Abstract
Dramatic evolutionary transitions in morphology are often assumed to be adaptive in a new habitat. However, these assumptions are rarely tested because such tests require intermediate forms, which are often extinct. In vertebrates, the evolution of an elongate, limbless body is generally hypothesized to facilitate locomotion in fossorial and/or cluttered habitats. However, these hypotheses remain untested because few studies examine the locomotion of species ranging in body form from tetrapod to snake-like. Here, we address these functional hypotheses by testing whether trade-offs exist between locomotion in surface, fossorial and cluttered habitats in Australian Lerista lizards, which include multiple intermediate forms. We found that snake-like species penetrated sand substrates faster than more lizard-like species, representing the first direct support of the adaptation to fossoriality hypothesis. By contrast, body form did not affect surface locomotion or locomotion through cluttered leaf litter. Furthermore, all species with hindlimbs used them during both fossorial and surface locomotion. We found no evidence of a trade-off between fossorial and surface locomotion. This may be either because Lerista employed kinematic strategies that took advantage of both axial- and limb-based propulsion. This may have led to the differential occupation of their habitat, facilitating diversification of intermediate forms.
Keywords: body form evolution, evolutionary transition, sand-penetration
1. Background
The tree of life is filled with dramatic evolutionary transitions such as the invasion of land by plants and animals [1–3], the evolution of powered flight in insects and vertebrates [4,5], the re-invasion of marine habitats by whales [6,7], and the evolution of snake-like body forms in fossorial and/or cluttered habitats [8–11]. These transitions are characterized by major shifts in phenotype and ecology, as organisms adapt and radiate into novel adaptive zones [12,13]. However, the absence of extant intermediate forms [14] hampers our understanding of these transitions. While many intermediate forms exist in the fossil record [15], how they function can be inferred only indirectly [16]. Furthermore, the selective pressures that led to the transitions changed as the clades subsequently radiated [13,17,18].
A snake-like form has evolved dozens of times across most major vertebrate lineages, and this transition involves the elongation of the body and the reduction of the limbs [9,19,20]. The prevailing hypotheses for the evolution of this body form are that it is an adaptation for fossoriality and/or inhabiting cluttered habitats because such bodies experience less drag and can fit through narrower gaps than limbed forms [10,21–23]. Although snake-like forms are often correlated with locomotion through fossorial or cluttered habitats [21,24], it remains unknown whether snake-like forms are better at fossorial locomotion or moving through cluttered habitats than tetrapodal forms.
Snake-like forms have evolved independently at least 25 times in squamates (lizards, amphisbaenians and snakes), as two ecomorphs: a short-tailed fossorial morph and a long-tailed surface-dweller that lives in cluttered habitats [8,24]. Some of these lineages have many extant intermediate forms [25–30], allowing tests of functional hypotheses about the origin of snake-like forms directly. The Australian skink genus Lerista contains species belonging to 12 forms, ranging from pentadactyl to limbless [29,31–33]. Most Lerista are semi-fossorial—swimming through loose sand and inhabiting Acacia leaf litter mats that lie atop the sand [34]. The shed leaves that comprise the litter mats are stiff and slender [35] and interdigitate to form small crawl spaces. Thus, Lerista uses both fossorial and cluttered habitats and have intermediate tail lengths, allowing testing of both hypotheses about the evolution of snake-like forms [33,36].
Here, we test both the fossoriality and the cluttered habitat hypotheses using 12 species of Lerista, representing nearly the full range of extant body forms (electronic supplementary material, table S1). If a snake-like form is an adaptation for fossoriality or cluttered habitats in Lerista, then we expect that more snake-like species will achieve greater velocities while moving through a sand substrate or leaf litter, respectively, than more lizard-like species. A corollary of this prediction is that more lizard-like species should achieve higher velocities during surface locomotion than more snake-like species [37,38], resulting in a performance trade-off between surface and fossorial locomotion that is related to body form (figure 1). We also test the hypothesis that Lerista partition their habitat in relation to their body form. Multiple species of Lerista that differ in body form can be found in sympatry, often under the same Acacia bush [39,40]. Some evidence suggests that more snake-like and smaller species tend to occupy the periphery of leaf litter mats, where the litter is shallower [40], and inhabit less compacted sands [41]. If body form is related to habitat preferences, this may lend further support to hypotheses of snake-like forms being adaptations for particular habitats.
Figure 1.
