Abstract
Objective
This study aims to assess the mood changes induced by mild acute inflammatory stimulation (typhoid vaccination).
Methods
Using a double blind study design, 26 healthy volunteers underwent baseline assessments of mood, financial strain and work stress and were randomised to injection of Salmonella typhi vaccine or placebo injection. Mood, symptoms and body temperature was assessed by a modified version of the Profile of Mood States at 1, 2, 3, 4, 6 and 8 h post injection.
Results
Typhoid vaccination induces no increases in physical symptoms or temperature. Mood improved over the day in the placebo but not in the vaccine condition. Negative changes in mood following injection were correlated with chronic stress (financial strain) in the vaccination condition (r=−.65, P<.025).
Conclusion
A mild acute inflammatory stimulus induces transient negative mood, and responses were modulated by chronic stress. Implications for depressed mood in physical illness are discussed.
Keywords: Inflammation, Cytokines, Chronic stress, Mood, Financial, Vaccination
Introduction
People with infections or inflammation can exhibit profound changes in behaviour. Initially, this was thought to be a psychological response to feeling unwell, but more recently the cytokines released as part of the inflammatory response have been implicated. “Cytokine associated sickness behaviour” is the term used for this collection of symptoms such as malaise, lethargy, anorexia, hyposomnia, inactivity, impaired cognitive function and anhedonia [1], [2]. This theory is supported by the fact that the syndrome can be reproduced by infusion of either recombinant cytokines, or molecules that induce cytokine synthesis (e.g., lipopolysaccharide) [3], [4], [5]. Agents which antagonise cytokines abolish the syndrome [2]. In animal models, the cytokine interleukin-1 (IL-1) plays a central role, but IL-6 and tumour necrosis factor alpha (TNFα) are also involved.
Inflammatory responses may also be relevant to depression. It has been known for several years that major depression is frequently associated with activation of the inflammatory response system, involving increased production of IL-1, IL-1 receptor antagonist (IL-1Ra), IL-6 and TNFα [6], although the evidence relating subclinical depressive symptoms with inflammatory responses is equivocal [7], [8]. Proinflammatory cytokines affect the brain's metabolism of 5 hxdroxy tryptophan (5-HT) by either directly or indirectly stimulating the enzyme indoleamine 2,3-dioxygenase leading to a peripheral depletion of 5-HT providing a possible intermediate in the link between inflammation and depressive symptoms [9]. These inflammatory responses may not be epiphenomena, but actively contribute to depressive symptoms and negative mood states. This has been documented most consistently in studies of mood responses following cytokine-based treatments for cancer. Immunotherapy with IL-2 or interferon α causes sadness, loss of interest and other depressive symptoms, and responses correlate with the magnitude of responses of endogenous cytokines [10], [11]. Depressive symptoms have also been reported following interferon α treatment of hepatitis C [12]. These effects are rapid, and may be mediated by the effects of cytokines on the hypothalamic–pituitary–adrenocortical (HPA) axis [13]. There is also evidence that antidepressants have antiinflammatory effects [14]. Taken together, these findings raise the intriguing possibility that depressive symptoms in as disparate illnesses as coronary heart disease, multiple sclerosis and rheumatoid arthritis may be due in part to cytokine activation.
The effects of cytokines on mood can be investigated experimentally by administering stimuli that acutely induce inflammatory cytokine release. Reichenberg et al. [15] assessed the effects on mood and cognitive function of Salmonella abortus equi endotoxin injection in volunteers. Rapid increases of 50- to 100-fold in IL-6 and TNFα occurred within 1–4 h of administration in endotoxin but not placebo-injected groups. Increases in anxiety and depressed mood were reported over the same time-scale in the endotoxin group, and the changes were correlated with the magnitude of cytokine responses. However, a problem with this model is that endotoxin administration leads to fever and malaise. A larger increase in body temperature was recorded by Reichenberg et al. [15] in the endotoxin than placebo groups and although no symptoms of sickness were reported, appetite was substantially inhibited. Many participants report flu-like symptoms in response to endotoxin. This makes it difficult to know whether behavioural changes were due to the inflammatory challenge or mild illness induced by the endotoxin itself.
