Abstract
Baculovirus infects insects in nature and is non-pathogenic to humans, but can transduce a broad range of mammalian and avian cells. Thanks to the biosafety, large cloning capacity, low cytotoxicity and non-replication nature in the transduced cells as well as the ease of manipulation and production, baculovirus has gained explosive popularity as a gene delivery vector for a wide variety of applications. This article extensively reviews the recent understandings of the molecular mechanisms pertinent to baculovirus entry and cellular responses, and covers the latest advances in the vector improvements and applications, with special emphasis on antiviral therapy, cancer therapy, regenerative medicine and vaccine.
Keywords: Baculovirus, Vaccine, Immune responses, Tissue engineering, Cancer therapy, Antiviral therapy
1. Introduction
Baculoviruses are a diverse group of DNA viruses capable of infecting more than 600 insects, among which Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is the best characterized and most extensively employed, thus baculovirus discussed in this article refers to AcMNPV unless otherwise noted. AcMNPV contains a circular dsDNA genome of ≈ 134 kb and replicates in a bi-phasic fashion. The viral proteins polyhedrin and p10 are expressed abundantly in infected cells and are dispensable for virus replication, thus recombinant baculovirus can be constructed by placing the foreign gene under the control of polyhedrin or p10 promoter, and used to infect insect cells for foreign gene expression. Such baculovirus–insect cell expression system has been exhaustively utilized for the production of numerous recombinant proteins (Kost and Condreay, 1999) and commercial vaccine products such as Cervarix® and Provenge® (Hitchman et al., 2009, Lin et al., 2011).
In addition to insect cells, baculovirus is able to transduce animal cells of human, rodent, rabbit, porcine, bovine, fish and avian origins (Hu, 2005, Hu, 2006) as well as relatively primitive cells including embryonic stem cells, adult stem cells (Table 1 ) and induced pluripotent stem cells (Fig. 1 ). Within the baculovirus-transduced cells, the transgene can be expressed as long as it is driven by an appropriate promoter (e.g. cytomegalovirus immediate-early (CMV) or hybrid CAG promoter consisting of the CMV early enhancer and chicken β-actin promoter). Baculovirus cloning capacity is as large as 38 kb (Cheshenko et al., 2001), thus allowing for the insertion of multiple genes and regulatory elements (Kost et al., 2005, Kost and Condreay, 2002). Baculovirus neither replicates inside the transduced cells nor integrates its DNA into host chromosomes in the absence of selective pressure (Chen et al., 2011a, Merrihew et al., 2001), hence easing the safety concerns. Humans do not possess pre-existing antibody and T-cells specifically against baculovirus (Strauss et al., 2007), therefore baculovirus may circumvent the pre-existing immunity problem faced by other viral vectors. Finally, recombinant baculovirus can be readily constructed and propagated to high titers in biosafety level 1 facilities by infecting its natural host insect cells (Hu, 2008). These attributes have fueled growing interests to explore baculovirus for a wide variety of applications, ranging from protein production (Jardin et al., 2008, Liu et al., 2010), virus production (Huang et al., 2007, Lesch et al., 2008, Lesch et al., 2011, Lucifora et al., 2008, McCormick et al., 2002, Nakowitsch et al., 2006, Zheng et al., 2010), virus-like particle production (Chen et al., 2005, Matsuo et al., 2006, Wang et al., 2005), eucaryotic protein display (Ernst et al., 2006, Grabherr and Ernst, 2010), vaccine development (Hu et al., 2008, Madhan et al., 2010, Tani et al., 2008), cancer therapy (Wang and Balasundaram, 2010), cell-based assay development (Condreay et al., 2006, Condreay and Kost, 2007, Kost et al., 2010) to tissue engineering (Lin et al., 2010b).
Table 1.
Selected types of cells permissive to baculovirus transduction.
Fig. 1.
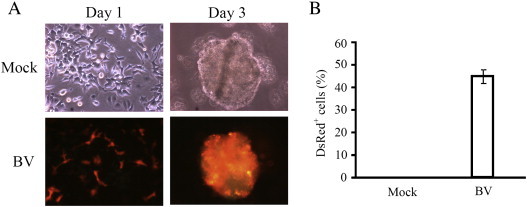
Baculovirus transduction of mouse induced pluripotent stem cells (iPSCs). (A) Microscopic observations. (B) Flow cytometry analyses. The cells were mock-transduced (Mock group) or transduced with a baculovirus expressing DsRed (red fluorescent protein) under the elongation factor-1α promoter at a multiplicity of infection (MOI) of 100 (BV group). The cells were observed under the phase contrast microscope or fluorescence microscope at 1 and 3 days post-transduction or detached for flow cytometry at 1 day post-transduction. The microscopic observations illustrated that baculovirus efficiently transduced iPSCs and expressed DsRed, without impairing the formation of embryonic body (EB) at day 3. The baculovirus transduction efficiency reached ≈ 45% as depicted by the flow cytometry data.
This article will focus on recent understandings of intracellular events after baculovirus transduction and latest applications of baculovirus for antiviral therapy, cancer therapy, tissue regeneration and vaccine development.
2. What happens after baculovirus transduction?
2.1. Baculovirus entry and intracellular transport
Baculovirus entry into mammalian cells was initially suggested to depend on electrostatic interactions (Duisit et al., 1999), heparin sulfate (Duisit et al., 1999) and phospholipids (Tani et al., 2001), but the exact cell surface molecules for baculovirus docking remained unknown. It was also proposed that clathrin-mediated endocytosis (Long et al., 2006, Matilainen et al., 2005) and macropinocytosis (Matilainen et al., 2005) play roles in baculovirus entry. Contradictorily, a recent study (Laakkonen et al., 2009) discovered that (1) baculovirus entered HEK293 and HepG2 cells along fluid-phase markers from the raft areas into vesicles devoid of clathrin; (2) macropinocytosis-related regulators (e.g. EIPA, Pak1, Rab34 and Rac1) imparted no significant effects on virus transduction and (3) the internalization and nuclear uptake were affected by the regulators of clathrin-independent entry. These data unveiled a baculovirus entry pathway independent of clathrin-mediated endocytosis and macropinocytosis and suggested that phagocytosis might play a role (Laakkonen et al., 2009), which echoed the observations reported previously (Abe et al., 2005). Moreover, other recent studies reported that baculovirus transduction related to direct fusion pathway induced by a short pH trigger (Dong et al., 2010, Paul and Prakash, 2010). These conflicting data underlined the need for more in-depth studies to elucidate the underlying mechanism and might suggest that baculovirus entry pathway varies with cell types. Nevertheless, one consensus is that baculovirus envelope protein gp64 is pivotal for entry because blocking gp64 can abrogate the baculovirus ability to transduce mammalian cells (Abe et al., 2005, Niu et al., 2008) and activate dendritic cells (DCs) (Schutz et al., 2006).
Once inside the cells, baculovirus is transported to the endosome, followed by endosomal escape via acid-triggered gp64 fusion (Kukkonen et al., 2003) and subsequent nuclear transport (Laakkonen et al., 2008, van Loo et al., 2001) with the aid of actin filament (Matilainen et al., 2005, Salminen et al., 2005). A major component of type III intermediate filaments, vimentin, also participates in intracellular trafficking (Mahonen et al., 2010). Vimentin is reorganized in the optimized culture medium and is linked to enhanced nuclear entry of baculovirus, underscoring the importance of culture medium in the cytoskeleton network assembly and in baculoviral gene delivery.
2.2. Expression of baculoviral genes in permissive cells
Baculovirus encodes ≈ 155 genes and a number of viral genes (e.g. orf149, ie0, p35 and gp64) are expressed in transduced mammalian cells (Fujita et al., 2006, Kitajima et al., 2006). Among the baculoviral genes, ie1 is expressed early in insect cells and transactivates downstream gene expression. Forced expression of ie1 by the minimal CMV promoter in Vero E6 cells also markedly activates gp64 and pe38, and upregulates ie2, he65, pcna, orf16, orf17 and orf25 (Liu et al., 2007). The critical role of ie1 for transactivating downstream genes was further unraveled in a recent study (Efrose et al., 2010), which showed that baculovirus deficient in ie1 gene mitigates residual baculoviral gene expression 10- to 100-fold (when compared with wild-type baculovirus) in transduced mammalian cells, thus enhancing safety features to baculovirus-based gene therapy.
In contrast to ie1, ie2 overexpression driven by the CMV promoter only upregulates 2 baculoviral genes (pe38 and orf17), but baculovirus-mediated co-expression of ie1 and ie2 acts in concert to upregulate 59 out of 155 baculovirus genes in mammalian cells (Liu et al., 2007). Strikingly, IE2 is a strong transactivator of CMV promoter in both Vero E6 and U-2OS cells (Liu et al., 2009a). When overexpressed within the baculovirus context, IE2 forms the nuclear foci and develops into large nuclear bodies (NBs) with a hollow center. The IE2 NBs structure contains abundant G-actin, closely associates with RNA polymerase II, promyelocytic leukemia (PML) and small ubiquitin-like modifier-1 (SUMO1) and is the site of active transcription, thereby contributing to the IE2-associated gene stimulation (Liu et al., 2009a). Furthermore, the NBs formation and CMV promoter activation require the N-terminal RING finger and C-terminal coiled-coil domains of IE2 (Liu et al., 2009a). Since CMV promoter is exhaustively used for baculovirus-mediated gene transfer, the transactivating activity of IE2 may be useful for baculovirus-mediated protein production in mammalian cells (Liu et al., 2010).
2.3. Innate immune responses elicited by baculovirus
Due to the complex cascade of events during baculovirus transduction, it is not surprising that baculovirus can alter the cell morphology and trigger cellular responses. For instance, baculovirus transduction of HepG2 cells alters the size of PML NBs, induces remodeling of the host cell chromatin (Laakkonen et al., 2008) and arouses extensive ruffle formation on the cell surface (Laakkonen et al., 2009). Shotgun proteomics also attests that baculovirus-transduced HepG2 cells exhibit a slight induction of proteins related to inflammation, cell survival and chromatin function (Gerner et al., 2010).
The most well-characterized baculovirus-induced cellular response is the innate immune response, as manifested by the induction of such cytokines as interferon α/β (IFN-α/β), interleukin-6 (IL-6), IL-8, IL-1β and tumor necrosis factor-α (TNF-α) (Abe and Matsuura, 2010, Tani et al., 2008). Baculovirus transduction of rat chondrocytes also elicits transient expression of IFN-α/β, which attenuates the transgene expression (Lee et al., 2009). Not only the cytokine secretion, in vitro baculovirus transduction also activates human (Schutz et al., 2006) and mouse (Abe et al., 2005) DCs. Moreover, wild-type baculovirus transduction of mouse bone marrow-derived DCs (BMDCs) upregulates the major histocompatibility complex (MHC) I and II and co-stimulation molecules (e.g. CD40, CD80 and CD86) (Suzuki et al., 2010a). The activated BMDCs can further stimulate natural killer (NK) cells upon co-culture, as evidenced by the IFN-γ production, CD69 upregulation and cell proliferation.
In contrast to these differentiated, specialized cells, stem cells appear to be less sensitive to baculovirus transduction. Upon wild-type baculovirus transduction, human bone marrow-derived mesenchymal stem cells (BMSCs) secret IL-6 and IL-8, but no detectable levels of IFN-γ, IFN-β, TNF-α, TNF-β, IL-1β, IL-2, IL-4, IL-5, IL-10 and IL-12 (Chen et al., 2009b). Human leukocyte antigen I (HLA-I) is slightly upregulated, but the expression of HLA-II and other surface markers are barely disturbed (Bak et al., 2010, Chuang et al., 2009). Neither does baculovirus transduction compromise the immunosuppressive properties of BMSCs (Chuang et al., 2009). Conversely, wild-type baculovirus transduction of mouse induced pluripotent stem cells (iPSCs) elicits only the chemokine IP-10, but not other well-known cytokines (unpublished data). BMSCs and iPSCs are promising cell sources for regenerative medicine. The lack of these cytokine responses reduces the risk of mounting strong immune responses after transplantation of baculovirus-transduced BMSCs and iPSCs.
In vivo, baculovirus administration triggers innate immune responses, activates macrophages (Abe et al., 2005), DCs (Hervas-Stubbs et al., 2007, Schutz et al., 2006) and NK cells (Facciabene et al., 2004, Kitajima et al., 2008). The baculovirus-induced innate immunity gives rise to antitumor activity (Suzuki et al., 2010a) and is sufficient to confer protection against influenza virus in mice (Abe et al., 2003), infectious bronchitis virus in chickens (Niu et al., 2008) and foot-and-mouth-disease virus in mice (Molinari et al., 2010). The innate immunity also confers baculovirus the adjuvant activity to promote humoral and cellular immune responses against co-administered antigens (Hervas-Stubbs et al., 2007). Moreover, intramuscular (i.m.) injection of baculovirus triggers T-cell responses against the vector, but the magnitude of anti-baculovirus T cells response is lower than that of anti-adenovirus response (Hervas-Stubbs et al., 2007).
2.4. Baculovirus recognition and associated signaling pathways
Toll-like receptors (TLRs) are a family of pattern recognition receptors essential for initiating the innate immunity and substantiating the adaptive immunity (Ishii et al., 2008). For instance, TLR3 recognizes virus-derived dsRNA while TLR9 recognizes unmethylated CpG DNA motifs. Upon engagement with cognate ligands, TLRs are activated and recruit adaptor molecules such as myeloid differentiating factor 88 (MyD88) and TIR domain-containing adaptor inducing IFN-β (TRIF) to transduce signals to downstream molecules. Besides, stimulator of interferon genes (STING) is a cytoplasmic sensor that activates IRF3 and IFN-α/β in response to viral dsDNA while retinoic acid-inducible gene 1 (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) are TLR-independent cytoplasmic RNA detectors that induce the IFN-α/β production through IFN promoter-stimulator-1 (IPS-1).
Baculovirus-induced innate immunity has been ascribed to the recognition of CpG motifs in viral DNA and ensuing activation of TLR9/MyD88-dependent pathway (Abe et al., 2005). The detection activates nuclear factor-κB (NF-κB) and leads to subsequent IFN-α/β production. However, IFN-α/β are still produced in peritoneal cells derived from MyD88- or TLR9-deficient mice (Abe et al., 2005). In mouse embryonic fibroblasts (MEFs), baculovirus triggers IFN-β and IFN-inducible chemokines through TLR-independent and IRF3-dependent pathways and endosomal maturation is required for induction (Abe et al., 2009). These data suggest that baculovirus DNA might be recognized by at least two different pathways: TLR9-dependent endosomal recognition and TLR9-independent cytoplasmic recognition. The latter was suggested to be related to STING (Abe and Matsuura, 2010) because baculovirus-induced IFN-α/β production was impaired in STING-deficient MEFs (Ishikawa et al., 2009), yet IFN-α/β stimulation was found to be independent of other cytoplasmic RNA detectors such as RIG-I and MDA5 (Abe et al., 2009). However, a recent study noted that RIG-I and MDA5 mRNA levels were elevated in baculovirus-transduced cells (Wang et al., 2010). Addition of DNA methytransferase inhibitors (DNMTi) prior to transduction retarded such upregulation and enhanced baculovirus-mediated gene expression, suggesting that DNMTi may somehow facilitate the baculovirus evasion from the cellular recognition and thus ameliorate the transgene expression.
