Abstract
The marine sponge Petrosia weinbergi was found to contain isofucosterol and clionasterol as its major sterols. The rare cyclopropyl sterol (24S,28S)-24,28-methylenestigmast-5-en-3β-ol, previously detected as only 0.07% of the total sterols of a pelagophytic alga Pulvinaria sp., made up 6.6% of the total sterols. These sterols are believed to be the biosynthetic precursors of the antiviral orthoesterols and weinbersterols found in the same sponge. Based on the side chains of the isolated sterols, the absolute configurations of the antiviral steroid side chains are assigned to be (24R,28S)- for orthoesterol B, (24R)- for orthoesterol C, and (24S,28S)- for weinbersterols A and B.
Keywords: Sterols, Cyclopropyl sterols, Marine natural products, Antiviral steroids, Petrosia weinbergi
1. Introduction
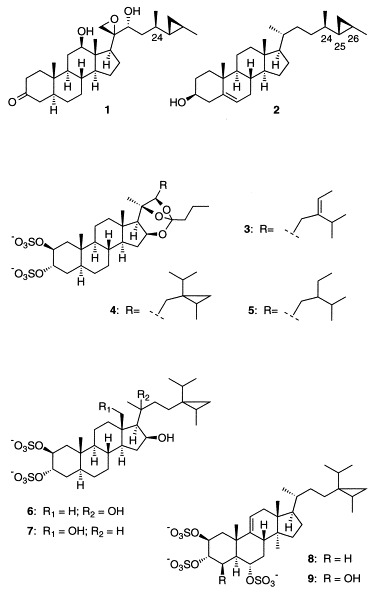
Recently, a number of biologically active steroids bearing unusual side chains have been isolated from marine sponges. Whereas NMR methods can generally be applied to solve the stereochemical structure of the steroidal nucleus, the conformationally flexible side chain is less tractable. For this reason, the structures of many new biologically active marine steroids have been reported without full stereochemical assignment of the side chain [1], [2], [3], [4], [5], [6]. In the case of the potent antitumor compound aragusterol A (1), the stereochemical configuration at C-24 of the side chain was determined by the chemical synthesis of the possible isomers [7]. This work showed that the stereochemical configuration of the carbon framework of the side chain was identical with that of the sponge sterol petrosterol (2). Because steroids are biosynthetically derived from sterols, it appears likely that petrosterol (2) occurs in this sponge and serves as the precursor of 1. Petrosterol is a sponge sterol that has been extensively studied by the Djerassi group. Because petrosterol (2) as well as the unnatural C-24,25,26 stereoisomers are known compounds [8], sterol analysis of the sponge may have been useful in assigning the C-24 configuration and could have saved some synthetic effort in the structure determination of aragusterol A (1).
The Caribbean sponge Petrosia weinbergi is another source of structurally unusual, biologically active steroids. Orthoesterols A, B, and C (3–5) contain bicyclic orthoesters between the side chain and ring D, and have been reported to be active against feline leukemia virus, mouse influenza virus, and mouse coronavirus [9]. Weinbersterols A and B (6,7), also isolated from this sponge, were shown to be active against feline leukemia virus and human immunodeficiency virus with EC50 values in the micromolar range [10]. Both weinbersterols A and B and orthoesterol B bear a rare cyclopropyl side chain that has also been reported for ibisterol (8), a human immunodeficiency virus-inhibitory steroid from a deep water Topsentia sp. [11], [12], and topsentiasterol E (9), a similar steroid from a shallow water Topsentia sp. [13]. The complete stereochemical configuration of the cyclopropyl side chain has not been reported for any of these compounds. This rare cyclopropyl side chain has previously only been detected once before, in a trace sterol (0.07% of total sterols) from a marine alga [14]. However, all four 24,28-stereoisomers of this cyclopropyl sterol have been synthesized and stereochemically assigned [15], [16]. Based on the precursor–product relationship between sterols and steroids, we have undertaken an analysis of the sterols of Petrosia weinbergi to shed light on the stereochemical configurations of the side chains of the antiviral steroidal metabolites.
