Abstract
Knowledge of implicated food vehicles and contributing factors derived from foodborne disease outbreak (FBDO) investigations allows consumers to be educated on decreasing high-risk behavior to reduce the risk of being affected by foodborne diseases. Food safety regulatory authorities also need summary of outbreak data, as these data indicate where the existing food supply system should be improved. To obtain information on epidemiology of FBDOs in China, FBDOs reported to the China National Foodborne Diseases Surveillance Network by 12 surveillance provinces that include 43% of the Chinese population was summarized. Between 2003 and 2008, 2795 FBDOs were reported, resulting in 62559 illnesses, 31261 hospitalizations, and 330 deaths. Outbreak size ranged from 2 to 464 cases, with a median of 14 cases. The outbreak rate had decreased from 1.37 per 1 million population in 2003 to 0.46 per 1 million population in 2008. Of the 2176 outbreaks with a single known etiology, bacteria (1051 outbreaks, 48%), man-made chemical hazards (550 outbreaks, 25%), and animal and plant toxins (536 outbreaks, 25%) were the main courses. Only one outbreak was caused by virus. Of the 1930 outbreaks with a single commodity, plant-based foods were the most common reported (930 outbreaks, 48%), followed by animal-based foods (590 outbreaks, 31%). Outbreaks most frequently occurred in private residences (32%), workplace cafeterias (21%), and restaurants (17%). The most common factor reported in the 2190 outbreaks with known contributing factors were improper cooking (510 outbreaks, 23%), contaminated ingredient (503 outbreaks, 23%), cross contamination (475 outbreaks, 22%) and improper storage (295 outbreaks, 13%). It is considered that FBDOs continue to be an important public health problem in China.
Keywords: China, Epidemiology, Foodborne disease, Outbreaks, Surveillance
Highlights
-
•
This is up to now the most comprehensive summary on foodborne disease outbreaks in China.
-
•
We described the epidemiology of foodborne disease outbreaks in China.
-
•
This summary reviews data which was reported to the China National Foodborne Diseases Surveillance Network.
-
•
Practical advice for improving foodborne disease outbreak surveillance in China is provided.
1. Introduction
Foodborne disease is a significant public health concern in both developed and developing countries. Although the disease is usually mild and self-limiting, due to the high number of individuals affected each year, foodborne disease exerts a substantial socioeconomic burden on the population and healthcare system. In the USA, foodborne disease causes about 47.8 million illnesses, 127839 hospitalizations, and 3037 deaths per year (Scallan, Griffin, Angulo, Tauxe, & Hoekstra, 2011). In Australia, foodborne gastroenteritis causes an estimated 5.4 million illnesses, 14700 hospitalizations, and 76 deaths each year (Hall et al., 2005). In England and Wales, foodborne gastroenteritis caused an estimated 2.4 million illnesses, 21138 hospitalizations, and 718 deaths in 1995 (Adak, Long, & O’Brien, 2002). A study of acute gastrointestinal illness (AGI) in China estimated 209 million episodes of foodborne disease occurred in 2010–2011, and the relative incidence in China appears to be within the range of incidence reported in the above-mentioned countries (Chen et al., 2013). However, the method used to estimate the burden of foodborne disease in the United States, England and Wales and Australia was different from that in China. In the above-mentioned studies, pathogen-specific data were used to calculate the foodborne proportion of AGI. However, due to the lack of pathogen-specific data used to calculate the foodborne proportion of AGI, the Chinese study only used the foodborne proportions calculated by those studies to roughly estimate the foodborne disease in China.
Cases of foodborne disease are typically under-reported by traditional surveillance, which needs cases to present to the healthcare system. Only a small part of foodborne diseases, hospitalizations, and deaths happen as portion of identified outbreaks (Dewey-Mattia, Roberts, Vieira, & Fullerton, 2016). However, the outbreak report is essential for a better understanding of the epidemiology of foodborne diseases. Although the summary of the outbreaks cannot lead to a clear conclusion of the disease trend, by identifying the implicated food vehicles and contributing factors, the health administration could be encouraged to evaluate and adopt suitable measures to prevent and control outbreaks in the future. Knowledge of implicated food vehicles and contributing factors allows consumers to be educated on decreasing high-risk behaviors, thereby reducing the risk of being affected by foodborne diseases. Up to date outbreak data can help food safety regulatory authorities to identify problems in the existing food supply system. Several countries systematically review foodborne disease outbreaks (FBDOs) in order to develop strategies to reduce the disease burden (Dalton et al., 2004, Gould et al., 2013, Lindqvist et al., 2000, O’Brien et al., 2002).
The formation of a set of reporting, investigation and analysis system with high efficiency is essential to the efficient management and minimization of foodborne disease. The primary source of information relating to foodborne disease in China mainly captures information on outbreaks, although occasionally information on sporadic illnesses was collected. Before the establishment of the National Foodborne Diseases Surveillance Network (NFDSN), there had been no systematic collection of detailed and standardized information on FBDO in China. Previous summaries of data reported to the China NFDSN were published for 1992–2001 (Liu, Chen, Wang, & Ji, 2004), 2003 (Liu, Chen, Fan, & Wang, 2006), 2004 (Chen, Liu, Fan, & Wang, 2008), 2005 (Liu, Chen, Guo, & Wang, 2008), and 2006 (Chen et al., 2010). The objective of this study was to summarize epidemiologic data on FBDOs reported to the China NFDSN between 2003 and 2008.
