Ebola virus (EV) is a filovirus which causes viral haemorrhagic fever (VHF) in humans (World Health Organization (WHO), 2014a). Fruit bats of the family Pteropodidae are thought to be the natural reservoir and humans are thought to acquire the disease through direct contact with non-human primates (NHP) (Leroy et al., 2005). The first cases of Ebola virus disease (EVD) were reported in 1976 in the Democratic Republic of Congo and since then sporadic cases and small scale outbreaks have occurred in central African countries (World Health Organization, 2014d, World Health Organization, 2014e, World Health Organization, 2014f). There are five strains of EV but the Zaire strain is the most severe, with a case-fatality rate up to 90% (World Health Organization (WHO), 2014a). The unprecedented scale of the current outbreak of EVD in Sierra Leone, Guinea, Liberia and Nigeria, led to the World Health Organization, 2014d, World Health Organization, 2014e, World Health Organization, 2014f declaring an international public health emergency on August 8th 2014. The outbreak has since spread to Senegal, and a reportedly unrelated outbreak has since occurred in the Democratic Republic of Congo (World Health Organization (WHO), 2014b). As of 22nd August 2014, the West African outbreak has resulted in 2615 cases and 1427 deaths and is unprecedented because it has continued for more than double the length of time of the largest previous outbreak in Uganda in 2000 (3 months vs. 8 months), has resulted in more than six times as many cases (425 cases vs. 2615 cases), and has for first time occurred in more than one country simultaneously and in capital cities (Okware et al., 2002, World Health Organization, 2014d, World Health Organization, 2014e, World Health Organization, 2014f). Among the total cases, 1251 have been laboratory confirmed, and genetic sequencing has showed that the similarity of the virus to the Zaire EV is 97% (Baize et al., 2014). Unlike past outbreaks, the current outbreak of EVD has not been contained and has resulted in social unrest, breakdown in law and order, shortages of personal protective equipment (PPE) and depletion of the healthcare workforce, with over 240 healthcare workers (HCWs) becoming infected and 120 HCW deaths as of 25th August 2014 (World Health Organization (WHO), 2014c). The inability to contain this outbreak has been blamed variously on lapses in infection control, shortages of PPE and other supplies, myths and misconceptions about EVD, and the fact that it is occurring in large cities rather than small villages.
HCWs, many of whom are nurses, are on the frontline of the response, and their occupational health and safety is critical to control of the outbreak and maintenance of the health workforce during a crisis. The WHO, the US Centers for Disease Control (1998) and several other countries recommend surgical masks for HCWs treating Ebola (Centers for Disease Control and Prevention, 2014a, Centers for Disease Control and Prevention, 2014b, Centers for Disease Control and Prevention, 2014c, World Health Organization, 2014d, World Health Organization, 2014e, World Health Organization, 2014f) whilst other countries (The Department of Health UK, 2014) and Médecins Sans Frontières (MSF) have recommend the use of respirators (Sterk, 2008) (Table 1 ). We question the recommendations for surgical masks and outline evidence on the use of respiratory protection for HCWs, and the issues that must be considered when selecting the most appropriate type of protection.
Table 1.
Recommendations around the use of mask/respirators to protect healthcare workers from Ebola Virus Disease (EVD).