2. Experimental
2.1. Sponge material
The sponge Petrosia weinbergi Van Soest 1980 (Phylum Porifera, Class Demospongiae, Order Haploslerida, Family Petrosiidae) [17] was collected by scuba at a depth of 18 m on the fore reef escarpment off Crooked Island, Bahamas. The sponge was plate-shaped, approximately 8 mm thick. The external color of the live sponge was olive green; the internal color was cream. The sponge was brittle in consistency. Scattered oscular sieve areas, 3–5 mm in diameter, occurred only on the upper surface. A sample of the sponge was preserved in ethanol as a taxonomic voucher; the remainder of the sponge was stored frozen at −20°C. The voucher specimen is deposited at the Harbor Branch Oceanographic Museum, catalog no. 003:00928 (DBMR no. 25-V-93-4-004). Scheme 1 Scheme 2
Scheme 1.
Scheme 2.
2.2. Sterol analysis
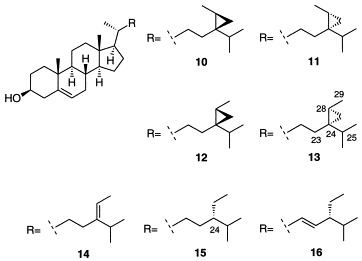
Frozen sponge material (15.6 g wet weight) was extracted at room temperature with acetone (20 ml) followed by dichloromethane/methanol 2:1 (75 ml). The dry weight of the extracted sponge was 1.514 g. The combined extracts were concentrated to dryness under reduced pressure and extracted with two 20-ml portions of ethyl acetate. Silica gel chromatography of the ethyl acetate extracts yielded 7.0 mg of free sterols. Purification was performed by reverse-phase HPLC (Waters 6000A pump, R401 differential refractometer, two Altex Ultrasphere ODS 5-μm 10 × 250-mm columns in series, 3 ml/min MeOH). The isolated sterols were identified by comparison of their 1H-NMR spectra (300 MHz, CDCl3) with those of authentic samples. References for NMR data are found in Table 1 and data for the 24,28-methylene-stigmast-5-en-3-ols 10–13 can be found in Table 2.
Table 1.
Sterols of Petrosia weinbergi
| Sterol | HPLC retention time (min) | Abundance |
|---|---|---|
| Isofucosterol (14) [22] | 51.8 | 38% |
| Clionasterol (15) [23] | 63.2 | 29% |
| Campesterol [24] | 57.6 | 8% |
| Crinosterol [24] | 46.4 | 7% |
| (24S,28S)-24,28-Methylene-stigmast-5-en-3-ol (13) [15] | 60.8 | 7% |
| Poriferasterol (16) [23] | 55.8 | 4% |
| Brassicasterol [24] | 48.4 | 3% |
| 22-Dehydrocholesterol [24] | 42.4 | 2% |
| Occelasterol [25] | 41.0 | 1% |
| (24S)-24-Isopropenylcholesterol [26] | 54.0 | tr. |
| 4-Demethyl-5-dehydrodinosterol [27] | 54.0 | tr. |
Table 2.
1H-NMR data for 24,28-methylene-stigmast-5-en-3-ols
| (24R,28S)- (10) | (24S,28R)- (11) | (24R,28R)- (12) | (24S,28S)- (13) | |
|---|---|---|---|---|
| C-3 | 3.523 (m) | 3.523 (m) | 3.523 (m) | 3.523 (m) |
| C-5 | 5.351 (m) | 5.351 (m) | 5.351 (m) | 5.351 (m) |
| C-18 | 0.672 (s) | 0.672 (s) | 0.664 (s) | 0.662 (s) |
| C-19 | 1.005 (s) | 1.005 (s) | 1.004 (s) | 1.005 (s) |
| C-21 | 0.917 (d, J = 6.4) | 0.914 (d, J = 6.5) | 0.881 (d, J = 6.5) | 0.881 (d, J = 6.4) |
| C-26,27 | 0.806 (d, J = 6.9) | 0.825 (d, J = 6.9) | 0.956 (d, J = 6.9) | 0.954 (d, J = 6.9) |
| 0.833 (d, J= 6.9) | 0.832 (d, J = 6.9) | 0.984 (d, J = 6.9) | 0.985 (d, J = 6.9) | |
| C-28 | 0.590 (m) | 0.601 (m) | 0.634 (m) | 0.635 (m) |
| C-29 | 1.032 (d, J = 6.3) | 1.042 (d, J = 6.3) | 1.075 (d, J = 6.3) | 1.080 (d, J = 6.3) |
| C-30 | −0.232 | −0.245 | −0.242 | −0.247 |
| (dd, J = 4.3, 5.4) | (dd, J = 4.3, 5.4) | (dd, J = 4.2, 5.4) | (dd, J = 4.2, 5.4) | |
| 0.385 | 0.359 | 0.416 | 0.415 | |
| (dd, J = 8.7, 4.3) | (dd, J = 8.6, 4.2) | (dd, J = 8.6, 4.2) | (dd, J = 8.6, 4.2) |
3. Results
Sterol analysis of Petrosia weinbergi was performed with the goal of isolating the rare cyclopropyl sterol that is the biosynthetic precursor to antiviral steroids 4, 6, and 7. The sterols of Petrosia weinbergi were separated by reverse-phase high performance liquid chromatography and analyzed by 1H-NMR spectroscopy. The structures of all sterols were unambiguously assigned by comparison of the spectra with those of authentic samples (Table 1).
