Abstract
Since the first case of human infection by the Middle East respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia in June 2012, more than 2260 cases of confirmed MERS-CoV infection and 803 related deaths have been reported since the 16th of October 2018. The vast majority of these cases (71%) were reported in Saudi Arabia but the epidemic has now spread to 27 countries and has not ceased 6 years later, unlike SARS-CoV that disappeared a little less than 2 years after emerging. Due to the high fatality rate observed in MERS-CoV infected patients (36%), much effort has been put into understanding the origin and pathophysiology of this novel coronavirus to prevent it from becoming endemic in humans. This review focuses in particular on the origin, epidemiology and clinical manifestations of MERS-CoV, as well as the diagnosis and treatment of infected patients. The experience gained over recent years on how to manage the different risks related to this kind of epidemic will be key to being prepared for future outbreaks of communicable disease.
Keywords: Emerging disease, MERS-CoV, Coronavirus, Pneumonia
Résumé
Depuis le premier cas d’infection liée au Middle East Respiratory Syndrome – Coronavirus (MERS-CoV), détecté en Arabie Saoudite en juin 2012, le MERS-CoV a donné lieu, au 16 octobre 2018, à plus de 2260 cas d’infections confirmées et à 803 décès. La grande majorité des cas (71 %) ont été déclarés en Arabie Saoudite mais l’épidémie a depuis touché 27 pays et n’est toujours pas enrayée 6 ans après son émergence, contrairement au SRAS-CoV qui a disparu un peu moins de deux ans après sa première détection. En raison du taux important de décès observé parmi les patients infectés par le MERS-CoV (36 %), beaucoup d’efforts ont été déployés pour comprendre l’origine et la physiopathologie de ce nouveau coronavirus ainsi que pour lutter contre une éventuelle installation endémique de ce virus au sein de la population humaine. Cette revue s’attache plus particulièrement à retracer l’origine et l’épidémiologie du MERS-CoV à décrire la clinique observée chez les patients ainsi que la prise en charge diagnostique et thérapeutique des patients infectés. L’expérience acquise au cours des dernières années dans la gestion des différents risques liés à ce type d’épidémie est importante pour pouvoir faire face à la prochaine émergence d’infection transmissible.
Mots clés: Maladies émergentes, MERS-CoV, Coronavirus, Pneumonie
1. Introduction
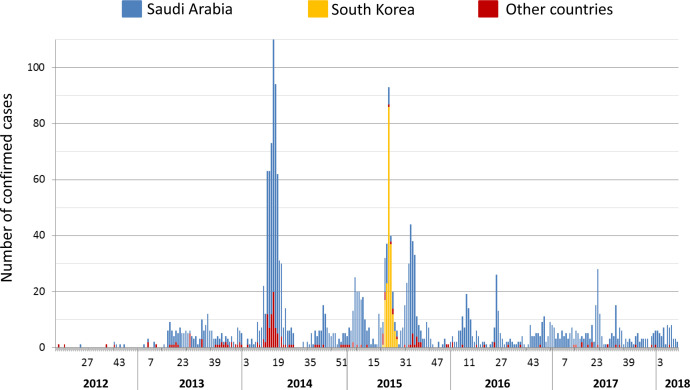
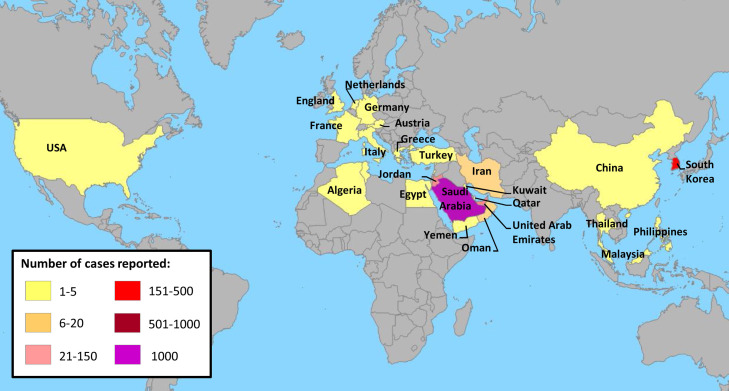
The first case of infection attributed to Middle East respiratory syndrome coronavirus (MERS-CoV) was detected in Saudi Arabia in June 2012 [1]. MERS-CoV then spread to several neighboring countries, mainly Jordan and Qatar (see Fig. 2), and imported cases of the disease were reported throughout the world in Asia, Africa, Europe and the Americas [2]. By the 16th of October 2018, 2260 confirmed cases of infection with MERS-CoV had been documented in 27 countries by the World Health Organization (WHO) and were associated with 803 deaths [2]. The vast majority of the cases (73%) were reported in Saudi Arabia and only one widespread outbreak was observed outside of the Arabian peninsula in South Korea in 2015 [3] (Fig. 1, Fig. 2 ). Due to the disease's high fatality rate (36%) [2], much effort has been put into understanding the origin and pathophysiology of this novel coronavirus to prevent it from becoming endemic in humans.
Fig. 2.
Distribution over time of confirmed cases of MERS-CoV infection worldwide. World Health Organization (WHO) data on September 10th, 2018.
Fig. 1.
Geographical distribution of confirmed cases of MERS-CoV infection. World Health Organization (WHO) data on September 10th, 2018.
This review focuses in particular on tracking down the origin of MERS-CoV, its epidemiology and clinical manifestations, as well as the diagnosis and treatment of infected patients.
2. Origin and emergence of the virus
2.1. Human coronaviruses
The first two coronaviruses demonstrated to cause respiratory infections in humans, the coronaviruses 229E and OC43, were identified in the 1960s. They were held responsible for respiratory infections of moderate severity in humans. Despite these viruses being identified in several reports as causing lower respiratory tract infections, it was generally accepted that coronaviruses were of low pathogenicity until the emergence of SARS-CoV (Severe Acute Respiratory Syndrome Coronavirus) in 2002, a virus with a fatality rate estimated at 10%. The SARS outbreak that resulted in more than 8400 cases was finally contained two years later, in 2004, and the virus has not been detected again since [4]. There was renewed interest in coronavirus research following the SARS epidemic, and two novel endemic human coronaviruses were identified, NL63 and HKU1 respectively in 2004 and 2005, but could not be replicated in cell culture. Both of these new viruses were responsible for respiratory infections of moderate seriousness like the coronaviruses 229E and OC43. Great effort has been made to identify coronaviruses in animal populations, both before and after the SARS outbreak, in order to better understand and control the risk of animal-to-human transmission. This resulted in the discovery of coronaviruses in numerous animal species, with a few exceptions such as sheep and goats, fish and non-human primates [5].
