometry was performed using a MasterScope spirometer (Erich Jager, Wurzburg, Germany) and the methacholine challenge test was performed using the Aerosol Provocation System (Viasys Healthcare GmbH, Hoechberg, Germany). Exhaled NO was measured according to ATS/ERS recommendations [20] using a chemiluminescence analyser (NIOX Flex; Aerocrine AB, Solna, Sweden).
2.4. Collection of nasopharyngeal aspirates, nucleic acid extraction and real-time PCR methods
NPAs were collected as previously described and stored at −80 °C [21]. Total viral nucleic acids were extracted with QIAamp MinElute Virus Spin Kit (QIAGEN, USA) according to the manufacturer's protocol with 200 μL of NPA eluted into 50 μL of extract. Real-time PCR assays for detection of HRV (including species A, B, and C), hCoV (OC43, 229E, HKU1, and NL63), influenza A and B, and RSV were performed on an ABI 7500 Real-Time PCR System (Applied Biosystems) [22], and both plasmids and viral RNA were used as positive controls.
2.5. Blood analyses
Venous blood samples were drawn for blood cell counts, and for preparation of serum and plasma (EDTA) samples that were stored at −80 °C. For serum, blood was allowed to clot for 60 min at 22 °C. Blood leukocyte counts were determined using routine methods (Cell-Dyn Sapphire, Abbott, IL, USA) at the Department of Clinical Chemistry, Uppsala University Hospital. Measurements of IgE antibodies against a mix of aeroallergens (Phadiatop; cat, dog, horse, Dermatophagoides pteronyssinus, Dermatophagoides farinae, Cladosporium herbarum, birch, timothy grass, and mugwort) and food allergens (fx5; egg white, milk, cod fish, wheat, peanut, and soybean) were performed, and subjects were identified as atopic if Phadiatop or fx5 was ≥0.35 kUA/L. IgE antibodies, total IgE and serum eosinophil cationic protein (S-ECP) were measured using ImmunoCAP System (Thermo Fisher Scientific, Uppsala, Sweden). C-X-C motif chemokine 10 (CXCL10, or IP-10) was measured with a sandwich ELISA (R&D Systems, Minneapolis, MN, USA). CXCL10 measurements were performed in all HRV+ asthmatics (n = 16) and in 17 HRV− asthmatics that were matched for sex, age and atopy: females 43.8% vs 47.1% (p = 0.85), age 23.4 ± 6.8 (mean ± SD) vs 24 ± 6.4 years (p = 0.81), and atopy 87.5% vs 88.2% (p = 0.95).
2.6. Statistical analyses