2. Materials and methods
2.1. Outbreak definition
A FBDO is defined as the occurrence of ≥2 cases of a similar illness resulting from the ingestion of the same type of food, or if the food vehicle was undetermined, sharing a common meal or food facility (Wu, Wen, Ma, Ma, & Chen, 2014). Food poisoning diagnostic criteria are mainly based on epidemiological survey data, the incubation period and the unique clinical features of the patients, while the aim of laboratory diagnosis is to determine the cause of poisoning (Ministry of Health of the People’s Republic of China, 1994). Outbreaks that did not meet these criteria were not reported to the NFDSN by surveillance provinces.
2.2. Data source
To better understand foodborne disease epidemiology in China, China National Center for Food Safety Risk Assessment (previously known as the Food Safety Section of the National Institute for Nutrition and Food Safety, Chinese Center for Disease Control and Prevention) established the NFDSN in 2001 in 13 surveillance provinces. Since 2003, surveillance provinces began reporting FBDO data to the NFDSN through the web-based National Food Safety Surveillance Information System. According to the Food Safety Law of the People’s Republic of China issued in 2009, a national food safety risk surveillance system that includes foodborne disease surveillance was built. Since then, foodborne disease surveillance had expanded to the entire nation, and the work of NFDSN was shifted to the new system. Thus, the research period of this study was limited to 2003–2008.
Between 2003 and 2008, outbreak reports were available from 12 surveillance provinces including Beijing, Neimenggu, Jilin, Shanghai, Jiangsu, Zhejiang, Fujian, Henan, Hubei, Guangdong, Guangxi, and Chongqing. In 2006, the surveillance provinces represent about 43% of the Chinese population (1314 million). Information collected for each outbreak included reporting province, date of illness onset, incubation period, number of cases, hospitalizations and deaths, etiology, suspected food vehicle, setting of food preparation or consumption and contributing factors.
2.3. Food vehicle classification
Food vehicles were coded into four levels of classification. Level one foods include animal-based foods, plant-based foods, other foods, mixed dishes, multiple foods, and unknown. Level two breaks the foods into a second level, such as animal-based foods, divided into aquatic products, dairy and dairy products, egg and egg products, meat and meat products, and other animal-based foods. Level three breaks the foods into a third level, such as aquatic products, divided into crustacean, fish, molluscs, etc. Level four breaks the foods into a fourth level, such as crustacean, divided into crayfish, lobsters, crabs, etc.
2.4. Statistical analysis
Data were entered into Microsoft Access and analysed in Excel 2010. The per capita rate of FBDOs during 2003 and 2008 was calculated for each province by using population data from the National Bureau of Statistics of China. We used 2006 population as the denominator since it was in the middle of the study period (National Bureau of Statistics of China, 2007).
3. Results
3.1. Time and province of outbreak
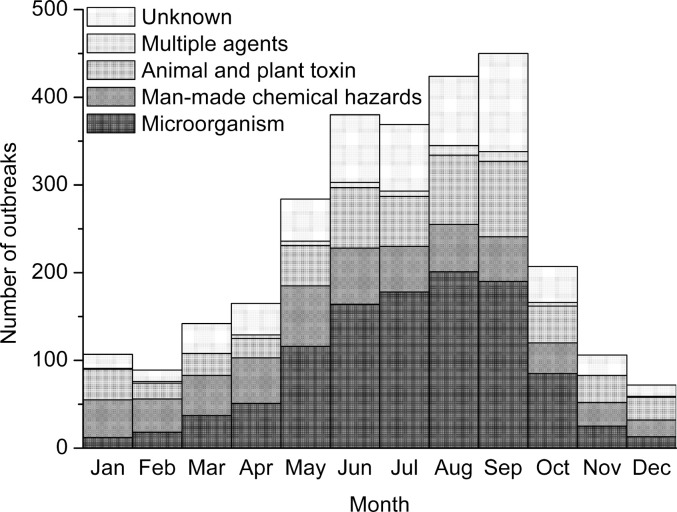
Between 2003 and 2008, a total of 2795 FBDOs were reported, which resulted in 62559 illnesses, 31261 hospitalizations, and 330 deaths (Table 1 ). Reports of outbreaks peaked in 2003 (772 outbreaks), falling to 262 outbreaks in 2008, with an average annual number of 466. The average annual rate of outbreaks and cases was 0.8 and 18.4 per 1 million population, respectively. Outbreaks are markedly seasonal, peaking in warmer months (Fig. 1 ). The seasonal trend of microbial outbreaks was similar to that of FBDOs, but other causes of outbreaks did not have such seasonal trend.
Table 1.
Number of reported foodborne disease outbreaks, cases, hospitalizations, and deaths, by year, China, 2003 to 2008.
| Year | Outbreaks |
Cases |
Hospitalizations |
Deaths |
||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| 2003 | 772 | 28 | 17163 | 27 | 11330 | 36 | 95 | 29 |
| 2004 | 569 | 20 | 14441 | 23 | 7553 | 24 | 48 | 15 |
| 2005 | 454 | 16 | 9401 | 15 | 3877 | 12 | 45 | 14 |
| 2006 | 432 | 15 | 9461 | 15 | 5555 | 18 | 45 | 14 |
| 2007 | 306 | 11 | 6555 | 10 | 1435 | 5 | 60 | 18 |
| 2008 | 262 | 9 | 5538 | 9 | 1511 | 5 | 37 | 11 |
| Total | 2795 | 100 | 62559 | 100 | 31261 | 100 | 330 | 100 |
Fig. 1.
Seasonality of foodborne disease outbreaks in 12 selected provinces in China, by etiology, 2003 to 2008.
Province-specific rate of outbreaks and cases ranged from 0.2–3.1 and 7.1–51.9 per 1 million population, respectively (Table 2 ). Guangxi, Hubei and Zhejiang recorded the highest number of outbreaks during the six-year period; the average number of outbreaks per 1 million population by province for the six-year period was highest in Beijing, Guangxi and Shanghai (Table 2). No outbreak occurs in multiple provinces was reported.