2.2. Emergence of the MERS epidemic
The first case of MERS-CoV infection was reported in Jeddah, Saudi Arabia, in June 2012 [1]. The patient, a 60-year-old man, died from lung and kidney failure 11 days after being admitted to hospital. Very shortly afterwards, in September 2012, a second patient was admitted to hospital in the United Kingdom for severe respiratory infection related to a novel coronavirus following travel to the Middle East. The new virus was found to replicate in a tissue culture model and was rapidly isolated and identified for both cases [6], [7]. Retrospectively, other cases of the disease were found to have occurred before the 2 aforementioned cases: in April 2012, an outbreak at Zarqa hospital in Jordan affected the staff of the intensive care unit, with two fatal cases. The respiratory samples collected were later confirmed to be positive for MERS-CoV [8].
These initial cases were rapidly followed by a series of outbreaks in all Saudi Arabian provinces that were characterized by the infection of health professionals in direct contact with the patients. Other similar outbreaks were observed in several neighboring countries: Qatar, Bahrain, Kuwait, Jordan and Tunisia. Health authorities reacted quickly to the reports of these epidemics and the strong resemblance with observed clinical features of SARS-CoV infections. Indeed, although a few patients developed mild infections, the fatality rate for patients infected with MERS-CoV was over 30% [2].
2.3. Natural reservoir of MERS-CoV
Following the identification of MERS-CoV, great effort was put into finding which animal species it originated from in order to stop the further spread of the disease to humans. MERS-CoV was very rapidly determined to be genotypically closely related to the betacoronavirus lineage C viruses identified in bats [9]. Based on these findings, and the major role of bats in the genetic diversity and spread of coronaviruses, much of the initial work aiming at finding the natural reservoir of MERS-CoV focused on bats. However, no conclusive evidence demonstrating that bats were the natural reservoir of MERS-CoV in the Arabian peninsula were found, despite the identification of closely related viruses in bats in Sub-Saharan Africa [10], far from the existing outbreaks. Very strong epidemiological links were identified between the human cases and camels and resulted in the isolation in camels of viruses that were directly related to MERS-CoV and that could replicate in cultured human cells [11]. The investigation of dromedary camel serum collections, some of which collected as early as 1983, demonstrated that the virus was already widespread (seropositivity rate > 80%) in the East African countries (Somalia, Sudan and Egypt). These countries export dromedary camels to Arabian countries, but also in Kenya, Nigeria, Tunisia, Ethiopia, Burkina Faso and Morocco [12], [13], [14]. Phylogenetic analysis revealed 5 distinct coronavirus lineages in dromedary camels, including one recombinant lineage that led to the MERS-CoV epidemic in humans [15].
3. Virus structure and cycle
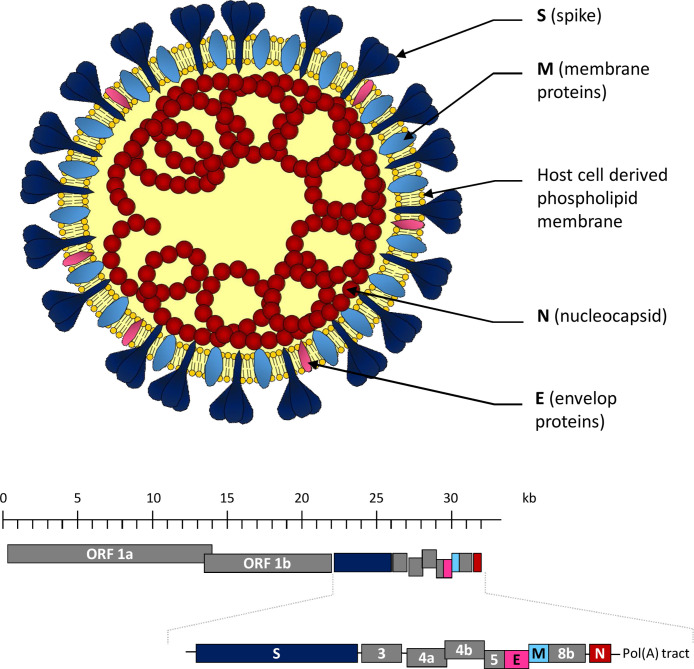
MERS-CoV is a betacoronavirus belonging to lineage C. It is an enveloped virus with a positive-sense single-stranded RNA genome of about 30 kb. Under electron microscopy, virions are generally spherical with surface projections (spikes) formed by the surface protein S creating an image reminiscent of a crown or solar corona. The positive-sense single-stranded RNA genome acts as messenger RNA (mRNA) with a 5′ cap and a 3′ polyadenylated tail. It plays three roles during the host cell cycle: (i) it acts as the initial RNA molecule for the infection cycle; (ii) it is the template for replication and transcription; (iii) it is the substrate that is packaged into the newly assembled viral particles [16].
The MERS-CoV genome is organized in the same way as other coronavirus species. The first two thirds of the MERS-CoV genome contain two overlapping reading frames (ORF1a and ORF1b) that translate into the replication-transcription complex including 16 non-structural proteins. The remaining third of the genome encodes the four structural proteins, the spike (S), envelop (E), membrane (M) and nucleocapsid (N) proteins, as well as five accessory proteins (ORF3, ORF4a, ORF4b, ORF5 and ORF8b) that are not required for genome replication but are probably involved in virulence. The flanking sequences, on both ends of the genome, contain untranslated 5′ and 3′ regions (UTR) (Fig. 3 ) [17].
Fig. 3.
Structure and genomic organization of MERS-CoV.