Using the cDNA microarray, Wang and coworkers discovered that baculovirus injection into the striatum in the rat brain perturbed the expression of 628 genes, which represented 3.45% of the total gene probes on the microarray (Boulaire et al., 2009). The same study also identified 532 gene probes (1.99%) in human astrocytes and 1219 gene probes (4.32%) in human neuronal cells that were disturbed in response to baculovirus. Despite the disparity between cell types, in all 3 samples baculovirus altered the expression of genes associated with TLR signaling pathway (e.g. TLR2, TLR3, CCL5, CXCL10 and STAT1) and cytokine–cytokine receptor interaction (e.g. CXCL10, CXCL11 and CCL5). Moreover, genes associated with interferon induction-related genes (e.g. CXCL10, MX1, MX2, OAS1 and STAT1) and antigen processing and presentation pathway (e.g. CD74 and RT-1Ba) were affected. As such, Wang and coworkers proposed that baculovirus recognition by TLRs triggers the expression of IFN-α/β, which initiates the subsequent signaling cascade involving STATs, upregulating the expression of IFN-responsive genes and hence confers the cells the antiviral state. Concurrent with the aforementioned findings, we also discovered that baculovirus transduction of human BMSCs disturbed the expression of 816 genes, most of which were related to 5 signaling pathways: cell adhesion molecules, TLR, Jak-STAT, apoptosis as well as antigen processing and presentation (Chen et al., 2009b). Of note, baculovirus triggered the TLR3 pathway, resulting in downstream NF-κB and IRF-3 activation and IL-6/IL-8 production. However, how baculovirus containing the dsDNA genome activated the dsRNA sensor TLR3 remains an enigma. Also, the transduction did not arouse the secretion of IFN-β (Chen et al., 2009b), a signature cytokine associated with TLR3 activation, implying a signaling cascade somewhat distinct from that in immune cells.
Interestingly, the budded form of another baculovirus, Antheraea pernyi nuclear polyhedrosis virus (ApNPV), triggers the TLR21 signaling in chicken macrophage-like cells (HD11) but not in chicken B cell-like cells (Han et al., 2010). ApNPV transduction of HD11 cells concomitantly induces the production of IFN-γ, IL-12p40 and nitric oxide (NO) (Niu et al., 2008), which is accompanied by the phosphorylation of extracellular signal-related kinase 1/2 (ERK 1/2), p38 mitogen activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) as well as activation of p65-NF-κB (Han et al., 2009). Inhibition of p38 MAPK and NF-κB by their respective inhibitors abrogates the expression of cytokines and NO, whereas inhibition of JNK abolishes only the induction of cytokines. Since in mammals TLRs signaling activates the downstream NF-κB and MAPK cascade comprised of at least p38 MAPK, ERK 1/2, and JNK (Ishii et al., 2008), these data altogether suggest that ApNPV transduction of HD11 cells activates TLR21 and signals though NF-κB, p38 MAPK and JNK pathways, and chicken TLR21 might play a role similar to mammalian TLR9 (Han et al., 2009, Han et al., 2010).
3. Modifications of baculovirus for improved transduction
3.1. Surface display via gp64 fusion or heterologous protein
Baculovirus envelope protein gp64 comprises an N-terminal signal peptide and a mature domain that encompasses the transmembrane domain and cytoplasmic domain (CTD). Heterologous protein/peptide has been inserted in between the signal peptide and mature domain, which after expression under the polyhedrin or p10 promoter as a fusion protein is translocated to the plasma membrane and incorporated into the viral envelope upon virus budding. Such feature has been exploited for surface display of protein/peptide to improve the virus transduction (Grabherr and Ernst, 2010, Grabherr et al., 2001, Raty et al., 2004) or for ligand-directed targeting if an appropriate ligand is chosen (Kitagawa et al., 2005, Makela et al., 2006, Makela et al., 2008). For instance, baculovirus poorly transduces B lymphocytes (Cheng et al., 2004, Condreay et al., 1999). Via gp64 fusion, the transduction has been enhanced by displaying the short peptide motif from gp350/220 of Epstein–Barr virus (EBV, which naturally infects B cells) on the baculovirus envelope (Ge et al., 2007). Alternatively, the cytoplasmic transduction peptide (CTP) has been fused to gp64 to enhance the baculovirus transduction of Vero E6, U-2OS and CHO-RD cells (Chen et al., 2011b). Another paradigm is the display of the fragment crystallisable (Fc) region of antibody on the baculovirus surface (Martyn et al., 2009). Fc receptors (FcRs) are membrane proteins that bind to the Fc region of antibody and mediate the phagocytosis and antigen presentation. The Fc display allows for specific baculovirus targeting to cell lines and antigen presenting cells (APCs) expressing FcRs, hence augmenting the vaccine effect (Martyn et al., 2009). The display system also allows for the surface presentation of functional membrane proteins to simplify subsequent isolation (Zhang et al., 2008).
Aside from the gp64-aided display, expression of vesicular stomatitis virus G protein (VSVG) (Chapple and Jones, 2002, Makela and Oker-Blom, 2006), influenza virus neuraminidase (Borg et al., 2004), Spodoptera exigua multiple nucleopolyhedrovirus F protein (Yu et al., 2009), single chain antibody fragments (Kitidee et al., 2010) and human endogenous retrovirus envelope protein (Lee et al., 2010) in insect cells also leads to incorporation of the protein into baculovirus envelope. Among these strategies, display of VSVG or heterologous peptide/protein via the VSVG anchor is the most widely adopted and can tremendously enhance baculovirus transduction in vitro and in vivo (Facciabene et al., 2004, Kaikkonen et al., 2006, Kaneko et al., 2006, Kitagawa et al., 2005, Lu et al., 2006, Tani et al., 2003, Tani et al., 2001, Zhou and Blissard, 2008).
Serum complement proteins (e.g. C5b-9) inactivate baculovirus, hence constituting a major hurdle in the in vivo use of baculovirus. The inactivation problem has been circumvented by the use of complement inhibitors such as compstatin (Georgopoulos et al., 2009) and soluble complement inhibitor 1 (Hoare et al., 2005, Hofmann et al., 1999), avoiding exposure to the complement (Airenne et al., 2000) or administrating the vector to immunoprivileged sites (Kinnunen et al., 2009, Lehtolainen et al., 2002). Alternatively, the baculoviral vector can become complement-resistant by displaying human DAF (decay accelerating factor) via gp64 fusion (Huser et al., 2001, Kaname et al., 2010). More recently, baculoviral vectors displaying different complement regulatory proteins (e.g. DAF, factor H-like protein-1, C4b-binding protein and membrane cofactor protein) were generated by fusion with the membrane anchor of VSVG (Kaikkonen et al., 2010). These surface-modified vectors exhibited varying degrees of complement resistance in vitro and DAF yielded the highest level of protection. Intraportal delivery of the DAF-displaying baculovirus increased the survival and gene expression in immunocompetent mice. The DAF-displaying baculovirus provoked lower levels of inflammatory cytokines IL-1β, IL-6, and IL-12p40 in macrophages and mitigated liver inflammation in mice when compared with the control virus. These results prove that DAF display offers protection to the baculoviral vector against complement inactivation and attenuates complement-mediated inflammation injury.
3.2. Surface modification via capsid display, chemical coupling or electrostatic interactions
Other than the display on the envelope, heterologous protein has been displayed on the capsid by fusion with the major capsid protein VP39. The VP39 fusion with enhanced green fluorescent protein (EGFP) neither interferes with the virus assembly nor affects the virus tier, thereby enabling intracellular baculovirus trafficking and biodistribution monitoring (Kukkonen et al., 2003). Similarly, the ZnO binding peptide has been fused to the N-terminus of VP39 while retaining the viral infectivity and conferring the ability to bind nanosized ZnO powders (Song et al., 2010). Besides, by fusing the protein transduction domain (PTD) of human immunodeficiency virus (HIV) TAT protein (a protein responsible for nuclear import of HIV genome) with VP39, the engineered baculovirus results in improved transduction of various mammalian cells (Chen et al., 2011b).
In addition to surface modification by genetic engineering, baculovirus has been labeled with tracers for tracking (Li et al., 2004) or biodistribution imaging (Raty et al., 2006, Raty et al., 2007). Chemical coupling of antigenic peptides also enables rapid modification of baculovirus particles and delivery of multiple epitopes (Wilson et al., 2008). Baculovirus can also be chemically conjugated with polyethylene glycol (PEG) alone (Kim et al., 2006) or together with folate (Kim et al., 2007, Kim et al., 2010) to improve the transduction of folate receptor-positive KB cells. Additionally, baculoviral vectors have been coated with positively charged polyethylenimine (25 kDa) through electrostatic interactions (Yang et al., 2009). The modification imparts baculoviral vectors resistance to human and rat serum-mediated inactivation in vitro and elevates in vivo transduction in the liver and spleen after tail vein injection into mice.
4. Baculovirus as an RNA interference (RNAi) mediator
RNA interference (RNAi) is a phenomenon that mediates sequence-specific post-transcriptional gene silencing and can be artificially elicited by the expression of short hairpin RNAs (shRNA) or microRNA (miRNA) precursors from an expression vector (Garzon et al., 2010). Since the initial demonstrations of baculovirus-mediated shRNA delivery (Nicholson et al., 2005, Ong et al., 2005), baculovirus-mediated RNAi has been explored for antiviral therapy. One baculovirus expressing the shRNA specific for the C-terminal nucleoprotein of porcine reproductive and respiratory syndrome virus (PRRSV) genome inhibits the viral replication in vitro (Lu et al., 2006). The baculovirus harboring a bispecific shRNA targeted against influenza virus A and B can inhibit the production of both virus types in transduced cell lines (Suzuki et al., 2009b). Another baculovirus expressing shRNA against the peste des petits ruminants virus (PPRV) represses PPRV replication in vitro and the inhibition effect is superior to that mediated by the adenovirus expressing the same shRNA (Nizamani et al., 2011). Hepatitis B virus (HBV) covalently closed circular (CCC) DNA is the source of HBV transcripts in chronically infected patients. Baculovirus expressing the HBV-specific shRNA is able to hinder the formation of HBV CCC DNA (Starkey et al., 2009). Additionally, the baculovirus expressing shRNAs specific for the highly conserved core region of hepatitis C virus (HCV) genome dramatically impedes the target protein expression (Suzuki et al., 2009c).
The replication and segregation of EBV genomes to daughter cells is coordinated by the binding of EBV nuclear antigen 1 (EBNA1) to oriP, an origin of replication derived from EBV. To prolong the transgene expression, baculoviral vectors incorporating the oriP/EBNA1 sequences were developed (Shan et al., 2006, Wang et al., 2008). Based on this concept, Suzuki et al. devised a baculoviral vector that accommodated the oriP/EBNA1 sequence and encoded the HCV-specific shRNA. This vector inhibited HCV core protein expression for > 14 days, which considerably outlasted the 3 days of inhibition conferred by the conventional vector (Suzuki et al., 2009a).
Recently, we also exploited the baculovirus vector for miRNA delivery and gene regulation. The baculovirus vectors carried artificial gene-specific miRNA sequences within the miR155 scaffold, which after expression under the CMV promoter could undergo the miRNA processing pathway and knocked down the target gene (e.g. EGFP and TNF-α) expression (unpublished data). The gene suppression effect was extended by flanking the miRNA expression cassette with the inverted terminal repeat sequences/direct repeats sequences (IR/DR) recognized by the Sleeping Beauty (SB) transposase. Co-transduction of cells with the hybrid baculovirus/transposon vector and another SB transposase-expressing baculovirus resulted in the integration of miRNA expression cassette into the chromosome, giving rise to prolonged target gene suppression (unpublished data). These data altogether implicate the potential of baculovirus-based RNAi shuttle for antiviral therapy and treatment of indications necessitating prolonged gene regulation (e.g. arthritis).
5. Baculovirus as a vector for cancer gene therapy
5.1. Direct baculovirus injection for cancer therapy
The feasibility of baculovirus-mediated cancer therapy was first explored by Wang et al. (2006), who developed a baculoviral vector expressing the Diphtheria Toxin A and demonstrated that the baculovirus impeded the growth of cultured glioma cells and glioma xenograft in the rat brain. Since then, baculovirus has been utilized for the treatment of mouse hepatoma (Kitajima et al., 2008), murine melanoma, lung cancer and brain cancer (Kim et al., 2007). Recently, Wang and coworkers constructed another baculovirus that selectively expressed herpes simplex virus thymidine kinase (HSVtk) in tumors for glioma suicide gene therapy (Balani et al., 2009). The HSVtk gene was driven by a truncated human high mobility group box2 (HMGB2) promoter, which allowed HSVtk expression in glioblastoma tissues but not in normal brain tissues. This vector triggered death of glioblastoma cells in the presence of ganciclovir, but did not affect the survival of human astrocytes and neurons. In a mouse xenograft model, intratumoral injection of this baculovirus at 7 days after tumor inoculation suppressed the growth of human glioblastoma and prolonged the mouse survival. However, the tumor mass was not completely eradicated after one single baculovirus injection, presumably due to the transient HSVtk expression (Balani et al., 2009). Alternatively, the HSVtk gene was driven by the GFAP (glial fibrillary acidic protein) promoter whose activity is similar in normal and tumor cells of the same lineage (Wu et al., 2009a). To limit the transgene expression in glioma cells, repeated target sequences of 3 miRNAs (has-miR31, has-miR127 and has-miR143) that are enriched in astrocytes but are sparse in glioblastoma cells were appended to the 3′ untranslated region of the HSVtk gene. The baculovirus vector markedly improved in vivo selectivity when compared with the control vector without miRNA regulation, effectively inhibited human glioma xenograft and imparted negligible toxicity to normal astrocytes. The incorporation of selected miRNA sponge thus provides an additional safety switch to prevent off-target transgene expression (Wu et al., 2009a).
In addition to HSVtk, the baculovirus expressing NES1 (normal epithelial cell specific-1) can inhibit the growth of gastric cancer cells (SGC-7901) in vitro and repress the SGC-7901 xenografted tumor growth after intratumoral injection, implicating the possibility for the therapy of gastric and colon cancers (Huang et al., 2008). Baculovirus can also carry tumor suppressor genes such as p53 or pdcd4 (programmed cell death 4) for cancer treatment. In the mouse glioma xenograft models, the antitumor effect of the baculovirus-expressed p53 was substantiated by surface coating of the baculovirus envelope with polyethylenimine (Yang et al., 2009) or by co-administering sodium butyrate (Guo et al., 2011), a histone deacetylase inhibitor that ameliorates baculovirus-mediated gene expression (Guo et al., 2010, Hu et al., 2003, Yin et al., 2010, Zhou et al., 2010). The PDCD4-expressing baculovirus conjugated with folate-PEG efficiently expressed PDCD4 protein and induced apoptosis in human epidermal carcinoma cells (Kim et al., 2010). In a tumor xenograft, intratumoral injection of the PDCD4-expressing baculovirus significantly suppressed tumor growth and induced apoptosis. Moreover, apoptin is a chicken anemia virus-derived protein that specifically triggers apoptosis in tumors. The VSVG-pseudotyped baculovirus expressing apoptin efficiently provoked apoptosis in mammalian cells, repressed the growth of xenograft tumors and prolonged the mice survival after intratumoral injection (Pan et al., 2010).
One determinant to the baculovirus-mediated antitumor effects is the innate immune responses elicited by the virus (Suzuki et al., 2010b). Takaku and coworkers demonstrated that wild-type baculovirus, after intravenous (i.v.) injection into immunocompetent B6 mouse, was taken up by the liver and spleen, preferentially entered DCs and B cells, activated DCs, induced NK cells proliferation in the liver and spleen, and enhanced the antitumor immunity in mice with B16 liver metastases (Kitajima et al., 2008). Baculovirus administration also increased the survival of C57BL/6, Jα281−/− and IFN-γ−/− mice bearing the B16 tumor, but did not enhance the survival of NK cell-depleted mice. These results prove that wild-type baculovirus efficiently induces NK cell-mediated antitumor immunity (Kitajima et al., 2008).
