Abstract
Recently, a few insects, including the caterpillar larva of the greater wax moth Galleria mellonella, have been identified as avid ‘plastivores’. These caterpillars are able to ingest and metabolize polyethylene at unprecedented rates. While it appears that G. mellonella plays an important role in the biodegradation process, the contribution of its intestinal microbiome remains poorly understood and contested. In a series of experiments, we present strong evidence of an intricate relationship between an intact microbiome, low-density polyethylene (LDPE) biodegradation and the production of glycol as a metabolic by-product. First, we biochemically confirmed that G. mellonella larvae consume and metabolize LDPE, as individual caterpillars fed on polyethylene excreted glycol, but those excretions were reduced by antibiotic treatment. Further, while the gut bacterial communities remained relatively stable regardless of diet, we showed that during the early phases of feeding on LDPE (24–72 h), caterpillars exhibited increased microbial abundance relative to those starved or fed on their natural honeycomb diet. Finally, by isolating and growing gut bacteria with polyethylene as their exclusive carbon source for over 1 year, we identified microorganisms in the genus Acinetobacter that appeared to be involved in this biodegradation process. Taken collectively, our study indicates that during short-term exposure, the intestinal microbiome of G. mellonella is intricately associated with polyethylene biodegradation in vivo.
Keywords: polyethylene biodegradation, microbiome, waxworm, metabolism
1. Introduction
The global production of plastics rose to 348 million tonnes in 2017, with the majority being single-use convenience products that eventually end up littering our environment [1,2]. Indeed, most petroleum-derived plastic polymers, including polyethylene, can persist in the environment for extended periods of time [3,4]. Consequently, an active area of research has focused on accelerating the natural degradative processes. One promising approach gaining traction is through the use of bacteria and fungi to alter and breakdown the chemical structure of polyethylene and other plastic polymers [5,6]. Plastic-degrading microorganisms are more ubiquitous than previously thought, and have been isolated from a variety of terrestrial, freshwater and marine environments [6–8]. The rate of biodegradation by microorganisms is strongly influenced by the properties of the plastic polymer, such as its molecular weight and density (e.g. HDPE versus LDPE). Polyethylene is particularly resilient, with its biodegradation by isolated microorganisms in vitro taking weeks to years, and requiring prior physical processing to accelerate the process [4,9–11]. Furthermore, it is likely that polyethylene biodegradation in vivo involves microbial communities rather than single organisms [12,13].
Recently, several insect species, most notably coleopterans and lepidopterans, were found to possess the remarkable ability to consume and degrade different polymers [4,14–18]. For example, Plodia interpunctella (Indian mealworms) can consume polyethylene and harbours several polyethylene-degrading intestinal bacteria [4]. Similarly, the larvae of Tenebrio molitor (yellow mealworm) can ingest and metabolize polyethylene and polystyrene [19,20]. Interestingly, polyethylene biodegradation seems dramatically accelerated in vivo, inside the insect host, as opposed to when bacterial species are isolated and grown in vitro [19]. Therefore, it is probable that the accelerated breakdown of plastics in insects is a complex process that is interdependent on both the microbiome and its host.
Recently, Bombelli et al. [10] described the extraordinary capacity of caterpillar larvae of the greater wax moth, Galleria mellonella (colloquially called waxworms), to ingest and degrade polyethylene at unprecedented rates. Indeed, G. mellonella larvae consumed polyethylene and presumably excreted ethylene glycol as a by-product, as evidenced by Fourier-transform infrared spectroscopy. Although G. mellonella does not actively feed on plastics in its natural environment, it is a ubiquitous pest of apiaries, where the fast-growing caterpillars voraciously consume honeycomb prior to pupation and metamorphosis into the adult moth [21]. Similar to polyethylene, the structure of honeycomb is rich in long aliphatic chains [22,23]. Therefore, it is possible that its natural ecology provides the caterpillar with a unique microbiota and set of physiological adaptations to derive energy from such a complex diet [10,23].