Table 2.
Number of reported foodborne disease outbreaks and cases, by province, China, 2003 to 2008.
| Province | Outbreaks |
Cases |
||
|---|---|---|---|---|
| n | Average annual rate per million population | n | Average annual rate per million population | |
| Beijing | 291 | 3.1 | 4721 | 49.8 |
| Neimenggu | 39 | 0.3 | 1480 | 10.3 |
| Jilin | 122 | 0.7 | 3193 | 19.5 |
| Shanghai | 204 | 1.9 | 5647 | 51.9 |
| Jiangsu | 222 | 0.5 | 5416 | 12.0 |
| Zhejiang | 320 | 1.1 | 8535 | 28.6 |
| Fujian | 172 | 0.8 | 3116 | 14.6 |
| Henan | 114 | 0.2 | 5200 | 9.2 |
| Hubei | 425 | 1.2 | 8003 | 23.4 |
| Guangdong | 188 | 0.3 | 3968 | 7.1 |
| Guangxi | 587 | 2.1 | 10946 | 38.7 |
| Chongqing | 111 | 0.7 | 2334 | 13.9 |
| Total | 2795 | 0.8 | 62559 | 18.4 |
3.2. Etiologic agent
A total of 2227 (80%) outbreaks had a known etiology and these outbreaks accounted for 79% (49422/62559) of illnesses (Table 3 ). Of the 2176 outbreaks with a single known etiology, bacterial disease was responsible for 48% (1051/2176) of outbreaks, 66% (31810/48044) of cases and 6% (18/299) of deaths (Table 3). Of the 2176 outbreaks with a single known etiology, Vibrio parahaemolyticus (322 outbreaks, 15%), non-typhoidal salmonellae (115 outbreaks, 5%), Bacillus cereus (111 outbreaks, 5%), and Proteus spp. (110 outbreaks, 5%) were the most frequent etiologic agents. There was no report of outbreak caused by Campylobacter spp., Clostridium perfringens and Listeria monocytogenes. Vibrio cholera infection is not reported through the foodborne disease reporting system. Burkholderia gladioli was responsible for 17 deaths among 27 cases, much higher case-fatality rate than any other etiologic agent.
Table 3.
Number of reported foodborne disease outbreaks, cases, and deaths, by etiology, China, 2003 to 2008.
| Etiology | Outbreaks |
Cases |
Deaths |
Outbreak size |
|||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | Median (range) | |
| Microorganism | 1090 | 39 | 32983 | 53 | 19 | 6 | 19 (2–464) |
| Bacteria | 1051 | 38 | 31810 | 51 | 18 | 5 | 19 (2–464) |
| Bacillus cereus | 111 | 4 | 2656 | 4 | 0 | 0 | 16 (2–134) |
| Burkholderia gladioli | 6 | 0 | 27 | 0 | 17 | 5 | 4 (3–9) |
| Clostridium botulinum | 1 | 0 | 3 | 0 | 0 | 0 | – |
| Escherichia coli | 60 | 2 | 2766 | 4 | 0 | 0 | 26 (4–288) |
| Plesiomons shigelloides | 5 | 0 | 150 | 0 | 0 | 0 | 15 (9–86) |
| Proteus spp. | 110 | 4 | 3093 | 5 | 0 | 0 | 22 (3–240) |
| Salmonella spp. | 115 | 4 | 3718 | 6 | 0 | 0 | 17 (3–206) |
| Shigella spp. | 11 | 0 | 581 | 1 | 0 | 0 | 15 (4–379) |
| Staphylococcus aureus | 94 | 3 | 2438 | 4 | 0 | 0 | 16 (2–185) |
| Vibrio parahaemolyticus | 322 | 12 | 9041 | 14 | 0 | 0 | 20 (2–244) |
| Other bacteria | 216 | 8 | 7337 | 12 | 1 | 0 | 19 (2–464) |
| Virus | 1 | 0 | 40 | 0 | 0 | 0 | – |
| Rotavirus | 1 | 0 | 40 | 0 | 0 | 0 | – |
| Fungi | 10 | 0 | 387 | 1 | 0 | 0 | 23 (2–144) |
| Other microorganism | 28 | 1 | 746 | 1 | 1 | 0 | 18 (2–123) |
| Man-made chemical hazards | 550 | 20 | 6776 | 11 | 163 | 49 | 6 (2–128) |
| Food additives | 146 | 5 | 1931 | 3 | 29 | 9 | 6 (2–116) |
| Nitrite | 139 | 5 | 1795 | 3 | 28 | 8 | 6 (2–116) |
| Other food additives | 7 | 0 | 136 | 0 | 1 | 0 | 19 (3–41) |
| Heavy metals | 7 | 0 | 129 | 0 | 5 | 2 | 12 (4–52) |
| Organic compounds | 14 | 1 | 187 | 0 | 7 | 2 | 7 (2–81) |
| Methanol | 6 | 0 | 40 | 0 | 7 | 2 | 6 (3–13) |
| Other organic compounds | 8 | 0 | 147 | 0 | 0 | 0 | 9 (2–81) |
| Pesticides | 312 | 11 | 3394 | 5 | 106 | 32 | 6 (2–86) |