In the present study, we used a milder model of experimental inflammation, involving standard typhoid (Salmonella typhi) vaccination. This method has been used by Vallance et al. to induce inflammatory responses that have been studied in relation to atherogenesis [16]. Increases of four- to sixfold in IL-6 occur, together with IL-1Ra increases of averaging 30-fold after 3 h [17]. Importantly, body temperature is not affected, and participants do not report any sickness or malaise. The use of vaccine rather than endotoxin may afford a better insight into the changes in mood in response to immune challenge in human healthy volunteers. The first aim of this study was therefore to examine the effects of typhoid vaccination on the subsequent mood of healthy volunteers in a placebo-controlled double blind trial. Based on the evidence linking proinflammatory cytokine release with negative moods, we hypothesised that typhoid vaccination would induce a negative mood response. Since moods are not constant over the day, changes in mood following vaccination cannot be definitively ascribed to the effects of an intervention in the absence of a comparison group. Half the participants were therefore injected with placebo, and the mood changes in this group taken as the reference against which the active vaccination group could be compared. In the light of the kinetics of proinflammatory cytokine responses observed in earlier studies, we hypothesised that compared with placebo, vaccination would induce transient negative moods in the 1–4 h following treatment, in the absence of rises in systemic body temperature or symptoms of illness.
Psychological stress plays an important role in the way the immune system functions [18]. Plasma concentrations of inflammatory cytokines vary with acute stress and with chronic stressors such as care-giving and work strain, and increase with acute stress [19], [20], [21], [22]. In a study of experimentally induced upper respiratory infection, background stress was found to correlate positively with IL-6 responses to infection [23]. It is conceivable that chronic stress might influence cytokine responses to vaccination, and thereby moderate mood responses. The supplementary aim of this study was therefore to assess associations between chronic stress and mood responses to typhoid vaccination. There are many aspects of chronic stress that could potentially be investigated, but in the interests of brevity and appropriateness for the population we limited measurement to two commonly assessed variables—financial strain and job demands. We hypothesised that background chronic stress would modulate cytokine responses, and thereby associations with mood responses in the vaccination but not placebo conditions.
Methods
Participants
Twenty-six volunteers (20 female, 6 male) between 20 and 35 years old were recruited from university staff. Volunteers were all nonsmokers, had no recent illnesses, were on no regular medication, and were not pregnant. None had any history of any mental illness or had received typhoid vaccination within the last 6 months. No volunteers had any allergies or had had any previous problems with vaccinations. All felt well on the morning of the study and had had no stressful events that morning. Volunteers refrained from excessive exercise and from any caffeine or alcohol from the day before the study. The study was presented to participants as an examination of immune responses to vaccination (as assessed by saliva samples). Ethical approval was given by the University College London medical research ethics committee.
Measures
Two measures of chronic stress were included in this study. The first was the financial strain questionnaire originally developed by Pearlin et al. [24] and utilized in the Whitehall II and other studies [25], [26]. Eight items (e.g., “Do you have enough money for the kind of clothing you and your family should have?”) were presented, with response options ranging from 1=no difficulty to 3=very great difficulty. Ratings were summed, so scores could range from 8 to 24, with higher ratings indicating greater financial strain. The second measure was an index of work demands derived from the demand/control model of work stress, as used in the Whitehall II study [26], [27]. Four items (e.g., “Do you have to work very intensively?”) were administered, each of which was rated on a four-point scale ranging from 0=often to 3=never/almost never. Scores were converted to a scale from 0–100, where 0 indicates minimum and 100 maximum demands.
