Abstract
Background
Community acquired viruses (CRVs) may cause severe disease in cancer patients. Thus, efforts should be made to diagnose CRV rapidly and manage CRV infections accordingly.
Methods
A panel of 18 clinicians from the Infectious Diseases Working Party of the German Society for Haematology and Medical Oncology have convened to assess the available literature and provide recommendations on the management of CRV infections including influenza, respiratory syncytial virus, parainfluenza virus, human metapneumovirus and adenovirus.
Results
CRV infections in cancer patients may lead to pneumonia in approximately 30% of the cases, with an associated mortality of around 25%. For diagnosis of a CRV infection, combined nasal/throat swabs or washes/aspirates give the best results and nucleic acid amplification based-techniques (NAT) should be used to detect the pathogen. Hand hygiene, contact isolation and face masks have been shown to be of benefit as general infection management. Causal treatment can be given for influenza, using a neuraminidase inhibitor, and respiratory syncytial virus, using ribavirin in addition to intravenous immunoglobulins. Ribavirin has also been used to treat parainfluenza virus and human metapneumovirus, but data are inconclusive in this setting. Cidofovir is used to treat adenovirus pneumonitis.
Conclusions
CRV infections may pose a vital threat to patients with underlying malignancy. This guideline provides information on diagnosis and treatment to improve the outcome.
Keywords: Upper respiratory tract infection, Pneumonia, Superinfection, Influenza, Respiratory syncytial virus, Parainfluenza
Highlights
-
•
Community acquired viral respiratory tract infections can be life-threatening in cancer patients.
-
•
Respiratory virus infections need early and appropriate management to improve outcome and avoid outbreaks.
-
•
This guideline summarises recommendations by the AGIHO on community acquired respiratory viruses in cancer patients.
1. Introduction
The importance of community acquired respiratory virus (CRV) infections is increasingly recognised. CRV are responsible for respiratory infections, which usually present as a common cold in the immunocompetent individual but may be life-threatening in the immunocompromised host. Usually, orthomyxoviridae (influenza A, B and C), paramyxoviridae (including parainfluenza 1–4 [PIV], respiratory syncytial virus A and B [RSV], and human metapneumovirus [hMPV]), coronaviridae, picornaviridae (including >100 different serotypes of rhinovirus and enterovirus), adenoviridae, polyomavirus type 1 and bocavirus are regarded as potential causes of CRV infection. This guideline is intended to give haematologists and oncologists a broad overview with regard to clinical relevance and diagnosis of CRV infection and management of cancer patients affected by CRV. Detailed information on respective viruses including emerging resistance is not the scope of this guideline. Most data on this topic originate from patients following allogeneic stem cell transplantation (allo-SCT), and we know little about CRV infections in cancer patients outside the setting of allo-SCT. However, in recent years increasing evidence has been gathered about other cancer patients, revealing clinical relevance of CRV infections in non-transplant patients. Therefore, this guideline discusses CRV infections in all cancer patients with ongoing relevant immunosuppression. It is left to the treating physician to assess the degree and relevance of immunosuppression in the individual patient.
2. Methods
This guideline has been developed by a panel from the Infectious Diseases Working Party (AGIHO) of the German Society of Haematology and Medical Oncology including 17 experts certified in internal medicine, haematology/oncology, infectious diseases, microbiology/virology or radiology and one medical student. First, predefined topics were delivered by the designated coordinator (MvLT) to all participants of the panel to form subgroups. Data were extracted and tabulated after a systematic literature search by subgroup members and revised in several steps by the members of the panel on the basis of an email-based discussion process and a face-to-face meeting. Finally, preliminary recommendations of the panel were discussed, revised and approved by the AGIHO assembly.
In May 2014, the first literature search was performed for CRV and immunosuppression using the terms ‘-virus’ and ‘immunocompromised’ (for example: ‘adenovirus immunocompromised’). This search was performed for adenovirus, bocavirus, coronavirus, enterovirus, hMPV, influenza, PIV, parechovirus, RSV and rhinovirus. The references were then screened by the subgroup members and relevant articles retrieved as full papers. Wherever applicable, additional papers were identified in the reference lists and treated as described. In February 2016, an update of the literature search was performed.
For grading, the system applied by the European Society of Clinical Microbiology and Infectious Diseases as proposed by Ullmann et al., in 2012 [1] was used (Table 1 ) with one modification: other than Ullmann et al., we used the same grading of the strength of recommendation for diagnostic measures as for interventions (Table 1). The results of the literature search and the following grading process were used to develop recommendations wherever possible. Recommendations and evidence were then presented at and approved by the AGIHO assembly during the spring meeting on 6th March 2015. Following the update of the literature search in February 2016 no relevant changes were made.
Table 1.
Grading of evidence as suggested by the ESCMID [1].
| Strength of recommendation | |
| A | Strongly support a recommendation for use |
| B | Moderately support a recommendation for use |
| C | Marginally support a recommendation for use |
| D | Support a recommendation against use |
| Quality of evidence for interventions—level | |
| I | Evidence from at least one properly designed randomized, controlled trial |
| II* | Evidence from at least one well-designed clinical trial, without randomization; from cohort- or case-control analytic studies (preferably from more than one centre); from multiple time series; or from dramatic results from uncontrolled experiments. |
| III | Evidence from opinion of respected authorities, based on clinical experience, descriptive case studies, or report of expert committees |
| *Added index | |
| R | Meta-analysis or systematic review of randomized controlled trials |
| T | Transferred evidence, i.e. results from different patients' cohorts or similar immune status situation |
| H | Comparator group is a historical control |
| U | Uncontrolled trial |
| A | Abstract published at an international meeting |
| Quality of evidence for diagnostic measures—level | |
| I | Evidence from at least one properly designed multicentre cross-sectional or cohort study |
| II | Evidence from
|
| III | Evidence from opinion of respected authorities, based on clinical experience, descriptive case studies, or report of expert committees |
3. Diseases caused by CRV
CRVs cause respiratory tract infections, which can be divided into upper respiratory tract infection (URTI), influenza-like illness (ILI) and lower respiratory tract infection/pneumonia (LRTI). Commonly, URTI can be assumed, if a patient has a new onset of symptoms including at least one of cough, coryza, sore throat or shortness of breath which is deemed to be due to an infection by the treating physician. LRTI requires clinical or radiological evidence of pneumonia [2]. ILI is diagnosed in patients with a sudden onset of new symptoms including at least one of fever or feverishness, malaise, headache or myalgia and at least one of the respiratory symptoms cough, sore throat or shortness of breath [3]. To be certain of the viral origin, the detection of the virus from respiratory samples like swabs, nasopharyngeal aspirates or bronchoalveolar lavage fluid is required. Of note, surveillance studies showed some patients to be asymptomatic but still shedding the virus [2], [4], [5], [6], [7]. For that reason, some authors distinguish between respiratory infection (detection of virus independent of symptoms) and respiratory infection disease (detection of virus and respective symptoms) [8]. However, for the purpose of this guideline, we omit this distinction and define URTI, LRTI and ILI as described above.
4. Epidemiology and clinical relevance
Some CRV like influenza or RSV have a seasonality with most infections occurring during the winter months [2], [9], [10]. Others like rhinovirus or parainfluenza are independent of seasonality [11]. Thus, an appropriate diagnostic work-up and clinical management is warranted in any patient presenting with typical symptoms regardless of the time of the year. As may be expected considering the nature of the disease, CRV frequently cause outbreaks in health care settings [7], [12], [13], [14], [15]. Importantly, outbreaks may also occur in outpatient settings [16] emphasising the need for awareness during all periods of cancer treatment.
Generally, viral URTI in cancer patients has some impact on the clinical course because the patients are symptomatic to a degree that frequently requires postponement of chemotherapy [17]. However, critical illness and mortality due to viral URTI are rare. In contrast, most patients who died were suffering from LRTI, which thus poses the biggest threat to cancer patients. Rates of LRTI and mortality differ amongst the respective CRV [18] and exact estimation is hampered by the fact that fatal cases are probably over-reported. We have tried to deduce reliable information on LRTI and mortality from the literature for various CRV: influenza appears to have a high rate of LRTI with approximately 30% and an associated mortality rate of approximately 25% [19], [20], [21], [22]. RSV appears to be at least as dangerous with a rate of LRTI of approximately 33% and an associated mortality rate of 27% [16]. However, it has to be kept in mind, that most data regarding RSV originate from SCT-recipients and very little is known regarding patients with other forms of malignancy. On the other hand, there are several reports of outbreaks in general haematology/oncology units, which showed a significant disease burden even in patients not undergoing stem cell transplantation [12].
