Abstract
The main objective of this study is to standardize an ELISA for the diagnosis of feline sporotrichosis. Sporothrix schenckii is the etiological agent of human and animal sporotrichosis. Cats may act as reservoirs for S. schenckii and can transmit the infection to humans by a bite or scratch. There are few methods for the serological diagnosis of fungal diseases in animals. In this paper, an ELISA test for the diagnosis of cat sporotrichosis is proposed, which detects S. schenckii-specific antibodies in feline sera. Two different kinds of antigens were used: “SsCBF”, a specific molecule from S. schenckii that consists of a Con A-binding fraction derived from a peptido-rhamnomannan component of the cell wall, and a S. schenckii crude exoantigen preparation. The ELISA was developed, optimized, and evaluated using sera from 30 cats with proven sporotrichosis (by culture isolation); 22 sera from healthy feral cats from a zoonosis center were used as negative controls. SsCBF showed 90% sensitivity and 96% specificity in ELISA; while crude exoantigens demonstrated 96% sensitivity and 98% specificity. The ELISA assay described here would be a valuable screening tool for the detection of specific S. schenckii antibodies in cats with sporotrichosis. The assay is inexpensive, quick to perform, easy to interpret, and permits the diagnosis of feline sporotrichosis.
Keywords: Sporothrix schenckii, Sporotrichosis, ELISA, SsCBF antigen, Crude exoantigens, Feline, Cat
1. Introduction
Sporothrix schenckii is composed of a complex mixture of antigens (Penha and Lopes-Bezerra, 2000), including cell wall peptido-rhamnomannan (CWPR) that can be fractionated by affinity chromatography on Sepharose 4B-Concanavalin A (Con A). This gives rise to two fractions: a Con A-binding fraction (SsCBF) and a Con A-nonbinding fraction (SsNBF). The SsCBF fraction was tested using ELISA for the diagnosis of human sporotrichosis and reacted specifically with 100% of the sera from patients with cutaneous sporotrichosis (Penha and Lopes-Bezerra, 2000). More recently, the same group tested the SsCBF fraction, by ELISA, against different clinical presentations of sporotrichosis (lymphocutaneous, fixed, disseminated, and extracutaneous forms) and found high levels of specificity and sensitivity (Bernardes-Engemann et al., 2005, Bernardes-Engemann et al., 2009). This specificity was correlated with the presence in the SsCBF antigen of epitopes comprised of O-linked mannose-containing oligosaccharides with non-reducing ends of α-L-Rha 1 > 4 α-D-GlcA and α-L-Rha 1 > 4 [α-L-Rha 1 > 2]α-D-GlcA (Lopes-Alves et al., 1994).
As in humans, cats may acquire systemic mycotic diseases, including blastomycosis (Blondin et al., 2007), histoplasmosis (Johnson et al., 2004), cryptococcosis (Malik et al., 1992), candidiasis (Pressler et al., 2003), aspergillosis (Hamilton et al., 2000), coccidioidomycosis (Graupmann-Kuzma et al., 2008), sporotrichosis (Barros et al., 2003, Barros et al., 2004), and dermatophytosis, a superficial mycosis (Pinheiro et al., 1997). In most cases, the diagnosis is based on traditional methods; serological diagnosis is rarely used in pets (Soltys and Sumner-Smith, 1971).
The incidence of sporotrichosis among animals, and particularly cats, is increasing in Brazil, especially in Rio de Janeiro where over the course of 6 years (1998–2004), 1503 cats, 64 dogs, and 759 humans were diagnosed with sporotrichosis, which is considered the largest epidemic of sporotrichosis due to zoonotic transmission (Barros et al., 2004, Schubach et al., 2004). The present study aimed to standardize an ELISA test for the serological diagnosis of cat sporotrichosis, using the specific S. schenckii antigen, SsCBF, proposed by Penha and Lopes-Bezerra (2000), as well as our own preparation of crude S. schenckii exoantigens.
