Abstract
Asynchronous fluctuations in abundance between species with similar ecological roles can stabilize food webs and support coexistence. Sardine (Sardinops spp.) and anchovy (Engraulis spp.) have long been used as an example of this pattern because low-frequency variation in catches of these species appears to occur out of phase, suggesting that fisheries and generalist predators could be buffered against shifts in productivity of a single species. Using landings data and biomass and recruitment estimates from five regions, we find that species do not have equivalent peak abundances, suggesting that high abundance in one species does not compensate for low abundance in the other. We find that globally there is a stronger pattern of asynchrony in landings compared to biomass, such that landings data have exaggerated the patterns of asynchrony. Finally, we show that power to detect decadal asynchrony is poor, requiring a time series more than twice the length of the period of fluctuation. These results indicate that it is unlikely that the dynamics of these two species are compensatory enough to buffer fisheries and predators from changes in abundance, and that the measurements of asynchrony have largely been a statistical artefact of using short time series and landings data to infer ecology.
Keywords: compensatory dynamics, small pelagic fishes, food webs, asynchrony, fisheries ecology, time series modelling
1. Introduction
How do species with similar ecological roles coexist? This most fundamental question in ecology has few accepted general answers. Most coexistence mechanisms (except neutrality; [1]) rely on either local niche partitioning or niche partitioning in space or time. Spatial and temporal niche partitioning mechanisms rely on environmental variation and the presence of stabilizing factors such as differential responses to environmental conditions among species, trade-offs between competitive and dispersal abilities, and other asymmetries in dispersal such as source–sink dynamics [2–6]. Such mechanisms achieve coexistence by producing asynchronous fluctuations between species in space or time. Similar mechanisms also facilitate the stability of larger food webs with multiple interaction types, where species synchrony is typically destabilizing [7]. Thus, the extent to which synchrony or asynchrony is observed in nature can shape researchers' perspectives on the nature of interactions and which particular coexistence mechanism may be driving their dynamics. Ecology textbooks contain numerous examples of strongly interacting species with clearly synchronous or asynchronous dynamics (e.g. microbial communities, hare–lynx predation), yet such patterns are likely difficult to detect from the noisy ecological data that emerges from most ecosystems. Hence, there is a need to assess what the strength of these relationships is by comparing them to a realistic null expectation (rather than simply asking whether species vary independently or not), and by determining if the existing data are sufficient to detect synchrony or the lack thereof.
While the mechanisms of species coexistence continue to be explored, a parallel line of investigation has identified the consequences of coexistence among species that play similar ecological roles. Evidence generally indicates that functional redundancy (species richness within a functional group) supports ecological stability, productivity and ecosystem services by reducing variation in community structure and ecological function [8–10], particularly if the redundant species have asynchronous dynamics [11,12]. Some highly productive marine ecosystems have little functional redundancy among secondary consumers in food webs, a group typically comprised of small, productive pelagic fishes called forage fish [13]. Determining the degree of asynchrony between these few forage species is critical to understanding and predicting the dynamics of entire ecosystems.
Sardine (Sardinops spp.) and anchovy (Engraulis spp.) are commonly the predominant forage species occupying the critical intermediate trophic position in coastal marine food webs [13,14], and prevailing paradigms assert that they are essentially interchangeable central nodes of the food web whose dynamics are asynchronous (out of phase). Evidence that sardine and anchovy populations are asynchronous includes rapid increases in anchovy abundance when sardine abundance is declining [15–18] and correlation between oceanographic phase shifts and alternating cycles of abundance between species [19]. Apparent response diversity to environmental drivers tends to lead to replacement of the species as the dominant forage fish in communities as the environment shifts to favour one species over another [20–22]. This in turn drives changes in the composition of catches and diets of high-trophic-level species, as the two genera constitute at least 50% of total marine fishery landings [23] and are important prey for a number of marine predators [23]. Other research has begun to erode this story: Baumgartner et al. [24] originally showed that sardine and anchovy regimes do not occur out of phase at multidecadal timescales, and this has been supported by other palaeorecord studies using scale deposition in sediments [25,26]. A recent study using landings patterns did not find evidence that sardine–anchovy asynchrony was a global pattern [27]. Despite these developments, the paradigm of sardine–anchovy asynchrony has largely persisted. As one of the pairs of species with the most documented asynchrony in marine systems, sardine and anchovy make a great case study of a natural system where we can critically evaluate asynchrony and why it matters for coexistence and reducing variability in ecosystem function and fishery production.
There are two components of sardine and anchovy co-dynamics that correspond to their causes and consequences: comparability and asynchrony. Comparability refers to the possibility that sardine and anchovy are ecologically or economically interchangeable [17]. This is particularly important for predators and fisheries of forage fish, which will have consistent dietary needs even as the prey community shifts. Evidence for comparability includes alternating but similar catches of sardine and anchovy [28] and similar predator consumption rates between periods when one or the other species is not available, e.g. in South Africa, where cape gannet (Morus capensis) diet composition is thought to reflect changes in relative forage biomass ([29] and references therein). If sardine and anchovy biomasses are comparable in marine ecosystems where they co-occur, predators and fisheries should be able to sustain a more stable supply of food or catches by switching between the two. The need for particular life-history strategies to cope with fluctuating environmental conditions may be obviated if critical prey fluctuate out of phase. Here we define asynchrony as species fluctuating out of phase, synchrony as in-phase fluctuations and independence as fluctuations that occur at different frequencies or are otherwise uncorrelated in the long term. Here we focus on sardine and anchovy because they are globally distributed and tend to be numerically dominant where they are present. Asynchrony in sardine and anchovy should result in a dampening of variation in forage resources available for predators, to the degree that their abundances are comparable.
