Abstract
This report describes the application of real-time PCR for testing antivirals against highly pathogenic viruses such as Lassa virus, SARS coronavirus and Ebola virus. The test combines classical cell culture with a quantitative real-time PCR read-out. The assay for Lassa virus was validated with ribavirin, which showed an IC50 of 9 μg/ml. Small-scale screening identified a class of imidazole nucleoside/nucleotide analogues with antiviral activity against Lassa virus. The analogues contained either dinitrile or diester groups at the imidazole 4,5-positions, and many of which possessed an acyclic sugar or sugar phosphonate moiety at the imidazole 1-position. The IC50 values of the most active compounds ranged from 5 to 21 μg/ml. The compounds also inhibited replication of SARS coronavirus and Ebola virus in analogous assays, although to a lesser extent than Lassa virus.
Keywords: Lassa virus, SARS coronavirus, Ebola virus, Real-time PCR, Imidazole nucleoside analogues
Lassa virus belongs to the family Arenaviridae and is the etiologic agent of Lassa fever in humans. Transmission of the virus from its natural host – the African rodent Mastomys natalensis – to humans causes a systemic disease with bleeding and organ failure. Lassa fever is endemic in the West African countries of Sierra Leone, Guinea, Liberia, and Nigeria. A variety of compounds have shown antiviral activity against arenaviruses in cell culture (Huggins et al., 1984, Rodriguez et al., 1986, Burns et al., 1988; Andrei and De Clercq, 1990, Andrei and De Clercq, 1993; De Clercq et al., 1991, Nair and Ussery, 1992, Smee et al., 1992, Candurra et al., 1996, Garcia et al., 2000, Wachsman et al., 2000, Bartolotta et al., 2001). The only drug with a proven therapeutic effect in humans with Lassa fever is the broad-spectrum nucleoside analogue ribavirin (McCormick et al., 1986), which inhibits replication of related arenaviruses in cell culture (Huggins et al., 1984, Rodriguez et al., 1986, Andrei and De Clercq, 1990) and has beneficial effects in experimental animal models of Lassa fever (Stephen and Jahrling, 1979, Jahrling et al., 1980, Dvoretskaia et al., 1991). However, the drug is effective only if administered early after infection (McCormick et al., 1986). Several cases of Lassa fever recently imported into Europe have demonstrated that even state-of-the-art intensive care cannot prevent a fatal outcome if ribavirin treatment is initiated too late (Schmitz et al., 2002). This report describes assays for testing potential antiviral compounds against Lassa virus and other highly pathogenic viruses, such as severe acute respiratory syndrome-associated coronavirus (SARS-CoV) (Drosten et al., 2003) and Ebola hemorrhagic fever virus (Feldmann et al., 2003). The test combines classical cell culture with real-time PCR. A class of imidazole nucleoside/nucleotide analogues was identified which showed inhibitory activity against Lassa virus and, to a lesser extent, against SARS-CoV and Ebola virus.
The flowchart of the test is shown in Fig. 1(A) . Vero cells were plated at a density of 4 × 104/well of a 24-well plate. Twenty-four hours later, the cells were infected in the biosafety level 4 laboratory with Lassa virus strain AV at a multiplicity of infection (MOI) of 0.01, SARS-CoV at an MOI of 0.01, or Ebola virus subtype Zaire at an MOI of 0.1 in 100 μl. The inoculum was removed after 1 h and replaced by fresh medium complemented with different concentrations of compound. None of the three viruses showed cytopathic effect under our culture conditions. Virus RNA concentration in supernatant was measured by real-time PCR during the exponential growth phase of the viruses (Fig. 1B), i.e. after 2 days for Lassa virus and SARS-CoV, and after 3 days for Ebola virus. During this time, the viruses were growing by several orders of magnitudes if no inhibitor was added. For RNA preparation, a simple and inexpensive method (Boom et al., 1990) was adopted. A 140-μl aliquot of supernatant was mixed with 560 μl chaotropic lysis buffer AVL (Qiagen) and incubated at room temperature for 15 min. The lysate was added to 100 mg diatomaceous silica (Sigma–Aldrich) suspended in 560 μl ethanol and incubated with agitation for 30 min at room temperature. The diatomaceous silica were pelleted by centrifugation and the pellet was washed with 500 μl AW1 buffer (Qiagen), subsequently with 500 μl AW2 buffer (Qiagen), and finally with 400 μl acetone. The pellet was dried at 56 °C and the RNA eluted with 100 μl water. Quantitative real-time PCR assays for Lassa virus, SARS-CoV, and Ebola virus Zaire were performed with the purified RNA based on previously published protocols (Gibb et al., 2001, Drosten et al., 2003, Asper et al., 2004). The detailed reaction conditions are given in Table 1 . In vitro transcripts of the PCR target regions were amplified in the PCR to generate standard curves for quantification of the virus RNA in supernatant.