Chi-square test was used to compare proportions. Non-parametric statistics were used in the whole study due to the low number of cases of HRV+ subjects. The Mann-Whitney U test was used to compare medians of continuous variables. A multiple logistic regression model was created to confirm the effect of risk factors for HRV positivity after adjustment for confounding factors. STATA IC 12.1 (StataCorp LP, College Station, Texas, USA) was used for statistical analyses.
2.7. Ethics
The Regional Ethics Committee in Uppsala approved the study (reg no 2009/349). All the subjects and, if appropriate, their legal guardians gave their written informed consent.
3. Results
3.1. Study population
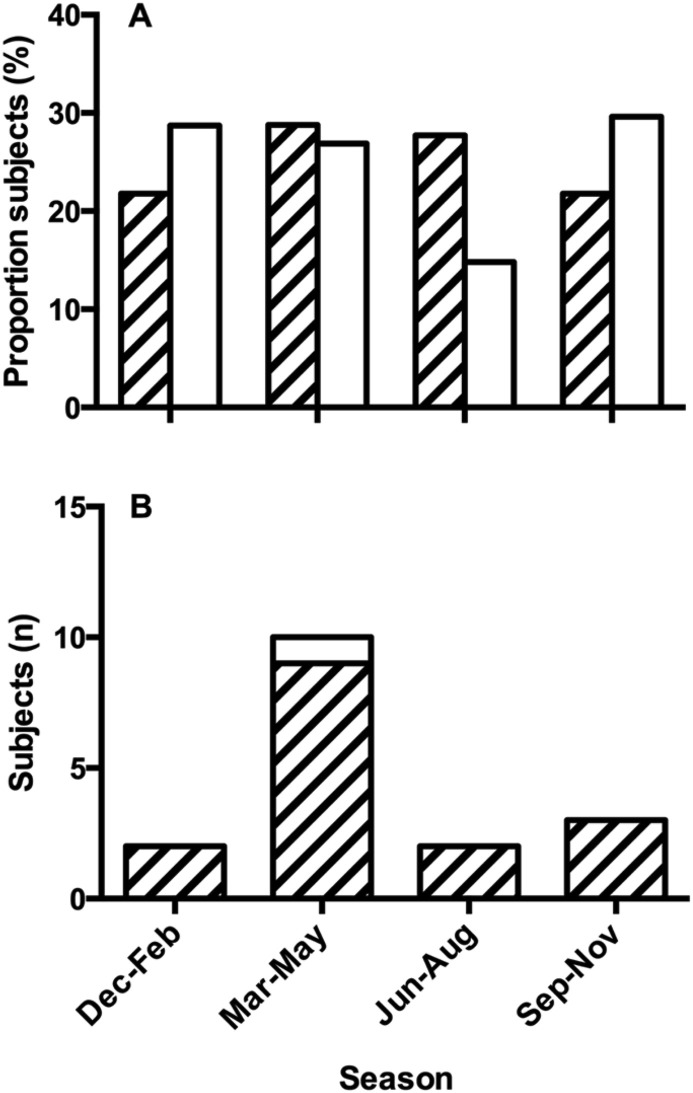
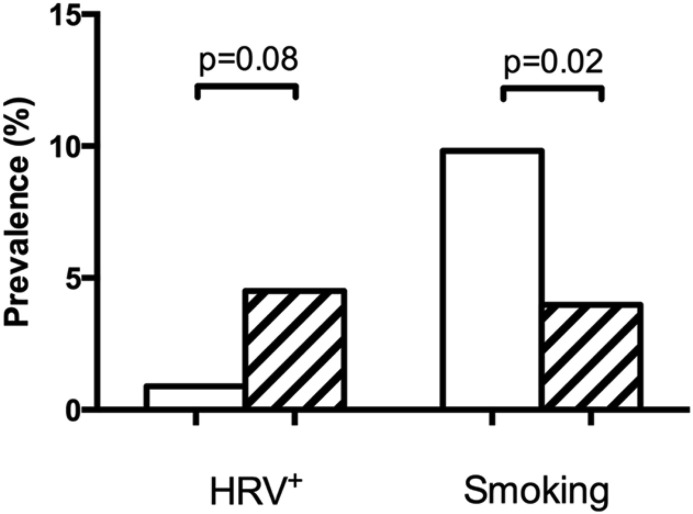
Subject characteristics are presented in Table 1 . Patients with asthma differed from healthy controls with regard to lung function, levels of biomarkers and IgE sensitisation. A slightly larger proportion of asthmatics was recruited during the summer (June–August) compared to controls (Fig. 2 A). The proportion of asthmatics recruited during the common cold seasons (March-May and September-November) was lower than that for controls but this difference was not significant (59% vs 65%, p = 0.31). A smaller proportion of asthmatics reported cigarette smoking compared to controls (Fig. 3 ).
Table 1.
Characteristics of study subjects presented as % or median (IQR).
| Asthma (n = 354) | Controls (n = 108) | p value | |
|---|---|---|---|
| Female | 52.0 | 54.6 | 0.63 |
| Age (years) | 20 (15, 26) | 20 (15, 26) | 0.85 |
| BMI (kg/m2) | 22.0 (19.8, 24.6) | 21.5 (20.1, 24.6) | 0.50 |
| ICS | 82.8 | – | NA |
| LTRA | 18.0 | – | NA |
| FEV1% | 91 (83, 101) | 96 (87, 106) | 0.007 |
| PD20 MCh (mg) | 0.27 (0.07, 1.81) | 1.27 (0.43, 7.2) | <0.001 |
| FeNO (ppb) | 15 (8.8, 27) | 12 (8.3, 17) | 0.003 |
| Eosinophils (109/L) | 0.2 (0.1, 0.3) | 0.1 (0.05, 0.2) | <0.001 |
| Neutrophils (109/L) | 3.1 (2.4, 4) | 3 (2.4, 3.9) | 0.98 |
| Monocytes (109/L) | 0.5 (0.4, 0.5) | 0.4 (0.4, 0.6) | 0.97 |
| S-ECP (μg/L) | 9.0 (6.0, 12.7) | 12.7 (7.9, 21.4) | <0.001 |
| Atopy | 80.1 | 33.3 | <0.001 |
| Total IgE (kU/L) | 152.3 (46.6, 420.4) | 31.8 (12.1, 76.1) | <0.001 |
| Aeroallergen IgE (kU/L) | 11 (0.90, 36) | 0.07 (0.04, 0.95) | <0.001 |
| Food IgE (kU/L) | 0.14 (0.07, 0.74) | 0.06 (0.04, 0.09) | <0.001 |
NOTE. P values less than 0.05 in bold. Abbreviations: ICS, inhaled corticosteroids; NA, not applicable; LTRA, leukotriene-receptor antagonists; PD20, provocative dose causing a 20% fall in FEV1; MCh, methacholine challenge; FeNO, fraction of exhaled nitric oxide; S-ECP, eosinophil cationic protein.
