Abstract
Flavonoids are dietary components and the most ubiquitous phenolic compounds found in nature, showing a range of pharmacological activities including antiviral action. This study describes the antiviral screening of 60 different flavones and flavonols against human rotavirus (Wa-1 strain) as well as their cytotoxicity in MA104 cells. Cytotoxicity was investigated by cell morphology assessment and antirotavirus activity by cytopathic effect inhibition. Results were expressed as CC50 and IC50, respectively, in order to calculate the selectivity index (SI = CC50/IC50) of each compound. Structure–activity relationships (SAR) were proposed based on antirotavirus activity.
Keywords: Antiviral screening, Flavonoids, Rotavirus, SAR
Graphical Abstract
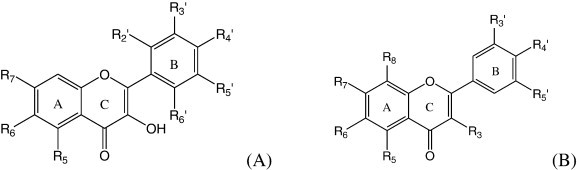
Basic structures of flavonols (A) and flavones (B).
1. Introduction
Flavonoids are polyphenolic compounds that can be found as dietary components such as food products, beverages and herbal medicines with different health benefits shown in a large number of studies [1]. Several pharmacological activities have been described for flavonoids such as antitumor [2], antibacterial [2], [3], [4], antifungal [3], [4], antiallergic, estrogenic, anti-inflammatory [2], prevention of cardiovascular diseases [5] and antiviral [3], [4], [6]. In addition, they are well-known antioxidants and metal ion-chelators [4], [7].
Natural products have shown to be an important source of useful compounds in antiviral chemotherapy [8], [9]. Compounds with promising activity can be used directly as drugs or as leads for the synthesis of new drugs. During the last few years, efforts have been made to increase the number of compounds with antiviral activity. Clinical use of the currently available antiviral drugs has use restrictions as narrow spectrum of activity, limited therapeutic usefulness and variable degrees of toxicity [10]. Furthermore, the therapeutic potency of most of the antiviral agents encountered so far is counterbalanced by their severe side effects in humans and, in some cases; the efficacy of these drugs is limited by increase of viral resistance [11]. Therefore, the search for antiviral compounds with high efficacy, low toxicity and minor side effects must continue to improve drug therapy.
Flavonoids have been investigated for antiviral activity, for instance, against human cytomegalovirus [12], [13], herpes simplex virus types 1 and 2 [14], [15], [16], [17], influenza virus, respiratory syncitial virus, adenovirus, varicella zoster virus [12], poliovirus [14], [18], [19], rhinovirus [19], sindbis virus [20], coronavirus, parainfluenzavirus, coxsackievirus B [8], HIV [21] and rotavirus [6].
Among enteric viruses, rotaviruses are the major cause of severe diarrhea and it is believed that they would account for about 30 to 80% of pediatric hospitalizations for acute gastroenteritis. Rotaviruses affect nearly the population regardless of level of hygiene, quality of water, food and sanitation. Generally, the estimated incidences of rotaviruses in developed and developing countries are similar. The infections may be asymptomatic or may present mild vomiting and/or diarrhea, but can also cause severe disease leading to potentially fatal dehydration. Currently, the goals of treatment of rotaviruses gastroenteritis are to prevent and treat dehydration, and prevent nutritional damage during and after diarrhea episodes [21]. Recently, two rotavirus vaccines have entered in the market: RotaRix (GlaxoSmithKline) and RotaTeq (Merck/CSL) and several issues could be resolved with these new vaccines [22].
Rotavirus is a nonenveloped virus which has a segmented, double-stranded RNA genome surrounded by three concentric protein layers [23]. According to the Coulson and Liakatos et al. [24], [25] the rotavirus entry into host cell is a multi-step process, requiring at least three virus host-cell interactions. The rotavirus initially binds to host cell-surface glycoconjugates, followed by interactions independent of sialic acid, believed to be part of the lipid domains in which integrins are thought to play an important role. The understanding of these interaction mechanisms between virus and cell is relevant, since it can contribute to a rational selection of compounds to be tested in antiviral assays.