5.2. Baculovirus in conjunction with cell therapy for cancer treatment
Besides direct injection, baculovirus has been employed in conjunction with cell therapy for cancer treatment. Bone marrow-derived DCs (BMDCs), after ex vivo transduction with wild-type baculovirus and i.v. injection into mice, significantly suppressed the lung cancer (Suzuki et al., 2010a). In a mouse melanoma model, baculovirus-transduced BMDCs inhibited tumor growth and improved animal survival at least partly due to the induction of CD8+ T cell- and NK cell-dependent, CD4+ T cell-independent antitumor immunity. Importantly, BMDCs administration did not provoke evident signs of damage to the liver or kidney, as judged from the negligible disturbance of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and creatine levels (Suzuki et al., 2010a). Another promising cell courier is BMSCs thanks to their intrinsic tumor homing property. In this regard, BMSCs were transduced with a baculovirus expressing HSVtk and injected via tail vein into the mice pre-inoculated with human U87 glioma cells (Bak et al., 2010). After ganciclovir injection, tumor growth was significantly repressed and the life span of animals was considerably prolonged. More recently, the same group generated MSC-like cells from human embryonic stem (ES) cells which, after transduction with the baculovirus expressing HSVtk and injection into the brain, were capable of inhibiting tumor growth and prolonging the survival of tumor-bearing mice in the presence of ganciclovir (Bak et al., 2011). These data implicated the feasibility of human ES cells-derived MSCs as a viable and attractive alternative for cancer therapy.
6. Baculoviral vector for regenerative medicine
Gene therapy has converged with tissue engineering, by which the therapeutic genes stimulating tissue regeneration can be administered to cells either in vivo or ex vivo with an appropriate vector. Given the highly efficient gene delivery, baculovirus has been used for cartilage and bone engineering (Lin et al., 2010b).
6.1. Baculovirus-mediated cartilage regeneration
Articular cartilage is a weight-bearing tissue comprising chondrocytes and extracellular matrix (ECM) composed of proteins (e.g. collagen II) and glycosaminoglycans (GAGs) such as aggrecan. Articular cartilage may be damaged due to direct trauma or joint diseases, but its ability to self-repair is limited, ultimately leading to debilitating pain and physical impairment. Therefore, cartilage tissue engineering that combines cells, scaffolds and biological signals has emerged for cartilage repair.
Given that chondrocytes are pivotal in the synthesis, composition modulation, structural arrangement of ECM components and hence the mechanical properties, we developed a protocol for baculovirus transduction of rat (Ho et al., 2004) and rabbit (Sung et al., 2007) chondrocytes. This protocol involves the incubation of cells with the virus at 25–27 °C for 4–8 h using Dulbecco's phosphate-buffered saline (D-PBS) as the surrounding solution and confers efficiencies higher than 90% (Chen et al., 2006). The key to such high transduction efficiencies is the absence of NaHCO3 in D-PBS, which hinders the baculovirus transduction (Shen et al., 2007). Consequently, the surrounding solution has been replaced with NaHCO3-deficient DMEM medium (Lo et al., 2009). Critically, chondrocytes transduced with an EGFP-expressing baculovirus remain capable of producing cartilage-specific collagen II and GAGs, and grow into cartilaginous tissues when seeded into the porous, poly (l-lactide-co-glycolide) (PLGA) scaffold and cultivated dynamically (Chen et al., 2006). These data demonstrate that baculovirus transduction of chondrocytes does not obstruct the chondrocytes differentiation. Therefore, we further constructed 3 baculovirus vectors each expressing one growth factor (TGF-β1, insulin-like growth factor-1 (IGF-1) or bone morphogenetic protein-2 (BMP-2)) known to stimulate chondrogenesis, and confirmed the protein expression in chondrocytes isolated from New Zealand White (NZW) rabbits (Sung et al., 2007). Among the 3 baculovirus vectors, only the BMP-2-expressing baculovirus (designated Bac-CB) remarkably enhanced the aggrecan and collagen II production by partially de-differentiated passage 3 (P3) cells and restored the differentiation. The baculovirus expressing TGF-β1 (designated Bac-CT) modestly augmented the chondrogenesis but was insufficient to reverse the de-differentiation status of P3 cells (Sung et al., 2007). Nonetheless, co-transduction of de-differentiated P3 chondrocytes with Bac-CB and Bac-CT synergistically modulated the re-differentiation program (Sung et al., 2009).
Albeit the chondrogenic potential, Bac-CB transduction alone was insufficient to support uniform 3D cartilage growth in the static culture due to the lack of mechanical stimulation and poor oxygen/nutrient transfer. To tackle these problems, P3 chondrocytes transduced with Bac-CB were seeded into PLGA scaffolds and cultured in a rotating-shaft bioreactor (RSB), which grew into cartilage-like tissues after 3-week dynamic culture (Chen et al., 2008). Implantation of the engineered cartilages into the osteochondral defects of NZW rabbits resulted in the regeneration of hyaline cartilages at week 8 and improved the integration of the host and engineered cartilages (Chen et al., 2009c).
6.2. Baculovirus-mediated bone regeneration
Massive segmental bony defects often occur following trauma or tumor resection and remain a clinical problem. For bone regeneration, BMSCs have evolved to be a promising cell source as they are immunoprivileged, immunosuppressive and capable of differentiating into osteoblasts (Kumar et al., 2010). In this regard, baculovirus transduces BMSCs with efficiencies exceeding ≈ 95% under optimized conditions (Lo et al., 2009), but the transduction efficiencies for BMSCs-derived progenitors varies widely with the differentiation states at which the committed progenitors are transduced (Ho et al., 2006, Lee et al., 2007b).
Furthermore, transduction of human BMSCs with Bac-CB (which expresses the potent osteogenic factor BMP-2) directed in vitro commitment of naïve BMSCs into osteoblasts (Chuang et al., 2007). After injection into the back subcutis of immunodeficient nude mice, the transduced BMSCs resulted in progressive mineralization and ectopic bone formation (Chuang et al., 2007). Implantation of the Bac-CB-engineered human BMSCs into rat calvarial defects stimulated mineralized bone matrix deposition and initiated the bone island formation at week 4, but without immunosuppression the xenogeneic cells were rejected and eradicated at week 12 (Chuang et al., 2010). To circumvent the rejection, BMSCs isolated from NZW rabbits were used for baculovirus transduction and allotransplantation. Given the important roles of vascular endothelial growth factor (VEGF) in angiogenesis, ossification and callus maturation, rabbit BMSCs were transduced with Bac-CV (expressing VEGF) or Bac-CB, mixed at a number ratio of 1:4, seeded into PLGA scaffolds and implanted to the critical-size (10 mm in length) femoral segmental defects of allogeneic, immunocompetent NZW rabbits, which represent a fairly rigorous bone healing scenario (Lin et al., 2010a). X-ray radiography, positron emission tomography (PET), micro computed tomography (μCT), immunohistochemical staining and biomechanical testing illustrated that the baculovirus-engineered cell/scaffold constructs not only accelerated the bone healing, but also gave rise to prominent angiogenesis and improved mechanical properties at week 8.
Adipose-derived stem cells (ASCs) are another promising cell source for regenerative medicine thanks to the ease of isolation in abundance and capacity of osteogenic differentiation (Levi et al., 2010). However, ASCs appear to be inferior to BMSCs in osteogenic differentiation and ASCs engineered by the conventional baculovirus transiently expressing BMP-2/VEGF (S group) led to significantly poorer healing of segmental femoral bone defects than BMSCs engineered by the same vectors (Fig. 2 ). To use ASCs for repairing large, segmental bone defects, we surmised that sustained expression of factors promoting osteogenesis (BMP-2) and angiogenesis (VEGF) are necessary. As such, we employed a hybrid baculovirus system developed previously for persistent expression (Lo et al., 2009). The dual vector system constitutes two baculoviruses whereby one expresses the flippase recombination enzyme (FLP) while the other accommodates the BMP-2 or VEGF cassette flanked by the flippase recognition target (Frt) sequences. Within the mammalian cells co-transduced with the hybrid baculoviruses, FLP catalyzes the recombination of the Frt-flanking cassette, resulting in the cassette excision off the baculovirus genome, formation of episomal circle and substantially prolonged transgene expression (Lo et al., 2009). Likewise, the FLP/Frt-mediated recombination occurred efficiently in the NZW rabbit ASCs, enabling persistent transgene expression for > 28 days (Lin et al., 2011). Allotransplantation of the NZW rabbit ASCs transduced with the hybrid baculoviruses expressing BMP-2/VEGF into the critical-size femoral segmental defects accelerated the healing, improved the bone quality and angiogenesis when compared with transplanting ASCs engineered with the conventional baculoviruses (Fig. 2). Therefore, ASCs engineered by the hybrid baculovirus vector hold promise for repairing massive segmental defects (Lin et al., 2011).
Fig. 2.
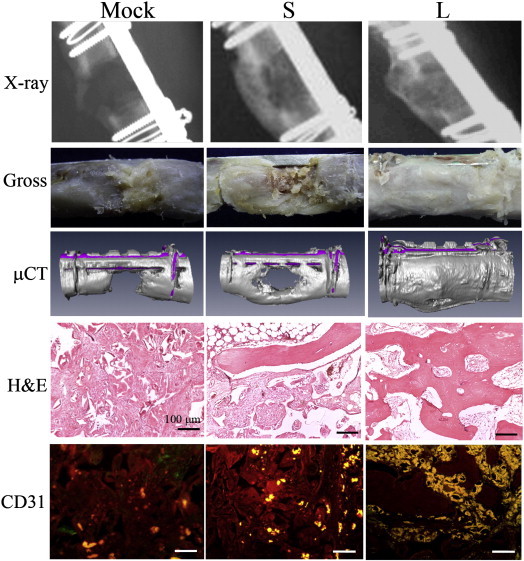
The ASCs engineered with the hybrid baculovirus augment the healing of massive bone defects. The NZW rabbit ASCs were transduced with the hybrid baculovirus vectors conferring sustained expression of BMP-2 or VEGF, mixed at a number ratio of 4:1, loaded into cylindrical PLGA scaffolds (1.5 × 106 cells/scaffold) and implanted to the critical-size segmental defects at the femora of NZW rabbits (2 constructs/defect, designated L group). The S group contained ASCs that were transduced with conventional baculoviruses transiently expressing BMP-2/VEGF and implanted in a similar fashion. The Mock group comprised the mock-transduced ASCs as the negative control. X-ray radiography, gross appearance examination, micro computed tomography (μCT), hematoxylin and eosin (H&E) staining and CD 31-specific immunohistochemical staining (to detect blood vessel formation) performed at 12 weeks post-implantation collectively demonstrated that the L group (persistently expressing BMP-2 and VEGF) resulted in significantly improved bone healing and angiogenesis in comparison with the S group (transiently expressing BMP-2 and VEGF) and Mock group. Stars indicate the new bone while arrows indicate the blood vessel formation.
The safety profile of the hybrid baculovirus vectors was recently assessed using human BMSCs as the host (Chen et al., 2011a). Transduction of human BMSCs with the hybrid baculovirus neither compromised the cell viability/differentiation, nor resulted in transgene integration into the host chromosome. The transduction did not disrupt the BMSCs karyotype, nor did it disturb the expression of well-known proto-oncogenes (c-myc, N-ras, K-ras and H-ras) and tumor suppressor genes (p53 and p16). Furthermore, the transduced BMSCs did not induce tumor formation in nude mice. These data altogether ensure the safe use of the FLP/Frt-based hybrid vector for stem cell engineering and tissue regeneration.
7. Baculoviruses as vaccine vectors
As discussed in Section 2.3, wild-type baculovirus triggers the innate immunity and potentiates the adaptive immune responses, which protect the animals from the infection of several viruses. These attributes have sparked explosive interests to develop baculovirus as a vector vaccine candidate, in which the antigens can be (1) expressed by the vector within the host cells, (2) displayed on the baculovirus surface or (3) displayed and expressed by the vector (Table 2 ).
Table 2.
Strategies for baculovirus display/expression.
| Strategies | Antigens | Pathogens | References |
|---|---|---|---|
| Expression | Hemagglutinin protein | Influenza virus | (Wu et al., 2009b) |
| E envelope glycoprotein | Japanese encephalitis virus | (Li et al., 2009b) | |
| gB, gC, and gD glycoproteins | Pseudorabies virus | (Grabowska et al., 2009) | |
| Spike and nucleocapsid proteins | SARS-like coronavirus | (Bai et al., 2008) | |
| Capsid protein | Porcine circovirus type 2 | (Fan et al., 2008) | |
| Capsid protein | Food-and-mouth disease virus | (Cao et al., 2011) | |
| GP5 and M proteins | Porcine reproductive and respiratory syndrome virus | (Wang et al., 2007) | |
| HPV-16L1 protein | Human papillomavirus | (Lee et al., 2010) | |
| SAG1 protein | Toxoplasma gondii | (Fang et al., 2010) | |
| Display | Hemagglutinin protein | Influenza virus | (Jin et al., 2008, Tang et al., 2010, Yang et al., 2007) |
| Peptides of hemagglutinin protein | Influenza virus | (Wilson et al., 2008) | |
| E envelope glycoprotein | Japanese encephalitis virus | (Xu et al., 2011) | |
| Glycoprotein D | Bovine herpesvirus-1 | (Peralta et al., 2007) | |
| σB and σC proteins | Avian reovirus | (Lin et al., 2008) | |
| Erns, E2 and NS3 proteins | Classical swine fever virus | (Xu et al., 2008, Xu et al., 2009, Xu and Liu, 2008) | |
| 19-kDa carboxyl terminus of merozoite surface protein 1 | Plasmodium yoelii | (Yoshida et al., 2010) | |
| Pfs25 protein | Plasmodium falciparum | (Mlambo et al., 2010) | |
| Dual | Hemagglutinin protein | Influenza virus | (Chen et al., 2010, He et al., 2008, Lu et al., 2007, Prabakaran et al., 2008, Prabakaran et al., 2010a, Prabakaran et al., 2010b, Prabakaran et al., 2010c) |
| VP28 protein | White spot syndrome virus | (Syed Musthaq et al., 2009) | |
| E2 protein | Classical swine fever virus | (Li et al., 2009a) | |
| Pvs25 protein | Plasmodium vivax | (Blagborough et al., 2010) | |
| Circumsporozoite protein (PbCSP) | Plasmodium berghei | (Yoshida et al., 2009) | |
| Circumsporozoite protein | Plasmodium falciparum | (Strauss et al., 2007) |
7.1. Baculovirus as an antigen expression vector
The feasibility of using baculovirus as a vaccine expression vector was first tested by in vivo inoculation of the baculovirus expressing the glycoprotein gB of pseudorabies virus (Aoki et al., 1999) or hemagglutinin (HA) of H1N1 influenza virus (Abe et al., 2003) into mice, which raised antigen-specific antibody. Moreover, baculovirus expressing the E2 glycoprotein of HCV or carcinoembryonic antigen could elicit antigen-specific T cell responses (Facciabene et al., 2004). Similarly, subcutaneous (s.c.) and intraperitoneal (i.p.) immunizations of mice with a baculovirus expressing the antigens of severe acute respiratory syndrome coronavirus (SARS-CoV) induced humoral immune responses and Th1-biased cellular immunity (Bai et al., 2008).
