Abstract
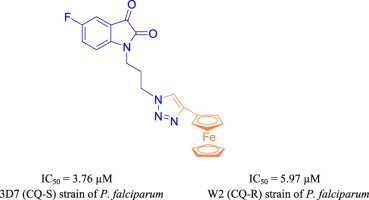
1H-1,2,3-triazole tethered isatin-ferrocene conjugates were synthesized and evaluated for their antiplasmodial activities against chloroquine-susceptible (3D7) and chloroquine-resistant (W2) strains of Plasmodium falciparum. The conjugates 5f and 5h with an optimum combination of electron-withdrawing halogen substituent at C-5 position of isatin ring and a propyl chain, introduced as linker, proved to be most potent and non-cytotoxic among the series with IC50 values of 3.76 and 4.58 μM against 3D7 and W2 strains, respectively.
Keywords: Isatin-ferrocene conjugates, Click chemistry, Structure activity relationship, Antimalarial evaluation, Cytotoxicity
Graphical abstract
Synthesis and antimalarial evaluation of Isatin-ferrocene conjugates.
Highlights
-
•
Synthesis of Isatin-ferrocene conjugates.
-
•
Antiplasmodial evaluation against 3D7 and W2 stains of P. falciparum.
-
•
The most active and non-cytotoxic conjugates exhibited IC50 values of 3.76 and 4.58 μM.
1. Introduction
Malaria is one of the most dangerous infectious diseases transmitted through the bite of infected female Anopheles mosquito [1]. Five species of Plasmodium viz. Plasmodium falciparum, Plasmodium ovale, Plasmodium malariae, Plasmodium vivax, and Plasmodium knowlesi are responsible for the spread of malaria, out of which P. falciparum is considered as the most virulent form [1]. According to World Health Organization (WHO) factsheet 2013, out of 3.4 billion people at malarial risk, 1.2 billion are at high risk with more than one case per 1000 people, especially in tropical areas with transmission over 97 countries [2]. In 2012, more than 207 million cases were reported globally with an estimated 6,27,000 deaths; 90% of them occurred in sub-Saharan Africa in which 77% were children under the age of five [3]. Since 2006, artemisinin-based combination therapies (ACTs) have been recommended for the treatment of malaria, however, the development of resistance in the recent times to ACT in many parts of Southeast Asian regions is of great concern [4], [5].
Isatin (1H-indole-2,3-dione) is a versatile heterocyclic scaffold with vast possibility of chemical modifications at C-3, C-5 and at N-1 position [6]. The endogenous molecule displays a wide range of biological and pharmacological activities such as anti-tumor [7], [8], [9], anti-HIV [10], antiviral [11], anti-angiogenic [12], antifungal [13], [14], anti-Parkinson’s disease therapeutic [15], anticonvulsants [16], and effective SARS coronavirus 3CL protease inhibitor [17] along with excellent tolerance in humans. Recently, Chibale and co-workers have disclosed the synthesis and antimalarial activity of a series of thiolactone–isatin conjugates and their tetracyclic by-products [18]. One of the most potent conjugate displayed an IC50 value of 6.92 μM against chloroquine resistant (CQ-R) W2 strain of P. falciparum. Further, Raval et al. described the synthesis of tetrahydropyrimidine–isatin hybrids and their in vitro activity against 3D7 strain of P. falciparum [19]. The most potent hybrid exhibited minimum inhibitory concentration (MIC) of 0.035 μg/mL which was better than the standard CQ (MIC = 0.125 μg/mL).
Last few years have witnessed a close association between the classical organometallic chemistry to medicine, biology, and molecular biotechnology [20]. The preparation of stable metal complexes with predictable structures, ability to tune ligand affinities along with efficient biological targeting are the major advantages associated with the synthesis of organometallics (c), [21], (a), (b). Among all the metals used, ferrocene (Fc) has played a distinctive role in the contemporary medicine because of its unique features including non-toxicity and stability under physiological conditions [22]. The replacement of the organic functionality with Fc has now been recognized as a useful strategy for the development of new and effective drugs viz. anti-androgen nilutamide and ferrocenyl analogs of commercial anti-estrogen tamoxifen displayed higher cytotoxicity against breast and prostate cancer cells respectively in comparison to the reference drugs (c), [23], (a), (b). Indeed, the most symbolic example of contribution of ferrocene with enhanced biological activity into a drug molecule is that of Ferroquine (FQ); derived from chloroquine (CQ) with ferrocene appended side chain which is 22 times more active than CQ against CQ-R strain of P. falciparum (a), (b), [24].