The role (if any) of the intestinal microbiome in the biodegradation of polyethylene is controversial. While several studies have implicated the intestinal microbiome in plastic breakdown in insects, including polystyrene degradation in T. molitor and T. obsucurus [15,16,19,24,25], there is no supportive evidence currently available for polyethylene degradation by G. mellonella. On the contrary, recent work indicated that larvae of G. mellonella metabolized intestinal beeswax and polyethylene with or without an intact microbiome [23]. However, without further testing of animals of diverse provenance, it cannot be ruled out that both the microbiome and caterpillar host can degrade plastic independently, and that the biodegradative process is accelerated due to synergistic effects.
To better explore the role of the caterpillar intestinal microbiome in the early process of polyethylene degradation, we used various molecular and physiological approaches to manipulate and characterize this microbiome. First, antibiotic treatments revealed a strong link between polyethylene biodegradation, glycol production and an intact microbiome. Secondly, 3 days on a polyethylene diet was sufficient to elicit an increased abundance of G. mellonella gut bacteria compared to a starved or natural honeycomb diet. In addition, 16S sequencing indicated that metabolizing polyethylene has relatively nominal impacts on the community structure of intestinal bacteria. Finally, using polyethylene as a sole source of carbon, we were able to culture microorganisms isolated from the caterpillars' intestine, confirming the ability of these microorganisms to metabolize this polymer. Taken together, our study suggests that while the host caterpillar may play a role in polyethylene biodegradation, the microbiome is an important driver of the early stages of the process.
2. Material and methods
(a). Colony maintenance
Experiments were carried out using a laboratory colony of G. mellonella established in 2017 from fourth and fifth instar larvae provided by The Worm Lady (Chatham, ON). To maintain genetic diversity, the colony has been subjected to multiple, ongoing introductions of larvae. Larval G. mellonella were reared in 40 × 20.5 × 22 cm cages (approx. 350 per cage) and fed a diet of honeycomb from apiaries near Dauphin, MB and Souris, MB. Cages were kept under controlled conditions of 22°C and 85% relative humidity in the dark. Upon pupation, approximately 100 pupae were transferred to new oviposition cylinders (9425 cm3) where metamorphosis took place. Eggs were laid on crumpled wax paper within a few weeks, dead moths were removed, and new honeycomb substrate was added.
For several of the experiments described below, collections consisted of up to three treatments: G. mellonella that were honeycomb-fed (HF), polyethylene-fed (PF) and starved (S). Fifth instar caterpillars (identified according to [26]) were selected from maintenance colonies and placed into individual 50 ml conical tubes containing 250 mg of fine ground honeycomb (HF), 100 cm2 of sterile Type 4 LDPE plastic (Real Canadian Superstore, Brandon, MB) (PF), or no added substrate (S). For the PF treatment, the polyethylene was replaced daily and only caterpillars that fed appreciably on plastic each day were used in the experiments. The HF food source was replenished every 4 days, where applicable. All animals were starved for a 24 h period prior to commencement of feeding assays. For intestinal dissections, individual G. mellonella were tethered (Safer's Sticky Stiks Flying Insect Traps), surface sterilized with 75% EtOH, and their guts were sampled using Type 12 razor blades. All collections were done at the same time (14.00 CDT) to circumvent potential influences of diurnal changes. Replicates comprised larvae from different cohorts and multiple maintenance cages.
(b). Polyethylene material
The LDPE used in all experiments was sent to Jordi Labs (Mansfield, MA) for analysis. The relative molecular weight distribution was characterized by high-temperature gel permeation chromatography (GPC-H) using a Viscotek HT-GPC Module 350A detector array (Malvern Panalytical Ltd, Malvern, UK). Data acquisition and handling were made with OmniSec software. Filler content of the bag was characterized using a TGA 500 thermogravimetric analyser in combination with Universal Analysis software (TA Instruments, New Castle, DE). The LDPE bag film had PE with Mw of 289 kDa and polydispersity index (Mw/Mn) of 8.57 or Mn 33 700. Thermogravimetric analysis indicates that the LDPE film possessed about 3.2% inorganic or incombustible content.
(c). Glycol assays
A total of 100 fifth instar larvae were reared for 24 h on a sterile artificial diet (adapted from [27]), which consisted of 22% corn meal, 22% wheat germ, 11% dry yeast, 17.5% beeswax, 16.5% honey (pasteurized) and 11% water. An additional 100 larvae were fed the same artificial diet that included broad-spectrum antibiotics streptomycin and tetracycline (100 µg ml−1, Bio Basic, Markham, ON; [28]). Subsequently, caterpillars were housed individually and provided polyethylene as their exclusive food source. After 48 h, liquid excreta were collected and stored at −80°C. Samples were pooled and spectrophotometrically assayed for glycol (Molecular Devices, CA) using glycerol dehydrogenase (0.05 U ml−1) from Cellulomonas sp. (Sigma-Aldrich, Oakville, ON), as previously described [29].