With regard to hMPV and PIV, exact information on the clinical relevance is even more difficult to obtain. However, although both viruses may cause asymptomatic infection [6], [23], the available evidence suggests a similar overall rate of LRTI and mortality [7], [11], [13], [24], [25], [26], [27], [28] compared with influenza and RSV. In contrast, despite case reports of fatal outcomes of infections with rhinovirus and coronavirus, these viruses as well as bocavirus appear to be rarely the cause of LRTI and dangerous only when patients are coinfected with other pathogens [2], [5], [29]. Herpesviridae like herpes simplex virus, human herpes virus 6, cytomegalovirus, varicella zoster and Epstein–Barr virus as well as polyomaviruses or parechoviruses usually do not cause CRV infection. Pneumonia due to reactivation of herpesviridae in severely immunocompromised patients is not an infection by CRV and thus not covered by this guideline.
In CRV infection, coinfection with bacteria, fungi or even other viruses appears to occur in approximately 30% of the patients [10], [11], [28]. They play a vital role with regard to outcome of patients since patients with bacterial or fungal superinfection have a dramatically higher mortality rate than those with viral infection only [11], [28]. Therefore, possible co- or superinfection should be considered when managing cancer patients with CRV. In addition to LRTI and bacterial or fungal superinfection, other risk factors for adverse outcome include haematological malignancy [22], severe immunosuppression such as steroid use or graft versus host disease or cytopenias [30], [31], [32] or low immunoglobulins [12].
Epidemiology of adenoviruses is somewhat different from the other CRV: often, the source of infection is a childhood infection in children under 5 years [33] and reactivation as well as new infection have been described to be the cause of disease [34], [35]. Other than CRVs like RSV or influenza, adenoviruses can cause a variety of symptoms such as conjunctivitis, haemorrhagic cystitis, gastroenteritis, and URTI in immunocompetent patients and hepatitis, colitis, nephritis, meningoencephalitis and LRTI in the immunocompromised host. In adult allo-SCT recipients, DNAemia occurs in up to 20% [36], [37], [38], but symptomatic disease by adenovirus is much less common with T-cell suppression being the predominant risk factor [36]. Again, coinfection is an important risk factor for severe illness [39].
5. Diagnosis of CRV infection
5.1. Virology—material
Cancer patients presenting with symptoms consistent with CRV infection should be diagnosed using material from the respiratory tract (Table 2 ). Serology is not useful to detect ongoing CRV infection and thus not recommended (D III). Regarding material used for microbiological diagnosis, a variety of approaches are used in various centres. As a general rule, a close collaboration with the local microbiology laboratory is highly recommended because this may determine which material should be used preferably since commercially available test kits are licensed for specific materials. For example testing for viral antigens usually requires more thorough sampling like combined nasal/throat swabs than testing for viral nucleic acids which can often be performed reliably on gargles alone. It is therefore essential for the clinician to be aware of the tests used in the respective laboratory. The overall evidence in the literature is best for combined nasal/throat swabs and nasopharyngeal aspirates (A IIt, Table 2).
Table 2.
Recommendations regarding diagnostic approaches in cancer patients with symptoms of CRV infection.
| Population | Intention | Intervention | SoR | QoE | Reference |
|---|---|---|---|---|---|
| Symptomatic IS | To detect viral pathogen and diagnose infection | Serology | D | III | [94] |
| Symptomatic IS | To detect viral pathogen | Combined nasal/throat swabs or washes/aspirates | A | II | [95], [96], [97], [98], [99], [100], [101], [102], [103] |
| Symptomatic IS | To detect viral pathogen | NAT | A | II | [42], [94], [104], [105], [106], [107], |
| Symptomatic IS | To detect LRTI in patients with CRV infection | Chest X-ray | D | II | [108] |
| Symptomatic IS | To detect LRTI in patients with CRV infection | CT scan | A | II | [44], [46], [47], [69], [108], [109], [110], [111], [112], [113], [114], [115], |
SoR, strength of recommendation; QoE, quality of evidence; IS, immunosuppressed cancer patients; NAT, nucleic acid amplification techniques; LRTI, lower respiratory tract infection; CRV, community acquired respiratory virus; CT, computer tomography.
5.2. Virology—test
The best evidence for reliable detection of a CRV present in respiratory samples exists for nucleic acid amplification based-techniques (NAT) like PCR. Therefore, the use of NAT is highly recommended (A II, Table 2) and any methods involving the detection of antigen appear to be second best in immunosuppressed cancer patients (C II [40], [41], [42]). Also, culture methods are not commonly used anymore and cannot be recommended for general diagnosis, but they are essential in individual cases in which no known virus can be detected or results of PCR-analysis are inconclusive (A II, [43]). In the era of multiplex-test kits, it is difficult to make a definite recommendation with regard to which viruses should be looked for. In the absence of any reliable data regarding this question, the panel feels that it is wise to search for influenza, RSV, PIV and viruses currently prevalent in the local environment in all immunosuppressed cancer patients presenting with symptoms. Patients with more severe disease (for example pneumonia or critical illness) may have the panel broadened to include hMPV and adenovirus and even viruses that only rarely cause LRTI like rhinovirus and coronavirus. However, evidence for this approach is low and it is strongly advisable to define local guidelines on this topic. It should be kept in mind, that herpesviridae are not the cause of CRV infection. Therefore, there is no rationale to look for cytomegalovirus, Epstein–Barr virus, herpes simplex virus or varicella zoster in patients with typical symptoms of CRV infection only.
5.3. Radiology
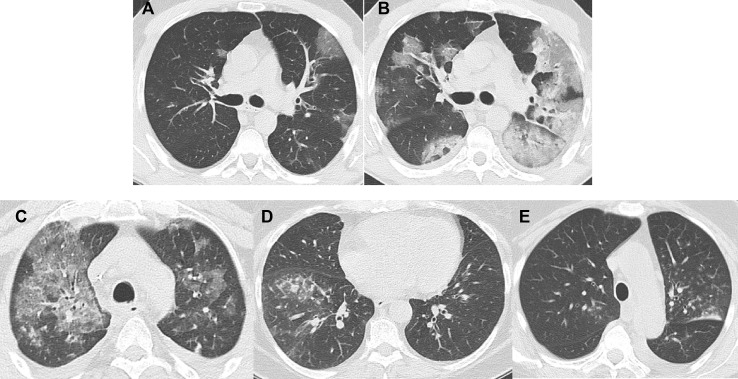
In patients with symptoms of LRTI, it is essential to determine the degree of pulmonary involvement. CRV can affect the tracheobronchial system or the lung parenchyma [44]. Generally, a chest X-ray has been proven to be unhelpful to diagnose pathologic changes in this setting because of lack of sensitivity. It is therefore not recommended (D II, see Table 2). In contrast, there is good evidence to recommend a CT scan of the chest to detect LRTI in patients with CRV infection (A II, see Table 2). Bronchial wall thickening as well as interstitial infiltrates presenting as ground-glass opacities may be detected. These are defined as increased lung density, whereas underlying lung architecture is still detectable. Ground-glass opacities may be patchy or diffuse [44], [45], [46], [47] and can be well distinguished from consolidations, which show higher density obscuring e.g. the pulmonary vessels and which are typical for other differential diagnoses including bacterial infection. Affection of the terminal bronchioles might lead to visibility of those usually invisible structures at CT as small centrilobular nodules or ‘tree-in-bud’ sign [45] or evidence of bronchiolitis causing air-trapping. To reliably detect air-trapping while inspiratory CT is normal, a CT scan in expiration is necessary [44]. In addition to good diagnostic accuracy with regard to the diagnosis of a viral LRTI, the CT scan may also reveal evidence for an outbreak, since specific viruses tend to present with a typical pattern in the CT scan [45]. For an example see Fig. 1 .
Fig. 1.
A–B: pneumonia caused by influenza, first CT scan (A) and follow-up scan after 4 d (B). The bilateral diffuse ground-glass opacities progress over time to cover most parts of the lung. In addition, consolidations with positive bronchopneumogram develop, indicative of possible bacterial superinfection. C–E: CT scans from three different patients with pneumonia caused by RSV. Again, ground-glass opacities can be found but are of a more patchy character (Fig. 1C). They are often combined with centrilobular nodules (tree-in-bud, Fig. 1D). In some cases, only nodules with a ground-glass character are detected (Fig. 1E). RSV, respiratory syncytial virus.
6. Management of CRV infection
6.1. Infection control
In the light of the danger of outbreaks with fatal consequences, the most important measure in the management of cancer patients with CRV infection is infection control (Table 3 ). Local authorities should give exact guidance on the necessary precautions in the respective institutions. The following statements intend to give a general overview.
Table 3.