| Organization/country | Developed by/year | Type of HCWs | Recommendation |
|---|---|---|---|
| WHO | World Health Organization (World Health Organization, 2014d, World Health Organization, 2014e, World Health Organization, 2014f) | Hospital HCWs | Routine care - Medical masks AGPs – N95 respirators or powered air purifying respirators (PAPRs). |
| World Health Organization (World Health Organization, 2014d, World Health Organization, 2014e, World Health Organization, 2014f) | Lab workers | N95 respirators or powered air purifying respirators (PAPRs). | |
| CDC US | Centers for Disease Control and Prevention (CDC) August 2014 (Centers for Disease Control and Prevention (CDC)) | Hospital HCWs | Routine care – Medical masks Fit-tested AGPs – N95 filtering face piece respirators or higher (e.g., powered air purifying respiratory or elastomeric respirators) |
| Centers for Disease Control and Prevention (CDC) August 2014 (Centers for Disease Control and Prevention (CDC)) | Lab workers | Appropriate respirators or a full body suit | |
| WHO/CDC | World Health Organization and Centers for Disease Control and Prevention (CDC) December 1998 (Centers for Disease Control and Prevention and World Health Organization) | Hospital HCWs and Lab workers | Respirators were recommended for HCWs. Medical and cloth masks were also recommended in cases respirators were not available |
| MSF | Médecins Sans Frontières (MSF) 2007 (Sterk, 2008) | Hospital HCWs and Lab workers | High Efficiency Particulate filtration (HEPA) masks |
| Australia | The Department of Health, August 2014 (The Department of Health. Australia 2014) | Hospital HCWS | Routine care – Medical masks AGPs - P2 (N95) respirators |
| Department of Health, September 2005 (The Department of Health Australia, 2014) | Lab workers | P2 (N95) respirators | |
| United Kingdom (UK) | Department of Health August 2014 (The Department of Health UK, 2014) | Hospital HCWs and Lab workers | Low possibility of VHF infection – Medical masks High possibility of VHF infection but patient does NOT have extensive bruising, active bleeding, uncontrolled diarrhoea, uncontrolled vomiting – Medical masks High possibility of VHF infection but patient does have extensive bruising, active bleeding, uncontrolled diarrhoea, uncontrolled vomiting – FFP3 respirators Confirmed VHF infection or AGPs in any situation – FFP3 respirators |
| Canada | Public Health Agency of Canada, August 2014 (Public Health Agency of Canada, 2014b) | Hospital HCWS | Medical masks; fit-tested respirators (seal-checked NIOSH approved N95 at a minimum) for AGPs |
| Public Health Agency of Canada August 2014 (Public Health Agency of Canada, 2014a) |
Lab workers | Particulate respirators (e.g., N95, or N100) or powered air purifying respirators (PAPRs) | |
| Belgium | Superior Health Council July 2014 (Superior Health Council, Belgium 2014) | Hospital HCWs and Lab workers | Patients categorized as ‘possibility of EMD – Surgical mask for routine care and FFP3 respirator or EN certified equivalent for AGPs Patients categorized as ‘high possibility’ or ‘confirmed EMD’ – FFP3 respirators |
| South Africa | Department of Health (Draft guidelines) August 2014 (Department of Health, South Africa 2014) | Hospital HCWS | Preferably N95 respirators |
CDC = Centers for Disease Control; HCW = Health Care Workers; MSF = Médecins Sans Frontières; WHO = World Health Organization.
1. Background controversy about face masks
There is ongoing debate and lack of consensus around the use of respiratory protection for HCWs for respiratory diseases, including influenza, which is reflected in inconsistencies between policies and guidelines across healthcare organizations and countries (Chughtai et al., 2013). In the healthcare setting facemasks (medical/surgical masks) are generally used to protect wearers from splashes and sprays of blood or body fluids and to prevent spread of infection from the wearer, while a respirator is intended for respiratory protection (Siegel et al., 2007). The mode of disease transmission is one factor which influences the selection of facemasks or respirators – for example, facemasks are recommended for infections transmitted through contact and droplets, while respirators are recommended for airborne infections. Such guidelines are based on often tenuous theoretical principles informed by limited experimental evidence, given the lack of data drawn from the complex clinical environment. Transmission is not fully elucidated for many infections, spread can occur by multiple modes and the relative contribution of each mode may not be precisely quantified. Further, host related factors can mediate the severity of the disease. Some diseases exclusively transmit through the airborne route in natural setting (e.g. tuberculosis), while other diseases mainly transmit through the droplet or contact modes but short range respiratory aerosols are generated during high risk procedures which increases the risk of infection transmission (Roy and Milton, 2004). For example, the primary mode of influenza transmission is thought to be droplet (reflected in guidelines which largely recommend surgical masks), but there is increasing evidence that it is also spread by short-range respiratory aerosols (Bischoff et al., 2013, Tellier, 2009). For Severe Acute Respiratory Syndrome (SARS), data supported both droplet and airborne transmission (Centers for Disease Control and Prevention, 2014a, Centers for Disease Control and Prevention, 2014b, Centers for Disease Control and Prevention, 2014c, Yu et al., 2004). Airborne precautions have even been recommended for measles and varicella-zoster viruses despite a lack of data (Siegel et al., 2007).