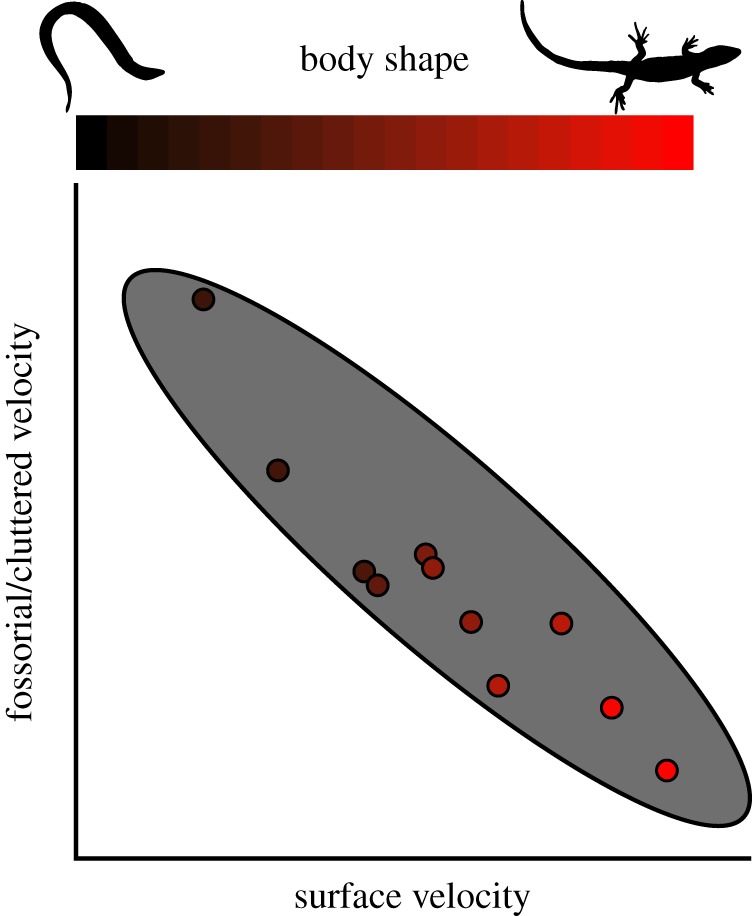
Theoretical predictions of the hypothesis that a snake-like form is an adaptation for fossoriality or moving in cluttered habitats. The colour of the dots indicates body form. Snake-like species should be better locomotors in fossorial or cluttered habitats, but worse surface locomotors, than lizard-like species, resulting in a performance trade-off related to body shape. (Online version in colour.)
2. Methods
(a). Specimen and microhabitat data collection
We sampled 102 adult and subadult specimens belonging to 12 species of Lerista, including 8 of the 12 evolved body forms (electronic supplementary material, tables S1 and S2). We sampled nine species in October–December 2014 in coastal Western Australia, and three species were laboratory specimens at the University of Adelaide. We collected lizards by hand while raking Acacia leaf litter [40,42]. We measured the depth of leaf litter where each animal was captured, the area of each leaf litter mat, and the relative distance each animal was from the trunk of the Acacia (electronic supplementary material, methods). We measured head length, snout–vent length, body width, tail length, fore- and hindlimb lengths, and counted the number of digits on the fore- and hindlimbs [33]. We only collected data from specimens with original tails or long, regenerated tails. We vouchered two specimens of each species at the Western Australian Museum and released the rest at the site of capture.
(b). Locomotion trials
We painted points on animals along their dorsal midline using non-toxic white paint at the level of the occiput, pectoral girdle, mid-body, pelvic girdle, cloaca and mid-tail. In the laboratory, we kept lizards in an incubator at 33°C prior to trials. In the field, we conducted trials when the ambient temperature was 30–33°C between 1200 and 1700 h, the preferred temperature of Lerista [43]. We conducted surface locomotion trials on approximately 5 mm deep mixed particle sand and sand-penetrating trials in approximately 25 mm deep coarse and fine sand ([44]; electronic supplementary material, methods). We also did trials with animals moving into approximately 25 mm deep Acacia leaf litter for lizards we caught in the field (electronic supplementary material, figures S2–4).
We recorded at least two good locomotor trials per individual per treatment from dorsal view at 240 Hz and a 518 × 384-pixel resolution using a Casio Exilim Ex-ZR1000 camera (Tokyo, Japan). We measured sand-penetrating force in the coarse and fine substrates with a custom-built tunnel attached to a Kistler Type 9203 uniaxial piezoelectric force transducer (Kistler Instrumente, Winterthur, Switzerland) [45,46] (electronic supplementary material, methods). We filled the tunnel with approximately 50 mm of the substrate, coaxing the animal to move into the sand while being careful not to push the animal to avoid erroneous force readings. We measured body temperature following each trial using a Raytek MT6 infrared thermometer (Santa Cruz, CA, USA).
(c). Quantifying locomotion
We digitized painted points using DLTdv5 [46] in MatLab 2016A (Mathworks, Natick, MA, USA) to get their xy coordinates in each video frame. For surface locomotion trials, we digitized the mid-body point. For sand-penetrating and leaf litter locomotion trials, we digitized the pelvic point, allowing us to track locomotion from the moment the tip of the snout penetrated the substrate to when the pelvic point disappeared into the substrate. We converted pixels to metres and frames to time, and calculated the cumulative distance moved for each frame [44]. We fitted a quintic spline with the Curve Fitting toolbox in MatLab to the cumulative distance and time data [44,47]. We used the first derivative of the spline, representing frame-by-frame velocity, and calculated average velocity from the moment of penetration of the substrate until the pelvic point disappeared. We tallied whether each specimen used its hindlimbs during each mode of locomotion. We then calculated the proportion of individuals that used their hindlimbs for each species (electronic supplementary material, table S3).
(d). Statistical analysis
We conducted all statistical analyses while taking phylogeny into account using R v. 3.5.2 [48]. We ln-transformed all morphometric measurements prior to analysis. We only included one trial with the highest maximum velocity or force for each individual on each substrate to calculate species mean performance (electronic supplementary material, methods).
