Abstract
For social bees, an understudied step in evaluating pesticide risk is how contaminated food entering colonies affects residing offspring development and maturation. For instance, neurotoxic insecticide compounds in food could affect central nervous system development predisposing individuals to become poorer task performers later-in-life. Studying bumblebee colonies provisioned with neonicotinoid spiked nectar substitute, we measured brain volume and learning behaviour of 3 or 12-day old adults that had experienced in-hive exposure during brood and/or early-stage adult development. Micro-computed tomography scanning and segmentation of multiple brain neuropils showed exposure during either of the developmental stages caused reduced mushroom body calycal growth relative to unexposed workers. Associated with this was a lower probability of responding to a sucrose reward and lower learning performance in an olfactory conditioning test. While calycal volume of control workers positively correlated with learning score, this relationship was absent for exposed workers indicating neuropil functional impairment. Comparison of 3- and 12-day adults exposed during brood development showed a similar degree of reduced calycal volume and impaired behaviour highlighting lasting and irrecoverable effects from exposure despite no adult exposure. Our findings help explain how the onset of pesticide exposure to whole colonies can lead to lag-effects on growth and resultant dysfunction.
Keywords: Bombus terrestris, imidacloprid, micro-computed tomography scanning, mushroom body calyces, neonicotinoid, sublethal
1. Introduction
Insect pollinator declines are of worldwide concern [1,2], and safeguarding this important functional group requires a deep understanding of the driving factors [3,4]. Social bees are important insect pollinators, and the threat posed by pesticide exposure is a widespread issue [5,6]. A growing number of studies have highlighted how foragers directly exposed to insecticide compounds can lead to sublethal effects on behaviour (e.g. [7–9]) with possible knock-on effects to colony function [10–12]. However, with insecticide residues detected inside colonies across the globe [13–18], we know less as to how pesticide-contaminated pollen and nectar brought back by foragers [19–24] place developing individuals being reared and residing inside colonies at risk [25–27]. To date, many studies chronically exposing whole colonies to certain insecticides have reported reductions in colony growth to manifest multiple weeks after onset of exposure [11,24,28–31]. A possible mechanistic explanation for this ‘lag-effect’ is that in-hive exposure is affecting the physiological development of brood and early-stage adults (a.k.a. callows—a cohort representing the future generation of the colony's workforce), predisposing these individuals to exhibit lower performance of tasks important for colony function as older adults [32–34]. In this paper, we test this hypothesis.
Of the neurotoxic insecticides found inside colonies [13,17], many are detected in the food stores and wax that brood (developing larvae and pupae) and residing callows feed-upon and come into contact with. This route of exposure to neurotoxic compounds could potentially affect the developmental plasticity of the central nervous system, with the growth of the brain being at particular risk [35]. Indeed, regions of bee brains are highly plastic early in development [36–38], and volume can increase with task experience [39–42] and correlate with the level of task performance ([43]; but see [44]). Therefore, if pesticide exposure were to affect early brain growth, by for instance reducing brain size, we might predict behavioural task performance, such as learning, to be impaired. However, while recognized as a research priority [32–34,45], no study to date has investigated how pesticide exposure during early-stage development affects brain developmental plasticity and its association with behavioural performance in older adulthood.
Here, we investigated if bumblebees (Bombus terrestris audax) developing inside colonies provisioned with imidacloprid (a neonicotinoid pesticide) treated nectar substitute (figure 1), show impaired learning behaviour as adults, and using micro-computed tomography (μCT) scanning [46–49] tested whether this is associated with reduced growth of brain regions during early-stage development (figure 2). Implementing a factorial experiment, we provisioned colonies with treated food at different stages to compare the responses of workers that experienced in-hive exposure during either their brood development stage, early-adult stage or both stages (figure 1). Comparing responses between these three treatments (pre-eclosion, post-eclosion or continual exposure, respectively) relative to unexposed workers (control), we investigated which developmental stage was more vulnerable to exposure in terms of later adult performance and physiology. By tracking worker development, we tested two controlled age cohorts of adults at 3 and 12 days old (see electronic supplementary material, figure S1), each of which we attempted to limit variation in prior experience and sensory input. Our comparison of young (3-day) versus older (12-day) workers within and between treatments allowed us to: (i) distinguish the effects of exposure from variation caused by potential innate effects of age (experience independent change); and (ii) test whether developmental plasticity (in behaviour or tissue growth) allows any potential impact from brood exposure to be recovered during the unexposed adult phase.
Figure 1.
Graphic showing the developmental and exposure periods of individuals inside colonies for the four colony treatments (control, pre-eclosion, post-eclosion and continual) and the eight cohorts of workers tested. ‘Brood development’ represents the larval and pupal stages of workers, with ‘Adult development’ representing the number of days after eclosion from the pupal case. Colonies were provisioned neonicotinoid untreated/treated sucrose solution with the blue solid line representing worker development in the presence of untreated and red dashed in the presence of treated sucrose solution. Developing brood spend the first ca 10 days feeding (direct exposure) and the last ca 11 days as non-feeding pupae but may be exposed to residues accumulated inside the pupal case or larval tissue (indirect exposure). White circles and individual bee symbols depict removal of these controlled aged adult workers at 3 or 12-days after eclosion for immediate involvement in the behavioural assay followed by decapitation for μCT scanning of the brain. (Online version in colour.)
Figure 2.
Three-dimensional rendering of one of the studied bumblebee brains using our μCT imaging method. (a) Focal neuropils considered in this study are shown in dark purple (optic lobes: medulla (Me); lobula (Lo); antennal lobes (AL); central body (CB); mushroom body: calyces (including lateral calyx (LC) and medial calyx (MC) and lobe (MBL)), surrounded by remaining brain tissue in transparent yellow. (b–d) Isolated three-dimensional structure of the mushroom body which has been rotated to show (b) frontal, (c) lateral and (d) dorsal views. (Online version in colour.)
2. Material and methods
(a). Study system and animal husbandry
Twenty-two Bombus terrestris audax colonies were ordered from the commercial supplier Biobest and distributed by Agralan Ltd. Each colony possessed a queen and mean (±s.e.m.) of 14.5 ± 1.1 workers on arrival (electronic supplementary material, table S2) and housed in an aerated plastic box (29 × 22.5 × 13 cm). Throughout the experiment, colonies were kept under a controlled environment (23°C; 60% humidity) red light room and provisioned honeybee collected pollen (distributed by Agralan Ltd) ad libitum in a petri-dish and 40/60% sucrose/water solution in a gravity feeder. Food was replenished every 2 days, and feeders thoroughly cleaned prior to refill (electronic supplementary material, table S3). Throughout the experiment, colonies were checked daily for males, gynes or dead individuals which were removed and frozen.