Mood and symptoms were assessed with a modified version of the Profile of Mood States (POMS) [28] as described elsewhere [29]. This consisted of 36 items, each of which was rated on a five-point scale from 0=not at all to 4=extremely. Six high loading items were taken from the vigour, tension–anxiety, depression–dejection, and confusion scales of the POMS, and five items from the fatigue scale. In addition, there were four symptoms (feverish, aching joints, nauseated, and headache), and three filler items. Participants were asked to rate how they felt at that moment. Body temperature was measured with a sublingual digital thermometer, and blood pressure and heart rate using an electronic sphygmomanometer.
Procedure
All studies began at the same time (9 a.m.) to ensure uniformity. The study was performed in a double blind, placebo controlled manner. After initial measurements of temperature, blood pressure, heart rate and mood, randomisation to either vaccine or placebo (0.5 ml of normal saline) was performed by a coordinator and 0.025 mg Salmonella typhi vaccine (Typhim Vi, Aventis Pasteur MSD) or placebo in identical 2-ml syringes was administered intramuscularly by a qualified nurse or doctor into the nondominant deltoid muscle of the volunteers. Both vaccine and placebo were stored in the fridge before administration. There were no complications of vaccination or placebo injection. Thereafter administration of questionnaires and clinical observations was performed by researchers blinded to the experimental group of participants.
Volunteers were assessed at 1, 2, 3, 4, 6 and 8 h after vaccination. At each of these times, they filled in the modified POMS scale and had temperature, blood pressure and pulse measurements.
Statistical analysis
Scores for the five POMS scales were computed for the baseline sample by summing ratings on individual items. The scores on the fatigue dimension were scaled up to conform with those of the other moods. Ratings of the four symptoms were summed to produce a total score. Vaccine and placebo groups were compared on baseline mood, symptoms, chronic stress and physiological measures using t tests.
Changes in symptoms, body temperature, blood pressure and heart rate over the study were analysed with repeated measures analysis of variance with group (vaccine, placebo) as the between-subject factor, and time (base, 1, 2, 3, 4, 6 and 8 h) as the within-subject factor. To avoid the multiple comparisons when assessing mood responses, we calculated total mood scores as recommended in the POMS manual, by summing the negative items (tension, depression, confusion, and fatigue), and subtracting them from the positive vigour score. Changes in mood from baseline were then computed and analysed by repeated measures analysis of variance. Finally, correlations between chronic stress measures and changes in mood were computed separately for the vaccine and placebo groups. All analyses were carried out using SPSS v.10. Results are presented in terms of means±standard deviation.
Results
The background characteristics and baseline measures are summarized in Table 1 . There were no significant differences between vaccine and placebo groups in age, financial strain, work demands, or in physiological measures. The mood scores showed a high positive vigour score coupled with low ratings on negative scales, indicating that participants were generally in positive moods. Mood scores did not differ significantly between groups, with the exception of fatigue (t=15.3, P<.05), where ratings were higher in the placebo than vaccine group. However, the scores in both groups were extremely low (possible range 0–24), so the difference was trivial. None of the participants reported appreciable symptoms preinjection.
Table 1.
Background characteristics and baseline measures
| Vaccine (n=13) | Placebo (n=13) | |
|---|---|---|
| Men/Women | 3/10 | 3/10 |
| Age (years) | 29.4±5.8 | 25.6±4.3 |
| Financial strain | 12.8±3.5 | 12.6±3.0 |
| Work demands | 64.1±16.6 | 57.7±8.9 |
| Vigour | 12.5±5.8 | 12.3±5.2 |
| Tension–anxiety | 2.8±1.6 | 3.7±2.6 |
| Depression–dejection | 0.7±1.2 | 0.9±1.4 |
| Confusion | 3.2±2.4 | 4.1±2.6 |
| Fatigue | 0.4±0.3 | 1.0±0.9 |
| Symptoms | 0.5±0.8 | 0.7±1.1 |
| Body temperature (°C) | 36.2±0.5 | 36.4±0.4 |
| Heart rate (bpm) | 71.5±11.7 | 78.0±9.6 |
| Systolic pressure (mm Hg) | 119.5±18.2 | 120.4±14.4 |
| Diastolic pressure (mm Hg) | 75.7±11.4 | 70.5±6.1 |
Physiological and symptom responses to vaccination
The mean symptom ratings and body temperature assessed at the seven time points are shown in Fig. 1 . There was no significant change over time or difference between groups in either variable. The absence of any rise in body temperature with vaccination indicates that the procedure did not induce even mild illness. The mean symptom scores never exceeded one at any time. Since the symptom scores theoretically range from a low of zero to a maximum of 16, it is evident that neither treatment elicited physical symptoms throughout the study.
