Abstract
In addition to controlling pest organisms, the systemic neurotoxic pesticide fipronil can also have adverse effects on beneficial insects and other non-target organisms. Here, we report on the sublethal effects of fipronil on the farmland butterfly Pieris brassicae. Caterpillars were reared on plants that had been grown from seeds coated with fipronil or on leaf discs topically treated with a range of fipronil dosages (1–32 µg kg−1 on dry mass basis). Females that had developed on fipronil plants laid ca half the number of eggs than females that had developed on control plants. In the bioassay with leaf discs, longevity and lifetime egg production declined with increasing fipronil dosage. Remarkably, exposure to fipronil during larval development primarily affected the adult stage. Chemical analyses of leaf tissues collected from seed-treated plants revealed concentrations of fipronil and its degradation products close to the analytical limit of detection (less than or equal to 1 µg kg−1). The effective dosage was fivefold higher in the leaf-disc than in the whole-plant experiment. In the whole plant, degradation of fipronil to products that are more toxic than fipronil may explain this discrepancy. Neurotoxicity of insecticides at the level of detection decreases the probability of pinpointing insecticides as the causal agent of harmful effects on non-target organisms.
Keywords: insect decline, pesticide, sublethal effects, toxicity
1. Introduction
Recent findings regarding declines in the abundance of insects [1–4] and insectivorous birds [5,6] have fuelled the debate about possible driving mechanisms. While there is little disagreement about the importance of agricultural intensification as a major factor causing these declines, there are four main components of this intensification that need to be disentangled [7]. First, there is the spatial component of habitat loss and fragmentation that restricts the persistence of insect populations (e.g. 8,9). The second component is a reduction in quality of remaining habitat through the influence of eutrophication and acidification from chemical fertilisers [10,11]. The third component stems from inappropriate management of potential habitat [12], which includes the abandonment of marginal lands. And finally, there is the influence of pesticides on survival of non-target insects and other organisms [13,14].
With respect to pesticides there is great concern regarding the effects of neurotoxic systemic pesticides, in particular neonicotinoids and fipronil [13,15]. Since their first commercial use in the 1990s, neonicotinoids and fipronil have become the most frequently used insecticides globally [16]. These chemicals are widely used to protect crops against insect herbivory, by spraying or seed treatment resulting in the translocation of the chemicals through the entire plant. Target insects feeding from the treated crop are effectively killed, but negative effects may occur by accumulation of these persistent chemicals in the soil, as well as in ground and surface water [17,18]. Beneficial insects, such as pollinators and biological control agents, can be exposed to these chemicals on treated crop plants and wild and untreated crop plants in adjacent fields as a result of dust drifts caused by drilling of chemically coated seeds and the uptake of contaminated drainage water by field margin plants [17,19]. These negative effects are not restricted to invertebrates, but can also affect vertebrates that consume treated seeds/crops or contaminated prey or are exposed following spraying [14].
Fipronil (5-amino-1-(2,6-dichloro-α,α,α-trifluoro-p-tolyl)-4-trifluoromethylsulfinyl pyrazole-3-carbonitrile) belongs to the phenylpyrazole class of insecticides. This compound and its major metabolite fipronil sulfone, noncompetitively bind to gamma-aminobutyric acid (GABA)-gated chloride channels, thereby blocking the inhibitory action of GABA in the central nervous system [20]. By contrast, neonicotinoids act selectively on insect nicotinic acetylcholine receptors in the central nervous system [21,22]. At sufficiently high dosages, fipronil leads to excessive neural excitation, paralysis, and death [13,16]. As the affinity of fipronil for mammalian GABA receptors is much lower than for arthropod GABA receptors, it is used as a broad-spectrum insecticide of agricultural pests of crop plants and in veterinary products against ectoparasites. Its toxicity is substantially more potent than that of other insecticides, e.g. the LD50 for honeybees of fipronil is greater than 6000 times lower than for dichlorodiphenyltrichloroethane (DDT) [13]. In developed countries the most common application is through prophylactic seed coating, but in developing countries, it is also applied as foliar sprays. Its typical application dosage is about 50 g ha−1 [13,23].
Most research regarding the impact of systemic pesticides on non-target organisms has focused on neonicotinoids. Increased mortality from neonicotinoids has been repeatedly found as a direct short-term effect, but also through a range of delayed indirect effects, which may be difficult to detect, but could be of a far greater significance [13,15]. Sublethal effects of neonicotinoid exposure, which have been primarily investigated in bees, include impaired reproductive activity and performance [24–26], reduced flight capacity and foraging activity [27,28], and reduced hygienic behaviour [29,30]. Moreover, there is mounting evidence on synergistic negative effects of neonicotinoids in combination with other chemical, pathogenic, or nutritional stressors [27,31,32]. The synthesis of these findings by the European Food and Safety Authority (EFSA) has led to a partial ban on the use of three types of neonicotinoids and fipronil in the European Union [33,34].
For fipronil, there is also ample evidence of its direct toxicity to both vertebrates and invertebrates [15]. Indeed, it has been postulated that the introduction of fipronil, and not the neonicotinoid imidacloprid, caused the mass mortality of bees in France during the 1990s [35]. Still, the evidence for sublethal effects of fipronil on non-target insects remains scant. Sublethal effects of fipronil have only been found for Odonata, where larval feeding activity and growth was significantly reduced in two Sympetrum species [36]. Here, we report the findings of an experimental study of fipronil exposure at a range of dosages on growth, survival, and reproduction of the insect herbivore and flower visitor, the butterfly Pieris brassicae. Application of fipronil on cabbage seeds is allowed only for the control of cabbage root fly Delia radicum L. (Diptera: Anthomyiidae) in The Netherlands, designating P. brassicae a non-target organism. Pieris brassicae has been reported as a pest on brassicaceous crops in the Mediterranean area and eastern Europe [37,38]. We raised and studied caterpillars in two experimental settings. In the first, we followed larval development until adult emergence on Brassica oleracea (Brussels sprouts) host plants grown from seeds that were either untreated or treated with fipronil against cabbage root fly, Delia radicum. In the second experiment, we subjected the caterpillars to a range of fipronil dosages topically applied to cabbage leaf discs. After emergence of the butterflies, both adult longevity and lifetime egg production were determined in both experiments. Based on an earlier experience, where two of our P. brassicae rearing populations crashed after successful larval development on Brassica plants grown from fipronil-coated seeds, we anticipated delayed detrimental effects of fipronil on reproductive performance.