We reduced the dimensionality of our data using a phylogenetic principal component analysis (pPCA) on the trait correlation matrix while accounting for phylogenetic signal as λ [49] using ‘phytools’ [50]. The pPCA included ln-transformed linear morphometric measurements and digit counts. The first two pPCs together explained 84% of variance (electronic supplementary material, table S4; figure 2). All variables loaded strongly positively on pPC-1. On pPC-2, limb variables loaded strongly positively while head length, SVL, body width, and tail length loaded strongly negatively. We used pPC-2 in subsequent analyses because species with low scores were more snake-like, while those with high scores were more lizard-like. For L. labialis and L. praepedita, we could only sample one specimen. To ensure that the specimens we collected were typical of each species, we ran a pPCA with a dataset supplemented with additional specimens of L. labialis and L. praepedita (eight each) from the Western Australian Museum. We then compared pPC scores each specimen to the supplemented specimens, confirming that our singletons were representative of each species (electronic supplementary material, figure S5).
Figure 2.
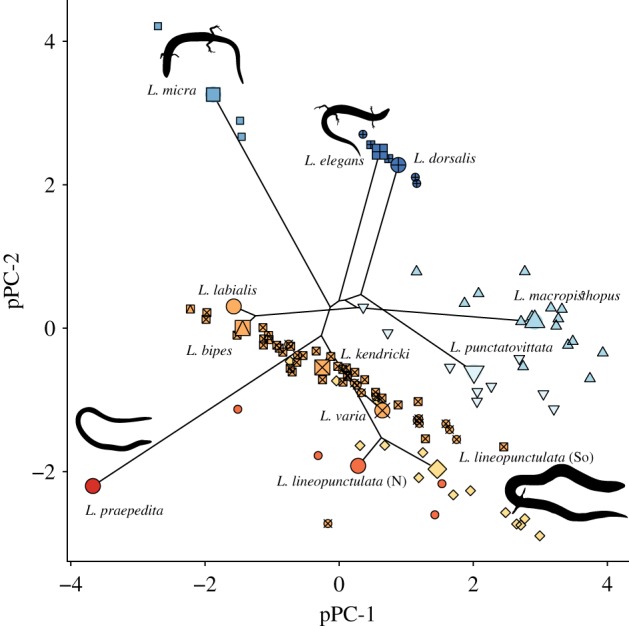
Phylomorphospace of Lerista characterized by pPC-1 and pPC-2. Colours indicate digit configuration, with warmer colours representing species with more digits and cooler colours representing species with fewer. Larger symbols are pPC scores of species means and smaller symbols are for individuals. ‘So’ and ‘N’ differentiate the southern and northern populations of L. lineopunctulata, respectively. (Online version in colour.)
We tested our hypotheses using phylogenetic generalized least-squares (PGLS) multiple regression, as implemented in ‘phylolm’ [51] while estimating phylogenetic signal of the residuals [52]. To test whether surface and fossorial/cluttered habitat performance were related to body form and size, we used regressions with each measure of performance as the response and pPC-2 and ln-mass as explanatory variables. We did the same with the other locomotor and habitat variables. Further, we regressed average sand-penetrating and leaf litter locomotor velocity on average surface locomotor velocity to test for the predicted trade-off between them. Our analyses included 12 species, except for leaf litter locomotion and habitat, which had 9 and 8 species, respectively. We reduced all regressions to the minimum adequate model [53].
3. Results
We found that more snake-like species moved at higher average velocities than more lizard-like species in both coarse (slope = −0.148 ± 0.039, p = 0.004, ) and fine (slope = −0.109 ± 0.038, p = 0.019, ) substrates (figure 3; and electronic supplementary material, table S5), lending the first functional support to the adaptation for fossoriality hypothesis. We found no relationship between body form and velocity during locomotion in leaf litter or surface locomotion (electronic supplementary material, table S5). We also found no relationship between velocity during surface locomotion and sand-penetration or moving through leaf litter (electronic supplementary material, table S6). Taken together, these results lend support to the fossoriality hypothesis and not the cluttered habitat hypothesis in Lerista but without the expected performance trade-off. Body mass did not affect any of these modes of locomotion (electronic supplementary material, table S5). However, we did find that larger species produced more force during sand-penetration into coarse (slope = 1. 652 ± 0.346, p = 0.001, ) and fine substrates (slope = 1.657 ± 0.461, p = 0.005, ; electronic supplementary material, table S7 and figure S6), but body form and sand-penetration force were unrelated (electronic supplementary material, table S7). In considering locomotor kinematics, we found that more lizard-like species used their hindlimbs more frequently during sand-penetration and moving into leaf litter but not during surface locomotion (electronic supplementary material, table S8 and figure S7). Body mass did not affect limb use (electronic supplementary material, table S8).
Figure 3.
