Abstract
Pneumonia virus of mice (PVM) infection of BALB/c mice induces bronchiolitis leading to a fatal pneumonia in a dose-dependent manner, closely paralleling the development of severe disease during human respiratory syncytial virus infection in man, and is thus a recognised model in which to study the pathogenesis of pneumoviruses. This model system was used to investigate delivery of the internal structural proteins of PVM as a potential vaccination strategy to protect against pneumovirus disease. Replication-deficient recombinant human adenovirus serotype 5 (rAd5) vectors were constructed that expressed the M or N gene of PVM pathogenic strain J3666. Intranasal delivery of these rAd5 vectors gave protection against a lethal challenge dose of PVM in three different mouse strains, and protection lasted for at least 20 weeks post-immunisation. Whilst the PVM-specific antibody response in such animals was weak and inconsistent, rAd5N primed a strong PVM-specific CD8+ T cell response and, to a lesser extent, a CD4+ T cell response. These findings suggest that T-cell responses may be more important than serum IgG in the observed protection induced by rAd5N.
Keywords: PVM, HRSV, Intranasal immunisation, Vaccine, Adenovirus vector
1. Introduction
Viruses classified within the genus Pneumovirus include the human (HRSV) and bovine (BRSV) respiratory syncytial viruses and pneumonia virus of mice (PVM) [1]. HRSV is an important respiratory pathogen, causing approximately 30 million cases of acute lower respiratory tract disease in children under 5 annually, some 10% of which require hospitalisation [2]. Most infants recover from a natural HRSV infection but some suffer a fatal outcome [3]. Since long-term immunity is not established, infections reoccur throughout life [4] and in elderly or immunocompromised individuals, these can lead to serious complications [5].
An early clinical trial of a formalin-inactivated HRSV vaccine led to exacerbation of disease on subsequent infection [6], associated with induction of low affinity, HRSV-specific antibodies [7], immune complex deposition and complement activation [8], and a Th2-biased immune response [9]. Subsequently, many HRSV vaccine strategies have been investigated [10], [11], [12], [13], [14], however no clinically successful candidate has emerged. HRSV vaccine candidates are often investigated in mice, although very high challenge doses are required to give disease and even then, pathogenesis does not match that seen in severely affected human infants [15]. In contrast, low doses of PVM in its natural rodent host give clinical signs ranging from upper respiratory tract infection to fatal pneumonia [16] with pathogenesis that closely resembles severe HRSV disease in humans, making it an appropriate system for investigating pneumovirus pathogenesis and immune responses [17], [18].
HRSV vaccine strategies have focused on stimulating a strong systemic humoral response against the F and G glycoproteins [11], [19]. However, the natural response that resolves pneumovirus infection is primarily mediated through CD8+ T-cells [20], [21], [22], [23], [24]. Recombinant adenoviruses (rAd) have been widely developed as vaccine candidates, including to deliver HRSV glycoproteins [13], [19], [25], [26], and typically elicit potent TH1-biased responses [27]. We therefore evaluated intranasal (i.n.) delivery of human rAd type 5 (rAd5) PVM recombinants in the mouse as a model for protection against pneumoviruses. We selected this route since it was superior to intramuscular (i.m) delivery in providing protection against either lethal influenza A virus challenge or HRSV replication in the lung [13], [28]; it is also now an established route for human vaccination [29]. We focused on the internal proteins M and N since they contain potent cytotoxic T-cell (CTL) epitopes in HRSV [30], [31] and H-2Db and H-2Kb restricted CD8+ T cell epitopes in PVM [32], [33]. Furthermore, vaccinating calves with N protein vectors primed BRSV-specific T cells and conferred partial protection against BRSV challenge [34], [35] and mucosal immunisation of mice with HRSV N protein nanoparticles induced both specific antibody and T cell responses, and reduced HRSV pulmonary replication [36]. We show here that rAd5 containing the M or N gene of PVM is able to elicit long-term protection against lethal PVM infection in mice, correlating with the stimulation of PVM-specific CD4 and CD8 T-cell populations.
2. Materials and methods
2.1. Cell and virus culture
A line of persistently PVM-infected cells (designated P2-2) was established by infecting BSC-1 African green monkey kidney cells with PVM strain 15 at a multiplicity of infection of 0.01 pfu per cell [37]. Following the initial appearance of cytopathic effect and loss of cells, medium was replaced every 2–3 days. Within 4 weeks, growth of persistently infected cells was detected and these were subsequently passaged as normal. P2-2 cells show PVM gene expression by immunofluorescence and western blotting, and continuously produce infectious virus which is detectable in the growth medium.
BS-C-1 cells, P2-2 cells and HEK293 cells were cultured in Glasgow minimal essential media (GMEM) plus 10% foetal bovine serum (FBS), GMEM plus 15% FBS or Dulbecco's modified Eagle media (DMEM) plus 10% FBS, respectively. PVM strain J3666 stocks were prepared and titrated in BS-C-1 cells as described previously [17]. Ad5 dl327 [38] was propagated in HEK293 cells cultured in DMEM plus 2% FBS. rAd5 stocks were amplified and titrated by plaque assay on HEK293 cells.
2.2. Construction and characterisation of recombinant Ad5 vectors
rAd5 with E3 deletions and E1 genes replaced by transgenes were generated using the AdEasy™ Adenoviral Vector System (Stratagene) according to the manufacturer's protocol. pShuttle-CMV plasmids, containing the M or N gene of PVM strain J3666 (Genbank AY743909) amplified by PCR from cDNAs using primers that included appropriate restriction enzyme sites, were used to generate rAd5M and rAd5N; the CMV promoters and transgene regions were verified by sequencing [39], [40]. A plasmid containing the Escherichia coli lacZ gene was used similarly to generate rAd5Z. Recombinant viruses were purified twice by caesium chloride density gradient centrifugation and dialysed against 20% glycerol in phosphate-buffered saline (PBS).