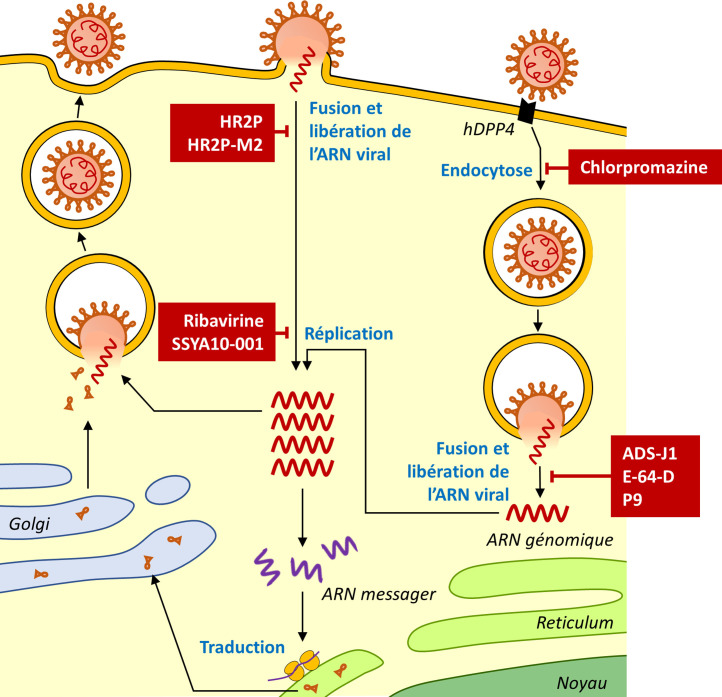
The viral particle can enter the cell in two ways, which probably contribute to the broad tissue tropism of this virus that replicates mainly in respiratory epithelial cells but can also infect many other cell types. Via the endosomal pathway, the S1 domain of the MERS-CoV spike protein (S) binds its receptor, dipeptidyl peptidase 4 (DPP4) [18], induces endocytosis of the viral particle and a change in the conformation of the S2 subunit of the S protein that then mediates virus-host membrane fusion and uncoating of virus RNA. MERS-CoV can also enter host cells via a non-endosomal mechanism by direct fusion of the virus with the plasma membrane following S protein cleavage by human proteases [19].
Following entry into the cytoplasm and uncoating of the virus nucleocapsid, the viral genomic RNA is translated to produce two polypeptides, pp1a and pp1b, that form the replicase-transcriptase complex. This initial replicase-transcriptase complex uses the genomic RNA to produce 16 non-structural proteins that assemble into the replication complex. The replication complex then replicates the genomic RNA and produces other subgenomic RNAs that ensure the translation of the structural proteins.
Virions are assembled at the endoplasmic reticulum membrane as viral proteins and genomic RNA are grouped together and then bud into the lumen of the endoplasmic reticulum. The virions are then exported via the secretory pathway of the endoplasmic reticulum into the Golgi intermediate compartment and then into the extracellular environment. The M protein drives the packaging process by selecting and organizing the viral envelop components at the assembly sites and interacting with the nucleocapsid to allow budding [20].
4. Transmission mechanisms and preventive measures
4.1. Transmission mechanisms
Several large serology studies suggest that cases of asymptomatic or mild MERS-CoV infection occur regularly, although infrequently. The importance of such cases is difficult to assess [10]. It is therefore difficult to determine whether these cases are due to or take part in human-to-human transmission. Several studies suggest that less than 50% of infected patients transmit the virus to individuals they come into contact with, even at the beginning of an outbreak [10]. The disease therefore seems to spread due to frequent animal-to-human transmission, from camels to humans, with limited subsequent human-to-human transmissions [21]. There are unfortunately exceptions to this observation and local outbreaks caused by human-to-human transmission have been observed on a regular basis, mostly in hospitals. To date, the most poignant example is the outbreak that occurred in South Korea in which the index case caused 185 secondary cases, among whom 30 were care providers, leading to 24 fatalities [3]. This outbreak was characterized by the key role of a few “super spreaders”, delayed diagnosis, high doctor shopping behavior and the importance of confined spaces (waiting room, hospital room, ambulance). In this example, the resemblance with SARS-CoV's spreading mechanisms is striking, despite lower degrees of transmission to care providers for MERS-CoV [22]. These regular cases of imported-MERS, the most recent was reported in England in August 2018 [23], represent a real threat of local epidemics outside Saudi Arabia and special screening and isolation procedures need to be implemented in units likely to receive patients suspected of MERS-CoV infections.
4.2. Preventive measures for travelers
When possible, the first measure to be taken is to delay departure, in particular for individuals over 65 or with chronic disease, and for pregnant women or children. Such measures are nevertheless challenging to maintain today as that the virus is still present 6 years after its apparition.
All other preventive measures aim at preventing both animal-to-human transmission and human-to-human transmission. It is therefore recommended to avoid any contact with domestic animals (firstly dromedary camels), their secretions, raw milk and insufficiently cooked meat. It is also advised to avoid eating fruit and vegetables that might have been in contact with animal secretions if not washed and peeled by oneself.
To avoid human-to-human transmission, the usual recommendations for preventing the spread of any respiratory virus should be applied: hand washing with soapy water or an alcohol-based solution, covering one's nose and mouth when sneezing, refraining from shaking hands and touching one's mouth and nose with one's hands, avoiding contact with people with respiratory symptoms.
Finally, a last series of recommendations focus on how to behave in case of suspicious symptoms: (i) consult a doctor as soon as symptoms occur during travel and delay the return until symptoms disappear; (ii) if symptoms occur with 14 days of returning home, consult a doctor and tell him/her about the recent travel [24].
5. Diagnostic laboratory testing
PCR-based detection methods are currently the preferred option for detecting the virus in respiratory samples and making a diagnosis of MERS-CoV infection. Serology tests can also be performed and are often used for second-line diagnostic investigation in patients with a high suspicion of MERS-CoV but negative results by direct PCR testing.
5.1. Direct PCR detection
Various respiratory matrices can be used: nasopharyngeal swabs, nasopharyngeal or tracheal aspirates, bronchoalveolar lavage (BAL), and even in some cases, induced sputum. The deepest samples, tracheal aspirates and BALs, show the greatest sensitivity and significantly higher viral loads [25].
The genome amplification and detection methods used (PCR) were initially mostly developed in situ and performed in biosafety level 3 (BSL-3) reference facilities. The time to results is generally relatively long, 24–48 h, due to the usual time required for conventional PCR testing to which must be added the additional preparation and sample neutralization time needed to protect the laboratory staff against this virus. The PCR methods used are generally semi-quantitative and some studies suggest a correlation between the amount of virus detected and the severity of the symptoms [26]. Nevertheless, no consensus has been reached yet regarding a threshold level that could actually predicts clinical severity. Targeting the envelop gene upE is recommended with confirmatory testing for ORF 1A or 1B or the N gene. If results diverge, sequencing is sometimes required to obtain conclusive results [27]. Today, an increasing number of commercial tests are becoming available (Altona Diagnostics, Fast Track Diagnostics, Primerdesing Ltd.) some even with a time to results of less than 1 hour (BioFire–bioMérieux). Some of these tests are point-of-care, or can be performed in BSL3 facilities or a standard laboratory following sample neutralization in a BSL3 facility. These commercial tests must always be validated before use to check their sensitivity and compare their performance with reference methods.