(b). Experimental set-up
In-hive exposure to developing individuals was achieved by spiking the sucrose solution provisioned to each colony with 5 parts per billion (ppb) imidacloprid (see electronic supplementary material for preparation). This is a neonicotinoid that: (i) is used across the globe [13,50–52]; (ii) targets nACh receptors found in insect brains [53,54]; (iii) has been shown to affect bee foraging and navigation reliant on aspects of learning behaviour [7–9]; (iv) is at a concentration approximating that found in pollen and nectar of crop and wild flowers that bees forage on [20,55,56]. Colonies were assigned to one of four treatments which determined the two periods of time (phase I or II) that colonies were provisioned treated and/or untreated sucrose (exposure): control = phases I and II both unexposed (n = 5 colonies); pre-eclosion = phase I exposed, phase II unexposed (n = 6); post-eclosion = phase I unexposed, phase II exposed (n = 6); continual = phases I and II exposed (n = 5). Phase I started 2 days after colonies arrived and lasted for a period of 21 days as this approximates the development time from an egg or very small larva to adult eclosion [57–59] (electronic supplementary material, figure S1). During phase I (figure 1; electronic supplementary material, figure S1), we conducted daily checks of all newly eclosed bees and marked each using a white paint pen (uni Posca, PC.5M 1.8–2.5 mm) so we could later distinguish these workers (not-tested in this study) from the workers eclosing after day 21 (individuals we did test in this study). Phase I colony exposure was thus what we used to investigate the effects of exposure during brood development. Phase II started on day 22 and ended on day 45 when the experiment stopped and colonies were frozen, and was the period allowing us to investigate the effects of exposure during early-adult development (up to 3 and 12 days old in this study). For the first 11 days of phase II, we checked daily for callow workers (adults recently eclosed from the pupal case) and attached a unique numbered Opalith tag using superglue. This tagging period (days 22–33; electronic supplementary material, table S4) approximates the time of pupal development in which individuals evacuate their gut and stop feeding [59]. This means that, for example, any worker eclosing on day 22 will have been directly feeding as larvae on treated/untreated sucrose during the first ca 10 days of phase I, with any worker eclosing on day 33 having fed as larvae in the last 10 days of phase I. This staggered design allowed us to standardized pesticide exposure as best possible while providing us with appropriate sample sizes to test our hypotheses.
For each tagging day, we took the total number of workers per colony that had eclosed on that day and randomly assigned half to the 3-day cohort and remaining half to the 12-day cohort. Using tag ID we could, therefore, remove adult workers for testing 3 or 12 days later accordingly without bias. Brain and behavioural development has been reported to occur both during brood and early-adult stages [38,60].
(c). Assessing olfactory learning performance using proboscis extension reflex conditioning
Proboscis extension reflex (PER) conditioning paradigm [61] is established as an appropriate discerning test for assessing pesticide effects on learning behaviour in bumblebees exposed as adults [62–64], and here we adapted it from a reported bumblebee set-up [62]. On removal from the colony, workers were taken to a neighbouring laboratory, cooled on ice and harnessed (13.00–14.00) using a modified 2 ml centrifuge tube and split pin yoke, under natural light and left to settle for 2 h (electronic supplementary material, figure S2). All bees were then fed 40% sucrose solution to satiety using a Gilmont® syringe by presenting droplets directly to the mouthparts. The bee was then taken to a separate controlled environment room and left for 17 h (overnight and under identical conditions as the rearing room with all further testing also done under red light). For unknown reasons, 24 workers did not survive overnight and so were excluded from the analyses (see electronic supplementary material, table S4).
Prior to the learning assay, between 08.00 and 09.00, we tested whether harnessed workers (total = 413; control = 110; pre-eclosion = 116; post-eclosion = 108; continual = 79; electronic supplementary material, table S4) showed a PER in response to their antenna being touched by a 50% sucrose solution droplet (‘responsive test’; electronic supplementary material, figure S2). Immediately after and 15 min before starting the PER test, each bee was fed a small droplet (0.8 μl) of 40% sucrose solution for motivation (electronic supplementary material, figure S2). PER conditioning was conducted in front of a filtered ventilation system (Expo Drills & Tools AB500 Extractor fan), preventing the odour coming into contact with neighbouring harnessed bees. Each bee was initially conditioned by exposure to clean air for 5 s, followed by scented air for 10 s. A harnessed bee was positioned 3 cm away from a glass odour tube, with airflow delivered at a constant rate of 80 ml s−1 (Tetra APS – 100) channelled through either a ‘clean’ unscented odour tube or diverted through a ‘scented’ odour tube containing a piece of filter paper (5 × 20 mm) impregnated with 1 μl of lemon essential oil (Naturally Thinking Ltd). Airflow diversion was controlled by a solenoid valve (Nass Magnet 108-030-0257 24vAC/12vDC) connected to a Raspberry Pi 2 (Model B) computer ensuring standardized exposure to clean and scented air volume. To develop an association between the lemon odour and reward, the bee's antennae was touched with a droplet of 0.8 μl of 50% sucrose 6 s into the 10 s odour delivery phase and allowed the bee to feed.
Following trial 1, the odour and reward presentation sequence was repeated to each adult an additional nine times. The inter-trial interval per individual was 10 min allowing PER testing in batches of up to 20 workers. We waited 15 s after the odour and reward presentation sequence before moving to the next individual [65], recording if each bee showed a PER to the odour stimulus prior or after the reward and defining as a learnt or non-learnt response, respectively. This provided a tally of learnt responses achieved by each worker over the nine trials. Bees were excluded if they responded to the initial conditioning trial (trial 1) before presenting the reward (n = 24). Bees were removed from testing and categorized as a non-learner if over three consecutive trials no PER (did not feed) was exhibited even after the reward was provided.
Following the PER assay, bees in their harness were placed on ice to immobilize the bee and allow swift decapitation using a disposable surgery scalpel. Heads were immediately submerged in a 70/30% ethanol/de-ionized water solution in separate 1.5 ml centrifuge tubes and stored at 5°C. Thorax inter-tegula distance of the remaining bodies were measured as a proxy for body size [66] by taking the mean of two repeat measurements using a digital calliper (Workzone® 150 mm, accuracy 0.01 mm).
(d). Micro-computed tomography scanning
Micro-computed tomography scanning enables us to accurately and non-destructively investigate the brain tissue in situ (within headcase) at high resolution [46–49]. Headcase preparation followed precisely the published protocol by Smith et al. [47] with the internal soft brain tissue being stained for 7 days with phosphotungstic acid before being CT scanned at a voxel size of 3.5–4 μm using a Nikon Metrology HMX ST 225 system (Nikon Metrology, Tring, UK). Raw μCT data for each brain scan was reconstructed using CTPro 2.1 software (Nikon Metrology, Tring, UK) and processed using VG Studio Max 2.1 (Volume Graphics GmbH, Heidelberg, Germany). Each three-dimensional reconstructed scan was re-oriented to the same optimum plane-of-view for visualization, and for each neuropil, we re-sliced into a new series of two-dimensional images. For each sample, scan images were exported as 8-bit BMP image series at a standardized voxel size of 4 μm. In total, 92 worker brains were μCT scanned and based on staining quality and the success of segmenting both left and right structures per individual, we considered the mushroom bodies for 78, central body for 88, antennal lobes for 89, medullas for 71 and lobulas for 71 workers (electronic supplementary material, table S5).