The timescale of regimes, their degree of asynchrony and the metric in which it appears are important to our understanding of the causes and consequences of sardine and anchovy dynamics. Cycles in landings represent consequences for change in resource use and benefits to human communities, whereas cycles in biomass reflect change in resources available to predators. Finally, recruitment dynamics reflect the underlying production processes that generate biomass and indicates prey availability for predators that specialize on earlier life stages [30,31]. Generalist predators and fishing communities that exploit both species interchangeably should be robust to changes in forage fish community composition if sardine and anchovy dynamics are asynchronous. The timescale of asynchrony influences the likelihood that it can be detected and the potential for assigning possible mechanisms.
Mechanisms for sardine–anchovy asynchrony have been discussed at length in the literature. These include differences in the ideal temperature range for each species (e.g. [32]) and density dependence [18] as well as extrinsic factors that occur at similar timescales [33,34]. For more comprehensive summaries of these potential mechanisms, we refer the reader to this literature [19,22,35]. Despite the substantial attention paid to mechanism, there have been few attempts to characterize this pattern across regions [22,27,36] and to our knowledge none have examined the timescale and prevalence of this pattern across metrics.
Establishing null expectations for asynchrony and comparing the pattern across ecosystems should mitigate the tendency to look for simple patterns when data are limited [37]. The most recent global analysis of this phenomenon focused on teleconnections between distinct ecosystems using only landings [27]. Here, we analyse landings, biomass and recruitment independently as we seek to identify ecological relationships within each ecosystem, including the potential for each species to replace the other from the perspective of fisheries and generalist predators.
In this paper, we synthesize data from five large marine ecosystems to quantitatively test the hypothesis that anchovies and sardines are asynchronous in marine coastal ecosystems. Specifically, we ask whether one species frequently replaces the other, and at what timescales observed the patterns of asynchrony are stronger than expected by chance.
Finally, we examine the data requirements for detecting asynchrony by asking:
-
(1)
If anchovy and sardine were truly asynchronous, how many years of data would be needed to robustly detect this pattern?
-
(2)
What is the probability of falsely detecting asynchrony in these populations?
We expect that asynchrony at short timescales will be most robustly detectable than asynchrony at longer timescales, because of the inherent limits on the number of cycles that can be observed in a given time series.
2. Methods
We collected sardine and anchovy time series from global databases to assemble the longest and most complete datasets available for five marine ecosystems. We used simple metrics to determine the extent to which sardine and anchovy are comparable in landings and biomass. We also estimated sardine–anchovy covariance and used a randomization test to assess whether the observed degree of asynchrony in each ecosystem was stronger than expected by chance. Finally, to determine the joint roles of time series length and the magnitude and frequency of variation in generating spurious asynchrony, we simulated sardine and anchovy populations with known dynamics and quantified the probability of falsely detecting asynchrony using conventional correlation methods.
(a). Data
We collated sardine and anchovy time series from five regions: The Kuroshio–Oyashio, California, Humboldt, Benguela Currents and the Northeast Atlantic (electronic supplementary material, figure S1; table S1). All are coastal upwelling ecosystems except for the Kuroshio–Oyashio Current, which is a western boundary current, and the Northeast Atlantic, which is a shelf ecosystem. Time series data were compiled from the Ransom A. Myers Legacy Stock Assessment database [RAM; 38,39], the Food and Agriculture Organization landings database [FAO; 40] and a collection of stock assessments and individual studies published in Barange et al. 2009 [41] (48 total time series; electronic supplementary material, table S2). These time series consist of data (landings) and estimated values from stock assessments (biomass and recruitment). This dataset includes more metrics than the most globally comprehensive studies of trends so far [27,36] and has a broader geographical scope than studies focused on dynamics in one region (e.g. [24]). We defined the dominant sardine and anchovy species for each region as the species in each group with the highest median biomass. We assessed comparability using the log-ratio of median anchovy to sardine landings and biomass in each ecosystem. This is a simple way of showing whether one species is much more abundant than the other.
(b). Evaluation of asynchrony
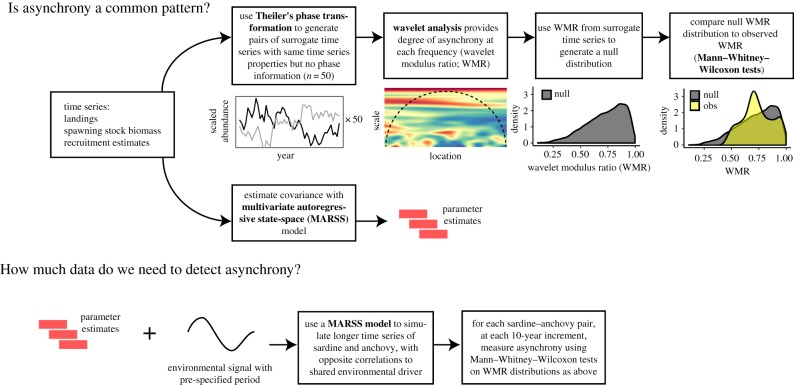
We assessed asynchrony using two approaches: (i) a time series approach, in which the strength and sign of the covariance were estimated by fitting a multivariate autoregressive state-space (MARSS) model and (ii) a wavelet approach, which decomposes time series into variation at different frequencies (figure 1; electronic supplementary material, figure S2). Specifically, the wavelet modulus ratio provides a measure of asynchrony at multiple frequencies [42] and has been used to analyse compensatory dynamics in ecological communities (e.g. [43]), including sardine and anchovy in the California Current [35].
Figure 1.
A schematic showing the study design. Methods are shown in bold. To evaluate asynchrony for each region, we used state-space models to estimate covariance and used wavelet methods to see whether asynchrony at three timescales was stronger than expected by chance from similar time series. To determine how much data are needed to detect asynchrony, we simulated longer time series driven oppositely by a shared environmental driver, then used the same wavelet methods to try to detect asynchrony at each of several observation lengths. (Online version in colour.)