Recommendations regarding general management of cancer patients with CRV.
| Population | Intention | Intervention | SoR | QoE | Reference |
|---|---|---|---|---|---|
| IS, infected persons, Contact persons | Infection control—prevent transmission | Hand hygiene | A | IIt | [49], [116] |
| IS, Infected persons, Contact persons | Infection control—prevent transmission | Face mask | B | IIt | [49], [116] |
| Infected persons | Infection control—prevent outbreak | Contact isolation | A | III | [117] |
| allo-SCT and evidence of CRV | Prevent disease, improve survival | Delay conditioning | A | II | [17] |
| All other chemotherapy and CRV | Prevent disease, improve survival | Delay chemotherapy if possible | C | III | [61] |
| allo-SCT and LRTI due to adenovirus | Prevent disease, shorten duration | Reduce immunosuppression | A | II | [36], [62] |
| allo-SCT and LRTI due to CRV | Prevent disease, shorten duration | Reduce immunosuppression | A | IIt | [36], [62] |
| allo-SCT and URTI | Prevent disease, shorten duration | Reduce immunosuppression | C | III | |
| IS with evidence of CRV | Reduce morbidity | Steroids >2 mg/kg | D | III | [10] |
| IS with evidence of RSV | Prevent LRTI, improve survival | IVIG | B | III | [30], [82] |
| IS with evidence of influenza, PIV, hMPV | prevent LRTI, improve survival | IVIG | C | III | [118], [119], [92], [69] |
SoR, strength of recommendation; QoE, quality of evidence; IS, immunosuppressed cancer patients; URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection; CRV, community acquired respiratory virus; IVIG intravenous immunoglobulins; allo-SCT, allogeneic stem cell transplantation; RSV, respiratory syncytial virus; PIV, parainfluenza virus; hMPV, human metapneumovirus.
There is sufficient evidence to recommend stringent hand hygiene (A IIt), the use of face masks (B IIt) and contact isolation (A III, Table 3). Importantly, shedding of CRV in cancer patients often lasts 2 weeks or longer [5], [10], [48]. It is therefore wise to perform follow-up testing of respiratory material in index patients and stop contact isolation only when they became negative. Of note, early implementation of infection control appears to be more effective than late implementation [49] which gives reason to recommend infection control as soon as symptoms appear and not only after evidence of CRV.
6.2. Supportive measures
Almost anybody who catches a cold applies some form of home remedies convinced that they ease the symptoms and positively influence the course of the disease. In contrast to this widespread use, there is very little evidence to recommend any such measures. In particular with regard to cancer patients, evidence is too poor to give a sound recommendation in favour of the use of vitamin C [50], echinacea [51], garlic [52], zinc [53], humidified hot air [54] or Chinese herbal medicine [55]. Surprisingly, even painkillers [56] and non-steroidal anti-inflammatory drugs [57] may ease the pain but have little influence on severity and duration of the CRV infection. However, as there is some evidence towards a considerable placebo effect [58], it can be argued that patients should be allowed to continue their home remedies provided there is no reason to assume harmfulness as may be the case for some Chinese herbal medicines contaminated with heavy metals [59] or the possibility to contract invasive fungal infection from inhaling contaminated air.
Needless to say, there is little to be gained from treating a viral infection with antibiotics [60], which is also true for cancer patients (D IIr,t for treating viral infections with antibiotics). However, having in mind the high rate of superinfection, cancer patients with viral LRTI and suspected or proven bacterial/fungal coinfection have to be treated accordingly (A III).
In cancer patients who present with symptoms consistent with CRV infection prior to initiation of chemotherapy, delaying treatment should be considered. Since a retrospective study with 2 groups showed a benefit, if treatment was delayed in patients undergoing allo-SCT, we clearly recommend delaying conditioning in those patients who are scheduled for allo-SCT and have evidence of CRV infection (A II, see Table 3). The situation is less clear for patients with less aggressive treatment, since there have been reports of uneventful courses of even high-dose chemotherapy in patients with initial CRV infection [61]. Therefore, the recommendation to delay the chemotherapy if possible to be on the safe side is merely a weak one (Table 3). Similarly, reducing immunosuppression in patients with LRTI due to adenovirus-infection after allo-SCT is well founded [36], [62] on retrospective cohort studies with 2 groups and is thus clearly recommended (A II, Table 3). Data can be transferred to the situation of other LRTI caused by CRV, therefore, reduction of immunosuppression is also recommended in allo-SCT recipients with LRTI caused by other CRV (A IIt, Table 3). In contrast, the situation is less clear in patients with URTI and therefore only a very weak recommendation can be made (C III, Table 3).
With regard to supportive application of systemic medication other than antivirals, no recommendation can be made for the use of steroids, since they show no effect and prolong viral shedding (D III, Table 3). In contrast, intravenous immunoglobulins (IVIGs) are a therapeutic option in RSV infection (B III) and may also be beneficial in influenza, PIV and hMPV infection (C III, Table 3). Because of lack of data, no recommendation can be made regarding the use of IVIG in other CRV infections.
7. Causal treatment
7.1. Influenza
If causal treatment was deemed necessary, influenza A was traditionally treated with amantadine or rimantadine. Nowadays, resistance rates are so high that neither can be recommended (D II [63], [64], [65], [66]). In contrast, despite ongoing discussion regarding the balance of efficacy and side effects [67], [68] the treatment of choice appears to be a neuraminidase inhibitor, be it oseltamivir, zanamivir or peramivir. They are recommended as prophylaxis as well as for treatment (for example http://www.rki.de/DE/Content/InfAZ/I/Influenza/IPV/IPV_Node.html or [63], [69], [70]). However, data regarding the efficacy of prophylactic use of neuraminidase inhibitors in cancer patients are very weak and almost exclusively in the setting of stem cell transplantation [71], [72]. Therefore, the authors believe it is not justified to give any recommendation in favour of or against their use in cancer patients in general. Thus, prevention of influenza by application of neuraminidase inhibitors remains one of the unresolved issues requiring further study. Still, we have included information on dose and duration in Table 5 .
Table 5.
Information on specific drugs.
| Name | Class | Indication | Dose | Application mode | Duration | Comment | Reference |
|---|---|---|---|---|---|---|---|
| Oseltamivir | Neuraminidase inhibitor | Prophylaxis influenza | 75 mg/d | Oral | As needed in seasonal prophylaxis; 10d in post-exposure prophylaxis | Caveat: data too weak to make a recommendation, local strategies needed | [63], [69], [71] |
| Oseltamivir | Neuraminidase inhibitor | Treatment influenza | 2 × 75-150 mg/d | Oral | 5–10 d | [10], [74], [76], [77] | |
| Zanamivir | Neuraminidase inhibitor | Prophylaxis influenza | 10 mg/d | Inhalation | As needed in seasonal prophylaxis; 10d in post-exposure prophylaxis | Caveat: data too weak to make a recommendation, local strategies needed | [63], [69] |
| Zanamivir | Neuraminidase inhibitor | Treatment influenza | 2 × 10 mg/d | Inhalation | Until negativity | [75] | |
| Peramivir | Neuraminidase inhibitor | Treatment influenza | 600 mg/d | Intravenous | Not available in Germany | [78] | |
| Ribavirin | Nucleoside inhibitor | Treatment RSV, PIV, hMPV | Daily dose: 2 g for 2 h every 6 h or 6 g over 18 h | Inhalation | 7–10 d | Be aware of potential teratogenic effect—special precautions needed | [82] |
| Ribavirin | Nucleoside inhibitor | Treatment RSV, PIV, hMPV | Different schedulesa | Oral | Be aware of potential hepatic and renal toxicity, haemolysis | [12], [30], [80], [81], [82], [86], [87] | |
| Ribavirin | Nucleoside inhibitor | Treatment RSV, PIV, hMPV | 10–30 mg/kg/d | Intravenous | Be aware of potential hepatic and renal toxicity, haemolysis | [87] | |
| Cidofovir | DNA polymerase inhibitor | Treatment adenovirus | Cidofovir 3–5 mg/kg iv once weekly for 2 weeks, then once every week | Intravenous | To reduce cidofovir toxicity, add at least 2 l of iv Prehydration and probenecid 2 g po 3 h prior and 1 g 2 and 8 h following cidofovir | [120], [121], [122], [123], [124] |
RSV, respiratory syncytial virus; PIV, parainfluenza virus; hMPV, human metapneumovirus.