2.3. Animal immunisation and processing
Male and female BALB/c mice from the breeding colony at the University of Warwick were confirmed PVM-free by serology and used at 5–7 weeks old. C3H/He-mg animals were similarly sourced in-house [41]; 5-week old C57BL/6 mice were purchased from Charles River. Animal care was carried out in accordance with the UK Animal Scientific Procedures Act 1986 and was approved by the University of Warwick Ethical Review Board. Mice were allocated to experimental groups with equal sex ratios, anaesthetised by intraperitoneal injection with ketamine (75 μg/g bodyweight) and xylazine (15 μg/g bodyweight) and inoculated i.n. with rAd doses in 50 μl PBS or PBS alone (control animals); repeat immunisations were at two week intervals. Animals were challenged i.n. with a lethal dose of 250 pfu PVM strain J3666 in 50 μl PBS. Clinical signs were assessed as previously described [17] and bodyweight measured daily. Animals were sacrificed for welfare reasons, or at the end of the experiment, by cervical dislocation.
2.4. ELISA
Flat-bottom microtitre plates were coated overnight at 4 °C with 1 μg/ml of either purified Ad5 dl327, or P2-2 or BS-C-1 cell lysate (cell material harvested and fragmented by agitation with glass beads, then sonicated), then incubated for 2 h with blocking buffer (5% low fat dried milk in PBS containing 0.02% (w/v) Tween 20 (PBS-T)). Serum, or broncho-alveolar lavage (BAL) fluid obtained using 1.0 ml PBS per animal, was titrated in threefold dilutions after an initial 1:85 dilution in blocking buffer. Antibody was bound for 2 h at room-temperature (r.t.), wells washed 3× with PBS-T, then horseradish peroxidise conjugated goat anti-mouse (Sigma) IgG—specific antibodies (Santa Cruz), diluted in blocking buffer, were added for 2 h at r.t. After 3× washing, 100 μl/well of 0.01 mg/ml 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) di-ammonium salt (ABTS) solution (Sigma) was added and absorbance measured at 405 nm using a Labsystems multiskan RC plate reader. Antibody levels were determined by linear regression analysis and values expressed as log10 endpoint titres. Statistical analysis was performed using the Mann Whitney U test with Prism software.
2.5. In vitro stimulation and analysis of PVM-specific lymphocytes
Spleens or lungs in 2 ml PBS were passed through cell strainers, generating single-cell suspensions that were centrifuged at 1200 × g over Histopaque 1086 (Sigma, Poole, UK). Mononuclear cells at the interface were harvested, washed 3× in PBS and resuspended in RPMI 1640 medium (Life Technologies) containing 10% FBS, 0.1% β-mercaptoethanol, 10 U/ml penicillin G and 10 μg/ml streptomycin sulfate (RPMI/10). Cells were re-stimulated in vitro with PVM-infected or mock-infected antigen-presenting cells (APCs) at a ratio of 10:1 in 96-well U-bottom plates containing 200 μl of RPMI/10 further supplemented with 5 U/ml recombinant human IL-2 (Roche) and 10 μg/ml Brefeldin A (Calbiochem®) for 16 h at 37 ̊C. APCs were naïve spleen cells, either PVM-infected (1 pfu/cell) or mock-infected with BS-C-1 cell lysate for 90 min at 37 ̊C, irradiated (3000 rad) and washed. After re-stimulation, cells were surface stained with rat anti-mouse CD8α allophycocyanin or rat anti-mouse CD4 phycoerythrin mAbs (BD Pharmingen). Intracellular staining used CytoFix/CytoPerm solution and Perm/Wash buffer according to the manufacturer's instructions (BD Biosciences), and rat anti-mouse IFNγ-FITC (BD Biosciences). Cells were analysed on a FACSCalibur flow cytometer (BD Biosciences) and data analysed using CellQuest software.
3. Results
3.1. Immunisation with rAd5N or rAd5M protects mice against lethal PVM infection
Expression of PVM M and N by rAd5 in cell culture was confirmed by immunofluorescence or Western blotting, respectively (data not shown). To evaluate protection by rAd5M, rAd5N or rAd5Z, mice were immunised i.n. on day 0 and day 14, then challenged on day 28 with a lethal dose of PVM and monitored for 2–3 weeks for clinical signs and bodyweight loss as previously described [17]. Animals receiving 106 pfu of rAd5M or rAd5N developed severe clinical signs of disease and significant weight loss upon challenge, similar to PBS mock-immunised animals and were therefore not protected (data not shown). In contrast, all animals that received higher doses of rAd5M or rAd5N survived (Fig. 1 ). Animals receiving 107 pfu of either recombinant showed transient signs of infection and weight loss but made a complete recovery (Fig. 1A and B) whereas animals immunised with rAd5Z developed severe disease after challenge, equivalent to mock-immunised animals (Fig. 1C). Thus, at the 107 pfu dose, there was clear evidence of antigen-specific protection elicited by rAd5N and rAd5M. Survival data from these and subsequent experiments are summarised in Table 1 .
Fig. 1.
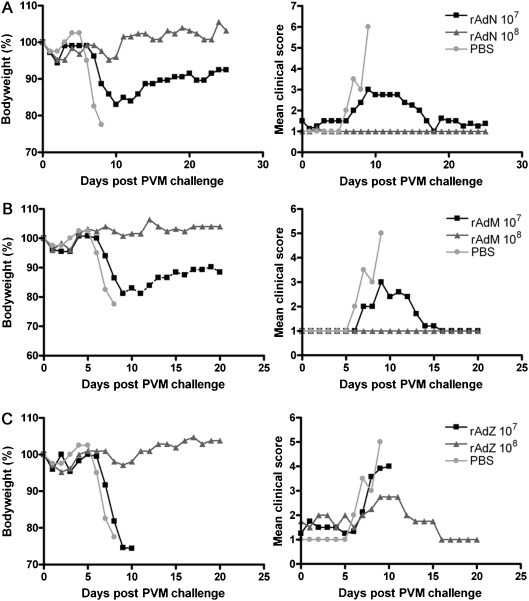
rAd5M and rAd5N protect BALB/c mice from lethal PVM challenge. Mice were immunised i.n. at 0 and 14 days with either 107 or 108 pfu of rAd5N (A), rAd5M (B), or rAd5Z (C) or mock-immunised with PBS as a control. All animals were challenged at 28 days with a lethal dose of PVM, and then weighed (left panels) and assessed for clinical signs of PVM disease (right panels) daily for the duration of the experiment. Weights are expressed as a proportion of the initial weight of the relevant group of mice with the weight on day zero taken as 100%. Clinical signs are expressed on a scale of 1 (healthy) to 5 (moribund) [17]. Results are the means from five mice for each group and are representative of two separate experiments. A single PBS control group was studied within each experiment and data from this group are reproduced in panels A–C for ease of comparison.