(d). Context-dependent quantification of the gut microbiome
Intestinal bacteria were quantified in three independent assays to compare changes in relative abundance between: (i) sterile media and antibiotic-treated (streptomycin and tetracycline) PF G. mellonella after 24 h; (ii) PF G. mellonella that produced and did not produce excreta after 24 h; and (iii) HF, PF and S G. mellonella collected at 24 and 72 h. For each experiment, replicates consisted of five pooled guts and five replicates per treatment/time point. Samples were stored at −80°C until further processing.
(i). DNA isolation and qPCR
Intestinal DNA from caterpillars was extracted using the One-4-All Genomic DNA Miniprep Kit (Bio Basic) and assessed for quantity and purity on a nanophotometer NP80 (Implen Inc., Westlake Village, CA). We then quantified bacterial abundance using a qPCR assay targeted against the V8 region of the 16S DNA sequence (1369F: 5′-CGGTGAATACGTTCYCGG-3′ and 1492R: 5′-GGTTACCTTGTTACGACTT-3′) [30]. The G. mellonella 28S rDNA was used to normalize microbial abundance to intestinal tissue DNA content (F: 5′-GGCACAACGAAAGTGAAGGC-3′ and R: 5′-TTCCCCTGACTTCGACCTG-3′). Duplicate reactions (15 µl) were performed using QuantiNova Sybr Green master mix (Qiagen, Mississauga, ON) on a Rotor-Gene-Q thermocycler (Qiagen). Conditions were as follows: 95°C followed by 40 cycles of 5 s denaturation at 95°C and combined annealing and extension of 60°C for 10 s. The critical threshold (Ct) for each assay was computed using the Rotor-Gene Q software (Qiagen). A negative control was included in each run and a melt-curve analysis was carried out routinely to ensure single target amplification. Relative microbial abundance was then calculated by using the 2−ΔΔCt method [31,32].
(ii). Statistical analysis
Raw data were tested for normality and homogeneity of variance on R (Tidyverse package) using the Shapiro–Wilk and Levene's test, respectively. After log-transforming the data, normality was confirmed (p > 0.05) and thus the data were suitable for running a two-way analysis of variance (ANOVA) for each diet. The two-way ANOVA test and Holm-Sidak post hoc analyses were carried out on SigmaPlot software (v. 11.0).
(e). Metagenomics analysis
(i). Experimental design
As described above, G. mellonella were starved for 24 h, provided with their respective diets (PF, HF and S) for 24 and 72 h before sampling. Replicates consisted of five excised caterpillar guts, and each treatment/time point was replicated five times (30 samples in total). Samples were flash-frozen in liquid nitrogen and stored at −80°C prior to DNA isolation.
(ii). DNA extraction and amplicon sequencing
For each replicate, gDNA was isolated from G. mellonella gut samples using the Genomic DNA Isolation Kit (Norgen Biotek Corp., Thorold, ON). DNA samples were sent to Génome Québec Innovation Centre (McGill University, Montreal, QC) for PCR validation and reaction, amplicon barcoding, and normalization. The 16S primer sequences used were 515F: GTGCCAGCMGCCGCGGTAA and 806R: GGACTACHVGGGTWTCTAAT [33]. The amplicon libraries were assessed for quality using the BioAnalyzer 2100 (Agilent Technologies, Santa Clara, CA) and sequenced on the Illumina MiSeq platform in paired-end 300 bp fashion. Only the R1 reads were used in subsequent analysis due to low pairing frequencies. The raw sequence reads can be accessed from the NCBI short sequence read archive (SRA) under the accession number PRJNA552585.
