Abstract
Conspicuous coloration displayed by animals that express sexual colour dimorphism is generally explained as an adaptation to sexual selection, yet the interactions and relative effects of selective forces influencing colour dimorphism are largely unknown. Qualitatively, colour dimorphism appears more pronounced in marine fishes that live on coral reefs where traits associated with strong sexual selection are purportedly more common. Using phylogenetic comparative analysis, we show that wrasses and parrotfishes exclusive to coral reefs are the most colour dimorphic, but surprisingly, the effect of habitat is not influenced by traits associated with strong sexual selection. Rather, habitat-specific selective forces, including clear water and structural refuge, promote the evolution of pronounced colour dimorphism that manifests colours less likely to be displayed in other habitats. Our results demonstrate that environmental context ultimately determines the evolution of conspicuous coloration in colour-dimorphic labrid fishes, despite other influential selective forces.
Keywords: conspicuous coloration, coral reefs, natural selection, phylogenetic comparative method, sexual selection, visual environment
1. Introduction
Secondary sexual traits such as colour dimorphism evolve in the context of complex interactions between natural and sexual selection. Ornamentation, elaborate displays and conspicuous coloration evolve in response to intersexual mate choice, while intrasexual competition for access to mates favours body size dimorphism, weaponry and badges of status [1–3]. Pressures imposed by natural selection often conflict with those of sexual selection via substantial mortality costs on conspicuousness, ultimately favouring cryptic coloration and reduced ornamentation [1,4]. The interplay among these forms of selection and their relative effects can vary the expression of sexual colour dimorphism within and between species, thereby generating phenotypic diversity [4–6]. Whether this leads to predictable macroevolutionary patterns of sexual colour dimorphism remains largely unexplored (but see [7–10]).
Comparative studies confirm that indices of sexual selection, such as mating system [11–15], extra-pair paternity [12,16] and sexual size dimorphism [17], are correlated with the evolution of sexual colour dimorphism across a broad range of taxa. More recently, research has focused on the ecological drivers of visual signal evolution [18–20]. Visual signals adapt to physical properties of the microhabitat including the medium of signal transmission, ambient light conditions and the background against which the signal is perceived [19–22]. Habitat structural complexity may also be an important environmental component in the evolution of visual signals through its effects on light and background conditions [23–25], as well as through the availability of shelter [10]. Therefore, habitat-related selective pressures can play an important role in the evolution of conspicuous, sexually selected visual signals by acting on both signal transmission and predation vulnerability [6]. Alternatively, differential habitat use by males and females (sexual niche partitioning) may result in sexual colour dimorphism in the absence of sexual selection [26,27].
Adding to the complexity, sexual colour dimorphism may be fixed, dynamic or expressed ontogenetically. Ontogenetic colour change that occurs with size or life-stage in one or both sexes resulting in sexual colour dimorphism has been documented in invertebrates [28,29], fishes [30,31], amphibians [32] and reptiles [33], but remains a poorly understood phenomenon. Predation pressure and vulnerability also change with size and life-stage; therefore, predator-mediated selection may be particularly important in the evolution of ontogenetic sexual colour dimorphism. Alternatively, in species with distinct juvenile and adult colour phases, a change in chromatophore sensitivity to either male or female sex hormones could result in the loss of ontogenetic colour change in only one sex [32], rendering the evolution of ontogenetic sexual colour dimorphism non-adaptive. The relative roles of natural and sexual selection, and their interaction, in the expression of ontogenetic sexual colour dimorphism has not been quantified. We explore this in an iconic group of marine fishes, the Labridae.
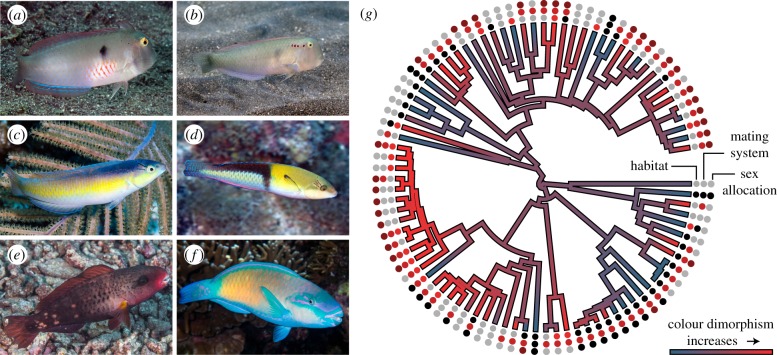
Wrasses and parrotfishes (family: Labridae) form a monophyletic assemblage [34]; the second largest family of marine fishes with a global distribution spanning tropical and temperate waters. The intraspecific colour diversity of labrids is dramatic enough that many colour phases were initially described as separate species. Later, colour phases were synonymized upon the realization that they comprised sequential phases of the same species separated by size [30,31] (figure 1a–f). Individuals transition to terminal phase coloration with increasing size and transitions are often, but not always accompanied by protogynous sex reversal from female to male [31]. The conspicuous coloration displayed by larger, usually male, terminal phase fish is thought to be the result of sexual selection [35–38]. Studies of the distinctive sex and mating systems expressed by labrids (figure 1g) have produced valuable advances [38–41]. Previous work predicted how sex allocation and mating system interact with habitat [42–46] to influence colour dimorphism [35,37,42,43] via effects on one or more of the aforementioned selective forces. Specifically, colour dimorphism in wrasses and parrotfishes has been linked to sex allocation pathways (protogyny) and mating systems (polygyny) that are purportedly more common on coral reefs [42,43]. Evidence supporting these associations is limited to qualitative assessments and the claims have not been subject to rigorous comparative analysis incorporating evolutionary history. Furthermore, predicted trait interactions and effects on colour dimorphism are often complicated by exceptions exemplified by certain taxa [44,45,47,48], and the interactions and relative effects of selective pressures driving the evolution of colour dimorphism remain unclear.
Figure 1.
Colour dimorphism and associated trait diversity. (a–f) The magnitude of colour dimorphism between initial (a,c,e) and terminal phase (b,d,f) wrasses and parrotfishes ranges from none, as in Iniistius pentadactylus (a,b), to moderate, as in Halichoeres garnoti (c,d), to extreme, as in Chlorurus spilurus (e,f). (g) Phylogenetic reconstruction of the magnitude of colour dimorphism. Habitat association (black, non-coral reef; light grey, coral reef associated; dark grey/red, coral reef exclusive), mating system (black, promiscuous; light grey, haremic; dark grey/red, lek-like) and sex allocation (black, gonochorous; light grey, monandric; dark grey/red, diandric) are depicted at the tips of the phylogeny. Photos with permission from Rickard Zerpe (a), Mark Rosenstein (b), François Libert (c,e,f) and Carlos Estape (d). (Online version in colour.)