| Organophosphorus pesticides | 195 | 7 | 2051 | 3 | 33 | 10 | 6 (2–61) |
| Rodenticides | 95 | 3 | 1013 | 2 | 69 | 21 | 4 (2–86) |
| Tetramine | 84 | 3 | 968 | 2 | 67 | 20 | 5 (2–86) |
| Other pesticides | 22 | 1 | 330 | 1 | 4 | 1 | 9 (2–83) |
| Veterinary drugs | 11 | 0 | 128 | 0 | 1 | 0 | 5 (3–34) |
| Clenbuterol hydrochloride | 8 | 0 | 86 | 0 | 0 | 0 | 8 (3–22) |
| Other veterinary drugs | 3 | 0 | 42 | 0 | 1 | 0 | 5 (3–34) |
| Other man-made chemical hazards | 60 | 2 | 1007 | 2 | 15 | 5 | 10 (2–128) |
| Animal and plant toxins | 536 | 19 | 8285 | 13 | 117 | 35 | 7 (2–218) |
| Aconitine | 3 | 0 | 21 | 0 | 4 | 1 | 6 (4–11) |
| Animal thyroxine | 3 | 0 | 70 | 0 | 0 | 0 | 30 (8–32) |
| Cassava | 3 | 0 | 17 | 0 | 1 | 0 | 4 (3–10) |
| Colchicine | 3 | 0 | 66 | 0 | 0 | 0 | 28 (4–34) |
| Datura | 7 | 0 | 53 | 0 | 0 | 0 | 5 (3–19) |
| Histamine | 12 | 0 | 338 | 1 | 0 | 0 | 21 (2–90) |
| Koumine | 4 | 0 | 17 | 0 | 6 | 2 | 5 (2–6) |
| Phytohemagglutinin | 163 | 6 | 3779 | 6 | 0 | 0 | 15 (3–191) |
| Mushroom poisioning | 158 | 6 | 765 | 1 | 59 | 18 | 4 (2–21) |
| Saxitoxin | 3 | 0 | 12 | 0 | 2 | 1 | 2 (2–8) |
| Solanen | 3 | 0 | 56 | 0 | 0 | 0 | 16 (16–24) |
| Tetrodotoxin | 25 | 1 | 106 | 0 | 16 | 5 | 3 (2–21) |
| Toxic honey | 7 | 0 | 36 | 0 | 9 | 3 | 4 (4–8) |
| Trypsin inhibitor | 3 | 0 | 64 | 0 | 0 | 0 | 11 (5–48) |
| Tung oil | 32 | 1 | 614 | 1 | 0 | 0 | 11 (5–79) |
| Other animal and plant toxin | 107 | 4 | 2271 | 4 | 20 | 6 | 11 (2–218) |
| Multiple agents | 51 | 2 | 1378 | 2 | 2 | 1 | 22 (2–88) |
| Unknown | 568 | 20 | 13137 | 21 | 29 | 9 | 16 (2–275) |
| Total | 2795 | 100 | 62559 | 100 | 330 | 100 | 14 (2–464) |
Of the 2176 outbreaks with a single known etiology, man-made chemical hazards were responsible for 25% of outbreaks, 14% of cases and 55% of deaths (Table 3). Of the 2176 outbreaks with a single known etiology, pesticides were responsible for 14% of outbreaks, 7% of cases, but 35% of deaths. Among them, organophosphorus pesticide alone was linked to 9% of outbreaks with a single known etiology and responsible for 4% of cases and 11% of deaths. Rodenticides were linked to 4% of outbreaks with a single known etiology, and 2% of cases, but 23% of deaths. Among them, Tetramine, was found in 4% of outbreaks with a single known etiology, and accountable for 2% of cases, but 22% of deaths. Food additives, mainly Nitrite, were linked to 7% of outbreaks with a single known etiology, 4% of cases, and 10% of deaths.
Of the 2176 outbreaks with a single known etiology, animal and plant toxins were responsible for 25% of outbreaks, 17% of cases and 39% of deaths (Table 3). Phytohemagglutinin was responsible for 7% of outbreaks with a single known etiology, 8% of cases, but no death reported. Mushroom toxins were linked to 7% of outbreaks with a single known etiology, and responsible for 2% of cases and 20% of deaths.
The incubation period was reported in 142 (25%) of the 568 outbreaks that had an unknown etiology: <1 h (7 outbreaks, 5%), 1–7 h (60 outbreaks, 42%), 8–14 h (42 outbreaks, 30%), and ≥15 h (33 outbreaks, 23%).
3.3. Food vehicles
A food vehicle was reported for 2073 (74%) of the 2795 outbreaks, 1930 (69%) of which were attributed to a single food commodity (Table 4 ). Plant-based foods were the most common single commodity reported (930 outbreaks, 48%), followed by animal-based foods (590 outbreaks, 31%), and other foods (283 outbreaks, 15%).
Table 4.
Number of reported foodborne disease outbreaks, cases, and deaths, by food vehicle implicated, China, 2003 to 2008.