(iii). QIIME analysis
Adapter and quality (quality limit = 0.00316, minimum length = 15, default parameters herein) trimming of amplicon sequencing reads were carried out using CLC Genomics Workbench v. 11.0.1 (Qiagen). Processed reads were then imported into the QIIME v. 1.9.1 pipeline [34] for further analysis. First, the operational taxonomic unit (OTU) picking was performed using USEARCH with a sequence similarity threshold of 97% [35]. The cluster seed was selected as a representative sequence, and OTUs were aligned and phylogeny inferred against the Greengenes core set using PyNAST [36]. Taxonomy was then assigned to each OTU using UCLUST [35], considering the top-3 database hits to the corresponding to the Greengenes database release 13_8 at 97% sequence similarity [37]. Chimeric sequences were identified and filtered using ChimeraSlayer [38]. To reduce false-positives and to omit singletons, OTUs accounting for less than 0.05% of the total number of sequences were removed from subsequent analysis.
To compare bacterial community diversity across the different treatments/time points, samples were rarefied to the smallest dataset (7972 sequences) and alpha diversity indices (Shannon, Simpson and observed OTUs) were calculated in QIIME based on OTUs. Beta diversity was estimated at the genus level using the non-phylogenetic and quantitative Bray–Curtis index. The resulting distance matrices were used for principal coordinates analyses (PCoA) and visualized in PCoA plots. Categorical (treatment) differences were then tested using permutational multivariate analysis of variance (PERMANOVA) based on Bray–Curtis distances. Follow-up testing of homogeneity of dispersion (PERMDISP) was used to deduce pairwise differences among the treatments/time points. Finally, we identified bacterial phyla and genera that significantly differed between treatments and time points using pairwise non-parametric t-tests with Monte Carlo simulation. Statistical significance was set at p < 0.05 for all statistical tests unless otherwise denoted.
(iv). CLC analysis
The CLC Microbial Genomics Module 3.5 (Qiagen, Aarhus, Denmark) was used for 16S analysis. Briefly, OTU clustering was performed on processed reads using a reference-based OTU picking at 97% to the SILVA 16S release 128 database [39]. The resulting OTU abundance tables were filtered to remove chimeric sequences and OTUs detected in less than 0.005% of the total reads for each dataset. To obtain significantly different phyla and genera, reads without an assigned taxonomy were filtered out and the remaining reads were square-root transformed and normalized using the scaling method. Pairwise t-tests were done on normalized reads to identify differentially abundant taxa.
(f). Isolation of polyethylene-degrading bacteria
(i). Preparation of inoculum
A total of 60 fifth instar G. mellonella larvae were placed into two containers (700 cm3 each) containing sterile polyethylene and incubated at 26°C in the dark. After 4 days, larvae were surface sterilized (75% EtOH), and their intestine were dissected and placed in 15 ml conical tubes containing 400 µl of sterile saline water per sample. The tubes were mixed by inverting for 1 min, and then the intestines were discarded.
(ii). Enrichment and selection of polyethylene-degrading bacteria
Sterile Petri plates containing potato glucose medium and polyethylene (25 cm2) were inoculated with 100 µl of gut suspension and incubated at 30°C (electronic supplementary material, figure S1a). Non-inoculated plates served as negative controls. After a series of streak-plate transfers, individual colonies in contact with polyethylene were placed in fresh medium and grown at room temperature. After 5 days, isolated bacterial colonies were transferred to 15 ml conical tubes containing 5 ml liquid carbon-free medium (5.14 mM KH2PO4, 4.02 mM K2HPO4, 2.84 mM MgSO4 · 7H2O, 12.37 mM NH4NO3, 85.6 µM NaCl, 7.19 µM FeSO4 · 7H2O, 6.96 µM ZnSO4 · 7H2O, 5.92 µM MnSO4·H2O) [4] and 42 mg of sterile polyethylene dots (0.28 cm2) (electronic supplementary material, figure S1b). The inoculated tubes were incubated at 30°C and 120 r.p.m. for 60 weeks.
(iii). DNA extraction, PCR and Sanger sequencing
Genomic DNA was isolated from bacteria cultured in liquid carbon-free medium using the One-4-All Genomic DNA Mini Prep Kit (Bio Basic). PCR was performed using 16S primers (515F/806R) and the Platinum SuperFi PCR Master Mix (Thermo Fisher Scientific, Winnipeg, MB), using the following conditions: 98°C for 30 s, 30 cycles of 98°C for 10 s, 55°C for 45 s, 72°C for 30 s, followed by 72°C for 5 min. Amplified PCR product was sent to Génome Québec for Sanger sequencing using the Applied Biosystem 3730xl DNA Analyzer.