Fig. 2.
(A) Proportion (%) of examined asthmatics (hatched bars) and healthy controls (open bars) according to season. The distribution was different between groups (p = 0.023, Chi-square test). (B) Frequency of HRV positivity in asthmatics (hatched bars) and controls (open bar) according to season.
Fig. 3.
Prevalence of HRV positivity and current cigarette smoking in healthy controls (open bars) and patients with asthma (hatched bars). Statistical analyses by Chi-square test.
3.2. Prevalence of respiratory viruses
HCoV, RSV, and influenza A and B were not found in any subject. However, HRV was found in a total of 17 subjects with uneven distribution between the groups (16 asthmatics and one healthy control) (Fig. 3). This resulted in a prevalence of 4.5% in subjects with asthma and 0.9% in controls (OR 5.1, p = 0.08). Furthermore, the presence of HRV was unevenly distributed by season. Thirteen out of 17 HRV+ subjects (76.5%) were sampled in March-May and September-November (Fig. 2B).
3.3. Basal characteristics in HRV+ vs HRV− patients with asthma
There was no difference with regard to sex and BMI between HRV+ (n = 16) and HRV− (n = 338) asthmatics (Table 2 ). However, HRV+ patients tended to be older (23.4 vs 20.4 years, p = 0.09) and to smoke more (12.5 vs 3.6%, p = 0.07) than those who were HRV−. No difference with regard to lung function, bronchial responsiveness, use of anti-inflammatory asthma medication, asthma control, or asthma-related quality of life could be detected between these two groups of asthmatics (Table 2).
Table 2.
Patient characteristics, current cigarette smoking, lung function, medication use, asthma control (ACQ) and quality of life (mAQLQ) scores in HRV+vs HRV− patients with asthma. Presented as %, mean ± SD or median (IQR)].
| HRV− (n = 338) | HRV+ (n = 16) | p value | |
|---|---|---|---|
| Female | 52.4 | 43.8 | 0.50 |
| Age (years) | 20.4 ± 7.0 | 23.4 ± 6.8 | 0.09 |
| BMI (kg/m2) | 22.6 ± 4.4 | 22.4 ± 2.9 | 0.85 |
| Current smoking | 3.6 | 12.5 | 0.07 |
| FEV1% | 96 (87, 106) | 91 (83, 101) | 0.99 |
| PD20 MCh (mg) | 0.27 (0.07, 1.81) | 0.26 (0.05, 3.01) | 0.95 |
| ICS (μg daily)a | 320 (200, 400) | 400 (320, 640) | 0.20 |
| LTRA | 18.3 | 12.5 | 0.55 |
| ACT | 21 (19, 23) | 20.5 (18.5, 23) | 0.92 |
| mAQLQ | 6.0 (5.3, 6.5) | 6.1 (4.9, 6.5) | 0.82 |
NOTE. P values less than 0.10 in italic. Abbreviations: HRV, human rhinovirus; PD20, provocative dose causing a 20% fall in FEV1; MCh, methacholine challenge; ICS, inhaled corticosteroids; LTRA, leukotriene-receptor antagonists; mAQLQ, Juniper Mini Asthma-related Quality of Life Questionnaire.
Budesonide equivalents.
3.4. Biomarkers, blood leukocyte counts and IgE sensitisation in HRV+ vs HRV− patients with asthma
HRV+ patients with asthma had higher FeNO compared to their HRV− counterparts, but this did not reach significance (17.2 vs 14.4 ppb, p = 0.15; Table 3 ). Further, S-ECP showed a statistical trend towards elevated levels in HRV+ subjects (17.2 vs 12.6 μg/L, p = 0.07), whereas blood eosinophil count did not differ between groups (Table 3, Table 4 ). There was also a trend towards statistical significance for increased neutrophil (4.0 vs 3.1 109/L, p = 0.09) and monocyte (0.5 vs 0.5, p = 0.06) absolute counts in HRV+ asthmatics (Table 4). When analysing the differential cell count (percentage of total leukocyte count), all comparisons were non-significant (p > 0.10). Interestingly, HRV+ asthmatics were characterised by a more than 3-fold greater degree of IgE sensitisation against aeroallergens compared to HRV− patients (37.7 vs 10.4 kU/L, p = 0.04; Table 3). These results were confirmed when only atopic asthmatics (n = 281) were included in the analysis: 49.5 (IQR 13.5–79.5) kU/L in HRV+ (n = 14) vs 16.9 (IQR 5.1–45.5) kU/L in HRV− subjects (n = 267; p = 0.04). The degree of IgE sensitisation against food allergens and total IgE did not differ between groups (Table 3). HRV+ (n = 16) and matched HRV− (n = 17) patients with asthma had similar plasma levels of CXCL10 (μg/L): 140.3 (108.0, 178.7) vs 126.9 (94.5, 179.0), p = 0.90.