To date, only four randomized controlled clinical trials (RCTs) and five papers on the clinical efficacy of facemasks in the healthcare setting have been published (Jacobs et al., 2009, Loeb et al., 2009, MacIntyre et al., 2011, MacIntyre et al., 2013, MacIntyre et al., 2014b). One of these had only 32 subjects (Jacobs et al., 2009), and one had 446 subjects (Loeb et al., 2009). The largest RCTs conducted (by authors CRM, HS and colleagues) on N95 respirators and masks, with 1669 and 1441 subjects, respectively, showed a benefit associated with using N95 respirators and failed to show any benefit of surgical masks (MacIntyre et al., 2011, MacIntyre et al., 2013). In one of the trials, the majority of laboratory confirmed infections were with respiratory syncytial virus and influenza, neither of which are thought to be predominantly airborne (MacIntyre et al., 2013). These data support the concept that transmission of viruses is multimodal and caution against dogmatic paradigms about pathogens and their transmission, particularly when the disease in question has a high case-fatality rate and no proven pharmaceutical interventions.
Respirators are designed for respiratory protection and are indicated for infections transmitted by aerosols (MacIntyre et al., 2011, MacIntyre et al., 2013). However, this is based purely on the fact that they have superior filtration capacity, and can filter smaller particles. The guidelines fail to consider that respirators offer the additional benefit of being fitted, therefore creating a seal around the face. It is also possible that the seal achieved by a respirator may be an additional benefit over and above the superior filtration that they offer. Respirators are not regulated by fit however, only on filtration capacity (with filtration of airborne particles being the sole consideration in guidelines), but the seal offered by a respirator adds to the protection when compared to other mask types. The risk of infection with respiratory pathogens increases three-fold during aerosol-generating procedures (AGPs) such as intubation and mechanical ventilation (MacIntyre et al., 2014a). Respirators are generally recommended in these situations for diseases that are known to be transmitted though the droplet route such as influenza and SARS (Chughtai et al., 2013), so the fact that they are not recommended more broadly for a disease with a much higher case-fatality rate such as EVD, is concerning.
2. Modes of transmission of Ebola
The inability to control the West African Ebola outbreak has led to debate around the mode of transmission of EV, with some public health agencies suggesting aerosol transmission (Murray et al., 2010). Current evidence suggests that human to human transmission occurs predominantly though direct contact with blood and body secretions, (World Health Organization (WHO), 2014a) and this is the basis of the WHO and the CDC recommendations for facemasks to protect HCWs from EVD.
However, like influenza and SARS, there is some evidence of aerosol transmission of EVD. In an observational study from The Democratic Republic of Congo, of the 19 EVD cases who visited the home of an EVD patient, 14 had contact with the infected case while the remaining five had no history of any contact, which points to transmission through some other mode (Roels et al., 1999). There is some evidence from experimental animal studies that EVD can be transmitted without direct contact; however these studies generally do not differentiate between droplet and airborne transmission (Dalgard et al., 1992, Jaax et al., 1995, Johnson et al., 1995). In one study, six monkeys were divided into three groups and each group was exposed to low-dose or high-dose aerosolized EV and aerosolized uninfected cell culture fluid (control), respectively. All four monkeys exposed to EV developed infection (Johnson et al., 1995). Jaax et al. found that two of three control monkeys caged in the same room as monkeys with EVD, 3 m apart, died of EVD (Jaax et al., 1995).
Studies have also shown that pigs may transmit EV though direct contact or respiratory aerosols (Kobinger et al., 2011). In one study, monkeys without direct contact contracted EBV from infected pigs in separate enclosures (Weingartl et al., 2012). It was not clear whether transmission was due to respiratory aerosols or large droplets. The first infection occurred in a monkey caged near the air ventilation system and positive air samples identified through real time polymerase chain reaction (PCR), which raised the possibility of airborne transmission. However, pigs cough and sneeze more than humans and thus have more capacity to generate aerosols. Furthermore, in pigs EVD mainly affects the lungs while in primates, it mainly affects the gastrointestinal tract and is excreted in the faeces. As with influenza, the transmission characteristics of EVD may also change due to temperature and humidity, and it should be noted that the experimental studies on EV transmission were conducted at low temperature and humidity, which might have favoured aerosol transmission. A recent study has shown that nonhuman primate to nonhuman primate transmission is mainly through contact, with airborne transmission being unlikely (Alimonti et al., 2014).