| Foods | Outbreaks |
Cases |
Deaths |
Outbreak size |
|||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | Median (range) | |
| Animal-based foods | 590 | 21 | 14098 | 23 | 51 | 15 | 16 (2–402) |
| Aquatic products | 137 | 5 | 2617 | 4 | 29 | 9 | 11 (2–139) |
| Crustacean | 13 | 0 | 286 | 0 | 0 | 0 | 9 (3–139) |
| Fish | 35 | 1 | 413 | 1 | 10 | 3 | 6 (2–67) |
| Molluscs | 11 | 0 | 119 | 0 | 2 | 1 | 8 (2–27) |
| Other aquatic productsa | 78 | 3 | 1799 | 3 | 17 | 5 | 13 (2–99) |
| Dairy and dairy products | 12 | 0 | 374 | 1 | 0 | 0 | 20 (2–152) |
| Egg and egg products | 18 | 1 | 211 | 0 | 4 | 1 | 5 (2–47) |
| Meat and meat products | 385 | 14 | 10069 | 16 | 17 | 5 | 18 (2–402) |
| Beef | 10 | 0 | 233 | 0 | 0 | 0 | 21 (11–45) |
| Cured meat | 8 | 0 | 96 | 0 | 2 | 1 | 13 (2–23) |
| Deli meat | 27 | 1 | 746 | 1 | 1 | 0 | 21 (2–105) |
| Internal organs | 14 | 1 | 197 | 0 | 0 | 0 | 11 (3–42) |
| Mutton | 3 | 0 | 26 | 0 | 1 | 0 | 7 (4–15) |
| Pork | 23 | 1 | 455 | 1 | 0 | 0 | 12 (4–66) |
| Poultry | 42 | 2 | 1105 | 2 | 3 | 1 | 11 (2–402) |
| Other meat and meat products | 258 | 9 | 7211 | 12 | 10 | 3 | 21 (2–240) |
| Other animal-based foods | 38 | 1 | 827 | 1 | 1 | 0 | 15 (2–84) |
| Plant-based foods | 930 | 33 | 16451 | 26 | 155 | 47 | 9 (2–464) |
| Bean products | 40 | 1 | 1004 | 2 | 2 | 1 | 20 (3–98) |
| Cereals | 214 | 8 | 4647 | 7 | 52 | 16 | 11 (2–464) |
| Flour products | 52 | 2 | 1167 | 2 | 9 | 3 | 9 (2–199) |
| Fruits | 40 | 1 | 413 | 1 | 6 | 2 | 7 (2–48) |
| Mushrooms | 161 | 6 | 828 | 1 | 58 | 18 | 4 (2–31) |
| Vegetable | 339 | 12 | 6981 | 11 | 9 | 3 | 14 (2–191) |
| Kidney beans | 136 | 5 | 3058 | 5 | 0 | 0 | 16 (3–190) |
| Other plant-based foods | 84 | 3 | 1411 | 2 | 19 | 6 | 10 (2–92) |
| Other foods | 283 | 10 | 6137 | 10 | 60 | 18 | 12 (2–379) |
| Edible oil | 18 | 1 | 545 | 1 | 1 | 0 | 10 (2–218) |
| Honey | 5 | 0 | 20 | 0 | 6 | 2 | 4 (3–5) |
| Instant noodles | 5 | 0 | 91 | 0 | 2 | 1 | 5 (2–59) |
| Liquor | 24 | 1 | 126 | 0 | 23 | 7 | 5 (2–13) |
| Seasonings | 43 | 2 | 409 | 1 | 13 | 4 | 6 (2–62) |
| Soft drinks | 20 | 1 | 755 | 1 | 3 | 1 | 15 (2–379) |
| Sugar | 2 | 0 | 35 | 0 | 0 | 0 | 18 (13–22) |
| Other unclassified foods | 166 | 6 | 4156 | 7 | 12 | 4 | 16 (2–244) |
| Mixed dishes | 127 | 5 | 3421 | 5 | 8 | 2 | 21 (2–123) |
| Multiple foods | 143 | 5 | 4584 | 7 | 2 | 1 | 21 (2–288) |
| Unknown | 722 | 26 | 17868 | 29 | 54 | 16 | 15 (2–464) |
| Total | 2795 | 100 | 62559 | 100 | 330 | 100 | 14 (2–464) |
Includes aquatic products in above categories that may be mixed together and aquatic products not in above categories, or where type of aquatic product was unknown. It is the same for other meat and meat products, other animal-based foods, and other plant-based foods.
In the category of plant-based foods, vegetable was the most common commodity reported (339 outbreaks, 36%), followed by cereals (214 outbreaks, 23%) and mushrooms (161 outbreaks, 17%). Among outbreaks associated with vegetable, the most common food vehicle reported was kidney beans (136 outbreaks, 40%).
In the category of animal-based foods, meats were the most common commodity reported (385 outbreaks, 65%), followed by aquatic products (137 outbreaks, 23%). Among outbreaks associated with aquatic products, the most common food vehicle reported was fish (35 outbreaks, 26%).
3.4. Settings
Setting of food prepared or consumed was provided in the majority (98%) of the outbreak reports (Table 5 ). Outbreaks most frequently occurred in private residences (32%), workplace cafeterias (21%), and restaurants (17%). Workplace cafeteria was responsible for the highest number of cases (15337 cases, 25%), while private residence (254 deaths, 77%) was associated with the majority of deaths. The median number of cases per outbreak was much higher for take-away (27 cases) and school cafeteria (24 cases) than for private residence (6 cases) and food supermarket (5 cases).
Table 5.
Number of reported foodborne disease outbreaks, cases, and deaths, by setting, China, 2003 to 2008.
| Settings | Outbreaks |
Cases |
Deaths |
Outbreak size |
|||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | Median (range) | |
| Private residence | 889 | 32 | 11185 | 18 | 254 | 77 | 6 (2–188) |
| Workplace cafeteria | 583 | 21 | 15337 | 25 | 2 | 1 | 18 (2–281) |
| Restaurant | 472 | 17 | 12499 | 20 | 7 | 2 | 18 (2–275) |
| School cafeteria | 358 | 13 | 14163 | 23 | 1 | 0 | 24 (3–464) |
| Street vendor | 119 | 4 | 2300 | 4 | 4 | 1 | 10 (2–138) |
| Fast food service | 66 | 2 | 1291 | 2 | 3 | 1 | 14 (2–76) |
| Takeaway | 25 | 1 | 732 | 1 | 2 | 1 | 27 (2–134) |
| Food supermarket | 11 | 0 | 363 | 1 | 1 | 0 | 5 (3–100) |
| Other | 225 | 8 | 4069 | 7 | 35 | 11 | 10 (2–169) |
| Unknown | 47 | 2 | 620 | 1 | 21 | 6 | 5 (2–80) |
| Total | 2795 | 100 | 62559 | 100 | 330 | 100 | 14 (2–464) |
3.5. Contributing factors
At least one contributing factor was reported for 2190 (78%) of the 2795 outbreaks (Table 6 ). The most common factor reported in the 2190 outbreaks with known contributing factors were improper cooking (510 outbreaks, 23%), contaminated ingredient (503 outbreaks, 23%), cross contamination (475 outbreaks, 22%) and improper storage (295 outbreaks, 13%).