The magnitude of colour dimorphism is expected to increase with the strength of sexual selection, driven by increased variation in male reproductive success associated with evolutionary transitions to polygynous mating [1] or protogynous sex change [39,49]. Labrids exhibit multiple types of polygyny and protogyny that have evolved synergistically based on the degree of male size advantage (see table 1 for definitions) [51]. The type of polygynous sex change expressed depends on the ability of large males to monopolize mates [51,52]. In species with lek-like mating, differential dominance relationships between males exist, creating the potential for primary males to achieve reproductive fitness and enabling conditions conducive to diandric protogyny [44–46,51,52]. This combination of character states increases variation in mating success through both male–male competition for mates and female mate choice and is predicted to enable the strongest sexual selection [1,52], and the most pronounced colour dimorphism. When males have strong social control over females, as is the case with haremic mating, or when sex allocation is monandric, the strength of sexual selection should be reduced [35,37]. In addition to reciprocated effects, sex allocation and mating system can also be influenced by habitat-dependent variables, including the dispersion of resources [36,52], water clarity [42] and the ability of small males to hide [42,44].
Table 1.
Definitions of key terms.
| terms | definition |
|---|---|
| habitat | |
| non-coral reef | associated with any number of habitats not including coral reefs |
| coral reef associated | associated with a wide range of habitats, including coral reefs |
| coral reef exclusive | associated exclusively with coral reefs |
| mating system | |
| promiscuity | terminal phase males do not defend territories with the purpose of attracting mates [47] |
| lek-like polygyny | terminal phase males establish temporary territories visited by females for the purpose of reproduction [45,47] |
| haremic polygyny | terminal phase males monopolize one or more females within a permanent territory [45,47] |
| sex allocation | |
| gonochorism | individuals reproduce exclusively as either male or female throughout their lives [50] |
| diandric protogyny | males are either born into the population (primary males) or derived from sex-changed females (secondary males) [50] |
| monandric protogyny | all males are derived via sex change from functional females (secondary males) [50] |
Aside from effects of sex allocation and mating system, colour dimorphism should evolve adaptively dependent on habitat-specific selection regimes that impact the efficacy of visual signals in communication and concealment [18]. Physical properties of coral reefs, including their clear oligotrophic water, bright ambient light [53] and background radiance [54,55], may facilitate the enhancement of visual perception and signalling that could result in the adaptive evolution of pronounced colour dimorphism [10,56]. Coral reefs are colourful environments and brightly coloured fishes may be less conspicuous when viewed against a coral reef background [37,57]. Alternatively, the structural complexity of coral reefs may provide important refuge in the form of solid, physical barriers for conspicuous fishes capable of entering the cavernous construction [37,56].
Here, we untangle the relative importance of sex allocation, mating system and habitat, and explore the ways in which they interact to shape the evolution of colour dimorphism in wrasses and parrotfishes. To do this, we first quantified colour dimorphism by scoring seven different body and fin regions as either primarily the same or different in colour between initial and terminal phase fishes using photographs from online repositories and scholarly identification guides (see electronic supplementary material, table S1). This produced a binary matrix that we reduced to a primary axis of colour-dimorphic variation using logistic principal component analysis. We also compiled data on sex allocation, mating system and habitat association from the literature and online repositories (see table 1 for definitions of each character state). Using the most recently reconstructed phylogenetic hypothesis for the family [51,58], we applied comparative methods to: (i) test for predicted associations between character states that would promote the evolution of pronounced colour dimorphism; (ii) determine whether colour dimorphism has evolved according to stochastic or trait-dependent processes, and quantify the relative influence of sex allocation, mating system and habitat association on the evolution of colour dimorphism; and (iii) determine whether the dominant form of selection produces consistent effects between colour phases and across body regions.
2. Material and methods
(a). Trait data compilation
Colour dimorphism was quantified by visually scoring seven different body regions (the head, flank, and the pectoral, pelvic, dorsal, anal and caudal fins) as either primarily the same or different in colour between photographs of living and preserved initial and terminal phase fishes (electronic supplementary material, table S1). When available, multiple photographs of each species and phase were scored. When it was unclear whether a region was primarily the same or different in colour no score was recorded for that region. We note that our quantification of colour dimorphism is limited to human visual perception—it is possible that labrids do not perceive coloration or colour dimorphism in the same way. Although data on fish visual perception is becoming increasingly available, data on the visual perception of labrid species are too few to incorporate into a comparative study of this scale. Our approach draws some support from comparisons of human and avian visual perception of colour dimorphism and the finding that human perception can provide a meaningful estimate for the purposes of comparative analyses [59].
We compiled colour dimorphism data for 346 labrid species, 17% of which we scored as completely colour dimorphic, 34% were scored as colour monomorphic and 50% were scored between the two extremes. To simultaneously maximize the variance in colour dimorphism and reduce the dimensionality of the binary matrix we performed a logistic principal component analysis using the R package logisticPCA [60]. We used cross validation to determine the value used to approximate the natural parameters from the saturated model and we reduced the matrix to two dimensions. Cumulatively, the two PC axes explained 78.9% of the deviance in colour dimorphism. We considered the first principal component as the primary axis of variation in colour dimorphism (see electronic supplementary material, table S2 for the variable loadings) and performed all further analyses on the resultant PC1 scores. Colour dimorphism becomes more pronounced from positive to negative along the axis.
We classified species as either coral reef exclusive, coral reef associated, or non-coral reef based on occurrence data catalogued in FishBase [61] and the IUCN Red List of Threatened Species [62] (table 1; electronic supplementary material, table S1). We used an existing dataset of species-specific sex allocation and mating systems [51,58]. Classifications focused on the mating systems reported for terminal phase males and did not consider the mating strategies of initial phase males. Sex allocation data were restricted to accounts of protogyny that were distinguishable as either monandric or diandric based on gonad histology, population demographics or both. Habitat association, mating system and sex allocation data were available for 89 labrid species (electronic supplementary material, table S1; deposited in the Dryad Digital Repository [63]). We present colour dimorphism data for only those species with corresponding habitat association, mating system and sex allocation data (n = 89; 22% completely colour dimorphic, 33% colour monomorphic and 45% in between).