The efficacy of baculovirus-based vaccines has been potentiated by surface display of VSVG protein on the envelope. The VSVG-pseudotyped baculovirus expressing the GP5 and M proteins of porcine reproductive and respiratory syndrome virus (PRRSV) under the CMV promoter elicited anti-PRRSV neutralizing antibodies and IFN-γ, and conferred better immunogenicity than the DNA vaccine expressing the same antigens (Wang et al., 2007). Conversely, i.m. immunizations of a mixture of VSVG-pseudotyped baculovirus expressing pseudorabies virus (PRV) proteins triggered Th1-biased immune responses, as manifested by the induction of IFN-γ and PRV-specific IgG2a antibodies (Grabowska et al., 2009). The immunization also provoked NK cell activity (accompanied by the production of IFN-α and IFN-γ) and protected mice against lethal PRV challenge. I.m. immunization of chickens with another VSVG-pseudotyped baculovirus expressing HA of H5N1 avian influenza virus (AIV) also evoked significantly higher levels of H5-specific antibody and cellular immunity than those receiving DNA vaccines, and conferred protection against lethal challenge with the homologous virus strain (Wu et al., 2009b). Similar VSVG-pseudotyped baculovirus vectors have been constructed to express the Japanese encephalitis virus (JEV) envelope protein (Li et al., 2009b), the capsid protein of porcine circovirus type 2 (PCV2) (Fan et al., 2008), and the Toxoplasma gondii SAG1 protein (Fang et al., 2010). All these studies showed that i.m. immunization of the VSVG-pseudotyped baculovirus vector results in stronger vaccine effects than the DNA vaccine counterparts, which can be attributable to the adjuvant properties of baculovirus and more efficient transduction of muscle cells in vivo. The effective transduction of muscle cells is desired as the chance of antigen presentation to DCs is increased and baculovirus-mediated expression is considerably prolonged in myogenic cells (Shen et al., 2008). Note, however, that VSVG pseudotyping induces cytotoxicity and impairs the baculovirus titer, hence a truncated version of VSVG comprised of the 21 amino acid ectodomain, the transmembrane and CTD domains was designed for baculovirus pseudotyping (Kaikkonen et al., 2006). The truncated VSVG reduced the cytotoxicity and was exploited to construct two pseudotyped baculoviruses: one expressing the capsid protein of foot-and-mouth disease virus (FMDV) and the other encoding the capsid and a T-cell immunogen (Cao et al., 2011). I.m. inoculation of the baculoviruses into mice induced the FMDV-specific neutralizing antibodies and IFN-γ, and expression of the additional T-cell immunogen augmented the immunogenicity.
Besides VSVG, human endogenous retrovirus (HERV) envelope protein was used for pseudotyping the baculovirus (designated AcHERV-HP16L1) that encoded the L1 capsid protein of human papillomavirus 16 (HPV 16) (Lee et al., 2010). After i.m. injection and 2 booster injections at 2-week intervals, the mice developed similarly high levels of IgG, IgA and neutralization titers, as well as tremendously higher levels of IFN-γ when compared with the mice immunized with the commercial virus-like particle vaccine Gardasil® (25 μL/dose). Thus AcHERV-HP16L1 holds promise as a cost-effective and efficient HPV vaccine.
7.2. Baculovirus as an antigen display vector
As mentioned in Section 3.1, surface display of heterologous protein/peptide has been achieved by fusion with gp64 gene and expression driven by either polyhedrin or p10 promoter. Taking advantage of this technology, baculovirus displaying the rodent malaria Plasmodium berghei circumsporozoite protein (PbCSP) (Yoshida et al., 2003), the antigenic site A of FMDV VP1 protein (Tami et al., 2004) or the SARS-CoV spike protein (Chang et al., 2004, Feng et al., 2006) has been shown to induce potent immune responses. More recently, baculovirus vectors displaying the Pfs25 protein of Plasmodium falciparum (Mlambo et al., 2010) and 19-kDa carboxyl terminus of merozoite surface protein 1 (PyMSP119) of Plasmodium yoelii (Yoshida et al., 2010) were also developed as potential vaccines against malaria. I.m. and intranasal (i.n) immunizations of mice with the PyMSP119-displaying baculovirus induced mixed Th1/Th2-type immune responses, but i.n. immunization yielded higher PyMSP119-specific antibody titers and natural boosting after challenge. I.n. immunization also conferred complete protection thanks to the Th1/Th2-type immunity associated with TLR9-dependent pathway (Yoshida et al., 2010).
HA protein is the major antigen of influenza virus and, similar to gp64, is a class I transmembrane protein on the viral envelope. gp64-based fusion was harnessed to display the HA of AIV using the CTD derived from HA or from gp64 (Yang et al., 2007). In comparison with the HA CTD, gp64 CTD endowed more efficient HA display and stimulated stronger hemagglutination inhibition (HI) titers crucial for neutralizing the live AIV (Yang et al., 2007). The profound effect of gp64 CTD on HA display was attested in a recent study which delineated that the signal peptide and CTD of gp64 could enhance the display of influenza HA on baculovirus surface, while the gp64 transmembrane domain impaired HA display (Tang et al., 2010). Based on the finding, a baculovirus simultaneously displaying 4 HAs derived from 4 subclades of H5N1 AIV was constructed. I.m. immunization of mice with this tetravalent baculovirus elicited HI titers against all 4 homologous H5N1 viruses, significantly reduced viral lung titers of challenged mice, raised high levels of IFN-γ-secreting and HA-specific CD8+ T cells, and provided 100% protection against lethal challenge with homologous H5N1 viruses (Tang et al., 2010). The same design concept exploiting the gp64 CTD was also adopted to display the σC and σB proteins of avian reovirus (Lin et al., 2008), Erns envelope glycoprotein (Xu et al., 2008), E2 (Xu and Liu, 2008) or NS3 protein (Xu et al., 2009) of classical swine fever virus (CSFV) and the E glycoprotein of JEV (Xu et al., 2011). These studies collectively attested that the antigen can be efficiently displayed on the baculoviral envelope and induce humoral/cellular immune responses against the lethal viral challenge.
Other than displaying the antigen via genetic engineering, CD4+ T helper epitope (SFERFEIPKE) and the major B cell epitope (WLTEKEGSYP) derived from the HA of influenza virus A/PR/8/34 have also been chemically conjugated to baculovirus envelope (Wilson et al., 2008). I.n. administration of such baculovirus to mice elicited antigen-specific IgG1a/IgG2a, IgA and IFN-γ, thus chemical coupling allows for the delivery of multiple epitopes to baculovirus. In addition, HA of AIV (H5N1) has been displayed on another baculovirus Bombyx mori NPV (BmNPV) via fusion with gp64 (Jin et al., 2008). The virus was produced and purified from silkworm pupae infected with the recombinant virus. Immunization of rhesus monkeys with aluminum hydroxide as the adjuvant at doses of 2 mg/kg and 0.67 mg/kg elicited neutralizing antibodies and protected monkeys against influenza virus challenge. The vaccine did not cause appreciable toxicity at the dose as large as 3.2 mg/kg in cynomolgus monkeys and 1.6 mg/kg in mice, indicating the safe vaccine doses (Jin et al., 2008).
7.3. Baculovirus as a dual vector for antigen expression/display
It is perceivable that the antigen-expressing baculovirus mimicks the DNA vaccine. Following vector injection into the hosts, the antigen is expressed and presented via MHC I pathway in the transduced cells, and activates CD8+ T cells. Conversely, the antigen-displaying baculovirus mimicks the subunit vaccine. Antigens on the envelope are internalized by professional antigen presenting cells (APCs) such as macrophages and DCs, and presented by the MHC II pathway to stimulate humoral immune responses.
To fully exploit both mechanisms, a dual baculovirus vector that simultaneously displays and expresses the Plasmodium falciparum circumsporozoite (CS) protein was developed as a human malaria vaccine (Strauss et al., 2007). This vector contained two expression cassettes, one encoding the CS-gp64 fusion protein under the polyhedrin promoter while the other accommodating the CS gene under the CMV promoter, such that CS can be displayed on the envelope and expressed after transduction into mammalian cells. Upon i.m. injection into mice, the dual vector induced higher anti-CS antibody titers and higher frequencies of CS-specific CD4+ and CD8+ T cells than the vectors that only displayed or only expressed CS.
Recently, we adopted a similar approach and constructed 3 vectors: Bac-HA64 harbored the HA gene of AIV under the p10 promoter for HA display; Bac-CHA expressed HA under the CMV promoter while the dual vector Bac-CHA/HA64 encompassed both expression cassettes in opposite orientations, such that Bac-CHA/HA64 displayed and expressed HA in the transduced cells (Chen et al., 2010). All 3 vectors, after administration (i.m., i.n. and s.c.) into BALB/c mice, provoked HA-specific humoral (IgG1, IgG2a and HI titers), mucosal (IgA titers) and cellular (IFN-γ and IL-4 producing T cells and IFN-γ+/CD8+ T cells) immune responses. The strong cellular immunity provoked by Bac-HA64, which in theory favors the MHC II pathway and preferentially elicits humoral immune responses, was likely due to the potent adjuvant effects of baculovirus. Regardless, via either administration route the dual vector Bac-CHA/HA64 triggered superior or at least comparable HA-specific immune responses than the other two vaccine forms, demonstrating the advantages of the dual form for vaccine design (Chen et al., 2010).
Instead of using two separate cassettes as above, Yoshida et al. (2009) devised a single cassette system whereby the PbCSP–gp64 fusion gene was driven by a tandem promoter consisting of the CMV and polyhedrin promoters, so that PbCSP was expressed in insect cells and mammalian cells. I.m. immunization with this baculovirus elicited both Th1 and Th2 responses as evidenced by the high PbCSP-specific IgG1/IgG2a titers and PbCSP-specific CD8+ T-cells responses, and conferred 100% protection against sporozoite challenge (Yoshida et al. 2009). The same dual expression system was subsequently utilized to express Plasmodium vivax transmission-blocking immunogen (Pvs25) for the development of malarial transmission-blocking vaccines against the sexual stages of the parasite (Blagborough et al., 2010). Both i.n. and i.m. immunizations of mice induced a mixed Th1/Th2 response as evidenced by high Pvs25-specific IgG1 and IgG2a/IgG2b titers as well as a strong transmission-blocking response after challenge.
Another simple approach to developing the dual vector is to drive the antigen expression using the white spot syndrome virus (WSSV) ie1 promoter, which is active in insect, mammalian and avian cells (Gao et al., 2007, He et al., 2008). Baculovirus encoding the WSSV VP28 envelope protein driven by the WSSV ie1 promoter was able to display VP28 on the envelope (Syed Musthaq et al., 2009). Immunization of shrimp with this baculovirus resulted in VP28 expression in shrimp tissues, elevated the survival rate and reduced the WSSV viral load after WSSV infection. WSSV ie1 promoter was also used in conjunction with VSVG pseudotyping. I.m. injection of the VSVG-pseudotyped baculovirus that expressed the E2 protein of CSFV under the ie1 promoter induced CSFV-specific neutralizing antibodies and lymphoproliferative responses in mice (Li et al., 2009a).
WSSV ie1 promoter has been most extensively employed by the Kwang group to drive the expression of HA of H5N1 AIV, which enabled HA display on the envelope and conferred the viral particle hemagglutination activity (Lu et al., 2007). I.m. injection of this vector (designated BacHA) into mice elicited the antibodies with HI titers against the tested influenza virus. In Sf-9 cells, WSSV ie1 promoter was more active than CMV promoter, thereby giving rise to more efficient HA incorporation into the baculoviral envelope (He et al., 2008). The ie1 promoter also resulted in strong HA expression in the lung (after i.n. inoculation) and thymus (after i.m. inoculation) in chickens. As a result, immunization of chickens with the baculovirus bearing the ie1 promoter (BacHA) elicited higher anti-HA antibody levels than that bearing the CMV promoter (He et al., 2008). However, i.n. inoculation of BacHA stimulated low anti-HA IgG titers (Prabakaran et al., 2008). To enhance the vaccine efficacy via the i.n. route, BacHA was co-administered with recombinant cholera toxin B subunit (rCTB) as the adjuvant, which significantly enhanced the serum IgG, mucosal IgA and serum microneutralization titers. With the adjuvant, BacHA also triggered higher HA-specific humoral and mucosal immune responses than the inactivated H5N1 virus adjuvanted with the same dose of rCTB, and conferred complete protection against challenge with homologous and heterologous H5N1 strains (Prabakaran et al., 2008).
Additionally, gastrointestinal delivery of BacHA into mice by oral gavage led to transduction in vivo and remarkably boosted the HA-specific IgG, HI and mucosal IgA titers (Prabakaran et al., 2010c). The live BacHA triggered stronger cross-clade neutralization against heterologous H5N1 strains than the inactivated BacHA, and provided 100% protection against challenge with lethal doses of homologous and heterologous H5N1. Moreover, after challenge the immunized mice exhibited only minimal bronchitis in lungs and regained their body weight more rapidly (Prabakaran et al., 2010c).
The oral vaccine efficacy was further potentiated by encapsulating the live BacHA within a reverse micelle structure of phosphatidylcholine, which provided protection against the destructive environment in the intestinal lumen (Prabakaran et al., 2010b). In comparison with the non-encapsulated BacHA, gastrointestinal delivery of the encapsulated baculovirus into mice led to significantly ameliorated HA-specific IgG and IgA responses, and higher HI titers. The encapsulated vaccine induced strong cross-clade neutralization titers against heterologous H5N1 strains and conferred protection against infection with highly pathogenic, heterologous H5N1 viruses (Prabakaran et al., 2010b).
Using the same WSSV ie1 approach, more recently Kwang and coworkers selected 3 HA proteins from different vaccine strains for expression and display on the baculovirus surface (Prabakaran et al., 2010a). The 3 HA proteins covered the entire variants in the neutralizing epitopes among the H5N1 lineages. S.c. immunization of mice with a mixture of 3 baculoviruses displaying the 3 HA proteins (tri-BacHA) induced antibodies capable of neutralizing viruses from clades 1, 2.1, 2.2, 4, 7, and 8 of H5N1 viruses. In contrast, s.c. immunization with a single HA-displaying baculovirus (mono-BacHA) or a single strain of inactivated whole virus vaccine neutralized only clade 1 (homologous), clade 2.1, and clade 8.0 viruses. Also, the tri-BacHA vaccine protected 100% of the mice against challenge with three different clades (clades 1.0, 2.1, and 7.0) of H5N1 viruses.
8. Conclusions
Since the discovery in 1995 that baculovirus effectively transduces mammalian cells and mediates transgene expression, baculovirus has emerged as a promising gene delivery vector. Albeit the rapidly growing lists of permissive cells and applications, relatively little is known about what happens inside the cells after baculovirus transduction. Evidence accumulating in recent years has indicated that after entry, baculovirus can translocate into the nucleus through a complex cascade of steps and express baculoviral genes and the transgene. The entire process results in the recognition of baculovirus by the cells via the TLR9-dependent and -independent pathways, and disturbs the expression of a small percentage of host genes, particularly those pertaining to the innate immune responses. However, disparity does exist between reports and the exact intracellular events remain elusive. The lack of comprehensive knowledge about the events governing the virus entry, intracellular transport and cellular responses will constitute a roadblock to future applications in the clinical setting, thus entailing extensive research to elucidate the underlying mechanisms.