Table 6.
Number of reported foodborne disease outbreaks, cases, and deaths, by contributing factor, China, 2003 to 2008.a
| Contributing factors | Outbreaks |
Cases |
Deaths |
Outbreak size |
|||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | Median (range) | |
| Improper cooking | 510 | 18 | 12759 | 20 | 18 | 5 | 17 (2–244) |
| Contaminated ingredient | 503 | 18 | 13509 | 22 | 30 | 9 | 16 (2–379) |
| Cross contamination | 475 | 17 | 15054 | 24 | 6 | 2 | 22 (2–464) |
| Improper storage | 295 | 11 | 9695 | 15 | 13 | 4 | 23 (2–244) |
| Contaminated equipment or utensil | 117 | 4 | 3992 | 6 | 0 | 0 | 24 (3–185) |
| Infected food handler (s) | 116 | 4 | 3812 | 6 | 2 | 1 | 24 (2–145) |
| Poisonous substance intentionally added | 41 | 1 | 566 | 1 | 17 | 5 | 6 (2–58) |
| Other | 216 | 8 | 4749 | 8 | 40 | 12 | 13 (2–464) |
| Unknown | 605 | 22 | 14174 | 23 | 40 | 12 | 15 (2–288) |
| Total | 2795 | 100 | 62559 | 100 | 330 | 100 | 14 (2–464) |
More than one contributing factor identified for some outbreaks.
4. Discussion
This is the most comprehensive summary of FBDOs in China since the publication of a summary from 1992 to 2001, which was published in Chinese (Liu et al., 2004). The annual number of FBDOs reported during 2003–2008 (466 outbreaks) was similar to the average annual number reported during 1992–2001 in the same 12 surveillance provinces (499 outbreaks, data not shown), indicating FBDOs continue to be an important public health problem in China.
The decrease in the number of outbreaks reported since 2003 might be true but it could also suggest reporter fatigue. The severe acute respiratory syndrome outbreak probably increased effort in the detection, investigation and reporting of outbreaks, thus led to the peak in 2003 (Feng, Li, & Varma, 2011). It is possible that the reliability of the data changes over time as, for example, methods or technical skills in the investigating laboratories, improved.
Seasonality of microbial foodborne outbreaks was observed in this summary, with a peak in the summer. Summer is associated with increased rates of Salmonella and Vibrio infection in China. The seasons might affect the incidence of infectious foodborne disease, investigating of the reasons for the summer dominance of FBDOs should be the focus of surveillance in the future. However, the seasonal peak of infection attributed to some foodborne pathogens is not in the summer. For instance, norovirus and rotavirus increase in autumn or in winter (Ahmed et al., 2013, Zhang et al., 2017). Seasonality may be related to climate conditions (e.g. temperature, humidity and rainfall), all of which may affect exposure frequency, host immune status, and virulence.
The number of outbreaks per million population differs greatly across provinces, a finding similar to Australia and the USA (Dalton et al., 2004, Gould et al., 2013). Such discrepancy may indicate differences in the management of FBDOs in the surveillance provinces rather than true disparities in outbreak event. These differences could also be due to differences in lifestyle, education or public health infrastructure in these provinces. A limitation of this summary is that it relies on the outbreak data reported by surveillance provinces while no supplementary study was carried out to determine how reliable the surveillance systems are between provinces, and how well resourced and how competent the laboratory staff are to unambiguously identify sources of illness in outbreaks. However, the population-based ratio may serve as a useful tool for evaluation the reliability of the surveillance and outbreak investigation systems in each of the provinces for which data are included.
An additional important factor that will affect reporting is the limitation of laboratory capacity to identify a full range of potential foodborne pathogens. Norovirus is the main cause of foodborne disease in the USA, leading to about 58% of indigenous foodborne disease with known etiology (Scallan, Hoekstra, et al., 2011). During 2003–2008, the virus replaced the bacterial pathogens as the most common cause of the reported FBDOs in the USA. As the number of outbreaks of viral causes increase, the number of outbreaks of unknown etiology is proportionally reduced (Gould et al., 2013). During the same period, probably due to lack of molecular biological and immunological techniques by local health sectors, only one rotavirus outbreak was reported to China NFDSN. The percentage of outbreaks with unknown etiology was almost doubled during the period 2003–2008 (20%) compared with that of 1992–2001 (10%). In the present study, about 20% of these outbreaks had an incubation period of equal or greater than 15 h, suggesting that many were of viral causes (Olsen, MacKinnon, Goulding, Bean, & Slutsker, 2000). It is not enough to rely on routine culture of intestinal bacteria during the outbreak of foodborne disease, and it is necessary to promote the local health sectors to improve the diagnostic capacity for virus detection and enumeration.
V. parahaemolyticus is widely found in estuaries and marine environments around the world and is the leading cause of human gastroenteritis in the USA (Iwamoto, Ayers, Mahon, & Swerdlow, 2010). It is also a common cause of foodborne diseases in Asian countries (Alam et al., 2002, Raghunath et al., 2008). Consistent with the 1992–2001 report, V. parahaemolyticus remains the number one cause of foodborne disease in 2003–2008 in the area covered by the China NFDSN. Consumers can reduce the risk of shellfish-related infections caused by V. parahaemolyticus by not eating raw or uncooked shellfish; doctors and individuals need to use antibiotics with care, since blind use of antibiotics may increase V. parahaemolyticus infection risk (Yan et al., 2015).