To estimate covariance between sardine and anchovy, we fit a MARSS model to the dominant sardine and anchovy stock in each region, using the MARSS package in R [44,45]. We assumed that species interactions (independent of the environment) and observation error were similar across ecosystems, but that sardine–anchovy covariance was region-specific, an assumption supported by model fits (see electronic supplementary material for full model structure).
To determine whether sardine and anchovy were more asynchronous than expected by chance, we generated 50 surrogate time series with the same length and spectral properties as the observations, but without phase information. Surrogate time series provide a ‘null expectation’ for the degree of asynchrony that spectral analysis would detect in unrelated time series with similar autocorrelation and error. Surrogate time series were generated using Theiler's phase randomization, via the fractal package in R [46]. For each pair of sardine and anchovy surrogates, we calculated the wavelet modulus ratio (WMR). The WMR is bounded between 0 and 1, where lower values are characteristic of asynchronous or compensatory dynamics, higher values are characteristic of synchronous dynamics, and intermediate values are characteristic of independent dynamics. WMR values were calculated using the mvcwt package in R [47]. WMR returns values for every timescale; we pooled WMR values in less than 5-yr, 5–10-yr and greater than 10-yr timescales.
To compare observed WMR values to the null expectation, we used Mann–Whitney–Wilcoxon tests and associated estimates of the median difference (d) and effect size, testing the null hypothesis that the null and observed WMR distributions at each timescale differ by a location parameter of 0. In order to compare the observed WMR to a null distribution, we ignored the arrangement of values in the time domain and binned the information by frequency.
(c). The amount of data needed to detect asynchrony
We explored two cases: the possibility that asynchrony would not be identified when it was present (Type II error) and the possibility of a false detection of asynchrony when it was not present (Type I error). To examine the potential for each of these, we used the fitted parameters from the state-space model described above to simulate two autocorrelated time series xt which respond to a shared driver ct. The time series were generated as follows:
| 2.1 |
where xt is an m × 1 vector of the natural log of biomass for sardine and anchovy at time t and B is an m × m matrix containing the effects of each species on itself along the diagonal and the effect of one species on the other on the off-diagonal (here, m = 2). C is an m × 1 vector relating sardine and anchovy to an environmental driver c, which here is described by a sine wave with a period T of 60 yr (similar to the period of fluctuations hypothesized for sardine and anchovy, although this exercise could be repeated for any period; [15]):
| 2.2 |
The variance–covariance matrix Q is
| 2.3 |
Values of were based on estimates from real time series of biomass (electronic supplementary material, equation S5). Because we were interested in the detectability of a known degree of synchrony, we did not simulate observation error; additional error would increase the length of the time series needed for detection.
To test the power of our method to detect asynchrony, we investigated a hypothetical condition in which the two species were correlated to a shared environmental driver (asynchronous at time period T; ). For each of 100 simulations, we generated one long time series of sardine and anchovy. We compared the value of d from increasing sample sizes (n = 30–150 yr) to the value from the full dataset.
To determine the potential for falsely detecting asynchrony (Type I error), we investigated a second hypothetical condition in which both species were positively correlated to a low-frequency environmental driver . We simulated 1,000 pairs of sardine and anchovy biomass and calculated the Spearman correlation at each sample length.
3. Results
We found no consistent pattern of comparability (the potential for sardine and anchovy to be ecologically or economically interchangeable) in landings between sardine and anchovy across the five ecosystems. Each ecosystem had one forage species with higher median landings, with differences of up to 196% (Humboldt Current, dominated by anchovy). Sardine dominated landings in the Northeast Atlantic and Kuroshio-Oyashio Current, and were nearly comparable in the California Current (figure 2) where median anchovy landings were only 13% lower than median sardine landings. Sardine and anchovy landings were most comparable in the California Current, where median anchovy landings were only 13% lower than median sardine landings ([48]; electronic supplementary material, figure S3).
Figure 2.
Log10 ratio of anchovy to sardine median landings, biomass and recruitment. The dotted line indicates a log-ratio of zero, where points would fall if the median values were comparable (i.e. sardine and anchovy have similar median landings, biomass, and recruitment). Points to the left of the dotted line are ecosystems where sardine have higher medians landings, biomass, and recruitment. Grey points indicate values from different data sources and coloured points indicate medians across all data sources. (Online version in colour.)
For three of the five ecosystems, sardine and anchovy were more comparable in landings than they were in biomass. Biomass also indicated one dominant forage species in each ecosystem, with differences in median biomass of up to 160% (Northeast Atlantic, dominated by sardine). Sardine and anchovy biomass were most replaceable in the California Current, where the maximum biomass was only 7% different (RAM). These results were consistent among datasets. Comparability in recruitment was in the same direction as biomass (except in the Northeast Atlantic), with much larger differences in the median. Sardine had higher recruitment than anchovy in Kuroshio–Oyashio and California Currents, and anchovy had higher recruitment in the Northeast Atlantic, Humboldt and Benguela Currents. Long-term maxima of landings and biomass showed similar signals.