1H-1,2,3-triazoles are privileged class of five membered nitrogen containing heterocyclic systems which attracted the attention of both synthetic and medicinal chemists due to their synthetic applications and biological potential (b), (c), (d), [25], (a). The systems are highly stable under the basic, acidic, reductive and oxidative conditions because of high aromatization. Moreover, high dipole moment, rigidity, stability and capability to form hydrogen bonding under in vivo conditions are their favorable characteristics in the binding with biological targets.
Previous reports from our group has described the synthesis and in vitro antimalarial potential of 1H-1,2,3-triazole tethered mono- and bis-ferrocenylchalcone-β-lactam conjugates [26] as well as 7-chloroquinoline-isatin conjugates [27] against chloroquine-susceptible (CQ-S) 3D7 and chloroquine-resistant (CQ-R) W2 strains of P. falciparum. In continuation with our interest, the present report describes the synthesis and in vitro antimalarial evaluation of 1H-1,2,3-triazole tethered isatin-ferrocene conjugates as depicted in Fig. 1 .
Fig. 1.

Design of target hybrids.
2. Result and discussion
2.1. Synthetic chemistry
C-5 substituted N-alkylazido-isatins 3 were prepared following our previously reported protocol [28] involving an initial base-promoted alkylation of isatins 1 with dibromoalkanes at 60 °C in DMF to yield the corresponding C-5 substituted N-alkylbromo-isatins 2. The subsequent treatment of 2 with sodium azide at 60 °C resulted in the formation of the desired precursors 3 as depicted in Scheme 1 . The precursors' 3 and ethnylferrocene 4 were subsequently utilized in the copper promoted azide-alkyne cycloaddition reaction to yield the desired 1H-1,2,3-triazole tethered isatin-ferrocene conjugates 5 [29].
Scheme 1.
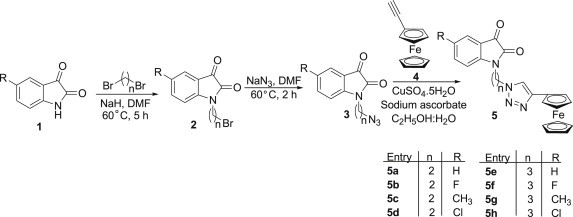
Synthesis of isatin-ferrocene based conjugates.
The structure assigned to the hybrids 5 was confirmed on the basis of spectral data and analytical evidences. Compound 5f, for example, showed a molecular ion peak at m/z 458.0833 [M]+ while its 1H NMR spectrum exhibited a singlet at δ 4.08 corresponding to 5H (cyclopentadiene ring of ferrocene) along with singlets at δ 4.31 (2H) and δ 4.72 (2H) due to the ferrocene ring protons. The presence of a characteristic singlet at δ 7.63 corresponding to the triazole ring proton ascertained the assigned structure which was further corroborated with the number of carbon atoms in the 13C NMR spectrum.
2.2. Antimalarial evaluation
The synthesized conjugates were evaluated for their antimalarial potential against chloroquine-susceptible 3D7 and chloroquine-resistant W2 strains of P. falciparum and the corresponding inhibitory concentration 50% (IC50s) and 95% confident interval (95%CI) values are enlisted in Table 1 . The synthetic precursor viz. N-alkylbromo-isatins were previously evaluated for their antimalarial potential against chloroquine-resistant W2 strains of P. falciparum and have displayed IC50 values >20 μM [18]. As evident from Table 1, the synthesized conjugates were not as active as standard drug chloroquine (CQ) but displayed considerable activity against both the strains. The conjugates 5a–d with an ethyl chain introduced as linker failed to inhibit the growth of 3D7 even at the highest concentration tested; exception being 5a (R = H) displaying an IC50 value of 24.43 μM. Increasing the chain length from ethyl to propyl seemed to have considerable influence on the activity profiles as the conjugates 5e–h displayed IC50s in the range 3.76–16.20 μM. The most potent conjugate 5f with a fluoro substituent at C-5 position and a propyl linker displayed an IC50 value of 3.76 μM against 3D7 strain. Similar comparison of structure activity relationship of the synthesized conjugates against W2 strain revealed the ethyl linked conjugates (5a–d) to be inactive even at highest tested concentration. The replacement of the ethyl chain with propyl chain again improved the activity profiles with little influence of the nature of substituent introduced at C-5 position of the isatin ring. The conjugate 5n with electron withdrawing Cl-substituent at C-5 position and n = 3 proved to be the most potent with an IC50 of 4.58 μM.