The importance of this health public problem and the significant amount of data available on rotavirus replication, as well the promising biological effects of flavonoids, led us to use this virus as a system to study the antiviral activity of different compounds. Thus, the aim of this study was to evaluate the potential antirotavirus of 60 flavonols and flavones (Fig. 1 , Table 1, Table 2 ) and to investigate structure–activity relationships (SAR).
Fig. 1.
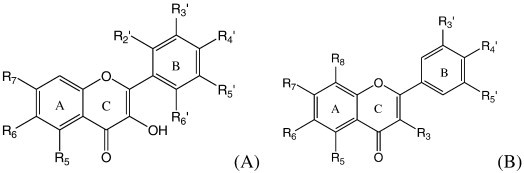
Basic structures of flavonols (A) and flavones (B).
Table 1.
Substitution patterns of tested flavonols and their cytotoxic (CC50) and viral inhibitory (IC50) concentrations, as well as their selective indices (SI = CC50/IC50).
| Number | R2′ | R3′ | R4′ | R5′ | R6′ | R5 | R6 | R7 | MW | CC50 | IC50 | SI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | OH | H | H | H | H | H | H | H | 254.24 | 61.36 | NA | – |
| 2 | H | OH | H | H | H | H | H | H | 254.24 | 61.36 | 27.53 | 2.2 |
| 3 | H | H | OH | H | H | H | H | H | 254.24 | 61.36 | NA | – |
| 4 | H | OH | OH | H | H | H | H | H | 270.24 | 57.73 | 14.43 | 4.0 |
| 5 | OH | H | H | OH | H | H | H | H | 270.24 | 57.73 | NA | – |
| 6 | H | OH | OH | OH | H | H | H | H | 286.24 | 27.25 | 18.17 | 1.5 |
| 7 | H | H | OCH3 | H | H | H | H | H | 268.26 | 29.08 | NA | – |
| 8 | H | H | H | H | OCH3 | H | H | H | 268.26 | 44.73 | NA | – |
| 9 | OCH3 | H | OCH3 | H | H | H | H | H | 298.29 | 52.30 | 26.15 | 2.0 |
| 10 | H | H | OCH3 | OCH3 | H | H | H | H | 298.29 | 52.30 | 13.07 | 4.0 |
| 11 | H | OCH3 | OCH3 | OCH3 | H | H | H | H | 328.38 | 47.51 | 11.88 | 4.0 |
| 12 | H | H | OCH3 | OCH3 | OCH3 | H | H | H | 328.38 | 23.75 | 6.09 | 3.9 |
| 13 | H | H | OCH2C6H5 | H | H | H | H | H | 344.36 | 22.65 | 3.78 | 6.0 |
| 14 | H | OCH2CH3 | H | H | H | H | H | H | 282.29 | 110.52 | NA | – |
| 15 | OCH2CH3 | H | H | H | H | H | H | H | 282.29 | 110.52 | NA | – |
| 16 | OCH2CH3 | H | OCH2CH3 | H | H | H | H | H | 326.34 | 47.80 | NA | – |
| 17 | OCH2CH3 | H | H | OCH2CH3 | H | H | H | H | 326.34 | 23.90 | 11.95 | 2.0 |