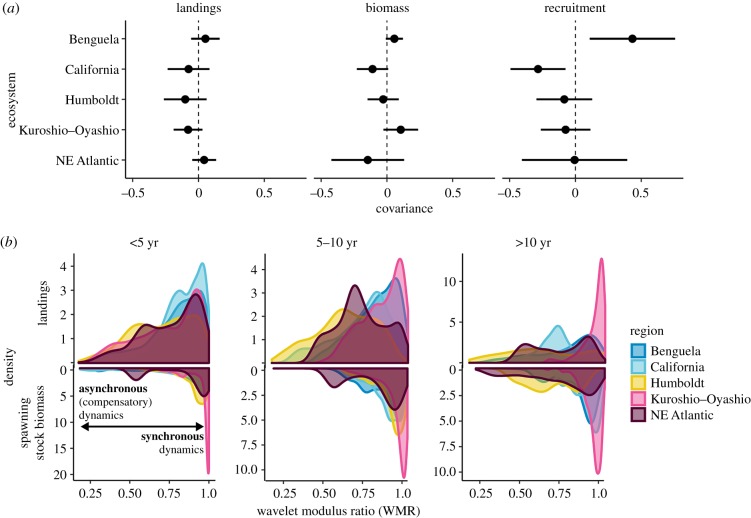
Covariance between sardine and anchovy landings and biomass was weak and the sign varied across ecosystems, whereas covariance in recruitment was stronger. The Humboldt Current exhibited the strongest negative covariance in landings (qsa = −0.10; 95% CI [−0.262, 0.0617]) (figure 3a). The confidence intervals for the covariance estimate overlapped zero in all five ecosystems. Covariance in biomass was similarly weak and occurred in the same direction as covariance in landings in every ecosystem except the Kuroshio–Oyashio Current and the Northeast Atlantic. Estimates of the elements of B suggest that there is a small global negative effect of sardine on anchovy (bsa = −0.10 [−0.19, −0.02]), but it is not strong enough to produce negative covariance in biomass where it is not indicated by Q (see electronic supplementary material). Confidence intervals for covariance overlapped among ecosystems. The strongest covariance between sardine and anchovy (both negative and positive) was in recruitment (Benguela Current covariance was 0.5 [0.166, 0.834]; California Current covariance was −0.275 [−0.471, −0.080]). Estimates of the elements of B indicate that density dependence is nearly absent for sardine (bs = 0.98 [0.93,1.0]) and stronger for anchovy (ba = 0.67 [0.55, 0.78]), where b = 1 indicates density independence [49].
Figure 3.
(a) Covariance between sardine and anchovy in each region, estimated from a MARSS model. Points indicate the maximum-likelihood estimate for covariance and lines indicate 95% confidence intervals. (b) Asynchrony expressed as the density of the WMR between sardine and anchovy, based on time series of landings (top) and biomass (bottom) at each timescale (columns). Time series with higher density at low WMR show more asynchronous (compensatory) dynamics. All data here are from Barange et al. (2009) [41]. WMR distributions from surrogate time series are in electronic supplementary material, figures S6–S8. (Online version in colour.)
There was slightly stronger evidence of asynchrony at longer timescales, particularly for landings. For example, in the Humboldt Current, landings were more asynchronous than expected by chance while biomass only indicated asynchrony at the longest timescale (10+ yr; p < 0.001, median of differences (observed − null) d = −0.056 [−0.079, −0.033], proportion of samples where null < observed r = 0.04) (electronic supplementary material, table S3). This pattern also occurred in the Kuroshio–Oyashio Current, for which asynchrony in landings was present at short (less than 5 yr) timescales (p < 0.001, d = −0.034 [−0.043, −0.025], r = 0.32), but not in biomass at any timescale.
Across all ecosystems, WMR distributions indicated stronger asynchrony in landings than biomass (higher density at lower WMR values; figure 3b, electronic supplementary material, table s3). There was not a strong signal of asynchrony in recruitment time series, except in the Northeast Atlantic, which had the strongest signal at short timescales (less than 5 yr; p < 0.001, d = −0.323 [−0.338, −0.308], r = 0.10). The Benguela Current was more synchronous than expected by chance, across all metrics. Finally, the California Current had more synchronous dynamics in recruitment and spawning biomass than expected by chance at every timescale.
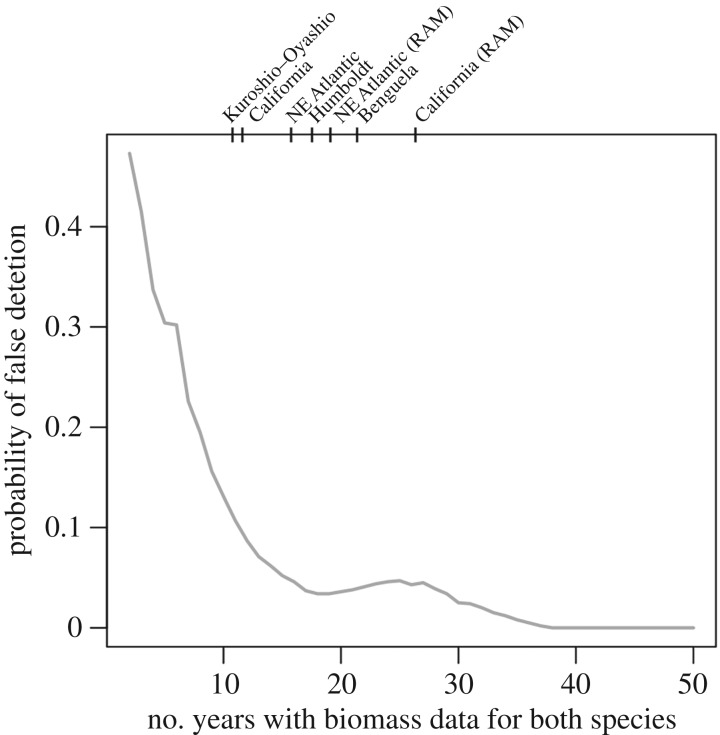
We found that statistical power to detect asynchrony at decadal scales was low. Our power analysis indicates that if sardine and anchovy were asynchronous at a 60-yr scale, detecting asynchrony at long (10+ yr) timescales could require several more decades of data than are currently available (greater than 120 yr to detect asynchrony in our example; electronic supplementary material, table S5). Dynamics at shorter (less than 10-yr) timescales accurately characterized in much shorter time series (40–80 yr of data; electronic supplementary material, figure S9). We also found a non-zero probability that using Spearman correlation can falsely detect asynchrony in shorter time series (a Type I error), even those that are synchronous at a decadal scale. If both species were similarly correlated to an environmental driver, the probability of falsely detecting asynchrony between those two species remains above 10% until there are at least 12 yr of data (figure 4). The number of years of data required increases when one or more species is less strongly correlated to the shared driver.