Fig. 1.
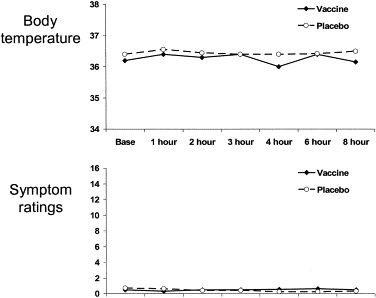
Average temperature and symptom scores for the vaccine and placebo group before injection (base) and then at 1, 2, 3, 4, 6, and 8 h postinjection.
There were some variations in cardiovascular measures. Heart rate showed a significant cubic trend over the study period [F(1,24)=31.8, P<.001]. The mean heart rate at baseline (74.8±11.0 bpm) decreased to a low of 63.0±12.0 bpm after 2 h. This was followed by a modest rise to 69.0±12.8 bpm after 6 h, with a final fall to 65.7±12.0 bpm after 8 h. These fluctuations did not differ by experimental condition, and are likely to have been related to variations in physical activity over the day. Similarly, there were no differences between groups in systolic or diastolic pressure over the day.
Mood responses to vaccination
The analysis of changes in total mood score between baseline and later samples show a significant quadratic effect of time [F(1,24)=4.62, P<.05], and a significant group by time cubic interaction [F(1,24)=4.17, P<.05]. These results are summarized in Fig. 2 . Mood became more positive in the early part of the experiment in the placebo group, but remained at baseline levels in the vaccination group. Both groups showed a diminution in positive mood later in the day. In post hoc analyses, changes from baseline averaged over the first, second and third hours after injection were greater in the placebo than vaccination conditions [F(1,24)=4.47, P<.05]. By contrast, changes between baseline and hours 4, 6 and 8 did not differ in the two groups [F(1,24)=2.07, P=.16].
Fig. 2.
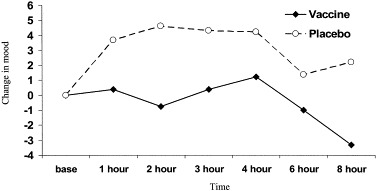
Changes in mood score for vaccine and placebo groups group before injection (base) and then at 1, 2, 3, 4, 6, and 8 h postinjection.
Background chronic stress and mood responses to vaccination
Product moment correlations between mood change and the two background stress variables (financial strain and job demands) were computed separately for the vaccination and placebo groups. No significant effects were found in placebo condition. In the vaccine group, there was a significant negative correlation between change in mood over the first hour following injection and financial strain (r=−.65, P<.025). This effect is illustrated in Fig. 3 . Participants who reported high financial strain showed negative changes in mood over the first hour following vaccination, while positive mood changes were apparent in most participants who reported low financial strain. This suggests that background chronic stress levels may have affected the mood responses to typhoid vaccination.
Fig. 3.
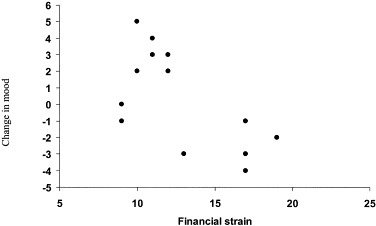
Mood change in response to vaccination according to preexisting financial stress in the hour after vaccination.