(b). Trait correlations
To establish whether mating or sex allocation systems are evolutionarily correlated with habitat we compared the fit of independent and dependent models of trait evolution using the Discrete package implemented in BayesTraits [64,65] and a sample of 1000 trees drawn from the posterior distribution of the most recent phylogenetic reconstruction of the family (deposited in the Dryad Digital Repository [58]). The independent, or null, model of evolution assumes there is no correlation between two traits and that they have evolved independently. The dependent model describes the correlated evolution of two traits such that the rate of change in one trait depends on the state of the other. As Discrete accepts only binary trait data we performed a series of tests of each state combination based on purported associations. To establish whether mating systems or sex allocation pathways that promote the evolution of colour dimorphism are associated with coral reef habitats, we tested the correlation between diandric protogyny and coral reef exclusivity (data coded as either coral reef exclusive or non-coral reef exclusive and diandric or non-diandric) and lek-like polygyny and coral reef exclusivity (data coded as either coral reef exclusive or non-coral reef exclusive and lek-like or non-lek-like).
Continuous-time Markov models were fit to each set of discrete character data using a reversible-jump Markov chain Monte Carlo (rjMCMC) analysis to derive posterior distributions of the ancestral state and transition rates. An exponential reverse jump hyperprior (0 10) was specified for the rate parameter distributions, and the trees were scaled to have a mean branch length of 0.1. Markov chains were run three times across a random sample of 1000 time-calibrated phylogenies for four-million iterations, sampling every 4000 steps, following a burn-in of one-million iterations. We monitored the average acceptance rates to ensure the values were between 20% and 40%, indicating that the rjMCMC was mixing well. We examined traces of the likelihood and parameters in Tracer [66] to ensure that convergence and effective sample sizes above 200 were obtained across the three independent runs.
We determined the most probable evolutionary model by calculating log Bayes factors (BF) for each pair of models as twice the difference in the log marginal likelihood of the dependent model minus the independent model [67]. Marginal likelihoods were estimated using the stepping stone sampler implemented in BayesTraits [64,65,68], where independent runs sampled 100 stones, each with 10 000 iterations. We calculated log BFs for three independent runs and then averaged them. The log BF quantifies the weight of evidence against the null hypothesis (the independent model), where values less than two indicate little evidence, values from two to five indicate positive evidence and values greater than five indicate strong evidence for the dependent model over the independent model [69]. We calculated Z-scores for each transition parameter as the proportion of transitions assigned to zero across three independent, concatenated runs. The Z-score provides an additional descriptor of the likelihood distribution of the transition rate. Low Z-scores indicate that transitions were rarely assigned to zero and are likely to occur, whereas Z-scores close to one describe transitions that were frequently assigned to zero, indicating that they are unlikely to occur.
(c). Evolutionary model fitting
We simulated character histories for habitat association, mating system and sex allocation across a random sub-sample of 121 trees (drawn from the sample of 1000 trees used in the preceding analyses; deposited in the Dryad Digital Repository [63]) using stochastic character mapping implemented in SIMMAP [70]. We specified an empirical prior on the bias parameter and a branch length prior on the rate parameter, with the branches rescaled. One map was simulated for each tree topology by a single draw from the prior distribution, resulting in 121 stochastic character maps for each dataset. Mapped trees were scaled back to their original height using the applyBranchLengths function in the R package phytools [71].
To determine whether habitat, mating system and sex allocation have influenced the evolution of colour dimorphism separately, and to determine which set of traits best explains the evolution of colour dimorphism, we fit evolutionary models using the R package OUwie [72]. The models included, with increasing complexity, single-rate Brownian motion (BM1), single-optimum Ornstein–Uhlenbeck (OU1), multi-peak Ornstein–Uhlenbeck (OUM) and multi-peak Ornstein–Uhlenbeck with separate estimates of rates of trait evolution (OUMV). The models differ in whether they incorporate effects of the predictor variable on the evolution of colour dimorphism. Both BM1 and OU1 allow colour dimorphism to evolve independently of the predictor variable. Thus, if colour dimorphism has evolved under stochastic processes without an effect of habitat association, mating system or sex allocation, we would expect the best-fit model to be either BM1 or OU1. Two multi-peak models allow colour dimorphism to assume separate, regime dependent optimal values, but they differ in whether the rate of trait evolution varies between regimes. If either habitat association, mating system or sex allocation has influenced the evolution of colour dimorphism we would expect the best-fit model to have multiple optima (OUM or OUMV), and potentially state-dependent rates of trait evolution (OUMV).
To determine the best overall predictor of colour dimorphism we compared eight models; two of the models included no effect of any categorical trait (BM1, OU1), and six of the models included an effect of one of the three categorical traits (OUM, OUMV). We selected the best overall model, as well as the best-fit model for each dataset individually, using the Akaike information criterion corrected for small sample size (AICC and AICC weights) [73,74]. All OUwie analyses were run with the assumption that the value of the trait at the root was distributed according to the stationary distribution of the OU process (root.station = TRUE). We examined the eigen-decomposition of the Hessian to ensure the analyses returned the maximum likelihood estimates, and discarded iterations (model results and trees) with negative eigenvalues or unrealistic parameter estimates (values well outside the realm of possibility). ΔAICc values were calculated for each retained iteration and averaged across iterations for each model. Mean ΔAICc values were used to calculate AICc weights, and the model with the highest AICc weight was selected as the best model.
We generated 95% confidence intervals for all best-fit model parameters using the parametric bootstrapping function OUwie.boot in the R package OUwie [72]. For each dataset we performed 100 bootstrap replicates per stochastic character map using the parameters originally estimated under the best-fit model. We also performed simulations to ensure that model complexity did not exceed the information contained in our dataset, and to demonstrate statistical power. For each retained iteration, data were simulated using the parameters originally estimated under the best fit model and recursively run through all models in OUwie. This allowed us to determine how well the algorithm could identify the correct model, or class of model (i.e. single-peak versus multi-peak), as well as the data parameters.