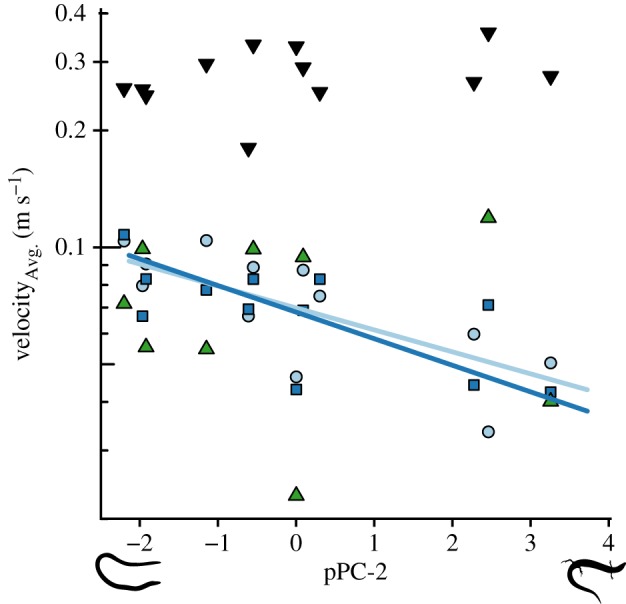
Relationship between pPC-2 and average velocity during surface locomotion (black, inverted triangles, n = 12), sand-penetration in coarse (light blue circles, n = 12) and fine sand (dark blue squares, n = 12), and in leaf litter (green triangles, n = 9). Lines are only shown for significant PGLS slopes and correspond to symbol colours. (Online version in colour.)
We found evidence to suggest that Lerista species partitioned the leaf litter habitat according to body form. More snake-like species tended to occupy smaller leaf litter mats than more lizard-like species (slope = 6.285 ± 1.956, p = 0.034, ). More snake-like species also tended to occupy peripheral parts of the leaf litter mat (slope = −0.042 ± 0.016, p = 0.034, ). We found no effect of body form on leaf litter depth, nor of body mass on any of the habitat variables (electronic supplementary material, table S9).
4. Discussion
(a). Snake-like body forms are an adaptation for fossoriality
We are the first to empirically test the hypotheses that a snake-like form is an adaptation for fossoriality and for locomotion in cluttered habitats [24,54]. We found strong support for the adaptation for fossoriality hypothesis but not for the cluttered habitat hypothesis. This is exemplified by more snake-like species of Lerista achieving higher velocities when sand-swimming but not when moving in litter composed of stiff leaves (figure 3). These findings add an additional line of evidence to support the hypothesis that snakes themselves may have evolved from a fossorial ancestor [54–56]. However, this hypothesis is not easily testable in snakes because of the lack of extant intermediate forms, which are numerous in Lerista skinks [33].
That body form did not affect the velocity of moving in leaf litter was surprising because a narrow, limbless body should facilitate negotiation of tight spaces [10,21]. However, this lack of support for the cluttered habitat hypothesis may be related to the tail. Despite strong correlations between elongate lizards with exceptionally long tails and cluttered habitats [8,24], there is considerable variation in relative tail length among snake-like species, and Lerista has an intermediate tail length ([25,36]; electronic supplementary material, table S2). Intermediate tail lengths may result from a balance between sand-swimming and moving through cluttered habitats. It is unknown whether the tail contributes to propulsion during sand-swimming in Lerista. While increasing total length improves the cost of transport during sand-swimming, this benefit diminishes at greater total lengths and incurs greater drag [23,57]. Simultaneously, the tail can provide points of static friction to push against features in a cluttered habitat [22,58,59]. However, consistent with our results, other work on semi-fossorial skinks, including Lerista, with intermediate tail lengths suggested that the tail exerted negligible propulsive forces [39,60,61].
(b). Absence of a performance trade-off and the persistence of intermediate forms
The persistence of intermediate forms with reduced limbs in Lerista and other lizards suggests that these intermediate forms are not transitory, but altogether different optima on the fitness landscape [8,25,62]. In Lerista, the intermediate forms comprise the majority of species diversity, having evolved and diversified multiples times [29,33]. However, it has long been a mystery why these forms persist and how their phenotype might be adaptive.
Contrary to predictions (figure 1), we showed a definitive lack of trade-off between fossorial and surface locomotion, and other aspects of the phenotype of the intermediate forms may be the reason for this. The lack of a trade-off in Lerista results from a relatively high surface locomotor speed that is independent of body form (figure 3). There has long been an appreciation that the transition to snake-like body form involves a dramatic shift in locomotion from limb-based to axial propulsion [21,22,63]. We found that even species with highly reduced limbs used them during both surface and fossorial locomotion (electronic supplementary material, figure S7 and table S8), and so intermediate species may run fast by effectively combining axial and limbed propulsion.
Intermediate species of Lerista are physically larger than the diminutive snake-like and lizard-like species [33]. This may contribute to higher surface locomotor velocities and help explain their persistence, eliminating any possible trade-off. Although we found no relationship between sand-penetration force and body form, there was a strong positive relationship with body mass (electronic supplementary material, figure S6). Since the diameter is an important predictor of how much force is needed to penetrate a substrate [57,64], the large intermediate species require more force to penetrate sand but also produce that required force. By contrast, small pentadactyl species do not produce much force for sand-swimming and are likely further hindered by their limbs. However, work on amphisbaenians shows that some sand-penetration forces are exerted vertically and laterally as well as in the direction of travel [65,66], so it would be interesting to quantify these forces in sand-swimming lizards as well.