5.2. Indirect serological testing
As with any other acute viral infection, antibodies can only generally be detected about 10 days after the onset of symptoms. In some patients, especially those with severe infections, the time interval to antibody detection may be even longer [28]. Serological testing is therefore of little help for the initial diagnosis of symptomatic patients, but can be useful for epidemiological investigations.
The highly immunogenic S and N viral proteins are widely used targets for serological tests and are found on all coronaviruses. Various approaches have been developed: serum neutralization assays [29], microarrays [30], or more recently ELISA confirmed by a microneutralization test [31]. All methods are technically complex and require a high level of expertise that restrict their use to a few highly specialized facilities.
6. Clinical presentation
The first cases of infection with MERS-CoV were reported in 2012 [1]. Hospital-acquired MERS-CoV infections have been described worldwide and represented a third of all cases reported in Saudi Arabia in the early stages of the epidemic [1], [32], [33]. Clustered hospital-acquired infections were frequently observed during the first outbreaks and probably contributed to spreading the disease from the primary site of virus infection to the whole Arabian peninsula, the most striking example of hospital-acquired outbreak being the Korean outbreak in 2015 [34]. Care providers are often affected and represent 15–22% of cases [33], [34], [35], [36], [37], [38], [39].
Most of the cases are described in Middle East countries, in particular Saudi Arabia (73%), with a predominance of male patients (66–69% in various studies) and a mean patient age ranging from 40 to 55 years [34], [38], [40].
Comorbidities are found in 46–68.6% of patients, in particular diabetes and high blood pressure, followed by other heart conditions and finally obesity [34], [37], [38], [41].
The mean incubation time is 5 to 6.5 days. The generation interval (time between the onset of symptoms of the first case and those of the second case) is 7.6 days, which is identical to that of the respiratory syncytial virus (RSV) but threefold more than the influenza virus [36], [42], [43], [44].
6.1. Clinical symptoms
The main challenge of MERS-CoV infection is the absence of specific clinical features for differential diagnosis with other viral respiratory diseases [37], [45]. This difficulty, combined with precautionary action taken to avoid potential secondary contamination with MERS-CoV [46], can result in medical confusion and inappropriate patient management due to prolonged, difficult isolation that makes it impossible to perform the necessary complementary tests while waiting for PCR results [47].
The clinical features of MERS-CoV infection are extremely variable, ranging from an absence of symptoms (14–80% of cases) to a flu-like syndrome, pneumonia and acute respiratory distress syndrome (ARDS) [37], [48].
The three most frequent symptoms are: fever (77% [IQR: 59–82]), cough (90% [52–69]), and dyspnea (68% [22–69]).
Many other secondary symptoms have been reported, such as sputum production (40%), odynophagia (39%), digestive system signs (20%), hemoptysis (4.3%), myalgia (43%) and headache (20%) [34], [37], [41], [42].
Diarrhea is significantly more frequent in patients infected with MERS-CoV than in patients with another acute, febrile respiratory conditions [45].
6.2. Severe MERS
Severe MERS is characterized predominantly by ARDS, acute kidney failure, and in the most severe cases, by multiple organ failure that can be fatal [49], [50]. One third of patients develop pneumonia and 20% develop ARDS [51].
The median time to respiratory failure is 12 days after the onset of symptoms. Depending on studies, 53 to 89% of hospitalized patients are admitted to an intensive care unit (ICU) [43], [52].
6.3. Fatality rate
Since the first MERS outbreak, WHO had documented, in October 2018, 2260 cases of MERS-CoV infection confirmed by laboratory testing and 803 related deaths in 27 different countries.
The retrospective fatality rate varies between outbreaks, ranging from 36.5 to 60% [33], [35], [37], [38], [42]. The mortality rate of 20.4% observed for the Korean outbreak is probably the most reliable epidemiologically due to the comprehensive investigations carried out [34]. The death rate is highest among patients admitted to an ICU, ranging from 58% to 90% [49], [53]. In the only cohort study performed in Saudi Arabia, the fatality rate for MERS-CoV patients was of only 10% (8/80). However, the patients of this cohort were younger, had less symptoms, showed less radiological features and only 17% were admitted to an ICU [37]. The findings of the latter study diverge therefore with the situations observed in other hospitals, but are perhaps a better reflection of the infection profile in the general population in which younger subjects are less symptomatic and therefore less frequently admitted to hospital.
The time interval between the onset of symptoms and death ranges from 11.5 to 27 days [34], [44], [54].
Finally, co-infection with other respiratory viruses, in particular influenza, has been described although the impact of such combined infections have not been evaluated [44], [55]. Co-infections with bacteria have also been reported in the patients developing the most severe disease [49], [51].
6.4. Laboratory findings
There are no specific laboratory findings related to MERS-CoV infection. Nevertheless in patients with acute respiratory infection in MERS-endemic areas, MERS-CoV infections have been associated with normal leukocyte and/or polymorphonuclear neutrophil counts but elevated transaminases [37], [45].
Moreover, hyperleukocytosis, lymphocytopenia, thrombocytopenia, hypoalbuminemia, elevated serum creatinine, LDH and CRP levels, and hypoxemia (PaO2/FiO2 < 300) have been repeated reported in MERS-CoV infected patients and are associated with severity and death [34], [45].
Imaging (chest X-ray and sometimes chest CT) has revealed infection-related features in 51–75% of cases. The lesions observed are uni- or multi-focal ground glass opacifications, of subpleural and lower lobe predominance, with sometimes bilateral bi-basal involvement or features of organizing pneumonia [34], [37], [42], [45].
6.5. Risk factors for mortality
Mortality is highest in elderly, male patients with comorbidities, especially diabetes [38], [45], [56]. Patients from Saudi Arabia and the Middle East have an increased mortality rate compared with patients from Korea or other countries [38], [40]. In contrast, being a medical professional significantly reduces the risk of mortality [38], [45].
Other factors associated with a higher mortality risk have been described in various studies: digestive symptoms, prolonged delay between the onset of symptoms and admission to hospital, smoking, low blood pressure, impaired gas exchange, leukopenia, anemia, disturbance of liver or kidney function, use of mechanical ventilation and prolonged stay in the ICU [42], [57].
For the Korean outbreak in 2015, the independent risk factors for mortality were: age > 55 years, dyspnea, diabetes, chronic lung disease, systolic blood pressure at admission < 90 mmHg, hyperleukocytosis at admission (> 10,000/mm3) and the use of mechanical ventilation [34].
6.6. Risk factors for severe MERS
Positive PCR results for MERS-CoV in blood at diagnosis are associated with an increased risk of requiring mechanical ventilation, extracorporeal membrane oxygenation (ECMO) or to lead to death [58], [59].