2. Material and methods
(a). Insects and plants
Pieris brassicae L. (Lepidoptera: Pieridae) is a specialist herbivore of which the caterpillars feed on plants containing glucosinolates, a group of secondary plant metabolites that is restricted to plant species in a few families including Brassicaceae. This plant family contains the majority of the herbivore's food plant species including cabbage and oil seed crops. As P. brassicae caterpillars feed gregariously and later instars feed voraciously, they are considered an important pest species of cabbage crops in some regions. After adult eclosion, females have to mate in order to lay viable eggs. Following egg maturation and mating, females usually initiate egg laying within 3 days after adult eclosion. Females lay their eggs in batches of on average 30 eggs, but batches greater than 100 eggs are produced as well. Virgin butterflies lay unviable eggs in much smaller numbers and a scattered distribution. The larvae go through five instars before they pupate usually in a secluded area away from the plant.
Pieris brassicae used in the experiments originated from cabbage fields near Wageningen University and caterpillars were reared on Brussels sprouts plants (Brassica oleracea, var. gemmifera, cv. Cyrus (seeds were purchased from Syngenta, Enkhuizen, The Netherlands). Insects were reared in a climate-controlled room at 21 ± 2°C, a light-dark regime of 16:8 h, and 60–70% relative humidity (r.h.). Plants used for insect rearing and the leaf-disc experiment (see below) were grown from untreated seeds at the Unifarm greenhouse facilities at Wageningen University and were four to 6 weeks old. Neonate caterpillars used in the experiment were obtained from the general rearing.
(b). Fipronil from coated seeds (whole plants)
Fipronil-treated and untreated seeds (both from Syngenta) were germinated in moist peat soil and seedlings were transferred to 2.5 l pots filled with potting soil (Lentse potgrond #4, Lent, The Netherlands). Plants, 20 of each of the two treatments, were randomly distributed in a greenhouse set at 21 ± 2°C, 50–70% r.h., and a photoperiod of at least 16 h. If the light conditions dropped below 500 µmol photons m−2 s−1 during the photoperiod, light was supplemented by high-pressure SON-T lamps. When plants were 3 weeks old, nutrients were added once by administering approximately 4 g Floranid Permanent (NPK 16-7-15(+2)) granules to the soil.
When the plants were 6 weeks old, neonate P. brassicae caterpillars were introduced onto the plants (five caterpillars per plant, 20 plants grown from untreated seeds, 19 plants from treated seeds). One of the seed-treated plants that did not develop normally was discarded. Caterpillars were allowed to move and feed freely on their assigned food plant. When food is not limiting, P. brassicae caterpillars do not leave their food plant until the final fifth instar and, therefore, do not need to be confined until this point. When caterpillars reached the fifth instar before they started to wander, plants with caterpillars were enclosed using netted sleeves (48 × 60 cm, 104 × 94 mesh inch−2, Bugdorm, Taiwan) supported by a wooden stick, to prevent final instar caterpillars from escaping when searching for pupation sites. Plants were watered regularly according to their needs. Pupae were collected and weighed on an analytical balance to the nearest mg. Pupae that had developed on the same individual plant, which is considered a replicate, were maintained together in a 10 cm Petri dish lined with filter paper. Pupae were transferred to netted cages (40 × 40 × 60 cm, Vermandel, Hulst, The Netherlands) separated according to dish, a few days before expected adult eclosion. When all adults had eclosed, one male and one female were selected, whereas the other butterflies were removed from the cages. Females were discarded, whereas surplus males were maintained together in two cages, one with control males and one with males reared on fipronil plants. These males were used to replace males that died (described below). One replicate of control and two replicates of fipronil-treated caterpillars only produced male butterflies reducing the final number of females, of which lifetime egg production was recorded, to 19 control and 17 fipronil-exposed individuals.
Adult butterflies were provided with 10% sugar water administered to cotton wool in blue caps, which attract the butterflies to feed. In each cage with a female butterfly, a single 4-week-old plant grown from an untreated seed was added as an oviposition substrate. Plants were checked daily for eggs. Leaves with eggs were removed, or whole plants were replaced by new ones. Females that had not produced eggs within 4 days since adult eclosion were provided with a new male collected from the cage with surplus males. This was repeated one more time if necessary. Mortality of both males and females was recorded and dead males were replaced by males from the surplus-male cages. Females of P. brassicae can mate more than once. The experiment was ended at day 24 post-eclosion when most females had died and females that were still alive produced no or very few eggs. All eggs were counted. Viability of the eggs was checked on leaves with eggs that were collected on the 2nd or 3rd day since initiation of egg laying. Usually, neonate caterpillars hatch from eggs within 4–5 days under the conditions of the experiment. Leaves were maintained in Petri dishes for a maximum of 7 days. If the larvae did not hatch within this period, checking of egg viability was continued two more times on consecutive leaves with eggs. The experimental design is summarized in electronic supplementary material, table S1.
(c). Fipronil applied to leaf discs
Ten mg of fipronil (CAS no. 1200068-37-3; Sigma-Aldrich, The Netherlands) was dissolved in 100% ethanol and subsequently diluted in tap water in a stepwise manner to obtain concentrations of 48, 24, 12, 6, 3, and 1.5 fipronil ng ml−1, respectively. The tap water used for preparation of the fipronil solutions contained 2% Tween 80 to lower the surface tension, improving distribution of the solution on the waxy surface of the cabbage leaves. The Tween 80 solution was applied as a control treatment. A volume of 2.12 µl test solution was applied per cm2 of cabbage leaf. Leaf discs taken from plants grown from untreated seeds were either 2.4 (used for first to third instar caterpillars) or 7 cm (used for fourth and fifth instar caterpillars) in diameter. Solutions were distributed evenly over the leaf surface using a fine paint brush and discs were left to dry for about 30 min before they were placed in the Petri dishes with the caterpillars. To determine leaf tissue dry mass (DM) equivalents of the treatment dosages, 20 7 cm diameter discs were dried in an oven for 3 days at 60°C and weighed (mean DM ± s.e.: 3.2 ± 0.2 mg cm−2). The estimated concentrations applied per cm2 were divided by the mean DM to obtain proximate DM equivalents of 1.0, 2.0, 4.0, 8.0, 16.1, and 32.2 µg fipronil kg−1 leaf tissue (DM), respectively.