Although the innate immune responses stir up concerns regarding the safety of baculovirus in gene therapy, these responses represent opportunities to harness baculovirus as a vector to defend against infectious agents and tumors. Indeed, baculovirus-based vaccine has captured explosive interests over the past 3 years and in one case has entered into trials in primates (Jin et al., 2008). Thanks to the intrinsic adjuvant properties, in most studies baculovirus-based vaccines were able to trigger potent immune responses in the absence of additional adjuvants. Due to the widespread use and encouraging preclinical data in comparison with other vaccine forms (e.g. DNA vaccine, virus-like particle vaccine and whole virus vaccine), it is envisioned that baculovirus-based vaccines can progress into next phase in the near future. The capability of shRNA/miRNA delivery also confers baculovirus an edge for target gene regulation and for future applications in antiviral therapy and immunotherapy. Furthermore, baculovirus transduction of neural cells (Kenoutis et al., 2006) and adult stem cells (Bak et al., 2010, Ho et al., 2006, Zeng et al., 2009) does not markedly alter the inherent properties and mitigate the differentiation capacity, warranting baculovirus a promising vector for cell therapy and tissue engineering. Recent progresses in the baculovirus vectors engineering with respect to surface modification, minimization of in vivo inactivation, transgene targeting and prolongation of expression further corroborate the potentials of baculovirus in these applications.
To advance the baculovirus technology from bench to bedside, other roadblocks still stand in the way. Over the past few years, a wealth of literature has addressed the problems in baculoviral vector production (Bernal et al., 2009, Carinhas et al., 2009, Carinhas et al., 2010, Dojima et al., 2010, Tsai et al., 2007), quantification (Chan et al., 2006, Ferris et al., 2011, Kärkkäinen et al., 2009, Lo et al., 2011, Lo and Chao, 2004, Roldao et al., 2009), purification (Chen et al., 2009a, Kaikkonen et al., 2008, Transfiguracion et al., 2007, Vicente et al., 2009, Vicente et al., 2010a, Vicente et al., 2010b, Wu et al., 2007) and quality assurance (Ihalainen et al., 2010, Jorio et al., 2006). New baculoviral vectors taking advantage of hybrid promoters (Gao et al., 2007, Keil et al., 2009, Lackner et al., 2008, Pan et al., 2009) and new regulatory elements (Du et al., 2010, Mahonen et al., 2007) are also being developed and evaluated for their potential applications. Additionally, the transduction conditions (Keil et al., 2009, Pan et al., 2009), supplements (Guo et al., 2010, Yin et al., 2010) and parameters (Lee et al., 2007a, Lee et al., 2007b, Shen et al., 2007, Shen et al., 2008) dictating the transduction efficiencies have been evaluated. These technological progresses undoubtedly will facilitate the production of baculoviral vectors in compliance of cGMP regulations, and advance baculovirus from a research tool to clinical applications.
Acknowledgments
The authors acknowledge the financial support from the National Tsing Hua University Booster Program (99N2544E1, 98N2901E1, 97N2511E1), CGMH–NTHU Joint Research Program (99N2419E1, CMRPG380101, CMRPG390141) and National Science Council (99-2221-E-007-025-MY3, 98-2221-E-007-047-MY3, NSC99-2622-E-007-012-CC2, NSC 98-2627-B-007-006), Taiwan.
References
- Abe T., Matsuura Y. Host innate immune responses induced by baculovirus in mammals. Curr Gene Ther. 2010;10:226–231. doi: 10.2174/156652310791321279. [DOI] [PubMed] [Google Scholar]
- Abe T., Takahashi H., Hamazaki H. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J Immunol. 2003;171:1133–1139. doi: 10.4049/jimmunol.171.3.1133. [DOI] [PubMed] [Google Scholar]
- Abe T., Hemmi H., Miyamoto H. Involvement of the toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J Virol. 2005;79:2847–2858. doi: 10.1128/JVI.79.5.2847-2858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T., Kaname Y., Wen X. Baculovirus induces type I interferon production through toll-like receptor-dependent and -independent pathways in a cell-type-specific manner. J Virol. 2009;83:7629–7640. doi: 10.1128/JVI.00679-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airenne K.J., Hiltunen M.O., Turunen M.P. Baculovirus-mediated periadventitial gene transfer to rabbit carotid artery. Gene Ther. 2000;7:1499–1504. doi: 10.1038/sj.gt.3301269. [DOI] [PubMed] [Google Scholar]
- Aoki H., Sakoda Y., Jukuroki K. Induction of antibodies in mice by a recombinant baculovirus expressing pseudorabies virus glycoprotein B in mammalian cells. Vet Microbiol. 1999;68:197–207. doi: 10.1016/s0378-1135(99)00110-8. [DOI] [PubMed] [Google Scholar]
- Bai B., Lu X., Meng J. Vaccination of mice with recombinant baculovirus expressing spike or nucleocapsid protein of SARS-like coronavirus generates humoral and cellular immune responses. Mol Immunol. 2008;45:868–875. doi: 10.1016/j.molimm.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak X.-Y., Yang J., Wang S. Baculovirus-transduced bone marrow mesenchymal stem cells for systemic cancer therapy. Cancer Gene Ther. 2010;17:721–729. doi: 10.1038/cgt.2010.32. [DOI] [PubMed] [Google Scholar]
- Bak X.Y., Dang H.L., Yang J. Human embryonic stem cell-derived mesenchymal stem cells as cellular delivery vehicles for prodrug gene therapy of glioblastoma. Hum Gene Ther. 2011 doi: 10.1089/hum.2010.212. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Balani P., Boulaire J., Zhao Y. High mobility group box2 promoter-controlled suicide gene expression enables targeted glioblastoma treatment. Mol Ther. 2009;17:1003–1011. doi: 10.1038/mt.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal V., Carinhas N., Yokomizo A.Y. Cell density effect in the baculovirus–insect cells system: a quantitative analysis of energetic metabolism. Biotechnol Bioeng. 2009;104:162–180. doi: 10.1002/bit.22364. [DOI] [PubMed] [Google Scholar]
- Blagborough A.M., Yoshida S., Sattabongkot J. Intranasal and intramuscular immunization with Baculovirus Dual Expression System-based Pvs25 vaccine substantially blocks Plasmodium vivax transmission. Vaccine. 2010;28:6014–6020. doi: 10.1016/j.vaccine.2010.06.100. [DOI] [PubMed] [Google Scholar]
- Borg J., Nevsten P., Wallenberg R. Amino-terminal anchored surface display in insect cells and budded baculovirus using the amino-terminal end of neuraminidase. J Biotechnol. 2004;114:21–30. doi: 10.1016/j.jbiotec.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Boulaire J., Zhao Y., Wang S. Gene expression profiling to define host response to baculoviral transduction in the brain. J Neurochem. 2009;109:1203–1214. doi: 10.1111/j.1471-4159.2009.06015.x. [DOI] [PubMed] [Google Scholar]
- Cao Y., Lu Z., Sun P. A pseudotype baculovirus expressing the capsid protein of foot-and-mouth disease virus and a T-Cell immunogen shows enhanced immunogenicity in mice. Virol J. 2011 doi: 10.1186/1743-422X-8-77. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carinhas N., Bernal V., Yokomizo A.Y. Baculovirus production for gene therapy: the role of cell density, multiplicity of infection and medium exchange. Appl Microbiol Biotechnol. 2009;81:1041–1049. doi: 10.1007/s00253-008-1727-4. [DOI] [PubMed] [Google Scholar]
- Carinhas N., Bernal V., Monteiro F. Improving baculovirus production at high cell density through manipulation of energy metabolism. Metab Eng. 2010;12:39–52. doi: 10.1016/j.ymben.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Chan Z.-R., Lai C.-W., Lee H.-P. Determination of the baculovirus transducing titer in mammalian cells. Biotechnol Bioeng. 2006;93:564–571. doi: 10.1002/bit.20749. [DOI] [PubMed] [Google Scholar]
- Chang Y.-J., Liu C.-Y., Chiang B.-L. Induction of IL-8 release in lung cells via activator protein-1 by recombinant baculovirus displaying severe acute respiratory syndrome-coronavirus spike proteins: Identification of two functional regions. J Immunol. 2004;173:7602–7614. doi: 10.4049/jimmunol.173.12.7602. [DOI] [PubMed] [Google Scholar]
- Chapple S.D.J., Jones I.M. Non-polar distribution of green fluorescent protein on the surface of Autographa californica nucleopolyhedrovirus using a heterologous membrane anchor. J Biotechnol. 2002;95:269–275. doi: 10.1016/s0168-1656(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Chen Y.-H., Wu J.-C., Wang K.-C. Baculovirus-mediated production of HDV-like particles in BHK cells using a novel oscillating bioreactor. J Biotechnol. 2005;118:135–147. doi: 10.1016/j.jbiotec.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Chen H.-C., Lee H.-P., Ho Y.-C. Combination of baculovirus-mediated gene transfer and rotating-shaft bioreactor for cartilage tissue engineering. Biomaterials. 2006;27:3154–3162. doi: 10.1016/j.biomaterials.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Chen H.-C., Sung L.-Y., Lo W.-H. Combination of baculovirus-mediated BMP-2 expression and rotating-shaft bioreactor culture synergistically enhances cartilage formation. Gene Ther. 2008;15:309–317. doi: 10.1038/sj.gt.3303087. [DOI] [PubMed] [Google Scholar]
- Chen G.-Y., Chen C.-Y., Chang M.D.-T. Concanavalin A affinity chromatography for efficient baculovirus purification. Biotechnol Prog. 2009;25:1669–1677. doi: 10.1002/btpr.253. [DOI] [PubMed] [Google Scholar]
- Chen G.-Y., Shiah H.-C., Su H.-J. Baculovirus transduction of mesenchymal stem cells triggers the toll-like receptor 3 (TLR3) pathway. J Virol. 2009;83:10548–10556. doi: 10.1128/JVI.01250-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.-C., Chang Y.-H., Chuang C.-K. The repair of osteochondral defects using baculovirus-mediated gene transfer with de-differentiated chondrocytes in bioreactor culture. Biomaterials. 2009;30:674–681. doi: 10.1016/j.biomaterials.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Chen C.-Y., Liu H.-J., Tsai C.-P. Baculovirus as an avian influenza vaccine vector: differential immune responses elicited by different vector forms. Vaccine. 2010;28:7644–7651. doi: 10.1016/j.vaccine.2010.09.048. [DOI] [PubMed] [Google Scholar]
- Chen C.-Y., Wu H.-H., Chen C.-P. Biosafety assessment of human mesenchymal stem cells engineered by hybrid baculovirus vectors. Mol Pharm. 2011 doi: 10.1021/mp100368d. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Chen H.-Z., Liu C.Y.-Y., Wu C.-P. Membrane penetrating peptides greatly enhance baculovirus transduction efficiency into mammalian cells. Biochem Biophys Res Commun. 2011;405:297–302. doi: 10.1016/j.bbrc.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T., Xu C.-Y., Wang Y.-B. A rapid and efficient method to express target genes in mammalian cell by baculovirus. World J Gastroenterol. 2004;10:1612–1618. doi: 10.3748/wjg.v10.i11.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko N., Krougliak N., Eisensmith R.C. A novel system for the production of fully deleted adenovirus vectors that does not require helper adenovirus. Gene Ther. 2001;8:846–854. doi: 10.1038/sj.gt.3301459. [DOI] [PubMed] [Google Scholar]
- Chuang C.-K., Sung L.-Y., Hwang S.-M. Baculovirus as a new gene delivery vector for stem cells engineering and bone tissue engineering. Gene Ther. 2007;14:1417–1424. doi: 10.1038/sj.gt.3302996. [DOI] [PubMed] [Google Scholar]
- Chuang C.-K., Wong T.-H., Hwang S.-M. Baculovirus transduction of mesenchymal stem cells: In vitro responses and in vivo immune responses after cell transplantation. Mol Ther. 2009;17:889–896. doi: 10.1038/mt.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang C.-K., Lin K.-J., Lin C.-Y. Xenotransplantation of human mesenchymal stem cells into immunocompetent rats for calvarial bone repair. Tissue Eng Part A. 2010;16:479–488. doi: 10.1089/ten.TEA.2009.0401. [DOI] [PubMed] [Google Scholar]
- Condreay J.P., Kost T.A. Baculovirus expression vectors for insect and mammalian cells. Curr Drug Targets. 2007;8:1126–1131. doi: 10.2174/138945007782151351. [DOI] [PubMed] [Google Scholar]
- Condreay J.P., Witherspoon S.M., Clay W.C. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc Natl Acad Sci U S A. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condreay J.P., Ames R.S., Hassan N.J. Baculoviruses and mammalian cell-based assays for drug screening. Adv Virus Res. 2006;68:255–286. doi: 10.1016/S0065-3527(06)68007-X. [DOI] [PubMed] [Google Scholar]
- Dojima T., Nishina T., Kato T. Production of scFv-displaying BmNPV in silkworm larvae and its efficient purification. Biotechnol Appl Biochem. 2010;57:63–69. doi: 10.1042/BA20100173. [DOI] [PubMed] [Google Scholar]
- Dong S.C., Wang M.L., Qiu Z.J. Autographa californica multicapsid nucleopolyhedrovirus efficiently infects Sf9 cells and transduces mammalian cells via direct fusion with the plasma membrane at low pH. J Virol. 2010;84:5351–5359. doi: 10.1128/JVI.02517-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Zeng J.M., Zhao Y. The combined use of viral transcriptional and post-transcriptional regulatory elements to improve baculovirus-mediated transient gene expression in human embryonic stem cells. J Biosci Bioeng. 2010;109:1–8. doi: 10.1016/j.jbiosc.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Duisit G., Saleun S., Douthe S. Baculovirus vector requires electrostatic interactions including heparan sulfate for efficient gene transfer in mammalian cells. J Gene Med. 1999;1:93–102. doi: 10.1002/(SICI)1521-2254(199903/04)1:2<93::AID-JGM19>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Efrose R., Swevers L., Iatrou K. Baculoviruses deficient in ie1 gene function abrogate viral gene expression in transduced mammalian cells. Virology. 2010;406:293–301. doi: 10.1016/j.virol.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Ernst W., Schinko T., Spenger A. Improving baculovirus transduction of mammalian cells by surface display of a RGD-motif. J Biotechnol. 2006;126:237–240. doi: 10.1016/j.jbiotec.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Facciabene A., Aurisicchio L., La Monica N. Baculovirus vectors elicit antigen-specific immune responses in mice. J Virol. 2004;78:8663–8672. doi: 10.1128/JVI.78.16.8663-8672.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H.-Y., Pan Y.-F., Fang L.-R. Construction and immunogenicity of recombinant pseudotype baculovirus expressing the capsid protein of porcine circovirus type 2 in mice. J Virol Methods. 2008;150:21–26. doi: 10.1016/j.jviromet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Fang R., Feng H., Nie H. Construction and immunogenicity of pseudotype baculovirus expressing Toxoplasma gondii SAG1 protein in BALB/c mice model. Vaccine. 2010;28:1803–1807. doi: 10.1016/j.vaccine.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Feng Q., Liu Y.-Y., Qu X.-X. Baculovirus surface display of SARS coronavirus (SARS-CoV) spike protein and immunogenicity of the displayed protein in mice models. DNA Cell Biol. 2006;25:668–673. doi: 10.1089/dna.2006.25.668. [DOI] [PubMed] [Google Scholar]