2. Materials and methods
2.1. Cat sera
In 2008, 30 cats with sporotrichosis underwent clinical examinations at the Laboratory of Clinical Research of Dermatophytosis of Domestic Animals of the Institute Evandro Chagas – Fiocruz, Rio de Janeiro, Brazil. To confirm the infection by S. schenckii, samples of lesions were collected, after local anesthesia, by a swab of cutaneous or mucosal lesions and/or biopsy (Schubach et al., 2004). The biological samples were inoculated into Sabouraud-dextrose agar (DIFCO) plus chloranfenicol and Mycobiotic agar (DIFCO) and incubated at 25 °C to isolate the S. schenckii in culture. The dimorphism was observed by conversion at 37 °C in Brain Heart Infusion agar (DIFCO). During consultation, blood was collected by vein puncture, and then stored in an incubator for 1 h; serum was obtained by centrifugation and stored at −20 °C, until needed. These 30 sera from cats with proven sporotrichosis are now referred to as “positive sera” (sera from cats with active sporotrichosis). The control group (negative sera) was composed of 22 sera from healthy feral cats from the Zoonosis Municipal Center of São Paulo city. These animals were examined by a veterinarian and were considered apparently healthy with no evidence of sporotrichosis or other diseases. They were housed in accordance with the Guide for Care and Use of Laboratory Animals, National Research Council, 1996. Also, 20 sera from cats with other pathologies were studied to verify cross-reactions: Feline Infectious Peritonitis – coronavirus (5 sera), Feline Leukemia Virus – Felv (3 sera), Feline Immunodeficiency Virus – Fiv (2 sera), Leptospirosis (3 sera), Ricketsiosis (2 sera), Elichiosis (3 sera), and Leishmaniasis (2 sera).
2.2. S. schenckii antigens
The S. schenckii SsCBF-specific molecule was prepared according to a previously described method (Penha and Lopes-Bezerra, 2000), using the 1099-18 isolate. The S. schenckii crude preparation was prepared similarly to that described by Camargo et al. (2003) for Paracoccidioides brasiliensis, but using S. schenckii strain #118, which was isolated from a human case of lymphocutaneous sporotrichosis. Table 1 shows the main characteristics of the cats with sporotrichosis, including identification, age, sex, time of infection, clinical form, castrated or not, outdoor or indoor, and the results of ELISA: O.D. 492 nm sera versus two different S. schenckii antigens (SsCBF and crude antigen).
Table 1.
Main characteristics of the cats with sporotrichosis.
| Identification N. Cat. | Sex | Time of infection (weeks) | Clinical form | Age (months) | Castrated | Outdoor | O.D. 492 nm |
|
|---|---|---|---|---|---|---|---|---|
| SsCBF | Exoantigen | |||||||
| 1 | M | 4 | Lymphocutaneous (L3) | 30 | No | Yes | 0.578 | 1.892 |
| 2 | F | 16 | Lymphocutaneous (L3) | 42 | Yes | Yes | 0.658 | 2.499 |
| 3 | M | 5 | Lymphocutaneous (L3) | 36 | No | No | 1.816 | 2.289 |
| 4 | M | 12 | Lymphocutaneous (L3) | 36 | No | No | 1.691 | 2.181 |
| 5 | M | 5 | Lymphocutaneous (L3) | 42 | No | Yes | 0.888 | 2.193 |
| 6 | M | 8 | Lymphocutaneous (L3) | 20 | No | Yes | 1.417 | 1.003 |
| 7 | M | 8 | Lymphocutaneous (L3) | 24 | No | Yes | 2.316 | 2.314 |
| 8 | M | 6 | Fixed (L1) | 24 | No | Yes | 1.204 | 1.710 |
| 9 | M | 4 | Lymphocutaneous (L3) | 24 | No | Yes | 2.290 | 1.305 |
| 10 | M | 12 | Lymphocutaneous (L3) | 18 | Yes | No | 0.699 | 1.733 |
| 11 | M | 2 | Lymphocutaneous (L2) | 12 | No | Yes | 2.258 | 2.156 |