Finally it must be emphasized that EV transmission in high-risk situations is not well studied, particularly during AGPs, in the handling of human remains or exposure to surgical smoke due to new surgical technologies like laser or diathermy. Although the CDC does recommend a respirator during AGPs for EVD patients, aerosols may be created in the absence of aerosol-generating procedures. Evidence suggests that aerosols from vomitus can transmit norovirus, and SARS was likely transmitted via faecal aerosols (Barker et al., 2004, Marks et al., 2003, McKinney et al., 2006, Yu et al., 2004). Staff contacts of two HCWs infected with Ebola in 1996, who were treated in South Africa, took universal precautions, with respirators used for high-risk procedures, and no further cases occurred in 300 potential contacts (Richards et al., 2000). The report of this outbreak (by author GAR) has been cited in support of the WHO and CDC guidelines (Klompas et al., 2014), however in South Africa one HCW contracted EBV when using normal surgical attire during placement of a central line in a patient with undiagnosed EBV. This occurred despite no obvious lapse in infection control. In contrast, once EBV had been diagnosed in the HCW, respirators, impermeable one-piece suits and visors were used (according to South African guidelines), and no further infections occurred despite procedures such as intubation, mechanical ventilation, dialysis, central line placement and the insertion of a Swan Ganz catheter (Richards et al., 2000).
3. Factors to consider in guidelines
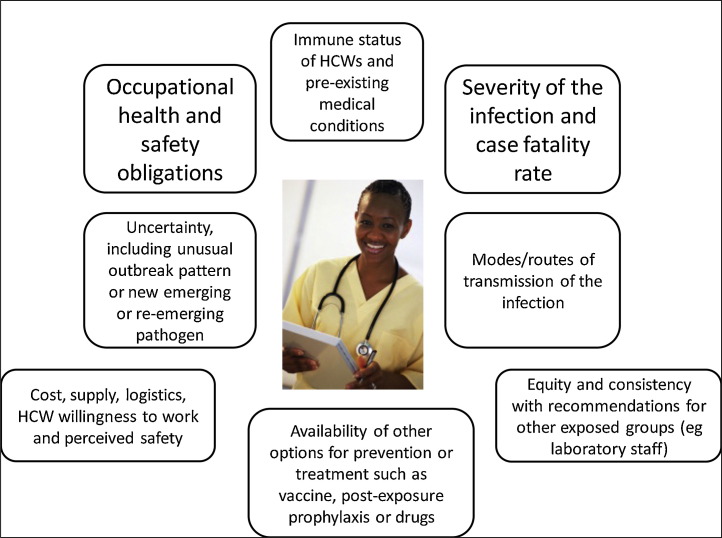
When determining recommendations for the protection of HCWs, guidelines should not be based solely on one parameter, the presumed mode of transmission. A risk-analysis approach is required that takes into account all relevant factors which could impact on the occupational health and safety of HCWs (Fig. 1 ). The severity of the outcome (case-fatality rate and disease severity) must be considered. Any level of uncertainty around modes of transmission must also be evaluated, particularly if the disease has a high case-fatality rate. In addition, the availability of pre- and post-exposure prophylaxis or treatment must be considered. The immune status and co-morbidities in HCWs should also be considered, as some HCWs may be innately more vulnerable to infection. As the ageing of the nursing workforce occurs in developed countries, there is likely to be a high proportion of HCWs with chronic conditions. In this case, facemasks have been recommended for HCWs by CDC and WHO because of the assumption that EV is not transmitted via the airborne route. However, there is uncertainty about transmission, the consequences of EVD infection are severe, there is no proven treatment, vaccine or post-exposure prophylaxis. Recommending a surgical mask for EVD has much more serious implications than for influenza, which has a far lower case-fatality rate and for which there are easily accessible vaccines and antiviral therapy. Further, numerous HCWs have succumbed to EVD during this epidemic, including senior physicians experienced in treating EVD and presumably less likely to have suffered lapses in infection control (World Health Organization, 2014d, World Health Organization, 2014e, World Health Organization, 2014f). Aside from these factors, it is also important to consider the perspectives of the staff member. In this highly stressful situation, staff members will want to be reassured that they are using the highest level of protection and are not putting themselves and their families/colleagues at risk. This is especially important if the outbreak escalates and additional staff members are required to assist. Staff may refuse to treat patients unless they feel adequately protected.
Fig. 1.
Factors to consider in making recommendations for respiratory protection of health workers*. *Cost, supply and logistics may affect implementation of guidelines, but should not drive recommendations for best practice.
We feel the recommendations for masks do not apply risk analysis methods appropriately, and are solely based on the low probability of non-contact modes of EV spread. Previous guidance provided by the WHO and CDC for “Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting” in 1999 were more conservative, with both organizations recommending the preferred use of respirators first line and surgical masks and cloth masks as a last option (Centers for Disease Control, 1998). Why then, during the worst outbreak of EVD in history, with the most virulent EV strain and with hundreds of HCWs succumbing to the disease is it considered adequate for them to wear surgical masks? The high case-fatality rate warrants the use of better protection such as a respirator and full body suit with face shield, where it can be provided.