Figure 4.
Probability of Type I error (i.e. detecting a negative correlation when asynchrony is not the dominant pattern), using Spearman correlations. Points show the length of biomass time series available from all the datasets in this study. All data are from Barange et al. (2009)[41] except the two indicated for the RAM legacy database.
4. Discussion
Asynchronous and comparable dynamics between species are often used to draw conclusions about ecological relationships, functional redundancy and system resilience. These considerations are important in a conservation context, particularly when human activities such as fishing can affect the abundance of one or more species in a food web. In marine systems, inferring these relationships can be even more difficult given our limited ability to observe them. Sardine and anchovy are an archetypal example of two marine species whose ecological relationship is inferred from patterns in abundance. We show that these two abundant and commercially important taxa are neither strongly asynchronous, nor are they comparable in abundance. We find that the observations of asynchrony in landings are not indicative of underlying patterns in biomass or recruitment. Furthermore, we find that conventional methods can falsely detect asynchrony in shorter time series. Together these results indicate that there is limited potential for sardine and anchovy to effectively replace each other as key forage species in the ecosystems where they occur.
Overall, we found very limited statistical support for asynchrony. Significant asynchrony in biomass and recruitment only occurred in the Northeast Atlantic and Humboldt ecosystems, a pattern that was unexpected given the volume of literature documenting asynchrony ([16] and others). Cases where we observed synchronous dynamics using our method may have been a limitation of our method, which can indicate synchrony in shorter datasets. In the Benguela Current, our finding of synchrony is in agreement with Izquierdo-Peña et al.'s finding that the stocks began to covary positively in the last two decades after declining to low productivity [27]. We also found that even when their dynamics are compensatory, sardine and anchovy are unlikely to be ecologically replaceable because their abundances are not comparable. We do not interpret our results to mean that asynchrony between sardine and anchovy does not exist, rather that it is difficult to detect in the systems where it occurs.
What makes asynchrony in biomass so difficult to detect for these two groups? Our results indicate two possibilities: (i) the strength of asynchrony is exaggerated by the use of landings data to make ecological inference and (ii) the timescale of fluctuations is too long to detect asynchrony at low frequencies. Across ecosystems, we found that landings indicated less comparable abundance but stronger asynchrony than was indicated by biomass, suggesting that fishery-dependent data exaggerate the appearance of compensatory dynamics.
Stronger asynchrony in landings than biomass could result from different regulatory frameworks and effort dynamics; effort in the fishery for one species could shift to another when abundance was declining, exaggerating asynchrony in landings relative to abundance. This would be consistent with a pattern of risk mitigation in which fishermen shift among target species as they are available and profitable [50]. Asynchrony in landings at all timescales was a particularly strong pattern in the Humboldt Current, an ecosystem where fishing effort shifts to sardine when anchoveta are less available. Stronger asynchrony in landings could also be a result of the schooling behaviour of sardine and anchovy, whose range contracts at low population sizes, allowing for large catches relative to abundance (the ‘basin model’; [51]). In both of these cases, human behaviour enhances natural patterns of asynchrony.
Our results indicate that perceived global asynchrony may be an artefact of the low frequency, strongly autocorrelated biomass of both species. Our analysis indicates that using correlations (like Spearman correlation) to infer dynamics can falsely indicate asynchrony when time series are short, including at the time series lengths currently available for our study regions. This is a useful example of a case where using simple correlation metrics on autocorrelated time series can result in incorrect classification [52]. In cases where low-frequency asynchrony is present, there are significant data requirements for detecting it, of which our estimates represent a minimum. That asynchrony is detectable in some long time series [36] but not others [24] indicates that more data are necessary but not always sufficient for detection, including detection via our method. The issue of statistical power has been brought up previously by MacCall [33] who noted that, ‘we still have not experienced more than two well-documented episodes of sardine or anchovy abundance in any of these systems, which is an absurdly small sample size by any statistical standard…it will most likely require at least a third episode, presumably sometime in the next few decades, for science to make further progress.’ We find support for this statement in our analysis.
Though preferable to landings for finding ecological patterns, biomass estimates may still obscure asynchronous drivers between the two species. For example, if sardine and anchovy filter environmental signals at different timescales, storage effects detectable in age structured data would not appear in biomass. Finally, the assessments of asynchrony could be affected by poor data quality or other issues with abundance estimation [53]. A full simulation study that explicitly models observation error could identify the specific roles of process versus observation error in generating the patterns we found ([36] presents one possible approach).
Even in regions where there is stronger evidence of asynchrony, inferences about functional replacement between sardine and anchovy should be made with caution. Comparable abundance indicates ecological replaceability only for predators with access to both species, and sardine and anchovy can occupy different areas depending on density, habitat suitability and prey availability ([54,55] and others). The impact of sardine–anchovy asynchrony on predators will depend on the composition of the forage guild [56–58], which is diverse even in systems where these species are dominant [59]. Assessments of the ecological impact of asynchrony should account for forage guild diversity where possible.
A primary issue in the management of forage species is how to adapt to big, often unanticipated changes in abundance. The degree to which compensatory dynamics buffer the forage category from change is unknown in most ecosystems. If sardine and anchovy were asynchronous, generalist predators and fisheries would be able to exploit sardine when anchovy abundance is low and vice versa. Our results show that clear patterns of asynchrony are the exception rather than the rule, so management should not rely on asynchrony to buffer populations of these two important forage species. In lieu of buffering from asynchrony, fluctuations in abundance of the dominant species are likely to be the predominant driver influencing the productivity of predators and fisheries, especially in places where sardine and anchovy abundance are more synchronous than expected by chance. The low potential for replacement indicates that even highly asynchronous dynamics will not be sufficient to stabilize forage fish availability for fisheries or predators. Together, these results suggest that effective management would involve quantifying, preserving and enhancing buffering effects instead of assuming they are present. Ideally, these management strategies would also be robust to non-stationarity in the community and in the relationship between these species and the environment [60]. Another option for better balancing trade-offs would be to implement management strategies that respond quickly to changes in abundance [61]. If productivity fluctuates on timescales even longer than those examined here (100+ yr; see e.g. [26,62]), management decisions based on shorter term patterns could be ill-informed.