- Ferris M.M., Stepp P.C., Ranno K.A. Evaluation of the Virus Counter® for rapid baculovirus quantitation. J Virol Methods. 2011;171:111–116. doi: 10.1016/j.jviromet.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita R., Matsuyama T., Yamagishi J. Expression of Autographa californica multiple nucleopolyhedrovirus genes in mammalian cells and upregulation of the host β-actin gene. J Virol. 2006;80:2390–2395. doi: 10.1128/JVI.80.5.2390-2395.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., McCormick C.J., Arthur M.J.P. High efficiency gene transfer into cultured primary rat and human hepatic stellate cells using baculovirus vectors. Liver. 2002;22:15–22. doi: 10.1046/j.0106-9543.2001.01555.x. [DOI] [PubMed] [Google Scholar]
- Gao H., Wang Y., Li N. Efficient gene delivery into mammalian cells mediated by a recombinant baculovirus containing a whispovirus ie1 promoter, a novel shuttle promoter between insect cells and mammalian cells. J Biotechnol. 2007;131:138–143. doi: 10.1016/j.jbiotec.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Garzon R., Marcucci G., Croce C.M. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J., Huang Y.-S., Hu X.-T. A surface-modified baculovirus vector with improved gene delivery to B-lymphocytic cells. J Biotechnol. 2007;129:367–372. doi: 10.1016/j.jbiotec.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Georgopoulos L.J., Elgue G., Sanchez J. Preclinical evaluation of innate immunity to baculovirus gene therapy vectors in whole human blood. Mol Immunol. 2009;46:2911–2917. doi: 10.1016/j.molimm.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner C., Haudek-Prinz V.J., Lackner A. Indications for cell stress in response to adenoviral and baculoviral gene transfer observed by proteome profiling of human cancer cells. Electrophoresis. 2010;31:1822–1832. doi: 10.1002/elps.200900753. [DOI] [PubMed] [Google Scholar]
- Gilbert L., Valilehto O., Kirjavainen S. Expression and subcellular targeting of canine parvovirus capsid proteins in baculovirus-transduced NLFK cells. FEBS Lett. 2005;579:385–392. doi: 10.1016/j.febslet.2004.11.101. [DOI] [PubMed] [Google Scholar]
- Grabherr R., Ernst W. Baculovirus for eukaryotic protein display. Curr Gene Ther. 2010;10:195–200. doi: 10.2174/156652310791321297. [DOI] [PubMed] [Google Scholar]
- Grabherr R., Ernst W., Oker-Blom C., Jones I. Developments in the use of baculoviruses for the surface display of complex eukaryotic proteins. Trends Biotechnol. 2001;19:231–236. doi: 10.1016/s0167-7799(01)01610-9. [DOI] [PubMed] [Google Scholar]
- Grabowska A.K., Lipinska A.D., Rohde J. New baculovirus recombinants expressing Pseudorabies virus (PRV) glycoproteins protect mice against lethal challenge infection. Vaccine. 2009;27:3584–3591. doi: 10.1016/j.vaccine.2009.03.067. [DOI] [PubMed] [Google Scholar]
- Grassi G., Kohn H., Dapas B. Comparison between recombinant baculo- and adenoviral-vectors as transfer system in cardiovascular cells. Arch Virol. 2006;151:255–271. doi: 10.1007/s00705-005-0636-4. [DOI] [PubMed] [Google Scholar]
- Guo R., Zhang Y.-F., Liang S. Sodium butyrate enhances the expression of baculovirus-mediated sodium/iodide symporter gene in A549 lung adenocarcinoma cells. Nucl Med Commun. 2010;31:916–921. doi: 10.1097/MNM.0b013e32833dedd7. [DOI] [PubMed] [Google Scholar]
- Guo H., Choudhury Y., Yang J. Antiglioma effects of combined use of a baculovirual vector expressing wild-type p53 and sodium butyrate. J Gene Med. 2011;13:26–36. doi: 10.1002/jgm.1522. [DOI] [PubMed] [Google Scholar]
- Han Y., Niu M.-S., An L.-J. Upregulation of proinflammatory cytokines and NO production in BV-activated avian macrophage-like cell line (HD11) requires MAPK and NF-κB pathways. Int Immunopharmacol. 2009;9:817–823. doi: 10.1016/j.intimp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Han Y., Niu M.-S., An L.-J. Involvement of TLR21 in baculovirus-induced interleukin-12 gene expression in avian macrophage-like cell line HD11. Vet Microbiol. 2010;144:75–81. doi: 10.1016/j.vetmic.2009.11.001. [DOI] [PubMed] [Google Scholar]
- He F., Ho Y.-F., Yu L. WSSV ie1 promoter is more efficient than CMV promoter to express H5 hemagglutinin from influenza virus in baculovirus as a chicken vaccine. BMC Microbiol. 2008;8 doi: 10.1186/1471-2180-8-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervas-Stubbs S., Rueda P., Lopez L. Insect baculoviruses strongly potentiate adaptive immune responses by inducing type I IFN. J Immunol. 2007;178:2361–2369. doi: 10.4049/jimmunol.178.4.2361. [DOI] [PubMed] [Google Scholar]
- Hitchman R.B., Possee R.D., King L.A. Baculovirus expression systems for recombinant protein production in insect cells. Recent Pat Biotechnol. 2009;3:46–54. doi: 10.2174/187220809787172669. [DOI] [PubMed] [Google Scholar]
- Ho Y.-C., Chen H.-C., Wang K.-C. Highly efficient baculovirus-mediated gene transfer into rat chondrocytes. Biotechnol Bioeng. 2004;88:643–651. doi: 10.1002/bit.20239. [DOI] [PubMed] [Google Scholar]
- Ho Y.-C., Chung Y.-C., Hwang S.-M., Wang K.-C., Hu Y.-C. Transgene expression and differentiation of baculovirus-transduced human mesenchymal stem cells. J Gene Med. 2005;7:860–868. doi: 10.1002/jgm.729. [DOI] [PubMed] [Google Scholar]
- Ho Y.-C., Lee H.-P., Hwang S.-M. Baculovirus transduction of human mesenchymal stem cell-derived progenitor cells: variation of transgene expression with cellular differentiation states. Gene Ther. 2006;13:1471–1479. doi: 10.1038/sj.gt.3302796. [DOI] [PubMed] [Google Scholar]
- Hoare J., Waddington S., Thomas H.C. Complement inhibition rescued mice allowing observation of transgene expression following intraportal delivery of baculovirus in mice. J Gene Med. 2005;7:325–333. doi: 10.1002/jgm.671. [DOI] [PubMed] [Google Scholar]
- Hofmann C., Huser A., Lehnert W. Protection of baculovirus-vectors against complement-mediated inactivation by recombinant soluble complement receptor type 1. Biol Chem. 1999;380:393–395. doi: 10.1515/BC.1999.052. [DOI] [PubMed] [Google Scholar]
- Hu Y.-C. Baculovirus as a highly efficient expression vector in insect and mammalian cells. Acta Pharmacol Sin. 2005;26:405–416. doi: 10.1111/j.1745-7254.2005.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.-C. Baculovirus vectors for gene therapy. Adv Virus Res. 2006;68:287–320. doi: 10.1016/S0065-3527(06)68008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.-C. Baculoviral vectors for gene delivery: a review. Curr Gene Ther. 2008;8:54–65. doi: 10.2174/156652308783688509. [DOI] [PubMed] [Google Scholar]
- Hu Y.-C., Tsai C.-T., Chang Y.-J. Enhancement and prolongation of baculovirus-mediated expression in mammalian cells: focuses on strategic infection and feeding. Biotechnol Prog. 2003;19:373–379. doi: 10.1021/bp025609d. [DOI] [PubMed] [Google Scholar]
- Hu Y.-C., Yao K., Wu T.-Y. Baculovirus as an expression and/or delivery vehicle for vaccine antigens. Expert Rev Vaccines. 2008;7:363–371. doi: 10.1586/14760584.7.3.363. [DOI] [PubMed] [Google Scholar]
- Huang K.-S., Lo W.-H., Chung Y.-C. Combination of baculovirus-mediated gene delivery and packed-bed reactor for scalable production of adeno-associated virus. Hum Gene Ther. 2007;18:1161–1170. doi: 10.1089/hum.2007.107. [DOI] [PubMed] [Google Scholar]
- Huang W., Tian X.-L., Wu Y.-L. Suppression of gastric cancer growth by baculovirus vector-mediated transfer of normal epithelial cell specific-1 gene. World J Gastroenterol. 2008;14:5810–5815. doi: 10.3748/wjg.14.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Cao S., Cui X. Efficient gene delivery into fish cells by an improved recombinant baculovirus. J Virol Methods. 2011;173:294–299. doi: 10.1016/j.jviromet.2011.02.022. [DOI] [PubMed] [Google Scholar]
- Huser A., Rudolph M., Hofmann C. Incorporation of decay-accelerating factor into the baculovirus envelope generates complement-resistant gene transfer vectors. Nat Biotechnol. 2001;19:451–455. doi: 10.1038/88122. [DOI] [PubMed] [Google Scholar]
- Ihalainen T.O., Laakkonen J.P., Paloheimo O. Morphological characterization of baculovirus Autographa californica multiple nucleopolyhedrovirus. Virus Res. 2010;148:71–74. doi: 10.1016/j.virusres.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Ishii K.J., Koyama S., Nakagawa A. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardin B.A., Zhao Y., Selvaraj M. Expression of SEAP (secreted alkaline phosphatase) by baculovirus mediated transduction of HEK 293 cells in a hollow fiber bioreactor system. J Biotechnol. 2008;135:272–280. doi: 10.1016/j.jbiotec.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Jin R., Lv Z., Chen Q. Safety and immunogenicity of H5N1 influenza vaccine based on baculovirus surface display system of Bombyx mori. PLoS One. 2008;3:e3933. doi: 10.1371/journal.pone.0003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorio H., Tran R., Meghrous J. Analysis of baculovirus aggregates using flow cytometry. J Virol Methods. 2006;134:8–14. doi: 10.1016/j.jviromet.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Kaikkonen M.U., Raty J.K., Airenne K.J. Truncated vesicular stomatitis virus G protein improves baculovirus transduction efficiency in vitro and in vivo. Gene Ther. 2006;13:304–312. doi: 10.1038/sj.gt.3302657. [DOI] [PubMed] [Google Scholar]
- Kaikkonen M.U., Viholainen J.I., Narvanen A. Targeting and purification of metabolically biotinylated baculovirus. Hum Gene Ther. 2008;19:589–600. doi: 10.1089/hum.2007.177. [DOI] [PubMed] [Google Scholar]
- Kaikkonen M.U., Maatta A.I., Yla-Herttuala S. Screening of complement inhibitors: shielded baculoviruses increase the safety and efficacy of gene delivery. Mol Ther. 2010;18:987–992. doi: 10.1038/mt.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaname Y., Tani H., Kataoka C. Acquisition of complement resistance through incorporation of CD55/decay-accelerating factor into viral particles bearing baculovirus GP64. J Virol. 2010;84:3210–3219. doi: 10.1128/JVI.02519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H., Suzuki H., Abe T., Miyano-Kurosaki N., Takaku H. Inhibition of HIV-1 replication by vesicular stomatitis virus envelope glycoprotein pseudotyped baculovirus vector-transduced ribozyme in mammalian cells. Biochem Biophys Res Commun. 2006;349:1220–1227. doi: 10.1016/j.bbrc.2006.08.184. [DOI] [PubMed] [Google Scholar]
- Kärkkäinen H.R., Lesch H.P., Määttä A.I. A 96-well format for a high-throughput baculovirus generation, fast titering and recombinant protein production in insect and mammalian cells. BMC Res Notes. 2009;23:63. doi: 10.1186/1756-0500-2-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G.M., Klopfleisch C., Giesow K. Novel vectors for simultaneous high-level dual protein expression in vertebrate and insect cells by recombinant baculoviruses. J Virol Methods. 2009;160:132–137. doi: 10.1016/j.jviromet.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Kenoutis C., Efrose R.C., Swevers L. Baculovirus-mediated gene delivery into mammalian cells does not alter their transcriptional and differentiating potential but is accompanied by early viral gene expression. J Virol. 2006;80:4135–4146. doi: 10.1128/JVI.80.8.4135-4146.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-K., Park I.-K., Jiang H.-L. Regulation of transduction efficiency by pegylation of baculovirus vector in vitro and in vivo. J Biotechnol. 2006;125:104–109. doi: 10.1016/j.jbiotec.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Kim C.-H., Yoon J.-S., Sohn H.-J. Direct vaccination with pseudotype baculovirus expressing murine telomerase induces anti-tumor immunity comparable with RNA-electroporated dendritic cells in a murine glioma model. Cancer Lett. 2007;250:276–283. doi: 10.1016/j.canlet.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Kim Y.-K., Kwon J.-T., Choi J.-Y. Suppression of tumor growth in xenograft model mice by programmed cell death 4 gene delivery using folate-PEG-baculovirus. Cancer Gene Ther. 2010;17:751–760. doi: 10.1038/cgt.2010.28. [DOI] [PubMed] [Google Scholar]
- Kinnunen K., Kalesnykas G., Mahonen A.J., Laidinen S., Holma L., Heikura T. Baculovirus is an efficient vector for the transduction of the eye: Comparison of baculovirus- and adenovirus-mediated intravitreal vascular endothelial growth factor D gene transfer in the rabbit eye. J Gene Med. 2009;11:382–389. doi: 10.1002/jgm.1311. [DOI] [PubMed] [Google Scholar]
- Kitagawa Y., Tani H., Kwang C. Ligand-directed gene targeting to mammalian cells by pseudotype baculoviruses. J Virol. 2005;79:3639–3652. doi: 10.1128/JVI.79.6.3639-3652.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Hamazaki H., Miyano-Kurosaki N. Characterization of baculovirus Autographa californica multiple nuclear polyhedrosis virus infection in mammalian cells. Biochem Biophys Res Commun. 2006;343:378–384. doi: 10.1016/j.bbrc.2006.02.167. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Abe T., Miyano-Kurosaki N. Induction of natural killer cell-dependent antitumor immunity by the Autographa californica multiple nuclear polyhedrosis virus. Mol Ther. 2008;16:261–268. doi: 10.1038/sj.mt.6300364. [DOI] [PubMed] [Google Scholar]
- Kitidee K., Nangola S., Gonzalez G. Baculovirus display of single chain antibody (scFv) using a novel signal peptide. BMC Biotechnol. 2010;10:80. doi: 10.1186/1472-6750-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost T.A., Condreay J.P. Recombinant baculoviruses as expression vectors for insect and mammalian cells. Curr Opin Biotechnol. 1999;10:428–433. doi: 10.1016/s0958-1669(99)00005-1. [DOI] [PubMed] [Google Scholar]
- Kost T.A., Condreay J.P. Recombinant baculoviruses as mammalian cell gene delivery vectors. Trends Biotechnol. 2002;20:173–180. doi: 10.1016/s0167-7799(01)01911-4. [DOI] [PubMed] [Google Scholar]
- Kost T.A., Condreay J.P., Jarvis D.L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost T.A., Condreay J.P., Ames R.S. Baculovirus gene delivery: a flexible assay development tool. Curr Gene Ther. 2010;10:168–173. doi: 10.2174/156652310791321224. [DOI] [PubMed] [Google Scholar]