For example: loading dose: 10 mg/kg, then 3 × 400 mg d2, 3 × 600 mg from d3 [30]; 1800 mg/d [87]; <65 kg body weight: 2 × 400 mg/d; 65–80 kg body weight: 2 × 500 mg/d; >80 kg body weight: 2 × 600 mg/d [12]; <75 kg body weight: 2 × 600 mg/d and ≥75 kg body weight: 2 × 800 mg/d [81]; 20 mg/kg/d in four divided doses increasing every 24–48 h to 60 mg/kg/d in four divided doses, if tolerated [86].
Treatment of influenza is usually recommended in symptomatic patients at high risk preferably <48h after the onset of symptoms [63]. Cancer patients might be regarded as high-risk patients per se, which is why neuraminidase inhibitors (usually oseltamivir) are often routinely given to patients with malignancies and influenza [69], [70], [73], since mostly retrospective data show a benefit of (early) antiviral treatment with regard to development of LRTI or further complications [10], [20], [74], [75], [76]. Thus, we do recommend the use of oseltamivir or zanamivir (B II, Table 4, Table 5). There is evidence to recommend early initiation of treatment, but that does not necessarily mean later treatment is futile [77] and therefore many authors recommend treatment regardless of timepoint [70]. However, we do not think either dose (150–300 mg/d) nor duration (5–10 d or longer) are well defined from the available evidence and need determination by local specialists. The reasons usually given for higher doses and longer treatment duration in high-risk patients is the prolonged viral shedding observed in cancer patients and a thus deduced susceptibility to develop resistances if not treated effectively [69], [70].
Table 4.
Recommendations regarding causal treatment of influenza, RSV, parainfluenza and adenovirus.
| Population | Intention | Intervention | SoR | QoE | Reference |
|---|---|---|---|---|---|
| IS and influenza | Shorten duration and prevent LRTI | Oseltamivir | B | II | [10], [74], [76], [77] |
| IS and influenza | Shorten duration and prevent LRTI | Zanamivir | B | II | [75], [76] |
| IS and RSV | Prevent LRTI and improve survival | Ribavirin | B | II | [12], [30], [80], [81], [82], [86], [87] |
| IS and PIV | Prevent LRTI and improve survival | Ribavirin | C | III | [11], [24], [79], [86], [88], [89] |
| Adenovirus-associated pneumonitis | Cure | Cidofovir | B | II | [120], [121], [122] |
SoR, strength of recommendation; QoE, quality of evidence; IS, immunosuppressed cancer patients; LRTI, lower respiratory tract infection; CRV, community acquired respiratory virus; RSV, respiratory syncytial virus; PIV parainfluenza virus.
Peramivir has received FDA approval during the 2009 pandemic and is recommended for patients with H1N1 infection unable to take oral medication [78]. It is not available in Germany but included in this guideline for the sake of completeness (Table 5). The authors advise its use in severe cases, when oral intake or inhalation is not possible (CIII). Another salvage option may be the combination of zanamivir or oseltamivir with ribavirin, since that has shown some efficacy in older studies [79].
7.2. RSV
RSV is usually treated with intravenous immunoglobulins (IVIG, B III, Table 3) and ribavirin. In Europe, the monoclonal antibody palivizumab is licensed for prevention of RSV in children only. In addition, the benefit over polyclonal IVIG is not entirely conclusive [80]. For these reasons, we do not make a clear recommendation for or against its use in cancer patients, since we regard the question whether to use palivizumab instead of IVIG as an unresolved question requiring further study.
Ribavirin is the agent of choice in the treatment of RSV infection. Most available data concern allo-SCT recipients [80], but recent evidence also suggests a benefit in less severely immunosuppressed cancer patients [12], [81]. It appears to lower the progression rate to LRTI [82] and is reported to have a positive influence on survival [12]. However, some authors report favourable outcome of RSV infections without any causal treatment [61], [83], [84]. Traditionally, it is used as an aerosol (see Table 5), but this application mode is cumbersome and may be associated with a teratogeneic effect [85]. Also, patients may not be able to inhale for such a long time or they may react with bronchospasm. Thus, oral application has been used increasingly with a similar efficacy [12], [30], [81], [86], [87] and even intravenous application is reported [87]. Despite some reports with a good outcome without treatment, we believe the available evidence justifies a recommendation for the use of ribavirin in cancer patients with RSV infection (B II, Table 4). Also, at least in high-risk patients the treatment should be given at the stage of URTI, since this has shown a benefit (B II [82]).
7.3. Parainfluenza (PIV)
Experience with antiviral therapy (generally ribavirin) in patients with parainfluenza infection is not very large and the efficacy is not entirely convincing [11], [24], [79], [86], [88], [89]. This may be partly because causal treatment is started too late in the course of the disease and partly because the cause of death often is a coinfection requiring antibiotic therapy [7], [28]. Nonetheless, it may be reasonable to attempt therapy with ribavirin in patients with parainfluenza infection (C III, Table 4).
7.4. Adenovirus
Causal therapy with cidofovir is justified in immunosuppressed cancer patients with LRTI caused by adenovirus (B II, Table 4, Table 5). More experimental therapies, which are employed in the setting of allo-SCT include donor-lymphocyte infusions [90] or adaptive transfer of specific T-cells [91]. However, to date evidence is too weak to justify a recommendation in favour of or against the use of these treatment modalities.
7.5. Human metapneumovirus (hMPV), rhinovirus, coronavirus and others
Causal therapy with ribavirin has been attempted in patients with infections caused by hMPV [92], [93], albeit with unconvincing results. There is not enough evidence to make a definitive recommendation for or against the use of any specific antiviral drug or other causal treatment approaches like interferon for any of the CRV other than the ones discussed above.
8. Conclusion and outlook
Early diagnosis and general infection prevention may improve the outcome of cancer patients with CRV infections. Despite some data regarding some viruses (influenza, RSV) and patient populations (HSCT-recipients), there is still a lack of information on most CRV and on other patient populations (for example those with solid tumours). Also, almost no prospective randomised trials have been performed for the treatment of CRV infections in cancer patients. Thus, most recommendations have to be deduced from other populations and further study is urgently needed.
Funding
None. Travel expenses and costs for group meetings were reimbursed by the German Society for Haematology and Medical Oncology.
Conflict of interest statement
MvLT has received honoraria and travel support from Gilead, MSD, Pfizer, Celgene and Janssen Cilag, has received travel support from Astellas Pharma and has received research support from MSD. She is member of the advisory board to MSD.
MC has received research funding from Deutsche Forschungsgemeinschaft (DFG) and Erich und Gertrud Roggenbuck Stiftung, been a speaker for MSD and Basilea, has been a consultant for MSD and Basilea, received travel grants from Celgene, Takeda, Gilead and MSD and is a recipient of the MSD stipend oncology 2013.
MH served on advisory boards of Gilead, Roche Pharma and Takeda and served on the speakers' bureau for Celgene, Novartis, Janssen and Amgen.
CPH is a stock owner of Stada and GSK and has received consultation fees and/or honoraria from Schering-Plough, Pfizer, Basilea, Boehringer Ingelheim, Novartis, Roche, Astellas, Gilead, MSD, Lilly, Intermune, Fresenius, Olympus, Gilead, AstraZeneca, Bracco, MEDA Pharma, Chiesi, Siemens, Covidien, Pierre Fabre, Grifols and research funding from Siemens, Pfizer, MeVis and Boehringer Ingelheim.
MK has received honoraria and travel support and served on the speakers' bureau for Gilead, MSD, Pfizer.
OP has received honoraria and travel support from Astellas, Gilead, Jazz, MSD, Neovii Biotech and Pfizer. He has received research support from Bio Rad, Gilead, Jazz, Neovii Biotech, Pierre Fabre, Sanofi and Takeda. He is member of the advisory board to Alexion, Jazz, Gilead and MSD.
MJGTV is a consultant to: Berlin Chemie, MSD/Merck and Astellas Pharma; has served at the speakers' bureau of: Pfizer, Merck/MSD, Gilead Sciences, Organobalance and Astellas Pharma; received research funding from: 3M, Astellas Pharma, DaVolterra and Gilead Sciences.
GS has received grant/research support from: MSD Sharp & Dohme GmbH, Haar, Germany, Pfizer, Berlin, Germany, GILEAD Sciences, Martinsried, Germany and Astellas Pharma GmbH, München, Germany. She has served as a consultant to: MSD Sharp & Dohme GmbH, Haar, Germany and Basilea Pharmaceutical Internatio Ltd; Switzerland.
All remaining authors have declared no conflicts of interest.
Acknowledgements
The authors thank Ramona Kraft for technical assistance with the retrieval of full papers.