The cyclopropyl sterol 13 was isolated as 6.6% of the total Petrosia weinbergi sterols. The identity of this sterol with the (24S,28S)-isomer (13) was determined by comparison with authentic synthetic samples. The (24R,28S)- and (24S,28R)- (10,11) configurations of the cyclopropyl side chain were easily ruled out based on differences in the 1H-NMR spectra Table 2, [15]. The 1H-NMR spectra of the (24R,28R)- and (24S,28S)-isomers (12 and 13), however, are very similar, but can be distinguished by the C-29 methyl signals, which are found at 1.075 ppm and 1.080 ppm, respectively (Fig. 1). Comparison of the isolated cyclopropyl sterol with authentic 13 by 1H-NMR showed that these were identical [15].
Fig. 1.
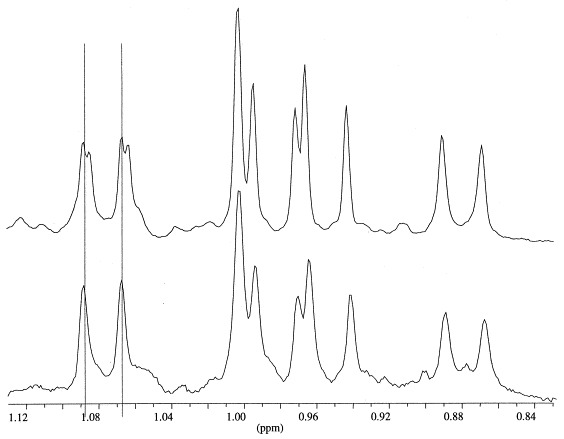
1H-NMR (300 MHz) spectra of cyclopropyl sterols 12 and 13. Top spectrum: Methyl region of mixed cyclopropyl sterols 12 and 13 (synthetic). Bottom spectrum: Methyl region of cyclopropyl sterol 13 from Petrosia weinbergi.
4. Discussion
A wealth of information is available for the structures of marine sterols [18]. Because of the precursor–product relationship between sterols and steroids, knowledge of structure of the sterols found in an organism can be used to assign the stereochemical configuration of the side chains of the biosynthetically derived steroids. Unlike many metabolites found in sponges, sterols are not produced by prokaryotic symbionts, but are produced largely via de novo biosynthesis and are characteristic for the sponge species where they are found [18], [19]. Although the stereochemical assignment based on reasonable biosynthetic relationships cannot be considered a rigorous proof, knowledge of the stereochemical configuration of the sterol side chains found in a sponge provides useful information in the assigning the side chain configurations of the steroids found in the same sponge.
In Petrosia weinbergi, the major sterol was found to be isofucosterol (14, 37.6%), which has the same carbon framework of the side chain as has been assigned to orthoesterol A (3) [9]. The next most abundant sterol was the 24-ethyl sterol clionasterol (15, 29.3%). The configuration at C-24 of the 24-ethyl steroid orthoesterol C (5) had not previously been specified. Because both of the 24-ethyl sterols (15 and 16) found in Petrosia weinbergi share the same configuration at C-24, 5 can be assigned to share the same configuration, in this case (24R)-. The isolation of the rare cyclopropyl sterol 13 in Petrosia weinbergi as a single stereoisomer implies that the cyclopropyl antiviral steroids isolated from this sponge (4,6,7) also share the same configuration. This configuration is (24S,28S)- in the case of weinbersterols A and B (6,7) and (24R,28S)- for orthoesterol B (4).