Table 1.
Antiplasmodial activity against the 3D7 and W2 strains of P. falciparum.
| Compound | R | N | Strains |
|||
|---|---|---|---|---|---|---|
| 3D7 |
W2 |
|||||
| IC50 (μM) | 95% CI | IC50 (μM) | 95% CI | |||
| 5a | H | 2 | 24.43 | 20.70–29.90 | 26.50 | 21.10–33.40 |
| 5b | F | 2 | >100 | _ | >100 | _ |
| 5c | CH3 | 2 | >100 | _ | >100 | _ |
| 5d | Cl | 2 | >100 | _ | >100 | _ |
| 5e | H | 3 | 6.35 | 4.78–8.44 | 7.41 | 6.13–8.96 |
| 5f | F | 3 | 3.76 | 2.75–7.13 | 5.97 | 5.53–6.45 |
| 5g | CH3 | 3 | 16.20 | 13.70–19.20 | 9.68 | 9.05–10.36 |
| 5h | Cl | 3 | 8.49 | 7.83–9.21 | 4.58 | 3.86–5.44 |
| CQ | 0.021 | 0.018–0.025 | 0.49 | 0.37–0.63 | ||
IC50 values are means of 5 experiments. 95%CI: 95% confident interval.
The improvement in activity profiles of conjugates 5e–h, over precursors' viz. N-alkylbromo-isatins against both CQ-S and CQ-R strains of P. falciparum clearly established the importance of amalgamating a ferrocene nucleus among the well established isatin family via triazole-linkers with reputed physicochemical profiles. Although the in vitro antimalarial activities of the synthesized isatin-ferrocene conjugates is lower than ferroquine in laboratory strains (2.1–13.4 nM) [30], the conjugates are equipotent as that of ferrocene-ciprofloxacin conjugates [31], [32] and ferrocenyl-chalcones such as 1-ferrocenyl-3-(4-nitrophenyl)prop-2-en-1-one [33], [34] or mono- and bis-ferrocenylchalcones-β-lactam conjugates [26].
Cytotoxicity of two most potent conjugates viz. 5f and 5h was also determined against mammalian HeLa cells and is listed in Table 2 . As evident, the conjugates were non-cytotoxic against mammalian cells and therefore had the selectivity for inhibition of P. falciparum.
Table 2.
Cytotoxicity and selective index of conjugates 5f and 5h.
| Strains | |||||
|---|---|---|---|---|---|
| Compound | Cytotoxicity |
3D7 |
W2 |
||
| (IC50 μM) | (IC50 μM) | SIa | (IC50 μM) | SIa | |
| 5f | 82.14 | 3.76 | 21.85 | 5.97 | 13.76 |
| 5h | 86.68 | 8.49 | 10.21 | 4.58 | 18.93 |
Selectivity index.
3. Conclusion
In conclusion, a series of isatin-ferrocene conjugates have been synthesized via Cu-promoted azide-alkyne cyloaddition reactions and evaluated for their antimalarial activities against the 3D7 and W2 strains of P. falciparum. The improvement in antimalarial efficacy of N-alkylbromo-isatins with the introduction of ferrocene nucleus clearly validated the potential of introducing organometallics for improving biological efficacies in present day drug discovery paradigm. The non-cytotoxic conjugates 5f and 5h proved to be the most potent among the series with IC50 values of 3.76 and 4.58 μM against 3D7 and W2 strain of P. falciparum respectively.