Table 1.
Survival data for animals challenged with PVM.
| Immunogen | BAlb/c† | C3H–He-mg | C57BL/6 | |||
|---|---|---|---|---|---|---|
| 107 pfu (×1) | 108 pfu (×1) | 107 pfu (×2) | 108 pfu (×2) | 107 pfu (×2) | 107 pfu (×2) | |
| rAd5-N | 6/6 | 5/5 | 3/3 | 6/6 | 5/5 | 5/5 |
| rAd5-M | 3/6 | 6/6 | 5/6 | 6/6 | 3*/5 | 5/5 |
| rAd5-Z | 1/6 | 6/6 | 0/6 | 4/4 | 2*/5 | 5*/5 |
| PBS | 0/2 | 0/2 | 0/4 | 2*/4 | 0/4 | 0/4 |
Mice were inoculated i.n. with one (×1) or two (×2) doses of Ad5 immunogens, at day 0 and 14, and were challenged i.n. at day 42 (×1) or day 28 (×2) with a lethal dose of PVM.
Mice exhibited elevated clinical signs of disease.
Animals receiving 108 pfu of rAd5M or rAd5N remained healthy after challenge (Fig. 1A and B). Surprisingly however, 108 pfu of control rAd5Z was also protective (Fig. 1C) although, in contrast to those receiving specific immunogen, rAd5Z animals developed some signs of illness. Thus the 108 pfu dose protected mice effectively from lethal challenge but a significant component of that protection was antigen-nonspecific. This non-specific protection is clearly distinct from that previously shown to be produced by defective-interfering (DI) influenza A virus against PVM. DI-based protection was undetectable 7 days post-administration of the protective virus and was interferon-dependent [41] whereas rAd5Z-based protection lasted for at least two weeks. Under our prime-boost protocol, and in contrast to its effect on PVM, 108 pfu rAd5Z afforded no protection against influenza A virus (data not shown).
To investigate single dose efficacy, mice were immunised and challenged with PVM six weeks later. Mice given 107 pfu rAd5 M or rAd5N developed transient weight loss and elevated clinical scores but made a full recovery, stabilising with bodyweights only a few percent from the starting weight (Fig. 2A) whilst rAd5Z gave no protection. These data indicate that even a single i.n. dose of 107 pfu of rAd5M or rAd5N elicits PVM-specific protection, although rAd5N was clearly more protective than rAd5 M in this regimen. Similar to the two-dose regimen, one 108 pfu dose of rAd5M or rAd5N gave full protection against disease whilst an equivalent single dose of rAd5Z also protected against lethal outcome, albeit with some signs of disease (Fig. 2B).
Fig. 2.
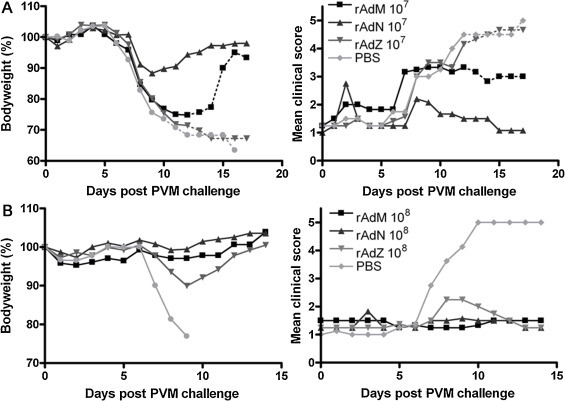
A single intranasal immunisation of rAd5M or rAd5N confers protection against PVM challenge. Mice were immunised with 107 pfu (A) or 108 pfu (B) of either rAd5M, rAd5N or rAd5Z, or mock-immunised with PBS. Animals were challenged with PVM six weeks post immunisation and then weighed (left panels) and assessed for clinical signs of PVM disease (right panels) daily for the duration of the experiment. Dotted lines: mean weights or scores after the loss of one or more animals from the group. Other details as for Fig. 1.
Antigen-specific protection implied involvement of adaptive responses. Such responses vary in outbred populations because epitopes must be presented by highly polymorphic major histocompatibility complex (MHC) antigens. We therefore tested whether protection in BALB/c mice (MHC haplotype H2d) could be replicated in other mouse strains. C57BL/6 (H2b) and C3H/He-mg (H2k) mice, immunised with 107 pfu rAd in a two-dose regime, were fully protected from PVM by rAd5N immunisation, as were C57BL/6 animals by rAd5M; in each case animals remained healthy following challenge (data not shown). rAd5M only partially protected C3H/He-mg animals with two of five succumbing during the challenge period, although one of these occurred without prior elevation in clinical score and so may not have been due to the challenge (data not shown). Thus, protection against lethal PVM infection by rAd PVM recombinants extends to multiple MHC haplotypes.