It is noteworthy that a cis relationship had originally been proposed for the cyclopropyl methyl and C-23 in 4 [9]. However, in sterol 13 a trans relationship is found, consistent with its probable biosynthesis from isofucosterol (14) [20]. The cis assignment had been made based on NOE difference spectra of 4, which showed an enhancement of signals at 1.13 ppm (cyclopropyl methyl) and 1.30 ppm (assigned as H-23) after irradiation of the high-field cyclopropyl proton at −0.20 ppm [9]. In our NOE experiments with 13, we observed enhancement of similar signals at 1.080 ppm (cyclopropyl methyl) and 1.266 ppm after irradiation of the high-field C-30 cyclopropyl proton (−0.247 ppm). However, COSY cross peaks between the 26- and 27-methyl signals (0.985 and 0.954 ppm) and 1.266 ppm show that this signal does not correspond to H-23, but to H-25. It should be noted that our assignment of structure 13 is not made on the basis of a NOE experiments, but is based on comparison with synthetic samples whose absolute stereochemical configurations rest on X-ray crystallography [15], [21].
Acknowledgments
We thank the American Society of Pharmacognosy Foundation for support of this project and David J. Kiemle for his expert assistance with the NMR experiments.
References
- 1.Fusetani N., Matsunaga S., Konosu S. Halistanol sulfate, an antimicrobial novel steroid sulfate from the marine sponge Halichondria cf. moorei Bergquist. Tetrahedron Lett. 1981;22:1985–1988. [Google Scholar]
- 2.Makar’eva T.N., Shubina L.K., Kalinovskii A.I., Stonik V.A., Elyakov G.B. Steroid derivatives from two sponges of the family Halichondriidae: sokotrasterol sulfate, a marine steroid with a new pattern of side chain alkylation. Steroids. 1983;42:267–281. doi: 10.1016/0039-128x(83)90039-9. [DOI] [PubMed] [Google Scholar]
- 3.Kanazawa S., Fusetani N., Matsunaga S., Halistanol A-E. New steroids sulfates, from a marine sponge, Epipolasis sp. Tetrahedron. 1992;48:5467–5472. [Google Scholar]
- 4.Li H., Matsunaga S., Fusetani N., Fujiki H., Murphy P.T., Willis R.H., Baker J.T. Echinoclasterol sulfate phenethylammonium salt, a unique steroid sulfate from the marine sponge, Echinoclathria subhispida. Tetrahedron Lett. 1993;34:5733–5736. [Google Scholar]
- 5.Bifulco G., Bruno I., Minale L., Riccio R. Novel HIV-inhibitory halostanol sulfates F-H form a marine sponge Pseudoaxinissa Digitata. J Nat Prod. 1994;57:164–167. doi: 10.1021/np50103a026. [DOI] [PubMed] [Google Scholar]
- 6.Sperry S., Crews P. Haliclostanone sulfate and halistanol sulfate from an Indo-Pacific Haliclona sponge. J Nat Prod. 1997;60:29–32. doi: 10.1021/np960592s. [DOI] [PubMed] [Google Scholar]
- 7.Iguchi K., Fujita M., Nakaoka H., Mitome H., Yamada Y. Aragusterol A: a potent antitumor marine steroid from the Okinawan sponge of the genus, Xestospongia. Tetrahedron Lett. 1993;34:6277–6280. [Google Scholar]
- 8.Proudfoot J.R., Djerassi C. Stereochemical effects in cyclopropane ring openings: synthesis and isomerization of petrosterol and all three of its trans cyclopropane diastereomers. J Am Chem Soc. 1984;106:5613–5622. [Google Scholar]
- 9.Koehn F.E., Gunasekera M., Cross S.S. New antiviral sterol disulfate ortho esters from the marine sponge Petrosia weinbergi. J Org Chem. 1991;56:1322–1325. [Google Scholar]
- 10.Sun H.H., Cross S.S., Gunasekera M., Koehn F.E. Weinbersterol disulfates A and B, antiviral steroid sulfates from the sponge Petrosia weinbergi. Tetrahedron. 1991;47:1185–1190. [Google Scholar]