4. Experimental section
Ethynylferrocene was procured from Sigma–Aldrich. Melting points were determined by open capillary using Veego Precision Digital Melting Point apparatus (MP-D) and are uncorrected. 1H NMR spectra were recorded in deuterochloroform and dimethylsulfoxide-d6 with Jeol 300 (300 MHz) spectrometers using TMS as an internal standard. Chemical shift values are expressed as parts per million downfield from TMS and J values are in hertz. Splitting patterns are indicated as s: singlet, d: doublet, t: triplet, m: multiplet, dd: double doublet, ddd: doublet of a doublet of a doublet, and br: broad peak. 13C NMR spectra were recorded on Jeol 300 (75 MHz) spectrometers in deuterochloroform and dimethylsulfoxide-d6 using TMS as internal standard. Elemental analyses were performed on Heraus CHN–O-Rapid Elemental Analyzer. Mass spectra were recorded on a BRUCKER high resolution mass spectrometer (micrOTOF-QII). Column chromatography was performed using silica gel (60–120 mesh).
4.1. Procedure for the preparation of isatin ferrocene hybrids (5a–h)
Copper sulfate (0.05 mmol) and sodium ascorbate (0.13 mmol) was added to a stirred solution of ethynyl-ferrocene 4 (1 mmol) and N-alkylated azido-isatins 3 (1 mmol) in ethanol-water (9:1) mixture. The reaction mixture was allowed to stir at room temperature for 7–10 h. After completion of reaction as evidenced by TLC, water (20 mL) was added and the reaction mixture was extracted twice with dichloromethane (2 × 30 mL). The combined organic layers were dried over anhydrous sodium sulfate, concentrated under reduced pressure and purified via column chromatography using 40:60 (EtOAc: hexane) mixture.
4.2. Methods for assessment of antimalarial activity of test compounds
The two strains viz. 3D7, the CQ-susceptible strain (isolated in West Africa; obtained from MR4, VA, USA), and W2 (isolated in Indochina; obtained from MR4, VA, USA), the CQ-resistant strain, were maintained in culture in RPMI 1640 (Invitrogen, Paisley, United Kingdom), supplemented with 10% human serum (Abcys S.A. Paris, France) and buffered with 25 mM HEPES and 25 mM NaHCO3. Parasites were grown in A-positive human blood (Etablissement Français du Sang, Marseille, France) under controlled atmospheric conditions that consisted of 10% O2, 5% CO2 and 85% N2 at 37 °C with a humidity of 95%.
The two strains were synchronized twice with sorbitol before use [35], and clonality was verified every 15 days through PCR genotyping of the polymorphic genetic markers msp1 and msp2 and microsatellite loci [36], [37]; additionally, clonality was verified each year by an independent laboratory from the Worldwide Anti-malarial Resistance Network (WWARN).
Chloroquine diphosphate (CQ) was purchased from Sigma (Saint Louis, MO). CQ was resuspended in water in concentrations ranging between 5 and 3200 nM. The synthetic compounds were resuspended in DMSO and then diluted in RPMI-DMSO (99v/1v) to obtain final concentrations ranging from 1 nM to 100 μM.
For in vitro isotopic microtests, 25 μL/well of antimalarial drug and 200 μL/well of the parasitized red blood cell suspension (final parasitemia, 0.5%; final hematocrit, 1.5%) were distributed into 96 well plates. Parasite growth was assessed by adding 1 μCi of tritiated hypoxanthine with a specific activity of 14.1 Ci/mmol (Perkin–Elmer, Courtaboeuf, France) to each well at time zero. The plates were then incubated for 48 h in controlled atmospheric conditions. Immediately after incubation, the plates were frozen and thawed to lyse erythrocytes. The contents of each well were collected on standard filter microplates (Unifilter GF/B; Perkin–Elmer) and washed using a cell harvester (Filter-Mate Cell Harvester; Perkin–Elmer). Filter microplates were dried, and 25 μL of scintillation cocktail (Microscint O; Perkin–Elmer) was placed in each well. Radioactivity incorporated by the parasites was measured with a scintillation counter (Top Count; Perkin–Elmer).