Table 3.
Levels of biomarkers and IgE sensitisation in HRV+vs HRV− patients with asthma. Presented as % or median (IQR).
| HRV− (n = 338) | HRV+ (n = 16) | p value | |
|---|---|---|---|
| FeNO (ppb) | 14.4 (8.7–26.7) | 17.2 (12.3–34.5) | 0.15 |
| S-ECP (μg/L) | 12.6 (7.8, 21.1) | 17.2 (12.0, 25.4) | 0.07 |
| Atopy (%)a | 79.7 | 87.5 | 0.45 |
| Total IgE (kU/L) | 151.3 (44.9, 401.1) | 206.8 (58.1, 544.9) | 0.47 |
| Aeroallergen IgE (kUA/L) | 10.4 (0.71, 33.5) | 37.7 (8.3, 69.8) | 0.04 |
| Food IgE (kUA/L) | 0.13 (0.07, 0.70) | 0.18 (0.06, 2.52) | 0.96 |
NOTE. P values less than 0.10 in italic, p values less than 0.05 in bold. Abbreviations: HRV, human rhinovirus; FeNO, fraction of exhaled nitric oxide; S-ECP, eosinophil cationic protein.
Food or aeroallergen IgE ≥ 0.35 kUA/L.
Table 4.
Blood leukocyte counts (109/L) in HRV+vs HRV− asthmatics [median (IQR)].
| HRV− (n = 338) | HRV+ (n = 16) | p value | |
|---|---|---|---|
| Total leukocytes | 6.1 (5.2, 7.1) | 6.9 (5.3, 8.3) | 0.18 |
| Eosinophils | 0.3 (0.1, 0.4) | 0.2 (0.1, 0.3) | 0.70 |
| Neutrophils | 3.1 (2.4, 4.0) | 4.0 (2.6, 4.7) | 0.09 |
| Basophils | 0.05 (0.05, 0.05) | 0.05 (0.05, 0.05) | 0.98 |
| Lymphocytes | 2.1 (1.8, 2.6) | 2.1 (1.7, 2.4) | 0.76 |
| Monocytes | 0.5 (0.4, 0.5) | 0.5 (0.5, 0.6) | 0.06 |
NOTE. Abbreviations: HRV, human rhinovirus. P values less than 0.10 in italic.
In a multiple logistic regression model including all subjects with asthma, the association between the degree of aeroallergen IgE sensitisation and HRV positivity was consistent (p = 0.03) after adjustment for age, sex, height, weight, FEV1% and current smoking. Higher age (p = 0.01) was also a significant determinant in this model. A similar analysis in subjects with atopic asthma yielded similar results with the degree of IgE sensitisation against aeroallergens (p = 0.04), higher age (p = 0.01) and male sex (p = 0.03) being significant.
4. Discussion
In this cross-sectional study we detected a higher frequency of HRV in the upper respiratory tract of young subjects with asthma without ongoing or recent symptoms of respiratory tract infections, as compared to healthy controls. In contrast, RSV, influenza A and B, and HCoV RNA were not detected in any of the subjects. HRV+ asthmatic subjects were characterised by a higher degree of aeroallergen IgE sensitisation and higher levels of S-ECP than HRV− subjects, whereas blood eosinophils, FeNO, CXCL10, asthma control and asthma-related quality of life did not differ.
This study assessed the prevalence of common respiratory tract viruses in a large number of asymptomatic asthmatic patients that were collected year-round and compared with age- and sex-matched healthy controls. Although the prevalence of HRV was higher in the asthmatic patients in our study the difference only showed a trend towards statistical significance. However, a slightly larger proportion of the asthmatic subjects were examined outside the common cold season, which may have reduced the chance of finding HRV in asthmatics relative to controls. Furthermore, the difference in HRV prevalence between the groups was seen in spite of the fact that a significantly larger proportion of control subjects than asthmatics were smokers, a factor that may increase susceptibility to HRV infections [23], [24]. Accordingly, there was a trend towards higher prevalence of smokers in HRV+ asthmatics versus HRV− asthmatics in our study. Cigarette smoke has been shown to down-regulate bronchial epithelial release of antiviral proteins, for example CXCL10, in response to rhinovirus infection [25], [26], via the inhibition of multiple transcriptional pathways linked to innate immune responses [27]. Wos and colleagues have previously demonstrated that HRV can be detected in the lower respiratory tract of asthmatic patients more frequently than in healthy controls, in the absence of recent asthma exacerbations or signs of common cold [7]. However, the asthmatic subjects in their study had undergone bronchoscopy on clinical indications, and were thus not representative of a general asthma population. A small study in schoolchildren during symptom-free periods indicated greater prevalence of HRV in asthmatics compared to control children, but a statistical comparison between groups was not presented and time of the year for sampling was not described [6]. In contrast, a recent relatively large study showed similar prevalence of HRV in children with stable asthma and healthy controls [28].