References
- 1.Ullmann A.J., Cornely O.A., Donnelly J.P., Akova M., Arendrup M.C., Arikan-Akdagli S. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: developing European guidelines in clinical microbiology and infectious diseases. Clin Microbiol Infect. 2012;18(Suppl. 7):1–8. doi: 10.1111/1469-0691.12037. [DOI] [PubMed] [Google Scholar]
- 2.Mikulska M., Del Bono V., Gandolfo N., Dini S., Dominietto A., Di Grazia C. Epidemiology of viral respiratory tract infections in an outpatient haematology facility. Ann Hematol. 2014;93(4):669–676. doi: 10.1007/s00277-013-1912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control. ECDC Influenza case definitions. http://ecdc.europa.eu/en/healthtopics/influenza/surveillance/Pages/influenza_case_definitions.aspx. [Accessed 13 May 2016].
- 4.Shachor-Meyouhas Y., Zaidman I., Kra-Oz Z., Arad-Cohen N., Kassis I. Detection, control, and management of a respiratory syncytial virus outbreak in a pediatric hematology-oncology department. J Pediatr Hematol Oncol. 2013;35(2):124–128. doi: 10.1097/MPH.0b013e3182756edc. [DOI] [PubMed] [Google Scholar]
- 5.Milano F., Campbell A.P., Guthrie K.A., Kuypers J., Englund J.A., Corey L. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood. 2010;115(10):2088–2094. doi: 10.1182/blood-2009-09-244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peck A.J., Englund J.A., Kuypers J., Guthrie K.A., Corey L., Morrow R. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood. 2007;110(5):1681–1688. doi: 10.1182/blood-2006-12-060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvala H., Gaunt E., McIntyre C., Roddie H., Labonte S., Curran E. Epidemiology and clinical characteristics of parainfluenza virus 3 outbreak in a Haemato-oncology unit. J Infect. 2012;65(3):246–254. doi: 10.1016/j.jinf.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch H.H., Martino R., Ward K.N., Boeckh M., Einsele H., Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56(2):258–266. doi: 10.1093/cid/cis844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall C.B. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 10.Nichols W.G., Guthrie K.A., Corey L., Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39(9):1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 11.Nichols W.G., Corey L., Gooley T., Davis C., Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98(3):573–578. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 12.Lehners N., Schnitzler P., Geis S., Puthenparambil J., Benz M.A., Alber B. Risk factors and containment of respiratory syncytial virus outbreak in a hematology and transplant unit. Bone Marrow Transplant. 2013;48(12):1548–1553. doi: 10.1038/bmt.2013.94. [DOI] [PubMed] [Google Scholar]
- 13.Hoellein A., Hecker J., Hoffmann D., Gottle F., Protzer U., Peschel C. Serious outbreak of human metapneumovirus in patients with hematologic malignancies. Leuk Lymphoma. 2015:1–5. doi: 10.3109/10428194.2015.1067699. [DOI] [PubMed] [Google Scholar]
- 14.Abdallah A., Rowland K.E., Schepetiuk S.K., To L.B., Bardy P. An outbreak of respiratory syncytial virus infection in a bone marrow transplant unit: effect on engraftment and outcome of pneumonia without specific antiviral treatment. Bone Marrow Transplant. 2003;32(2):195–203. doi: 10.1038/sj.bmt.1704116. [DOI] [PubMed] [Google Scholar]
- 15.Jones B.L., Clark S., Curran E.T., McNamee S., Horne G., Thakker B. Control of an outbreak of respiratory syncytial virus infection in immunocompromised adults. J Hosp Infect. 2000;44(1):53–57. doi: 10.1053/jhin.1999.0666. [DOI] [PubMed] [Google Scholar]
- 16.Chu H.Y., Englund J.A., Podczervinski S., Kuypers J., Campbell A.P., Boeckh M. Nosocomial transmission of respiratory syncytial virus in an outpatient cancer center. Biol Blood Marrow Transplant. 2014;20(6):844–851. doi: 10.1016/j.bbmt.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peck A.J., Corey L., Boeckh M. Pretransplantation respiratory syncytial virus infection: impact of a strategy to delay transplantation. Clin Infect Dis. 2004;39(5):673–680. doi: 10.1086/422994. [DOI] [PubMed] [Google Scholar]
- 18.Ljungman P., Ward K.N., Crooks B.N., Parker A., Martino R., Shaw P.J. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28(5):479–484. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 19.Ljungman P., de la Camara R., Perez-Bercoff L., Abecasis M., Nieto Campuzano J.B., Cannata-Ortiz M.J. Outcome of pandemic H1N1 infections in hematopoietic stem cell transplant recipients. Haematologica. 2011;96(8):1231–1235. doi: 10.3324/haematol.2011.041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chemaly R.F., Vigil K.J., Saad M., Vilar-Compte D., Cornejo-Juarez P., Perez-Jimenez C. A multicenter study of pandemic influenza A (H1N1) infection in patients with solid tumors in 3 countries: early therapy improves outcomes. Cancer. 2012;118(18):4627–4633. doi: 10.1002/cncr.27447. [DOI] [PubMed] [Google Scholar]
- 21.Choi S.M., Xie H., Campbell A.P., Kuypers J., Leisenring W., Boudreault A.A. Influenza viral RNA detection in blood as a marker to predict disease severity in hematopoietic cell transplant recipients. J Infect Dis. 2012;206(12):1872–1877. doi: 10.1093/infdis/jis610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnell D., Mayaux J., de Bazelaire C., Legoff J., Feuillet S., Scieux C. Risk factors for pneumonia in immunocompromised patients with influenza. Respir Med. 2010;104(7):1050–1056. doi: 10.1016/j.rmed.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Debiaggi M., Canducci F., Sampaolo M., Marinozzi M.C., Parea M., Terulla C. Persistent symptomless human metapneumovirus infection in hematopoietic stem cell transplant recipients. J Infect Dis. 2006;194(4):474–478. doi: 10.1086/505881. [DOI] [PubMed] [Google Scholar]
- 24.Marcolini J.A., Malik S., Suki D., Whimbey E., Bodey G.P. Respiratory disease due to parainfluenza virus in adult leukemia patients. Eur J Clin Microbiol Infect Dis. 2003;22(2):79–84. doi: 10.1007/s10096-002-0864-4. [DOI] [PubMed] [Google Scholar]
- 25.Williams J.V., Martino R., Rabella N., Otegui M., Parody R., Heck J.M. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis. 2005;192(6):1061–1065. doi: 10.1086/432732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renaud C., Xie H., Seo S., Kuypers J., Cent A., Corey L. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant. 2013;19(8):1220–1226. doi: 10.1016/j.bbmt.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debur M.C., Vidal L.R., Stroparo E., Nogueira M.B., Almeida S.M., Takahashi G.A. Human metapneumovirus infection in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2010;12(2):173–179. doi: 10.1111/j.1399-3062.2009.00465.x. [DOI] [PubMed] [Google Scholar]
- 28.Ustun C., Slaby J., Shanley R.M., Vydra J., Smith A.R., Wagner J.E. Human parainfluenza virus infection after hematopoietic stem cell transplantation: risk factors, management, mortality, and changes over time. Biol Blood Marrow Transplant. 2012;18(10):1580–1588. doi: 10.1016/j.bbmt.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parody R., Rabella N., Martino R., Otegui M., del Cuerpo M., Coll P. Upper and lower respiratory tract infections by human enterovirus and rhinovirus in adult patients with hematological malignancies. Am J Hematol. 2007;82(9):807–811. doi: 10.1002/ajh.20974. [DOI] [PubMed] [Google Scholar]
- 30.Khanna N., Widmer A.F., Decker M., Steffen I., Halter J., Heim D. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin Infect Dis. 2008;46(3):402–412. doi: 10.1086/525263. [DOI] [PubMed] [Google Scholar]
- 31.Shah D.P., Ghantoji S.S., Ariza-Heredia E.J., Shah J.N., El Taoum K.K., Shah P.K. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123(21):3263–3268. doi: 10.1182/blood-2013-12-541359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo S., Xie H., Campbell A.P., Kuypers J.M., Leisenring W.M., Englund J.A. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis. 2014;58(10):1357–1368. doi: 10.1093/cid/ciu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper R.J., Hallett R., Tullo A.B., Klapper P.E. The epidemiology of adenovirus infections in Greater Manchester, UK 1982–96. Epidemiol Infect. 2000;125(2):333–345. doi: 10.1017/s0950268899004550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Tol M.J., Kroes A.C., Schinkel J., Dinkelaar W., Claas E.C., Jol-van der Zijde C.M. Adenovirus infection in paediatric stem cell transplant recipients: increased risk in young children with a delayed immune recovery. Bone Marrow Transplant. 2005;36(1):39–50. doi: 10.1038/sj.bmt.1705003. [DOI] [PubMed] [Google Scholar]