| 12 | M | 8 | Lymphocutaneous (L2) | 60 | No | Yes | 0.314 | 0.568 |
| 13 | M | 4 | Lymphocutaneous (L3) | 12 | No | No | 1.962 | 1.584 |
| 14 | M | 4 | Lymphocutaneous (L3) | 12 | No | Yes | 1.232 | 2.199 |
| 15 | M | 6 | Lymphocutaneous (L3) | 18 | No | Yes | 1.587 | 2.254 |
| 16 | M | 16 | Lymphocutaneous (L3) | 24 | No | Yes | 1.738 | 2.306 |
| 17 | M | 4 | Lymphocutaneous (L3) | – | Yes | Yes | 2.341 | 1.350 |
| 18 | M | 4 | Lymphocutaneous (L3) | 17 | Yes | Yes | 1.124 | 1.978 |
| 19 | M | 4 | Lymphocutaneous (L3) | 24 | No | Yes | 2.239 | 2.016 |
| 20 | M | 12 | Lymphocutaneous (L3) | 14 | No | Yes | 0.383 | 1.890 |
| 21 | M | 8 | Lymphocutaneous (L3) | – | No | Yes | 1.487 | 2.228 |
| 22 | M | 5 | Lymphocutaneous (L3) | 14 | No | Yes | 1.370 | 1.776 |
| 23 | F | 3 | Lymphocutaneous (L3) | – | No | No | 0.385 | 1.298 |
| 24 | M | 5 | Lymphocutaneous (L2) | 36 | No | Yes | 0.831 | 1.675 |
| 25 | M | 8 | Lymphocutaneous (L3) | 24 | Yes | Yes | 2.688 | 2.450 |
| 26 | M | 9 | Lymphocutaneous (L3) | 48 | No | Yes | 2.048 | 1.804 |
| 27 | M | 6 | Lymphocutaneous (L3) | 12 | No | Yes | 1.444 | 1.909 |
| 28 | M | 4 | Lymphocutaneous (L3) | 12 | No | Yes | 1.377 | 2.246 |
| 29 | M | 4 | Lymphocutaneous (L3) | 36 | No | Yes | 2.356 | 2.132 |
| 30 | M | 24 | Lymphocutaneous (L2) | 30 | No | Yes | 1.813 | 1.749 |
| Mean | 1.484 | 1.890 | ||||||
| S.D. | 0.678 | 0.445 | ||||||
| Median | 1.466 | 1.944 | ||||||
L1 = one lesion; L2 = two lesions; L3 = three or more lesions.
2.3. ELISA method
High binding microtiter plates (Corning Costar, Corning, New York, USA) were sensitized with SsCBF antigen (3.6 μg/ml; 100 μl per well in 0.1 M carbonate-bicarbonate buffer (CBB), pH 9.6, concentration previously determined by the check board titration), for 2 h at 37 °C and overnight at 4 °C in a refrigerator. Then, the plates were blocked with 5% skim milk in phosphate-buffered saline (PBS) for 2 h at 37 °C in an incubator. Plates were washed three times with PBS + 0.05% Tween 20 (PBS-T). Then, 100 μl of cat sera, diluted 1:800 (dilution previously determined in pilot tests; data not shown) in PBS-T-0.5% gelatin (PBS-T-G), were added to each well (in triplicate), and stored at 37 °C for 2 h in an incubator. The plates were washed again three times with PBS-T, and 100 μl of goat anti-feline IgG conjugated to horseradish peroxidase (US Biological) (1:2000 dilution) was added to each well for 2 h at 37 °C in an incubator. After washing three times with PBS-T, substrate (H2O2/Ortho-Phenylenediamine – OPD) was added under dark conditions for 8 min; then, the reaction was stopped by adding 50 μl of 4N H2SO4. Absorbance was read at 492 nm with an ELISA reader (TECAN model Sunrise; TECAN Austria GmbH). When crude exoantigen preparations were used for ELISA, the plates were coated with 40.0 μg/ml (100 μl/well) of exoantigens in CBB (concentration previously determined by check board titration), and steps were performed as described above.
2.4. Validation tests
In our laboratory the ELISA tests were performed on three different occasions by three different people. Also, the tests were performed in the same way in another laboratory.
2.5. Immunodiffusion test
The test was performed as previously described by Camargo et al. (2003) for paracoccidioidomycosis, but using S. schenckii crude exoantigens as a reagent. Serum was collected from a rabbit previously immunized with S. schenckii antigen was used as a positive control.