| 18 | H | OCH2CH3 | OCH2CH3 | H | H | H | H | H | 326.34 | 47.80 | 7.97 | 6.0 |
| 19 | OCH2C6H5 | H | H | H | H | H | H | H | 344.36 | 45.30 | 7.55 | 6.0 |
| 20 | H | H | H | H | OCH2C6H5 | H | H | H | 344.6 | 45.27 | 5.51 | 8.2 |
| 21 | H | H | CH2CH3 | H | H | H | H | H | 266.29 | 14.65 | 3.64 | 4.0 |
| 22 | H | O(CH2)3CH3 | H | H | H | H | H | H | 310.34 | 25.13 | 6.12 | 4.1 |
| 23 | O(CH2)3CH3 | H | H | H | H | H | H | H | 310.34 | 301.93 | 75.40 | 4.0 |
| 24 | H | H | O(CH2)3CH3 | H | H | H | H | H | 310.34 | 201.39 | 40.60 | 5.0 |
| 25 | H | O(CH2)3CH3 | O(CH2)3CH3 | H | H | H | H | H | 382.45 | 40.79 | 6.80 | 6.0 |
| 26 | O(CH2)3CH3 | H | O(CH2)3CH3 | H | H | H | H | H | 382.45 | 40.79 | 10.20 | 4.0 |
| 27 | H | H | H | H | H | H | H | OH | 254.24 | 61.36 | 15.73 | 3.9 |
| 28 | H | OH | OH | H | H | OH | H | OH | 302.24 | 103.23 | NA | – |
| 29 | H | H | H | H | H | H | OCH3 | H | 268.26 | 29.08 | NA | – |
| 30 | H | H | H | H | H | H | H | OCH3 | 268.26 | 29.08 | NA | – |
| 31 | H | H | OCH3 | H | H | OCH3 | H | H | 298.29 | 26.15 | 4.69 | 5.6 |
| 32 | H | H | OCH3 | H | H | H | OCH3 | H | 298.29 | 104.60 | 17.43 | 6.0 |
| 33 | H | H | OCH3 | H | H | H | H | OCH3 | 298.29 | 26.15 | 6.37 | 4.1 |
| 34 | OCH3 | H | OCH3 | H | H | H | H | OCH3 | 329.12 | 47.40 | 11.85 | 4.0 |
| 35 | H | H | H | H | H | H | H | OCH2CH3 | 282.29 | 110.52 | NA | – |
| 36 | H | H | OCH2CH3 | H | H | H | H | OCH2CH3 | 326.34 | 47.80 | 11.95 | 4.0 |
| 37 | H | OCH2CH3 | H | H | H | H | H | OCH2CH3 | 326.34 | 47.80 | 7.97 | 6.0 |
| 38 | H | H | H | H | H | H | H | O(CH2)3CH3 | 310.34 | 50.27 | 8.38 | 6.0 |
| 39 | H | H | OCH2C6H5 | H | H | H | H | OCH2C6H5 | 450.49 | 69.26 | 17.31 | 4.0 |
MW = molecular weight; NA = no antiviral activity; CC50 (μM), MA104 cells; IC50 (μM), human rotavirus. Substances 8, 10, 12, 20, 21, 28, 29, 31, 32 and 34 were purchased from Sigma and others were synthesized [26].
Table 2.
Substitution patterns of tested flavones and their cytotoxic (CC50) and viral inhibitory (EC50) concentrations, as well as their selective indices (SI = CC50/IC50).