Antigen-specific protection against PVM was observed at four and six weeks after the primary immunisation. To determine the duration of protection, BALB/c mice were challenged at 8, 11, 14 or 20 weeks after initial immunisation with 107 pfu rAd in a two-dose protocol (Fig. 3 ). Older animals are slightly less susceptible to PVM-induced disease [42] and this was reflected in the survival of some PBS mock-immunised animals. However, these control animals showed significantly greater weight loss and clinical scores than immunised animals following PVM challenge at any time-point. rAd5N or rAd5M protected against lethal PVM challenge up to 20 weeks post-immunisation (Fig. 3A and B). Whilst rAd5M-immunised animals showed some weight loss, particularly following prolonged delay between immunisation and challenge, this was transient and less severe than in rAd5Z-immunised mice, which showed severe disease similar to the mock-immunised animals (Fig. 3C). Thus, immunisation with 107 pfu rAd5M or rAd5N can elicit long-lasting, antigen-specific protection in mice against severe or lethal PVM disease.
Fig. 3.
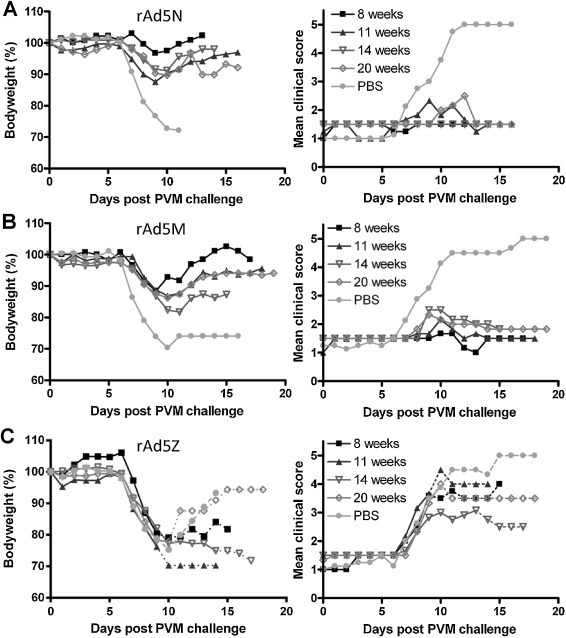
Protection from lethal PVM challenge by rAd5M and rAd5N is long lasting. Mice were immunised i.n. at 0 and 14 days with 107 pfu doses of rAd5N (A), rAd5M (B) or rAd5Z (C) or mock-immunised with PBS, and groups of six immunised animals plus two mock-immunised animals were challenged with a lethal dose of PVM at each time point. Following challenge, mice were weighed (left panels) and assessed for clinical signs of PVM disease (right panels) daily. The graphs for PBS mock-immunised animals were generated by taking an average of the data from the mice challenged at the various time points. Dotted lines: mean weights or scores after the loss of one or more animals from the group. A single experiment was conducted because of the numbers of animals involved. Other details as for Fig. 1.
3.2. Immunisation with rAd5 PVM vectors induces a variable PVM-specific antibody response
Vaccination with 107 pfu rAd5N elicited no detectable anti-PVM serum IgG by day 14 (Fig. 4A), and although a few mice showed some response at day 28, 2 weeks after the second immunisation, most still had no detectable PVM-specific antibody. Vaccination with 108 pfu rAd5N generated a greater anti-PVM IgG response, with one animal responding at day 14 and an increased average titre among day 28 responding animals (Fig. 4B) although not all animals developed detectable antibody. This weak PVM-specific antibody response did not reflect an intrinsic problem in eliciting serum antibody responses by this vector and route of administration since robust anti-Ad5 serum antibody responses were induced by both 107 and 108 pfu rAd (Fig. 4C and D). It was also not due to the assay failing to detect antibody directed against internal proteins of the virus, since the assay was highly effective in detecting binding of N-specific mAbs in comparison with either F-specific mAbs or polyclonal sera from PVM-infected mice (Fig. 4E).
Fig. 4.
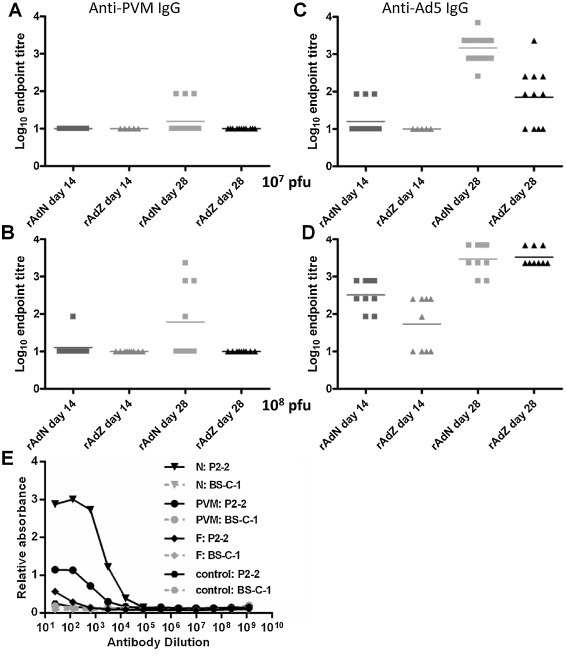
Antibody titres in sera from rAd5N-immunised mice. Mice were immunised i.n. at 0 and 14 days with either 107 pfu (A and C) or 108 pfu (B and D) doses of rAd5N, rAd5Z or mock-immunised with PBS as control. Sera from immunised animals were analysed for anti-PVM IgG responses (A and B) and anti-Ad IgG responses (C and D) by ELISA. Results are expressed as the geometric means of the log10 endpoint titres, after correction of values for background, with the background taken as the mean values in the assay from PBS control animal sera. (E) Anti-N or anti-F monoclonal antibodies, or PVM-specific or control mouse sera, were titrated in the ELISA using either PVM antigen-containing (P2-2) or control (BS-C-1) cell lysate as antigens.