The lack or delayed detection of MERS antibodies (ELISA IgG and IgA, or PRNT) in the blood or airways is a poor prognostic factor [54], [60]. It should however be noted that no seroconversion is observed in asymptomatic MERS-infected patients [54]. Finally, the MERS-CoV viral loads in distal lung samples were higher among deceased patients [60].
In a study including 45 patients in a tertiary referral hospital in South Korea:
-
•
the predictive factors for pneumonia in MERS-CoV patients were: age > 45 years, body temperature > 37.5 °C on day 3, platelet counts < 150,000/mm3, lymphocytopenia (< 1000/mm3), CRP ≥ 20 mg/L and high viral loads (Ct value < 28.5);
-
•
the predictive factors for respiratory failure were male sex, high blood pressure, thrombocytopenia, lymphocytopenia, hypoalbuminemia < 35 g/L and CRP ≥ 40 mg/L.
The patients with at least two, one and none of the predictive pneumonia factors developed pneumonia in 100%, 50% and 0% of cases, respectively [61].
7. Treatment of MERS
Several therapeutic options targeting various viral elements are currently available or under development (Fig. 4 ) [62]. The different classes of available treatment are (i) immunotherapy with specific anti-MERS-CoV antibodies, (ii) molecules with antiviral activity, (iii) symptomatic treatment. Few molecules have shown real curative action and the reports in the literature generally describe isolated cases or small series of cases. More studies have focused on associated treatment and supportive care. At this time, preventive therapies are still in preclinical stages.
Fig. 4.
Viral cycle of MERS-CoV and targets of the antiviral drugs available or under development.
7.1. Immunotherapy
7.1.1. Convalescent plasma
The efficacy and safety of plasma from convalescent patients have not been assessed. Three separate reports concluded that such therapeutic approaches were inappropriate [63]. One trial is listed on www.clinicaltrials.gov.
7.1.2. Intravenous immunoglobulins (IgGs)
Two cases of therapy with intravenous polyclonal IgGs have been reported. In one of them, the IgGs originated from donors in regions negative for MERS specific antibodies.
Several monoclonal antibodies were tested and seemed to show anti-MERS-CoV activity in vitro [64]. No clinical trials are currently underway. Recently, a phase I placebo-controlled, dose escalation study evaluated the efficacy of polyclonal IgGs produced by transchromosomal cattle with human immunoglobulin genes immunized with the MERS-CoV spike (S) protein [65]. The primary outcome of tolerance to a single dose was reached. The secondary pharmacodynamic endpoint (serum neutralization activity) showed efficacy with a dose of 50 mg/kg. No phase II trials are currently underway. A phase I study has been registered to assess the immunogenicity and tolerance of a combination of two monoclonal anti-MERS-CoV antibodies. The study has not yet started recruiting patients.
7.2. Antivirals
7.2.1. Interferons (IFN)
Infection with MERS-CoV reduces the host's interferon response. MERS-CoV is 100 times more sensitive to IFN-α. Treatment with IFN-α has been reported for many clinical cases and several retrospective cohort studies have been performed, in combination with ribavirin, lopinavir or mycophenolate mofetil (MMF). None of these studies have demonstrated increased overall survival. One study reported increased survival at D14 but not at D30 for critically ill intubated and ventilated patients [66]. A IFN/MMF combination trial is currently underway (see below).
7.2.2. Ribavirin
High doses of ribavirin have shown anti-MERS-CoV activity in vitro. Ribavirin has been used to treat patients in Saudi Arabia as well as in France for the most severe cases managed in ICUs [67]. No significant effects were demonstrated either on the mortality rate or the time spent in the ICU.
7.2.3. Protease inhibitors
Ritonavir-boosted lopinavir has shown efficacy against MERS-CoV in vitro. As a result, the FDA has extended the indications of lopinavir to patients infected with MERS-CoV. Two case reports (in Greece and Korea) have described improvement in patients treated with lopinavir, type 1 interferon and ribavirin [68]. A phase II-III clinical trial is registered on clinicaltrials.gov. The aim of this study is to evaluate the feasibility, efficacy and safety of the combination lopinavir/ritonavir/recombinant IFNβ-1b vs. a placebo in patients with confirmed MERS receiving optimal symptomatic care.
7.2.4. Chloroquine
Chloroquine is among the molecules approved by the FDA following in vitro studies. No clinical data or studies support its use in vivo at the present time.
7.2.5. Nitazoxamide
In vitro, anti-MERS-CoV activity has been demonstrated for doses of nitazoxamide that could be reached with two daily oral doses. No clinical data or studies support its use in vivo at the present time [69].
7.2.6. Mycophenolate mofetil (MMF)
In vitro, anti-MERS-CoV activity has been demonstrated for doses of MMF that are acceptable for use in humans. MMF seems to show a synergistic effect with IFN-β1b in vitro [70]. But in a non human primate common marmosets model, animals treated with MMF developed more severe lesions and showed a higher case fatality rate compared with untreated animals [70]. In contrast with animal model, the combination IFN-β1b/MMF was administered to 8 patients in Saudi Arabia. All the patients survived but had lower APACHE II scores that other patient groups [71].
7.2.7. Alisporivir
Alisporivir has been shown to provide additive in vitro anti-MERS-CoV activity when used in combination with ribavirin. No clinical data or studies support its use in vivo at the present time [72].
7.2.8. Silvestrol
Silvestrol is a molecule of the flavagline family found in plants. It binds to eIF4A and enhances the affinity of eIF4A for mRNA. This blocks helicase activity and inhibits protein translation. A recent in vitro study demonstrated that silvestrol has anti-MERS-CoV activity [73]. No clinical data or studies support its use in vivo at the present time.
7.2.9. Corticosteroids
Corticosteroid therapy is currently the most widely studied therapeutic option. In a retrospective study, Arabi et al. [67] compared the outcome of 309 patients with confirmed MERS-CoV infection managed in an ICU setting and treated with (151) or without (159) corticosteroid therapy. The overall fatality rate was 67%. Univariate analysis showed that mortality in the ICU, during the hospital stay or at 90 days was higher in the corticosteroid group. Then, following adjustment using a marginal structural model for causal inference, corticosteroid therapy was shown not to be associated with mortality, but delayed virus clearance. These findings, together with the absence of any description of the adverse effects caused by corticosteroid treatment, argue against the use of corticosteroids.