| Number | R3′ | R4′ | R5′ | R3 | R5 | R6 | R7 | R8 | MW | CC50 | IC50 | SI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40 | H | OH | H | H | OH | OCH3 | OCH3 | OCH3 | 344.0 | 22.67 | 7.56 | 3.0 |
| 41 | H | OH | H | H | OH | OCH3 | OCH3 | H | 316.0 | 49.37 | 12.34 | 4.0 |
| 42 | H | OH | H | H | OH | H | OCH3 | H | 284.6 | 54.81 | 18.27 | 3.0 |
| 43 | OH | OH | H | OCH3 | OH | H | OH | H | 316.26 | 49.33 | 12.33 | 4.0 |
| 44 | OH | OH | H | O-RHAMNOSE | OH | H | OH | H | 448.38 | 69.58 | NA | – |
| 45 | OH | OH | H | O-RHAMNO-GLUCOSE | OH | H | OH | H | 610.51 | 51.10 | NA | – |
| 46 | OH | OH | H | H | OH | H | OH | H | 286.24 | 27.25 | NA | – |
| 47 | OH | OH | H | OSO3 | OH | H | OH | H | 381.3 | 40.91 | 10.23 | 4.0 |
| 48 | H | OCH3 | H | H | H | OCH2C6H5 | H | H | 357.38 | 21.83 | NA | – |
| 49 | H | OCH3 | H | H | OCH3 | OCH3 | OCH3 | OCH3 | 372.37 | 167.84 | 28.20 | 6.0 |
| 50 | H | OCH3 | H | H | OH | H | OCH3 | H | 298.0 | 52.35 | 13.09 | 4.0 |
| 51 | H | OCH3 | H | H | OH | OCH3 | OCH3 | OCH3 | 358.0 | 43.58 | 7.26 | 6.0 |
| 52 | H | OCH3 | H | H | OH | OCH3 | OCH3 | H | 328.0 | 23.78 | 15.55 | 1.5 |
| 53 | H | OCH3 | OCH3 | H | OH | OCH3 | OCH3 | H | 342.24 | 45.58 | 17.53 | 2.6 |
| 54 | H | OCH3 | OCH3 | H | OCH3 | OCH3 | OCH3 | H | 372.37 | 41.89 | 6.98 | 6.0 |
| 55 | H | OCH3 | OCH3 | H | OCH3 | OCH3 | OCH3 | OCH3 | 402.40 | 38.77 | NA | – |
| 56 | H | OCH3 | OCH3 | OCH3 | OH | H | OCH3 | H | 342.24 | 45.58 | 30.10 | 1.5 |
| 57 | H | OCH3 | OCH3 | OCH3 | OCH3 | OCH3 | OCH3 | OCH3 | 432.42 | 36.08 | 18.04 | 2.0 |
| 58 | OCH3 | OCH3 | H | H | OH | OCH3 | OCH3 | OCH3 | 376.34 | 124.62 | 31.09 | 4.0 |
| 59 | OCH3 | OH | H | OCH3 | OH | H | OCH3 | OCH3 | 374.34 | 41.67 | 6.95 | 6.0 |
| 60 | OH | OCH3 | H | OCH3 | OH | OCH3 | OCH3 | OCH3 | 404.37 | 77.16 | 77.16 | 1.0 |
MW = molecular weight; NA = no antiviral activity; CC50 (μM), MA104 cells; IC50 (μM), human rotavirus. Substances 49, 54, 55 and 57 were synthesized [26] and others were purchased from Sigma.
2. Materials and methods
Flavonols identified as 8, 10, 12, 20, 21, 28, 29, 31, 32 and 34 were purchased from Sigma and others were synthesized [26]. Flavones identified as 49, 54, 55 and 57 were synthesized [26] and others were purchased from Sigma.
Compounds were assayed for their toxicity to MA104 cells by microscopical morphology evaluation [27], [28] and for their antirotavirus activity (Wa-1 strain) by cytopathogenicity inhibition [29]. They were dissolved in 1% of dimethyl sulfoxide (DMSO, Merck, Darmstadt, Germany) diluted in 199 medium (Sigma Chemical Co., St. Louis, MO, USA) and filtered through 0.22 μm membranes (Millipore, Bedfore, MA, USA). All stock solutions were stored at 4 °C protected from light until used. The used cell line was MA104 cells (Biological Science Institute, University of São Paulo, Brazil), grown in 199 Medium (Sigma Chemical Co., St. Louis, MO, USA) and supplemented with 10% fetal bovine serum (Gibco BRL, New York, USA), penicillin G (100 U/ml), streptomycin (100 μg/ml) and amphotericin B (0.025 μg/ml) (Gibco BRL, New York, USA). The cell cultures were maintained at 37 °C in a humidified 5% CO2 atmosphere. The human rotavirus Wa-1 (ATCC: VR2018) was used and it was propagated in MA104 cells in the presence of trypsin (Sigma, 5 μg/ml). Stock viruses were prepared as previously described by Barardi et al. [27] and the supernatant fluids were harvested, titrated and stored at − 80 °C until used. Virus titers were estimated from cytopathogenicity by the limit-dilution method and expressed as 50% tissue culture infectious dose per ml (TCID50/ml).