This study is the first to examine asynchrony in exploited marine forage species using multiple data types and to quantify the robustness of observed asynchrony at multiple temporal scales. Our results show that sardine and anchovy alone rarely fulfil the requirements of a compensatory forage portfolio for fisheries and ecosystems. Furthermore, we have shown that our ability to detect asynchrony is limited by data availability. In cases where dynamics are synchronous or independent, the assumption of asynchrony is particularly risky for managers to make, even if previous long-term patterns show that decreases in one species usually coincide with increases in the other. Forage fish management decisions are made at much shorter timescales and waiting to get information about synchrony from existing time series is unrealistic at the timescale of management decisions.
Supplementary Material
Acknowledgements
We thank Daniel Schindler, Trevor Branch, Tessa Francis, Jan Ohlberger and three anonymous reviewers for feedback on an earlier version of this manuscript.
Data accessibility
All data analysed in this article are already published in public databases. The source code to reproduce our analysis, as well as data files with the cleaned and original data, are available at https://github.com/mcsiple/sardine-anchovy.
Authors' contributions
M.C.S., T.E.E. and L.A.K.B. came up with the conceptual framework of the study, M.C.S. and L.A.K.B. designed the time series analysis, M.D.S. created the simulation model; M.C.S., T.E.E. and L.A.K.B. wrote the manuscript, and all authors contributed substantially to revisions.
Competing interests
The authors have no competing interests to declare.
Funding
This work was supported by a Pew fellowship in Marine Conservation to T.E.E. M.C.S. was funded by the same fellowship.
References
- 1.Hubbell SP. 2001. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Hutchinson GE. 1961. Paradox of the plankton. Am. Nat. 95, 137–145. ( 10.1086/282171) [DOI] [Google Scholar]
- 3.Hastings A. 1980. Disturbance, coexistence, history, and competition for space. Theor. Popul. Biol. 18, 363–373. ( 10.1016/0040-5809(80)90059-3) [DOI] [Google Scholar]
- 4.Chesson PL, Warner RR. 1981. Environmental variability promotes coexistence in lottery competitive systems. Am. Nat. 117, 923–943. ( 10.1086/283778) [DOI] [Google Scholar]
- 5.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366. ( 10.1146/annurev.ecolsys.31.1.343) [DOI] [Google Scholar]
- 6.Amarasekare P, Hoopes M, Mouquet N, Holyoak M. 2004. Mechanisms of coexistence in competitive metacommunities. Am. Nat. 164, 310–326. ( 10.1086/422858) [DOI] [PubMed] [Google Scholar]
- 7.Amarasekare P. 2008. Spatial dynamics of foodwebs. Annu. Rev. Ecol. Evol. Syst. 39, 479–500. ( 10.1146/annurev.ecolsys.39.110707.173434) [DOI] [Google Scholar]
- 8.Loreau M, et al. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808. ( 10.1126/science.1064088) [DOI] [PubMed] [Google Scholar]
- 9.Hooper DU, et al. 2005. Effects of biodiversity on ecosystem functioning: a consensis of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 10.Levin SA, Lubchenco J. 2008. Resilience, robustness, and marine ecosystem-based management. BioScience 58, 27–32. ( 10.1641/B580107) [DOI] [Google Scholar]
- 11.Doak DF, Bigger D, Harding EK, Marvier MA, O'Malley RE, Thomson D. 1998. The statistical inevitability of stability–diversity relationships in community ecology. Am. Nat. 151, 264–276. ( 10.1086/286117) [DOI] [PubMed] [Google Scholar]
- 12.Yachi S, Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468. ( 10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cury P, Bakun A, Crawford RJM, Jarre A, Quinones RA, Shannon LJ, Verheye HM. 2000. Small pelagics in upwelling systems: patterns of interaction and structural changes in ‘wasp-waist’ ecosystems. Ices J. Mar. Sci. 57, 603–618. ( 10.1006/jmsc.2000.0712) [DOI] [Google Scholar]
- 14.Shannon LJ, Cury PM, Jarre A. 2000. Modelling effects of fishing in the Southern Benguela ecosystem. ICES J. Mar. Sci. 57, 720–722. ( 10.1006/jmsc.2000.0716) [DOI] [Google Scholar]
- 15.Shackleton LY. 1987. A comparative study of fossil fish scales from three upwelling regions. South Afr. J. Mar. Sci. 5, 79–84. ( 10.2989/025776187784522270) [DOI] [Google Scholar]
- 16.Lluch-Belda D, Crawford RJM, Kawasaki T, MacCall AD, Parrish RH, Schwartzlose RA, Smith PE. 1989. World-wide fluctuations of sardine and anchovy stocks: the regime problem. South Afr. J. Mar. Sci. 8, 195–205. ( 10.2989/02577618909504561) [DOI] [Google Scholar]
- 17.Lluch-Belda D, Schwartzlose RA, Serra R, Parrish R, Kawasaki T, Hedgecock D, Crawford RJM. 1992. Sardine and anchovy regime fluctuations of abundance in four regions of the world oceans: a workshop report. Fish. Oceanogr. 1, 339–347. ( 10.1111/j.1365-2419.1992.tb00006.x) [DOI] [Google Scholar]
- 18.Schwartzlose RA, et al. 1999. Worldwide large-scale fluctuations of sardine and anchovy populations. South Afr. J. Mar. Sci.-Suid-Afr. Tydsk. Seewetens. 21, 289–347. ( 10.2989/025776199784125962) [DOI] [Google Scholar]