- 11.McKee T.C., Cardellina J.H.I., Riccio R., D’Auria M.V., Iorizzi M., Minale L., Moran R.A., Gulakowski R.J., McMahon J.B., Buckheit R.W.J., Snader K.M., Boyd M.R. Comparative studies of sulfated sterols from marine invertebrates. J Med Chem. 1994;37:793–797. doi: 10.1021/jm00032a012. [DOI] [PubMed] [Google Scholar]
- 12.McKee T.C., Cardellina J.H.I., Tischler M., Snader K.M., Boyd M.R. Ibisterol sulfate, a novel HIV-inhibitory sulfated sterol from the deep water sponge Topsentia sp. Tetrahedron Lett. 1993;34:389–392. [Google Scholar]
- 13.Fusetani N., Takahashi M., Matsunaga S. Topsentiasterol sulfates, antimicrobial sterol sulfates possessing novel side chains, from a marine sponge, Topsentia sp. Tetrahedron. 1994;50:7765–7770. [Google Scholar]
- 14.Kokke W.C.M.C., Shoolery J.N., Fenical W., Djerassi C. Mechanism of side chain alkylation in E-24-propylidenecholesterol by a Chrysophyte alga. J Org Chem. 1984;49:3742–3752. [Google Scholar]
- 15.Giner J.-L., Zimmerman M.P., Djerassi C. Synthesis of (24R,28R)- and (24S,28S)-24,28-methylene-5-stigmasten-3β-ol and biosynthetic implications of cyclopropyl cleavage to 24-substituted cholesterols. J Org Chem. 1988;53:5895–5902. [Google Scholar]
- 16.Lang R.W., Djerassi C. Synthesis of sterols with cyclopropane-containing side chains: spectroscopic properties and absolute configurations. J Org Chem. 1982;47:625–633. [Google Scholar]
- 17.Van Soest R.W.M. Haplosclerida. Stud Fauna Curacao Caribb Isl. 1980;62:1–173. [Google Scholar]
- 18.Djerassi C., Silva C.J. Sponge sterols: origin and biosynthesis. Acc Chem Res. 1991;24:371–378. [Google Scholar]
- 19.Silva C.J., Djerassi C. Biosynthetic studies of marine lipids 36: the origin of common sterol side chains in eleven sponges using [3-3H]-squalene. Comp Biochem Physiol B Biochem Mol Biol. 1992;101:255–268. doi: 10.1016/0305-0491(92)90188-w. [DOI] [PubMed] [Google Scholar]
- 20.Giner J.-L., Djerassi C. Evidence for a protonated cyclopropyl intermediate in the biosynthesis of 24-propylidenecholesterol. J Am Chem Soc. 1991;113:1386–1393. [Google Scholar]
- 21.Lang R.W., Djerassi C., Strong P.D., Swenson D.C., Duax W.L. Synthesis and absolute configuration of new trichloro steroids with cyclopropane-containing side chains. Helv Chim Acta. 1981;64:2853–2859. [Google Scholar]
- 22.Rohmer M., Kokke W.C.M.C., Fenical W., Djerassi C. Isolation of two new C30 sterols, (24E)-24-N-propylidenecholesterol and 24ξ-N-propylcholesterol, from a cultured marine chrysophyte. Steroids. 1980;35:219–231. doi: 10.1016/0039-128x(80)90104-x. [DOI] [PubMed] [Google Scholar]
- 23.Khalil M.W., Djerassi C., Sica D. Minor and trace sterols in marine invertebrates. XVII. (24R)-24,26-Dimethylcholesta-5,26-dien-3β-ol, a new sterol from the sponge Petrosia ficiformis. Steroids. 1980;35:707–719. doi: 10.1016/0039-128x(80)90095-1. [DOI] [PubMed] [Google Scholar]
- 24.Rubinstein I., Goad L.J., Clague A.D.H., Mulheirn L.J. The 220 MHz NMR Spectrum of phytosterols. Phytochemistry. 1976;15:195–200. [Google Scholar]
- 25.Kobayashi M., Mitsuhashi H. Isolation and structure of occelasterol, a new 27-norergostane-type sterol from an Annelida, Pseudopotamilla occelata. Steroids. 1974;24:399–410. doi: 10.1016/0039-128x(74)90037-3. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi T., Kadota S., Shima T. Effective separation and C-24 stereochemical assignment of epimeric 24-isopropenylcholesterols. Tetrahedron Lett. 1985;26:3817–3820. [Google Scholar]
- 27.Withers N.W., Kokke W.C.M.C., Fenical W., Djerassi C. Sterol patterns of cultured zooxanthellae isolated from marine invertebrates: synthesis of gorgosterol and 23-desmethylgorgosterol by aposymbiotic algae. Proc Natl Acad Sci USA. 1982;79:3764–3768. doi: 10.1073/pnas.79.12.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]