To determine whether anti-PVM IgG might develop or increase over a longer-term experiment, animals were immunised with 107 pfu rAd in a two-dose regime and serum analysed by ELISA at weeks 8, 11, 14 and 20. Although some rAd5M-immunised animals possessed anti-PVM IgG at weeks 6, 8 and 11 (Fig. 5A), at no time point did any more than a minority show a response. For rAd5N, only one animal developed detectable anti-PVM IgG by week 6 but the numbers responding and magnitude of response increased in weeks 8 and 11 (Fig. 5B); again, only a minority showed a detectable response at any time point and anti-PVM IgG antibodies were no longer detectable by week 20. As expected, rAd5Z elicited no detectable anti-PVM IgG at any time (Fig. 5C). In contrast, all animals mounted a detectable anti-Ad5 response, confirming delivery of the immunogen (Fig. 5D–F). The absence of consistent anti-PVM antibody responses in the context of consistent protection from lethal PVM challenge suggested that an anti-PVM IgG response was not the primary mechanism of protection.
Fig. 5.
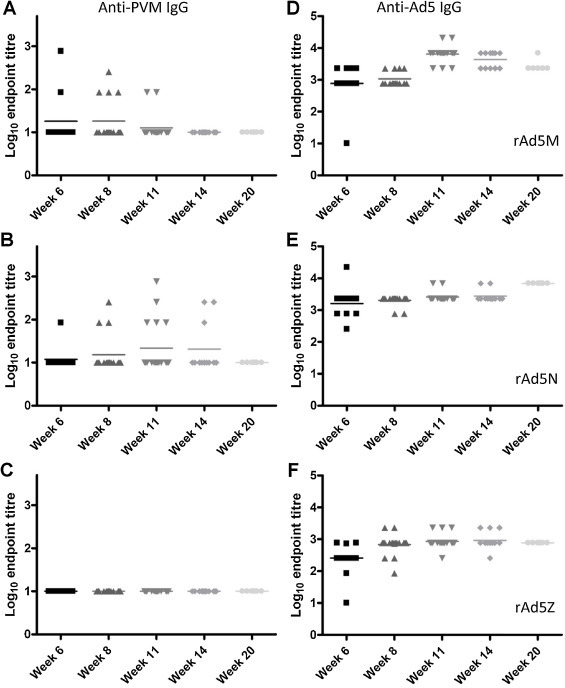
Longevity of antibody titres in sera of rAd5M and rAd5N immunised mice. Mice were immunised i.n. at 0 and 14 days with 107 pfu of rAd5M, rAd5N or rAd5Z or treated with PBS alone. Sera from immunised animals were analysed for anti-PVM IgG responses (A–C) and anti-Ad IgG responses (D–F) by ELISA. Antibody titres were calculated as for Fig. 4.
3.3. Immunisation with rAd5N vector elicits a PVM-specific T-cell response
rAd5N was selected to study T cell responses since it generated the more robust protection. Mice were immunised with 107 pfu in a two dose regimen and lymphocytes harvested for analysis on day 20. IFN-γ intracellular staining revealed that the majority of PVM-specific T-cells induced by rAd5N in the spleen and lungs were CD4+ cells (0.2% CD4+ IFNγ+ in spleen and 0.08% CD4+IFNγ+ in lungs). In contrast, no PVM-specific CD8+ T-cells were observed (data not shown). However, 7 days after immunisation with three doses of 107 pfu of rAd5N, 2 weeks apart, the majority of PVM-specific T-cells induced by rAd5N in the spleen were CD8+ T cells (9.2% CD8+IFNγ+ compared with 2.04% CD4+IFNγ+, Fig. 6 ). Notably, PVM-specific T-cells were not detected in lung lymphocytes from these mice (data not shown); this may have been related to the high background IFNγ response observed in lung lymphocytes from these animals. As PVM-specific T cells were not elicited by rAd5Z (Fig. 6), the PVM-specific CD4+ and CD8+ splenocytes in rAd5N-immunised animals demonstrate that rAd5N induces a significant PVM-specific T-cell response.
Fig. 6.
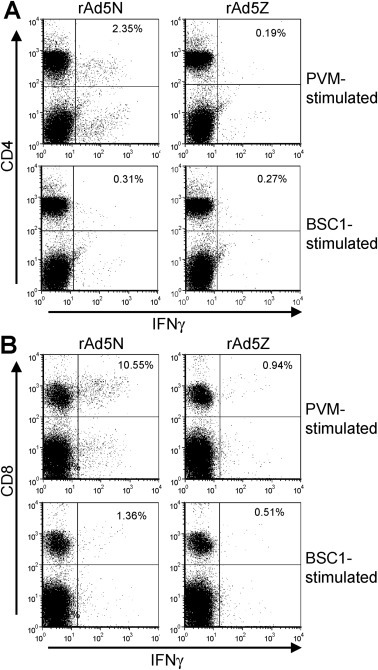
Mucosal immunisation with rAd5N primes a PVM-specific T-cell response. Mice were immunised i.n. with 107 pfu of rAd5N or rAd5Z on days 0, 14 and 28 and spleens were harvested on day 35. Splenocytes from two mice from each group were pooled and stimulated in vitro with PVM or control cell lysate (BSC1) for 16 h, and responses were analysed by intracellular IFNγ staining of: (A) CD4+ and (B) CD8+ cells by flow cytometry. Frequencies of IFNγ+ cells are shown as a percentage of total CD4+ or CD8 T+ cell numbers in the upper right quadrant. Representative data of groups of 4 mice are shown.
4. Discussion
Ad5 has a natural tropism for mucosal surfaces and is a potent stimulator of adaptive immunity [27], [43] making it an attractive candidate vector for recombinant pneumovirus vaccine development. We evaluated the properties of rAd5 vectors expressing PVM N or M proteins and found that i.n. immunisation with 107 pfu of either construct elicited protection against PVM lethal disease. This protection was maintained for 20 weeks in a two-dose regimen and for at least six weeks after a single immunisation. Protection also extended to mouse strains having three different MHC haplotypes.