Discussion
The principal hypothesis tested in this study was that the mild inflammatory response induced by typhoid vaccination would induce transient negative mood. It was necessary to test this hypothesis in a double-blind placebo-controlled fashion, since researchers' and participants' expectations might have influenced the pattern of results. In addition, mood can change over the day irrespective of stimuli such as vaccination, so the effects of this stimulus were tested against the fluctuations observed in the placebo group. Mood was assessed using a standard measure, and we analysed the total POMS rather than individual scales to provide a global impression of mood change. The results confirmed our expectations about the effects of the inflammatory stimulus. Mood improved over the day in the placebo group, reaching a plateau 2–4 h postvaccination (11:00 a.m. to 1:00 p.m.), before deteriorating slightly later in the day. The increase in positive mood from the start of the study may have been due partly to time of day, and partly to the relief at having completed the injection. By contrast, mood did not improve over the day in the vaccination condition. The difference between groups was greatest over the first 3 h following vaccination, with some convergence later in the day. By the end of the study (5:00 p.m.), mood had deteriorated below baseline in the vaccination group, while remaining elevated in the placebo condition. Thus, relative to the placebo condition, the vaccination group had a more negative mood following injection.
The mood differences were significant but modest. This compares with result of Reichenberg et al. [15], in which both depression and anxiety increased in absolute terms in response to endotoxin. The difference may be due to the greater intensity of the inflammatory stimulus in this earlier study. In the present study, there was no change in body temperature, no febrile responses, and symptom reports were low and stable. In addition, the study by Reichenberg et al. [15] was more invasive in nature, involving venous cannulation and continuous temperature measurement through a rectal probe. It is notable that anxiety increased in the placebo as well as endotoxin condition in this earlier study. Our results suggest that vaccination may be a preferable way of studying the relationship between immune challenges, mood and behavioural responses. A previous study of rubella vaccination has shown increases in depressed mood in a proportion of the young women tested who were initially seronegative [30].
Cytokines were not measured in this study, so it cannot be definitely concluded that mood changes were due to their activation. We are currently carrying out a study to address this issue. However, the time course of responses is consistent with the cytokine changes that would be expected. In previous studies using typhoid vaccination, the maximum rise in IL-6 and IL-1Ra occurred at 2- to 4-h postvaccination [16], [31]. Cytokine levels then fall gradually, but do not reach baseline values even 8-h postvaccination. These responses parallel the changes in mood observed in the present investigation.
The second important finding was that the magnitude of mood change following vaccination was associated with one of the two measures of chronic stress. Participants reporting greater financial strain showed larger negative changes in mood in the hour after vaccination. No such association was observed in the placebo group, so it is not a nonspecific response caused by negative affectivity reporting biases. There was also no association with the second measure of chronic stress, job demands. The explanation may be that job demands were relatively low, while financial strain was high on average in this sample compared with other population samples in which the measures have been used. The association with financial strain needs to be confirmed in a larger study involving cytokine measures, so as to discover whether inflammatory or some other responses to vaccine mediate effects. If the association is confirmed, it could be due to one of two processes; either background stress magnifies inflammatory responses to standard challenges such as vaccination thereby increasing the mood change, or it affects the extent of mood change following a fixed inflammatory response. Individual differences in mood response to rubella vaccination have also been reported, with greater depressed mood in young women of lower socioeconomic status [30]. A number of studies of responses to fixed doses of coronavirus or influenza have shown that the likelihood of infection is affected by psychosocial factors such as life stress, negative affect, and social isolation [32], [33]. The observation by Cohen et al. [23] that the IL-6 increases following infection with influenza were correlated with psychological stress is consistent with the possibility that the present finding was mediated through individual differences in IL-6 response.