Each concentration was tested in 10 replications in 10 cm glass Petri dishes. Each dish lined with moist filter paper initially contained two 2.4 cm leaf discs and 10 neonate caterpillars. Leaf discs and filter paper were replaced every other day during the first 8 days and the number of discs was gradually increased up to six small leaf discs according to the feeding needs of the caterpillars. At day 9, when most caterpillars had reached the fourth instar, the number of caterpillars was reduced to five per dish, by randomly selecting and removing caterpillars. Caterpillars were transferred to larger 13.5 cm dishes and freshly prepared leaf discs were now added daily. The number of discs was gradually increased to three 7 cm discs per dish until the caterpillars pupated. Dead caterpillars were removed and recorded throughout the experiment. One dish of the lowest fipronil concentration was discarded when all caterpillars had died on day 6 due to pathogen infection. This dish was not further considered in the data analysis as larval mortality in all other treatments was low (less than 10%). Pupae were weighed to the nearest mg and were maintained in 10 cm Petri dishes. A few days before expected adult eclosion all pupae exposed to the same fipronil concentration were placed together in netted cages (40 × 40 × 60 cm). Five times six butterflies, three females and three males, were randomly selected from a treatment group and transferred to new cages with a cabbage plant and 10% sugar water. Of the 4 µg kg−1 concentration only 2 cages were prepared, due to insufficient number of females. The plants were replaced by new ones every 4 days until day 12 and then every 2 days until day 35 when most females had died and only few eggs were laid by the females that were still alive. All eggs were counted. Dead butterflies were removed and recorded in both the oviposition cages and the cages with the remaining butterflies that were not released in the oviposition cages.
(d). Fipronil analyses
Leaf tissues were sampled from control plants and plants grown from fipronil-treated seed used in the whole-plant bioassay. Plants were sampled when the caterpillars had pupated and, at this point, were approximately 8 weeks old. All mature, green leaves were collected from five plants of each treatment. Tissues were frozen and stored at −20°C until they were dried for 3 days using a freeze drier (CHRIST ALPHA1-4 LDplus, Martin Christ Gefriertrocknungsanlagen GmbH, Germany). Leaf discs treated similarly as in the leaf disc bioassay were also chemically analysed. Seven cm leaf discs were each treated with the approximated dosage of 16.1 µg fipronil kg−1. There were three samples of fipronil-treated discs and three samples of control discs which were treated with water only containing 2% Tween 80. Each sample consisted of 21–22 leaf discs. Solutions were distributed evenly over the leaf surface using a fine paint brush and discs were left to dry for about 30 min. Samples were placed in an oven and dried for 48 h at 60°C and weighed. All samples (freeze-dried and oven-dried) were pulverized using a grinder (Cyclotec 1093 Sample Mill, Foss-Tecator AB, Sweden). In addition to leaf tissues, fipronil concentrations were also measured in two seed samples (1 g each).
The samples were analysed at the Wageningen Food Safety Research facilities. One gram of leaf disc material was weighed in a 50 ml Greiner tube (Greiner Bio-One B.V., Alphen a/d Rijn, The Netherlands), and 7.5 ml of acetonitril (Biosolve HPLC Supra Gradient) containing 1% formic acid (Sigma-Aldrich 25,736-4) was added. After thorough mixing (head over head) for 30 min, the resulting solution was centrifuged for 15 min at 3500 rpm (VWR Microstar 17). Finally, 500 µl of the clear supernatant was transferred to an liquid chromatography with tandem mass spectrometry (LC-MSMS) filter vial (mini-uniprep polytetrafluoroethylene (PTFE) filter vial (0.45 µm; Whatman, Maidstone, UK). Seed material was treated accordingly with an additional sample pre-treatment: the seed material was measured both ‘as is' and after homogenizing with an Ultra-Turrax.
Quantification of fipronil, and the metabolites fipronil-sulfone, fipronil-sulfide, fipronil-desulfinyl, and fipronil-carboxamide was performed by LC-MSMS. The calibration curves of each component were linear in the range from 0.1 to 50 ng ml−1 (see electronic supplementary material, figure S2). The identity of the compounds was confirmed by retention time and MSMS ratio. Quantitation was performed against solvent standards with good results. Quality control (QC) samples showed acceptable recoveries for all compounds: fipronil 76–84%, fipronil-sulfon 74–91%, fipronil-sulfide 77–95%, fipronil-desulfinyl 78–91%, and fipronil-carboxamide 77–96%. The average relative standard deviation for all compounds ranged from 3.9% to 9.5%. The limits of detection of the five compounds were: fipronil-sulfide and fipronil-desulfinyl 1 µg kg−1; fipronil and fipronil-carboxamide 0.7 µg kg−1; fipronil-sulfon 0.5 µg kg−1, respectively.
All identification and QC parameters complied to SANTE/11813/2017, guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed. If needed the sample extracts were diluted with acetonitril containing 1% formic acid. This was the case with both seed material samples.
(e). Data processing and statistical analysis
Whole plant experiment—Survival of the larvae, which was determined as the recovery of pupae out of the five neonate caterpillars that were initially introduced on the plants, was analysed using a generalized linear model with a binomial error distribution and a logit-link function. Plant treatment (control or seed-treated) was the explanatory variable in the analysis. Pupal masses were analysed with a general linear mixed model with plant treatment as the main factor and plant individual as a random factor (up to five pupae had developed on the same plant individual). Development times from egg hatching to adult eclosion on treated and control plants were compared using Kaplan–Meier survival analysis (log-rank test) with four groups (two sexes times two plant treatments). If the test results were significant, survival curves were analysed pair-wise using a Bonferroni correction for inflated type I errors (adjusted α = 0.008). Longevity of the females on control and treated plants was also compared using Kaplan–Meier survival analysis. Lifetime reproduction was analysed with a general linear model analysis of variance with plant treatment as the main factor.