Proteusspecies, which are usually found in soil and water, mostly known as conditioned human pathogens, have often been responsible for both food spoilage and FBDOs (Wang et al., 2010). In the present summary, Proteus spp. induced FBDOs represented 10% of microbial FBDOs, which falls within the same range as those reported previously (8%–14%) (Chen et al., 2010, Chen et al., 2008, Liu et al., 2004, Liu et al., 2006, Liu et al., 2008).
B. gladioli is an ’unexpected’ foodborne pathogen. However, due to common consumption of tempeh with which it is associated, and in which it produces bongkrekic acid, it is an important foodborne pathogen in China.
Generally, the contamination of chemical hazards in food is well regulated and managed in developed countries (Rocourt et al., 2003, World Health Organization, 2015). However, foodborne diseases caused by chemical hazards are still one of the important food safety problems faced by mainland China. In the present study, of the outbreaks with a single known etiology, man-made chemical hazards accounted for 25% of outbreaks, 14% of cases and 55% of deaths. The number of outbreaks and mortality attributed to man-made chemicals is remarkable, and all of these outbreaks are acute poisonings. To guarantee the chemicals such as food additives, pesticides and veterinary drugs are safely used, the Chinese government needs to develop stricter regulations on food and agricultural products, and to improve the administrative law enforcement mechanism.
In an outbreak situation investigators are more likely to identify a food vehicle, which makes outbreaks a valuable source of data on transmission. Mushrooms led to 6% of outbreaks and 18% of deaths. It is necessary to educate consumers to carefully consider the consumption of wild mushrooms which may contain biological toxins. Kidney beans caused 5% of outbreaks. Ingestion of the phytohemagglutinin present in raw or undercooked kidney beans can lead to AGI (Miyake, Tanaka, & McNeil, 2007).
Private residence led to 32% of outbreaks, 18% of cases and 77% of deaths, suggesting that food safety risk communication and education should be carried out for household food preparation. Company canteen led to 21% of outbreaks and 25% of cases. It is necessary to strengthen the supervision of such food service agencies, and to provide specialized education and training to food handlers working in these facilities.
It is noteworthy that the number of reported FBDOs summarized in the present study only represents a proportion of all FBDOs that actually occurred during the surveillance period. Underreporting of FBDOs is common in China for many factors, such as the awareness of consumers and physicians, lack of resources and capacity of foodborne disease surveillance in underdeveloped and faraway regions (Olsen et al., 2000, Xue and Zhang, 2012). Therefore, caution should be exercised before extrapolating the findings of these data for 43% of the Chinese population in 12 provinces to the risk of FBDO to all Chinese people and provinces.
Analysis of FBDO investigations has highlighted the epidemiological characteristics and the important control regulations of FBDOs in China, which has been useful in the improvement of public health control measures. Continued surveillance for FBDOs is crucial to understand changes in the etiologic agents, the implicated food vehicles, and contributing factors associated with illness.
Acknowledgements
The authors gratefully thank the participating Provincial Centers for Disease Control and Prevention personnel for their positive role in FBDO surveillance. The authors also thank Dr. Zumin Shi (University of Adelaide, Australia) for his providing advice on the manuscript. This work was financially supported by the National Natural Science Foundation of China (No. 81673175) and the National Health and Family Planning Commission of the People’s Republic of China (No. 201302005).
References
- Adak G.K., Long S.M., O’Brien S.J. Trends in indigenous foodborne disease and deaths, England and Wales: 1992 to 2000. Gut. 2002;51(6):832–841. doi: 10.1136/gut.51.6.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.M., Lopman B.A., Levy K. A systematic review and meta-analysis of the global seasonality of norovirus. PLoS One. 2013;8(10):e75922. doi: 10.1371/journal.pone.0075922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M.J., Tomochika K.I., Miyoshi S.I., Shinoda S. Environmental investigation of potentially pathogenic Vibrio parahaemolyticus in the Seto-Inland Sea, Japan. FEMS Microbiology Letters. 2002;208(1):83–87. doi: 10.1111/j.1574-6968.2002.tb11064.x. [DOI] [PubMed] [Google Scholar]
- Chen Y., Guo Y., Wang Z., Liu X., Liu H., Dai Y. Foodborne disease outbreaks in 2006 report of the national foodborne disease surveillance network, China. Wei Sheng Yan Jiu. 2010;39(3):331–334. [PubMed] [Google Scholar]
- Chen Y., Liu X.M., Fan Y.X., Wang M.Q. Foodborne diseases outbreaks in 2004-report of national foodborne diseases surveillance network in China. Chinese Journal of Food Hygiene. 2008;20(6):503–506. [Google Scholar]
- Chen Y., Yan W.X., Zhou Y.J., Zhen S.Q., Zhang R.H., Chen J. Burden of self-reported acute gastrointestinal illness in China: A population-based survey. BMC Public Health. 2013;13:456. doi: 10.1186/1471-2458-13-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton C.B., Gregory J., Kirk M.D., Stafford R.J., Givney R., Kraa E. Foodborne disease outbreaks in Australia, 1995 to 2000. Communicable Diseases Intelligence Quarterly Report. 2004;28(2):211–224. [PubMed] [Google Scholar]