Fig. 1.
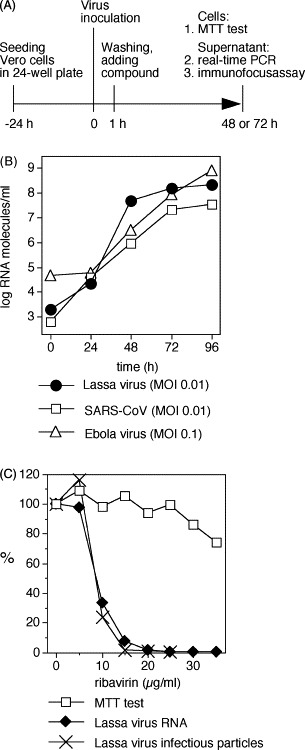
Real-time PCR-based assay for testing the effect of compounds on replication of Lassa virus, SARS-CoV, and Ebola virus. (A) Flowchart of the assay. (B) Growth kinetics of Lassa virus, SARS-CoV, and Ebola virus in Vero cells. The RNA concentration in supernatant was determined by real-time PCR as described in the text. (C) Validation of the Lassa virus assay using ribavirin. Inhibition of Lassa virus replication was tested by real-time PCR and immunofocus assay. Cell growth was measured by MTT test.
Table 1.
Quantitative real-time PCRs used in the study
| Assay and references | Reaction conditions | Cycling conditions |
|---|---|---|
| Lassa virus (Demby et al., 1994, Asper et al., 2004) | 20-μl reaction based on Brilliant single-step quantitative RT-PCR kit (Stratagene): 2 μl RNA, 1× buffer, 2.5 mM MgCl2, 800 μM dNTP, 0.8 μg bovine serum albumin, 5 μM SybrGreen-binding molecule SGS (Drosten, 2003), SybrGreen 1:10,000 (Molecular Probes), 1.25 U StrataScript RT, 1 U SureStart Taq polymerase, 200 nM primer 36E2 (ACC GGG GAT CCT AGG CAT TT), and 300 nM primer 80F2 (ATA TAA TGA TGA CTG TTG TTC TTT GTG CA) | LightCycler (Roche): 20 min at 45 °C; 12 min at 95 °C; 10 precycles with 10 s at 95 °C, 10 s at 60 °C with a decrease of 0.8 °C/cycle, and 20 s at 72 °C; 25 cycles with 5 s at 95 °C, 10 s at 55 °C, 30 s at 65 °C, and 20 s fluorescence read at 80 °C (F1 detection channel); melting curve analysis. |
| SARS-CoV (Drosten et al., 2003) | 25-μl reaction based on Superscript II RT/Platinum Taq polymerase one-step RT-PCR kit (Invitrogen): 5 μl RNA, 1× buffer, 3.6 mM additional MgSO4, 0.6 μl enzyme mixture, 240 nM probe BNITMSARP (FAM-TCG TGC GTG GAT TGG CTT TGA TGT-TAMRA), 200 nM primer BNITMSARS1 (TTA TCA CCC GCG AAG AAG CT), and 200 nM primer BNITMSARAs2 (CTC TAG TTG CAT GAC AGC CCT C) | 7000 SDS machine (Applied Biosystems): 15 min at 45 °C; 3 min at 95 °C; 40 cycles with 15 s at 95 °C and 30 s at 58 °C with fluorescence measured at 58 °C (FAM detection channel without passive reference dye) |
| Ebola virus (Gibb et al., 2001) | 20-μl reaction based on Brilliant single-step quantitative RT-PCR kit (Stratagene): 2 μl RNA, 1× buffer, 2.5 mM MgCl2, 800 μM dNTP, 1.25 U StrataScript RT, 1 U SureStart Taq polymerase, 250 nM probe EBOGP-1DZPrb (FAM-CTA CCA GCA GCG CCA GAC GG-TAMRA), 500 nM primer EBOGP-1D forward (TGG GCT GAA AAY TGC TAC AAT C), and 500 nM primer EBOGP-1D reverse (CTT TGT GMA CAT ASC GGC AC) | 7000 SDS machine (Applied Biosystems): 30 min at 50 °C; 10 min at 95 °C; 45 cycles with 15 s at 95 °C and 30 s at 58 °C with fluorescence measured at 58 °C (FAM detection channel without passive reference dye) |
Abbreviations: FAM, 6-carboxyfluorescein; TAMRA, 6-carboxy-N,N,N′,N′-tetramethylrhodamin; RT, reverse transcriptase.