3. Results
(a). Consistency of excreta changes with feeding regime
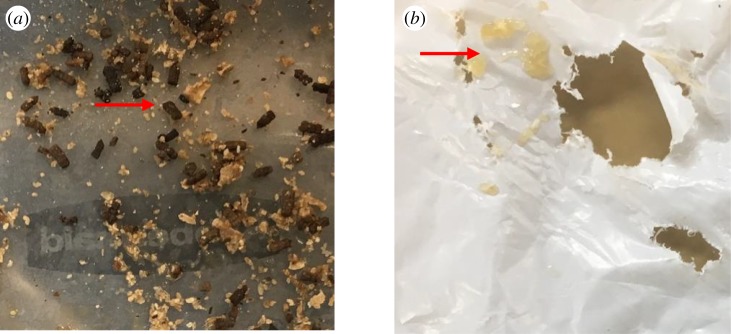
Within 24 h of feeding on polyethylene, we observed a distinct change in the consistency of G. mellonella excreta (i.e. frass) from solid (characteristic of HF caterpillars) to a liquid (figure 1). This phenotype was consistent and unique for caterpillars that actively ingested plastic, and thus was used as the basis of the subsequent antibiotic assays described below.
Figure 1.
The consistency of Galleria mellonella excreta was significantly impacted by feeding regime. Honeycomb-fed caterpillars showed a solid form (a), whereas polyethylene-fed caterpillars showed a liquid form within 24 h of feeding (b). (Online version in colour.)
(b). Glycol production in plastic-fed Galleria mellonella is associated with the intestinal microbiome
Since liquid excreta were unique to PF caterpillars, we speculated that these excreta were composed of glycol and produced as a by-product of the intestinal microbial degradation of polyethylene (see [10]). Thus, to biochemically confirm glycol presence in PF excreta and directly implicate the microbiome in its production, we first treated caterpillars with broad-spectrum antibiotics. Within 24 h, gut bacterial abundance was effectively reduced by 65% (p = 0.046, figure 2a), accompanied by a nearly threefold reduction in the proportion of caterpillars excreting liquid in the antibiotic-treated group (62% versus 22%), despite the fact that most animals in both groups ingested polyethylene (greater than 94%). Further, as a group, the antibiotic-treated PF caterpillars produced 40% less liquid excreta and considerably less glycol relative to untreated animals (27 versus 57 nmol larva−1). Finally, antibiotic-treated caterpillars that still excreted liquid had 71% higher gut bacterial abundance than those that did not excrete any observable waste (p = 0.0427) (figure 2b). Taken collectively, these findings establish a clear relationship between polyethylene degradation and glycol production, and a distinct role of the caterpillar intestinal microbiome in this process.
Figure 2.
Polyethylene degradation and Galleria mellonella intestinal microbiome after 24 h of antibiotic treatment. (a) Antibiotic-treated caterpillars (grey bar) had significantly lower abundance of gut bacteria than those fed sterile food only (white bar), n = 5. (b) Antibiotic-treated caterpillars fed polyethylene that produce liquid excreta (grey bar) have 71% higher gut bacterial abundance than those that did not produce liquid excreta (white bar), n = 6–10. *Denotes statistical significance between treatment groups.
(c). PF Galleria mellonella have considerably higher gut bacterial abundance
We next assessed the relative influence each feeding regime (HF, PF and S) had on the abundance of intestinal bacteria after 24 and 72 h. While we saw no interaction between time and diet (p = 0.489) or the effect of time (p = 0.216), diet had a significant impact on intestinal microbial abundance (p < 0.001). Indeed, at 24 h PF caterpillars had the highest microbial abundance of all treatments: sevenfold and 1.7-fold higher than S and HF caterpillars, respectively (p < 0.01; figure 3). Although we observed a similar trend at 72 h, the difference was only statistically significant between PF and S caterpillars (p < 0.01). While there was no significant effect of time, HF and PF G. mellonella showed marginally increased bacterial abundance over time while the opposite was true for food-deprived caterpillars.
Figure 3.