2.6. Statistical analysis
To determine the discriminatory power and accuracy of the ELISA test, ROC (Receiver Operating Characteristic) curves were prepared using the Stata program version 8.2 with data from the ELISA. The cutoff was obtained as: mean + 2× standard deviations (S.D.) from sera from healthy cats. All sera (from healthy cats and cats with sporotrichosis) were classified as TP = true positive (sera from cats with sporotrichosis that presented O.D. values above the cutoff in the serological test); FP = false positive (sera from healthy control cats that presented O.D. values above the cutoff in the serological test); TN = true negative (sera from healthy control cats that presented O.D. values below the cutoff in the serological test); and FN = false negative (sera from cats with sporotrichosis that presented O.D. values below the cutoff in the serological test). The parameters: sensitivity, specificity, positive and negative predictive values, and global efficiency were obtained with the following formulas: sensitivity: TP/(TP + FN); specificity: TN/(TN + FP); positive predictive value: TP/(TP + FP); negative predictive value: TN/(TN + FN); global efficiency: TP + TN/TP + TN + FP + FN.
3. Results
3.1. Sensitivity and specificity of ELISA using the SsCBF purified antigen
The sera from healthy cats (control sera) showed absorbances at 492 nm from 0.000 to 0.644. The mean absorbance plus two standard deviations for all control sera was A 492 = 0.485, which represented the cutoff. Of the confirmed infected cats, 27/30 (90%) (Fig. 1 ) were positive, with an absorbance greater than 0.485. The sera of cats with sporotrichosis had a mean O.D. of 1.484 ± 0.678, and a median of 1.466. The mean O.D. of the control cat sera was 0.157 ± 0.164 and the median was 0.117. Thus, for the ELISA assay: sensitivity = 90%, specificity = 96%, positive predictive value = 93%; negative predictive value = 94%, and global efficiency = 93%. The area under the curve (AUC) from the ROC curve (Fig. 2 ) was 0.9936 ± 0.0049 (confidence interval 95% (IC) 0.98400–1.00000). Sera from cats with other pathologies had O.D. values = 0.137 ± 0.091; median = 0.125.
Fig. 1.
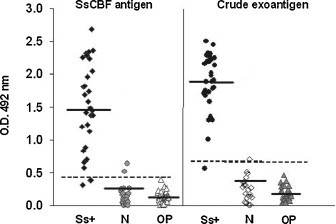
Results of the ELISA. O.D. obtained from the sera of cats with confirmed sporotrichosis (Ss+), healthy feral cats (N), and cats with other pathologies (OP) used as controls to detect IgG antibodies against the Sporothrix schenckii SsCBF purified antigen and crude exoantigens (sera dilution 1:800). Horizontal bars represent the median O.D. and dashed bars (- - -) represent the cutoff.
Fig. 2.
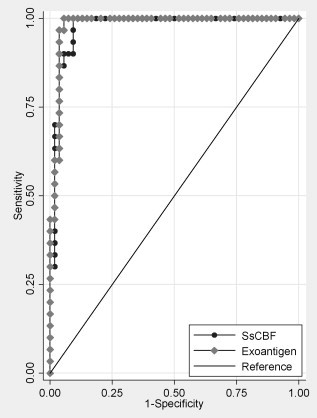
Receiver operating characteristics (ROC) curve for the SsCBF purified antigen and crude exoantigens. The ROC is plotted between the true-positive rate (sensitivity) on the y-axis, and the false-positive rate (1-specificity) on the x-axis. The area under the curve (AUC) represents the accuracy of the ELISA test, which was 0.9936 for the SsCBF (dark gray line) and 0.9994 for the crude exoantigens (light gray line).
3.2. Sensitivity and specificity of ELISA using crude antigens
The sera from healthy cats (control sera) had absorbances at 492 nm from 0.009 to 0.701. The mean absorbance plus two standard deviations for all of the control cats was A 492 = 0.609. Among cats with sporotrichosis, 29/30 (96.67%) (Fig. 1) were positive, with an absorbance greater than 0.609. The mean O.D. of sera from cats with sporotrichosis was 1.890 ± 0.445 and the median was 1.944. The mean O.D of healthy cat sera was 0.241 ± 0.184 and the median was 0.213. Thus, for this ELISA assay: sensitivity = 96%, specificity = 98%, positive predictive value = 96%, negative predictive value = 98%, and global efficiency = 97%. The AUC was 0.9994 ± 0.0009 (confidence interval 95% (IC) 0.99758–1.00000) (Fig. 2). The O.D. of sera from cats with other pathologies = 0.188 ± 0.109; median = 0.172.
3.3. Validation tests
The results of ELISA tests obtained in the same laboratory (intra laboratory) or in a different laboratory (inter laboratory) gave very similar results (data not shown).