(d). The contribution of depth
To explore whether depth may be an underlying factor driving the effect of coral reef association, we performed phylogenetic generalized least-squares regressions implemented in the R package caper [75]. The analyses used the maximum clade credibility tree and minimum, midpoint and maximum depths recorded from IUCN species pages [62] and FishBase [61] (deposited in the Dryad Digital Repository [63]). For each species, the shallowest minimum depth and deepest maximum depth values were used; substituted by the most common depths when available. The midpoint was calculated as half of the depth range plus the minimum depth. Each regression used maximum likelihood to optimize the strength of the phylogenetic signal by adjusting the branch lengths with the lambda transformation.
(e). Phase- and body-specific selective pressures
We recorded the presence of colours associated with different colour-producing molecules on initial and terminal phase fishes. The colour groups, associated pigments and chromatophores included: melanophores containing eumelanin that appears black or brown; leucophores containing purines that scatter light and appear whitish when illuminated by incident light; xanthophores containing pteridine pigments that appear yellow; erythrophores containing carotenoids that appear red or orange; and iridophores that reflect light producing blue colours (including purple) or green [76]. We used data for each phase to test whether the probability of observing each colour group differs with coral reef association.
Finally, using the colour dimorphism data we tested whether the probability of observing colour dimorphism for each body and fin region varies with coral reef association. For each of the aforementioned tests, we combined the data with the maximum clade credibility tree to perform phylogenetic generalized linear models using the phyloglm function in the R package phylolm [77,78]. We specified the ‘logistic_MPLE’ method to maximize the penalized likelihood of the logistic regression and used multiple starting points for alpha (0.1, 0.5 and 0.9) to ensure the results were returning the global maximum. For each set of results, we selected the one that returned the maximum penalized likelihood. We report the range when multiple starting values returned the same maximum penalized likelihood value.
3. Results and discussion
(a). Evolutionary correlations
Bayesian tests show no support for the correlated evolution of either lek-like polygyny or diandric protogyny and coral reef exclusivity (average log BF = –5.18 and –7.53, respectively; electronic supplementary material, figure S1 and table S3), contesting a common explanation for the appearance of pronounced colour dimorphism in fishes on coral reefs [42,43]. Ecological factors are known to influence both mating system [37,47,52] and sex allocation [39,45,50]. Our results show that these factors can vary across habitat types, and that habitat type itself does not determine the influence ecological factors may have on either mating system or sex allocation.
(b). Adaptive evolution of colour dimorphism
Evolutionary models best-fit to colour dimorphism indicate that within wrasses and parrotfishes, colour dimorphism has evolved adaptively dependent on sex allocation, mating system and habitat association (electronic supplementary material, table S4). Model estimates agree with predictions that diandric protogyny, lek-like polygyny and coral reef exclusivity favour the evolution of pronounced colour dimorphism (figure 2). Interestingly, our results indicate the most pronounced colour dimorphism evolves in lineages found exclusively on coral reefs (figure 2), and, of the three traits examined, habitat association best explains the evolution of colour dimorphism (Akaike information criterion corrected for small sample size (AICc) weight = 0.774; table 2). Collectively, these results show that selection pressures specific to coral reefs promote the evolution of colour dimorphism and that these effects are separate from any effects of sex allocation or mating system.
Figure 2.
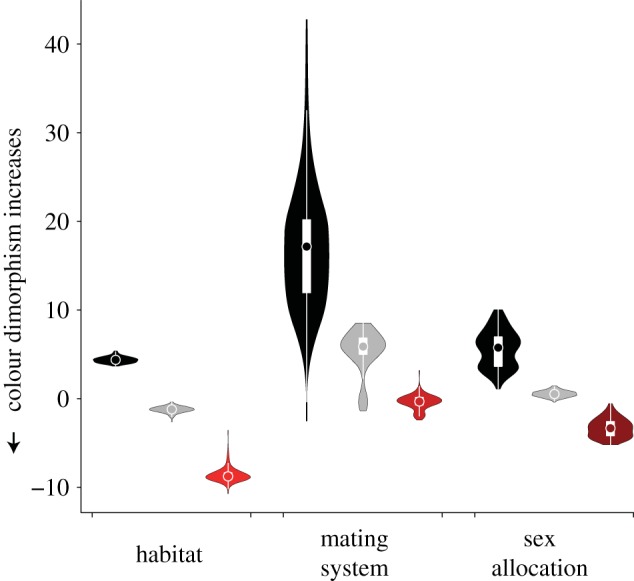
The adaptive evolution of colour dimorphism. Median optimal colour dimorphism (coloured circles), interquartile range (IQR, white rectangles), 1.5 × IQR (white lines), and the kernel density corresponding to habitat association (black, non-coral reef; light grey, coral reef associated; dark grey/red, coral reef exclusive), mating system (black, promiscuous; light grey, haremic; dark grey/red, lek-like), and sex allocation (black, gonochorous; light grey, monandric; dark grey/red, diandric). Values were estimated from best-fit evolutionary models. Of the three predictor traits, habitat best explains the evolution of colour dimorphism, whereby colour dimorphism becomes more pronounced with increasing coral reef association. (Online version in colour.)
Table 2.
Comparison of evolutionary models fit to colour dimorphism as predicted by habitat association, mating system and sex allocation. Eight models were compared; two of the models included no effect of a predictor variable (BM1, OU1), and six of the models included an effect of one of the predictor variables (OUM, OUMV).
| trait | model | ΔAICc | AICc weight |
|---|---|---|---|
| no effect | BM1 | 69.645 | 0.000 |
| OU1 | 8.144 | 0.015 | |
| habitat association | OUM | 0.246 | 0.774 |
| OUMV | 3.681 | 0.139 | |
| mating system | OUM | 8.745 | 0.011 |
| OUMV | 6.376 | 0.036 | |
| sex allocation | OUM | 8.108 | 0.015 |
| OUMV | 9.117 | 0.009 |
Habitat-specific selection pressures that influence the evolution of colour dimorphism act on both initial and terminal phase fishes. To determine how selection impacts each colour phase and the resultant magnitude of colour dimorphism expressed between them, we tested whether the probability of observing colours associated with different colour-producing molecules differs with coral reef association for each phase. We found no differences among initial phase fish (figure 3a; electronic supplementary material, table S5), suggesting that selection acting on the evolution of initial phase fish coloration does not vary with coral reef association. By contrast, the probability of observing white in terminal phase fishes significantly decreases with increasing coral reef association, while the probability of observing blue and green colours increases (figure 3b; electronic supplementary material, table S5). White was observed in 92% of all initial phase fishes—generally expressed as a key component of countershading or mottled patterning, both of which are known to be important tactics of animal camouflage [79–81]. Blue is an important signalling colour [54] that is the most spectrally contrasting against coral reef backgrounds [54,55]. Hence, upon transition to the terminal colour phase, wrasses and parrotfishes on coral reefs are more likely to lose a component of cryptic coloration and gain more conspicuous colours, opposing the idea that brightly coloured fishes appear less conspicuous when viewed against a coral reef background [37,57].