4. Consistency of guidelines
There appears to be a double standard in recommendations for laboratory scientists working with EV, who must adhere to the highest level of biocontainment (BSL4) when working with the virus. (Centers for Disease Control and Prevention, 2014a, Centers for Disease Control and Prevention, 2014b, Centers for Disease Control and Prevention, 2014c, Department of Health and Aging Australia, 2007) Further, in contrast to HCWs, laboratory workers are exposed to the virus in a highly controlled, sterile environment in which there is less risk of transmission than in the highly unstable, contaminated and unpredictable clinical environment. The perceived inequity inherent in these inconsistent guidelines may also reduce the willingness of HCWs to work during an EVD outbreak.
Table 1 shows recommendations of the selected organizations and countries regarding the use of masks/respirators for EVD for HCWs and laboratory workers. Only the UK and South African guidelines have consistent guidelines for HCWs and laboratory scientists, with respirators recommended for confirmed cases of Viral Haemorrhagic Fever (including EVD) (Department of Health, 2014, Superior Health Council, 2014, The Department of Health UK, 2014). Among healthcare organizations, only MSF recommends respirators for EVD, and notably, in contrast to other international agencies including WHO, no MSF worker has developed EVD during the West African outbreak (Thomson, 2007).
In conclusion, whilst EV is predominantly spread by contact with blood and body fluids, there is some uncertainty about the potential for aerosol transmission. There is RCT evidence for respirators (but not masks) providing protection against non-aerosolised infections, (MacIntyre et al., 2013) and an abundance of evidence that transmission of pathogens in the clinical setting is rarely unimodal. Where uncertainty exists, the precautionary principle (that action to reduce risk should not await scientific certainty) should be invoked and guidelines should be consistent and err on the side of caution. Moreover, a clear description of risk should be provided to HCWs (Jackson et al., 2014). Given the predominant mode of transmission, every HCW death from Ebola is a potentially preventable death. It is highly concerning that a recent commentary suggests HCWs do not need a mask at all “to speak with conscious patients, as long as a distance of 1–2 metres is maintained”(Martin-Moreno et al., 2014). This fails to consider the changeability and unpredictability of the clinical environment and disregards the rights of the HCW. It is also unrealistic to believe a HCW can constantly keep track of their distance from a patient in the hectic acute care setting. We accept that cost, supply and logistics may, in some settings, preclude the use of respirators, but guidelines should outline best practice in the ideal setting, with discussion about contingency plans should the ideal recommendation be unfeasible. Importantly, in the absence of sufficient evidence, recommendations should be conservative and estimation of risk considered. Recommendations should be developed using a risk analysis framework, with the occupational health and safety of HCWs being the primary consideration.
Conflict of interest statement
CR MacIntyre has conducted several investigator-driven trials of respirators vs face masks, one of which was funded by an Australian Research Council Linkage Grant, where the industry partner was 3M, a manufacturer of PPE. 3M also provided supplies of surgical masks and respirators for the investigator-driven trials in health workers in China. H Seale was also involved in this research as a co-investigator. A Chugtai has had filtration testing of masks for his PhD thesis conducted by 3M Australia.
Acknowledgement
We acknowledge Dr. Kathleen Harriman, PhD, MPH, RN, Chief, Vaccine Preventable Diseases Epidemiology Section, Immunization Branch, California Department of Public Health for comments and reviewing the final manuscript.