The IC50, the drug concentration able to inhibit 50% of parasite growth, was assessed by identifying the drug concentration corresponding to 50% of the uptake of tritiated hypoxanthine by the parasite in the drug-free control wells. The IC50 value was determined by non-linear regression analysis of log-based dose–response curves (Riasmart™, Packard, Meriden, USA). IC50 are expressed as geometric means of 5 experiments.
4.3. In vitro analysis of cytotoxicity on HeLa cells
HeLa cells were cultured in 60 × 15 mm tissue culture dishes containing 5 mL of Dulbecco's Modified Eagle's Medium (DMEM) supplemented with penicillin and streptomycin. Compounds were dissolved in DMSO to 100 μM concentrations. Once cell cultures reached 70% confluency, 5 μL of compound was added to the DMEM in the tissue culture dish for a final concentration of 100 μM. Cells were incubated for 24 h in a 37 °C CO2 incubator. After 24 h incubation, the media was removed from the HeLa cells and the cells were then washed with 5 mL of 1× PBS. The cells were then cleaved off of the bottom of the plate via 5-min incubation with 0.5 mL of 0.25% trypsin. Cells were re-suspended in 1 mL of 1× PBS and transferred to a micro-centrifuge tube. 100 μL of trypan blue solution was added to the re-suspended cells and allowed to incubate at room temperature for approximately 10 min. Viable and dead cells were visualized and counted with a hemacytometer. IC50 values were determined using GraphPad PRISM.
Acknowledgments
Financial assistance from Department of Science and Technology, New Delhi under Innovation in Science Pursuit for Inspired Research (INSPIRE) Fellowship with code IF-10482 (KK) is gratefully acknowledged.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejmech.2014.10.024.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.M. Njoroge, N. M. Njuguna, P. Mutai, D. S. B. Ongarora, P. W. Smith, K. Chibale, (2014) http://dx.doi.org/10.1021/cr500098f. [DOI] [PubMed]
- 2.Factsheet on the World Malaria Report. 2013. http://www.who.int/malaria/media/world_malaria_report_2013/en/ [Google Scholar]
- 3.World Health Organization . 2013. World Malaria Report. Geneva. [Google Scholar]
- 4.Wongsrichanalai C., Meshnick S.R. Emerg. Infect. Dis. 2008;14:716. doi: 10.3201/eid1405.071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondorp A.M., Nosten F., Yi P., Das D., Phyo A.P., Tarning J., Lwin K.M., Ariey F., Hanpithakpong W., Lee S.J., Ringwald P., Silamut K., Imwong M., Chotivanich K., Lim P., Herdman T., An S.S., Yeung S., Singhasivanon P., Day N.P., Lindegardh N., Socheat D., White N.J.N. Engl. J. Med. 2009;361:455. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newkome G.R., Pandler W.W. Wiley; New York: 1982. Contemporary Heterocyclic Chemistry. [Google Scholar]
- 7.Tripathy R., Reiboldt A., Messina P.A., Iqbal M., Singh J., Bacon E.R., Angeles T.S., Yang S.X., Albom M.S., Robinson C., Chang H., Ruggeri B.A., Mallamo J.P. Bioorg. Med. Chem. Lett. 2006;16:2158. doi: 10.1016/j.bmcl.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 8.Cane A., Tournaire M.C., Barritault D., Crumeyrolle-Arias M. Biochem.. Biophys. Res. Commun. 2000;276:379. doi: 10.1006/bbrc.2000.3477. [DOI] [PubMed] [Google Scholar]