Figure 3.
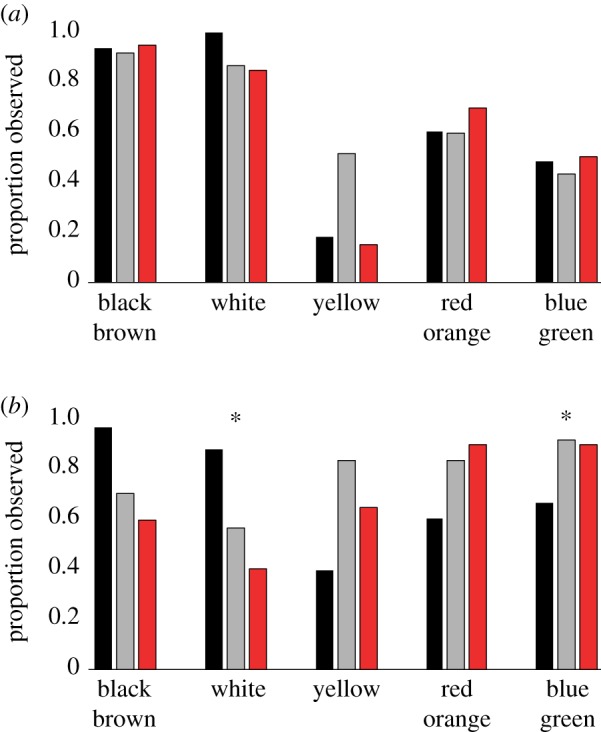
Variation in colour with coral reef association (black bars, non-coral reef; light grey bars, coral reef associated; dark grey/red bars, coral reef exclusive). (a) The proportion of each colour group observed in initial phase fishes. (b) The proportion of each colour group observed in terminal phase fishes. Asterisks denote significant differences in the probability of observing the corresponding colour group between categories of coral reef association. (Online version in colour.)
Species exclusive to coral reefs are habitat specialists whose sensory and signalling systems should be similarly specialized to match the environmental conditions [18], which feature shallow depths and clear, oligotrophic water. Light gradients driven by changes in depth and turbidity are known to influence the evolution of visual perception and colour signals in fishes [10,53,82]. Accounting for the effect of habitat, we find no significant effect of depth (minimum, midpoint or maximum) on colour dimorphism (electronic supplementary material, table S6). This reflects a lack of variation in depth within our dataset—95% of minimum depths were between 0 and 5 m, while 60% of maximum depths were between 10 and 30 m. Turbidity probably differs between our habitat categories; therefore, water clarity remains a potentially important underlying factor. Interestingly, fishes living in clear, tropical marine water tend to be more blue-sensitive in their colour perception than fishes living in turbid or tinted water [18]. Given that terminal phase fishes are more likely to display blue on coral reefs, this suggests that clear, oligotrophic water has played an important role in the evolution of pronounced colour dimorphism.
Despite the result that terminal phase fishes on coral reefs display colours that contrast against the background, many labrids lose the conspicuousness of their coloration with increasing viewing distance due to blurring of pattern elements and the additive effect of colours [55]. Therefore, when viewed by more distantly placed predators many labrids, regardless of habitat may be cryptic [18,55]. Colour-dimorphic fishes living in habitats other than coral reefs do, however, appear to bear a cost [37], which they offset by restricting colour dimorphism to more easily concealed body regions. Fishes not associated with coral reefs are less likely to have colour-dimorphic heads, bodies and caudal fins than fishes associated with coral reefs (electronic supplementary material, table S7) but are equally likely to have colour-dimorphic dorsal and anal fins, which typically remain folded against their bodies unless actively turning or signalling to conspecifics (electronic supplementary material, table S7). Similar results have been reported for lizards, where males living in closed habitats that afford greater protection from predators evolve conspicuous colours on exposed body regions [8]. Our results suggest that fishes living away from the structural refuge provided by coral reefs mitigate their risk of predation by restricting conspicuous colours to body regions that allow for controlled intermittent signalling [18].
5. Conclusion
We present a comprehensive analysis of the selective forces shaping the evolution of colour dimorphism in labrid fishes. Our results show that colour dimorphism is best adapted to habitat-specific conditions where coral reefs provide the ideal environment for the evolution of conspicuous visual signals. Clear water enhances the visual perception of blue colours that contrast against the background, while the structural complexity of coral reefs creates solid, physical barriers that provide refuge for conspicuously coloured fishes. Together, these unique features have led to the evolution of pronounced colour dimorphism in wrasses and parrotfishes on coral reefs and contributed to the diversity of the bold and bizarre colour patterns so long admired among coral reef fishes.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the many ichthyologists and naturalists who have contributed their photographs to scholarly identification guides and online repositories. Thanks to D. R. Robertson, T. Caro, S. A. Price, S. J. Longo, S. T. Friedman and S. J. Staples for helpful discussion and suggestions that improved this work; and T. Caro, T. C. Mendelson, G. L. Patricelli and S. J. Staples for comments on manuscript drafts.
Data accessibility
Data reported in this paper are presented in electronic supplementary material, table S1 and are also available from the Dryad Digital Repository: https://doi.org/10.25338/B8C60Z [63]. The phylogenetic tree, samples from the posterior distribution of trees, and the species-specific sex allocation and mating systems dataset are also available from the Dryad Digital Repository: https://doi.org/10.25338/B8GC91 [58].
Authors' contributions
J.R.H. and P.C.W. designed the research. J.R.H. compiled the data, performed the analyses and interpretations and wrote the paper with contributions from F.S. and P.C.W.
Competing interests
We declare we have no competing interests.
Funding
J.R.H. was supported by the National Science Foundation's Postdoctoral Research Fellowship in Biology for Research Using Biological Collections (DBI-1523934) and parts of this work were supported by National Science Foundation grant nos DEB-0717009 and DEB-1061981 to P.C.W.