The CC50 (cytotoxic concentration for 50% of cells) and IC50 (inhibitory concentration for 50% of infected cells) values were estimated from concentration–effect curves after linear regression analysis, and represent the mean values of three independent experiments.
The selectivity index (SI = CC50/IC50) was calculated for each tested flavonoid. According to Sidwell [30], when SI ≥ 4 promising antiviral activity must be considered.
3. Results
The selectivity indexes (SI) were calculated from the cytotoxic and inhibitor concentrations (Table 1, Table 2) and the values obtained were considered to propose a structure–activity relationship. In tests with non-cytotoxic concentrations, the compounds showed different degrees of antirotavirus activity, excepting the flavonols 1, 3, 5, 7, 8, 14–16 and 28–30, and the flavones 35, 44–46, 48 and 55. The majority of tested flavonols and flavones demonstrated SI ≥ 4, indicating a favorable activity against human rotavirus (Wa-1 strain).
4. Discussion
In the present study, the cytotoxicity and antiviral action of 60 flavonoids were evaluated on MA104 cells, which have susceptibility to human rotavirus. Taking into account these results, the selectivity index for each compound was calculated and these values were considered to describe SAR. The tested flavonoids were divided in groups according to their similar structural characteristics and some SAR were proposed considering the conformational effects exerted by the substituents.
For the flavonols, the A ring without radicals, compounds 1–26, with hydroxyl groups in orto position (1, 3 and 5) did not grant antiviral activity, but not when it was in meta position (2, 4 and 6). Considering just one methoxyl in B ring (7 and 8), antiviral action was not observed, in contrast to the existence of two (9 and 10) or three (11 and 12) methoxyl radicals in B ring. A considerable antirotavirus action was detected in the presence of benzyloxyl radical in the same ring (13, 19 and 20). In view of ethoxyl radicals in B ring (14–18), the promising antiviral activity was linked to the presence of two of this radical in meta position (18). Compounds with butoxyl radicals in B ring (22–26) also showed activity, with higher activity for orto/meta positions of this radical (25).
Antiviral activity was provided for compounds with one butoxyl group in A ring (38), one ethyl radical in B ring (21) and one benzyloxyl radical simultaneously in A and B rings (39).
When A ring has one methoxyl group (29–34), the antiviral activity was detected only if the B ring also has a methoxyl at the R4′ position (31–34).
With one substituent ethoxyl in A ring (35–37), an antiviral activity was detected only in the presence of one ethoxyl radical in B ring at the R3′ or R4′ position (36 and 37).
In view of the A and B rings containing two hydroxyl groups each one (28), the antiviral activity was not observed. The same result was observed when the flavonol comprised only one hydroxyl in B ring and no substituent in A ring (3).
For the flavonols, the presence of two or more methoxyl and ethoxyl radicals, one or two butoxyl radicals, and one benzyloxyl radical showed antiviral activity while compounds containing only one methoxyl or ethoxyl radical did not reduce the rotavirus infection.
In view of the role that sialic acid has in infection by rotavirus, Fazli et al. [31] evaluated the antiviral effect of lactose-based sialylmimetics, with different chemical variations in order to find new target for therapeutic intervention. The results of this study suggested that small alkyl groups are tolerated in the binding pocket of the viral adhesion proteins of the bovine and human strains, and that aryl groups may be too sterically demanding (or too hydrophobic) for the human (Wa) strain of rotavirus. These findings could contribute to explain the higher antirotavirus effect found for flavonols that had butoxyl groups or one benzyloxyl radical (compound 20 had the highest selectivity index of tested compounds).
Concerning the tested flavones, an antiviral action was shown when the B and C rings did not have methoxyl substituents (40–42) and had one hydroxyl and hydroxyl radicals in A ring.