References
- Alimonti J., Leung A., Jones S., Gren J., Qiu X., Fernando L., Balcewich B., Wong G., Stroher U., Grolla A., Strong J., Kobinger G. Evaluation of transmission risks associated with in vivo replication of several high containment pathogens in a biosafety level 4 laboratory. Sci. Rep. 2014;4:5824. doi: 10.1038/srep05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baize S., Pannetier D., Oestereich L., Rieger T., Koivogui L., Magassouba N., Soropogui B., Sow M.S., Keita S., De Clerck H., Tiffany A., Dominguez G., Loua M., Traore A., Kolie M., Malano E.R., Heleze E., Bocquin A., Mely S., Raoul H., Caro V., Cadar D., Gabriel M., Pahlmann M., Tappe D., Schmidt-Chanasit J., Impouma B., Diallo A.K., Formenty P., Van Herp M., Gunther S. Emergence of Zaire Ebola virus disease in Guinea – preliminary report. N. Engl. J. Med. 2014 doi: 10.1056/NEJMoa1404505. http://www.nejm.org/doi/full/10.1056/NEJMoa1404505 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Barker J., Vipond I., Bloomfield S. Effects of cleaning and disinfection in reducing the spread of Norovirus contamination via environmental surfaces. J. Hosp. Infect. 2004;58:42–49. doi: 10.1016/j.jhin.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Bischoff W.E., Swett K., Leng I., Peters T.R. Exposure to influenza virus aerosols during routine patient care. J. Infect. Dis. 2013;207:1037–1046. doi: 10.1093/infdis/jis773. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Infection Prevention and Control Recommendations for Hospitalized Patients with Known or Suspected Ebola Hemorrhagic Fever in U.S. Hospitals. Available at: http://www.cdc.gov/vhf/ebola/hcp/infection-prevention-and-control-recommendations.html (accessed 08.08.14.).
- Centers for Disease Control and Prevention (CDC). Recognizing the Biosafety Levels. Available at: http://www.cdc.gov/training/quicklearns/biosafety/ (accessed 18.08.14.).
- Centers for Disease Control and Prevention (CDC) 2004. Fact Sheet: Basic Information about SARS. Available at: http://www.cdc.gov/sars/about/fs-SARS.html (accessed 19.08.14.) [Google Scholar]
- Centers for Disease Control and Prevention; Atlanta: 1998. Centers for Disease Control and Prevention and World Health Organization Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting; pp. 1–198. [Google Scholar]
- Chughtai A.A., Seale H., MacIntyre C.R. Availability, consistency and evidence-base of policies and guidelines on the use of mask and respirator to protect hospital health care workers: a global analysis. BMC Res. Notes. 2013;6:216. doi: 10.1186/1756-0500-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgard D.W., Hardy R.J., Pearson S.L., Pucak G.J., Quander R.V., Zack P.M., Peters C.J., Jahrling P.B. Combined simian hemorrhagic fever and Ebola virus infection in cynomolgus monkeys. Lab. Anim. Sci. 1992;42:152–157. [PubMed] [Google Scholar]
- Department of Health, South Africa . 2014. National Guidelines for Recognition and Management of Viral Haemorrhagic Fevers (Draft Guidelines) Available at: http://www.caa.co.za/Documents/Avmed/National%20Guidelines%20for%20Viral%20Haemorrhagic%20Fevers.pdf (accessed 30.08.14.) [Google Scholar]
- Department of Health and Aging Australia . 2007. Guidelines for Certification of a Physical Containment Level 4 Facility – Version 2.1. Available at: http://www.ogtr.gov.au/internet/ogtr/publishing.nsf/content/certifications-1 (accessed 30.08.14.) [Google Scholar]
- Jaax N., Jahrling P., Geisbert T., Geisbert J., Steele K., McKee K., Nagley D., Johnson E., Jaax G., Peters C. Transmission of Ebola virus (Zaire strain) to uninfected control monkeys in a biocontainment laboratory. Lancet. 1995;346:1669–1671. doi: 10.1016/s0140-6736(95)92841-3. [DOI] [PubMed] [Google Scholar]
- Jackson C., Lowton K., Griffiths P. Infection prevention as “a show”: a qualitative study of nurses’ infection prevention behaviours. Int. J. Nurs. Stud. 2014;51:400–408. doi: 10.1016/j.ijnurstu.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Jacobs J.L., Ohde S., Takahashi O., Tokuda Y., Omata F., Fukui T. Use of surgical face masks to reduce the incidence of the common cold among health care workers in Japan: a randomized controlled trial. Am. J. Infect. Control. 2009;37:417–419. doi: 10.1016/j.ajic.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Johnson E., Jaax N., White J., Jahrling P. Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus. Int. J. Exp. Pathol. 1995;76:227–236. [PMC free article] [PubMed] [Google Scholar]