(e). Neuropil volume measurements
We analysed how the volumetric growth of six different functional components (neuropils) of the brain (see electronic supplementary material, table S1 for assigned functions) responded to exposure and reveal how an individual worker's learning performance is predicted by volume of the mushroom body calyces and how neonicotinoid exposure influences this relationship.
Segmentation and volume analysis of brain neuropils was undertaken using SPIERS 2.20 software. For segmentation, scan slices were converted to binary threshold images adjusted to achieve an optimum ratio of active white pixels comprising the structure of interest. Looped splines were placed around the active pixels at regular five slice intervals and then interpolated across all slices between intervals to define each structure for three-dimensional reconstruction and volumetric calculation (full segmentation protocol reported in Smith et al. [47]). Neuropil absolute volume was calculated using the voxel count function in SPIERS Edit, with relative volume calculated by dividing absolute volume by the worker's inter-tegula width. Volumes of the left and right paired structures per neuropil (except central body) were summed so a single value could be used in the analyses.
(f). Data analysis
Statistical analyses were conducted in R v. 3.5.1 (R Development Core Team 2018) using RStudio v. 1.1.463, with models implemented using lme4 package [67]. For all models, we included treatment as a fixed categorical factor. Bee body size (ITD) was considered a continuous variable and colony as a random factor in our models if inclusion increased the fit of the model (model comparisons assessed by AIC comparison) otherwise they were not retained. For responsiveness and learning, data were analysed using the proportion of individuals showing a response with a generalized linear model (glm) using a binomial distribution and included the categorical variable age (3 or 12-day) as an additional fixed factor with age * treatment interaction term removed as it showed no significant effect. For looking at the proportion of learners by trial we used a linear mixed-effects model (lmer) in which treatment consisted of two categories, control workers and pesticide workers (pooled from all three pesticide treatments because of the reduced sample sizes from the negative effects of exposure, see Results). We considered a second-order polynomial fit for trial number and individual ID as a random factor. For relative neuropil volumes, we used a linear mixed-effects model (lmer) that included age, ITD and colony (random effect). We used a binomial generalized linear model (glm) to analyse how calycal volume—by analysing the calycal volume * treatment interaction-influenced learning score, as a proportion of the maximum learning score.
3. Results
(a). Did pesticide exposure affect the proportion of workers responding to a sucrose reward prior to the learning test?
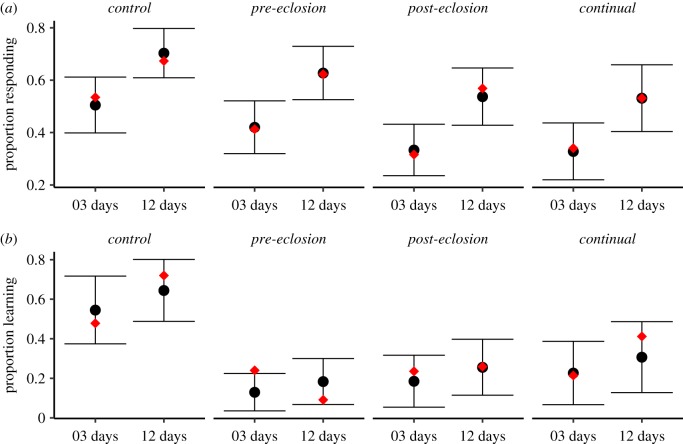
When considering the 413 harnessed workers across all treatments, we found that a significantly higher proportion of 12-day compared to 3-day workers responded (GLM: age: z = −4.10, p < 0.001). This effect of age enhancement did not differ between treatments as no age * treatment effect was detected (supported by overlapping CIs in figure 3 and the interaction term not being retained in the model; electronic supplementary material, table S5). However, when we then focused on the effect of treatment independently, we found consistent negative model estimates for all three pesticide treatments relative to control, and detected a significantly lower proportion of responsive workers from post-eclosion and continual exposed colonies (z = −2.53, p = 0.011 and z = −2.40, p = 0.016; figure 3; electronic supplementary material, table S6 for post hoc Tukey tests). Worker size had no significant effect (z = 1.41, p = 0.16).
Figure 3.
Proportion of (a) responsive workers and (b) learners between treatments. Intersecting circular points represent estimated model mean taken from model back-transformation (binomial GLM) with bars depicting associated ±95% confidence limits. Red diamond corresponds to the mean value taken from the raw response data. Sample sizes of 3- and 12-day worker cohorts for ‘responsive’: control = 58 and 52, pre-eclosion = 63 and 53; post-eclosion = 57 and 51; continual = 47 and 32; ‘learners’: control = 23 and 25, pre-eclosion = 25 and 33; post-eclosion = 17 and 27; continual = 14 and 17. (Online version in colour.)
(b). Did pesticide exposure affect learning performance?
For responsive workers, we analysed the proportion of workers exhibiting at least one learnt response over the 10 trials (n = 181 tested in PER assay; electronic supplementary material, figure S2 and table S4). Our model showed a positive estimate for the effect of age, but unlike responsiveness, no significant increase was detected which was consistent across treatments as evidenced by no age * treatment effect and the interaction term not being retained in the model (electronic supplementary material, table S5). However, we detected for each exposure treatment a significantly lower proportion of learners relative to the control (GLM: pre-eclosion: z = −4.38, p < 0.001; post-eclosion: z = −3.49, p < 0.001; continual: z = −2.78, p < 0.01; figure 3b; see electronic supplementary material, table S6 for post hoc Tukey tests), and worker size had a significant positive effect on the probability of being a learner (z = 2.31, p = 0.021).
Across all learners, we then looked at the proportion of learnt responses over the successive trials (trials 2–10 considered; by definition a naive worker cannot learn on the first trial). The strong negative effect of pesticide exposure discussed above heavily reduced sample sizes per pesticide treatment (pre-eclosion = 9, post-eclosion = 11, continual = 10), therefore, we pooled workers from the three treatments (n = 30 exposed workers) and compared them to control workers (n = 29) while not distinguishing between 3- and 12-day cohorts. The proportion of learnt responses increased over the trials (GLM polynomial: p1: t = 14.26, p < 0.001) with the incremental proportion decreasing in rate over the consecutive trials (p2: t = −2.48, p = 0.014; electronic supplementary material, table S5) driven primarily by the significant negative effect of pesticide exposure (t = −2.04, p = 0.046) in which exposed workers showed a lower proportion of learnt responses in the latter few trials relative to control (figures 4 and 5).
Figure 4.

Proportion of workers by trial exhibiting a learnt response. Workers from all three pesticide treatments were pooled (blue triangles; n = 30 workers) and compared against control workers (red circles; n = 29), with both age cohorts aggregated per treatment. Lines (blue dashed = pesticide treatment; red solid = control) represent the binomial model (LMER polynomial) estimates over the consecutive trials and shaded areas represent the 95% confidence intervals. (Online version in colour.)
Figure 5.