In the Nordic countries, symptomatic HRV infections occur year-round with peaks in the spring and fall [29]. In line with this, 77% of our asymptomatic HRV infections were detected in March-May and September-November. This indicates that we may have detected sub-acute infections rather than shedding from persistent infections. Our cross-sectional study design prevent analysis of the presence of chronic HRV infection and shedding among asthmatics, but one could speculate that if true, the prevalence of detectable HRV should have been higher as well as more evenly distributed over seasons. Furthermore, Jartti et al. recently showed that infants with prolonged or recurrent respiratory illnesses most often had a series of infections rather than a persistent infection with one virus strain [30]. However, given that HRV has been detected in consecutive samples in longitudinal studies [31], [32], [33], the short-term persistence (slower clearance) of rhinovirus is also possible.
In the study by Soto-Quiros and co-workers, 13% of schoolchildren with stable asthma were HRV+ [28]. Inclusion was primarily done during the common cold season, whereas we recruited year-round, which could explain the higher HRV prevalence compared to our study. The frequency of HRV infections is rather high in pre-school children, but decreases with age [34]. Parents of young children, however, seem to catch their colds primarily at home, and a hiatus in the decrease of HRV frequency has been observed in the age interval 20–29 years [35], coinciding with the time when people commonly establish a family. This could possibly explain why we found the median age of the HRV− asthmatic patients to be 19 years, whereas for the HRV+ patients it was 26 years. The lack of detectable viral RNA from RSV, influenza A and B, and hCoV is consistent with two other studies on asymptomatic asthmatic individuals [6], [28], and our data further support the unique relationship between asthma and susceptibility to HRV infections.
The HRV+ asthma patients showed a higher degree of IgE sensitisation against aeroallergens compared to HRV− asthmatics, whereas HRV positivity was not related to food allergen sensitisation. This would be in line with the previously shown relationship between the susceptibility to HRV infections and local increases in type 2 cytokines [8] but not to atopy per se [36]. Exhaled NO signals local type-2 cytokine activity in the airways, and mean FeNO was increased in HRV+ asthmatics but this did not reach significance. However, it must be noted that most of the asthmatics in our study used inhaled corticosteroids, and FeNO is most often rapidly reduced by this treatment [37], whereas restoration of viral host defence may be a slower process. Exhaled NO is also increased by HRV infections, but the increase is seen only for a few days after acute infection and should thus not have influenced our results [38], [39].
Our study also showed that serum levels of ECP tended to be elevated in HRV+ asthmatics, whereas B-Eos count was not. Instead, a trend towards increased numbers of blood neutrophils and monocytes was seen in HRV+ subjects. Previous studies have shown increased neutrophil counts in induced sputum by virus infections in both asthmatics and controls [40]. Jarjour et al. have shown an increase in B-Eos differential count acutely after HRV inoculation in patients with atopic asthma [41]. Further, Wos et al. have presented increased B-Eos count in atopic asthmatics with asymptomatic HRV positivity in the lower airways [7]. One study showed increased S-ECP acutely after HRV exposure in patients with atopic asthma and high IgE concentrations [42]. Elevated S-ECP, but not B-Eos count, have been associated with acute virus-induced exacerbations in children [43], [44]. This indicates that S-ECP may be a more sensitive marker of HRV-induced systemic eosinophilic activation than B-Eos count. Plasma levels of CXCL10 have previously been shown to be increased in acute asthma, presumably related to HRV infections, whereas CXCL10 was not elevated in stable asthma [43]. We could not detect any increase in plasma CXCL10 levels in the asymptomatic HRV+ asthmatics in our study, indicating a low rate of virus replication in these subjects.