Dish experiment—Larval survival until day 8 (out of 10 caterpillars per dish) and survival from day 8 until pupation (out of 5 caterpillars per dish) was analysed using generalized linear models with a binomial error distribution and a logit-link function. Fipronil concentration (7 levels) was entered as the explanatory variable and dishes served as experimental units. Adult survival was analysed using Kaplan–Meier survival analysis combining survival of butterflies in the oviposition cages and the collection cage. If the test results were significant, survival curves were analysed pair-wise using a Bonferroni correction for inflated type I errors (adjusted α = 0.0023). To obtain lifetime egg production per female, egg numbers were summated per cage and divided by three as each oviposition cage originally contained three females. Egg production did not differ for females reared on discs containing less than or equal to 4 µg fipronil kg−1 (general linear-model analyses of variance: F1, 15 = 0.10, p = 0.76). The overall mean egg production by these females was 703 ± 31 eggs per female which was consequently considered as lifetime egg production by females that were not affected by fipronil. To construct a sigmoidal relationship, we used logistic regression. Here, the response variable was the relative performance, i.e. egg production divided by 703. If egg production was higher than 703, the relative performance was set at 1. The approximated fipronil concentration (μg kg−1) was the explanatory variable in the model.
Kaplan–Meier survival analyses and logistic regression were conducted using Genstat 19, whereas the other analyses were performed using SAS 9.4.
3. Results
(a). Fipronil from coated seeds (whole plants)
The percentage of pupae that developed out of the five neonate caterpillars that were initially released on each plant was lower on plants grown from treated (81 ± 4%) than from untreated seeds (93 ± 2%) (GLM; F1,37 = 1.15, p = 0.011). However, pupal masses did not differ between the two plant treatments (residual maximum likelihood (REML); F1,36.5 = 0.15, p = 0.70). The average pupal mass (mean ± s.e.) was 424 ± 3 and 426 ± 4 mg on fipronil and control plants, respectively. All pupae eclosed as adult butterflies, except for one pupa from the fipronil plants. Development time from larval hatching to adult eclosion was marginally affected by plant treatment (Kaplan–Meier survival, log-rank test; χ2 = 9.15, d.f. = 3, p = 0.027), but, after Bonferroni correction, pair-wise comparisons were not significant. Longevity of the adult females was not affected by plant treatment (χ2 = 0.002, d.f. = 1, p = 0.96). However, lifetime egg production was significantly lower on fipronil than on control plants (GLM; F1,34 = 16.7, p < 0.001, figure 1). Females from fipronil plants laid less than half (45.0%) the number of eggs than females from control plants. Egg viability of fipronil butterflies was strongly reduced. Control females (n = 19) produced in total 15 393 eggs with viability close to 100%, whereas five out of the 17 females that had developed on fipronil plants did not produce any viable eggs. These five females produced 1383 out of the 6191 eggs laid in total, thereby reducing fitness of the fipronil-grown butterflies by an additional 22%. Moreover, more than 3100 eggs were produced by only three of the 17 females.
Figure 1.
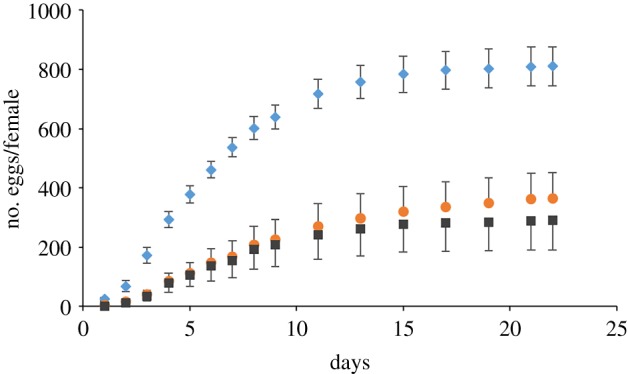
Cumulative egg production (mean ± s.e.) of female Pieris brassicae butterflies that had developed on control plants (blue diamonds, n = 19) and fipronil-treated plants (orange circles, n = 17). Egg production on fipronil-treated plants is also given excluding unviable eggs (dark grey squares, n = 17). Production of unviable eggs was negligible in control butterflies. (Online version in colour.)
(b). Fipronil applied to leaf discs
Larval survival until day 9, when most caterpillars had developed into the fourth instar was not affected by fipronil (GLM F1,67 = 3.20, p = 0.078). Mortality varied between 0% in the control and 10% at the highest concentration of 32 µg kg−1 DM. Mortality from day 9 to pupation was not affected by fipronil (GLM F1,67 = 0.67, p = 0.42). Approximately 90% of the caterpillars still alive at day 9 pupated successfully, irrespective of the fipronil dosage to which they had been exposed.
Out of the 313 pupae collected, 310 pupae eclosed as adults within 3 days. Pupal masses were similar irrespective of the fipronil concentration to which the larvae had been exposed (REML: F1,9.1 = 1.48, p = 0.28). The average pupal mass was 297 ± 2 mg. Adult longevity was significantly lower at higher fipronil dosages (Kaplan–Meier survival, log-rank test, χ2 = 490, p < 0.001; figure 2). There was no difference in survival rates between males and females (χ2 = 0.033, d.f.= 1, p = 0.85). Adults exposed to the highest concentration all died within 9 days since eclosion, whereas some of the adults at fipronil concentrations of 2 µg kg−1 or lower were still alive at day 35 when the experiment was ended. Longevity of butterflies exposed to dosages of 2 µg kg−1 or lower did not differ from control butterflies (figure 2). Lifetime egg production decreased significantly with fipronil concentration (χ2 = 154, d.f. = 30, p < 0.001, figure 3), with no eggs being produced at the highest concentration of 32 µg kg−1.
Figure 2.