In selected experiments, infectious Lassa virus particles in the supernatant were quantified by immunofocus assay. Vero cells in 24-well plates were inoculated with serial 10-fold dilutions of supernatant. The inoculum was removed after 1 h and replaced by a 1% methylcellulose-medium overlay. After 5 days of incubation, cells were fixed with 4% formaldehyde, permeabilized with 0.5% Triton X-100, blocked with 10% fetal calf serum, and washed. Infected cell foci were detected with Lassa virus nucleoprotein (NP)-specific monoclonal antibody L2F1 diluted 1:50 (Hufert et al., 1989). After washing and incubation with peroxidase-labelled anti-mouse IgG antibody 1:3000 (Dianova), foci were stained with 3,3′,5,5′-tetramethylbenzidine (TMB) and counted.
Cell growth inhibition or cytotoxicity was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazoliumbromide (MTT) method. MTT solution (50 μl, 5 mg/ml PBS) was added to the remaining supernatant of the cells from which an aliquot of supernatant was taken for measuring virus RNA or infectious particles concentration. The plate was incubated for 90 min at 37 °C. The supernatant was completely removed and the cells were fixed with 4% formaldehyde for 30 min. Tetrazolium crystals were dissolved in 1 ml ethanol for 10 min and the extinction was measured at 560 nm.
The concentrations required to inhibit virus replication by 50% (IC50) or 90% (IC90) were calculated by fitting a sigmoidal curve to the data following logarithmic transformation of the drug concentration. Curve fitting was performed by probit regression analysis using Statgraphics plus 5.0 software (Statistical Graphics Inc.).
Initial experiments were performed with the Lassa virus assay. It was validated using ribavirin as a positive control (Fig. 1C). In agreement with published data on related arenaviruses (Huggins et al., 1984, Rodriguez et al., 1986, Andrei and De Clercq, 1990), ribavirin inhibited Lassa virus replication with an IC50 of 9 μg/ml and an IC90 of 14 μg/ml. Furthermore, the inhibition kinetics measured with real-time PCR perfectly corresponded to the kinetics measured with the immunofocus assay (Fig. 1C), indicating that real-time PCR can be used to measure the effect of compounds on Lassa virus replication. About 100 nucleoside analogues of different chemical classes available at the Institute were tested using this assay. For screening, three different concentrations in the range between 10 and 50 μg/ml were tested. A class of imidazole nucleoside/nucleotide analogues was identified that showed inhibitory effects on Lassa virus without severely affecting cell growth and viability. Eighteen compounds of this class were tested (Fig. 2 and Table 2 ). Particularly active compounds (3, 8, 23, 24, 34, 35, 56, and 64) were analysed in detail using real-time PCR and immunofocus assay as a read-out (Fig. 3A ). Each of these compounds showed a similar effect on Lassa virus RNA and infectious particles, again demonstrating the validity of the PCR-based assay. IC50 values as determined with the RNA concentration data ranged from 5 to 21 μg/ml and IC90 values ranged from 27 to 58 μg/ml (Table 2). In the 48-h assay, compounds 8, 23, 24, 35, and 56 showed no significant influence on the rapidly growing Vero cells in concentrations up to 100 μg/ml (Fig. 3A). In order to test whether this class of nucleoside analogues shows broad-spectrum activity, the complete set of 18 compounds was also tested against SARS-CoV and Ebola virus (Table 2). Virus-specific effects were observed with the less toxic compounds 8, 10, 13, 23, 24, and 35 (Fig. 3B and C). However, the inhibiting effects against SARS-CoV and Ebola virus were considerably lower than observed for Lassa virus and overlapped with cell toxicity at higher concentrations. There was no evidence for a SARS-CoV- or Ebola virus-specific effect with compound 3 due to its toxicity.