- 19.Chavez FP, Ryan J, Lluch-Cota SE, Ñiquen M. 2003. From anchovies to sardines and back: multidecadal change in the Pacific ocean. Science 299, 217–221. ( 10.1126/science.1075880) [DOI] [PubMed] [Google Scholar]
- 20.Kuwae M, Yamamoto M, Sagawa T, Ikehara K, Irino T, Takemura K, Takeoka H, Sugimoto T. 2017. Multidecadal, centennial, and millennial variability in sardine and anchovy abundances in the western North Pacific and climate–fish linkages during the late Holocene. Prog. Oceanogr. 159, 86–98. ( 10.1016/j.pocean.2017.09.011) [DOI] [Google Scholar]
- 21.Kawasaki T, Nihon Seitai G. 1991. Long-term variability of pelagic fish populations and their environment: Proceedings of the International Symposium, Sendai, Japan, 14–18 November 1989, 1st edn Oxford, UK: Pergamon Press. [Google Scholar]
- 22.Alheit J, Bakun A. 2010. Population synchronies within and between ocean basins: apparent teleconnections and implications as to physical–biological linkage mechanisms. J. Mar. Sys. 79, 267–285. ( 10.1016/j.jmarsys.2008.11.029) [DOI] [Google Scholar]
- 23.Cury PM, et al. 2011. Global seabird response to forage fish depletion-one-third for the birds. Science 334, 1703–1706. ( 10.1126/science.1212928) [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner TR, Soutar A, Ferreira-Bartrina V. 1992. Reconstruction of the history of Pacific sardine and northern anchovy populations over the past two millennia from sediments of the Santa Barbara Basin, California. CalCOFI Rep 33, 24–40. [Google Scholar]
- 25.Finney BP, Alheit J, Emeis K-C, Field DB, Gutierrez D, Struck U. 2010. Paleoecological studies on variability in marine fish populations: a long-term perspective on the impacts of climatic change on marine ecosystems. J. Mar. Syst. 79, 316–326. ( 10.1016/j.jmarsys.2008.12.010) [DOI] [Google Scholar]
- 26.McClatchie S, Hendy IL, Thompson AR, Watson W. 2017. Collapse and recovery of forage fish populations prior to commercial exploitation. Geophys. Res. Lett. 44(2016G), L071751 ( 10.1002/2016GL071751) [DOI] [Google Scholar]
- 27.Izquierdo-Peña V, Lluch-Cota SE, Hernandez-Rivas ME, Martínez-Rincón RO. 2018. Revisiting the regime problem hypothesis: 25 years later. Deep Sea Res. Part II 159, 4–10. ( 10.1016/j.dsr2.2018.11.003) [DOI] [Google Scholar]
- 28.Moloney CL, Wickens PA. 1985. A simulation investigation of coexistence and species replacement between two competitors with common predators. S. Afr. J. Sci. 81, 703–704. [Google Scholar]
- 29.Cury P, Shannon L. 2004. Regime shifts in upwelling ecosystems: observed changes and possible mechanisms in the northern and southern Benguela. Prog. Oceanogr. 60, 223–243. ( 10.1016/j.pocean.2004.02.007) [DOI] [Google Scholar]
- 30.Lluch-Belda DA, Lluch-Cota DB, Hernandez-Vazquez SE, Salinas-Zavala CA, Schwartzlose RA. 1991. Sardine and anchovy spawning as related to temperature and upwelling in the California Current system. CalCOFI Rep. 32, 105–111. [Google Scholar]
- 31.Jacobson LD, MacCall AD. 1995. Stock-recruitment models for Pacific sardine (Sardinops sagax). Can. J. Fish. Aquat. Sci. 52, 566–577. ( 10.1139/f95-057) [DOI] [Google Scholar]
- 32.Bakun A, Broad K. 2003. Environmental ‘loopholes’ and fish population dynamics: comparative pattern recognition with focus on El Niño effects in the Pacific. Fish. Oceanogr. 12, 458–473. ( 10.1046/j.1365-2419.2003.00258.x) [DOI] [Google Scholar]
- 33.MacCall AD. 2009. Mechanisms of low-frequency fluctuations in sardine and anchovy populations. In Climate change and small pelagic fish, pp. 285–299. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 34.Alheit J, Roy C, Kifani S. 2009. Decadal-scale variability in populations. In Climate change and small pelagic fish, pp. 64–87. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 35.Lindegren M, Checkley DM, Rouyer T, MacCall AD, Stenseth NC. 2013. Climate, fishing, and fluctuations of sardine and anchovy in the California Current. Proc. Natl Acad. Sci. USA 110, 13 672–13 677. ( 10.1073/pnas.1305733110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosack GR, Trenkel VM, Dambacher JM. 2013. The relative importance of environmental stochasticity, interspecific interactions, and observation error: insights from sardine and anchovy landings. J. Mar. Sys. 125, 77–89. ( 10.1016/j.jmarsys.2012.09.003) [DOI] [Google Scholar]
- 37.Chater N. 1999. The search for simplicity: a fundamental cognitive principle? Q. J. Exp. Psychol. Sect A-Hum. Exp. Psychol. 52, 273–302. ( 10.1080/027249899391070) [DOI] [Google Scholar]
- 38.RAM Legacy Stock Assessment Database. 2018. RAM Legacy Stock Assessment Database v4.44. (doi:10.5281/zenodo.2542919)
- 39.Ricard D, Minto C, Jensen OP, Baum JK. 2012. Examining the knowledge base and status of commercially exploited marine species with the RAM Legacy Stock Assessment Database: The RAM Legacy Stock Assessment Database. Fish Fish. 13, 380–398. ( 10.1111/j.1467-2979.2011.00435.x) [DOI] [Google Scholar]
- 40.FAO. 2018. The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. See http://www.fao.org/3/i9540en/i9540en.pdf.