3.4. Immunodiffusion test
Sera from cats with sporotrichosis were positive in 14/30 (46.6%) samples by the immunodiffusion (ID) test, and all sera from healthy cats (control group) were negative.
4. Discussion
The highest prevalence of mycoses in animals is in the Americas, and known cases may represent only a fraction of the total, as reported by Soltys and Sumner-Smith (1971). The natural habitat of many pathogenic fungi is soil animals exposed to fungi may produce only a mild or undetectable immune response and in many cases, clinical disease is not evident. In the last three decades, domestic cats have been recognized in the transmission of sporotrichosis to man (Read and Sperling, 1982, Dunstan et al., 1986a, Dunstan et al., 1986b, Larsson et al., 1989, Reed et al., 1993, Werner and Werner, 1994, Schubach and Schubach, 2000). Since S. schenckii is a saprophyte found in soil and on plants, cats can be easily infected; the fungus is inoculated subcutaneously by implantation of spores in penetrating wounds caused by a thorn or splinter. Recently, Barros et al. (2004) reported the largest epidemic of sporotrichosis due to zoonotic transmission was in Rio de Janeiro, Brazil; over 6 years, 1503 cats, 64 dogs, and 759 humans had a diagnosis that was confirmed by the isolation of S. schenckii in culture (Lima Barros et al., 2001, Barros et al., 2003, Schubach and Schubach, 2000, Schubach et al., 2004). This sporotrichosis epidemic was described in areas with underprivileged socio-economic conditions and precarious health services (Barros et al., 2004, Schubach and Schubach, 2000, Schubach et al., 2004). Among the cats with sporotrichosis in this study, five were housed indoors; although, they could have had some contact with the environment or with cats with the mycosis. In our study, only cats with lesions that resulted in positive cultures for S. schenckii were considered as having sporotrichosis.
Although the sera from two healthy feral cats reacted positively against the S. schenckii SsCBF and the sera from one healthy cat reacted against the crude exoantigens, we cannot exclude previous contact with the fungus in the nature and cats presenting with subclinical infections. We believe these reactions occurred because the cats had prior contact with the fungus in nature. These animals live free in the outskirts of the city and had likely previously encountered the fungus, and produced a humoral response against S. schenckii antigenic components. On the other hand, other mycoses were not diagnosed in these animals. If they were infected by another fungus, they could present cross-reactivity in serological tests. This raises the question if an acute case of sporotrichosis might be impaired. From our point of view, sporotrichosis is suspected in felines only when lesions are observed in the skin or mucosa. In this situation, antibodies are produced over the time necessary for the lesion to develop in a higher quantity than when subclinical infection occurs due to contact with the fungus in nature.
In our opinion, both antigens presented very good results and this ELISA is a relatively simple and rapid assay for assessing infection by S. schenckii in cats. The use of a purified antigen such as the SsCBF molecule allows for better standardization than the use of crude preparations, but the choice of the antigen depends on the facilities found in each laboratory. In the case of the purified SsCBF antigen, only one epitope is the target for antibody recognition, while in the case of the crude exoantigens, many molecules and epitopes are involved in the antibody detection. Recently, Bernardes-Engemann et al. (2009) showed that SsCBF obtained from different isolates present different reactivity in ELISA assay and the isolate 1099-18 proved to be a more accurate diagnostic tool for the serodiagnosis of sporotrichosis. At this moment, there are no comparative studies for CWPR molecules in ELISA. On the other hand, a serological reaction involving crude antigens depends on the antigenic composition of the batch of the respective isolate. In the present study, the S. schenckii crude antigens (strain #118) performed well, but in a recent publication we described the great antigen variability among 23 S. schenckii isolates from different Brazilian regions (Fernandes et al., 2009). This variability in antigen composition may certainly affect an ELISA result. Therefore, a consensus among the scientific community working on the serology of sporotrichosis is necessary so that the same strain is used for serological studies, allowing results to be compared. Alternatively, a regional strain is used for this purpose. However, the traditional culture method is time-consuming, requiring at least a week for the initial growth of the fungus and the subsequent micro-morphological identification. Further, the simpler ID test showed less sensitivity. Thus, this ELISA test may be used in conjunction with more traditional diagnostic methods for the diagnosis of cat sporotrichosis.