Studies relating negative moods with inflammation may be relevant to depression in coronary heart disease. Inflammation is now thought to be a fundamental factor in the pathophysiology of vascular disease [34]. Acute coronary syndromes are also associated with marked inflammatory responses, with larger increases in IL-1β followed by raised IL-6 [35]. The acute increase in IL-1Ra and IL-6 over the first 2 days following hospitalization with unstable angina is associated with adverse clinical outcomes [36]. Depression in the days following admission with acute coronary syndromes has also been shown to predict poor outcome in many studies [37], and several mechanisms to explain the association are being investigated [38]. An additional possibility is that depressed mood is due to inflammation in these patients, and that heightened levels of inflammation underlie the association between depression and adverse cardiac outcomes.
We studied a relatively homogeneous group of university workers, the majority of whom were postgraduate academics. They were relatively young, physically fit and active. The study was small, with insufficient numbers to test sex differences in responses. Future studies of larger samples will involve cytokine as well as mood measures, to determine whether the magnitude of inflammatory responses is directly associated with mood changes following vaccination.
Acknowledgements
This study was supported by the British Heart Foundation and the Medical Research Council. We are grateful to Bev Murray, Lindsay Emmerson and Lena Brydon for assistance in vaccination.
References
- 1.Larson SJ, Dunn AJ. Behavioral effects of cytokines. Brain Behav Immun. 2001;15(4):371–387. doi: 10.1006/brbi.2001.0643. [DOI] [PubMed] [Google Scholar]
- 2.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25(3):154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 3.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15(1):7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 4.Maes M, Capuron L, Ravaud A, Gualde N, Bosmans E, Egyed B, Dantzer R, Neveu PJ. Lowered serum dipeptidyl peptidase IV activity is associated with depressive symptoms and cytokine production in cancer patients receiving interleukin-2-based immunotherapy. Neuropsychopharmacology. 2001;24(2):130–140. doi: 10.1016/S0893-133X(00)00168-8. [DOI] [PubMed] [Google Scholar]
- 5.Spath-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, Fehm HL, Born J. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998;83(5):1573–1579. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- 6.Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 7.Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J, Newman A, Hirsch C, Tracy RP. Inflammation and coagulation factors in Persons >65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89(4):419–424. doi: 10.1016/s0002-9149(01)02264-0. 15 Feb. [DOI] [PubMed] [Google Scholar]
- 8.Steptoe A, Kunz-Ebrecht S, Owen N. Lack of association between depressive symptoms and markers of immune and vascular inflammation in middle-aged men and women. Psychol Med. 2003;33:667–674. doi: 10.1017/s0033291702007250. [DOI] [PubMed] [Google Scholar]
- 9.Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int J Neuropsychopharmacol. 2002;5(4):375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- 10.Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med. 2001;63(3):376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Capuron L, Ravaud A, Gualde N, Bosmans E, Dantzer R, Maes M, Neveu PJ. Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology. 2001;26(8):797–808. doi: 10.1016/s0306-4530(01)00030-0. [DOI] [PubMed] [Google Scholar]
- 12.Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, Maes M. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. J Affect Disord. 2002;72(3):237–241. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 13.Capuron L, Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav Immun. 2003;17(1 Suppl):119–124. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 14.Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for “depression due to a general medical condition”, immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol. 2002;5(4):389–399. doi: 10.1017/S1461145702003152. [DOI] [PubMed] [Google Scholar]