Only weak and inconsistent anti-PVM serum IgG responses were detected in immunised animals that were reliably protected against lethal PVM challenge, indicating that there was no correlation between the humoral response and protection. Notably, anti-PVM IgG had declined to undetectable levels by 20 weeks post immunisation yet all animals were still protected. Specific protection was not explained by priming for a rapid PVM-specific antibody response as there was no difference in specific IgG titres between rAd5N-immunised and control vector or rAd5Z-immunised animals post-challenge (data not shown). PVM-specific antibody responses might be weak because PVM M and N proteins do not contain strong B-cell epitopes or because the route of administration could not elicit such responses. The latter is unlikely since rAd5 expressing HRSV antigens elicited good serum IgG responses when delivered by the i.n. route [13], [26] and our immunisations reliably elicited Ad5-specific IgG. We also considered whether our protocol might have elicited greater mucosal than serum antibody responses, however we did not detect any PVM-specific IgA in BAL fluid obtained from vaccinated mice. Therefore, whilst it remains possible that rAd5N and rAd5M each elicited some form of antibody that our assays could not detect and which was the basis of protection, we consider this unlikely.
In contrast to the weak PVM-specific antibody responses, rAd5N immunisation produced strong PVM-specific CD8+ and CD4+ T cell responses in the spleen. PVM-specific T cells are known to protect against challenge [23] and we propose that this cellular response is a major factor mediating specific protection in our study. Since rAd5N elicited protection against PVM challenge in three different mouse strains, this suggests that PVM N contains T-cell epitopes that are recognised in multiple MHC backgrounds.
Previous studies with rAd HRSV vaccine candidates have included vectors expressing the F protein [25], [26], [44], [45], G protein elements [13] or F, N and M2-1 proteins [46] which elicited strong anti-HRSV serum antibody and potent T-cell responses in mice. In contrast to PVM rAd5 studied here, vaccination with a chimpanzee rAd (PanAd3) expressing F, N and M2-1 caused accelerated onset of weight loss in mice following HRSV challenge, a difference most likely due to the high titre HRSV challenge inoculum encountering a strong CD8+ response induced by M2-1 [46]. However, whilst these studies demonstrate that various rAds and dosing regimens can inhibit HRSV replication in mouse lungs, the failure of even high-dose HRSV challenge of mice to fully replicate the pathogenesis of RSV infection in humans means the implications of these studies for protection of humans against HRSV disease are not clear.
Whilst 107 pfu rAd5N and rAd5M clearly elicited antigen-specific protection against PVM, significant non-specific protection against PVM disease was seen with a 10-fold higher dose rAd5Z, albeit less effective than equivalent doses of rAd5N or rAd5M. A similar finding of non-specific protection was reported previously in ferrets immunised with high dose rAd: though having no effect on SARS coronavirus replication in the nose, control rAd reduced virus penetration into the lung and consequent pathology [47]. Many other studies of rAd immunisation have not included both empty rAd and non-immunised controls so any non-specific protection would not have been detected. In one further study that incorporated both controls, non-specific protection in the airway was not seen after rAd delivery to mice i.m. [48]; this difference from our findings likely reflects the distinct immunisation and challenge target tissues preventing any non-specific local responses to the immunogen being protective. Further investigation is needed to understand the nature and scope of non-specific protection against respiratory infection following high-dose intranasal rAd delivery.
One potential issue in extrapolating the present study to protection against HRSV in humans is that widespread immunity to Ad5 might compromise immunisation of infants with rAd5 via maternal antibody [49]. However pre-existing circulating Ad5 antibody did not prevent a response to mucosally delivered rAd5-Ebola antigen that was sufficient to protect mice against lethal Ebolavirus challenge [50] so pre-existing antibody to rAd5 is not an absolute barrier to effective use. Alternatively, vectors based on either human Ad types of lower sero-prevalence, non-human Ads [45] or chimeric Ad5 vectors having substitutions in key antigenic regions [51] may be used, although these alternative Ad types may not share the strong immunogenicity of Ad5 [52].
In summary, we have shown that replication-deficient rAd5 vectors expressing internal antigens of PVM elicit robust protection against PVM disease in multiple mouse strains when delivered i.n. Protection can be generated with a single dose, and a two-dose regime provides long-term protection. As PVM pathogenesis in rodents is similar to HRSV infection in humans, our findings of successful protection against disease in this model suggest that a similar strategy could be applied to protect against HRSV disease in humans.
Conflicts of interest statement
The authors declare no conflicts of interest.
Acknowledgements
The authors gratefully acknowledge the technical support provided by staff of the Biomedical Support Unit, University of Warwick, Sara Wyld at The Pirbright Institute, Compton, and Drs Paul Scott and Susan Morris for valuable discussions. HEM was supported by a studentship from the Medical Research Council, U.K. The funder had no involvement in the design of the study.
Contributor Information
Helen E. Maunder, Email: H.Maunder@oxfordbiomedica.co.uk.
Geraldine Taylor, Email: geraldine.taylor@pirbright.ac.uk.
Keith N. Leppard, Email: keith.leppard@warwick.ac.uk.
Andrew J. Easton, Email: a.j.easton@warwick.ac.uk.