Being large and powerful may also allow the intermediate species to gain access to niches unavailable to diminutive species. More snake-like species occupied the periphery of smaller leaf litter mats (electronic supplementary material, table S9). These positions probably contain less compacted sand, which is easier to penetrate [41,67]. Indeed, these different microhabitat usages are mirrored by differences in diet. More snake-like species ate a greater proportion of termites, which are small and fossorial, while intermediate species fed more on other invertebrates that tended to be larger [40]. Lizard-like species, like L. elegans, fed on various arthropods that were not fossorial [40], suggesting that they may themselves spend less time under the substrate. These lines of evidence show that the different species of Lerista fill different niches, reflected by microhabitat use, diet and locomotor capacities, allowing persistence and coexistence.
(c). Diversity of snake-like forms
The hypothesis that a snake-like form is an adaptation for fossoriality is fundamentally a hypothesis about locomotor performance, and we offer the first such evidence in support of it. Snake-like species of Lerista are better sand-swimmers than more lizard-like ones. We also present data germane to understanding why intermediate forms between lizard- and snake-like persist and coexist. Species of Lerista are divergent in body size, microhabitat use, and diet. However, Lerista is but one of several clades with a range of body forms from lizard- to snake-like [8,25]. Recently, we showed that snake-like body shapes exhibit imperfect convergence (sensu [68]), with historical contingency likely playing an important role in how snake-like bodies evolved in each clade [25]. Likewise, the evolution of sand-swimming is likely historically contingent as short-bodied, robustly limbed lizards like Scincus scincus and Uma scoparia are capable of exhibiting similar levels of sand-penetrating performance [69,70]. However, these differences in evolutionary history remain largely unexplored in terms of organismal function and niche-use, which may have profound functional implications in how each lineage interacts with the environment.
Supplementary Material
Acknowledgements
We thank B. Goodman for providing us with space and animals during our stay in Adelaide. We thank P. Doughty for providing us with field equipment and insight on finding Lerista species. We thank A. Mitchell for assistance in the field. We thank S. Mann for help digitizing locomotion videos. We thank two anonymous reviewers for comments that improved the manuscript.
Ethics
D. Stefoni of Western Australia Department of Parks and Wildlife issued our collecting permits for Western Australia (licence SF009969 and CE004562). We conducted this study under Clark University IACUC approval (Protocol 017R).
Data accessibility
The dataset analysed in this article is available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.5hqbzkh2x [71].
Authors' contributions
G.M. and P.J.B. conceived and designed the study and wrote the manuscript. G.M. collected and analysed the data. Both authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by Clark University and the National Science Foundation (grant no. IOS-1353703) to P.J.B.
References
- 1.Bateman RM, Crane PR, DiMichele WA, Kenrick PR, Rowe NP, Speck T, Stein WE. 1998. Early evolution of land plants: phylogeny, physiology, and ecology of the primary terrestrial radiation. Annu. Rev. Ecol. Evol. Syst. 29, 263–292. ( 10.1146/annurev.ecolsys.29.1.263) [DOI] [Google Scholar]
- 2.Benton MJ. 2010. The origins of modern biodiversity on land. Phil. Trans. R. Soc. B 365, 3667–3679. ( 10.1098/rstb.2010.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coates MI, Ruta M, Friedman M. 2008. Ever since Owen: changing perspectives on the early evolution of tetrapods. Annu. Rev. Ecol. Evol. Syst. 39, 571–592. ( 10.1146/annurev.ecolsys.38.091206.095546) [DOI] [Google Scholar]
- 4.Dudley R, Yanoviak SP. 2011. Animal aloft: the origins of aerial behavior and flight. Integr. Comp. Biol. 51, 926–936. ( 10.1093/icb/icr002) [DOI] [PubMed] [Google Scholar]
- 5.Engel MS, Davis SR, Prokop J. 2013. Insect wings: the evolutionary development of Nature's first flyers. In Arthropod biology and evolution: molecules, development, morphology (eds Minelli A, Boxshall G, Fusco G), pp. 269–298. Berlin, Germany: Springer. [Google Scholar]
- 6.Thewissen JGM, Cooper LN, Clementz MT, Bajpai S, Tiwari BN. 2007. Whales originated from aquatic artiodactyls in the Eocene epoch of India. Nature 450, 1190–1194. ( 10.1038/nature06343) [DOI] [PubMed] [Google Scholar]
- 7.Thewissen JGM, Williams EM, Roe LJ, Hussain ST. 2001. Skeletons of terrestrial cetaceans and the relationship of whales to artiodactyls. Nature 413, 277–281. ( 10.1038/35095005) [DOI] [PubMed] [Google Scholar]
- 8.Brandley MC, Huelsenbeck JP, Wiens JJ. 2008. Rates and patterns in the evolution of snake-like body form in squamate reptiles: evidence for repeated re-evolution of lost digits and long-term persistence of intermediate body forms. Evolution 62, 2042–2064. ( 10.1111/j.1558-5646.2008.00430.x) [DOI] [PubMed] [Google Scholar]
- 9.Lande R. 1978. Evolutionary mechanisms of limb loss in tetrapods. Evolution 32, 73–92. ( 10.1111/j.1558-5646.1978.tb01099.x) [DOI] [PubMed] [Google Scholar]
- 10.Mehta RS, Ward AB, Alfaro ME, Wainwright PC. 2010. Elongation of the body in eels. Integr. Comp. Biol. 50, 1091–1105. ( 10.1093/icb/icq075) [DOI] [PubMed] [Google Scholar]