- 15.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58(5):445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 16.Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, Donald AE, Palacios M, Griffin GE, Deanfield JE, MacAllister RJ, Vallance P. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102(9):994–999. doi: 10.1161/01.cir.102.9.994. 29 Aug. [DOI] [PubMed] [Google Scholar]
- 17.Kharbanda RK, Walton B, Allen M, Klein N, Hingorani AD, MacAllister RJ, Vallance P. Prevention of inflammation-induced endothelial dysfunction. A novel vasculo-protective action of aspirin. Circulation. 2002;105:2600–2604. doi: 10.1161/01.cir.0000017863.52347.6c. [DOI] [PubMed] [Google Scholar]
- 18.Ader R, Felten DL, Cohen N. Psychoneuroimmunology. 3rd ed. Academic Press; San Diego (CA): 2001. [Google Scholar]
- 19.Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 20.Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong SY, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. J Gerontol A Biol Sci Med Sci. 1999;54(9):M434–M439. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steptoe A, Owen N, Kunz-Ebrecht S, Mohamed-Ali V. Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain Behav Immun. 2002;16(6):774–784. doi: 10.1016/s0889-1591(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 22.De Gucht V, Fischler B, Demanet C. Immune dysfunction associated with chronic professional stress in nurses. Psychiatry Res. 1999;85(1):105–111. doi: 10.1016/s0165-1781(98)00131-0. 18 Jan. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S, Doyle WJ, Skoner DP. Psychological stress, cytokine production, and severity of upper respiratory illness. Psychosom Med. 1999;61(2):175–180. doi: 10.1097/00006842-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. J Health Soc Behav. 1981;22(4):337–356. [PubMed] [Google Scholar]
- 25.Ross CE, Huber J. Hardship and Depression. J Health Soc Behav. 1985;26(4):312–327. [PubMed] [Google Scholar]
- 26.Marmot MG, Smith GD, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A. Health inequalities among British Civil Servants: the Whitehall II study. Lancet. 1991;337(8754):1387–1393. doi: 10.1016/0140-6736(91)93068-k. 8 Jun. [DOI] [PubMed] [Google Scholar]
- 27.Bosma H, Marmot MG, Hemingway H, Nicholson AC, Brunner E, Stansfeld SA. Low job control and risk of coronary heart disease in Whitehall II (prospective cohort) study. BMJ. 1997;314(7080):558–565. doi: 10.1136/bmj.314.7080.558. 22 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNair DM, Lorr N, Droppleman LF. Manual for the profile of mood states. Education and Industrial Testing Service; San Diego (CA): 1981. [Google Scholar]
- 29.Moses J, Steptoe A, Mathews A, Edwards S. The effects of exercise training on mental well-being in the normal population: a controlled trial. J Psychosom Res. 1989;33(1):47–61. doi: 10.1016/0022-3999(89)90105-0. [DOI] [PubMed] [Google Scholar]
- 30.Morag M, Yirmiya R, Lerer B, Morag A. Influence of socioeconomic status on behavioral, emotional and cognitive effects of rubella vaccination: a prospective, double blind study. Psychoneuroendocrinology. 1998;23(4):337–351. doi: 10.1016/s0306-4530(98)00012-2. [DOI] [PubMed] [Google Scholar]
- 31.Kharbanda RK, Walton B, Allen M, Klein N, Hingorani AD, MacAllister RJ, VAllance P. Prevention of inflammation-induced endothelial dysfunction: a novel vasculo-protective action of aspirin. Circulation. 2002;105(22):2600–2604. doi: 10.1161/01.cir.0000017863.52347.6c. 4 Jun. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. JAMA. 1997;277(24):1940–1944. 25 Jun. [PubMed] [Google Scholar]
- 33.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325(9):606–612. doi: 10.1056/NEJM199108293250903. 29 Aug. [DOI] [PubMed] [Google Scholar]
- 34.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. 14 Jan. [DOI] [PubMed] [Google Scholar]
- 35.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. 5 Mar. [DOI] [PubMed] [Google Scholar]
- 36.Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, Dinarello CA, Maseri A. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99(16):2079–2084. doi: 10.1161/01.cir.99.16.2079. 27 Apr. [DOI] [PubMed] [Google Scholar]
- 37.Ziegelstein RC. Depression in patients recovering from a myocardial infarction. JAMA. 2001;286(13):1621–1627. doi: 10.1001/jama.286.13.1621. 3 Oct. [DOI] [PubMed] [Google Scholar]
- 38.Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53(4):897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]