Fig. 2.
Chemical structures of imidazole nucleoside/nucleotide analogues tested. Abbreviations: Et, ethyl; Ac, acetyl; Bz, benzoyl; Tol, toluoyl.
Table 2.
Inhibition parameters of the compounds
| Compound | Lassa virusa |
SARS-CoVa |
Ebola virusa |
|||||
|---|---|---|---|---|---|---|---|---|
| IC50 (μg/ml) | IC90 (μg/ml) | CC50 (μg/ml) | IC50 (μg/ml) | CC50 (μg/ml) | IC50 (μg/ml) | CC50 (μg/ml) | ||
| A | 3 | 10 | 28 | 70 | 30 | 31 | 13 | 36 |
| A | 8 | 13 | 43 | >100 | 20 | >75 | 37 | >75 |
| A | 23 | 8 | 27 | >100 | 23 | >75 | 19 | >75 |
| A | 24 | 5 | 29 | >100 | 17 | >75 | 43 | >75 |
| A | 34 | 10 | 47 | 81 | 15 | 56 | 52 | >75 |
| A | 40 | 32 | >50 | >50 | 48 | >75 | 41 | >75 |
| A | 72 | >50 | >50 | >50 | 46 | 64 | 18 | >75 |
| B-1 | 10 | 25 | >50 | >50 | 32 | >75 | 12 | >75 |
| B-1 | 35 | 13 | 48 | >100 | 18 | >75 | 32 | 75 |
| B-1 | 39 | 30 | >50 | >50 | 28 | >75 | 17 | 75 |
| B-1 | 56 | 21 | 58 | >100 | 46 | >75 | 30 | >75 |
| B-1 | 64 | 12 | 38 | 97 | 40 | 75 | 19 | 75 |
| B-1 | 71 | 46 | >50 | >50 | 23 | 75 | 19 | >75 |
| B-1 | 83 | 2 | 7 | 12 | 17 | 29 | 10 | 30 |
| B-2 | 13 | 4 | >50 | >50 | 28 | >75 | 16 | >75 |
| B-2 | 36 | 18 | >50 | >50 | 49 | 75 | 41 | >75 |
| B-3 | 28 | 35 | >50 | >50 | 35 | >75 | 23 | >75 |
| B-4 | 54 | 10 | >50 | >50 | 26 | >75 | 28 | >75 |
| Ribavirin | 9 | 14 | >35 | n.t. | n.t. | n.t. | n.t. | |
Abbreviations: IC50, concentration causing 50% reduction of the viral RNA concentration in the supernatant; IC90, concentration causing 90% reduction of the viral RNA concentration in the supernatant; CC50, concentration causing 50% reduction of the MTT value; n.t., not tested.
Virus RNA measurement and MTT test were performed with cells and supernatant, respectively, of the same cell culture well. IC90 values were not calculated for SARS-CoV and Ebola virus because they were for most compounds above the concentration range tested.
Fig. 3.
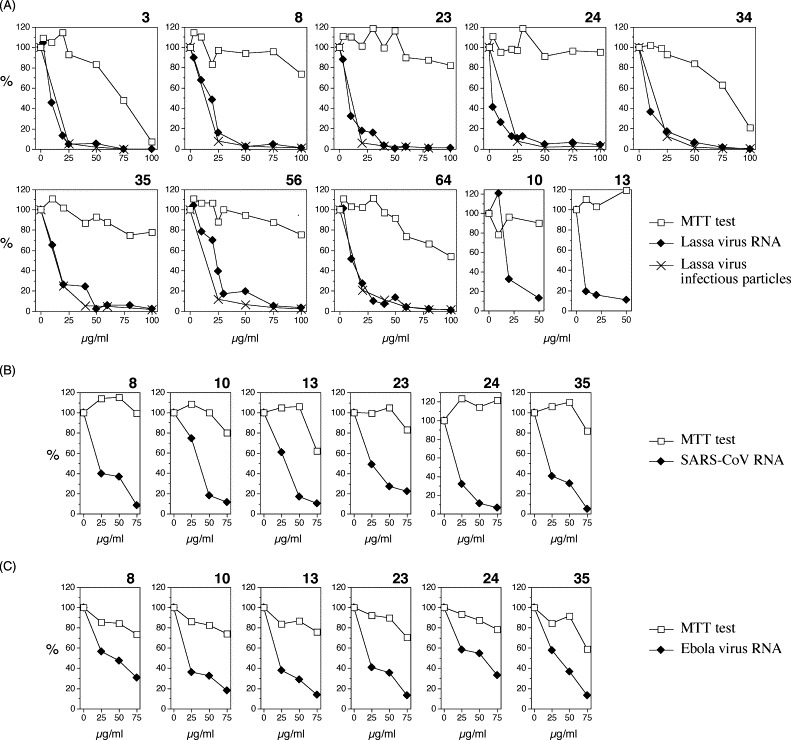
Antiviral effects exerted by selected compounds shown in Fig. 2. The compound is indicated above each graph. (A) Inhibition of replication of Lassa virus as tested by real-time PCR and immunofocus assay. Cell growth was measured by MTT test. (B) Inhibition of replication of SARS-CoV replication as tested by real-time PCR. Cell growth was measured by MTT test. (C) Inhibition of replication of Ebola virus replication as tested by real-time PCR. Cell growth was measured by MTT test. Note the increased toxicity of the compounds due to the prolonged assay time of 3 days.