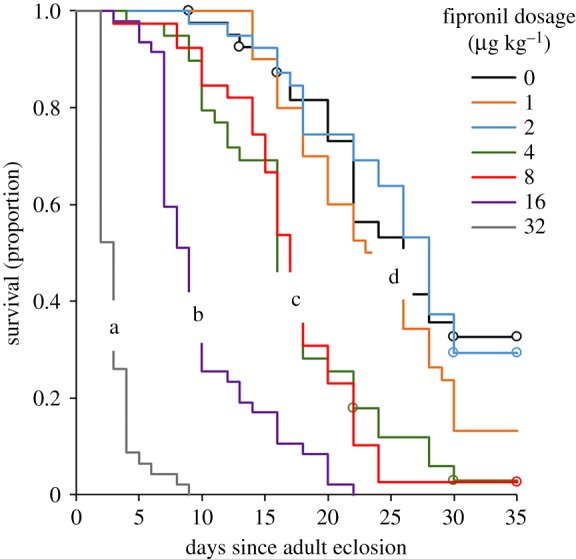
Survival curves of Pieris brassicae butterflies (males and females combined) that had been reared in Petri dishes on leaf discs treated with fipronil (0–32 µg kg−1 DM). Survival curves with the same letter are not significantly different (Kaplan–Meier log-rank test, α = 0.0023). Open circles denote censored individuals. (Online version in colour.)
Figure 3.
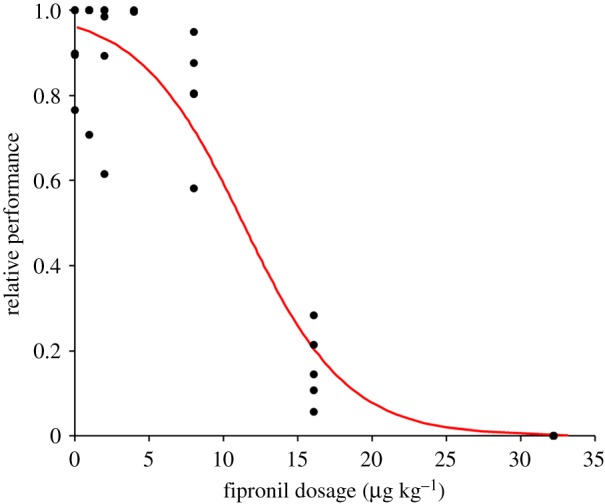
Relative reproductive performance of Pieris brassicae female butterflies that had been reared in Petri dishes on leaf discs treated with fipronil (0–32 µg kg−1 DM). Each point in the graph denotes the average lifetime egg production of three females that were placed together in an oviposition cage relative to the average lifetime egg production of females that were not affected by fipronil, which was estimated to be 703 ± 31 eggs per female. There were five cages per concentration, except for the 4 µg kg−1 DM dosage, which had only two replicates. The red line gives the fitted relationship between the relative reproductive performance and the fipronil dosage (ln (y/(1 – y)) = 3.228–0.2849x, see text for statistics). (Online version in colour.)
(c). Fipronil analyses
The seeds used in the experiments contained 33% of the original fipronil content according to the product information of the manufacturer. Fipronil-coated seeds are not commercially available anymore. Therefore, we relied on a stock of seeds that was kept in a storage room at 13°C at 30% r.h. in the dark. Long-term storage may have resulted in the degradation of fipronil in these seeds. Concentrations of fipronil calculated based on the concentration in a solvent-wash of intact fipronil-coated seeds were higher than in homogenized seeds which confirms that fipronil is primarily present in the seed coating. In plants grown from these seeds, only trace amounts of fipronil were found in leaf tissues harvested when the caterpillars had completed their development. Fipronil can be converted through photolysis into fipronil-desulfinyl, through oxidation into fipronil sulfone, through reduction into fipronil sulfide, and through hydrolysis into fipronil-carboxamide [39]. Fipronil in the seeds was relatively stable; only very low concentrations of the degradation products fipronil-sulfon (2.3% of total) and fipronil-carboxamide (0.06% of total) were found in the seeds (table 1). By contrast, degradation products in leaf tissues contributed significantly to the total amount, 26 and 38% for fipronil-sulfon and fipronil-carboxamide, respectively. No fipronil or metabolites were detected in leaf tissues collected from control plants. Fipronil was also measured in leaf discs topically treated with fipronil. Leaf discs were similarly prepared as the discs prepared for the bioassays. Based on the DM of the leaf discs and the dosage applied to the discs that were chemically analysed, the calculated fipronil concentration in the leaf discs was 17.9 ± 0.2 (mean ± s.e.) µg kg−1 DM leaf tissue. The measured concentration was 18.8 ± 1.6 µg kg−1 leaf tissue. This 5% difference could be caused by measuring inaccuracy when preparing stock solution and volumetric variation in the dilution steps of the treatment solution. No metabolites of fipronil were detected 24 h after surface application on leaf discs.
Table 1.
Fipronil and fipronil degradation products in seeds and leaf tissues.
| per seed | fipronil | fipronil-sulfon | fipronil-sulfide | fipronil-desulfinyl | fipronil-carboxamide | |
|---|---|---|---|---|---|---|
| tissue | (μg) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) |
| commercial dose in seeds | 125 | 24 644 | ||||
| exp. seeds (wash) | 8385 | 199 | 50 | 0.43 | 5.2 | |
| exp. seeds (homogenized) | 8039 | 207 | 51 | 0.41 | 5.1 | |
| exp. leaf tissues | 0.00094 ± 0.00011 | 0.00066 ± 0.00009 | <0.001 | <0.001 | 0.00096 ± 0.00011 |
4. Discussion
Acute lethal effects of the systemic neurotoxic insecticides fipronil and neonicotinoids are well documented [13,15]. Sublethal effects, such as changes in foraging and reproductive behaviour and performance are less well investigated and are primarily studied for neonicotinoids in bees [13,15]. We found that reproductive performance of adult butterflies of P. brassicae that had fed as larvae on cabbage plants grown from fipronil-treated seeds was seriously impaired. The females that had developed on fipronil plants laid approximately 55% fewer eggs than females that had developed on control plants. This reduced oviposition was not caused by a reduction in longevity. Remarkably, exposure to fipronil during larval development primarily affected the adult stage. In the disc bioassay using a range of fipronil dosages, we found that longevity and lifetime egg production declined with increasing fipronil dosage.