- Klompas M., Diekema D.J., Fishman N.O., Yokoe D.S. Ebola fever: reconciling Ebola planning with Ebola risk in US hospitals. Ann. Intern. Med. 2014 doi: 10.7326/M14-M1918. [DOI] [PubMed] [Google Scholar]
- Kobinger G.P., Leung A., Neufeld J., Richardson J.S., Falzarano D., Smith G., Tierney K., Patel A., Weingartl H.M. Replication, pathogenicity, shedding, and transmission of Zaire Ebola virus in pigs. J. Infect. Dis. 2011;204:200–208. doi: 10.1093/infdis/jir077. [DOI] [PubMed] [Google Scholar]
- Leroy E.M., Kumulungui B., Pourrut X., Rouquet P., Hassanin A., Yaba P., Delicat A., Paweska J.T., Gonzalez J.P., Swanepoel R. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- Loeb M., Dafoe N., Mahony J., John M., Sarabia A., Glavin V., Webby R., Smieja M., Earn D.J., Chong S., Webb A., Walter S.D. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- MacIntyre C.R., Wang Q., Cauchemez S., Seale H., Dwyer D.E., Yang P., Shi W., Gao Z., Pang X., Zhang Y., Wang X., Duan W., Rahman B., Ferguson N. A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respir. Viruses. 2011;5:170–179. doi: 10.1111/j.1750-2659.2011.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre C.R., Wang Q., Seale H., Yang P., Shi W., Gao Z., Rahman B., Zhang Y., Wang X., Newall A.T., Heywood A., Dwyer D.E. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am. J. Respir. Crit. Care Med. 2013;187:960–966. doi: 10.1164/rccm.201207-1164OC. [DOI] [PubMed] [Google Scholar]
- MacIntyre C.R., Seale H., Yang P., Zhang Y., Shi W., Almatroudi A., Moa A., Wang X., Li X., Pang X., Wang Q. Quantifying the risk of respiratory infection in healthcare workers performing high-risk procedures. Epidemiol. Infect. 2014;142:1802–1808. doi: 10.1017/S095026881300304X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre C.R., Wang Q., Rahman B., Seale H., Ridda I., Gao Z., Yang P., Shi W., Pang X., Zhang Y. Efficacy of face masks and respirators in preventing upper respiratory tract bacterial colonization and co-infection in hospital healthcare workers. Prev. Med. 2014;62:1–7. doi: 10.1016/j.ypmed.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P., Vipond I., Regan F., Wedgwood K., Fey R., Caul E. A school outbreak of Norwalk-like virus: evidence for airborne transmission. Epidemiol. Infect. 2003;131:727–736. doi: 10.1017/s0950268803008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Moreno J.M., Llinás G., Hernández J.M., Rodin G., Sharpe M., Walker J., Hansen C.H., Martin P., Symeonides S., Gourley C. Is respiratory protection appropriate in the Ebola response? Lancet. 2014;384(9946):856. doi: 10.1016/S0140-6736(14)61343-X. [DOI] [PubMed] [Google Scholar]
- McKinney K.R., Gong Y.Y., Lewis T.G. Environmental transmission of SARS at Amoy Gardens. J. Environ. Health. 2006;68:26–30. quiz 51-22. [PubMed] [Google Scholar]
- Murray M., Grant J., Bryce E., Chilton P., Forrester L. Facial protective equipment, personnel, and pandemics: impact of the pandemic (H1N1) 2009 virus on personnel and use of facial protective equipment. Infect. Control Hosp. Epidemiol. 2010;31:1011–1016. doi: 10.1086/656564. [DOI] [PubMed] [Google Scholar]
- Okware S.I., Omaswa F.G., Zaramba S., Opio A., Lutwama J.J., Kamugisha J., Rwaguma E.B., Kagwa P., Lamunu M. An outbreak of Ebola in Uganda. Trop. Med. Int. Health. 2002;7:1068–1075. doi: 10.1046/j.1365-3156.2002.00944.x. [DOI] [PubMed] [Google Scholar]
- Public Health Agency of Canada . 2014. Interim Biosafety Guidelines for Laboratories Handling Specimens from Patients Under Investigation for Ebola Virus Disease. Available at: http://www.phac-aspc.gc.ca/id-mi/vhf-fvh/ebola-biosafety-biosecurite-eng.php (accessed 22.08.14.) [Google Scholar]
- Public Health Agency of Canada . 2014. Interim Guidance – Ebola Virus Disease: Infection Prevention and Control Measures for Borders, Healthcare Settings and Self-monitoring at Home. Available at: http://www.phac-aspc.gc.ca/id-mi/vhf-fvh/ebola-ipc-pci-eng.php#tbl-3 (accessed 27.08.14.) [Google Scholar]