Diet influences gut bacterial abundance in Galleria mellonella. Caterpillars fed on polyethylene (grey bars) had increased intestinal bacteria compared to honeycomb-fed (gold bars) and starved (white bars) caterpillars. Different letters indicate statistically significant differences between treatments within a time point (p < 0.0.5, n = 4–5). (Online version in colour.)
(d). Microbial analysis
(i). Structure of gut bacterial communities
We characterized intestinal bacterial communities of G. mellonella associated with different feeding regimes using 16S amplicon sequencing. A total of 30 samples were collected across two time points (24 and 72 h) and the three dietary treatments (starved (S), honeycomb-fed (HF) and polyethylene-fed (PF)). Overall, sequencing generated 3 032 135 reads, of which approximately 30% were chimeric and removed from subsequent analysis. QIIME analysis identified 3905 OTUs, representing seven phyla, 10 classes, 27 orders, 35 families and 89 genera. For completeness, we conducted parallel CLC analyses; as the results were largely consistent between approaches, we focused on the QIIME results below.
(ii). Microbial phyla composition in the Galleria mellonella gut
To better infer the bacterial communities present in highest abundance in the caterpillar intestines, bacteria were filtered to the phylum level. Proteobacteria comprised the largest proportion (87.5%), followed by Cyanobacteria (4.4%) and Firmicutes (4.1%). Of all phyla, only Proteobacteria and Firmicutes were consistently present across samples. At the genus level, we identified 89 genera, of which Escherichia-Shigella (28.5%), Asaia (20.3%) and Acinetobacter (13.1%) predominated. There were 12 genera present in greater than or equal to 90% of samples: Asaia, Brevundimonas, Rhizobium, Aeromonas, Shewanella, Vibrio, Pseudomonas, Strenotrophomonas, Ralstonia, Bacillus, Escherichia-Shigella and Acinetobacter. Interestingly, five genera were specific to plastic-fed treatment, and one each for the other two treatments (electronic supplementary material, figure S2). However, in most cases, these treatment-specific genera were not shared by all replicates within the respective treatment.
(iii). Microbial community diversity is influenced by feeding regime
We further explored the differences in gut bacterial communities at the OTU and genus levels to provide a more focused look at their relative structure. Rarefaction curves of the samples approached saturation, indicating that bacterial diversity was sufficiently represented by the number of reads in the samples. Both the Simpson and Shannon Entropy diversity indices showed a similar trend across the treatments and time points (electronic supplementary material, figure S3). The only statistically significant difference was between plastic-fed and starved G. mellonella at 24 h, demonstrating that the abundance and evenness of the species present was relatively stable [40]. PERMDISP analyses also indicated that this was the only pairwise difference in bacterial communities at either 24 or 72 h (p = 0.02). Interestingly, alpha diversity showed a marginal decrease in plastic-fed G. mellonella with time, whereas the opposite was found in the other treatments.
Two-dimensional PCoA based on beta diversity were constructed to visualize the relationships among treatments and time points (electronic supplementary material, figure S4). Although the sample clustering was not discrete, the majority of HF samples clustered with the plastic samples, indicating honeycomb- and polyethylene-fed caterpillars have a more similar gut microbial profile than starved individuals. Some temporal separation was also apparent, particularly for the PF treatment. Overall the within-group differences, as assessed by non-parametric pairwise t-tests, was significantly lower than the between-group differences, indicating distinct microbial profiles of the treatments. PerMANOVA provided further support for this separation, as both treatment (p = 0.005) and the interaction factor (p = 0.003) were significant, whereas time was not (p = 0.86).
(iv). Microbial abundance differs across treatments and time
Pairwise comparisons of the relative bacterial abundance of the S, HF and PF treatments at 24 and 72 h were carried out at the genus level (electronic supplementary material, table S1). Statistical significance was set at p < 0.05 with a minimum log2fold change threshold greater than 1.5. As highlighted by the PERMDISP testing, the majority of differences were identified between PF and S treated G. mellonella at 24 h. This included relative increases in the genera Escherichia-Shigella, Pantoea, Pseudocitrobacter, Salmonella, Serratia and Citrobacter in plastic-fed caterpillars, whereas the genera Acetobacter, Asaia, Gluconacetobacter and Swaminathania showed reduced abundance. However, at 72 h the only significant differences were an increase in the genera Aeromonas, Ottowia and uncultured Burkholderiaceae in PF caterpillars. No significant differences were observed between PF and HF animals at 24 h; however, at 72 h both Acetobacter and Ottowia showed elevated abundance in the plastic-fed caterpillars.