Relative volumes of bumblebee worker mushroom body (a) calyces, (b) lobes. Intersecting circular point represents the estimated model mean taken from model back-transformation (lmer) with bars depicting associated ±95% confidence limits. Red diamond corresponds to the mean value taken from the raw response data. Sample sizes of 3- and 12-day worker cohorts for: ‘lobes’: control = 10 and 10, pre-eclosion = 11 and 11; post-eclosion = 13 and 11; continual = 13 and 9; ‘calyces’: control = 9 and 8, pre-eclosion = 11 and 11; post-eclosion = 10 and 10; continual = 11 and 8. (Online version in colour.)
(c). Did pesticide exposure affect brain neuropil volumes?
Focusing first on the mushroom body calyces, relative volumes were significantly smaller in workers from all three pesticide exposure treatments relative to control (pre-eclosion: t = −2.41, p = 0.049; post-eclosion: t = −3.83, p < 0.01; continual: t= −2.90, p = 0.021; figure 5; electronic supplementary material, tables S7, S8). This was consistent for 3- and 12-day workers as evidenced by no effect of age * treatment and the interaction term not being retained in the model (electronic supplementary material, table S8; see table S9 for post hoc Tukey tests). For the relative volume of the mushroom body lobes, we found negative model estimates for all three pesticide treatments relative to the control, however, unlike the calyces, we did not detect these comparisons as significantly lower (figure 5; electronic supplementary material, table S8). Analysis of the four other segmented neuropils (central body, antennal lobes, lobulas and medullas) showed workers from pesticide-treated colonies possessed no significant volumetric differences relative to control, although we did find consistent negative model estimates for the antennal lobes across all pesticide treatments (electronic supplementary material, table S10).
(d). Are bigger mushroom body calyces associated with higher learning scores and how does pesticide exposure affect this relationship?
For each responsive worker starting the PER learning assay that had their mushroom body successfully segmented, we investigated how the total number of demonstrated learnt responses (learning score) was associated with relative calycal volume (predictor). As for the learning-by-trial data, workers from the three pesticide exposure treatments were pooled and compared to control workers when not distinguishing age. We found a significant positive association between relative calycal volume and learning score (t = 4.51, p < 0.001; figure 6; electronic supplementary material, figure S3), but this relationship was driven by control workers as pesticide exposed workers showed no clear relationship as supported by a significant negative volume * treatment interaction (t = −3.96, p < 0.001; electronic supplementary material, tables S11, S12).
Figure 6.
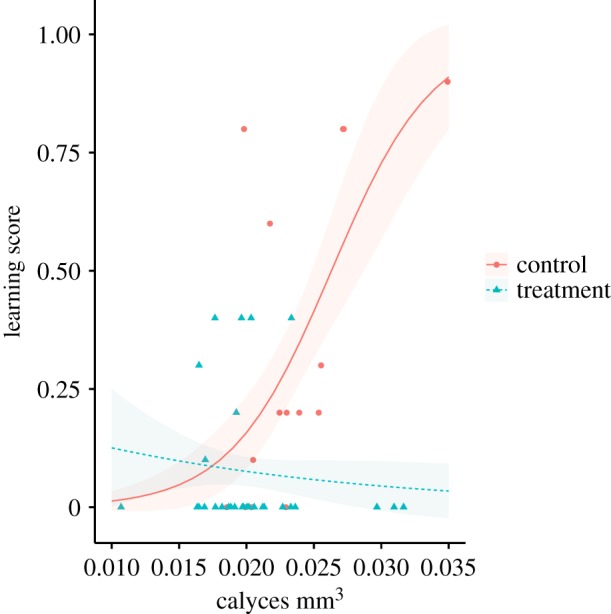
Relative mushroom body calycal volume plotted against the respective worker's learning score. Workers from all three pesticide treatments were pooled (blue triangles, n = 29; pre-eclosion = 11, post-eclosion = 12, continual = 6) and compared against control workers (red circles, n = 15), with fitted lines (blue dashed = pesticide treatment; red solid = control) representing binomial model (GLM) estimates and shaded areas representing the 95% confidence intervals. (Online version in colour.)
4. Discussion
In-hive exposure to imidacloprid-spiked nectar substitute caused workers to exhibit impeded developmental plasticity. The degree of impact from exposure during brood development appeared comparatively as detrimental as exposure during adulthood, with workers exhibiting lower responsiveness to the presentation of a sucrose reward and impaired learning performance coupled with possessing smaller mushroom body calyces. Furthermore, investigation of the relationship between each worker's respective calycal volume and learning performance revealed that while ‘bigger is better’ for control workers this positive relationship (i.e. larger calyces improves learning) was absent for pesticide exposed workers indicating functionally impaired mushroom bodies from exposure.
(a). Pesticide exposure during early development affected responsiveness and learning
Our findings of an impact on learning performance from direct adult neonicotinoid exposure conforms with previous studies showing negative effects on learning in honeybees [68–70] and bumblebees [62]. However, our study design allowed us to compare responses between young and older adults receiving different chronic exposure histories. Three- and 12-day workers from post-eclosion and continual exposure colonies (only adult stage exposed) showed a similar level of impaired behaviours despite differences in exposure length. Similarly, although 3-day adults from pre-eclosion colonies were exposed for up to two weeks during brood development compared to just 3 days for post-eclosion, learning appeared impaired to the same degree. Together these findings highlight that the first 72 h of adulthood must be important in behavioural development but also represents a susceptible developmental window to insecticide exposure [26,71] showing the importance of considering different life-stages when assessing pesticide risk.
Workers exposed during brood development (pre-eclosion) exhibited impaired learning performance at a similar reduced level as adult only exposed workers (post-eclosion) indicating a lag-effect from brood exposure on adult learning. Consideration of delayed effects of pesticide exposure is thus important when assessing pesticide risk. Indeed, studies on honeybees (Apis cerana and A. mellifera) and a stingless bee (Melipona quadrifasciata anthidioides) reported adult workers showing negative effects on adult learning and motor function when reared as larvae under topical or oral exposure to a neonicotinoid [32–34]. Our findings further show that comparison of 3- and 12-day adults from pre-eclosion colonies (exposed only as brood) exhibited a similar level of impaired learning performance showing that no recovery could be made during the 9-day interim period.
(b). Impaired learning in pesticide exposed workers was associated with reduced volumetric growth of the mushroom body calyces
Exposure at either stage of development was associated with workers possessing smaller mushroom body calyces. A focus on 3-day adults revealed that post-eclosion and continual exposed workers showed reduced volumes relative to control workers, highlighting that in just 3 days of adult exposure a key brain region was unable to grow as normal. With mushroom body growth rate in bumblebees considered to be highest during the first 72 h of adulthood [36,38], our findings indicate this to be a developmental period particularly susceptible to stress.
The average volume reductions of the mushroom body calyces and lobes showed a strikingly mirrored pattern to the reduced proportion of learners in each respective pesticide treatment. More tellingly, for workers in which we could pair relative volume with learning score, we found that bigger did equate to better learning performance for control workers. However, such a positive relationship was not the case for imdacloprid exposed workers, indicating impaired neuronal functioning of this brain region. While our study cannot explicitly test whether reduced calycal volume is the direct cause of lowered learning—for example, behaviour could be affected if exposure were to affect immune pathways [72] or demotivate individuals during harnessing prior to the PER assay (although similar learning performance between pre- and post-eclosion bees would not support this view), our findings do support for our clear a priori hypothesis that effects of exposure on the brain during early-stage development would be associated with impacts on behavioural performance.