7.3. Extracorporeal membrane oxygenation (ECMO)
A retrospective study was recently carried out in Saudi Arabia in MERS-CoV patients with refractory respiratory failure [74]. The patients were included in the study from 2014 to 2015 in five ICUs. The study consisted of two patient groups: ECMO versus conventional treatment. The primary endpoint was inhospital mortality. Secondary endpoints included the length of stay in the ICU and in hospital. Thirty-five patients were included: 17 were treated with ECMO and 18 received conventional care. Both groups had similar baseline characteristics. Inhospital mortality was lower in the ECMO group (65 vs. 100%; P = 0.02) although they stayed longer in the ICU (median stay of 25 days vs. 8 days; P < 0.01). The overall time in hospital was similar in both groups (median stay of 41 vs. 31 days; P = 0.421). In addition, patients in the ECMO group showed improved PaO2/FiO2 values at 7 and 14 days after admission into the ICU (124 vs. 63, and 138 vs. 36, respectively; P < 0.05), and lower levels of vasoactive amines at D1 and D14 (29 vs. 80%, and 36 vs. 93%, respectively; P < 0.05). The results of this study support the use of ECMO as salvage treatment for MERS patients with respiratory failure, as is the case for other respiratory infections.
8. Vaccine development
Two trials with candidate vaccines are currently registered at https://clinicaltrials.gov/ct2/home. A phase-I clinical trial on healthy volunteers was set up to evaluate the safety and immunogenicity of a plasmid DNA vaccine (GLS-5300) that expresses the S protein of MERS-CoV. This trial was planned to last one year and started in 2016. No results are available yet. A second phase-I trial was started by Oxford University in January 2018. It uses a chimpanzee adenovirus vector containing the MERS-CoV S protein gene [75]. Patient inclusion is currently underway. Many other candidate vaccines using various different technologies are at a less advanced stage of development.
9. Conclusions
The MERS epidemic started in 2012. In contrast with SARS-CoV that disappeared 2 years after it first appeared, MERS-CoV continues to persist in the Middle East 6 years later. Although the disease has not become pandemic, outbreaks have occurred worldwide. Today, it is impossible to predict with certainty whether MERS-CoV will disappear or continue to remain a threat for human populations. Efficient vaccine development for host animals and humans could play a key role in tilting the balance from potentially-pandemic to MERS-CoV elimination. Furthermore, the epidemiological and viral determinants of the emergence of MERS-CoV in the Middle East are difficult to comprehend, due to the high seropositivity rate of African dromedary camels but no similar disease in local human populations.
The constant increase of transcontinental travel, in particular towards the main focal points of MERS outbreaks with religious pilgrimages and mass tourism, raises the problem of the management of patients suspected of MERS-CoV infection and the absence of efficient treatment options to this date. The main problem in non-epidemic countries is to detect a MERS-CoV case among a great number of non-MERS patients. In France, with the exception of the first 2 cases, no further cases have been detected. The current strategy is to isolate any suspicious cases as rapidly as possible to contain the infection and prevent local outbreaks as seen in South Korea. The ability to rapidly test patients suspected to have MERS-CoV infection is the cornerstone of this strategy. The experience gained over the last few years by the health community will also help deal with any respiratory infections that will emerge in the future.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
The chapters on the origin, emergence, structure, transmission mechanisms, prevention and diagnostic methods were mainly written MB, NH, and BV.
BV produced the figures.
The chapters on clinical presentation, prognosis and available treatment options were mainly written by AB and CB.
All authors read, amended and agreed with the entire final manuscript.
References
- 1.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Middle East respiratory syndrome coronavirus (MERS-CoV). WHO; 2018. http://www.who.int/emergencies/mers-cov/en/.(accessed March 5, 2018).
- 3.Cho S.Y., Kang J.-M., Ha Y.E., Park G.E., Lee J.Y., Ko J.-H., et al. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: an epidemiological outbreak study. Lancet. 2016;388:994–1001. doi: 10.1016/S0140-6736(16)30623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). World Health Organization; 2003. https://apps.who.int/iris/handle/10665/70863.
- 5.Forni D., Cagliani R., Clerici M., Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol 2017; 2017;25:35–48. doi: 10.1016/j.tim.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Boheemen S., de Graaf M., Lauber C., Bestebroer T.M., Raj V.S., Zaki A.M., et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012;3 doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermingham A., Chand M.A., Brown C.S., Aarons E., Tong C., Langrish C., et al. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012;17:20290. [PubMed] [Google Scholar]
- 8.WHO | Background and summary of novel coronavirus infection–as of 21 December 2012. WHO; 2012. http://www.who.int/csr/disease/coronavirus_infections/update_20121221/en/(accessed March 21, 2018).
- 9.Woo P.C.Y., Wang M., Lau S.K.P., Xu H., Poon R.W.S., Guo R., et al. Comparative analysis of twelve genomes of three novel group 2c and group 2d coronaviruses reveals unique group and subgroup features. J Virol. 2007;81:1574–1585. doi: 10.1128/JVI.02182-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller M.A., Meyer B., Corman V.M., Al-Masri M., Turkestani A., Ritz D., et al. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological stud. Lancet Infect Dis. 2015;15:629. doi: 10.1016/S1473-3099(15)00029-8. [DOI] [PubMed] [Google Scholar]
- 11.Memish Z.A., Cotten M., Meyer B., Watson S.J., Alsahafi A.J., Al Rabeeah A.A., et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerging Infect Dis. 2014;20:1012–1015. doi: 10.3201/eid2006.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller M.A., Corman V.M., Jores J., Meyer B., Younan M., Liljander A., et al. MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983–1997. Emerging Infect Dis. 2014;20:2093–2095. doi: 10.3201/eid2012.141026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corman V.M., Jores J., Meyer B., Younan M., Liljander A., Said M.Y., et al. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992–2013. Emerging Infect Dis. 2014;20:1319–1322. doi: 10.3201/eid2008.140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miguel E., Chevalier V., Ayelet G., Ben Bencheikh M.N., Boussini H., Chu D.K., et al. Risk factors for MERS coronavirus infection in dromedary camels in Burkina Faso, Ethiopia, and Morocco, 2015. Euro Surveill. 2017:22. doi: 10.2807/1560-7917.ES.2017.22.13.30498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabir J.S.M., Lam T.T.-Y., Ahmed M.M.M., Li L., Shen Y., Abo-Aba S.E.M., et al. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science. 2016;351:81–84. doi: 10.1126/science.aac8608. [DOI] [PubMed] [Google Scholar]
- 16.van Boheemen S., de Graaf M., Lauber C., Bestebroer T.M., Raj V.S., Zaki A.M., et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012:3. doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menachery V.D., Mitchell H.D., Cockrell A.S., Gralinski L.E., Yount B.L., Graham R.L., et al. MERS-CoV Accessory ORFs Play Key Role for Infection and Pathogenesis. MBio. 2017:8. doi: 10.1128/mBio.00665-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Doremalen N., Miazgowicz K.L., Milne-Price S., Bushmaker T., Robertson S., Scott D., et al. Host species restriction of Middle East respiratory syndrome coronavirus through its receptor, dipeptidyl peptidase 4. J Virol. 2014;88:9220–9232. doi: 10.1128/JVI.00676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc Natl Acad Sci U S A. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuman B.W., Kiss G., Kunding A.H., Bhella D., Baksh M.F., Connelly S., et al. A structural analysis of M protein in coronavirus assembly and morphology. J Struct Biol. 2011;174:11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO | Middle East respiratory syndrome coronavirus (MERS-CoV)–update. WHO; 2014. http://www.who.int/csr/don/2014_05_15_mers/en/(accessed April 9, 2018).