References
- 1.Easton A.J., Ling R. Mononegavirales. In: Mahy B.W.J., Van Regenmortel M.H.V., editors. Encyclopedia of virology. 3rd ed. Elsevier; Oxford, UK: 2008. pp. 324–334. [Google Scholar]
- 2.Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall C.B., Weinberg G.A., Iwane M.K., Blumkin A.K., Edwards K.M., Staat M.A. The burden of respiratory syncytial virus infection in young children. New Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall C.B., Walsh E.E., Long C.E., Schnabel K.C. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 5.Cowton V.M., McGivern D.R., Fearns R. Unravelling the complexities of respiratory syncytial virus RNA synthesis. J Gen Virol. 2006;87:1805–1821. doi: 10.1099/vir.0.81786-0. [DOI] [PubMed] [Google Scholar]
- 6.Kim H.W., Canchola J.G., Brandt C.D., Pyles G., Chanock R.M., Jensen K. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 7.Delgado M.F., Coviello S., Monsalvo A.C., Melendi G.A., Hernandez J.Z., Batalle J.P. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polack F.P., Teng M.N., Collins P.L., Prince G.A., Exner M., Regele H. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med. 2002;196:859–865. doi: 10.1084/jem.20020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knudson C.J., Hartwig S.M., Meyerholz D.K., Varga S.M. RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog. 2015;11:e1004757. doi: 10.1371/journal.ppat.1004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karron R.A., Wright P.F., Belshe R.B., Thumar B., Casey R., Newman F. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191:1093–1104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 11.Murawski M.R., McGinnes L.W., Finberg R.W., Kurt-Jones E.A., Massare M.J., Smith G. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J Virol. 2010;84:1110–1123. doi: 10.1128/JVI.01709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H., Dennis V.A., Pillai S.R., Singh S.R. RSV fusion (F) protein DNA vaccine provides partial protection against viral infection. Virus Res. 2009;145:39–47. doi: 10.1016/j.virusres.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu J.-R., Kim S., Lee J.-B., Chang J. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. J Virol. 2008;82:2350–2357. doi: 10.1128/JVI.02372-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng R., Zhang Z., Mei X., Gong W., Wei L. Protective effect of a RSV subunit vaccine candidate G1F/M2 was enhanced by a HSP70-like protein in mice. Biochem Biophys Res Commun. 2008;377:495–499. doi: 10.1016/j.bbrc.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg H.F., Bonville C.A., Easton A.J., Domachowske J.B. The pneumonia virus of mice infection model for severe respiratory syncytial virus infection: identifying novel targets for therapeutic intervention. Pharmacol Ther. 2005;105:1–6. doi: 10.1016/j.pharmthera.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Horsfall F.L.J., Hahn R.G. A latent virus in normal mice mapable of producing pneumonia in its natural host. J Exp Med. 1940;71:391–408. doi: 10.1084/jem.71.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook P.M., Eglin R.P., Easton A.J. Pathogenesis of pneumovirus infections in mice: detection of pneumonia virus of mice and human respiratory syncytial virus mRNA in lungs of infected mice by in situ hybridization. J Gen Virol. 1998;79:2411–2417. doi: 10.1099/0022-1317-79-10-2411. [DOI] [PubMed] [Google Scholar]
- 18.Dyer K., Garcia-Crespo K., Glineur S., Domachowske J., Rosenberg H. The pneumonia virus of mice (PVM) model of acute respiratory infection. Viruses. 2012;4:3494–3510. doi: 10.3390/v4123494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao H.-Y., Yu S.-L., Sia C., Chen Y., Chitra E., Chen I.H. Immunogenic properties of RSV-B1 fusion (F) protein gene-encoding recombinant adenoviruses. Vaccine. 2009;27:5460–5471. doi: 10.1016/j.vaccine.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Claassen E.A., van der Kant P.A., Rychnavska Z.S., van Bleek G.M., Easton A.J., van der Most R.G. Activation and inactivation of antiviral CD8 T cell responses during murine pneumovirus infection. J Immunol. 2005;175:6597–6604. doi: 10.4049/jimmunol.175.10.6597. [DOI] [PubMed] [Google Scholar]
- 21.Ostler T., Ehl S., Pulmonary T. cells induced by respiratory syncytial virus are functional and can make an important contribution to long-lived protective immunity. Eur J Immunol. 2002;32:2562–2569. doi: 10.1002/1521-4141(200209)32:9<2562::AID-IMMU2562>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Simmons C.P., Hussell T., Sparer T., Walzl G., Openshaw P., Dougan G. Mucosal delivery of a respiratory syncytial virus CTL peptide with enterotoxin-based adjuvants elicits protective, immunopathogenic, and immunoregulatory antiviral CD8+ T cell responses. J Immunol. 2001;166:1106–1113. doi: 10.4049/jimmunol.166.2.1106. [DOI] [PubMed] [Google Scholar]
- 23.van Helden M.J.G., van Kooten P.J.S., Bekker C.P.J., Gröne A., Topham D.J., Easton A.J. Pre-existing virus-specific CD8+ T-cells provide protection against pneumovirus-induced disease in mice. Vaccine. 2012;30:6382–6388. doi: 10.1016/j.vaccine.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor G., Thomas L.H., Wyld S.G., Furze J., Sopp P., Howard C.J. Role of T-lymphocyte subsets in recovery from respiratory syncytial virus infection in calves. J Virol. 1995;69:6658–6664. doi: 10.1128/jvi.69.11.6658-6664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu Y., He J., Zheng X., Wu Q., Zhang M., Wang X. Intranasal immunization with a replication-deficient adenoviral vector expressing the fusion glycoprotein of respiratory syncytial virus elicits protective immunity in BALB/c mice. Biochem Biophys Res Commun. 2009;381:528–532. doi: 10.1016/j.bbrc.2009.02.075. [DOI] [PubMed] [Google Scholar]