(v). Bacteria isolated from Galleria mellonella intestine can grow on polyethylene as a sole carbon source
Selected bacterial colonies isolated from guts of PF caterpillars were grown with polyethylene fragments in carbon-free media. After 60 weeks at 30°C, we isolated DNA from the growing bacteria and performed 16S sequencing to identify these microorganisms. The bacteria belong to the Acinetobacter genus but could not be identified to the species-level in any available database. However, we confirmed its presence (100% sequence identity, 250 nt coverage) in the G. mellonella intestinal microbiome.
4. Discussion
Polyethylene is the most commonly used form of synthetic polymer worldwide, but its resilience to natural degradation has resulted in its accumulation in our landfills and in the environment [41–43]. Therefore, identifying effective means to accelerate its biodegradation process would be highly advantageous, and has been the focus of much research in recent years. Thus far there have been more than 56 species across 25 genera of bacteria and fungi associated with polyethylene biodegradation (see [44]; [18]). In addition, a handful of lepidopteran species has recently come under the spotlight as exceptional ‘plastivores’ [10,14,17,18]. By contrast to the typically slow rates of polyethylene degradation by microorganisms in vitro [9], the process appears considerably accelerated within an insect host [15]. However, the respective contributions of the host and its microbiome in this process are unclear. Here, we present evidence that commercially obtained caterpillar larvae of the greater wax moth, G. mellonella, can metabolize LDPE and excrete a liquid containing glycol within 24 h. Furthermore, our results strongly suggest that the microbiome is an important driver in this initial short-term biodegradation process.
As caterpillars, G. mellonella are voracious eaters and voluntarily feed on polyethylene substrate [10,18]. In our study, we confirmed that commercially procured G. mellonella larvae readily consume and metabolize high purity LDPE, as evidenced by the abrupt change in their waste excretion, from solid to liquid form. Although currently debated in the scientific community, recent work has identified ethylene glycol as a metabolic by-product of polyethylene degradation [10,45]. While we made no formal attempts to determine the exact chemical nature of the excreta, we used a broad biochemical test [46] to detect glycol as a major component of the liquid excretions. Similarly, recent studies have identified alcohol derivatives as part of the polyethylene biodegradative process for both T. molitor (mealworms) and Achroia grisella (lesser waxworms) [17,20]. We are, therefore, confident that we detected a glycol—a putative by-product of polyethylene metabolism—though more refined chemical detection techniques are warranted to identify with certainty the aliphatic diol and any other metabolic by-product(s) involved.
After confirming G. mellonella larvae readily breakdown polyethylene and produce glycol, we were interested in deciphering the contribution, if any, that the caterpillar intestinal microbiome had in this process. We found caterpillars with reduced gut microbiota abundance, attained through broad-spectrum antibiotic treatment, excreted considerably less than those possessing an intact microbiome. Further, the subset of antibiotic-treated worms that continued to metabolize polyethylene had elevated microbial abundance, consistent with (albeit not as high) that of untreated caterpillars. While it is unclear whether there is some resistance conferred to the caterpillars that did not respond to antibiotic treatment, our findings strongly suggest that a diminished intestinal microbiome dramatically impedes the capability of G. mellonella to metabolize polyethylene. Similarly, larvae of the coleopterans T. molitor and T. obscurus with reduced intestinal microbial abundance showed a lower capacity to breakdown another plastic polymer, polystyrene [24,25].