- 11.Ward AB, Mehta RS. 2010. Axial elongation in fishes: using morphological approaches to elucidate developmental mechanisms in studying body shape. Integr. Comp. Biol. 50, 1106–1119. ( 10.1093/icb/icq029) [DOI] [PubMed] [Google Scholar]
- 12.Burress ED, Wainwright PC. 2019. Adaptive radiation in labrid fishes: a central role for functional innovations during 65 My of relentless diversification. Evolution 73, 346–359. ( 10.1111/evo.13670) [DOI] [PubMed] [Google Scholar]
- 13.Simpson GG. 1953. Tempo and mode in evolution. Columbia Classics edn New York, NY: Columbia University Press. [Google Scholar]
- 14.Darwin C. 1859. On the origin of species: by means of natural selection, or, the preservation of favored races in the struggle for life. London, UK: John Murray. [Google Scholar]
- 15.Jablonski D, Shubin NH. 2015. The future of the fossil record: paleontology in the 21st century. Proc. Natl. Acad. Sci. 112, 4852–4858. ( 10.1073/pnas.1505146112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauder GV. 1995. On the inference of function from structure. In Functional morphology in vertebrate paleontology (ed. Thomason J.), pp. 1–18. New York, NY: Cambridge University Press. [Google Scholar]
- 17.Erwin DH. 2017. The topology of evolutionary novelty and innovation in macroevolution. Phil. Trans. R. Soc. B 372, 20160422 ( 10.1098/rstb.2016.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svensson E, Calsbeek R (eds). 2013. The adaptive landscape in evolutionary biology. Oxford, UK: Oxford University Press. [Google Scholar]
- 19.Ward AB, Brainerd EL. 2007. Evolution of axial patterning in elongate fishes. Biol. J. Linn. Soc. 90, 97–116. ( 10.1111/j.1095-8312.2007.00714.x) [DOI] [Google Scholar]
- 20.Law CJ, Slater GJ, Mehta RS. 2019. Shared extremes by ectotherms and endotherms: body elongation in mustelids is associated with small size and reduced limbs. Evolution 73, 735–749. ( 10.1111/evo.13702) [DOI] [PubMed] [Google Scholar]
- 21.Gans C. 1975. Tetrapod limblessness: evolution and functional corollaries. Am. Zool. 15, 455–467. ( 10.1093/icb/15.2.455) [DOI] [Google Scholar]
- 22.Gans C. 1962. Terrestrial locomotion without limbs. Am. Zool. 2, 167–182. ( 10.1093/icb/2.2.167) [DOI] [Google Scholar]
- 23.Sharpe SS, Koehler SA, Kuckuk RM, Serrano M, Vela PA, Mendelson J, Goldman DI. 2015. Locomotor benefits of being a slender and slick sand swimmer. J. Exp. Biol. 218, 440–450. ( 10.1242/jeb.108357) [DOI] [PubMed] [Google Scholar]
- 24.Wiens JJ, Brandley MC, Reeder TW. 2006. Why does a trait evolve multiple times within a clade? Repeated evolution of snakelike body form in squamate reptiles. Evol. Int. J. Org. Evol. 60, 123–141. ( 10.1554/05-328.1) [DOI] [PubMed] [Google Scholar]
- 25.Bergmann PJ, Morinaga G. 2019. The convergent evolution of snake-like forms by divergent evolutionary pathways in squamate reptiles. Evolution 73, 481–496. ( 10.1111/evo.13651) [DOI] [PubMed] [Google Scholar]
- 26.Carranza S, Arnold EN, Geniez Ph, Roca J, Mateo JA. 2008. Radiation, multiple dispersal and parallelism in the skinks, Chalcides and Sphenops (Squamata: Scincidae), with comments on Scincus and Scincopus and the age of the Sahara Desert. Mol. Phylogenet. Evol. 46, 1071–1094. ( 10.1016/j.ympev.2007.11.018) [DOI] [PubMed] [Google Scholar]
- 27.Kohlsdorf T, Wagner GP. 2006. Evidence for the reversibility of digit loss: a phylogenetic study of limb evolution in Bachia (Gymnophthalmidae: Squamata). Evolution 60, 1896–1912. ( 10.1111/j.0014-3820.2006.tb00533.x) [DOI] [PubMed] [Google Scholar]
- 28.Siler CD, Brown RM. 2011. Evidence for repeated acquisition and loss of complex body-form characters in an insular clade of southeast Asian semi-fossorial skinks: evolutionary simplification and reacquisition of complex body form in skinks. Evolution 65, 2641–2663. ( 10.1111/j.1558-5646.2011.01315.x) [DOI] [PubMed] [Google Scholar]
- 29.Skinner A, Lee MSY. 2009. Body-form evolution in the scincid lizard clade Lerista and the mode of macroevolutionary transitions. Evol. Biol. 36, 292–300. ( 10.1007/s11692-009-9064-9) [DOI] [Google Scholar]
- 30.Whiting A, Baur AM, Sites JW. 2003. Phylogenetic relationships and limb loss in sub-Saharan African scincine lizards (Squamata: Scincidae). Mol. Phylogenet. Evol. 29, 582–598. ( 10.1016/S1055-7903(03)00142-8) [DOI] [PubMed] [Google Scholar]
- 31.Greer AE. 1990. Limb reduction in the scincid lizard genus Lerista. 2. Variation in the bone complements of the front and rear limbs and the number of postsacral vertebrae. J. Herpetol. 24, 142 ( 10.2307/1564221) [DOI] [Google Scholar]
- 32.Greer AE. 1987. Limb reduction in the lizard genus Lerista. 1. Variation in the number of phalanges and presacral vertebrae. J. Herpetol. 21, 267 ( 10.2307/1563968) [DOI] [Google Scholar]
- 33.Morinaga G, Bergmann PJ. 2017. Convergent body shapes have evolved via deterministic and historically contingent pathways in Lerista lizards. Biol. J. Linn. Soc. 121, 858–875. ( 10.1093/biolinnean/blx040) [DOI] [Google Scholar]
- 34.Vanhooydonck B, Boistel R, Fernandez V, Herrel A. 2011. Push and bite: trade-offs between burrowing and biting in a burrowing skink (Acontias percivali). Biol. J. Linn. Soc. 102, 91–99. ( 10.1111/j.1095-8312.2010.01563.x) [DOI] [Google Scholar]
- 35.Maslin BR. (coordinator) 2018. WATTLE: interactive identification of Australian Acacia. Version 3. See https://apps.lucidcentral.org/wattle/ (accessed 11 November 2019).