- 22.Chowell G., Abdirizak F., Lee S., Lee J., Jung E., Nishiura H., et al. Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 2015;13:210. doi: 10.1186/s12916-015-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.New case of MERS-CoV identified in the United Kingdom. European Centre for Disease Prevention and Control 2018. http://ecdc.europa.eu/en/news-events/new-case-mers-cov-identified-united-kingdom (accessed October 22, 2018).
- 24.Pavli A., Tsiodras S., Maltezou H.C. Middle East respiratory syndrome coronavirus (MERS-CoV): prevention in travelers. Travel Med Infect Dis. 2014;12:602–608. doi: 10.1016/j.tmaid.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Memish Z.A., Al-Tawfiq J.A., Makhdoom H.Q., Assiri A., Alhakeem R.F., Albarrak A., et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis. 2014;210:1590–1594. doi: 10.1093/infdis/jiu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feikin D.R., Alraddadi B., Qutub M., Shabouni O., Curns A., Oboho I.K., et al. Association of Higher MERS-CoV Virus Load with Severe Disease and Death, Saudi Arabia, 2014. Emerging Infect Dis. 2015;21:2029–2035. doi: 10.3201/eid2111.150764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO | Laboratory testing for Middle East Respiratory Syndrome Coronavirus. WHO; 2018. http://www.who.int/csr/disease/coronavirus_infections/mers-laboratory-testing/en/(accessed April 9, 2018).
- 28.Modjarrad K., Moorthy V.S., Ben Embarek P., Van Kerkhove M., Kim J., Kieny M.-P. A roadmap for MERS-CoV research and product development: report from a World Health Organization consultation. Nat Med. 2016;22:701–705. doi: 10.1038/nm.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller M.A., Meyer B., Corman V.M., Al-Masri M., Turkestani A., Ritz D., et al. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis 2015; 2015;15:629. doi: 10.1016/S1473-3099(15)00029-8. [DOI] [PubMed] [Google Scholar]
- 30.Reusken C., Mou H., Godeke G.J., van der Hoek L., Meyer B., Müller M.A., et al. Specific serology for emerging human coronaviruses by protein microarray. Euro Surveill. 2013;18:20441. doi: 10.2807/1560-7917.es2013.18.14.20441. [DOI] [PubMed] [Google Scholar]
- 31.Trivedi S., Miao C., Al-Abdallat M.M., Haddadin A., Alqasrawi S., Iblan I., et al. Inclusion of MERS-spike protein ELISA in algorithm to determine serologic evidence of MERS-CoV infection. J Med Virol. 2018;90:367–371. doi: 10.1002/jmv.24948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guery B., Poissy J., el Mansouf L., Séjourné C., Ettahar N., Lemaire X., et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013;381:2265–2272. doi: 10.1016/S0140-6736(13)60982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alhamlan F.S., Majumder M.S., Brownstein J.S., Hawkins J., Al-Abdely H.M., Alzahrani A., et al. Case characteristics among Middle East respiratory syndrome coronavirus outbreak and non-outbreak cases in Saudi Arabia from 2012 to 2015. BMJ Open. 2017;7:e011865. doi: 10.1136/bmjopen-2016-011865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi W.S., Kang C.-I., Kim Y., Choi J.-P., Joh J.S., Shin H.-S., et al. Clinical Presentation and Outcomes of Middle East Respiratory Syndrome in the Republic of Korea. Infect Chemother. 2016;48:118–126. doi: 10.3947/ic.2016.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oboho I., et al. 2014 MERS-CoV outbreak in Jeddah--a link to health care facilities. NEJM. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ki M. 2015 MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol Health. 2015;37:e2015033. doi: 10.4178/epih/e2015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohd H.A., Memish Z.A., Alfaraj S.H., McClish D., Altuwaijri T., Alanazi M.S., et al. Predictors of MERS-CoV infection: A large case control study of patients presenting with ILI at a MERS-CoV referral hospital in Saudi Arabia. Travel Med Infect Dis. 2016;14:464–470. doi: 10.1016/j.tmaid.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed A.E. The predictors of 3- and 30-day mortality in 660 MERS-CoV patients. BMC Infect Dis. 2017;17:615. doi: 10.1186/s12879-017-2712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Memish Z.A., Zumla A.I., Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369:884–886. doi: 10.1056/NEJMc1308698. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed A.E. Estimating survival rates in MERS-CoV patients 14 and 45 days after experiencing symptoms and determining the differences in survival rates by demographic data, disease characteristics and regions: a worldwide study. Epidemiol Infect. 2017;2017:1–7. doi: 10.1017/S095026881700293X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherbini N., Iskandrani A., Kharaba A., Khalid G., Abduljawad M., Al-Jahdali H. Middle East respiratory syndrome coronavirus in Al-Madinah City, Saudi Arabia: Demographic, clinical and survival data. J Epidemiol Glob Health. 2017;7:29–36. doi: 10.1016/j.jegh.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A.T., et al. Hospital outbreak of middle east respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garbati M.A., Fagbo S.F., Fang V.J., Skakni L., Joseph M., Wani T.A., et al. A Comparative Study of Clinical Presentation and Risk Factors for Adverse Outcome in Patients Hospitalised with Acute Respiratory Disease Due to MERS Coronavirus or Other Causes. PLoS ONE. 2016;11:e0165978. doi: 10.1371/journal.pone.0165978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.eCDC, Rapid risk assessment: Severe respiratory disease associated with Middle East respiratory syndrome coronavirus (MERS-CoV), 22nd update. European Centre for Disease Prevention and Control 2018. http://ecdc.europa.eu/en/publications-data/rapid-risk-assessment-severe-respiratory-disease-associated-middle-east-11 (accessed September 16, 2018).