A limitation of the study may be that we did not perform any follow-up examinations. Thus, our data do not allow us to determine whether the increased prevalence of subclinical HRV infections in asthmatics relates to increased frequency of acute infections or a slower elimination of virus after an acute infection. Further, self-reporting of symptoms increases the risk of bias and we may have sampled individuals during the incubation phase of an acute HRV infection. However, the incubation time is very short for HRV, shorter than for other respiratory viruses, suggesting that this risk should be low [45]. Another potential limitation is that we did not diagnose which HRV species the patients were infected with, but regarding HRV positivity and its effect on levels of FeNO, a recent report showed no differences between HRV species [28]. Also, the cross-sectional study did not allow for prospectively assessing upcoming asthma exacerbations.
In conclusion, HRV, but not RSV, influenza A/B or hCoV, was detected in the airways of asthmatics that had been free from symptoms of infection for at least two weeks. The prevalence of HRV tended to be increased in adolescents and young adults with asthma compared to age-matched controls. The presence of HRV in asthmatics was significantly associated with the degree of IgE sensitisation against aeroallergens and weakly to S-ECP levels. Our data suggest that HRV infections are more common, and/or cleared at a slower rate, in asthmatics with signs of high degree of type-2-driven disease compared to healthy controls, and the difference seems to be related to presence of systemic eosinophilic inflammation despite inhaled corticosteroid treatment.
Funding
The MIDAS study was financially supported within the framework of an academy-industry collaboration initiated by the Swedish Governmental Agency for Innovation Systems (VINNOVA, SAMBIO program, grant number 2007-00084), where Aerocrine AB and Thermo Fisher Scientific, Immunodiagnostics were partners and co-financed the program. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Funding was also received from the Swedish Heart-Lung Foundation and the Swedish Asthma and Allergy Association's Research Foundation.
Conflict of interest
KA has received research support from Thermo Fisher Scientific and Aerocrine. The other authors have declared that no competing interests exist.
Author contributions
Conceived and designed the experiments: LÖ AM MW PL CJ KB KA. Performed the experiments: LÖ MW PL KA. Analyzed the data: LÖ AM MW PL CJ KB KA. Contributed reagents/materials/analysis tools: LÖ MA KB KA. Wrote the paper: LÖ AM MW PL CJ KB KA.
Acknowledgement
We are grateful to research nurses Pia Kalm-Stephens and Katarina Nisser for skilfully performing all examinations, and laboratory engineer Britt-Inger Nyberg for accurate blood analyses.
References
- 1.Heymann P.W., Carper H.T., Murphy D.D., Platts-Mills T.A., Patrie J. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J. Allergy Clin. Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grissell T.V., Powell H., Shafren D.R., Boyle M.J., Hensley M.J. Interleukin-10 gene expression in acute virus-induced asthma. Am. J. Respir. Crit. Care Med. 2005;172:433–439. doi: 10.1164/rccm.200412-1621OC. [DOI] [PubMed] [Google Scholar]
- 3.Wark P.A., Johnston S.L., Moric I., Simpson J.L., Hensley M.J. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur. Respir. J. 2002;19:68–75. doi: 10.1183/09031936.02.00226302. [DOI] [PubMed] [Google Scholar]
- 4.Dales R.E., Schweitzer I., Toogood J.H., Drouin M., Yang W. Respiratory infections and the autumn increase in asthma morbidity. Eur. Respir. J. 1996;9:72–77. doi: 10.1183/09031936.96.09010072. [DOI] [PubMed] [Google Scholar]
- 5.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Campbell M.J. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am. J. Respir. Crit. Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 6.Marin J., Jeler-Kacar D., Levstek V., Macek V. Persistence of viruses in upper respiratory tract of children with asthma. J. Infect. 2000;41:69–72. doi: 10.1053/jinf.2000.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wos M., Sanak M., Soja J., Olechnowicz H., Busse W.W. The presence of rhinovirus in lower airways of patients with bronchial asthma. Am. J. Respir. Crit. Care Med. 2008;177:1082–1089. doi: 10.1164/rccm.200607-973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Message S.D., Laza-Stanca V., Mallia P., Parker H.L., Zhu J. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc. Natl. Acad. Sci. U S A. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards M.R., Regamey N., Vareille M., Kieninger E., Gupta A. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013;6:797–806. doi: 10.1038/mi.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spurrell J.C., Wiehler S., Zaheer R.S., Sanders S.P., Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L85–L95. doi: 10.1152/ajplung.00397.2004. [DOI] [PubMed] [Google Scholar]