- 41.Barange M. 2009. Current trends in the assessment and management of stocks. In Climate change and small pelagic fish (eds Checkley DM, Alheit J, Oozeki Y, Roy C). Cambridge, UK: Cambridge University Press. [Google Scholar]
- 42.Keitt TH. 2008. Coherent ecological dynamics induced by large-scale disturbance. Nature 454, 331–334. ( 10.1038/nature06935) [DOI] [PubMed] [Google Scholar]
- 43.Vasseur DA, et al. 2014. Synchronous dynamics of zooplankton competitors prevail in temperate lake ecosystems. Proc. R. Soc. B 281, 20140633 ( 10.1098/rspb.2014.0633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmes EE, Ward EJ, Wills K. 2012. MARSS: multivariate autoregressive state-space models for analyzing time-series data. R J. 4, 11–19. ( 10.32614/RJ-2012-002) [DOI] [Google Scholar]
- 45.R Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 46.Constantine W, Percival D. 2016. fractal: Fractal time series modeling and analysis. See https://CRAN.R-project.org/package=fractal.
- 47.Keitt TH. 2014. mvcwt: Wavelet analysis of multiple time series. See https://CRAN.R-project.org/package=mvcwt.
- 48.Barange M, Coetzee J, Takasuka A, Hill K, Gutierrez M, Oozeki Y, Lingen Cvd, Agostini V. 2009. Habitat expansion and contraction in anchovy and sardine populations. Prog. Oceanogr. 83, 251–260. ( 10.1016/j.pocean.2009.07.027) [DOI] [Google Scholar]
- 49.Ives AR, Dennis B, Cottingham KL, Carpenter SR. 2003. Estimating community stability and ecological interactions from time series data. Ecol. Monogr. 73, 301–330. ( 10.1890/0012-9615(2003)073[0301:ECSAEI]2.0.CO;2) [DOI] [Google Scholar]
- 50.Cline TJ, Schindler DE, Hilborn R. 2017. Fisheries portfolio diversification and turnover buffer Alaskan fishing communities from abrupt resource and market changes. Nat. Commun. 8, ncomms14042 ( 10.1038/ncomms14042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacCall AD. 1990. Dynamic geography of marine fish populations: books in recruitment fishery oceanography. Seattle, WA: University of Washington Press. [Google Scholar]
- 52.Agiakloglou C, Tsimpanos A. 2012. An alternative approach for testing for linear association for two independent stationary AR(1) processes. Appl. Econ. 44, 4799–4803. ( 10.1080/00036846.2011.595695) [DOI] [Google Scholar]
- 53.Siple MC, Essington TE, Plagányi ÉE. 2018. Forage fish fisheries management requires a tailored approach to balance tradeoffs. Fish Fish. 20, 110–124. ( 10.1111/faf.12326) [DOI] [Google Scholar]
- 54.Bertrand A, Segura M, Gutiérrez M, Vásquez L. 2004. From small-scale habitat loopholes to decadal cycles: a habitat-based hypothesis explaining fluctuation in pelagic fish populations off Peru. Fish Fish. 5, 296–316. ( 10.1111/j.1467-2679.2004.00165.x) [DOI] [Google Scholar]
- 55.Bertrand A, Chaigneau A, Peraltilla S, Ledesma J, Graco M, Monetti F, Chavez FP. 2011. Oxygen: a fundamental property regulating pelagic ecosystem structure in the coastal Southeastern Tropical Pacific. PLoS ONE 6, e29558 ( 10.1371/journal.pone.0029558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takasuka A, Oozeki Y, Kubota H, Lluch-Cota SE. 2008. Contrasting spawning temperature optima: why are anchovy and sardine regime shifts synchronous across the North Pacific? Prog. Oceanogr. 77, 225–232. ( 10.1016/j.pocean.2008.03.008) [DOI] [Google Scholar]
- 57.Glaser SM. 2010. Interdecadal variability in predator–prey interactions of juvenile North Pacific albacore in the California Current system. Mar. Ecol. Prog. Ser. 414, 209–221. ( 10.3354/meps08723) [DOI] [Google Scholar]
- 58.Lindegren M, Checkley DM, Ohman MD, Koslow JA, Goericke R. 2016. Resilience and stability of a pelagic marine ecosystem. Proc. R. Soc. B 283, 20151931 ( 10.1098/rspb.2015.1931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koehn LE, Essington TE, Marshall KN, Kaplan IC, Sydeman WJ, Szoboszlai AI, Thayer JA. 2016. Developing a high taxonomic resolution food web model to assess the functional role of forage fish in the California Current ecosystem. Ecol. Modell 335, 87–100. ( 10.1016/j.ecolmodel.2016.05.010) [DOI] [Google Scholar]
- 60.Litzow MA, Ciannelli L, Puerta P, Wettstein JJ, Rykaczewski RR, Opiekun M. 2019. Nonstationary environmental and community relationships in the North Pacific ocean. Ecology 100, e02760 ( 10.1002/ecy.2760) [DOI] [PubMed] [Google Scholar]
- 61.Plagányi ÉE, Haywood MDE, Gorton RJ, Siple MC, Deng RA. 2019. Management implications of modelling fisheries recruitment. Fish. Res. 217, 169–184. ( 10.1016/j.fishres.2019.03.007) [DOI] [Google Scholar]
- 62.Rogers LA, et al. 2013. Centennial-scale fluctuations and regional complexity characterize Pacific salmon population dynamics over the past five centuries. PNAS 110, 1750–1755. ( 10.1073/pnas.1212858110) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analysed in this article are already published in public databases. The source code to reproduce our analysis, as well as data files with the cleaned and original data, are available at https://github.com/mcsiple/sardine-anchovy.