- 47.Bleibtreu A., Jaureguiberry S., Houhou N., Boutolleau D., Guillot H., Vallois D., et al. Clinical management of respiratory syndrome in patients hospitalized for suspected Middle East respiratory syndrome coronavirus infection in the Paris area from 2013 to 2016. BMC Infect Dis. 2018;18:331. doi: 10.1186/s12879-018-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aleanizy F.S., Mohmed N., Alqahtani F.Y., El Hadi Mohamed R.A. Outbreak of Middle East respiratory syndrome coronavirus in Saudi Arabia: a retrospective study. BMC Infect Dis. 2017;17:23. doi: 10.1186/s12879-016-2137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arabi Y.M., Arifi A.A., Balkhy H.H., Najm H., Aldawood A.S., Ghabashi A., et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 50.Joob B., Wiwanitkit V. Middle East respiratory syndrome coronavirus infection: a short note on cases with renal failure problem. Ren Fail. 2016;38:1749–1750. doi: 10.3109/0886022X.2015.1128772. [DOI] [PubMed] [Google Scholar]
- 51.Who Mers-Cov Research Group State of Knowledge and Data Gaps of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in Humans. PLoS Curr. 2013;5 doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Tawfiq J.A., Hinedi K., Ghandour J., Khairalla H., Musleh S., Ujayli A., et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014;59:160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A., et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ko J.-H., Müller M.A., Seok H., Park G.E., Lee J.Y., Cho S.Y., et al. Serologic responses of 42 MERS-coronavirus-infected patients according to the disease severity. Diagn Microbiol Infect Dis. 2017;89:106–111. doi: 10.1016/j.diagmicrobio.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alfaraj S.H., Al-Tawfiq J.A., Alzahrani N.A., Altwaijri T.A., Memish Z.A. The impact of co-infection of influenza A virus on the severity of Middle East Respiratory Syndrome Coronavirus. J Infect. 2017;74:521–523. doi: 10.1016/j.jinf.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arabi Y.M., Al-Omari A., Mandourah Y., Al-Hameed F., Sindi A.A., Alraddadi B., et al. Critically Ill Patients With the Middle East Respiratory Syndrome: A Multicenter Retrospective Cohort Study. Crit Care Med. 2017 doi: 10.1097/CCM.0000000000002621. [DOI] [PubMed] [Google Scholar]
- 57.Nam H.-S., Park J.W., Ki M., Yeon M.-Y., Kim J., Kim S.W. High fatality rates and associated factors in two hospital outbreaks of MERS in Daejeon, the Republic of Korea. Int J Infect Dis. 2017;58:37–42. doi: 10.1016/j.ijid.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shalhoub S., Farahat F., Al-Jiffri A., Simhairi R., Shamma O., Siddiqi N., et al. IFN-(2a or IFN-(1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129–2132. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim S.Y., Park S.J., Cho S.Y., Cha R.-H., Jee H.-G., Kim G., et al. Viral RNA in blood as Indicator of Severe Outcome in Middle East Respiratory Syndrome Coronavirus Infection. Emerging Infect Dis. 2016;22:1813–1816. doi: 10.3201/eid2210.160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Min C.-K., Cheon S., Ha N.-Y., Sohn K.M., Kim Y., Aigerim A., et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6:25359. doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ko J.-H., Park G.E., Lee J.Y., Lee J.Y., Cho S.Y., Ha Y.E., et al. Predictive factors for pneumonia development and progression to respiratory failure in MERS-CoV infected patients. J Infect. 2016;73:468–475. doi: 10.1016/j.jinf.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McKimm-Breschkin J.L., Jiang S., Hui D.S., Beigel J.H., Govorkova E.A., Lee N. Prevention and treatment of respiratory viral infections: presentations on antivirals, traditional therapies and host-directed interventions at the 5th ISIRV Antiviral Group conference. Antiviral Res. 2018;149:118–142. doi: 10.1016/j.antiviral.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arabi Y.M., Hajeer A.H., Luke T., Raviprakash K., Balkhy H., Johani S., et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerging Infect Dis. 2016;22:1554–1561. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mustafa S., Balkhy H., Gabere M.N. Current treatment options and the role of peptides as potential therapeutic components for Middle East Respiratory Syndrome (MERS): a review. J Infect Public Health. 2018;11:9–17. doi: 10.1016/j.jiph.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beigel J.H., Voell J., Kumar P., Raviprakash K., Wu H., Jiao J.-A., et al. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect Dis. 2018 doi: 10.1016/S1473-3099(18)30002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mo Y., Fisher D. A review of treatment modalities for Middle East Respiratory Syndrome. J Antimicrob Chemother. 2016;71:3340–3350. doi: 10.1093/jac/dkw338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Al Mekhlafi G.A., Hussein M.A., et al. Corticosteroid Therapy for Critically Ill Patients with the Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 68.Spanakis N., Tsiodras S., Haagmans B.L., Raj V.S., Pontikis K., Koutsoukou A., et al. Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int J Antimicrob Agents. 2014;44:528–532. doi: 10.1016/j.ijantimicag.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rossignol J.-F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Pub Health. 2016;9:227–230. doi: 10.1016/j.jiph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chan J.F.-W., Yao Y., Yeung M.-L., Deng W., Bao L., Jia L., et al. Treatment With Lopinavir/Ritonavir or Interferon-(1b Improves Outcome of MERS-CoV Infection in a Nonhuman Primate Model of Common Marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al Ghamdi M., Alghamdi K.M., Ghandoora Y., Alzahrani A., Salah F., Alsulami A., et al. Treatment outcomes for patients with Middle Eastern Respiratory Syndrome Coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect Dis. 2016;16:174. doi: 10.1186/s12879-016-1492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Wilde A.H., Falzarano D., Zevenhoven-Dobbe J.C., Beugeling C., Fett C., Martellaro C., et al. Alisporivir inhibits MERS- and SARS-coronavirus replication in cell culture, but not SARS-coronavirus infection in a mouse model. Virus Res. 2017;228:7–13. doi: 10.1016/j.virusres.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Müller C., Schulte F.W., Lange-Grünweller K., Obermann W., Madhugiri R., Pleschka S., et al. Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona- and picornaviruses. Antiviral Res. 2017;150:123–129. doi: 10.1016/j.antiviral.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alshahrani M.S., Sindi A., Alshamsi F., Al-Omari A., El Tahan M., Alahmadi B., et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8:3. doi: 10.1186/s13613-017-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alharbi N.K., Padron-Regalado E., Thompson C.P., Kupke A., Wells D., Sloan M.A., et al. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine. 2017;35:3780–3788. doi: 10.1016/j.vaccine.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]