- 11.Wark P.A., Bucchieri F., Johnston S.L., Gibson P.G., Hamilton L. IFN-gamma-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J. Allergy Clin. Immunol. 2007;120:586–593. doi: 10.1016/j.jaci.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cakebread J.A., Haitchi H.M., Xu Y., Holgate S.T., Roberts G. Rhinovirus-16 induced release of IP-10 and IL-8 is augmented by Th2 cytokines in a pediatric bronchial epithelial cell model. PLoS One. 2014;9:e94010. doi: 10.1371/journal.pone.0094010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roos A.B., Mori M., Gronneberg R., Osterlund C., Claesson H.E. Elevated exhaled nitric oxide in allergen-provoked asthma is associated with airway epithelial iNOS. PLoS One. 2014;9:e90018. doi: 10.1371/journal.pone.0090018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhakta N.R., Solberg O.D., Nguyen C.P., Nguyen C.N., Arron J.R. A qPCR-based metric of Th2 airway inflammation in asthma. Clin. Transl. Allergy. 2013;3:24. doi: 10.1186/2045-7022-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malinovschi Alving. Basic aspects of exhaled nitric oxide. Eur. Respir. Monogr. 2010;49:1–31. [Google Scholar]
- 16.Jackson D.J., Makrinioti H., Rana B.M., Shamji B.W., Trujillo-Torralbo M.B. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am. J. Respir. Crit. Care Med. 2014;190:1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patelis A., Janson C., Borres M.P., Nordvall L., Alving K. Aeroallergen and food IgE sensitization and local and systemic inflammation in asthma. Allergy. 2014;69:380–387. doi: 10.1111/all.12345. [DOI] [PubMed] [Google Scholar]
- 18.Heijkenskjold-Rentzhog C., Nordvall L., Janson C., Borres M.P., Alving K. Alveolar and exhaled NO in relation to asthma characteristics–effects of correction for axial diffusion. Allergy. 2014;69:1102–1111. doi: 10.1111/all.12430. [DOI] [PubMed] [Google Scholar]
- 19.Krantz C., Janson C., Borres M.P., Nordvall L., Alving K. Nasal nitric oxide is associated with exhaled NO, bronchial responsiveness and poor asthma control. J. Breath. Res. 2014;8:026002. doi: 10.1088/1752-7155/8/2/026002. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic S, European Respiratory S ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 21.Ohrmalm L., Wong M., Rotzen-Ostlund M., Norbeck O., Broliden K. Flocked nasal swab versus nasopharyngeal aspirate for detection of respiratory tract viruses in immunocompromised adults: a matched comparative study. BMC Infect. Dis. 2010;10:340. doi: 10.1186/1471-2334-10-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohrmalm L., Wong M., Aust C., Ljungman P., Norbeck O. Viral findings in adult hematological patients with neutropenia. PLoS One. 2012;7:e36543. doi: 10.1371/journal.pone.0036543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen S., Tyrrell D.A., Russell M.A., Jarvis M.J., Smith A.P. Smoking, alcohol consumption, and susceptibility to the common cold. Am. J. Public Health. 1993;83:1277–1283. doi: 10.2105/ajph.83.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller E.K., Linder J., Kraft D., Johnson M., Lu P. Hospitalizations and outpatient visits for rhinovirus-associated acute respiratory illness in adults. J. Allergy Clin. Immunol. 2016;137:734–743. doi: 10.1016/j.jaci.2015.06.017. e731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eddleston J., Lee R.U., Doerner A.M., Herschbach J., Zuraw B.L. Cigarette smoke decreases innate responses of epithelial cells to rhinovirus infection. Am. J. Respir. Cell Mol. Biol. 2011;44:118–126. doi: 10.1165/rcmb.2009-0266OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proud D., Hudy M.H., Wiehler S., Zaheer R.S., Amin M.A. Cigarette smoke modulates expression of human rhinovirus-induced airway epithelial host defense genes. PLoS One. 2012;7:e40762. doi: 10.1371/journal.pone.0040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudy M.H., Traves S.L., Proud D. Transcriptional and epigenetic modulation of human rhinovirus-induced CXCL10 production by cigarette smoke. Am. J. Respir. Cell Mol. Biol. 2014;50:571–582. doi: 10.1165/rcmb.2013-0129OC. [DOI] [PubMed] [Google Scholar]