With neonicotinoids acting as nACh receptor agonists [54] calycal functional impairment does make sense for adult exposed workers (post-eclosion and continual). However, with brood exposed workers (pre-eclosion) also being affected this indicates exposure to be affecting synaptic development and proliferation in the calyces. For instance, microglomeruli configuration is considered to be associated with learning and memory in bees [73–76], and reduced density from neonicotinoid exposure has been reported in honeybees [35]. It could also stem from impeded neurogenesis where neuronal precursor cells were prevented from giving rise to mushroom body Kenyon cells, which in honeybees occurs during development pre-eclosion [77,78], or reduced Kenyon cell size as shown in neonicotinoid exposure experiments on bumblebee cell cultures [79].
(c). Mushroom body calyces were disproportionately affected over the other neuropils
Mushroom body calyces are multisensory processors fed by afferent neurons, in contrast to the lobes that predominantly function as output regions with efferent neurons [36]. This functional difference could explain why calycal volumetric variation was more tightly associated with our measure of learning performance and seemingly more vulnerable to exposure when considering plasticity. But why was little effect also observed in the other neuropils? For instance, antennal lobes are involved in detecting and processing olfactory information (electronic supplementary material, table S1), are developmentally plastic during early adulthood [36,38], and exhibit reduced neuronal function under nicotinic agonists [80,81]. Considering exposed workers performed worse in the olfactory conditioning assay, we might have expected a pattern of impeded growth in the antennal lobes, but our analysis did not detect the negative model estimates as significant. Possible explanations for the disproportionate effect on the mushroom bodies include: (i) nACh receptors targeted by neonicotinoids are found in high density in the mushroom body Kenyon cells [82], and if Kenyon cell proliferation was affected by exposure this could lead to neuropil volumetric reductions; (ii) the high degree of mushroom body plasticity compared to other neuropils [36,38,60,83] requires a high level of neurogenesis and organization possibly increasing the risk of neurotoxic exposure interfering with this process; (iii) developing adult workers were stimulus deprived but not void, therefore, while mushroom body volumetric increase is likely to be more experience independent than dependent, we could not rule out investment in olfactory processing to compensate for a lack of visual information [38,39]; (iv) consistent volumetric reductions in non-mushroom body neuropils did occur as a result of exposure but we did not have the power to detect these small effect sizes or the resolution of our μCT technology was too low.
(d). Improved behavioural performance and mushroom body growth with age independent of experience
Previous bumblebee studies have reported no effect of age on aspects of learning ability [84,85], although these were carried out in foraging arenas whereby prior experiences were not controlled. Having kept our colonies under a stimulus deprived environment and compared between controlled age cohorts, we, in contrast, do provide evidence of a positive effect of B. terrestris age on responsiveness and learning indicative of experience independent age enhancement. In parallel, we show brain volume to increase which to our knowledge has only been shown once before in a histological study on the bumblebee Bombus impatiens by Jones et al. [38]. Jones' findings suggested ca 10% increase in mushroom body volume between workers of similar ages used in our study compare to just over 20% in our study. This difference perhaps stems from variation in methodological approaches, sample sizes (higher in our study) or taxonomic variation, but together supports an innate increase in neuropil volume over the first 12-days of adulthood.
(e). Conclusions
Our findings of early exposure affecting later adult behaviour can provide an explanation for why reduced colony growth has been detected two to three weeks after the onset of neonicotinoid exposure in previous studies [11,24,28–31]. If future generations of workers are predisposed to be inefficient functioning cohorts, this could lead to a density-dependent build-up of colony-level impairment increasing the risk of colony collapse [12]. Our results suggest that even if newly eclosed workers were to delay the age at which they start any specific task performance, such a strategy could be futile given we saw a little adult recovery in behaviour from 3 to 12 days of adulthood from pre-eclosion colonies. Our method of provisioning colonies with a treated nectar substitute may also represent a conservative level of exposure given that developing brood are more dependent on pollen for tissue growth than adults, and that concentrations of neonicotinoid residues in pollen are typically higher than found in nectar [20,55,56]. Future work could also look to compare responses between insect species that progressively feed their offspring during development, such as bumblebees, and those that mass provision offspring, such as pollen and nectar packages in the cells of solitary bees [71]. Importantly, our findings are unlikely to be exclusively applicable to: (i) workers, as newly reared males and queens are also at risk with possible implications for mating and hibernation; (ii) neonicotinoids, as many neurotoxic pesticides including cholinergic insecticides (e.g. sulfoxamines, butenolides) can build up inside bee colonies and induce sublethal effects on individual and colony-level traits [31,86].
Supplementary Material
Acknowledgements
We thank Peter Graystock for constructive advice on the manuscript, Russell Garwood for help using SPIERS software, Dan Sykes and Amin Garbout for help with the μCT scanning protocol, Paul Beasley for technical support, Alfredo Sánchez Tójar for advice on data analysis, and Richard Abel, Mark Brown, Inti-Pedroso, Nigel Raine and Seirian Sumner for advice on pilot work.
Data accessibility
Raw data and scripts used for statistical analysis and figure production are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.63xsj3tzp [87].
Authors' contributions
R.J.G. conceived the project; D.B.S. and R.J.G. designed the experiment; D.B.S., A.R.R. and P.H.B. conducted the experiment; D.B.S. and F.A. carried out the μCT scanning; D.B.S. and D.B. reconstructed and segmented the brains; A.N.A. and R.J.G. performed the data analyses; D.B.S., A.N.A. and R.J.G. wrote the manuscript.
Competing interests
We declare we have no competing interests
Funding
D.B.S. PhD studentship was supported by Imperial College's NERC SSCP DTP. The following NERC grant nos. NE/L00755X/1 and NE/P012574/1 awarded to R.J.G. further enabled this research and supported A.N.A. and A.R.R. The Masters of Research programme at Imperial College's Silwood Park supported P.H.B.