In the case of flavones without methoxyl substituents in A and B rings (43–47), the presence of O-rhamnose and O-rhamno-glucose at R3 position (44 and 45, respectively) or the absence of radical (46) in C ring did not provide antiviral activity. Bae et al. [32] tested the in vitro inhibitory action of different rhamnoglycosides on rotavirus infection in that dosmin and hesperidin had a higher activity (IC50 = 10 μM). These same authors reported a low inhibitory effect for rutin, which it could be related to its low solubility in water. The antirotavirus activity results found in our study for the compounds 45 (rutin) and 46 (quercetrin) were similar to those of Bae et al. [32]. On the other hand, Jolly et al. [33] considered the galactose an important component of the host cell receptor since applied galectins (galactose-recognising proteins) inhibited rotavirus infection. However, flavonoids containing this sugar were not synthesized in this study in order to evaluate the antirotavirus effect.
The presence of [–OSO3] radical (47) or methoxyl radical (43) in C ring conferred an antiviral activity. Takahashi et al. [23] evaluated the in vitro and vivo antiviral activity of sulfated sialyl lipid (NMSO3) and they suggested that this compound may bind to the specific sites of VP4 and/or VP7 of human rotavirus by either hydrophobic association of lipid chains or negative charge of sulfate residues followed by an interference with the binding of VP4 and/or VP7 to cellular receptor(s) by steric hindrance. Although only one compound containing [–OSO3] radical was tested in our study, this group appears to be important for an antiviral effect.
Taking into consideration just one methoxyl radical in R4′ and no radical in C ring (48–52), the presence of methoxyl radicals in A ring provided antiviral activity, independently of hydroxyl groups in this ring. However, an antiviral activity was not shown in the presence and absence of benzyloxyl and methoxyl radicals in A ring (48), respectively.
In the presence of two methoxyl radicals in B ring and no radicals in C ring (53–55 and 58), the addition of methoxyl radicals in A ring contributed to an antiviral activity, independently of hydroxyl groups in this last ring. On the other hand, an antiviral effect was not shown in the presence of four methoxyl substituents in B ring (55). For two methoxyl radicals in B ring and one methoxyl in C ring (56 and 57), a weak antirotavirus activity was verified.
The flavones 59 and 60 have similar characteristics in their B and C rings and they can be distinguished from other tested flavones by methoxyl and hydroxyl radicals in B ring. The absence of methoxyl in R6 position of A ring (59) could explain the antiviral activity when these compounds were compared.
Considering the same A and C rings (49 and 55), the absence of methoxyl in R5′ position of B ring (49) could explain the antiviral activity observed.
For the tested flavones, the SAR analysis suggests that the presence of methoxyl group in A ring is relevant to antirotavirus action, nevertheless, the characteristics about the other rings also need to be considered to each compound.
Thirty-four amongst 60 tested compounds showed moderate antiviral activity (SI ≥ 4) and these results encourage further exploration about this property of these flavonoids, as well as the confirmation of the detected antirotavirus activity by other methodologies and the investigation of their action mechanism. These effects in vitro from the structural aspects represent only preliminary results since the antiviral or cytotoxic activity of flavonoids is related to multiple factors. Concomitantly, metabolism studies should be carried out due to the fact that several flavonoids can degrade to variable extents in the digestive tract. A screening of bioactive compounds, synthetic or isolated from natural sources, and the evaluation of their SAR, allied to technological advances, are required to develop more effective and safer new drugs than those already available.
Acknowledgments
C.M.O. Simões, E.P. Schenkel, C.R.M. Barardi and T. Caon give thanks to CNPq/MCT/Brazil, and L.A. Savi and A.M. Sobottka thanks CAPES/MEC/Brazil for their research fellowships.