- 28.Soto-Quiros M., Avila L., Platts-Mills T.A., Hunt J.F., Erdman D.D. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J. Allergy Clin. Immunol. 2012;129:1499–1505. doi: 10.1016/j.jaci.2012.03.040. e1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kares S., Lonnrot M., Vuorinen P., Oikarinen S., Taurianen S. Real-time PCR for rapid diagnosis of entero- and rhinovirus infections using LightCycler. J. Clin. Virol. 2004;29:99–104. doi: 10.1016/s1386-6532(03)00093-3. [DOI] [PubMed] [Google Scholar]
- 30.Jartti T., Lee W.M., Pappas T., Evans M., Lemanske R.F., Jr. Serial viral infections in infants with recurrent respiratory illnesses. Eur. Respir. J. 2008;32:314–320. doi: 10.1183/09031936.00161907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winther B., Hayden F.G., Hendley J.O. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: association with symptomatic illness and effect of season. J. Med. Virol. 2006;78:644–650. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 32.Kling S., Donninger H., Williams Z., Vermeulen J., Weinberg E. Persistence of rhinovirus RNA after asthma exacerbation in children. Clin. Exp. Allergy. 2005;35:672–678. doi: 10.1111/j.1365-2222.2005.02244.x. [DOI] [PubMed] [Google Scholar]
- 33.Jartti T., Lehtinen P., Vuorinen T., Koskenvuo M., Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J. Med. Virol. 2004;72:695–699. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 34.Hendley J.O., Gwaltney J.M., Jr., Jordan W.S., Jr. Rhinovirus infections in an industrial population. IV. Infections within families of employees during two fall peaks of respiratory illness. Am. J. Epidemiol. 1969;89:184–196. doi: 10.1093/oxfordjournals.aje.a120928. [DOI] [PubMed] [Google Scholar]
- 35.Monto A.S., Bryan E.R., Ohmit S. Rhinovirus infections in Tecumseh, Michigan: frequency of illness and number of serotypes. J. Infect. Dis. 1987;156:43–49. doi: 10.1093/infdis/156.1.43. [DOI] [PubMed] [Google Scholar]
- 36.Sykes A., Macintyre J., Edwards M.R., Del Rosario A., Haas J. Rhinovirus-induced interferon production is not deficient in well controlled asthma. Thorax. 2014;69:240–246. doi: 10.1136/thoraxjnl-2012-202909. [DOI] [PubMed] [Google Scholar]
- 37.Anderson W.J., Short P.M., Williamson P.A., Lipworth B.J. Inhaled corticosteroid dose response using domiciliary exhaled nitric oxide in persistent asthma: the FENOtype trial. Chest. 2012;142:1553–1561. doi: 10.1378/chest.12-1310. [DOI] [PubMed] [Google Scholar]
- 38.de Gouw H.W., Grunberg K., Schot R., Kroes A.C., Dick E.C. Relationship between exhaled nitric oxide and airway hyperresponsiveness following experimental rhinovirus infection in asthmatic subjects. Eur. Respir. J. 1998;11:126–132. doi: 10.1183/09031936.98.11010126. [DOI] [PubMed] [Google Scholar]
- 39.Sanders S.P., Proud D., Permutt S., Siekierski E.S., Yachechko R. Role of nasal nitric oxide in the resolution of experimental rhinovirus infection. J. Allergy Clin. Immunol. 2004;113:697–702. doi: 10.1016/j.jaci.2004.01.755. [DOI] [PubMed] [Google Scholar]
- 40.Pizzichini M.M., Pizzichini E., Efthimiadis A., Chauhan A.J., Johnston S.L. Asthma and natural colds. Inflammatory indices in induced sputum: a feasibility study. Am. J. Respir. Crit. Care Med. 1998;158:1178–1184. doi: 10.1164/ajrccm.158.4.9712082. [DOI] [PubMed] [Google Scholar]
- 41.Jarjour N.N., Gern J.E., Kelly E.A., Swenson C.A., Dick C.R. The effect of an experimental rhinovirus 16 infection on bronchial lavage neutrophils. J. Allergy Clin. Immunol. 2000;105:1169–1177. doi: 10.1067/mai.2000.106376. [DOI] [PubMed] [Google Scholar]
- 42.Zambrano J.C., Carper H.T., Rakes G.P., Patrie J., Murphy D.D. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J. Allergy Clin. Immunol. 2003;111:1008–1016. doi: 10.1067/mai.2003.1396. [DOI] [PubMed] [Google Scholar]
- 43.Kato M., Yamada Y., Maruyama K., Hayashi Y. Serum eosinophil cationic protein and 27 cytokines/chemokines in acute exacerbation of childhood asthma. Int. Arch. Allergy Immunol. 2010;152(1):62–66. doi: 10.1159/000312127. [DOI] [PubMed] [Google Scholar]
- 44.Tang R.B., Chen S.J. Serum levels of eosinophil cationic protein and eosinophils in asthmatic children during a course of prednisolone therapy. Pediatr. Pulmonol. 2001;31:121–125. doi: 10.1002/1099-0496(200102)31:2<121::aid-ppul1019>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 45.Saraya T., Kurai D., Ishii H., Ito A., Sasaki Y. Epidemiology of virus-induced asthma exacerbations: with special reference to the role of human rhinovirus. Front. Microbiol. 2014;5:226. doi: 10.3389/fmicb.2014.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]