- 35.Veltrop-Duits L.A., van Vreeswijk T., Heemskerk B., Thijssen J.C., El Seady R., Jol-van der Zijde E.M. High titers of pre-existing adenovirus serotype-specific neutralizing antibodies in the host predict viral reactivation after allogeneic stem cell transplantation in children. Clin Infect Dis. 2011;52(12):1405–1413. doi: 10.1093/cid/cir231. [DOI] [PubMed] [Google Scholar]
- 36.Chakrabarti S., Mautner V., Osman H., Collingham K.E., Fegan C.D., Klapper P.E. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100(5):1619–1627. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- 37.Munoz-Cobo B., Solano C., Nieto J., de la Camara R., Remigia M.J., Garcia-Noblejas A. Surveillance for adenovirus DNAemia early after transplantation in adult recipients of unrelated-donor allogeneic stem cell transplants in the absence of clinically suspected infection. Bone Marrow Transplant. 2011;46(11):1484–1486. doi: 10.1038/bmt.2010.322. [DOI] [PubMed] [Google Scholar]
- 38.Ohrmalm L., Lindblom A., Omar H., Norbeck O., Gustafson I., Lewensohn-Fuchs I. Evaluation of a surveillance strategy for early detection of adenovirus by PCR of peripheral blood in hematopoietic SCT recipients: incidence and outcome. Bone Marrow Transplant. 2011;46(2):267–272. doi: 10.1038/bmt.2010.86. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy T., Lebeck M.G., Capuano A.W., Schnurr D.P., Gray G.C. Molecular typing of clinical adenovirus specimens by an algorithm which permits detection of adenovirus coinfections and intermediate adenovirus strains. J Clin Virol. 2009;46(1):80–84. doi: 10.1016/j.jcv.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drexler J.F., Helmer A., Kirberg H., Reber U., Panning M., Muller M. Poor clinical sensitivity of rapid antigen test for influenza A pandemic (H1N1) 2009 virus. Emerg Infect Dis. 2009;15(10):1662–1664. doi: 10.3201/eid1510.091186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ebihara T., Endo R., Ma X., Ishiguro N., Kikuta H. Detection of human metapneumovirus antigens in nasopharyngeal secretions by an immunofluorescent-antibody test. J Clin Microbiol. 2005;43(3):1138–1141. doi: 10.1128/JCM.43.3.1138-1141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camps Serra M., Cervera C., Pumarola T., Moreno A., Perello R., Torres A. Virological diagnosis in community-acquired pneumonia in immunocompromised patients. Eur Respir J. 2008;31(3):618–624. doi: 10.1183/09031936.00073807. [DOI] [PubMed] [Google Scholar]
- 43.Costa C., Libertucci D., Solidoro P., Sinesi F., Bergallo M., Margio S. Rapid shell vial culture for the detection of respiratory viruses from bronchoalveolar lavage in immunocompromised patients. Panminerva Med. 2007;49(1):1–6. [PubMed] [Google Scholar]
- 44.Kim E.A., Lee K.S., Primack S.L., Yoon H.K., Byun H.S., Kim T.S. Viral pneumonias in adults: radiologic and pathologic findings. Radiographics. 2002;22 doi: 10.1148/radiographics.22.suppl_1.g02oc15s137. Spec No:S137–49. [DOI] [PubMed] [Google Scholar]
- 45.Mayer J.L., Lehners N., Egerer G., Kauczor H.U., Heussel C.P. CT-morphological characterization of respiratory syncytial virus (RSV) pneumonia in immune-compromised adults. Rofo. 2014;186(7):686–692. doi: 10.1055/s-0033-1356353. [DOI] [PubMed] [Google Scholar]
- 46.Franquet T., Rodriguez S., Martino R., Gimenez A., Salinas T., Hidalgo A. Thin-section CT findings in hematopoietic stem cell transplantation recipients with respiratory virus pneumonia. AJR Am J Roentgenol. 2006;187(4):1085–1090. doi: 10.2214/AJR.05.0439. [DOI] [PubMed] [Google Scholar]
- 47.Franquet T., Rodriguez S., Martino R., Salinas T., Gimenez A., Hidalgo A. Human metapneumovirus infection in hematopoietic stem cell transplant recipients: high-resolution computed tomography findings. J Comput Assist Tomogr. 2005;29(2):223–227. doi: 10.1097/01.rct.0000157087.14838.4c. [DOI] [PubMed] [Google Scholar]
- 48.Lehners N., Tabatabai J., Prifert C., Wedde M., Puthenparambil J., Weissbrich B. Long-term shedding of influenza virus, parainfluenza virus, respiratory syncytial virus and nosocomial epidemiology in patients with hematological disorders. PLoS One. 2016;11(2):e0148258. doi: 10.1371/journal.pone.0148258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cowling B.J., Chan K.H., Fang V.J., Cheng C.K., Fung R.O., Wai W. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. 2009;151(7):437–446. doi: 10.7326/0003-4819-151-7-200910060-00142. [DOI] [PubMed] [Google Scholar]
- 50.Hemila H., Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980. doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karsch-Volk M., Barrett B., Kiefer D., Bauer R., Ardjomand-Woelkart K., Linde K. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev. 2014;2:CD000530. doi: 10.1002/14651858.CD000530.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lissiman E., Bhasale A.L., Cohen M. Garlic for the common cold. Cochrane Database Syst Rev. 2014;11:CD006206. doi: 10.1002/14651858.CD006206.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh M., Das R.R. Zinc for the common cold. Cochrane Database Syst Rev. 2013;6:CD001364. doi: 10.1002/14651858.CD001364.pub4. [DOI] [PubMed] [Google Scholar]
- 54.Singh M., Singh M. Heated, humidified air for the common cold. Cochrane Database Syst Rev. 2013;6:CD001728. doi: 10.1002/14651858.CD001728.pub5. [DOI] [PubMed] [Google Scholar]
- 55.Chen W., Lewith G., Wang L.Q., Ren J., Xiong W.J., Lu F. Chinese proprietary herbal medicine listed in 'China national essential drug list' for common cold: a systematic literature review. PLoS One. 2014;9(10):e110560. doi: 10.1371/journal.pone.0110560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li S., Yue J., Dong B.R., Yang M., Lin X., Wu T. Acetaminophen (paracetamol) for the common cold in adults. Cochrane Database Syst Rev. 2013;7:CD008800. doi: 10.1002/14651858.CD008800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim S.Y., Chang Y.J., Cho H.M., Hwang Y.W., Moon Y.S. Non-steroidal anti-inflammatory drugs for the common cold. Cochrane Database Syst Rev. 2013;6:CD006362. doi: 10.1002/14651858.CD006362.pub3. [DOI] [PubMed] [Google Scholar]
- 58.Hayden F.G., Diamond L., Wood P.B., Korts D.C., Wecker M.T. Effectiveness and safety of intranasal ipratropium bromide in common colds. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1996;125(2):89–97. doi: 10.7326/0003-4819-125-2-199607150-00002. [DOI] [PubMed] [Google Scholar]
- 59.Genuis S.J., Schwalfenberg G., Siy A.K., Rodushkin I. Toxic element contamination of natural health products and pharmaceutical preparations. PLoS One. 2012;7(11):e49676. doi: 10.1371/journal.pone.0049676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kenealy T., Arroll B. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev. 2013;6:CD000247. doi: 10.1002/14651858.CD000247.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aslan T., Fassas A.B., Desikan R., Siegel D., Munshi N., Mehta J. Patients with multiple myeloma may safely undergo autologous transplantation despite ongoing RSV infection and no ribavirin therapy. Bone Marrow Transplant. 1999;24(5):505–509. doi: 10.1038/sj.bmt.1701946. [DOI] [PubMed] [Google Scholar]
- 62.Avivi I., Chakrabarti S., Milligan D.W., Waldmann H., Hale G., Osman H. Incidence and outcome of adenovirus disease in transplant recipients after reduced-intensity conditioning with alemtuzumab. Biol Blood Marrow Transplant. 2004;10(3):186–194. doi: 10.1016/j.bbmt.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Fiore A.E., Fry A., Shay D., Gubareva L., Bresee J.S., Uyeki T.M. Antiviral agents for the treatment and chemoprophylaxis of influenza – recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60(1):1–24. [PubMed] [Google Scholar]
- 64.Bright R.A., Shay D.K., Shu B., Cox N.J., Klimov A.I. Adamantane resistance among influenza A viruses isolated early during the 2005-2006 influenza season in the United States. JAMA. 2006;295(8):891–894. doi: 10.1001/jama.295.8.joc60020. [DOI] [PubMed] [Google Scholar]