- Kukkonen S.P., Airenne K.J., Marjomaki V. Baculovirus capsid display: a novel tool for transduction imaging. Mol Ther. 2003;8:853–862. doi: 10.1016/j.ymthe.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Kumar S., Wan C., Ramaswamy G. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther. 2010;18:1026–1034. doi: 10.1038/mt.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakkonen J.P., Kaikkonen M.U., Ronkainen P.H.A. Baculovirus-mediated immediate-early gene expression and nuclear reorganization in human cells. Cell Microbiol. 2008;10:667–681. doi: 10.1111/j.1462-5822.2007.01074.x. [DOI] [PubMed] [Google Scholar]
- Laakkonen J.P., Makela A.R., Kakkonen E. Clathrin-independent entry of baculovirus triggers uptake of E. coli in non-phagocytic human cells. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner A., Genta K., Koppensteiner H. A bicistronic baculovirus vector for transient and stable protein expression in mammalian cells. Anal Biochem. 2008;380:146–148. doi: 10.1016/j.ab.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Lavdas A.A., Efrose R., Douris V., Gaitanou M., Papastefanaki F., Swevers L. Soluble forms of the cell adhesion molecule L1 produced by insect and baculovirus-transduced mammalian cells enhance Schwann cell motility. J Neurochem. 2010;115:1137–1149. doi: 10.1111/j.1471-4159.2010.07003.x. [DOI] [PubMed] [Google Scholar]
- Lee H.-P., Chen Y.-L., Shen H.-C. Baculovirus transduction of rat articular chondrocytes: roles of cell cycle. J Gene Med. 2007;9:33–43. doi: 10.1002/jgm.994. [DOI] [PubMed] [Google Scholar]
- Lee H.-P., Ho Y.-C., Hwang S.-M. Variation of baculovirus-harbored transgene transcription among mesenchymal stem cell-derived progenitors leads to varied expression. Biotechnol Bioeng. 2007;97:649–655. doi: 10.1002/bit.21261. [DOI] [PubMed] [Google Scholar]
- Lee H.-P., Matsuura Y., Chen H.-C. Baculovirus transduction of chondrocytes elicits interferon-α/β and suppresses transgene expression. J Gene Med. 2009;11:302–312. doi: 10.1002/jgm.1299. [DOI] [PubMed] [Google Scholar]
- Lee H.-J., Park N., Cho H.-J. Development of a novel viral DNA vaccine against human papillomavirus: AcHERV-HP16L1. Vaccine. 2010;28:1613–1619. doi: 10.1016/j.vaccine.2009.11.044. [DOI] [PubMed] [Google Scholar]
- Lehtolainen P., Tyynela K., Kannasto J., Airenne K.J., Yla-Herttuala S. Baculoviruses exhibit restricted cell type specificity in rat brain: a comparison of baculovirus- and adenovirus-mediated intracerebral gene transfer in vivo. Gene Ther. 2002;9:1693–1699. doi: 10.1038/sj.gt.3301854. [DOI] [PubMed] [Google Scholar]
- Lesch H.P., Turpeinen S., Niskanen E.A. Generation of lentivirus vectors using recombinant baculoviruses. Gene Ther. 2008;15:1280–1286. doi: 10.1038/gt.2008.76. [DOI] [PubMed] [Google Scholar]
- Lesch H.P., Laitinen A., Peixoto C. Production and purification of lentiviral vectors generated in 293T suspension cells with baculoviral vectors. Gene Ther. 2011;18(6):531–538. doi: 10.1038/gt.2010.162. [DOI] [PubMed] [Google Scholar]
- Levi B., James A.W., Nelson E.R. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang X., Guo H. Axonal transport of recombinant baculovirus vectors. Mol Ther. 2004;10:1121–1129. doi: 10.1016/j.ymthe.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Li M., Wang Y.-F., Wang Y. Immune responses induced by a BacMam virus expressing the E2 protein of classical swine fever virus in mice. Immunol Lett. 2009;125:145–150. doi: 10.1016/j.imlet.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Li Y.-M., Ye J., Cao S.-B. Immunization with pseudotype baculovirus expressing envelope protein of Japanese encephalitis virus elicits protective immunity in mice. J Gene Med. 2009;11:150–159. doi: 10.1002/jgm.1282. [DOI] [PubMed] [Google Scholar]
- Lin Y.-H., Lee L.-H., Shih W.-L. Baculovirus surface display of σC and σB proteins of avian reovirus and immunogenicity of the displayed proteins in a mouse model. Vaccine. 2008;26:6361–6367. doi: 10.1016/j.vaccine.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Lin C.-Y., Chang Y.-H., Lin K.-J. The healing of critical-sized femoral segmental bone defects in rabbits using baculovirus-engineered mesenchymal stem cells. Biomaterials. 2010;31:3222–3230. doi: 10.1016/j.biomaterials.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Lin C.-Y., Lu C.-H., Luo W.-Y. Baculovirus as a gene delivery vector for cartilage and bone tissue engineering. Curr Gene Ther. 2010;10:242–254. doi: 10.2174/156652310791321242. [DOI] [PubMed] [Google Scholar]
- Lin S.-Y., Chen G.-Y., Hu Y.-C. Recent patents on the baculovirus systems. Recent Pat Biotechnol. 2011;5:1–11. doi: 10.2174/187220811795655904. [DOI] [PubMed] [Google Scholar]
- Lin C.Y., Lin K.-J., Kao C.-Y. Adipose-derived stem cells engineered with the persistently expressing hybrid baculovirus augment the healing of massive bone defects. Spine. 2011;22:1–13. doi: 10.1016/j.biomaterials.2011.05.059. [DOI] [PubMed] [Google Scholar]
- Liu X., Li K., Song J. Efficient and stable gene expression in rabbit intervertebral disc cells transduced with a recombinant baculovirus vector. Spine. 2006;31:732–735. doi: 10.1097/01.brs.0000206977.61305.43. [DOI] [PubMed] [Google Scholar]
- Liu C.Y.-Y., Wang C.-H., Wang J.-C. Stimulation of baculovirus transcriptome expression in mammalian cells by baculoviral transcriptional activators. J Gen Virol. 2007;88:2176–2184. doi: 10.1099/vir.0.82664-0. [DOI] [PubMed] [Google Scholar]
- Liu C.Y.-Y., Wang C.-H., Hsiao W.-K. RING and Coiled-Coil domains of baculovirus IE2 are critical in strong activation of the cytomegalovirus major immediate-early promoter in mammalian cells. J Virol. 2009;83:3604–3616. doi: 10.1128/JVI.01778-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.-S., Xu Y.-F., Feng S.-W. Baculovirus-transduced mouse amniotic fluid-derived stem cells maintain differentiation potential. Ann Hematol. 2009;88:565–572. doi: 10.1007/s00277-008-0634-1. [DOI] [PubMed] [Google Scholar]
- Liu C.Y.-Y., Chen H.-Z., Chao Y.-C. Maximizing baculovirus-mediated foreign proteins expression in mammalian cells. Curr Gene Ther. 2010;10:232–241. doi: 10.2174/156652310791321215. [DOI] [PubMed] [Google Scholar]
- Lo H.-R., Chao Y.-C. Rapid titer determination of baculovirus by quantitative real-time polymerase chain reaction. Biotechnol Prog. 2004;20:354–360. doi: 10.1021/bp034132i. [DOI] [PubMed] [Google Scholar]
- Lo W.-H., Hwang S.-M., Chuang C.-K. Development of a hybrid baculoviral vector for sustained transgene expression. Mol Ther. 2009;17:658–666. doi: 10.1038/mt.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo W.-H., Chen C.-Y., Yeh C.-N. Rapid baculovirus titration based on regulatable green fluorescent protein expression in mammalian cells. Enzyme Microb Technol. 2011;48:13–18. doi: 10.1016/j.enzmictec.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Long G., Pan X., Kormelink R. Functional entry of baculovirus into insect and mammalian cells is dependent on clathrin-mediated endocytosis. J Virol. 2006;80:8830–8833. doi: 10.1128/JVI.00880-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L.-Q., Ho Y.-F., Kwang J. Suppression of porcine arterivirus replication by baculovirus-delivered shRNA targeting nucleoprotein. Biochem Biophys Res Commun. 2006;340:1178–1183. doi: 10.1016/j.bbrc.2005.12.133. [DOI] [PubMed] [Google Scholar]
- Lu L., Yu L., Kwang J. Baculovirus surface-displayed hemagglutinin of H5N1 influenza virus sustains its authentic cleavage, hemagglutination activity, and antigenicity. Biochem Biophys Res Commun. 2007;358:404–409. doi: 10.1016/j.bbrc.2007.04.148. [DOI] [PubMed] [Google Scholar]
- Lucifora J., Durantel D., Belloni L. Initiation of hepatitis B virus genome replication and production of infectious virus following delivery in HepG2 cells by novel recombinant baculovirus vector. J Gen Virol. 2008;89:1819–1828. doi: 10.1099/vir.0.83659-0. [DOI] [PubMed] [Google Scholar]
- Madhan S., Prabakaran M., Kwang J. Baculovirus as vaccine vectors. Curr Gene Ther. 2010;10:201–213. doi: 10.2174/156652310791321233. [DOI] [PubMed] [Google Scholar]
- Mahonen A.J., Airenne K.J., Purola S. Post-transcriptional regulatory element boosts baculovirus-mediated gene expression in vertebrate cells. J Biotechnol. 2007;131:1–8. doi: 10.1016/j.jbiotec.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Mahonen A.J., Makkonen K.E., Laakkonen J.P. Culture medium induced vimentin reorganization associates with enhanced baculovirus-mediated gene delivery. J Biotechnol. 2010;145:111–119. doi: 10.1016/j.jbiotec.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Makela A.R., Oker-Blom C. Baculovirus display: A multifunctional technology for gene delivery and eukaryotic library development. Adv Virus Res. 2006;68:91–112. doi: 10.1016/S0065-3527(06)68003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela A.R., Matilainen H., White D.J. Enhanced baculovirus-mediated transduction of human cancer cells by tumor-homing peptides. J Virol. 2006;80:6603–6611. doi: 10.1128/JVI.00528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela A.R., Enback J., Laakkonen J.P. Tumor targeting of baculovirus displaying a lymphatic homing peptide. J Gene Med. 2008;10:1019–1031. doi: 10.1002/jgm.1222. [DOI] [PubMed] [Google Scholar]
- Martyn J.C., Cardin A.J., Wines B.D. Surface display of IgG Fc on baculovirus vectors enhances binding to antigen-presenting cells and cell lines expressing Fc receptors. Arch Virol. 2009;154:1129–1138. doi: 10.1007/s00705-009-0423-8. [DOI] [PubMed] [Google Scholar]
- Matilainen H., Rinne J., Gilbert L. Baculovirus entry into human hepatoma cells. J Virol. 2005;79:15452–15459. doi: 10.1128/JVI.79.24.15452-15459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo E., Tani H., Lim C.k. Characterization of HCV-like particles produced in a human hepatoma cell line by a recombinant baculovirus. Biochem Biophys Res Commun. 2006;340:200–208. doi: 10.1016/j.bbrc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- McCormick C.J., Rowlands D.J., Harris M. Efficient delivery and regulable expression of hepatitis C virus full-length and minigenome constructs in hepatocyte-derived cell lines using baculovirus vectors. J Gen Virol. 2002;83:383–394. doi: 10.1099/0022-1317-83-2-383. [DOI] [PubMed] [Google Scholar]
- Merrihew R.V., Clay W.C., Condreay J.P. Chromosomal integration of transduced recombinant baculovirus DNA in mammalian cells. J Virol. 2001;75:903–909. doi: 10.1128/JVI.75.2.903-909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlambo G., Kumar N., Yoshida S. Functional immunogenicity of baculovirus expressing Pfs25, a human malaria transmission-blocking vaccine candidate antigen. Vaccine. 2010;28:7025–7029. doi: 10.1016/j.vaccine.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Molinari P., García-Nuñez S., Gravisaco M.J. Baculovirus treatment fully protects mice against a lethal challenge of FMDV. Antiviral Res. 2010;87:276–279. doi: 10.1016/j.antiviral.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Nakowitsch S., Kittel C., Ernst W. Optimization of baculovirus transduction on FreeStyle (™) 293 cells for the generation of influenza B/Lee/40. Mol Biotechnol. 2006;34:157–164. doi: 10.1385/mb:34:2:157. [DOI] [PubMed] [Google Scholar]
- Nicholson L.J., Philippe M., Paine A.J. RNA interference mediated in human primary cells via recombinant baculoviral vectors. Mol Ther. 2005;11:638–644. doi: 10.1016/j.ymthe.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Niu M.S., Han Y., Li W.L. Baculovirus up-regulates antiviral systems and induces protection against infectious bronchitis virus challenge in neonatal chicken. Int Immunopharmacol. 2008;8:1609–1615. doi: 10.1016/j.intimp.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Nizamani Z.A., Keil G.M., Albina E. Potential of adenovirus and baculovirus vectors for the delivery of shRNA against morbilliviruses. Antiviral Res. 2011;90(1):98–101. doi: 10.1016/j.antiviral.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Ong S.-T., Li F., Du J. Hybrid cytomegalovirus enhancer-H1 promoter-based plasmid and baculovirus vectors mediate effective RNA interference. Hum Gene Ther. 2005;16:1404–1412. doi: 10.1089/hum.2005.16.1404. [DOI] [PubMed] [Google Scholar]
- Pan Y., Zhao Q., Fang L. Efficient gene delivery into mammalian cells by recombinant baculovirus containing a hybrid cytomegalovirus promoter/Semliki Forest virus replicon. J Gene Med. 2009;11:1030–1038. doi: 10.1002/jgm.1390. [DOI] [PubMed] [Google Scholar]
- Pan Y., Fang L., Fan H. Antitumor effects of a recombinant pseudotype baculovirus expressing Apoptin in vitro and in vivo. Int J Cancer. 2010;126:2741–2751. doi: 10.1002/ijc.24959. [DOI] [PubMed] [Google Scholar]
- Paul A., Prakash S. Baculovirus reveals a new pH-dependent direct cell-fusion pathway for cell entry and transgene delivery. Future Virol. 2010;5:533–537. [Google Scholar]
- Peralta A., Molinari P., Conte-Grand D. A chimeric baculovirus displaying bovine herpesvirus-1 (BHV-1) glycoprotein D on its surface and their immunological properties. Appl Microbiol Biotechnol. 2007;75:407–414. doi: 10.1007/s00253-006-0825-4. [DOI] [PubMed] [Google Scholar]
- Ping W.-X., Ge J.-P., Li S.-X. Baculovirus-mediated gene expression in chicken primary cells. Avian Dis. 2006;50:59–63. doi: 10.1637/7418-080705R.1. [DOI] [PubMed] [Google Scholar]
- Prabakaran M., Velumani S., He F. Protective immunity against influenza H5N1 virus challenge in mice by intranasal co-administration of baculovirus surface-displayed HA and recombinant CTB as an adjuvant. Virology. 2008;380:412–420. doi: 10.1016/j.virol.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Prabakaran M., He F., Meng T. Neutralizing epitopes of influenza virus hemagglutinin: target for the development of a universal vaccine against H5N1 lineages. J Virol. 2010;84:11822–11830. doi: 10.1128/JVI.00891-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran M., Madhan S., Prabhu N. Reverse micelle-encapsulated recombinant baculovirus as an oral vaccine against H5N1 infection in mice. Antiviral Res. 2010;86:180–187. doi: 10.1016/j.antiviral.2010.02.315. [DOI] [PubMed] [Google Scholar]
- Prabakaran M., Madhan S., Prabhu N. Gastrointestinal delivery of baculovirus displaying influenza virus hemagglutinin protects mice against heterologous H5N1 infection. J Virol. 2010;84:3201–3209. doi: 10.1128/JVI.02175-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raty J.K., Airenne K.J., Marttila A.T. Enhanced gene delivery by avidin-displaying baculovirus. Mol Ther. 2004;9:282–291. doi: 10.1016/j.ymthe.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Raty J.K., Liimatainen T., Wirth T., Airenne K.J., Ihalainen T.O., Huhtala T. Magnetic resonance imaging of viral particle biodistribution in vivo. Gene Ther. 2006;13:1440–1446. doi: 10.1038/sj.gt.3302828. [DOI] [PubMed] [Google Scholar]