- 26.Kohlmann R., Schwannecke S., Tippler B., Ternette N., Temchura V.V., Tenbusch M. Protective efficacy and immunogenicity of an adenoviral vector vaccine encoding the codon-optimized F protein of respiratory syncytial virus. J Virol. 2009;83:12601–12610. doi: 10.1128/JVI.01036-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santra S., Seaman M.S., Xu L., Barouch D.H., Lord C.I., Lifton M.A. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J Virol. 2005;79:6516–6522. doi: 10.1128/JVI.79.10.6516-6522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park K.S., Lee J., Ahn S.S., Byun Y.H., Seong B.L., Baek Y.H. Mucosal immunity induced by adenovirus-based H5N1 HPAI vaccine confers protection against a lethal H5N2 avian influenza virus challenge. Virology. 2009;395:182–189. doi: 10.1016/j.virol.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Belshe R.B., Edwards K.M., Vesikari T., Black S.V., Walker R.E., Hultquist M. Live attenuated versus inactivated influenza vaccine in infants and young children. New Engl J Med. 2007;356:685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 30.Alwan W.H., Record F.M., Openshaw P.J. Phenotypic and functional characterization of T cell lines specific for individual respiratory syncytial virus proteins. J Immunol. 1993;150:5211–5218. [PubMed] [Google Scholar]
- 31.Cherrie A.H., Anderson K., Wertz G.W., Openshaw P.J. Human cytotoxic T cells stimulated by antigen on dendritic cells recognize the N, SH, F, M, 22K, and 1b proteins of respiratory syncytial virus. J Virol. 1992;66:2102–2110. doi: 10.1128/jvi.66.4.2102-2110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh K.B., Sidney J., Welch M., Fremgen D.M., Sette A., Oldstone M.B.A. CD8+ T-cell epitope mapping for pneumonia virus of mice in H-2b mice. J Virol. 2013;87:9949–9952. doi: 10.1128/JVI.00339-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claassen E.A.W., van der Kant P.A.A., Rychnavska Z.S., van Bleek G.M., Easton A.J., van der Most R.G. Activation and inactivation of antiviral CD8 T cell responses during murine pneumovirus infection. J Immunol. 2005;175:6597–6604. doi: 10.4049/jimmunol.175.10.6597. [DOI] [PubMed] [Google Scholar]
- 34.Taylor G., Thomas L.H., Furze J.M., Cook R.S., Wyld S.G., Lerch R. Recombinant vaccinia viruses expressing the F G or N, but not the M2, protein of bovine respiratory syncytial virus (BRSV) induce resistance to BRSV challenge in the calf and protect against the development of pneumonic lesions. J Gen Virol. 1997;78:3195–3206. doi: 10.1099/0022-1317-78-12-3195. [DOI] [PubMed] [Google Scholar]
- 35.Carine L., Mathieu B., Laurent R., Jean-Francois T., Karl W., Stefan R. Vaccination of calves using the BRSV nucleocapsid protein in a DNA prime-protein boost strategy stimulates cell-mediated immunity and protects the lungs against BRSV replication and pathology. Vaccine. 2008;26:4840–4848. doi: 10.1016/j.vaccine.2008.06.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roux X., Dubuquoy C., Durand G., Tran-Tolla T.L., Castagne N., Bernard J. Sub-nucleocapsid nanoparticles: a nasal vaccine against respiratory syncytial F virus. PLoS ONE. 2008;3:e1766. doi: 10.1371/journal.pone.0001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook P.M. University of Warwick; 1996. Pathogenesis and persistence of pneumonia virus of mice. (PhD thesis) [Google Scholar]
- 38.Thimmappaya B., Weinberger C., Schneider R.J., Shenk T., Adenovirus V.A.I. RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982;31:543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- 39.Barr J., Chambers P., Pringle C.R., Easton A.J. Sequence of the major nucleocapsid protein gene of pneumonia virus of mice: sequence comparisons suggest structural homology between nucleocapsid proteins of pneumoviruses, paramyxoviruses, rhabdoviruses and filoviruses. J Gen Virol. 1991;72:677–685. doi: 10.1099/0022-1317-72-3-677. [DOI] [PubMed] [Google Scholar]
- 40.Easton A.J., Chambers P. Nucleotide sequence of the genes encoding the matrix and small hydrophobic proteins of pneumonia virus of mice. Virus Res. 1997;48:27–33. doi: 10.1016/s0168-1702(96)01430-x. [DOI] [PubMed] [Google Scholar]
- 41.Easton A.J., Scott P.D., Edworthy N.L., Meng B., Marriott A.C., Dimmock N.J. A novel broad-spectrum treatment for respiratory virus infections: influenza-based defective interfering virus provides protection against pneumovirus infection in vivo. Vaccine. 2011;29:2777–2784. doi: 10.1016/j.vaccine.2011.01.102. [DOI] [PubMed] [Google Scholar]
- 42.Bonville C.A., Bennett N.J., Percopo C.M., Branigan P.J., Del Vecchio A.M., Rosenberg H.F. Diminished inflammatory responses to natural pneumovirus infection among older mice. Virology. 2007;368:182–190. doi: 10.1016/j.virol.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 43.Tatsis N., Ertl H.C.J. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu Y.-H., He J.-S., Qiao W., Jiao Y.-Y., Hua Y., Zhang Y. Intranasal immunization with a helper-dependent adenovirus vector expressing the codon-optimized fusion glycoprotein of human respiratory syncytial virus elicits protective immunity in BALB/c mice. Virol J. 2013;10:183. doi: 10.1186/1743-422X-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma A., Wendland R., Sung B., Wu W., Grunwald T., Worgall S. Maternal immunization with chimpanzee adenovirus expressing RSV fusion protein protects against neonatal RSV pulmonary infection. Vaccine. 2014;32:5761–5768. doi: 10.1016/j.vaccine.2014.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pierantoni A., Esposito M.L., Ammendola V., Napolitano F., Grazioli F., Abbate A. Mucosal delivery of a vectored RSV vaccine is safe and elicits protective immunity in rodents and nonhuman primates. Mol Ther—Methods Clin Dev. 2015;2:15018. doi: 10.1038/mtm.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.See R.H., Petric M., Lawrence D.J., Mok C.P.Y., Rowe T., Zitzow L.A. Severe acute respiratory syndrome vaccine efficacy in ferrets: whole killed virus and adenovirus-vectored vaccines. J Gen Virol. 2008;89:2136–2146. doi: 10.1099/vir.0.2008/001891-0. [DOI] [PubMed] [Google Scholar]
- 48.Steitz J., Barlow P.G., Hossain J., Kim E., Okada K., Kenniston T. A Candidate H1N1 pandemic influenza vaccine elicits protective immunity in mice. PLoS ONE. 2010;5:e10492. doi: 10.1371/journal.pone.0010492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mast T.C., Kierstead L., Gupta S.B., Nikas A.A., Kallas E.G., Novitsky V. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2009;28:950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 50.Croyle M.A., Patel A., Tran K.N., Gray M., Zhang Y., Strong J.E. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS ONE. 2008;3:e3548. doi: 10.1371/journal.pone.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts D.M., Nanda A., Havenga M.J.E., Abbink P., Lynch D.M., Ewald B.A. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 52.Abbink P., Lemckert A.A.C., Ewald B.A., Lynch D.M., Denholtz M., Smits S. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