References
- 1.Vanbergen AJ. 2013. Threats to an ecosystem service: pressures on pollinators. Front. Ecol. Environ. 11, 251–259. ( 10.1890/120126) [DOI] [Google Scholar]
- 2.Potts SG, et al. 2016. Safeguarding pollinators and their values to human well-being. Nature 540, 220–229. ( 10.1038/nature20588) [DOI] [PubMed] [Google Scholar]
- 3.Gill RJ, et al. 2016. Protecting an ecosystem service: approaches to understanding and mitigating threats to wild insect pollinators. Adv. Ecol. Res. 54, 135–206. ( 10.1016/bs.aecr.2015.10.007) [DOI] [Google Scholar]
- 4.Goulson D, Nicholls E, Botías C, Rotheray EL. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 ( 10.1126/science.1255957) [DOI] [PubMed] [Google Scholar]
- 5.Woodcock BA, et al. 2017. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 356, 1393–1395. ( 10.1126/science.aaa1190) [DOI] [PubMed] [Google Scholar]
- 6.Brittain C, Potts SG. 2011. The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic Appl. Ecol. 12, 321–331. ( 10.1016/j.baae.2010.12.004) [DOI] [Google Scholar]
- 7.Fischer J, Mueller T, Spatz AK, Greggers U, Gruenewald B, Menzel R. 2014. Neonicotinoids interfere with specific components of navigation in honeybees. PLoS ONE 9, e91364 ( 10.1371/journal.pone.0091364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill RJ, Raine NE. 2014. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Funct. Ecol. 28, 1459–1471. ( 10.1111/1365-2435.12292) [DOI] [Google Scholar]
- 9.Samuelson EEW, Chen-Wishart ZP, Gill RJ, Leadbeater E. 2016. Effect of acute pesticide exposure on bee spatial working memory using an analogue of the radial-arm maze. Sci. Rep. 6, 38957 ( 10.1038/srep38957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crall JD, et al. 2018. Neonicotinoid exposure disrupts bumblebee nest behavior, social networks, and thermoregulation. Science 362, 683–686. ( 10.1126/science.aat1598) [DOI] [PubMed] [Google Scholar]
- 11.Gill RJ, Ramos-Rodriguez O, Raine NE. 2012. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491, 105–108. ( 10.1038/nature11585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryden J, Gill RJ, Mitton RAA, Raine NE, Jansen VAA. 2013. Chronic sublethal stress causes bee colony failure. Ecol. Lett. 16, 1463–1469. ( 10.1111/ele.12188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell EAD, Mulhauser B, Mulot M, Mutabazi A, Glauser G, Aebi A. 2017. A worldwide survey of neonicotinoids in honey. Science 358, 109–111. ( 10.1126/science.aan3684) [DOI] [PubMed] [Google Scholar]
- 14.Daniele G, Giroud B, Jabot C, Vulliet E. 2018. Exposure assessment of honeybees through study of hive matrices: analysis of selected pesticide residues in honeybees, beebread, and beeswax from French beehives by LC-MS/MS. Environ. Sci. Pollut. Res. 25, 6145–6153. ( 10.1007/s11356-017-9227-7) [DOI] [PubMed] [Google Scholar]
- 15.Calatayud-Vernich P, Calatayud F, Simó E, Picó Y. 2018. Pesticide residues in honey bees, pollen and beeswax: assessing beehive exposure. Environ. Pollut. 241, 106–114. ( 10.1016/j.envpol.2018.05.062) [DOI] [PubMed] [Google Scholar]
- 16.Valdovinos-Flores C, Alcantar-Rosales VM, Gaspar-Ramírez O, Saldaña-Loza LM, Dorantes-Ugalde JA. 2017. Agricultural pesticide residues in honey and wax combs from Southeastern, Central and Northeastern Mexico. J. Apic. Res. 56, 667–679. ( 10.1080/00218839.2017.1340798) [DOI] [Google Scholar]
- 17.Sanchez-Bayo F, Goka K. 2014. Pesticide residues and bees—a risk assessment. PLoS ONE 9, e94482 ( 10.1371/journal.pone.0094482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis C, Park KJ, Whitehorn P, David A, Goulson D. 2017. The neonicotinoid insecticide thiacloprid impacts upon bumblebee colony development under field conditions. Environ. Sci. Technol. 51, 1727–1732. ( 10.1021/acs.est.6b04791) [DOI] [PubMed] [Google Scholar]
- 19.Pohorecka K, et al. 2012. Residues of neonicotinoid insecticides in bee collected plant materials from oilseed rape crops and their effect on bee colonies. J. Apic. Sci. 56, 115–134. ( 10.2478/v10289-012-0029-3) [DOI] [Google Scholar]
- 20.Botias C, David A, Horwood J, Abdul-Sada A, Nicholls E, Hill E, Goulson D, 2015. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 49, 12 731–12 740. ( 10.1021/acs.est.5b03459) [DOI] [PubMed] [Google Scholar]
- 21.David A, Botias C, Abdul-Sada A, Nicholls E, Rotheray EL, Hill EM, Goulson D. 2016. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Int. 88, 169–178. ( 10.1016/j.envint.2015.12.011) [DOI] [PubMed] [Google Scholar]
- 22.Kasiotis KM, Anagnostopoulos C, Anastasiadou P, Machera K. 2014. Pesticide residues in honeybees, honey and bee pollen by LC-MS/MS screening: reported death incidents in honeybees. Sci. Total Environ. 485, 633–642. ( 10.1016/j.scitotenv.2014.03.042) [DOI] [PubMed] [Google Scholar]
- 23.Chauzat MP, Faucon J-P, Martel A-C, Lachaize J, Cougoule N, Aubert M. 2006. A survey of pesticide residues in pollen loads collected by honey bees in France. J. Econ. Entomol. 99, 253–262. ( 10.1093/jee/99.2.253) [DOI] [PubMed] [Google Scholar]
- 24.Rundlöf M, et al. 2015. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80. ( 10.1038/nature14420) [DOI] [PubMed] [Google Scholar]
- 25.Pohorecka K, Szczęsna T, Witek M, Miszczak A, Sikorski P. 2017. The exposure of honey bees to pesticide residues in the hive environment with regard to winter colony losses. J. Apic. Sci. 61, 105–125. ( 10.1515/jas-2017-0013) [DOI] [Google Scholar]
- 26.Wu JY, Anelli CM, Sheppard WS. 2011. Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS ONE 6, e14720 ( 10.1371/journal.pone.0014720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu JY, Smart MD, Anelli CM, Sheppard WS. 2012. Honey bees (Apis mellifera) reared in brood combs containing high levels of pesticide residues exhibit increased susceptibility to Nosema (Microsporidia) infection. J. Invertebr. Pathol. 109, 326–329. ( 10.1016/j.jip.2012.01.005) [DOI] [PubMed] [Google Scholar]
- 28.Whitehorn PR, O'Connor S, Wackers FL, Goulson D. 2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351–352. ( 10.1126/science.1215025) [DOI] [PubMed] [Google Scholar]
- 29.Arce AN, David TI, Randall EL, Ramos Rodrigues A, Colgan TJ, Wurm Y, Gill RJ. 2017. Impact of controlled neonicotinoid exposure on bumblebees in a realistic field setting. J. Appl. Ecol. 54, 1199–1208. ( 10.1111/1365-2664.12792) [DOI] [Google Scholar]
- 30.Tsvetkov N, Samson-Robert O, Sood K, Patel HS, Malena DA, Gajiwala PH, Maciukiewicz P, Fournier V, Zayed A. 2017. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 356, 1395–1397. ( 10.1126/science.aam7470) [DOI] [PubMed] [Google Scholar]