This study shows that real-time PCR is a useful approach to determine the antiviral effect of compounds. The assay provides an impartial and quantitative value of virus growth in cells in the presence of a compound over a broad dynamic range (i.e. several orders of magnitudes), with the effect of the compound on cell growth and viability being measured with the same cells. However, it is more laborious than classical methods, such as plaque reduction tests, immunofluorescence tests, or tests based on inhibition of virus-induced cytopathogenicity.
The nucleoside analogues identified as inhibitors of Lassa virus replication contained a 5-membered heterocyclic ring, namely an imidazole. A number of nucleoside analogues containing 5-membered heterocyclic rings, such as imidazole, pyrazole, and triazole, have since long been documented as potent antiviral, antibacterial, and antitumor agents (De Clercq and Luczak, 1975, Srivastava et al., 1976, Korbukh et al., 1978, Smith and Kirkpatrick, 1980, Revankar et al., 1981). These include, but are not limited to, bredinin (Gerber et al., 1998), a potent nucleoside antibiotic containing an imidazole ring, and ribavirin (Sidwell et al., 1972, Witkowski et al., 1972, Smith and Kirkpatrick, 1980, Tam et al., 2001, Crotty et al., 2002, Lau et al., 2002), a broad-spectrum antiviral agent containing a triazole nucleus. In addition to possessing a 5-membered heterocyclic ring, the target compounds also contain acyclic sugar or sugar phosphonate groups attached to the heterocyclic ring, and thus mimic part or whole of the natural sugar or sugar phosphate of nucleosides and nucleotides. In this context, they are analogues of the widely known potent, broad-spectrum antivirals, namely acyclovir [9-(2-hydroxyethoxymethyl)guanine] (Schaeffer et al., 1978, Kaplan and Bain, 1999, Emmert, 2000, De Clercq et al., 2001) and PMEA/PMEG [9-(2-phosphonylmethoxyethyl)adenine/guanine] (Balzarini et al., 1997, Naesens and De Clercq, 1997, Naesens et al., 1997, Kramata et al., 1998, Compton et al., 1999, Noble and Goa, 1999). The observation that SARS-CoV and Ebola virus were also inhibited to a certain extent suggests that this class of analogues potentially has broad-spectrum antiviral activity. The mode of action is completely speculative and may be different depending on the chemical characteristics of a given compound. One possibility is direct inhibition of the virus replication machinery. Alternatively, the analogues might affect cellular pathways of nucleotide metabolism. The RNA synthesis of a highly replicating virus like Lassa virus probably is more susceptible to inhibition of nucleotide metabolism than the corresponding cellular processes. Inhibition of nucleotide metabolism could also account for the toxicity associated with some of the compounds.
Acknowledgements
The research was supported by a grant # 9RO1 AI55452 from the United States National Institute of Allergy and Infectious Diseases (NIAID/NIH) (to R.S.H.), grant E/B41G/1G309/1A403 from the Bundesamt für Wehrtechnik und Beschaffung (to S.G.), and a grant from the Concelho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (to L.K.S.L.). The Bernhard-Nocht-Institut is supported by the Bundesministerium für Gesundheit and the Freie und Hansestadt Hamburg.