- Richards G.A., Murphy S., Jobson R., Mer M., Zinman C., Taylor R., Swanepoel R., Duse A., Sharp G., De La Rey I.C. Unexpected Ebola virus in a tertiary setting: clinical and epidemiologic aspects. Crit. Care Med. 2000;28:240–244. doi: 10.1097/00003246-200001000-00041. [DOI] [PubMed] [Google Scholar]
- Roels T.H., Bloom A.S., Buffington J., Muhungu G.L., Mac Kenzie W.R., Khan A.S., Ndambi R., Noah D.L., Rolka H.R., Peters C.J., Ksiazek T.G. Ebola hemorrhagic fever, Kikwit, Democratic Republic of the Congo, 1995: risk factors for patients without a reported exposure. J. Infect. Dis. 1999;179(Suppl. 1):S92–S97. doi: 10.1086/514286. [DOI] [PubMed] [Google Scholar]
- Roy C.J., Milton D.K. Airborne transmission of communicable infection-the elusive pathway. N. Engl. J. Med. 2004;22(350 (17)):1710–1712. doi: 10.1056/NEJMp048051. [DOI] [PubMed] [Google Scholar]
- Siegel J.D., Rhinehart E., Jackson M., Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am. J. Infect. Control. 2007;35:S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterk E. 2008. Filovirus Haemorrhagic Fever Guideline. Médecins Sans Frontières. Available at: http://www.medbox.org/ebola-toolbox/filovirus-haemorrhagic-fever-guideline/preview (accessed 30.08.14.) [Google Scholar]
- Superior Health Council, Belgium . 2014. Practical Recommendations to the Attention of Healthcare Professionals and Health Authorities Regarding the Identification of and Care Delivered to Suspected or Confirmed Carriers of Highly Contagious Viruses (of the Ebola or Marburg type) in the Context of an Epidemic Outbreak in West Africa. Available at: www.shea-online.org/Portals/0/PDFs/Belgian-guidelines-ebola.pdf (accessed 30.08.14.) [Google Scholar]
- Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J. R. Soc. Interface. 2009;6(6):S783–S790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Department of Health, Australia . 2014. Ebola virus Disease (EVD) Outbreaks in West Africa. Important Information for Clinicians in Secondary or Tertiary Care. Available at: www.health.gov.au/internet/main/.nsf/../ebola-clinicians-20140811.pdf (accessed 30.08.14.) [Google Scholar]
- The Department of Health, UK . 2014. Management of Hazard Group 4 Viral Haemorrhagic Fevers and Similar Human Infectious Diseases of High Consequence. Advisory Committee on Dangerous Pathogens. Available at: www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1194947382005 (accessed 30.08.14.) [Google Scholar]
- Thomson P. 2007. Ebola & Marburg Outbreak Control Guidance Manual. Version 2.0. Médecins Sans Frontières (MSF) Available at: http://www.medbox.org/ebola-toolbox/ebola-marburg-outbreak-control-guidance-manual/preview?q= (accessed 30.08.14.) [Google Scholar]
- Weingartl H.M., Embury-Hyatt C., Nfon C., Leung A., Smith G., Kobinger G. Transmission of Ebola virus from pigs to non-human primates. Sci. Rep. 2012;2:811. doi: 10.1038/srep00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2014. Ebola and Marburg Virus Disease Epidemics: Preparedness, Alert, Control, and Evaluation. Available at: http://www.who.int/csr/disease/ebola/manual_EVD/en/ (accessed 28.08.14.) [Google Scholar]
- World Health Organization (WHO) 2014. Ebola Virus Disease – Democratic Republic of Congo. Available at: http://www.who.int/csr/don/2014_08_27_ebola/en/ (accessed 30.08.14.) [Google Scholar]
- World Health Organization (WHO) 2014. Unprecedented Number of Medical Staff Infected with Ebola. Available at: http://www.who.int/mediacentre/news/ebola/25-august-2014/en/ (accessed 28.08.14.) [Google Scholar]
- World Health Organization (WHO). Ebola Virus Disease. Available at: http://www.who.int/csr/disease/ebola/en/ (accessed 14.08.14.).
- World Health Organization (WHO). Ebola Virus Disease Update – West Africa. Available at: http://www.who.int/csr/don/2014_08_22_ebola/en/ (accessed 22.08.14.).
- World Health Organization (WHO) 2014. Infection Prevention and Control Guidance for Care of Patients with Suspected or Confirmed Filovirus Haemorrhagic Fever in Health-care Settings, With Focus on Ebola. Available at: http://www.who.int/csr/bioriskreduction/filovirus_infection_control/en/ (accessed 02.09.14.) [Google Scholar]
- Yu I.T., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H., Leung D.Y., Ho T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]