- 31.Siviter H, Brown MJF, Leadbeater E. 2018. Sulfoxaflor exposure reduces bumblebee reproductive success. Nature 561, 109–112. ( 10.1038/s41586-018-0430-6) [DOI] [PubMed] [Google Scholar]
- 32.Tan K, Chen W, Dong S, Liu X, Wang Y, Nieh JC. 2015. A neonicotinoid impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Sci. Rep. 5, 10989 ( 10.1038/srep10989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomé HVV, Martins GF, Lima MAP, Campos LAO, Guedes RNC. 2012. Imidacloprid-induced impairment of mushroom bodies and behavior of the native stingless bee Melipona quadrifasciata anthidioides. PLoS ONE 7, e38406 ( 10.1371/journal.pone.0038406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang EC, Chang HC, Wu WY, Chen YW. 2012. Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS ONE 7, e49472 ( 10.1371/journal.pone.0049472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng YC, Yang EC. 2016. Sublethal dosage of imidacloprid reduces the microglomerular density of honey bee mushroom bodies. Sci. Rep. 6, 19298 ( 10.1038/srep19298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riveros AJ, Gronenberg W. 2010. Brain allometry and neural plasticity in the bumblebee Bombus occidentalis. Brain Behav. Evol. 75, 138–148. ( 10.1159/000306506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galizia GC, Eisenhardt D, Giurfa M (eds) 2011. Honeybee neurobiology and behavior: a tribute to Randolf Menzel. Dordrecht, Netherlands: Springer Science & Business Media B.V. [Google Scholar]
- 38.Jones BM, Leonard AS, Papaj DR, Gronenberg W. 2013. Plasticity of the worker bumblebee brain in relation to age and rearing environment. Brain Behav. Evol. 82, 250–261. ( 10.1159/000355845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fahrbach SE, Moore D, Capaldi EA, Farris SM, Robinson GE. 1998. Experience-expectant plasticity in the mushroom bodies of the honeybee. Learn. Mem. 5, 115–123. [PMC free article] [PubMed] [Google Scholar]
- 40.Durst C, Eichmüller S, Menzel R. 1994. Development and experience lead to increased volume of subcompartments of the honeybee mushroom body. Behav. Neural. Biol. 62, 259–263. ( 10.1016/S0163-1047(05)80025-1) [DOI] [PubMed] [Google Scholar]
- 41.Withers GS, Fahrbach SE, Robinson GE. 1993. Selective neuroanatomical plasticity and division of labour in the honeybee. Nature 364, 238–240. ( 10.1038/364238a0) [DOI] [PubMed] [Google Scholar]
- 42.Maleszka J, Barron AB, Helliwell PG, Maleszka R. 2009. Effect of age, behaviour and social environment on honey bee brain plasticity. J. Comp. Physiol. 195, 733–740. ( 10.1007/s00359-009-0449-0) [DOI] [PubMed] [Google Scholar]
- 43.Gronenberg W, Couvillon MJ. 2010. Brain composition and olfactory learning in honey bees. Neurobiol. Learn. Mem. 93, 435–443. ( 10.1016/j.nlm.2010.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chittka L, Niven J. 2009. Are bigger brains better? Curr. Biol. 19, R995–R1008. ( 10.1016/j.cub.2009.08.023) [DOI] [PubMed] [Google Scholar]
- 45.Siviter H, Koricheva J, Brown MJF, Leadbeater E. 2018. Quantifying the impact of pesticides on learning and memory in bees. J. Appl. Ecol. 55, 2812–2821. ( 10.1111/1365-2664.13193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ribi W, Senden TJ, Sakellariou A, Limaye A, Zhang S. 2008. Imaging honey bee brain anatomy with micro-X-ray-computed tomography. J. Neurosci. Methods 171, 93–97. ( 10.1016/j.jneumeth.2008.02.010) [DOI] [PubMed] [Google Scholar]
- 47.Smith DB, Bernhardt G, Raine NE, Abel RL, Sykes D, Ahmed F, Pedroso I, Gill RJ. 2016. Exploring miniature insect brains using micro-CT scanning techniques. Sci. Rep. 6, 21768 ( 10.1038/srep21768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baird E, Taylor G. 2017. X-ray micro computed-tomography. Curr. Biol. 27, R289–R291. ( 10.1016/j.cub.2017.01.066) [DOI] [PubMed] [Google Scholar]
- 49.Gutiérrez Y, Ott D, Töpperwien M, Salditt T, Scherber C. 2018. X-ray computed tomography and its potential in ecological research: a review of studies and optimization of specimen preparation. Ecol. Evol. 8, 7717–7732. ( 10.1002/ece3.4149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cressey D. 2017. The bitter battle over the world's most popular insecticides. Nature 551, 156–158. ( 10.1038/551156a) [DOI] [PubMed] [Google Scholar]
- 51.Zhang W. 2018. Global pesticide use: profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 8, 1–27. [Google Scholar]
- 52.Casida JE. 2018. Neonicotinoids and other insect nicotinic receptor competitive modulators: progress and prospects. Annu. Rev. Entomol. 63, 125–144. ( 10.1146/annurev-ento-020117-043042) [DOI] [PubMed] [Google Scholar]
- 53.Jeschke P, Nauen R. 2008. Neonicotinoids—from zero to hero in insecticide chemistry. Pest Manag. Sci. 64, 1084–1098. ( 10.1002/ps.1631) [DOI] [PubMed] [Google Scholar]
- 54.Palmer MJ, Moffat C, Saranzewa N, Harvey J, Wright GA, Connolly CN. 2013. Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat. Commun. 4, 1634 ( 10.1038/ncomms2648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Godfray HCJ, David A, Horwood J, Abdul-Sada A, Nicholls E, Hill E, Goulson D. 2014. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B 281, 1786 ( 10.1098/rspb.2014.0558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wood TJ, Goulson D. 2017. The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ. Sci. Pollut. Res. 24, 17 285–17 325. ( 10.1007/s11356-017-9240-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alford D. 1975. Bumblebees. London, UK: Davis-Poynter. [Google Scholar]
- 58.Duchateau MJ, Velthuis HHW. 1988. Development and reproductive strategies in Bombus terrestris colonies. Behaviour 107, 186–207. ( 10.1163/156853988X00340) [DOI] [Google Scholar]
- 59.Cnaani J, Schmid-Hempel R, Schmidt JO. 2002. Colony development, larval development and worker reproduction in Bombus impatiens Cresson. Insectes Soc. 49, 164–170. ( 10.1007/s00040-002-8297-8) [DOI] [Google Scholar]
- 60.Farris SM, Robinson GE, Fahrbach SE. 2001. Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. J. Neurosci. 21, 6395–6404. ( 10.1523/JNEUROSCI.21-16-06395.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bitterman ME, Menzel R, Fietz A, Schäfer S. 1983. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp. Psychol. 97, 107–119. ( 10.1037/0735-7036.97.2.107) [DOI] [PubMed] [Google Scholar]