References
- Andrei G., De Clercq E. Inhibitory effect of selected antiviral compounds on arenavirus replication in vitro. Antiviral Res. 1990;14:287–299. doi: 10.1016/0166-3542(90)90009-v. [DOI] [PubMed] [Google Scholar]
- Andrei G., De Clercq E. Molecular approaches for the treatment of hemorrhagic fever virus infections. Antiviral Res. 1993;22:45–75. doi: 10.1016/0166-3542(93)90085-w. [DOI] [PubMed] [Google Scholar]
- Asper M., Sternsdorf T., Hass M., Drosten C., Rhode A., Schmitz H., Günther S. Inhibition of different Lassa virus strains by alpha and gamma interferons and comparison with a less pathogenic arenavirus. J. Virol. 2004;78:3162–3169. doi: 10.1128/JVI.78.6.3162-3169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J., Vahlenkamp T., Egberink H., Hartmann K., Witvrouw M., Pannecouque C., Casara P., Nave J.F., De Clercq E. Antiretroviral activities of acyclic nucleoside phosphonates [9-(2-phosphonylmethoxyethyl)adenine, 9-(2-phosphonylmethoxyethyl)guanine, (R)-9-(2-phosphonylmethoxypropyl)adenine, and MDL 74,968] in cell cultures and murine sarcoma virus-infected newborn NMRI mice. Antimicrob. Agents Chemother. 1997;41:611–616. doi: 10.1128/aac.41.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolotta S., Garcia C.C., Candurra N.A., Damonte E.B. Effect of fatty acids on arenavirus replication: inhibition of virus production by lauric acid. Arch. Virol. 2001;146:777–790. doi: 10.1007/s007050170146. [DOI] [PubMed] [Google Scholar]
- Boom R., Sol C.J., Salimans M.M., Jansen C.L., Wertheim-van Dillen P.M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns N.J., III, Barnett B.B., Huffman J.H., Dawson M.I., Sidwell R.W., De Clercq E., Kende M. A newly developed immunofluorescent assay for determining the Pichinde virus-inhibitory effects of selected nucleoside analogues. Antiviral Res. 1988;10:89–98. doi: 10.1016/0166-3542(88)90017-4. [DOI] [PubMed] [Google Scholar]
- Candurra N.A., Maskin L., Damonte E.B. Inhibition of arenavirus multiplication in vitro by phenotiazines. Antiviral Res. 1996;31:149–158. doi: 10.1016/0166-3542(96)06956-2. [DOI] [PubMed] [Google Scholar]
- Compton M.L., Toole J.J., Paborsky L.R. 9-(2-Phosphonylmethoxyethyl)-N6-cyclopropyl-2,6-diaminopurine (cpr-PMEDAP) as a prodrug of 9-(2-phosphonylmethoxyethyl)guanine (PMEG) Biochem. Pharmacol. 1999;58:709–714. doi: 10.1016/s0006-2952(99)00138-0. [DOI] [PubMed] [Google Scholar]
- Crotty S., Cameron C., Andino R. Ribavirin’s antiviral mechanism of action: lethal mutagenesis? J. Mol. Med. 2002;80:86–95. doi: 10.1007/s00109-001-0308-0. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Luczak M. Fluoroimidazoles as antiviral agents and inhibitors of polynucleotide biosynthesis. Life Sci. 1975;17:187–194. doi: 10.1016/0024-3205(75)90502-0. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Cools M., Balzarini J., Snoeck R., Andrei G., Hosoya M., Shigeta S., Ueda T., Minakawa N., Matsuda A. Antiviral activities of 5-ethynyl-1-beta-d-ribofuranosylimidazole-4-carboxamide and related compounds. Antimicrob. Agents Chemother. 1991;35:679–684. doi: 10.1128/aac.35.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Andrei G., Snoeck R., De Bolle L., Naesens L., Degreve B., Balzarini J., Zhang Y., Schols D., Leyssen P., Ying C., Neyts J. Acyclic/carbocyclic guanosine analogues as anti-herpesvirus agents. Nucleosides Nucleotides Nucleic Acids. 2001;20:271–285. doi: 10.1081/NCN-100002298. [DOI] [PubMed] [Google Scholar]
- Demby A.H., Chamberlain J., Brown D.W., Clegg C.S. Early diagnosis of Lassa fever by reverse transcription-PCR. J. Clin. Microbiol. 1994;32:2898–2903. doi: 10.1128/jcm.32.12.2898-2903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten, C., 2003. Echtzeitdetektion von DNA Amplifikationsprodukten, Patent no. 101 50 121.8, Deutsches Patentamt München, Germany.
- Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Müller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Dvoretskaia V.I., Bogatikov G.V., Pshenichnov V.A., Evseev A.A. The therapeutic efficacy of ribamidil and virazole in experimental Lassa fever in monkeys. Vopr. Virusol. 1991;36:55–57. [PubMed] [Google Scholar]
- Emmert D.H. Treatment of common cutaneous herpes simplex virus infections. Am. Fam. Physician. 2000;61(1697-1706):1708. [PubMed] [Google Scholar]
- Feldmann H., Jones S., Klenk H.D., Schnittler H.J. Ebola virus: from discovery to vaccine. Nat. Rev. Immunol. 2003;3:677–685. doi: 10.1038/nri1154. [DOI] [PubMed] [Google Scholar]
- Garcia C.C., Candurra N.A., Damonte E.B. Antiviral and virucidal activities against arenaviruses of zinc-finger active compounds. Antiviral Chem. Chemother. 2000;11:231–237. doi: 10.1177/095632020001100306. [DOI] [PubMed] [Google Scholar]
- Gerber D.A., Bonham C.A., Thomson A.W. Immunosuppressive agents: recent developments in molecular action and clinical application. Transplant. Proc. 1998;30:1573–1579. doi: 10.1016/s0041-1345(98)00361-3. [DOI] [PubMed] [Google Scholar]
- Gibb T.R., Norwood D.A., Jr., Woollen N., Henchal E.A. Development and evaluation of a fluorogenic 5′ nuclease assay to detect and differentiate between Ebola virus subtypes Zaire and Sudan. J. Clin. Microbiol. 2001;39:4125–4130. doi: 10.1128/JCM.39.11.4125-4130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufert F.T., Ludke W., Schmitz H. Epitope mapping of the Lassa virus nucleoprotein using monoclonal anti-nucleocapsid antibodies. Arch. Virol. 1989;106:201–212. doi: 10.1007/BF01313953. [DOI] [PubMed] [Google Scholar]
- Huggins J.W., Robins R.K., Canonico P.G. Synergistic antiviral effects of ribavirin and the C-nucleoside analogs tiazofurin and selenazofurin against togaviruses, bunyaviruses, and arenaviruses. Antimicrob. Agents Chemother. 1984;26:476–480. doi: 10.1128/aac.26.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling P.B., Hesse R.A., Eddy G.A., Johnson K.M., Callis R.T., Stephen E.L. Lassa virus infection of rhesus monkeys: pathogenesis and treatment with ribavirin. J. Infect. Dis. 1980;141:580–589. doi: 10.1093/infdis/141.5.580. [DOI] [PubMed] [Google Scholar]
- Kaplan C.P., Bain K.P. Cognitive outcome after emergent treatment of acute herpes simplex encephalitis with acyclovir. Brain Inj. 1999;13:935–941. doi: 10.1080/026990599121133. [DOI] [PubMed] [Google Scholar]
- Korbukh, I.A., Budanova, O.V., Preobrazhenskaya, M.N., 1978. 4-Amino-1-beta-d-ribofuranosylpyrazole-3-carboxamide: synthesis of pyrazole nucleosides by the fusion method without a catalyst, in: Townsend L.B., Tipson R.S. (Eds.), Nucleic Acid Chem., vol. 1. John Wiley, New York, p. 469.