- 65.Englund J.A., Champlin R.E., Wyde P.R., Kantarjian H., Atmar R.L., Tarrand J. Common emergence of amantadine- and rimantadine-resistant influenza A viruses in symptomatic immunocompromised adults. Clin Infect Dis. 1998;26(6):1418–1424. doi: 10.1086/516358. [DOI] [PubMed] [Google Scholar]
- 66.Hayden F.G., Sperber S.J., Belshe R.B., Clover R.D., Hay A.J., Pyke S. Recovery of drug-resistant influenza A virus during therapeutic use of rimantadine. Antimicrob Agents Chemother. 1991;35(9):1741–1747. doi: 10.1128/aac.35.9.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dobson J., Whitley R.J., Pocock S., Monto A.S. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385(9979):1729–1737. doi: 10.1016/S0140-6736(14)62449-1. [DOI] [PubMed] [Google Scholar]
- 68.Jefferson T., Jones M.A., Doshi P., Del Mar C.B., Hama R., Thompson M.J. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014;4:CD008965. doi: 10.1002/14651858.CD008965.pub3. [DOI] [PubMed] [Google Scholar]
- 69.Casper C., Englund J., Boeckh M. How I treat influenza in patients with hematologic malignancies. Blood. 2010;115(7):1331–1342. doi: 10.1182/blood-2009-11-255455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engelhard D., Mohty B., de la Camara R., Cordonnier C., Ljungman P. European guidelines for prevention and management of influenza in hematopoietic stem cell transplantation and leukemia patients: summary of ECIL-4 (2011), on behalf of ECIL, a joint venture of EBMT, EORTC, ICHS, and ELN. Transpl Infect Dis. 2013;15(3):219–232. doi: 10.1111/tid.12054. [DOI] [PubMed] [Google Scholar]
- 71.Vu D., Peck A.J., Nichols W.G., Varley C., Englund J.A., Corey L. Safety and tolerability of oseltamivir prophylaxis in hematopoietic stem cell transplant recipients: a retrospective case-control study. Clin Infect Dis. 2007;45(2):187–193. doi: 10.1086/518985. [DOI] [PubMed] [Google Scholar]
- 72.Tomblyn M., Chiller T., Einsele H., Gress R., Sepkowitz K., Storek J. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chemaly R.F., Shah D.P., Boeckh M.J. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;59(Suppl. 5):S344–S351. doi: 10.1093/cid/ciu623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Machado C.M., Boas L.S., Mendes A.V., da Rocha I.F., Sturaro D., Dulley F.L. Use of oseltamivir to control influenza complications after bone marrow transplantation. Bone Marrow Transplant. 2004;34(2):111–114. doi: 10.1038/sj.bmt.1704534. [DOI] [PubMed] [Google Scholar]
- 75.Johny A.A., Clark A., Price N., Carrington D., Oakhill A., Marks D.I. The use of zanamivir to treat influenza A and B infection after allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;29(2):113–115. doi: 10.1038/sj.bmt.1703343. [DOI] [PubMed] [Google Scholar]
- 76.Chemaly R.F., Torres H.A., Aguilera E.A., Mattiuzzi G., Cabanillas M., Kantarjian H. Neuraminidase inhibitors improve outcome of patients with leukemia and influenza: an observational study. Clin Infect Dis. 2007;44(7):964–967. doi: 10.1086/512374. [DOI] [PubMed] [Google Scholar]
- 77.Choi S.M., Boudreault A.A., Xie H., Englund J.A., Corey L., Boeckh M. Differences in clinical outcomes after 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood. 2011;117(19):5050–5056. doi: 10.1182/blood-2010-11-319186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Birnkrant D., Cox E. The emergency use authorization of peramivir for treatment of 2009 H1N1 influenza. N Engl J Med. 2009;361(23):2204–2207. doi: 10.1056/NEJMp0910479. [DOI] [PubMed] [Google Scholar]
- 79.Sparrelid E., Ljungman P., Ekelof-Andstrom E., Aschan J., Ringden O., Winiarski J. Ribavirin therapy in bone marrow transplant recipients with viral respiratory tract infections. Bone Marrow Transplant. 1997;19(9):905–908. doi: 10.1038/sj.bmt.1700752. [DOI] [PubMed] [Google Scholar]
- 80.Shah J.N., Chemaly R.F. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117(10):2755–2763. doi: 10.1182/blood-2010-08-263400. [DOI] [PubMed] [Google Scholar]
- 81.Marcelin J.R., Wilson J.W., Razonable R.R. Oral ribavirin therapy for respiratory syncytial virus infections in moderately to severely immunocompromised patients. Transpl Infect Dis. 2014;16(2):242–250. doi: 10.1111/tid.12194. [DOI] [PubMed] [Google Scholar]
- 82.Shah D.P., Ghantoji S.S., Shah J.N., El Taoum K.K., Jiang Y., Popat U. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. 2013;68(8):1872–1880. doi: 10.1093/jac/dkt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mendes E.T., Ramos J., Peixoto D., Dulley F., Alves T., Vilas Boas L.S. An outbreak of respiratory syncytial virus infection in hematopoietic stem cell transplantation outpatients: good outcome without specific antiviral treatment. Transpl Infect Dis. 2013;15(1):42–48. doi: 10.1111/j.1399-3062.2012.00764.x. [DOI] [PubMed] [Google Scholar]
- 84.Anaissie E.J., Mahfouz T.H., Aslan T., Pouli A., Desikan R., Fassas A. The natural history of respiratory syncytial virus infection in cancer and transplant patients: implications for management. Blood. 2004;103(5):1611–1617. doi: 10.1182/blood-2003-05-1425. [DOI] [PubMed] [Google Scholar]
- 85.Kilham L., Ferm V.H. Congenital anomalies induced in hamster embryos with ribavirin. Science. 1977;195(4276):413–414. doi: 10.1126/science.401547. [DOI] [PubMed] [Google Scholar]
- 86.Casey J., Morris K., Narayana M., Nakagaki M., Kennedy G.A. Oral ribavirin for treatment of respiratory syncitial virus and parainfluenza 3 virus infections post allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2013;48(12):1558–1561. doi: 10.1038/bmt.2013.112. [DOI] [PubMed] [Google Scholar]
- 87.Gueller S., Duenzinger U., Wolf T., Ajib S., Mousset S., Berger A. Successful systemic high-dose ribavirin treatment of respiratory syncytial virus-induced infections occurring pre-engraftment in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2013;15(4):435–440. doi: 10.1111/tid.12092. [DOI] [PubMed] [Google Scholar]
- 88.Maziarz R.T., Sridharan P., Slater S., Meyers G., Post M., Erdman D.D. Control of an outbreak of human parainfluenza virus 3 in hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2010;16(2):192–198. doi: 10.1016/j.bbmt.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hohenthal U., Nikoskelainen J., Vainionpaa R., Peltonen R., Routamaa M., Itala M. Parainfluenza virus type 3 infections in a hematology unit. Bone Marrow Transplant. 2001;27(3):295–300. doi: 10.1038/sj.bmt.1702776. [DOI] [PubMed] [Google Scholar]
- 90.Hromas R., Cornetta K., Srour E., Blanke C., Broun E.R. Donor leukocyte infusion as therapy of life-threatening adenoviral infections after T-cell-depleted bone marrow transplantation. Blood. 1994;84(5):1689–1690. [PubMed] [Google Scholar]
- 91.Geyeregger R., Freimuller C., Stemberger J., Artwohl M., Witt V., Lion T. First-in-man clinical results with good manufacturing practice (GMP)-compliant polypeptide-expanded adenovirus-specific T cells after haploidentical hematopoietic stem cell transplantation. J Immunother. 2014;37(4):245–249. doi: 10.1097/CJI.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 92.Egli A., Bucher C., Dumoulin A., Stern M., Buser A., Bubendorf L. Human metapneumovirus infection after allogeneic hematopoietic stem cell transplantation. Infection. 2012;40(6):677–684. doi: 10.1007/s15010-012-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Englund J.A., Boeckh M., Kuypers J., Nichols W.G., Hackman R.C., Morrow R.A. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144(5):344–349. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 94.Ison M.G., Michaels M.G. RNA respiratory viral infections in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl. 4):S166–S172. doi: 10.1111/j.1600-6143.2009.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Covalciuc K.A., Webb K.H., Carlson C.A. Comparison of four clinical specimen types for detection of influenza A and B viruses by optical immunoassay (FLU OIA test) and cell culture methods. J Clin Microbiol. 1999;37(12):3971–3974. doi: 10.1128/jcm.37.12.3971-3974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaiser L., Briones M.S., Hayden F.G. Performance of virus isolation and Directigen Flu A to detect influenza A virus in experimental human infection. J Clin Virol. 