References
- 1.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.McCullough EL, Miller CW, Emlen DJ. 2016. Why sexually selected weapons are not ornaments. Trends Ecol. Evol. 31, 742–751. ( 10.1016/J.TREE.2016.07.004) [DOI] [PubMed] [Google Scholar]
- 3.Clutton-Brock T. 2007. Sexual selection in males and females. Science 318, 1882–1885. ( 10.1126/science.1133311) [DOI] [PubMed] [Google Scholar]
- 4.Endler JA. 1983. Natural and sexual selection on color patterns in poeciliid fishes. Environ. Biol. Fishes 9, 173–190. ( 10.1007/BF00690861) [DOI] [Google Scholar]
- 5.Andersson M. 1982. Sexual selection, natural selection and quality advertisement. Biol. J. Linn. Soc. 17, 375–393. ( 10.1111/j.1095-8312.1982.tb02028.x) [DOI] [Google Scholar]
- 6.Endler JA. 2000. Evolutionary implications of the interaction between animal signals and the environment. In Animal signals: signalling and signal design in animal communication (eds Espmark Y, Amundsen T, Rosenqvist G), pp. 11–46. Trondheim, NO: Tapir Academic. [Google Scholar]
- 7.Badyaev AV, Hill GE. 2003. Avian sexual dichromatism in relation to phylogeny and ecology. Annu. Rev. Ecol. Evol. Syst. 34, 27–49. ( 10.1146/annurev.ecolsys.34.011802.132441) [DOI] [Google Scholar]
- 8.Stuart-Fox DM, Ord TJ. 2004. Sexual selection, natural selection and the evolution of dimorphic coloration and ornamentation in agamid lizards. Proc. R. Soc. Lond. B 271, 2249–2255. ( 10.1098/rspb.2004.2802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen CE, Zwaan BJ, Brakefield PM. 2011. Evolution of sexual dimorphism in the Lepidoptera. Annu. Rev. Entomol. 56, 445–464. ( 10.1146/annurev-ento-120709-144828) [DOI] [PubMed] [Google Scholar]
- 10.Bossu CM, Near TJ. 2015. Ecological constraint and the evolution of sexual dichromatism in darters. Evolution 69, 1219–1231. ( 10.1111/evo.12655) [DOI] [PubMed] [Google Scholar]
- 11.Kodric-Brown A. 1990. Mechanisms of sexual selection: insights from fishes. Ann. Zool. Fennici 27, 87–100. [Google Scholar]
- 12.Owens IPF, Hartley IR. 1998. Sexual dimorphism in birds: why are there so many different forms of dimorphism? Proc. R. Soc. Lond. B 265, 397–407. ( 10.1098/rspb.1998.0308) [DOI] [Google Scholar]
- 13.Figuerola J, Green AJ. 2000. The evolution of sexual dimorphism in relation to mating patterns, cavity nesting, insularity and sympatry in the Anseriformes. Funct. Ecol. 14, 701–710. ( 10.2307/2656522) [DOI] [Google Scholar]
- 14.Dunn PO, Whittingham LA, Pitcher TE. 2001. Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution 55, 161–175. ( 10.1554/0014-3820(2001)055[0161:msscat]2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 15.Bell RC, Webster GN, Whiting MJ. 2017. Breeding biology and the evolution of dynamic sexual dichromatism in frogs. J. Evol. Biol. 30, 2104–2115. ( 10.1111/jeb.13170) [DOI] [PubMed] [Google Scholar]
- 16.Møller AP, Birkhead TR. 1994. The evolution of plumage brightness in birds is related to extrapair paternity. Evolution 48, 1089–1100. ( 10.1111/j.1558-5646.1994.tb05296.x) [DOI] [PubMed] [Google Scholar]
- 17.Pérez i de Lanuza G, Font E, Monterde JL. 2013. Using visual modelling to study the evolution of lizard coloration: sexual selection drives the evolution of sexual dichromatism in lacertids. J. Evol. Biol. 26, 1826–1835. ( 10.1111/jeb.12185) [DOI] [PubMed] [Google Scholar]
- 18.Endler JA. 1992. Signals, signal conditions, and the direction of evolution. Am. Nat. 139, S125–S153. ( 10.1086/285308) [DOI] [Google Scholar]
- 19.Boughman JW. 2002. How sensory drive can promote speciation. Trends Ecol. Evol. 17, 571–577. ( 10.1016/S0169-5347(02)02595-8) [DOI] [Google Scholar]
- 20.Ciccotto PJ, Mendelson TC. 2016. The ecological drivers of nuptial color evolution in darters (Percidae: Etheostomatinae). Evolution 70, 745–756. ( 10.1111/evo.12901) [DOI] [PubMed] [Google Scholar]
- 21.Gamble S, Lindholm AK, Endler JA, Brooks R. 2003. Environmental variation and the maintenance of polymorphism: the effect of ambient light spectrum on mating behaviour and sexual selection in guppies. Ecol. Lett. 6, 463–472. ( 10.1046/j.1461-0248.2003.00449.x) [DOI] [Google Scholar]
- 22.Chunco AJ, McKinnon JS, Servedio MR. 2007. Microhabitat variation and sexual selection can maintain male color polymorphisms. Evolution 61, 2504–2515. ( 10.1111/j.1558-5646.2007.00213.x) [DOI] [PubMed] [Google Scholar]
- 23.Marchetti K. 1993. Dark habitats and bright birds illustrate the role of the environment in species divergence. Nature 362, 149–152. ( 10.1038/362149a0) [DOI] [Google Scholar]
- 24.Endler JA, Thery M. 1996. Interacting effects of lek placement, display behavior, ambient light, and color patterns in three neotropical forest-dwelling birds. Am. Nat. 148, 421–452. ( 10.2307/2463298) [DOI] [Google Scholar]
- 25.White TE, Vogel-Ghibely N, Butterworth NJ. 2020. Flies exploit predictable perspectives and backgrounds to enhance iridescent signal salience and mating success. Am. Nat. 195 ( 10.1086/707584) [DOI] [PubMed] [Google Scholar]
- 26.Shine R. 1989. Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q. Rev. Biol. 64, 419–461. ( 10.1086/416458) [DOI] [PubMed] [Google Scholar]
- 27.Shine R, Madsen T. 1994. Sexual dichromatism in snakes of the genus Vipera: a review and a new evolutionary hypothesis. Source J. Herpetol. 28, 114–117. [Google Scholar]
- 28.Taylor LA, Clark DL, McGraw KJ. 2014. From spiderling to senescence: ontogeny of color in the jumping spider, Habronattus pyrrithrix. J. Arachnol. 42, 268–276. ( 10.1636/0161-8202-42.3.268) [DOI] [Google Scholar]
- 29.Khan MK, Herberstein ME. 2020. Ontogenetic colour change signals sexual maturity in a non-territorial damselfly. Ethology 126, 51–58. ( 10.1111/eth.12959) [DOI] [Google Scholar]
- 30.Schultz LP. 1958. Review of the parrotfishes, family Scaridae. Bull. US Natl Museum 214, 1–143. ( 10.5479/si.03629236.214.1) [DOI] [Google Scholar]