- Raty J.K., Liimatainen T., Huhtala T., Kaikkonen M.U., Airenne K.J., Hakumaki J.M. SPECT/CT imaging of baculovirus biodistribution in rat. Gene Ther. 2007;14:930–938. doi: 10.1038/sj.gt.3302934. [DOI] [PubMed] [Google Scholar]
- Roldao A., Oliveira R., Carrondo M.J.T. Error assessment in recombinant baculovirus titration: evaluation of different methods. J Virol Methods. 2009;159:69–80. doi: 10.1016/j.jviromet.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Salminen M., Airenne K.J., Rinnankoski R., Reimari J., Valilehto O., Rinne J. Improvement in nuclear entry and transgene expression of baculoviruses by disintegration of microtubules in human hepatocytes. J Virol. 2005;79:2720–2728. doi: 10.1128/JVI.79.5.2720-2728.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L., Wang L.Y., Yin J., Zhong P., Zhong J. An OriP/EBNA-1-based baculovirus vector with prolonged and enhanced transgene expression. J Gene Med. 2006;8:1400–1406. doi: 10.1002/jgm.978. [DOI] [PubMed] [Google Scholar]
- Schutz A., Scheller N., Breinig T. The Autographa californica nuclear polyhedrosis virus AcNPV induces functional maturation of human monocyte-derived dendritic cells. Vaccine. 2006;24:7190–7196. doi: 10.1016/j.vaccine.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Shen H.-C., Lee H.-P., Lo W.-H. Baculovirus-mediated gene transfer is attenuated by sodium bicarbonate. J Gene Med. 2007;9:470–478. doi: 10.1002/jgm.1037. [DOI] [PubMed] [Google Scholar]
- Shen H.-C., Yeh C.-N., Chen G.-Y. Sustained baculovirus-mediated expression in myogenic cells. J Gene Med. 2008;10:1190–1197. doi: 10.1002/jgm.1245. [DOI] [PubMed] [Google Scholar]
- Sollerbrant K., Elmen J., Wahlestedt C., Acker J., Leblois-Prehaud H., Latta-Mahieu M. A novel method using baculovirus-mediated gene transfer for production of recombinant adeno-associated virus vectors. J Gen Virol. 2001;82:2051–2060. doi: 10.1099/0022-1317-82-9-2051. [DOI] [PubMed] [Google Scholar]
- Song S.-U., Shin S.-H., Kim S.-K. Effective transduction of osteogenic sarcoma cells by a baculovirus vector. J Gen Virol. 2003;84:697–703. doi: 10.1099/vir.0.18772-0. [DOI] [PubMed] [Google Scholar]
- Song J.-H., Liang C.-Y., Chen X.-W. Transduction of avian cells with recombinant baculovirus. J Virol Methods. 2006;135:157–162. doi: 10.1016/j.jviromet.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Song L., Liu Y.-Y., Chen J.-C. Baculoviral capsid display of His-tagged ZnO inorganic binding peptide. Cytotechnology. 2010;62:133–141. doi: 10.1007/s10616-010-9269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey J.L., Chiari E.F., Isom H.C. Hepatitis B virus (HBV)-specific short hairpin RNA is capable of reducing the formation of HBV covalently closed circular (CCC) DNA but has no effect on established CCC DNA in vitro. J Gen Virol. 2009;90:115–126. doi: 10.1099/vir.0.004408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R., Huser A., Ni S. Baculovirus-based vaccination vectors allow for efficient induction of immune responses against Plasmodium falciparum circumsporozoite protein. Mol Ther. 2007;15:193–202. doi: 10.1038/sj.mt.6300008. [DOI] [PubMed] [Google Scholar]
- Sung L.-Y., Lo W.-H., Chiu H.-Y. Modulation of chondrocyte phenotype via baculovirus-mediated growth factor expression. Biomaterials. 2007;28:3437–3447. doi: 10.1016/j.biomaterials.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Sung L.-Y., Chiu H.-Y., Chen H.-C. Baculovirus-mediated growth factor expression in dedifferentiated chondrocytes accelerates redifferentiation: Effects of combinational transduction. Tissue Eng Part A. 2009;15:1353–1362. doi: 10.1089/ten.tea.2008.0310. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Matsumoto N., Suzuki T. Stable replication of the EBNA1/OriP-mediated baculovirus vector and its application to anti-HCV gene therapy. Virol J. 2009;6:156–163. doi: 10.1186/1743-422X-6-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Saitoh H., Suzuki T. Baculovirus-mediated bispecific short-hairpin small-interfering RNAs have remarkable ability to cope with both influenza viruses A and B. Oligonucleotides. 2009;19:307–316. doi: 10.1089/oli.2009.0189. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Tamai N., Habu Y. Suppression of hepatitis C virus replication by baculovirus vector-mediated short-hairpin RNA expression. FEBS Lett. 2009;582:3085–3089. doi: 10.1016/j.febslet.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Chang M.O., Kitajima M. Baculovirus activates murine dendritic cells and induces non-specific NK cell and T cell immune responses. Cell Immunol. 2010;262:35–43. doi: 10.1016/j.cellimm.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Oo Chang M., Kitajima M. Induction of antitumor immunity against mouse carcinoma by baculovirus-infected dendritic cells. Cell Mol Immunol. 2010;7:440–446. doi: 10.1038/cmi.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Musthaq S., Madhan S., Hameed A.S.S. Localization of VP28 on the baculovirus envelope and its immunogenicity against white spot syndrome virus in Penaeus monodon. Virology. 2009;391:315–324. doi: 10.1016/j.virol.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Tami C., Peralta A., Barbieri R. Immunological properties of FMDV-gp64 fusion proteins expressed on SF9 cell and baculovirus surfaces. Vaccine. 2004;23:840–845. doi: 10.1016/j.vaccine.2004.03.070. [DOI] [PubMed] [Google Scholar]
- Tang X.C., Lu H.R., Ross T.M. Hemagglutinin displayed baculovirus protects against highly pathogenic influenza. Vaccine. 2010;28:6821–6831. doi: 10.1016/j.vaccine.2010.08.040. [DOI] [PubMed] [Google Scholar]
- Tani H., Nishijima M., Ushijima H. Characterization of cell-surface determinants important for baculovirus infection. Virology. 2001;279:343–353. doi: 10.1006/viro.2000.0699. [DOI] [PubMed] [Google Scholar]
- Tani H., Limn C.-K., Yap C.-C. In vitro and in vivo gene delivery by recombinant baculoviruses. J Virol. 2003;77:9799–9808. doi: 10.1128/JVI.77.18.9799-9808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H., Abe T., Matsunaga T.M. Baculovirus vector for gene delivery and vaccine development. Future Virol. 2008;3:35–43. [Google Scholar]
- Transfiguracion J., Jorio H., Meghrous J. High yield purification of functional baculovirus vectors by size exclusion chromatography. J Virol Methods. 2007;142:21–28. doi: 10.1016/j.jviromet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Tsai C.-T., Chan Z.-R., Lu J.-T. Factors influencing the production and storage of baculovirus for gene delivery: an alternative perspective from the transducing titer assay. Enzyme Microb Technol. 2007;40:1345–1351. [Google Scholar]
- van Loo N.D., Fortunati E., Ehlert E. Baculovirus infection of nondividing mammalian cells: mechanisms of entry and nuclear transport of capsids. J Virol. 2001;75:961–970. doi: 10.1128/JVI.75.2.961-970.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente T., Peixoto C., Carrondo M.J.T. Purification of recombinant baculoviruses for gene therapy using membrane processes. Gene Ther. 2009;16:766–775. doi: 10.1038/gt.2009.33. [DOI] [PubMed] [Google Scholar]
- Vicente T., Mota J.P.B., Peixoto C. Analysis of adsorption of a baculovirus bioreaction bulk on an ion-exchange surface by surface plasmon resonance. J Biotechnol. 2010;148:171–181. doi: 10.1016/j.jbiotec.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Vicente T., Peixoto C., Alves P.M. Modeling electrostatic interactions of baculovirus vectors for ion-exchange process development. J Chromatogr A. 2010;1217:3754–3764. doi: 10.1016/j.chroma.2010.03.059. [DOI] [PubMed] [Google Scholar]
- Wagle M., Jesuthasan S. Baculovirus-mediated gene expression in zebrafish. Mar Biotechnol. 2003;5:58–63. doi: 10.1007/s10126-002-0050-9. [DOI] [PubMed] [Google Scholar]
- Wang S., Balasundaram G. Potential cancer gene therapy by baculoviral transduction. Curr Gene Ther. 2010;10:214–225. doi: 10.2174/156652310791321251. [DOI] [PubMed] [Google Scholar]
- Wang K.-C., Wu J.-C., Chung Y.-C. Baculovirus as a highly efficient gene delivery vector for the expression of hepatitis delta virus antigens in mammalian cells. Biotechnol Bioeng. 2005;89:464–473. doi: 10.1002/bit.20385. [DOI] [PubMed] [Google Scholar]
- Wang C.-Y., Li F., Yang Y. Recombinant baculovirus containing the Diphtheria toxin A gene for malignant glioma therapy. Cancer Res. 2006;66:5798–5806. doi: 10.1158/0008-5472.CAN-05-4514. [DOI] [PubMed] [Google Scholar]
- Wang S.-P., Fang L.-R., Fan H.-Y. Construction and immunogenicity of pseudotype baculovirus expressing GP5 and M protein of porcine reproductive and respiratory syndrome virus. Vaccine. 2007;25:8220–8227. doi: 10.1016/j.vaccine.2007.09.069. [DOI] [PubMed] [Google Scholar]
- Wang L.Y., Shan L., Lo K.W., Yin J., Zhang Y.Y., Sun R. Inhibition of nasopharyngeal carcinoma growth by RTA-expressing baculovirus vectors containing oriP. J Gene Med. 2008;10:1124–1133. doi: 10.1002/jgm.1237. [DOI] [PubMed] [Google Scholar]
- Wang X., Yin J., Huang X.-X. DNA methyltransferase inhibitors increase baculovirus-mediated gene expression in mammalian cells when applied before infection. Anal Biochem. 2010;396:322–324. doi: 10.1016/j.ab.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Wilson S., Baird M., Ward V.K. Delivery of vaccine peptides by rapid conjugation to baculovirus particles. Vaccine. 2008;26:2451–2456. doi: 10.1016/j.vaccine.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Wu C., Soh K.Y., Wang S. Ion-exchange membrane chromatography method for rapid and efficient purification of recombinant baculovirus and baculovirus gp64 protein. Hum Gene Ther. 2007;18:665–672. doi: 10.1089/hum.2007.020. [DOI] [PubMed] [Google Scholar]
- Wu C., Lin J., Hong M. Combinatorial control of suicide gene expression by tissue-specific promoter and microRNA regulation for cancer therapy. Mol Ther. 2009;17:2058–2066. doi: 10.1038/mt.2009.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.-F., Fang L.-R., Wu X.-B. A pseudotype baculovirus-mediated vaccine confers protective immunity against lethal challenge with H5N1 avian influenza virus in mice and chickens. Mol Immunol. 2009;46:2210–2217. doi: 10.1016/j.molimm.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Xu X.-G., Liu H.-J. Baculovirus surface display of E2 envelope glycoprotein of classical swine fever virus and immunogenicity of the displayed proteins in a mouse model. Vaccine. 2008;26:5455–5460. doi: 10.1016/j.vaccine.2008.07.090. [DOI] [PubMed] [Google Scholar]
- Xu X.-G., Chiou M.-T., Zhang Y.-M. Baculovirus surface display of Erns envelope glycoprotein of classical swine fever virus. J Virol Methods. 2008;153:149–155. doi: 10.1016/j.jviromet.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Xu X.-G., Tong D.-W., Chiou M.-T. Baculovirus surface display of NS3 nonstructural protein of classical swine fever virus. J Virol Methods. 2009;159:259–264. doi: 10.1016/j.jviromet.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Xu X.-G., Wang Z.-S., Zhang Q. Baculovirus surface display of E envelope glycoprotein of Japanese encephalitis virus and its immunogenicity of the displayed proteins in mouse and swine models. Vaccine. 2011;29:636–643. doi: 10.1016/j.vaccine.2010.11.045. [DOI] [PubMed] [Google Scholar]
- Yan Y., Du J., Chen T.-S. Establishment of medakafish as a model for stem cell-based gene therapy: efficient gene delivery and potential chromosomal integration by baculoviral vectors. Exp Cell Res. 2009;315:2322–2331. doi: 10.1016/j.yexcr.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Yang D.-G., Chung Y.-C., Lai Y.-K. Avian influenza virus hemagglutinin display on baculovirus envelope: cytoplasmic domain affects virus properties and vaccine potential. Mol Ther. 2007;15:989–996. doi: 10.1038/mt.sj.6300131. [DOI] [PubMed] [Google Scholar]
- Yang Y., Lo S.-L., Yang J.-Y. Polyethylenimine coating to produce serum-resistant baculoviral vectors for in vivo gene delivery. Biomaterials. 2009;30:5767–5774. doi: 10.1016/j.biomaterials.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Yin H.-Y., Zhou X.-A., Wu H.-F. Baculovirus vector-mediated transfer of NIS gene into colon tumor cells for radionuclide therapy. World J Gastroenterol. 2010;16:5367–5374. doi: 10.3748/wjg.v16.i42.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kondoh D., Arai E. Baculovirus virions displaying Plasmodium berghei circumsporozoite protein protect mice against malaria sporozoite infection. Virology. 2003;316:161–170. doi: 10.1016/j.virol.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Kawasaki M., Hariguchi N. A baculovirus dual expression system-based malaria vaccine induces strong protection against Plasmodium berghei sporozoite challenge in mice. Infect Immun. 2009;77:1782–1789. doi: 10.1128/IAI.01226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Araki H., Yokomine T. Baculovirus-based nasal drop vaccine confers complete protection against malaria by natural boosting of vaccine-induced antibodies in mice. Infect Immun. 2010;78:595–602. doi: 10.1128/IAI.00877-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.L., Lin Y.C., Robinson J.H., Lung O. Transduction of vertebrate cells with Spodoptera exigua multiple nucleopolyhedrovirus F protein-pseudotyped gp64-null Autographa californica multiple nucleopolyhedrovirus. J Gen Virol. 2009;90:2282–2287. doi: 10.1099/vir.0.012138-0. [DOI] [PubMed] [Google Scholar]
- Zeng J.-M., Du J., Lin J.-K. High-efficiency transient transduction of human embryonic stem cell-derived neurons with baculoviral vectors. Mol Ther. 2009;17:1585–1593. doi: 10.1038/mt.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lv Z., Chen J. A novel method for isolation of membrane proteins: a baculovirus surface display system. Proteomics. 2008;8:4178–4185. doi: 10.1002/pmic.200800133. [DOI] [PubMed] [Google Scholar]
- Zheng H., Liu C., Zhuang J. Baculovirus expression of cloned porcine arterivirus generates infectious particles in both insect and mammalian cells. J Biotechnol. 2010;150:251–258. doi: 10.1016/j.jbiotec.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Blissard G.W. Display of Heterologous proteins on gp64null baculovirus birions and enhanced budding mediated by a vesicular stomatitis virus G-stem construct. J Virol. 2008;82:1368–1377. doi: 10.1128/JVI.02007-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Li B., Wang J. The feasibility of using a baculovirus vector to deliver the sodium-iodide symporter gene as a reporter. Nucl Med Biol. 2010;37:299–308. doi: 10.1016/j.nucmedbio.2009.12.007. [DOI] [PubMed] [Google Scholar]