Acknowledgments
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp), Conselho Nacional de Pesquisas (CNPq), Centro de Zoonosis de São Paulo, and FioCruz, RJ. The authors have no conflicts of interest. ZPC, LMLB, and GFF are fellows of CNPq.
References
- Barros M.B., Schubach A.O., Valle A.C., Gutierrez-Galhardo M.C., Conceição-Silva F., Schubach T.M., Reis R.S., Wanke B., Marzochi K.B., Conceição M.J. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin. Infect. Dis. 2004;38:529–535. doi: 10.1086/381200. [DOI] [PubMed] [Google Scholar]
- Barros M.B.L., Schubach A.O., Galhardo M.C., Schubach T.M.P., Reis R.S., Conceição M.J., Valle A.C. Sporotrichosis with widespread cutaneous lesions – a report of 24 cases related to transmission by domestic cats in Rio de Janeiro, Brazil. Int. J. Dermatol. 2003;42:581–677. doi: 10.1046/j.1365-4362.2003.01813.x. [DOI] [PubMed] [Google Scholar]
- Bernardes-Engemann A.R., Costa R.C., Miguens B.R., Penha C.V., Neves E., Pereira B.A., Dias C.M., Mattos M., Gutierrez M.C., Schubach A., Oliveira-Neto M.P., Lazéra M., Lopes-Bezerra L.M. Improved enzyme-linked immunosorbent assay using the SsCBF antigen for the serodiagnosis of several clinical forms of sporotrichosis. Med. Mycol. 2005;43:487–493. doi: 10.1080/13693780400019909. [DOI] [PubMed] [Google Scholar]
- Bernardes-Engemann A.R., Loureiro y Penha C.V., Benvenuto F., Braga J.U., Barros M.L., Orofino-Costa R., Lopes-Bezerra L.M. A comparative serological study of the SsCBF antigenic fraction isolated from three Sporothrix schenckii strains. Med. Mycol. 2009;47:874–878. doi: 10.3109/13693780802695520. [DOI] [PubMed] [Google Scholar]
- Blondin N., Baumgardner D.J., Moore G.E., Lawrence T. Glickman blastomycosis in indoor cats: Suburban Chicago, Illinois, USA. Mycopathology. 2007;163:59–66. doi: 10.1007/s11046-006-0090-1. [DOI] [PubMed] [Google Scholar]
- Camargo Z.P., Berzaghi R., Amaral C.C., Silva S.H.M. Simplified method for producing Paracoccidioides brasiliensis exoantigens for use in immunodiffusion tests. Med. Mycol. 2003;41:539–542. doi: 10.1080/13693780310001615358. [DOI] [PubMed] [Google Scholar]
- Dunstan R.W., Langham R.F., Reimann K.A., Wakenell P.S. Feline sporotrichosis: a report of five cases with transmission to humans. J. Am. Acad. Dermatol. 1986;15:37–45. doi: 10.1016/s0190-9622(86)70139-4. [DOI] [PubMed] [Google Scholar]
- Dunstan R.W., Reimann K.A., Langham R.F. Feline sporotrichosis. J. Am. Vet. Med. Assoc. 1986;189:880–883. [PubMed] [Google Scholar]
- Fernandes G.F., do Amaral C.C., Sasaki A., Godoy P.M., de Camargo Z.P. Heterogeneity of proteins expressed by Brazilian Sporothrix schenckii isolates. Med. Mycol. 2009;47:855–861. doi: 10.3109/13693780802713216. [DOI] [PubMed] [Google Scholar]
- Graupmann-Kuzma A., Valentine B.A., Shubitz L.F., Dial S.M., Watrous B., Tornquist S.J. Coccidioidomycosis in dogs and cats: a review. J. Am. Anim. Hosp. Assoc. 2008;44:226–235. doi: 10.5326/0440226. [DOI] [PubMed] [Google Scholar]
- Hamilton H.L., Whitley R.D., McLaughlin S.A. Exophthalmos secondary to aspergillosis in a cat. J. Am. Anim. Hosp. Assoc. 2000;36:343–347. doi: 10.5326/15473317-36-4-343. [DOI] [PubMed] [Google Scholar]