- 36.Bergmann PJ. 2015. Convergent evolution of body shape in squamate reptiles. In All animals are interesting: a Festschrift in honour of Anthony P. Russell (eds Bininda-Edmonds ORP, Powell GL, Jamniczky HA, Bauer AM, Theodor J), pp. 245–277. Oldenburg, Germany: BIS-Verlag. [Google Scholar]
- 37.Jayne BC, Bennett AF. 1989. The effect of tail morphology on locomotor performance of snakes: a comparison of experimental and correlative methods. J. Exp. Zool. 252, 126–133. ( 10.1002/jez.1402520204) [DOI] [Google Scholar]
- 38.Vanhooydonck B, Measey J, Edwards S, Makhubo B, Tolley KA, Herrel A. 2015. The effects of substratum on locomotor performance in lacertid lizards. Biol. J. Linn. Soc. 115, 869–881. ( 10.1111/bij.12542) [DOI] [Google Scholar]
- 39.Gans C, Fusari M. 1994. Locomotor analysis of surface propulsion by three species of reduced-limbed fossorial lizards (Lerista: Scincidae) from western Australia. J. Morphol. 222, 309–326. ( 10.1002/jmor.1052220308) [DOI] [PubMed] [Google Scholar]
- 40.Kendrick PG. 1991. The phylogenetics and comparative ecology of Lerista Bell, 1833: patterns of evolution in a genus of sand-swimming skinks. PhD dissertation, University of Western Australia, Perth, Australia. [Google Scholar]
- 41.Greenville AC, Dickman CR. 2009. Factors affecting habitat selection in a specialist fossorial skink. Biol. J. Linn. Soc. 97, 531–544. ( 10.1111/j.1095-8312.2009.01241.x) [DOI] [Google Scholar]
- 42.Bergmann PJ, Irschick DJ. 2010. Alternate pathways of body shape evolution translate into common patterns of locomotor evolution in two clades of lizards. Evolution 64, 1569–1582. ( 10.1111/j.1558-5646.2009.00935.x) [DOI] [PubMed] [Google Scholar]
- 43.Pough FH, Preest MR, Fusari MH. 1997. Prey-handling and the evolutionary ecology of sand-swimming lizards (Lerista: Scincidae). Oecologia 112, 351–361. ( 10.1007/s004420050320) [DOI] [PubMed] [Google Scholar]
- 44.Bergmann PJ, Pettinelli KJ, Crockett ME, Schaper EG. 2017. It's just sand between the toes: how particle size and shape variation affect running performance and kinematics in a generalist lizard. J. Exp. Biol. 220, 3706–3716. ( 10.1242/jeb.161109) [DOI] [PubMed] [Google Scholar]
- 45.Herrel A, Measey GJ. 2010. The kinematics of locomotion in caecilians: effects of substrate and body shape. J. Exp. Zool. A Ecol. Genet. Physiol . 313, 301–309. ( 10.1002/jez.599) [DOI] [PubMed] [Google Scholar]
- 46.Hedrick TL. 2008. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir. Biomim. 3, 034001 ( 10.1088/1748-3182/3/3/034001) [DOI] [PubMed] [Google Scholar]
- 47.Umberger CM, de Buron I, Roumillat WA, McElroy EJ.. 2013. Effects of a muscle-infecting parasitic nematode on the locomotor performance of their fish host: impact of a parasite on swimming performance. J. Fish Biol. 82, 1250–1258. ( 10.1111/jfb.12061) [DOI] [PubMed] [Google Scholar]
- 48.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 49.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884. ( 10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 50.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things): phytools: R package. Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 51.Tung Ho L si, Ané C. 2014. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 63, 397–408. ( 10.1093/sysbio/syu005) [DOI] [PubMed] [Google Scholar]
- 52.Revell LJ. 2010. Phylogenetic signal and linear regression on species data: phylogenetic regression. Methods Ecol. Evol. 1, 319–329. ( 10.1111/j.2041-210X.2010.00044.x) [DOI] [Google Scholar]
- 53.Crawley MJ. 2012. The R book, 2nd edn Chichester, UK: Wiley. [Google Scholar]
- 54.Rieppel O. 1988. A review of the origin of snakes. In Evolutionary biology (eds Hecht MK, Wallace B, Prance GT), pp. 37–130. Boston, MA: Springer US. [Google Scholar]
- 55.Simões BF, et al. 2015. Visual system evolution and the nature of the ancestral snake. J. Evol. Biol. 28, 1309–1320. ( 10.1111/jeb.12663) [DOI] [PubMed] [Google Scholar]