- 31.Roede MJ. 1972. Color as related to size, sex and behavior in seven Caribbean labrid fish species (genera Thalassoma, Halichoeres and Hemipteronotus). Stud. Fauna Curacao other Caribb. Islands 42, 1–264. [Google Scholar]
- 32.Bell RC, Zamudio KR. 2012. Sexual dichromatism in frogs: natural selection, sexual selection and unexpected diversity. Proc. R. Soc. B 279, 4687–4693. ( 10.1098/rspb.2012.1609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galán P. 2008. Ontogenetic and sexual variation in the coloration of the lacertid lizards Iberolacerta monticola and Podarcis bocagei. Do the females prefer the greener males? Anim. Biol. 58, 173–198. () [DOI] [Google Scholar]
- 34.Westneat MW, Alfaro ME. 2005. Phylogenetic relationships and evolutionary history of the reef fish family Labridae. Mol. Phylogenet. Evol. 36, 370–390. ( 10.1016/j.ympev.2005.02.001) [DOI] [PubMed] [Google Scholar]
- 35.Choat JH, Robertson DR. 1975. Protogynous hermaphroditism in fishes of the family Scaridae. In Intersexuality in the animal kingdom (ed. Reinboth R.), pp. 263–283. Berlin, Germany: Springer-Verlag. [Google Scholar]
- 36.Robertson DR. 1981. The social and mating systems of two labrid fishes, Halichoeres maculipinna and H. garnoti, off the Caribbean coast of Panama. Mar. Biol. 64, 327–340. ( 10.1007/BF00393634) [DOI] [Google Scholar]
- 37.Robertson DR, Hoffman SG. 1977. The roles of female mate choice and predation in the mating systems of some tropical labroid fishes. Z. Tierpsychol. 45, 298–320. ( 10.1111/j.1439-0310.1977.tb02123.x) [DOI] [Google Scholar]
- 38.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 39.Ghiselin MT. 1969. The evolution of hermaphroditism among animals. Q. Rev. Biol. 44, 189–208. ( 10.1086/406066) [DOI] [PubMed] [Google Scholar]
- 40.Robertson DR. 1972. Social control of sex reversal in a coral-reef fish. Science 177, 1007–1009. ( 10.1126/science.177.4053.1007) [DOI] [PubMed] [Google Scholar]
- 41.Muñoz RC, Warner RR. 2004. Testing a new version of the size-advantage hypothesis for sex change: sperm competition and size-skew effects in the bucktooth parrotfish, Sparisoma radians. Behav. Ecol. 15, 129–136. ( 10.1093/beheco/arg086) [DOI] [Google Scholar]
- 42.Warner RR. 1984. Mating behavior and hermaphroditism in coral reef fishes: the diverse forms of sexuality found among tropical marine fishes can be viewed as adaptations to their equally diverse mating systems. Am. Sci. 72, 128–136. [Google Scholar]
- 43.Streelman JT, Alfaro M, Westneat MW, Bellwood DR, Karl SA. 2002. The evolutionary history of the parrotfishes: biogeography, ecomorphology, and comparative diversity. Evolution 56, 961–971. ( 10.1111/j.0014-3820.2002.tb01408.x) [DOI] [PubMed] [Google Scholar]
- 44.Robertson DR, Warner RR. 1978. Sexual patterns in the labroid fishes of the western Caribbean, II: the parrotfishes (Scaridae). Smithson. Contrib. Zool. 255, 1–26. [Google Scholar]
- 45.Warner RR, Robertson DR. 1978. Sexual patterns in the labroid fishes of the western Caribbean, I: the wrasses (Labridae). Smithson. Contrib. Zool. 254, 1–27. [Google Scholar]
- 46.Robertson DR, Choat JH. 1974. Protogynous hermaphroditism and social systems in labrid fish. In Proc. of the 2nd Int. Coral Reef Symposium, Brisbane, 22 June–2 July 1973, pp. 217–226. Brisbane, Australia: Great Barrier Reef Committee. [Google Scholar]
- 47.Colin PL, Bell LJ. 1991. Aspects of the spawning of labrid and scarid fishes (Pisces: Labroidei) at Enewetak Atoll, Marshall Islands with notes on other families. Environ. Biol. Fishes 31, 229–260. ( 10.1007/BF00000690) [DOI] [Google Scholar]
- 48.Thresher RE. 1979. Social behavior and ecology of two sympatric wrasses (Labridae: Halichoeres spp.) off the coast of Florida. Mar. Biol. 53, 161–172. ( 10.1007/BF00389187) [DOI] [Google Scholar]
- 49.Taborsky M. 1998. Sperm competition in fish: ‘bourgeois' males and parasitic spawning. Trends Ecol. Evol. 13, 222–227. ( 10.1016/S0169-5347(97)01318-9) [DOI] [PubMed] [Google Scholar]
- 50.Sadovy de Mitcheson Y, Liu M.. 2008. Functional hermaphroditism in teleosts. FISH Fish. 9, 1–43. ( 10.1111/j.1467-2979.2007.00266.x) [DOI] [Google Scholar]
- 51.Hodge JR, Santini F, Wainwright PC. In press. Correlated evolution of sex allocation and mating system in wrasses and parrotfishes. Am. Nat. ( 10.1086/708764) [DOI] [PubMed] [Google Scholar]
- 52.Emlen ST, Oring LW. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223. ( 10.2307/1744497) [DOI] [PubMed] [Google Scholar]
- 53.Seehausen O, et al. 2008. Speciation through sensory drive in cichlid fish. Nature 455, 620–626. ( 10.1038/nature07285) [DOI] [PubMed] [Google Scholar]
- 54.Cheney K, Grutter AS, Blomberg SP, Marshall NJ. 2009. Blue and yellow signal cleaning behavior in coral reef fishes. Curr. Biol. 19, 1283–1287. ( 10.1016/j.cub.2009.06.028) [DOI] [PubMed] [Google Scholar]
- 55.Marshall NJ. 2000. Communication and camouflage with the same ‘bright’ colours in reef fishes. Phil. Trans. R. Soc. B 355, 1243–1248. ( 10.1098/rstb.2000.0676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kodric-Brown A. 1998. Sexual dichromatism and temporary color changes in the reproduction of fishes. Am. Zool. 38, 70–81. ( 10.1093/icb/38.1.70) [DOI] [Google Scholar]
- 57.Wallace AR. 1889. Darwinism: an exposition of the theory of natural selection, with some of its applications, 2nd edn London, UK: Macmillan. [Google Scholar]
- 58.Hodge JR, Santini F, Wainwright PC. 2020. Data from: Correlated evolution of sex allocation and mating system in wrasses and parrotfishes Dryad Digital Repository. ( 10.25338/B8GC91) [DOI] [PubMed]
- 59.Seddon N, Tobias JA, Eaton M, Ödeen A, Byers BE. 2010. Human vision can provide a valid proxy for avian perception of sexual dichromatism. Auk 127, 283–292. ( 10.1525/auk.2009.09070) [DOI] [Google Scholar]
- 60.Landgraf AJ, Lee Y. 2015. Dimensionality reduction for binary data through the projection of natural parameters. arXiv 1510.06112 http://arxiv.org/abs/1510.06112.