- Dewey-Mattia D., Roberts V.A., Vieira A., Fullerton K.E. Foodborne (1973-2013) and waterborne (1971-2013) disease outbreaks - United States. Morbidity and Mortality Weekly Report. 2016;63(55):79–84. doi: 10.15585/mmwr.mm6355a8. [DOI] [PubMed] [Google Scholar]
- Feng Z., Li W., Varma J.K. Gaps remain in China’s ability to detect emerging infectious diseases despite advances since the onset of SARS and avian flu. Health Affairs. 2011;30(1):127–135. doi: 10.1377/hlthaff.2010.0606. [DOI] [PubMed] [Google Scholar]
- Gould L.H., Walsh K.A., Vieira A.R., Herman K., Williams I.T., Hall A.J. Surveillance for foodborne disease outbreaks - United States, 1998-2008. Morbidity and Mortality Weekly Report. 2013;62(2):1–34. [PubMed] [Google Scholar]
- Hall G., Kirk M.D., Becker N., Gregory J.E., Unicomb L., Millard G. Estimating foodborne gastroenteritis, Australia. Emerging Infectious Diseases. 2005;11(8):1257–1264. doi: 10.3201/eid1108.041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M., Ayers T., Mahon B.E., Swerdlow D.L. Epidemiology of seafood-associated infections in the United States. Clinical Microbiology Reviews. 2010;23(2):399–411. doi: 10.1128/CMR.00059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist R., Andersson Y., de Jong B., Norberg P. A summary of reported foodborne disease incidents in Sweden, 1992 to 1997. Journal of Food Protection. 2000;63(10):1315–1320. doi: 10.4315/0362-028x-63.10.1315. [DOI] [PubMed] [Google Scholar]
- Liu X.M., Chen Y., Fan Y.X., Wang M.Q. Foodborne diseases occurred in 2003–report of the national foodborne diseases surveillance system, China. Wei Sheng Yan Jiu. 2006;35(2):201–204. [PubMed] [Google Scholar]
- Liu X.M., Chen Y., Guo Y.C., Wang Z.T. Foodborne diseases outbreaks in 2005-report of national foodborne diseases surveillance network in China. Chinese Journal of Food Hygiene. 2008;20(6):506–509. [Google Scholar]
- Liu X., Chen Y., Wang X., Ji R. Foodborne disease outbreaks in China from 1992 to 2001 national foodborne disease surveillance system. Wei Sheng Yan Jiu. 2004;33(6):725–727. [PubMed] [Google Scholar]
- Ministry of Health of the People’s Republic of China . China Standards Publishing House; Beijing: 1994. GB 14938-94 General principles of diagnostic criteria and technical management of food poisoning. [Google Scholar]
- Miyake K., Tanaka T., McNeil P.L. Lectin-based food poisoning: A new mechanism of protein toxicity. PLoS One. 2007;2(8):e687. doi: 10.1371/journal.pone.0000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Bureau of Statistics of China China statistical year book 2007. 2007. http://www.stats.gov.cn/tjsj/ndsj/2007/html/D0403e.htm
- O’Brien S.J., Elson R., Gillespie I.A., Adak G.K., Cowden J.M. Surveillance of foodborne outbreaks of infectious intestinal disease in England and Wales 1992-1999: Contributing to evidence-based food policy? Public Health. 2002;116(2):75–80. doi: 10.1038/sj.ph.1900835. [DOI] [PubMed] [Google Scholar]
- Olsen S.J., MacKinnon L.C., Goulding J.S., Bean N.H., Slutsker L. Surveillance for foodborne-disease outbreaks–United States, 1993-1997. Morbidity and Mortality Weekly Report. 2000;49(1):1–62. [PubMed] [Google Scholar]
- Raghunath P., Acharya S., Bhanumathi A., Karunasagar I. Detection and molecular characterization of Vibrio parahaemolyticus isolated from seafood harvested along the southwest coast of India. Food Microbiology. 2008;25(6):824–830. doi: 10.1016/j.fm.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Rocourt J., Moy G., Vierk K., Schlundt J. Food Safety Department, World Health Organization; 2003. The present state of foodborne disease in OECD countries.http://www.who.int/foodsafety/publications/foodborne_disease/oecd_fbd.pdf [Google Scholar]
- Scallan E., Griffin P.M., Angulo F.J., Tauxe R.V., Hoekstra R.M. Foodborne illness acquired in the United States–unspecified agents. Emerging Infectious Diseases. 2011;17(1):16–22. doi: 10.3201/eid1701.P21101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.A., Roy S.L. Foodborne illness acquired in the United States–major pathogens. Emerging Infectious Diseases. 2011;17(1):7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang S., Yu J., Zhang H., Yuan Z., Sun Y. An outbreak of Proteus mirabilis food poisoning associated with eating stewed pork balls in brown sauce, Beijing. Food Control. 2010;21(3):302–305. [Google Scholar]
- World Health Organization WHO estimates of the global burden of foodborne diseases: Foodborne disease burden epidemiology reference group 2007-2015. 2015. http://apps.who.int/iris/bitstream/10665/199350/1/9789241565165_eng.pdf?ua=1
- Wu Y., Wen J., Ma Y., Ma X., Chen Y. Epidemiology of foodborne disease outbreaks caused by Vibrio parahaemolyticus, China, 2003-2008. Food Control. 2014;46:197–202. [Google Scholar]
- Xue J., Zhang W. Understanding China’s food safety problem: An analysis of 2387 incidents of acute foodborne illness. Food Control. 2012;30(1):311–317. [Google Scholar]
- Yan W.X., Dai Y., Zhou Y.J., Liu H., Duan S.G., Han H.H. Risk factors for sporadic Vibrio parahaemolyticus gastroenteritis in east China: A matched case-control study. Epidemiology and Infection. 2015;143(5):1020–1028. doi: 10.1017/S0950268814001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Lai S., Yu J., Geng Q., Yang W., Chen Y. Etiology of acute diarrhea in the elderly in China: A six-year observational study. PLoS One. 2017;12(3):e0173881. doi: 10.1371/journal.pone.0173881. [DOI] [PMC free article] [PubMed] [Google Scholar]