The concentrations of fipronil and its degradation products in cabbage leaves collected from the experimental plants grown from fipronil-coated seeds were extremely low, i.e. close to or below the limit of detection (i.e. less than or equal to 1 µg kg−1). Similar patterns were found in maize plants grown from seeds treated with fipronil [40]. In general, approximately 5% of the fipronil in the seed coating is taken up by the plants and this can be enhanced using polymers [16]. The egg production of females on fipronil-plants relative to egg production on control plants was 0.44. Using the parameter estimates of the regression model shown in figure 3, the concentration of fipronil in the leaf-disc experiment causing a relative performance of 0.44 was 12.2 µg kg−1 (equivalent to 1.22 µg kg−1 on leaf fresh mass basis, based on an estimated 10% dry matter content of the leaves). This concentration is fivefold higher than that measured in leaf tissues that were collected after larvae had stopped feeding from plants grown from fipronil-coated seeds, taking fipronil and two metabolites into account. The difference in effective concentration could be explained by dilution of fipronil as a result of plant growth suggesting that the caterpillars were exposed to decreasing concentrations of fipronil during larval development. Few studies have estimated half-life values (t1/2) of fipronil in plant tissues. For cotton t1/2 varied between 2.4 and 7.3 days and for water hyacinth between 5.3 and 7.6. In both plant species, this was largely caused by oxidation [41–43]. An alternative explanation is that degradation of fipronil, including oxidation, resulted in the production of metabolites that are more toxic than fipronil itself, e.g. the desulfinyl derivative that is a more potent neurotoxin to the housefly Musca domestica L. than the parent compound [44]. In our study, two metabolites were detected in cabbage plant tissues, fipronil-sulfon through oxidation and fipronil-carboxamide through hydrolysis, and these represented 26 and 38% of the total content of fipronil metabolites. These metabolites might be more toxic than fipronil which could further explain the difference in effective concentration between leaf disc and whole-plant assays. Recently, fipronil has been suggested as a more likely suspect than the neonicotinoid imidacloprid in causing the mass mortality of French honeybees when these insecticides were first introduced [35]. This was based on the fact that fipronil, by contrast with imidacloprid, is bioaccumulating in honeybees even at trace dosages resulting in time-reinforced lethal toxicity [35]. If this also occurs in P. brassicae caterpillars, time-reinforced lethal toxicity may explain the similar behavioural responses of the butterflies when reared on seed-treated plants on which the fipronil concentrations declined over time and when reared on leaf discs with a constant concentration of fipronil.
Interestingly, feeding on fipronil-treated leaves (whole plant and leaf discs) did not significantly or only marginally affect larval development, pupation, pupal biomass, or adult eclosion. Clearly, the dosages applied here only affected the physiology and behaviour of P. brassicae at the adult stage. These results contrast with those found for the neonicotinoid imidacloprid [45]. Exposure to sublethal concentrations of imidacloprid decreased development time and reduced pupal mass [45]. Longevity and lifetime egg production of the adult females declined with increasing fipronil dosage. Although we did not quantify this, butterflies that had been exposed to fipronil were observed to be less active and less motivated to mate. In the whole-plant bioassay, we replaced males when females produced no offspring within 4 days since adult eclosion. All control butterflies initiated egg laying within 3 days, whereas this was the case for 7 out of the 17 females in the fipronil-exposed group. Moreover, whereas all control females produced viable offspring, five (29%) of the fipronil females did not. Pieris brassicae females that are not mated can produce low numbers of inviable eggs. Thus, reduced offspring production can partially be explained by inactivity, of which refraining to mate is an essential one. However, some females did produce viable eggs, albeit in lower numbers than females in the control group. This suggests that the effects of fipronil are not only behavioural, affecting neurological processes, but also physiological, affecting egg and or sperm production. The latter is likely to happen during larval development, when most of the resources for somatic and reproductive tissues for the adult stage are acquired. We do not know whether the effects of fipronil are sex-specific. To reveal the underlying mechanism explaining reduced lifetime egg production in P. brassicae further investigation is needed.
The recent concerns on insect declines involve all major classes of insects, including butterflies [2,4,46]. In the review by Sánchez-Bayo & Wyckhuys [2] agricultural intensification and the use of agrochemicals (pesticides and fertilisers) were assessed as the most important drivers of these declines. The decline of host-plant specialist butterflies is stronger than that of generalist butterfly species [47]. Modelling of changes in population indices from 1985 to 2012 for 17 common farmland butterfly species in the United Kingdom showed that the number of hectares on which neonicotinoid pesticide were used correlated negatively with butterfly population indices [48]. This negative association was also found for P. brassicae. A similar negative association was found for butterfly populations and increasing neonicotinoid application in lowland Northern California [49]. Restrictions on the use of neonicotinoids and fipronil in 2013 have increased the use of pyrethroids and neonicotinoids that have not been restricted in three out of the eight regions studied in Europe [50]. The present study as well as numerous other studies have demonstrated the unintended adverse effects of pesticide use and demonstrate the necessity to decrease the dependence on pesticides in agricultural practices and develop more sustainable and environmentally safe approaches [51]. Moreover, this study revealed harmful effects of the systemic insecticide fipronil and metabolites occurring in cabbage plants on reproduction of a butterfly at concentrations close to the analytical limit of detection. This finding highlights the increasing difficulty to pinpoint neurotoxic insecticides as the causal agent of harmful effects on non-target organisms.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Theo de Rijk and Loes van Vliet of Wageningen Food Safety Research for performing the chemical analysis.
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.7pvmcvdpr [52]. Supporting data are accessible in electronic supplementary material, table S1 and figure S1.