- 62.Stanley DA, Smith KE, Raine NE. 2015. Bumblebee learning and memory is impaired by chronic exposure to a neonicotinoid pesticide. Sci. Rep. 5, 16508 ( 10.1038/srep16508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piiroinen S, Goulson D. 2016. Chronic neonicotinoid pesticide exposure and parasite stress differentially affects learning in honeybees and bumblebees. Proc. R. Soc. B 283, 20160246 ( 10.1098/rspb.2016.0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tison L, Holtz S, Adeoye A, Kalkan Ö, Irmisch NS, Lehmann N, Menzel R. 2017. Effects of sublethal doses of thiacloprid and its formulation Calypso® on the learning and memory performance of honey bees. J. Exp. Biol. 220, 3695–3705. ( 10.1242/jeb.154518) [DOI] [PubMed] [Google Scholar]
- 65.Smith BH, Burden CM. 2014. A proboscis extension response protocol for investigating behavioral plasticity in insects: application to basic, biomedical, and agricultural research. J. Vis. Exp. 8, e51057 ( 10.3791/51057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cane JH. 1987. Estimation of bee size using intertegular span (Apoidea). J. Kansas Entomol. Soc. 60, 145–147. [Google Scholar]
- 67.Bates D, Machler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 68.Aliouane Y, El Hassani AK, Gary V, Armengaud C, Lambin M, Gauthier M. 2009. Subchronic exposure of honeybees to sublethal doses of pesticides: effects on behavior. Environ. Toxicol. Chem. 28, 113–122. ( 10.1897/08-110.1) [DOI] [PubMed] [Google Scholar]
- 69.Démares FJ, Crous KL, Pirk CWW, Nicolson SW, Human H. 2016. Sucrose sensitivity of honey bees is differently affected by dietary protein and a neonicotinoid pesticide. PLoS ONE 11, e0156584 ( 10.1371/journal.pone.0156584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Démares FJ, Pirk CWW, Nicolson SW, Human H. 2018. Neonicotinoids decrease sucrose responsiveness of honey bees at first contact. J. Insect. Physiol. 108, 25–30. ( 10.1016/j.jinsphys.2018.05.004) [DOI] [PubMed] [Google Scholar]
- 71.Sandrock C, Tanadini LG, Pettis JS, Biesmeijer JC, Potts SG, Neumann P. 2014. Sublethal neonicotinoid insecticide exposure reduces solitary bee reproductive success. Agric. For. Entomol. 16, 119–128. ( 10.1111/afe.12041) [DOI] [Google Scholar]
- 72.Riddell CE, Mallon EB. 2006. Insect psychoneuroimmunology: immune response reduces learning in protein starved bumblebees (Bombus terrestris). Brain Behav. Immun. 20, 135–138. ( 10.1016/j.bbi.2005.06.008) [DOI] [PubMed] [Google Scholar]
- 73.Hourcade B, Muenz TS, Sandoz JC, Rossler W, Devaud JM. 2010. Long-term memory leads to synaptic reorganization in the mushroom bodies: a memory trace in the insect Brain? J. Neurosci. 30, 6461–6465. ( 10.1523/JNEUROSCI.0841-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li L, Maboudi HD, Egertovã¡ M, Elphick MR, Chittka L, Perry CJ. 2017. A possible structural correlate of learning performance on a colour discrimination task in the brain of the bumblebee. Proc. R. Soc. B 284, 20171323 ( 10.1098/rspb.2017.1323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cabirol A, Brooks R, Groh C, Barron AB, Devaud JM. 2017. Experience during early adulthood shapes the learning capacities and the number of synaptic boutons in the mushroom bodies of honey bees (Apis mellifera). Learn. Mem. 24, 557–562. ( 10.1101/lm.045492.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Nest BN, Wagner AE, Marrs GS, Fahrbach SE. 2017. Volume and density of microglomeruli in the honey bee mushroom bodies do not predict performance on a foraging task. Dev. Neurobiol. 77, 1057–1071. ( 10.1002/dneu.22492) [DOI] [PubMed] [Google Scholar]
- 77.Fahrbach SE, Strande JL, Robinson GE. 1995. Neurogenesis is absent in the brains of adult honey bees and does not explain behavioral neuroplasticity. Neurosci. Lett. 197, 145–148. ( 10.1016/0304-3940(95)11913-H) [DOI] [PubMed] [Google Scholar]
- 78.Farris SM, Robinson GE, Davis RL, Fahrbach SE. 1999. Larval and pupal development of the mushroom bodies in the honey bee, Apis mellifera. J. Comp. Neurol. 414, 97–113. () [DOI] [PubMed] [Google Scholar]
- 79.Wilson DE, Velarde RA, Fahrbach SE, Mommaerts V, Smagghe G. 2013. Use of primary cultures of Kenyon cells from bumblebee brains to assess pesticide side effects. Arch. Insect. Biochem. Physiol. 84, 43–56. ( 10.1002/arch.21112) [DOI] [PubMed] [Google Scholar]
- 80.Thany SH, Gauthier M. 2005. Nicotine injected into the antennal lobes induces a rapid modulation of sucrose threshold and improves short-term memory in the honeybee Apis mellifera. Brain Res. 1039, 216–219. ( 10.1016/j.brainres.2005.01.056) [DOI] [PubMed] [Google Scholar]
- 81.Andrione M, Vallortigara G, Antolini R, Haase A. 2016. Neonicotinoid-induced impairment of odour coding in the honeybee. Sci. Rep. 6, 38110 ( 10.1038/srep38110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Déglise P, Grünewald B, Gauthier M. 2002. The insecticide imidacloprid is a partial agonist of the nicotinic receptor of honeybee Kenyon cells. Neurosci. Lett. 321, 13–16. ( 10.1016/S0304-3940(01)02400-4) [DOI] [PubMed] [Google Scholar]
- 83.Cabirol A, Cope AJ, Barron AB, Devaud JM. 2018. Relationship between brain plasticity, learning and foraging performance in honey bees. PLoS ONE 13, e0196749 ( 10.1371/journal.pone.0196749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riveros AJ, Gronenberg W. 2009. Olfactory learning and memory in the bumblebee Bombus occidentalis. Naturwissenschaften 96, 851–856. ( 10.1007/s00114-009-0532-y) [DOI] [PubMed] [Google Scholar]
- 85.Smith KE, Raine NE. 2014. A comparison of visual and olfactory learning performance in the bumblebee Bombus terrestris. Behav. Ecol. Sociobiol. 68, 1549–1559. ( 10.1007/s00265-014-1765-0) [DOI] [Google Scholar]
- 86.Tosi S, Nieh JC. 2019. Lethal and sublethal synergistic effects of a new systemic pesticide, flupyradifurone (Sivantow), on honeybees. Proc. R. Soc. B 286, 20190433 ( 10.1098/rspb.2019.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith DB, Arce AN, Ramos Rodrigues A, Bischoff PH, Burris D, Ahmed F, Gill RJ. 2020. Data from: Insecticide exposure during brood or early-adult development reduces brain growth and impairs adult learning in bumblebees Dryad Digital Repository. ( 10.5061/dryad.63xsj3tzp) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Smith DB, Arce AN, Ramos Rodrigues A, Bischoff PH, Burris D, Ahmed F, Gill RJ. 2020. Data from: Insecticide exposure during brood or early-adult development reduces brain growth and impairs adult learning in bumblebees Dryad Digital Repository. ( 10.5061/dryad.63xsj3tzp) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw data and scripts used for statistical analysis and figure production are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.63xsj3tzp [87].