References
- 1.Li N., Liu J.H., Zhang J., Yu B.Y. Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J Agric Food Chem. 2008;56:3876–3883. doi: 10.1021/jf073520n. [DOI] [PubMed] [Google Scholar]
- 2.Das S., Rosazza J.P. Microbial and enzymatic transformations of flavonoids. J Nat Prod. 2006;69:499–508. doi: 10.1021/np0504659. [DOI] [PubMed] [Google Scholar]
- 3.Cushnie T.P., Lamb A.J. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol Nutr Food Res. 2007;51:116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nardini M., Natella F., Scaccini C. Role of dietary polyphenols in platelet aggregation. A review of the supplementation studies. Platelets. 2007;18:224–243. doi: 10.1080/09537100601078083. [DOI] [PubMed] [Google Scholar]
- 6.Bae E.A., Han M.J., Lee M., Kim D.H. In vitro inhibitory effect of some flavonoids on rotavirus infectivity. Biol Pharm Bull. 2000;23:1122–1124. doi: 10.1248/bpb.23.1122. [DOI] [PubMed] [Google Scholar]
- 7.Stevenson D.E., Hurst R.D. Polyphenolic phytochemicals—just antioxidants or much more? Cell Mol Life Sci. 2007;64:2900–2916. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chattopadhyay D. Antivirals of ethnomedicinal origin: structure–activity relationship and scope. Mini Rev Med Chem. 2007;7:275–301. doi: 10.2174/138955707780059844. [DOI] [PubMed] [Google Scholar]
- 9.Potterat O., Hamburger M. Drug discovery and development with plant-derived compounds. Prog Drug Res. 2008;65:47–118. doi: 10.1007/978-3-7643-8117-2_2. [DOI] [PubMed] [Google Scholar]
- 10.Martin K.W., Ernst E. Antiviral agents from plants and herbs: a systematic review. Antivir Ther. 2003;8:77–90. [PubMed] [Google Scholar]
- 11.Balfour H.H. Antiviral drugs. N Engl J Med. 1999;340:1255–1268. doi: 10.1056/NEJM199904223401608. [DOI] [PubMed] [Google Scholar]
- 12.Lin Y.M., Flavin M.T., Schure R., Chen F.C., Sidwell R., Barnard D.L., Huffman J.H., Kern E.R. Antiviral activities of biflavonoids. Planta Med. 1999;65:120–125. doi: 10.1055/s-1999-13971. [DOI] [PubMed] [Google Scholar]
- 13.Evers D.L., Chao C.F., Wang X., Zhang Z., Huong S.M., Huang E.S. Human cytomegalovirus-inhibitory flavonoids: studies on antiviral activity and mechanism of action. Antiviral Res. 2005;68:124–134. doi: 10.1016/j.antiviral.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaul T.N., Middleton E., Ogra P.L. Antiviral effect of flavonoids on human viruses. J Med Virol. 1985;15:71–79. doi: 10.1002/jmv.1890150110. [DOI] [PubMed] [Google Scholar]
- 15.Amoros M., Simões C.M., Girre L., Sauvager F., Cormier M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J Nat Prod. 1992;55:1732–1740. doi: 10.1021/np50090a003. [DOI] [PubMed] [Google Scholar]
- 16.Khan M.T., Ather A., Thompson K.D., Gambari R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antiviral Res. 2005;67:107–119. doi: 10.1016/j.antiviral.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Savi L.A., Barardi C.R., Simões C.M. Evaluation of antiherpetic activity and genotoxic effects of tea catechin derivatives. J Agric Food Chem. 2006;54:2552–2557. doi: 10.1021/jf052940e. [DOI] [PubMed] [Google Scholar]
- 18.Vrijsen R., Everaert L., Van Hoof L.M., Vlietinck A.J., Vanden Berghe D.A., Boeyé A. The poliovirus-induced shut-off of cellular protein synthesis persists in the presence of 3-methylquercetin, a flavonoid which blocks viral protein and RNA synthesis. Antiviral Res. 1987;7:35–42. doi: 10.1016/0166-3542(87)90037-4. [DOI] [PubMed] [Google Scholar]