- 9.Silveira V.C., Luz J.S., Oliveira C.C., Graziani I., Ciriolo M.R., Costa-Ferreira A.M. J. Inorg. Biochem. 2008;102:1090. doi: 10.1016/j.jinorgbio.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 10.Ratan B.T., Anand B., Yogeeswari P., Sriram D. Bioorg. Med. Chem. Lett. 2005;15:4451. doi: 10.1016/j.bmcl.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 11.Jiang T., Kuhen K.L., Wolff K., Yin H., Bieza K., Caldwell J., Bursulaya B., Tuntland T., Zhang K., Karanewsky D., He Y. Bioorg. Med. Chem. Lett. 2006;16:2109. doi: 10.1016/j.bmcl.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 12.Maskell L., Blanche E.A., Colucci M.A., Whatmore J.L., Moody C.J. Bioorg. Med. Chem. Lett. 2007;17:1575. doi: 10.1016/j.bmcl.2006.12.108. [DOI] [PubMed] [Google Scholar]
- 13.Amal R.A., Raghunathan R., Sridevikumaria M.R., Raman N. Bioorg. Med. Chem. 2003;11:407. doi: 10.1016/s0968-0896(02)00439-x. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Arguelles M.C., Mosquera-Vazaquez S., Touron-Touceda P., Sanmartin-Matalobos J., Garcia-Deibe A.M., Belicchi-Ferraris M., Pelosi G., Pelizzi C., Zani F. J. Inorg. Biochem.. 2007;101:138. doi: 10.1016/j.jinorgbio.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Igosheva N., Lorz C., O'Conner E., Glover V., Mehmet H. Neurochem.. Int. 2005;47:216. doi: 10.1016/j.neuint.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Verma M., Nath P.S., Nand S.K., Stables J.P. Acta Pharm. 2004;54:49. [PubMed] [Google Scholar]
- 17.Chen L.R., Wang Y.C., Lin Y.W., Chou S.Y., Chen S.F., Liu L.T., Wu Y.T., Kuo C.J., Chen T.S.S., Juang S.H. Bioorg. Med. Chem. Lett. 2005;15:3058. doi: 10.1016/j.bmcl.2005.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hans R.H., Wiid I.J.F., van Helden P.D., Wan B., Franzblau S.G., Gut J., Rosenthal P.J., Chibale K. Bioorg. Med. Chem. Lett. 2011;21:2055. doi: 10.1016/j.bmcl.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Akhaja T.N., Raval J.P. Chin. Chem. Lett. 2012;23:785. [Google Scholar]
- 20.Gasser G., Metzler-Nolte N. Curr. Opin. Chem. Biol. 2012;16:84. doi: 10.1016/j.cbpa.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 21.(a) Biot C., Castro W., Botte C.Y., Navarro M. Dalton Trans. 2012;41:6335. doi: 10.1039/c2dt12247b. [DOI] [PubMed] [Google Scholar]; (b) Hartinger C.G., Dyson P.J. Chem. Soc. Rev. 2009;38:391. doi: 10.1039/b707077m. [DOI] [PubMed] [Google Scholar]; (c) Jaouen G., editor. Bioorganometallics: Biomolecules, Labeling, Medicine. Wiley- VCH Verlag GmbH & Co. KGaA; Weinheim: 2006. [Google Scholar]
- 22.Staveren D.R., Metzler-Nolte N. Chem. Rev. 2004;104:5931. doi: 10.1021/cr0101510. [DOI] [PubMed] [Google Scholar]
- 23.(a) Top S., Vessières A., Leclerq G., Quivy J., Tang J., Vaissermann J., Huché M., Jaouen G. Chem. Eur. J. 2003;9:5223. doi: 10.1002/chem.200305024. [DOI] [PubMed] [Google Scholar]; (b) Hillard E., Vessières A., Thouin L., Jaouen G., Amatore C. Angew. Chem. Int. Ed. 2006;45:285. doi: 10.1002/anie.200502925. [DOI] [PubMed] [Google Scholar]; (c) Payen O., Top S., Vessières A., Brulé E., Plamont M.A., McGlinchey M.J., Müller- Bunz H., Jaouen G. J. Med. Chem. 2008;51:1791. doi: 10.1021/jm701264d. [DOI] [PubMed] [Google Scholar]