- 56.Walls GL. 1942. The vertebrate eye and its adaptive radiation. Bloomfield Hills, MI: Cranbrook Institute of Science. [Google Scholar]
- 57.Albert I, Sample JG, Morss AJ, Rajagopalan S, Barabási A-L, Schiffer P. 2001. Granular drag on a discrete object: shape effects on jamming. Phys. Rev. E 64, 061303 ( 10.1103/PhysRevE.64.061303) [DOI] [PubMed] [Google Scholar]
- 58.Gans C, Gasc J-P. 1990. Tests on the locomotion of the elongate and limbless reptile Ophisaurus apodus (Sauria: Anguidae). J. Zool. 220, 517–536. ( 10.1111/j.1469-7998.1990.tb04731.x) [DOI] [Google Scholar]
- 59.Ward AB, Costa A, Monroe SL, Aluck RJ, Mehta RS. 2015. Locomotion in elongate fishes: a contact sport. Zoology 118, 312–319. ( 10.1016/j.zool.2015.06.002) [DOI] [PubMed] [Google Scholar]
- 60.Gans C, Morgan WK, Allen ES. 1992. Surface locomotion of the elongate and limbless lizard Anniella pulchra (Anguidae). Herpetologica 48, 246–262. [Google Scholar]
- 61.Mushinsky HR, Gans C. 1992. The role of the tail in channel passage by the sand skink, Neoseps reynoldsi. Amphib.-Reptil. 13, 393–403. ( 10.1163/156853892X00085) [DOI] [Google Scholar]
- 62.Skinner A, Lee MS, Hutchinson MN. 2008. Rapid and repeated limb loss in a clade of scincid lizards. BMC Evol. Biol. 8, 310 ( 10.1186/1471-2148-8-310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renous S, Hofling E, Gasc JP. 1998. Respective role of the axial and appendicular systems in relation to the transition to limblessness. Acta Biotheor. 46, 141–156. ( 10.1023/A:1001129920394) [DOI] [PubMed] [Google Scholar]
- 64.Clark AH, Behringer RP. 2013. Granular impact model as an energy-depth relation. EPL Europhys. Lett. 101, 64001 ( 10.1209/0295-5075/101/64001) [DOI] [Google Scholar]
- 65.Hohl LSL, Loguercio MFC, Buendía RA, Almeida-Santos M, Viana LA, Barros-Filho JD, Rocha-Barbosa O. 2014. Fossorial gait patterns and performance of a shovel-headed amphisbaenian: fossorial gaits of a shovel-headed amphisbaenian. J. Zool. 294, 234–240. ( 10.1111/jzo.12173) [DOI] [Google Scholar]
- 66.de Barros-Filho JD, Hohl L dos SL, Rocha-Barbosa O. 2008. Excavatory cycle of Leposternon microcephalum Wagler, 1824 (Reptilia, Amphisbaenia). Int. J. Morphol. 26, 411–414. ( 10.4067/s0717-95022008000200027) [DOI] [Google Scholar]
- 67.McDonald PJ, Pavey CR, Fyfe G. 2012. The lizard fauna of litter mats in the stony desert of the southern Northern Territory. Aust. J. Zool. 60, 166 ( 10.1071/ZO12068) [DOI] [Google Scholar]
- 68.Collar DC, Reece JS, Alfaro ME, Wainwright PC, Mehta RS. 2014. Imperfect morphological convergence: variable changes in cranial structures underlie transitions to durophagy in moray eels. Am. Nat. 183, E168–E184. ( 10.1086/675810) [DOI] [PubMed] [Google Scholar]
- 69.Baumgartner W, Fidler F, Weth A, Habbecke M, Jakob P, Butenweg C, Böhme W. 2008. Investigating the locomotion of the sandfish in desert sand using NMR-imaging. PLoS ONE 3, e3309 ( 10.1371/journal.pone.0003309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jayne BC, Daggy MW. 2000. The effects of temperature on the burial performance and axial motor pattern of the sand-swimming of the Mojave fringe-toed lizard Uma scoparia. J. Exp. Biol. 203, 1241–1252. [DOI] [PubMed] [Google Scholar]
- 71.Morinaga Gen, Bergmann Philip J. 2020. Data from: Evolution of fossorial locomotion in the transition from tetrapod to snake-like in lizards Dryad Digital Repository. ( 10.5061/dryad.5hqbzkh2x) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Morinaga Gen, Bergmann Philip J. 2020. Data from: Evolution of fossorial locomotion in the transition from tetrapod to snake-like in lizards Dryad Digital Repository. ( 10.5061/dryad.5hqbzkh2x) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The dataset analysed in this article is available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.5hqbzkh2x [71].