- Kramata P., Downey K.M., Paborsky L.R. Incorporation and excision of 9-(2-phosphonylmethoxyethyl)guanine (PMEG) by DNA polymerase delta and epsilon in vitro. J. Biol. Chem. 1998;273:21966–21971. doi: 10.1074/jbc.273.34.21966. [DOI] [PubMed] [Google Scholar]
- Lau J.Y., Tam R.C., Liang T.J., Hong Z. Mechanism of action of ribavirin in the combination treatment of chronic HCV infection. Hepatology. 2002;35:1002–1009. doi: 10.1053/jhep.2002.32672. [DOI] [PubMed] [Google Scholar]
- McCormick J.B., King I.J., Webb P.A., Scribner C.L., Craven R.B., Johnson K.M., Elliott L.H., Belmont-Williams R. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- Naesens L., De Clercq E. Therapeutic potential of HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues as broad-spectrum antiviral agents. Nucleosides Nucleotides. 1997;16:983–992. [Google Scholar]
- Naesens L., Snoeck R., Andrei G., Balzarini J., Neyts J., De Clercq E. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antiviral Chem. Chemother. 1997;8:1–23. [Google Scholar]
- Nair V., Ussery M.A. New hypoxanthine nucleosides with RNA antiviral activity. Antiviral Res. 1992;19:173–178. doi: 10.1016/0166-3542(92)90076-h. [DOI] [PubMed] [Google Scholar]
- Noble S., Goa K.L. Adefovir dipivoxil. Drugs. 1999;58:479–489. doi: 10.2165/00003495-199958030-00010. [DOI] [PubMed] [Google Scholar]
- Revankar, G.R., Solan, V.C., Robins, R.K., Witkowski, J.T., 1981. Synthesis and biological activity of certain 1,2,3-triazole carboxamide nucleosides related to bredinin and pyrazofurin. Nucleic Acids Symp. Ser. 65–68. [PubMed]
- Rodriguez M., McCormick J.B., Weissenbacher M.C. Antiviral effect of ribavirin on Junin virus replication in vitro. Rev. Argent. Microbiol. 1986;18:69–74. [PubMed] [Google Scholar]
- Schaeffer H.J., Beauchamp L., de Miranda P., Elion G.B., Bauer D.J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978;272:583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Schmitz H., Köhler B., Laue T., Drosten C., Veldkamp P.J., Günther S., Emmerich P., Geisen H.P., Fleischer K., Beersma M.F., Hoerauf A. Monitoring of clinical and laboratory data in two cases of imported Lassa fever. Microbes Infect. 2002;4:43–50. doi: 10.1016/s1286-4579(01)01508-8. [DOI] [PubMed] [Google Scholar]
- Sidwell R.W., Huffman J.H., Khare G.P., Allen L.B., Witkowski J.T., Robins R.K. Broad-spectrum antiviral activity of Virazole: 1-beta-d-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;177:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- Smee D.F., Morris J.L., Barnard D.L., Van Aerschot A. Selective inhibition of arthropod-borne and arenaviruses in vitro by 3′-fluoro-3′-deoxyadenosine. Antiviral Res. 1992;18:151–162. doi: 10.1016/0166-3542(92)90035-4. [DOI] [PubMed] [Google Scholar]
- Smith, R.A., Kirkpatrick, W., 1980. Ribavirin: a Broad Spectrum Antiviral Agent. Academic Press, New York.
- Srivastava P.C., Streeter D.G., Matthews T.R., Allen L.B., Sidwell R.W. Synthesis and antiviral and antimicrobial activity of certain 1-beta-d-ribofuranosyl-4,5-disubstituted imidazoles. J. Med. Chem. 1976;19:1020–1026. doi: 10.1021/jm00230a009. [DOI] [PubMed] [Google Scholar]
- Stephen E.L., Jahrling P.B. Experimental Lassa fever virus infection successfully treated with ribavirin. Lancet. 1979;1:268–269. doi: 10.1016/s0140-6736(79)90790-6. [DOI] [PubMed] [Google Scholar]
- Tam R.C., Lau J.Y., Hong Z. Mechanisms of action of ribavirin in antiviral therapies. Antiviral Chem. Chemother. 2001;12:261–272. doi: 10.1177/095632020101200501. [DOI] [PubMed] [Google Scholar]
- Wachsman M.B., Lopez E.M., Ramirez J.A., Galagovsky L.R., Coto C.E. Antiviral effect of brassinosteroids against herpes virus and arenaviruses. Antivir. Chem. Chemother. 2000;11:71–77. doi: 10.1177/095632020001100107. [DOI] [PubMed] [Google Scholar]
- Witkowski J.T., Robins R.K., Sidwell R.W., Simon L.N. Design, synthesis, and broad spectrum antiviral activity of 1- -d-ribofuranosyl-1,2,4-triazole-3-carboxamide and related nucleosides. J. Med. Chem. 1972;15:1150–1154. doi: 10.1021/jm00281a014. [DOI] [PubMed] [Google Scholar]