Authors' contributions
R.G., M.W., and J.L. conceived the study, R.G. and J.L. conducted the experiments. R.G. analysed the data. R.G. and M.W. wrote drafts of the manuscript, which were edited further in collaborative work involving all authors.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Hallmann CA, et al. 2017. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12, e0185809 ( 10.1371/journal.pone.0185809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sánchez-Bayo F, Wyckhuys KAG. 2019. Worldwide decline of the entomofauna: a review of its drivers. Biol. Conserv. 232, 8–27. ( 10.1016/j.biocon.2019.01.020) [DOI] [Google Scholar]
- 3.Powney GD, Carvell C, Edwards M, Morris RK, Roy HE, Woodcock BA, Isaac NJ. 2019. Widespread losses of pollinating insects in Britain. Nat. Commun. 10, 1018 ( 10.1038/s41467-019-08974-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Strien AJ, van Swaay CAM, van Strien-van Liempt WTFH, Poot MJM, WallisDeVries MF. 2019. Over a century of data reveal more than 80% decline in butterflies in the Netherlands. Biol. Conserv. 234, 116–122. ( 10.1016/j.biocon.2019.03.023) [DOI] [Google Scholar]
- 5.Bowler DE, Heldbjerg H, Fox AD, de Jong M, Böhning-Gaese K. 2019. Long-term declines of European insectivorous bird populations and potential causes. Conserv. Biol. 33, 1120–1130. ( 10.1111/cobi.13307) [DOI] [PubMed] [Google Scholar]
- 6.Lister BC, Garcia A. 2018. Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl Acad. Sci. USA 115, E10 397–E10 406. ( 10.1073/pnas.1722477115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habel JC, Ulrich W, Biburger N, Seibold S, Schmitt T. 2019. Agricultural intensification drives butterfly decline. Insect Conserv. Diver. 12, 289–295. ( 10.1111/icad.12343) [DOI] [Google Scholar]
- 8.Kleijn D, Rundlöf M, Scheper J, Smith HG, Tscharntke T. 2011. Does conservation on farmland contribute to halting the biodiversity decline? Trends Ecol. Evol. 26, 474–481. ( 10.1016/j.tree.2011.05.009) [DOI] [PubMed] [Google Scholar]
- 9.Tscharntke T, et al. 2012. Landscape moderation of biodiversity patterns and processes - eight hypotheses. Biol. Rev. 87, 661–685. ( 10.1111/j.1469-185X.2011.00216.x) [DOI] [PubMed] [Google Scholar]
- 10.WallisDeVries MF, Bobbink R. 2017. Nitrogen deposition impacts on biodiversity in terrestrial ecosystems: mechanisms and perspectives for restoration. Biol. Conserv. 212, 387–389. ( 10.1016/j.biocon.2017.01.017) [DOI] [Google Scholar]
- 11.Habel JC, Schmitt T. 2018. Vanishing of the common species: empty habitats and the role of genetic diversity. Biol. Conserv. 218, 211–216. ( 10.1016/j.biocon.2017.12.018) [DOI] [Google Scholar]
- 12.Bonari G, et al. 2017. Management of semi-natural grasslands benefiting both plant and insect diversity: the importance of heterogeneity and tradition. Agr. Ecosyst. Environ. 246, 243–252. ( 10.1016/j.agee.2017.06.010) [DOI] [Google Scholar]
- 13.Pisa LW, et al. 2015. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 22, 68–102. ( 10.1007/s11356-014-3471-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbons D, Morrissey C, Mineau P. 2015. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. 22, 103–118. ( 10.1007/s11356-014-3180-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pisa L, et al. 2017. An update of the worldwide integrated assessment (WIA) on systemic insecticides. Part 2: impacts on organisms and ecosystems. Environ. Sci. Pollut. Res. 10.1007/s11356-017-0341-3 ( 10.1007/s11356-017-0341-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon-Delso N, et al. 2015. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 22, 5–34. ( 10.1007/s11356-014-3470-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood TJ, Goulson D. 2017. The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ. Sci. Pollut. Res. 24, 17 285–17 325. ( 10.1007/s11356-017-9240-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonmatin J-M, et al. 2015. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 22, 35–67. ( 10.1007/s11356-014-3332-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botías C, David A, Hill EM, Goulson D. 2016. Contamination of wild plants near neonicotinoid seed-treated crops, and implications for non-target insects. Sci. Total Environ. 566–567, 269–278. ( 10.1016/j.scitotenv.2016.05.065) [DOI] [PubMed] [Google Scholar]
- 20.Gant DB, Chalmers AE, Wolff MA, Hoffman HB, Bushey DF. 1998. Fipronil: action at the GABA receptor. Rev. Toxicol. 2, 147–156. [Google Scholar]
- 21.Brown LA, Ihara M, Buckingham SD, Matsuda K, Sattelle DB. 2006. Neonicotinoid insecticides display partial and super agonist actions on native insect nicotinic acetylcholine receptors. J. Neurochem. 99, 608–615. ( 10.1111/j.1471-4159.2006.04084.x) [DOI] [PubMed] [Google Scholar]
- 22.Matsuda K, Kanaoka S, Akamatsu M, Sattelle DB. 2009. Diverse actions and target-site selectivity of neonicotinoids: structural insights. Mol. Pharmacol. 76, 1–10. ( 10.1124/mol.109.055186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goulson D. 2013. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 50, 977–987. ( 10.1111/1365-2664.12111) [DOI] [Google Scholar]
- 24.Straub L, et al. 2016. Neonicotinoid insecticides can serve as inadvertent insect contraceptives. Proc. R. Soc. B 283, 20160506 ( 10.1098/rspb.2016.0506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rundlöf M, et al. 2015. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80. ( 10.1038/nature14420) [DOI] [PubMed] [Google Scholar]
- 26.Whitehorn PR, Cook N, Blackburn CV, Gill SM, Green J, Shuker DM. 2015. Sex allocation theory reveals a hidden cost of neonicotinoid exposure in a parasitoid wasp. Proc. R. Soc. B 282, 20150389 ( 10.1098/rspb.2015.0389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanken LJ, van Langevelde F, van Dooremalen C. 2015. Interaction between Varroa destructor and imidacloprid reduces flight capacity of honeybees. Proc. R. Soc. B 282, 20151738 ( 10.1098/rspb.2015.1738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanley DA, Garratt MPD, Wickens JB, Wickens VJ, Potts SG, Raine NE. 2015. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 528, 548–550. ( 10.1038/nature16167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsvetkov N, Samson-Robert O, Sood K, Patel HS, Malena DA, Gajiwala PH, Maciukiewicz P, Fournier V, Zayed A. 2017. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 356, 1395–1397. ( 10.1126/science.aam7470) [DOI] [PubMed] [Google Scholar]