- 19.De Meyer N., Haemers A., Mishra L., Pandey H.K., Pieters L.A., Vanden Berghe D.A., Vlietinck A.J. 4′-Hydroxy-3-methoxyflavones with potent antipicornavirus activity. J Med Chem. 1991;34:736–746. doi: 10.1021/jm00106a039. [DOI] [PubMed] [Google Scholar]
- 20.Paredes A., Alzuru M., Mendez J., Rodríguez-Ortega M. Anti-Sindbis activity of flavanones hesperetin and naringenin. Biol Pharm Bull. 2003;26:108–109. doi: 10.1248/bpb.26.108. [DOI] [PubMed] [Google Scholar]
- 21.D'Agostino J. Considerations in assessing the clinical course and severity of rotavirus gastroenteritis. Clin Pediatr (Phila) 2006;45:203–212. doi: 10.1177/000992280604500301. [DOI] [PubMed] [Google Scholar]
- 22.Dennehy P.H. Rotavirus vaccines: an overview. Clin Microbiol Ver. 2008;21:198–208. doi: 10.1128/CMR.00029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi K., Ohashi K., Abe Y., Mori S., Taniguchi K., Ebina T., Nakagomi O., Terada M., Shigeta S. Protective efficacy of a sulfated sialyl lipid (NMSO3) against human rotavirus-induced diarrhea in a mouse model. Antimicrob Agents Chemother. 2002;46:420–424. doi: 10.1128/AAC.46.2.420-424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coulson B.S., Londrigan S.L., Lee D.J. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc Natl Acad Sci U S A. 1997;94:5389–5394. doi: 10.1073/pnas.94.10.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liakatos A., Kiefel M.J., Fleming F., Coulson B., von Itzstein M. The synthesis and biological evaluation of lactose-based sialylmimetics as inhibitors of rotaviral infection. Bioorg Med Chem. 2006;14:739–757. doi: 10.1016/j.bmc.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 26.Sobottka A.M., Werner W., Blaschke G., Kiefer W., Nowe U., Dannhardt G., Schapoval E.E., Schenkel E.P., Scriba G.K. Effect of flavonol derivatives on the carrageenin-induced paw edema in the rat and inhibition of cyclooxygenase-1 and 5-lipoxygenase in vitro. Arch Pharm (Weinheim) 2000;333:205–210. doi: 10.1002/1521-4184(20007)333:7<205::aid-ardp205>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 27.Barardi C.R., Emslie K.R., Vesey G., Williams K.L. Development of a rapid and sensitive quantitative assay for rotavirus based on flow cytometry. J Virol Methods. 1998;74:31–38. doi: 10.1016/s0166-0934(98)00061-5. [DOI] [PubMed] [Google Scholar]
- 28.Andrighetti-Fröhner C.R., Antonio R.V., Creczynski-Pasa T.B., Barardi C.R., Simões C.M. Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum. Mem Inst Oswaldo Cruz. 2003;98:843–848. doi: 10.1590/s0074-02762003000600023. [DOI] [PubMed] [Google Scholar]
- 29.Simões C.M., Amoros M., Girre L. Mechanism of antiviral activity of triterpenoid saponins. Phytother Res. 1999;13:323–328. doi: 10.1002/(SICI)1099-1573(199906)13:4<323::AID-PTR448>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 30.Sidwell R.W. Determination of antiviral activity. Drugs Pharm Sci. 1986;27:433–480. [Google Scholar]
- 31.Fazli A., Bradley S.J., Kiefel M.J., Jolly C., Holmes I.H., von Itzstein M.J. Synthesis and biological evaluation of sialylmimetics as rotavirus inhibitors. J Med Chem. 2001;44:3292–3301. doi: 10.1021/jm0100887. [DOI] [PubMed] [Google Scholar]
- 32.Bae E.A., Han M.J., Lee M., Kim D.H. In vitro inhibitory effect of some flavonoids on rotavirus infectivity. Biol Pharm Bull. 2000;23:1122–1124. doi: 10.1248/bpb.23.1122. [DOI] [PubMed] [Google Scholar]
- 33.Jolly C.L., Beisner B.M., Holmes I.H. Rotavirus infection of MA104 cells is inhibited by Ricinus lectin and separately expressed single binding domains. Virology. 2000;275:89–97. doi: 10.1006/viro.2000.0470. [DOI] [PubMed] [Google Scholar]