1999;14(3):191–197. doi: 10.1016/s1386-6532(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 97.Debyle C., Bulkow L., Miernyk K., Chikoyak L., Hummel K.B., Hennessy T. Comparison of nasopharyngeal flocked swabs and nasopharyngeal wash collection methods for respiratory virus detection in hospitalized children using real-time polymerase chain reaction. J Virol Methods. 2012;185(1):89–93. doi: 10.1016/j.jviromet.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heikkinen T., Marttila J., Salmi A.A., Ruuskanen O. Nasal swab versus nasopharyngeal aspirate for isolation of respiratory viruses. J Clin Microbiol. 2002;40(11):4337–4339. doi: 10.1128/JCM.40.11.4337-4339.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li L., Chen Q.Y., Li Y.Y., Wang Y.F., Yang Z.F., Zhong N.S. Comparison among nasopharyngeal swab, nasal wash, and oropharyngeal swab for respiratory virus detection in adults with acute pharyngitis. BMC Infect Dis. 2013;13:281. doi: 10.1186/1471-2334-13-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Macfarlane P., Denham J., Assous J., Hughes C. RSV testing in bronchiolitis: which nasal sampling method is best? Arch Dis Child. 2005;90(6):634–635. doi: 10.1136/adc.2004.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meerhoff T.J., Houben M.L., Coenjaerts F.E., Kimpen J.L., Hofland R.W., Schellevis F. Detection of multiple respiratory pathogens during primary respiratory infection: nasal swab versus nasopharyngeal aspirate using real-time polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 2010;29(4):365–371. doi: 10.1007/s10096-009-0865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sung R.Y., Chan P.K., Choi K.C., Yeung A.C., Li A.M., Tang J.W. Comparative study of nasopharyngeal aspirate and nasal swab specimens for diagnosis of acute viral respiratory infection. J Clin Microbiol. 2008;46(9):3073–3076. doi: 10.1128/JCM.01209-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Waris M., Osterback R., Lahti E., Vuorinen T., Ruuskanen O., Peltola V. Comparison of sampling methods for the detection of human rhinovirus RNA. J Clin Virol. 2013;58(1):200–204. doi: 10.1016/j.jcv.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 104.Kuypers J., Campbell A.P., Cent A., Corey L., Boeckh M. Comparison of conventional and molecular detection of respiratory viruses in hematopoietic cell transplant recipients. Transpl Infect Dis. 2009;11(4):298–303. doi: 10.1111/j.1399-3062.2009.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Falsey A.R., Formica M.A., Treanor J.J., Walsh E.E. Comparison of quantitative reverse transcription-PCR to viral culture for assessment of respiratory syncytial virus shedding. J Clin Microbiol. 2003;41(9):4160–4165. doi: 10.1128/JCM.41.9.4160-4165.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hindiyeh M., Hillyard D.R., Carroll K.C. Evaluation of the Prodesse Hexaplex multiplex PCR assay for direct detection of seven respiratory viruses in clinical specimens. Am J Clin Pathol. 2001;116(2):218–224. doi: 10.1309/F1R7-XD6T-RN09-1U6L. [DOI] [PubMed] [Google Scholar]
- 107.Chen K.F., Rothman R.E., Ramachandran P., Blyn L., Sampath R., Ecker D.J. Rapid identification viruses from nasal pharyngeal aspirates in acute viral respiratory infections by RT-PCR and electrospray ionization mass spectrometry. J Virol Methods. 2011;173(1):60–66. doi: 10.1016/j.jviromet.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abbo L., Quartin A., Morris M.I., Saigal G., Ariza-Heredia E., Mariani P. Pulmonary imaging of pandemic influenza H1N1 infection: relationship between clinical presentation and disease burden on chest radiography and CT. Br J Radiol. 2010;83(992):645–651. doi: 10.1259/bjr/53692814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chandler T.M., Leipsic J., Nicolaou S., Quiney B., Romney M., Muller N.L. Confirmed swine-origin influenza A(H1N1) viral pneumonia: computed tomographic findings in the immunocompetent and the immunocompromised. J Comput Assist Tomogr. 2011;35(5):602–607. doi: 10.1097/RCT.0b013e31822c56f1. [DOI] [PubMed] [Google Scholar]
- 110.El-Badrawy A., Zeidan A., Ebrahim M.A. 64 multidetector CT findings of influenza A (H1N1) virus in patients with hematologic malignancies. Acta Radiol. 2012;53(6):662–667. doi: 10.1258/ar.2012.120038. [DOI] [PubMed] [Google Scholar]
- 111.Elicker B.M., Schwartz B.S., Liu C., Chen E.C., Miller S.A., Chiu C.Y. Thoracic CT findings of novel influenza A (H1N1) infection in immunocompromised patients. Emerg Radiol. 2010;17(4):299–307. doi: 10.1007/s10140-010-0859-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ko J.P., Shepard J.A., Sproule M.W., Trotman-Dickenson B., Drucker E.A., Ginns L.C. CT manifestations of respiratory syncytial virus infection in lung transplant recipients. J Comput Assist Tomogr. 2000;24(2):235–241. doi: 10.1097/00004728-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 113.Laqmani A., Adam G., Regier M. Pulmonary manifestation of novel swine-origin influenza A (H1N1) virus (S-OIV) infection in immunocompromised patients: initial findings with multidetector computed tomography. Med Princ Pract. 2012;21(6):548–553. doi: 10.1159/000338399. [DOI] [PubMed] [Google Scholar]
- 114.Oikonomou A., Muller N.L., Nantel S. Radiographic and high-resolution CT findings of influenza virus pneumonia in patients with hematologic malignancies. AJR Am J Roentgenol. 2003;181(2):507–511. doi: 10.2214/ajr.181.2.1810507. [DOI] [PubMed] [Google Scholar]
- 115.Syha R., Beck R., Hetzel J., Ketelsen D., Grosse U., Springer F. Human metapneumovirus (HMPV) associated pulmonary infections in immunocompromised adults–initial CT findings, disease course and comparison to respiratory-syncytial-virus (RSV) induced pulmonary infections. Eur J Radiol. 2012;81(12):4173–4178. doi: 10.1016/j.ejrad.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 116.Jefferson T., Del Mar C.B., Dooley L., Ferroni E., Al-Ansary L.A., Bawazeer G.A. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207. doi: 10.1002/14651858.CD006207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.RSV Outbreak Investigation Team Contributing and terminating factors of a large RSV outbreak in an Adult Hematology and Transplant Unit. PLoS Curr. 2014:6. doi: 10.1371/currents.outbreaks.3bc85b2a508d205ecc4a5534ecb1f9be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Falsey A.R. Current management of parainfluenza pneumonitis in immunocompromised patients: a review. Infect Drug Resist. 2012;5:121–127. doi: 10.2147/IDR.S25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gokturk B., Pekcan S., Guner S.N., Artac H., Keles S., Kirac M. Efficacy of intravenous immunoglobulin treatment in immunocompromised children with H1N1 influenza: a clinical observation. Clin Respir J. 2016 Mar;10(2):223–230. doi: 10.1111/crj.12209. [DOI] [PubMed] [Google Scholar]
- 120.Ljungman P., Ribaud P., Eyrich M., Matthes-Martin S., Einsele H., Bleakley M. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2003;31(6):481–486. doi: 10.1038/sj.bmt.1703798. [DOI] [PubMed] [Google Scholar]
- 121.Robin M., Marque-Juillet S., Scieux C., Peffault de Latour R., Ferry C., Rocha V. Disseminated adenovirus infections after allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcome. Haematologica. 2007;92(9):1254–1257. doi: 10.3324/haematol.11279. [DOI] [PubMed] [Google Scholar]
- 122.Legrand F., Berrebi D., Houhou N., Freymuth F., Faye A., Duval M. Early diagnosis of adenovirus infection and treatment with cidofovir after bone marrow transplantation in children. Bone Marrow Transplant. 2001;27(6):621–626. doi: 10.1038/sj.bmt.1702820. [DOI] [PubMed] [Google Scholar]
- 123.Leruez-Ville M., Chardin-Ouachee M., Neven B., Picard C., Le Guinche I., Fischer A. Description of an adenovirus A31 outbreak in a paediatric haematology unit. Bone Marrow Transplant. 2006;38(1):23–28. doi: 10.1038/sj.bmt.1705389. [DOI] [PubMed] [Google Scholar]
- 124.Nicolasora N.P., Reddy P., Kaul D.R. Biopsy-proven adenoviral diarrhea responding to low-dose cidofovir. Transpl Infect Dis. 2008;10(5):346–350. doi: 10.1111/j.1399-3062.2008.00303.x. [DOI] [PubMed] [Google Scholar]