- Johnson R.J., Fry M.M., Anez K.L., Proctor B.M., Jang S.S. Histoplasmosis infection in two cats from California Johnson. J. Am. Anim. Hosp. Assoc. 2004;40:165–169. doi: 10.5326/0400165. [DOI] [PubMed] [Google Scholar]
- Larsson C.E., Gonçalves M.A., Araujo V.C., Dagli M.L., Correa B., Fava-Neto C. Feline sporotrichosis: clinical and zoonotic aspects. Rev. Inst. Med. Trop. São Paulo. 1989;31:353–358. doi: 10.1590/s0036-46651989000500010. [DOI] [PubMed] [Google Scholar]
- Lima Barros M.B., Schubach T.M., Galhardo M.C., Oliviera-Schubach A., Monteiro P.C., Reis R.S., Zancopé-Oliveira R.M., Santos L.M., Cuzzi-Maya T., Blanco T.C., Marzochi K.B., Wanke B., Valle A.C. Sporotrichosis: an emergent zoonosis in Rio de Janeiro. Mem. Inst. Oswaldo Cruz. 2001;96:777–779. doi: 10.1590/s0074-02762001000600006. [DOI] [PubMed] [Google Scholar]
- Lopes-Alves L., Travassos L.R., Previato J.O., Mendonça-Previato L. Novel antigenic determinants from peptidorhamnomannans of Sporothrix schenckii. Glycobiology. 1994;4:281–288. doi: 10.1093/glycob/4.3.281. [DOI] [PubMed] [Google Scholar]
- Malik R., Wigney D.I., Muir D.B., Gregory D.J., Love D.N. Cryptococcosis in cats: clinical and mycological assessment of 29 cases and evaluation of treatment using orally administered fluconazole. Med. Mycol. 1992;30:133–144. doi: 10.1080/02681219280000181. [DOI] [PubMed] [Google Scholar]
- Penha C.V.L., Lopes-Bezerra L.M. Concanavalin A-binding cell wall antigens of Sporothrix schenckii: a serological study. Med. Mycol. 2000;38:1–7. [PubMed] [Google Scholar]
- Pressler B.M., Vaden S.L., Lane I.F., Cowgill L.D., Dye J.A. Candida spp. urinary tract infections in 13 dogs and seven cats: predisposing factors, treatment, and outcome. J. Am. Anim. Hosp. Assoc. 2003;39:263–270. doi: 10.5326/0390263. [DOI] [PubMed] [Google Scholar]
- Pinheiro A.Q., Moreira J.L.B., Sidrim J.J.C. Dermatofitoses no meio urbano e a coexistência do homem com cães e gatos. Rev. Soc. Bras. Med. Trop. 1997;30:287–294. doi: 10.1590/s0037-86821997000400003. [DOI] [PubMed] [Google Scholar]
- Read S.I., Sperling L.C. Feline sporotrichosis. Transmission to man. Arch. Dermatol. 1982;118:429–431. [PubMed] [Google Scholar]
- Reed K.D., Moore F.M., Geiger G.E., Stemper M.E. Zoonotic transmission of sporotrichosis: case report and review. Clin. Infect. Dis. 1993;16:384–387. doi: 10.1093/clind/16.3.384. [DOI] [PubMed] [Google Scholar]
- Schubach T.M.P., Schubach A. Sporotrichosis in cat and dog – review. Clin. Vet. 2000;29:21–24. [Google Scholar]
- Schubach, T.M.P., Schubach, A., Okamoto, T., Barros, M.B.L., Figueiredo, F.B., Cuzzi, T., Fialho-Monteiro, P.C., Reis, R.S., Perez, M.A., Wanke, B., 2004. Evaluation of an epidemic of sporotrichosis in cats: 347 cases (1998–2001). J. Am. Vet. Med. Canine sporotrichosis in Rio de Janeiro, Brazil: Clinical presentation, laboratory diagnosis and therapeutic response in 44 cases (1998–2003). Med. Mycol. 44, 87–92.
- Soltys M.A., Sumner-Smith G. Systemic mycoses in dogs and cats. Can. Vet. J. 1971;12:191–199. [PMC free article] [PubMed] [Google Scholar]
- Werner A.H., Werner B.E. Sporotrichosis in man and animal. Int. J. Dermatol. 1994;33:692–700. doi: 10.1111/j.1365-4362.1994.tb01512.x. [DOI] [PubMed] [Google Scholar]