- 24.(a) Daher W., Biot C., Fandeur T., Jouin H., Pelinski L., Viscogliosi E., Fraisse L., Pradines B., Brocard J., Khalife J., Dive D. Malar. J. 2006;5:11. doi: 10.1186/1475-2875-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dubar F., Khalife J., Brocard J., Dive D., Biot C. Molecules. 2008;13:2900. doi: 10.3390/molecules13112900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(a) Hou J., Liu X., Shen J., Zhao G., Wang P.G. Expert Opin. Drug Discov. 2012;7:489. doi: 10.1517/17460441.2012.682725. http://informahealthcare.com/action/doSearch?action=runSearch&type=advanced&result=true&prevSearch=%2Bauthorsfield%3A%28Hou%2C+Jingli%29 http://informahealthcare.com/action/doSearch?action=runSearch&type=advanced&result=true&prevSearch=%2Bauthorsfield%3A%28Shen%2C+Jie%29 [DOI] [PubMed] [Google Scholar]; (b) Beal D.M., Jones L.H. Angew. Chem. Int. Ed. 2012;51:6320. doi: 10.1002/anie.201200002. [DOI] [PubMed] [Google Scholar]; (c) He X.P., Xie J., Tang Y., Li J., Chen G.R. Curr. Med. Chem. 2012;19:2399. doi: 10.2174/092986712800269245. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kumar K., Sagar S., Esau L., Kaur M., Kumar V. Eur. J. Med. Chem. 2012;58:153. doi: 10.1016/j.ejmech.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Kumar K., Pradines B., Madamet M., Amalvict R., Kumar V. Eur. J. Med. Chem. 2014;86:113. doi: 10.1016/j.ejmech.2014.08.053. [DOI] [PubMed] [Google Scholar]
- 27.Raj R., Biot C., Kremer S.C., Kremer L., Guerardel Y., Gut J., Rosenthal P.J., Forge D., Kumar V. Chem. Biol. Drug Des. 2014;83:622. doi: 10.1111/cbdd.12273. [DOI] [PubMed] [Google Scholar]
- 28.Singh P., Sharma P., Anand A., Bedi P.M.S., Kaur T., Saxena A.K., Kumar V. Eur. J. Med. Chem. 2012;55:455. doi: 10.1016/j.ejmech.2012.06.057. [DOI] [PubMed] [Google Scholar]
- 29.Thirumurugan P., Matosiuk D., Jozwiak K. Chem. Rev. 2013;113:4905. doi: 10.1021/cr200409f. [DOI] [PubMed] [Google Scholar]
- 30.Henry M., Briolant S., Fontaine A., Mosnier J., Baret E., Amalvict R., Fusai T., Fraisse L., Rogier C., Pradines B. Antimicrob. Agents Chemother. 2008;52:2755. doi: 10.1128/AAC.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubar F., Anquetin G., Pradines B., Dive D., Khalife J., Biot C. J. Med. Chem. 2009;52:7954. doi: 10.1021/jm901357n. [DOI] [PubMed] [Google Scholar]
- 32.Dubar F., Wintjens R., Martins-Duarte E.S., Vommaro R.C., Souza W., Dive D., Pierrot C., Pradines B., Wohlkonig A., Khalife J., Biot C. MedChemCom. 2011;2:430. [Google Scholar]
- 33.Wu X., Wilairat P., Go M.L. Bioorg. Med. Chem. Lett. 2002;12:2299. doi: 10.1016/s0960-894x(02)00430-4. [DOI] [PubMed] [Google Scholar]
- 34.Wu X., Tiekink E.R., Kostetski I., Kochorginsky N., Tan A.L., Khoo S.B., Wilairat P., Go M.L. Eur. J. Pharm. Sci. 2006;27:175. doi: 10.1016/j.ejps.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Lambros C., Vanderberg J.P. J. Parasitol. 1979;65:418. [PubMed] [Google Scholar]
- 36.Bogreau H., Renaud F., Bouchiba H., Durand P., Assi S.B., Henry M.C., Garnotel E., Pradines B., Fusai T., Wade B., Adehossi E., Parola P., Kamil M.O., Puijalon O., Rogier C. Am. J. Trop. Med. Hyg. 2006;74:953. [PubMed] [Google Scholar]
- 37.Henry M., Diallo I., Bordes J., Ka S., Pradines B., Diatta B., M'Baye P.S., Sane M., Thiam M., Gueye P.M., Wade B., Touze J.E., Debonne J.M., Rogier C., Fusai T. Am. J. Trop. Med. Hyg. 2006;75:146. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