- 61.Froese R, Pauly D.. 2017. FishBase. See www.fishbase.org .
- 62.IUCN. 2017. IUCN red list of threatened species, v. 2017.3. See http://www.iucnredlist.org.
- 63.Hodge JR, Santini F, Wainwright PC. 2020. Data from: Colour dimorphism in labrid fishes as an adaptation to life on coral reefs Dryad Digital Repository. ( 10.25338/B8C60Z) [DOI] [PMC free article] [PubMed]
- 64.Pagel M, Meade A. 2006. Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain Monte Carlo. Am. Nat. 167, 808–825. ( 10.1086/503444) [DOI] [PubMed] [Google Scholar]
- 65.Pagel M, Meade A, Barker D. 2004. Bayesian estimation of ancestral character states on phylogenies. Syst. Biol. 53, 673–684. ( 10.1080/10635150490522232) [DOI] [PubMed] [Google Scholar]
- 66.Rambaut A, Drummond AJ. 2009. Tracer v1.5 See http://beast.bio.ed.ac.uk.
- 67.Kass RE, Raftery AE. 1995. Bayes factors. J. Am. Stat. Assoc. 90, 773–795. ( 10.1080/01621459.1995.10476572) [DOI] [Google Scholar]
- 68.Xie W, Lewis PO, Fan Y, Kuo L, Chen M-H. 2011. Improving marginal likelihood estimation for Bayesian phylogenetic model selection. Syst. Biol. 60, 150–160. ( 10.1093/sysbio/syq085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raftery AE. 1996. Hypothesis testing and model selection. In Markov chain Monte Carlo in practice (eds Gilks WR, Richardson S, Spiegelhalter DJ), pp. 163–188. London, UK: Chapman & Hall. [Google Scholar]
- 70.Bollback JP. 2006. SIMMAP: stochastic character mapping of discrete traits on phylogenies. BMC Bioinf. 7, 1–7. ( 10.1186/1471-2105-7-88) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 72.Beaulieu JM, Jhwueng D-C, Boettiger C, O'Meara BC. 2012. Modeling stabilizing selection: expanding the Ornstein–Uhlenbeck model of adaptive evolution. Evolution 66, 2369–2383. ( 10.1111/j.1558-5646.2012.01619.x) [DOI] [PubMed] [Google Scholar]
- 73.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn New York, NY: Springer. [Google Scholar]
- 74.Burnham KP, Anderson DR, Huyvaert KP. 2011. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65, 23–35. ( 10.1007/s00265-010-1029-6) [DOI] [Google Scholar]
- 75.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2013. caper: comparative analysis of phylogenetics and evolution in R. R package version 0.5.2. See https://cran.r-project.org/pacckage=caper. [Google Scholar]
- 76.Fujii R. 2000. The regulation of motile activity in fish chromatophores. Pigment Cell Res. 13, 300–319. ( 10.1034/j.1600-0749.2000.130502.x) [DOI] [PubMed] [Google Scholar]
- 77.Ho LST, Ane C, Lachlan R, Tarpinian K, Feldman R, Yu Q, van der Bijl W.. 2016. Package ‘phylolm’. See http://cran.r-project.org/web/packages/phylolm/index.html. [Google Scholar]
- 78.Ives AR, Garland T. 2010. Phylogenetic logistic regression for binary dependent variables. Syst. Biol. 59, 9–26. ( 10.1093/sysbio/syp074) [DOI] [PubMed] [Google Scholar]
- 79.Hanlon RT, Chiao CC, Mäthger LM, Barbosa A, Buresch KC, Chubb C. 2009. Cephalopod dynamic camouflage: bridging the continuum between background matching and disruptive coloration. Philos. Trans. R. Soc. B 364, 429–437. ( 10.1098/rstb.2008.0270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cott HB. 1940. Adaptive coloration in animals. London, UK: Methuen. [Google Scholar]
- 81.Kelley JL, Taylor I, Hart NS, Partridge JC. 2017. Aquatic prey use countershading camouflage to match the visual background. Behav. Ecol. 28, 1314–1322. ( 10.1093/beheco/arx093) [DOI] [Google Scholar]
- 82.Seehausen O, van Alphen JJM, Witte F.. 1997. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277, 1808–1811. ( 10.1126/science.277.5333.1808) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hodge JR, Santini F, Wainwright PC. 2020. Data from: Correlated evolution of sex allocation and mating system in wrasses and parrotfishes Dryad Digital Repository. ( 10.25338/B8GC91) [DOI] [PubMed]
- Hodge JR, Santini F, Wainwright PC. 2020. Data from: Colour dimorphism in labrid fishes as an adaptation to life on coral reefs Dryad Digital Repository. ( 10.25338/B8C60Z) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data reported in this paper are presented in electronic supplementary material, table S1 and are also available from the Dryad Digital Repository: https://doi.org/10.25338/B8C60Z [63]. The phylogenetic tree, samples from the posterior distribution of trees, and the species-specific sex allocation and mating systems dataset are also available from the Dryad Digital Repository: https://doi.org/10.25338/B8GC91 [58].