- 30.Wu-Smart J, Spivak M. 2016. Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Sci. Rep. 6, 32108 ( 10.1038/srep32108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Prisco G, Cavaliere V, Annoscia D, Varricchio P, Caprio E, Nazzi F, Gargiulo G, Pennacchio F. 2013. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl Acad. Sci. USA 110, 18 466–18 471. ( 10.1073/pnas.1314923110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sgolastra F, et al. 2017. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Manage. Sci. 73, 1236–1243. ( 10.1002/ps.4449) [DOI] [PubMed] [Google Scholar]
- 33.European Commission. 2013. Commission Implementing Regulation (EU) No 781/2013. Official J. Eur. Union 219, 22–25. See https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:219:0022:0025:EN:PDF. [Google Scholar]
- 34.Euorpean Commission. 2013. Commission Implementing Regulation (EU) No 485/2013. Official J. Eur. Union 139, 12–26. See https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:139:0012:0026:EN:PDF. [Google Scholar]
- 35.Holder PJ, Jones A, Tyler CR, Cresswell JE. 2018. Fipronil pesticide as a suspect in historical mass mortalities of honey bees. Proc. Natl Acad. Sci. USA 115, 13 033–13 038. ( 10.1073/pnas.1804934115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jinguji H, Thuyet DQ, Uéda T, Watanabe H. 2013. Effect of imidacloprid and fipronil pesticide application on Sympetrum infuscatum (Libellulidae: Odonata) larvae and adults. Paddy Water Environ. 11, 277–284. ( 10.1007/s10333-012-0317-3) [DOI] [Google Scholar]
- 37.Cartea ME, Padilla G, Vilar M, Velasco P. 2009. Incidence of the major Brassica pests in northwestern Spain. J. Econ. Entomol. 102, 767–773. ( 10.1603/029.102.0238) [DOI] [PubMed] [Google Scholar]
- 38.Kowalska J. 2011. The possibilities of protection of organic cabbage crops in Poland. IOBC/WPRS Bull. 65, 77–83. [Google Scholar]
- 39.Gunasekara AS, Truong T, Goh KS, Spurlock F, Tjeerdema RS. 2007. Environmental fate and toxicology of fipronil. J. Pestic. Sci. 32, 189–199. ( 10.1584/jpestics.R07-02) [DOI] [Google Scholar]
- 40.Wang T, Hu J, Liu C. 2014. Simultaneous determination of insecticide fipronil and its metabolites in maize and soil by gas chromatography with electron capture detection. Environ. Monit. Assess. 186, 2767–2774. ( 10.1007/s10661-013-3577-5) [DOI] [PubMed] [Google Scholar]
- 41.Wu X, Yu Y, Xu J, Dong F, Liu X, Du P, Wei D, Zheng Y. 2017. Residue analysis and persistence evaluation of fipronil and its metabolites in cotton using high-performance liquid chromatography-tandem mass spectrometry. PLoS ONE 12, e0173690 ( 10.1371/journal.pone.0173690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu D, Liu D, Gu X, Diao J, Zhou Z. 2010. Stereoselective metabolism of fipronil in water hyacinth (Eichhornia crassipes). Pestic. Biochem. Physiol. 97, 289–293. ( 10.1016/j.pestbp.2010.04.009) [DOI] [Google Scholar]
- 43.Liu D, Wang P, Zhu W, Gu X, Zhou W, Zhou Z. 2008. Enantioselective degradation of fipronil in Chinese cabbage (Brassica pekinensis). Food Chem. 110, 399–405. ( 10.1016/j.foodchem.2008.02.017) [DOI] [PubMed] [Google Scholar]
- 44.Hainzl D, Casida JE. 1996. Fipronil insecticide: novel photochemical desulfinylation with retention of neurotoxicity. Proc. Natl Acad. Sci. USA 93, 12 764–12 767. ( 10.1073/pnas.93.23.12764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitehorn PR, Norville G, Gilburn A, Goulson D. 2018. Larval exposure to the neonicotinoid imidacloprid impacts adult size in the farmland butterfly Pieris brassicae. PeerJ. 6, e4772 ( 10.7717/peerj.4772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B. 2014. Defaunation in the Anthropocene. Science 345, 401–406. ( 10.1126/science.1251817) [DOI] [PubMed] [Google Scholar]
- 47.Brereton T, Roy DB, Middlebrook I, Botham M, Warren M. 2011. The development of butterfly indicators in the United Kingdom and assessments in 2010. J. Insect Conserv. 15, 139–151. ( 10.1007/s10841-010-9333-z) [DOI] [Google Scholar]
- 48.Gilburn AS, Bunnefeld N, Wilson JM, Botham MS, Brereton TM, Fox R, Goulson D. 2015. Are neonicotinoid insecticides driving declines of widespread butterflies? Peerj 3, e1402 ( 10.7717/peerj.1402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forister ML, et al. 2016. Increasing neonicotinoid use and the declining butterfly fauna of lowland California. Biol. Lett. 12, 20160475 ( 10.1098/rsbl.2016.0475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kathage J, Castañera P, Alonso-Prados JL, Gómez-Barbero M, Rodríguez-Cerezo E. 2018. The impact of restrictions on neonicotinoid and fipronil insecticides on pest management in maize, oilseed rape and sunflower in eight European Union regions. Pest Manage. Sci. 74, 88–99. ( 10.1002/ps.4715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harvey JA, et al. 2020. International scientists formulate a roadmap for insect conservation and recovery. Nat. Ecol. Evol. 4, 174–176. ( 10.1038/s41559-019-1079-8) [DOI] [PubMed] [Google Scholar]
- 52.Gols R, WallisDeVries MF, van Loon JJA. 2020. Data from: Reprotoxic effects of the systemic insecticide fipronil on the butterfly Pieris brassicae Dryad Digital Repository. ( 10.5061/dryad.7pvmcvdpr) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gols R, WallisDeVries MF, van Loon JJA. 2020. Data from: Reprotoxic effects of the systemic insecticide fipronil on the butterfly Pieris brassicae Dryad Digital Repository. ( 10.5061/dryad.7pvmcvdpr) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.7pvmcvdpr [52]. Supporting data are accessible in electronic supplementary material, table S1 and figure S1.