To better characterize how consuming and metabolizing polyethylene influences the intestinal microbial composition of G. mellonella, we examined the bacterial community structure of caterpillars fed polyethylene for 3 days relative to those provided a natural diet (honeycomb) or no substrate (starved). The lepidopteran gut microbiome has been shown to be influenced by several context-dependent factors, including environment [47], developmental stage [48,49] and diet [47,50,51]. Overall, the gut microbiota of G. mellonella was surprisingly stable across time and feeding regime within this short time frame. Not only was there minimal variability in species diversity and richness indices, but many taxa that are typically detected in other lepidopterans were also present and well represented in G. mellonella. For instance, the most abundant bacterial phylum (Proteobacteria) in our samples is ubiquitous in many lepidopterans [51]. Similarly, Enterococcus, Bacillus, Staphylococcus and Pseudomonas, genera commonly associated with lepidopterans [51], were also present across times/feeding regimes. Most of the discernable differences were attributed to a reduction in several genera in starved caterpillars after 1 day, but this treatment seemingly recovered by day 3. Taken together, diet appears to have little effect on the caterpillar's overall intestinal microbiota structure in the short term, suggesting the presence of a core gut microbiome in G. mellonella [51–54]. By contrast, recent work in T. molitor suggests that over longer periods, a plastic diet exerts a strong influence on bacterial community structures [55]. It would, therefore, be of interest to extend the plastic exposure period to assess the long-term effects of this diet on the caterpillar's microbial communities. Further, a direct comparison between various populations of the wax-eating G. mellonella and the omnivorous T. molitor larvae would enable testing whether the former species is naturally and ubiquitously gifted with adequate metabolic machinery to biodegrade long hydrocarbon chains as suggested previously [23].
Interestingly, while the microbial communities were only marginally affected by the caterpillar's diet, overall microbial abundance was rapidly and dramatically impacted. By contrast to other insect model studies, which indicate minimal changes in intestinal microbial abundance due to short-term starvation [56,57], starved G. mellonella larvae showed a substantial decrease in bacterial abundance, indicating that even short-term dietary restrictions can have drastic consequences on the enteric microbial abundance in this animal. Larvae fed on polyethylene demonstrated the highest microbial abundance of all diets, suggesting that the intestinal microbiome is responding favourably to the polyethylene diet, and presumably metabolically benefiting from this substrate. While this increased abundance appears attributed to the bacterial community at large rather than a net increase from a few isolated taxa, we have isolated putative plastic-degrading bacteria from the gut of G. mellonella that grow for an extended period on a polyethylene-only diet, as discussed below.
The caterpillar's gut microbiota seem to play a key role in the polyethylene biodegradation process. However, to this point the respective contribution of the microbial community and the lepidopteran host metabolic pathways is unclear. Consequently, we isolated individual bacterial colonies from the G. mellonella intestine and cultured them in liquid carbon-free medium containing polyethylene for 60 weeks. After this extensive growth period, we confirmed the proliferation of bacteria belonging to the genus Acinetobacter. While we were unable to provide resolution to species-level, the bacteria could be traced back to our 16S microbiome dataset, thereby unequivocally associating these microorganisms with the caterpillar's intestinal fauna. Interestingly, Acinetobacter was one of the few genera found in significantly higher abundance in caterpillars fed polyethylene relative to those provided a natural honeycomb diet. At the species-level, Acinetobacter sp. ACT126 was over 5000-fold more abundant in polyethylene-fed larvae compared to the other feeding regimes; thus, this species represents an excellent candidate for contributing to the biodegradation process. The genus Acinetobacter has been repeatedly associated with polyethylene degradation [13,58–63]. In particular, this genus encompasses strictly aerobic species, and oxidation of polyethylene's stable carbon–carbon double bond is necessary for its degradation [9]. Considering that these bacteria were able to grow for more than 1 year by using polyethylene as their sole carbon source, we speculate that it is intricately involved in the biodegradation process, though further characterization of the microbial associations are warranted. It is also probable that additional microorganisms housed in the caterpillars' gut are capable of polyethylene degradation. By altering our culturing techniques (e.g. pH of the liquid carbon-free medium) in the future, we may be able to uncover other bacterial or fungal species, and perhaps even expedite the isolation process.
The role of the intestinal microbiome in LDPE biodegradation by larvae of G. mellonella has been contested. While multiple studies have highlighted the key roles of lepidopteran gut microorganisms in metabolizing plastic polymers, a recent study suggested that G. mellonella larvae are able to carry out the biodegradation process independent of their gut microbiota [23]. In a series of molecular, microbiological, physiological and biochemical experiments, we provide compelling evidence that the intestinal microbiome, most notably the genus Acinetobacter, provides an important contribution to the polyethylene biodegradation process for this lepidopteran. A major phenotype observed from the polyethylene-fed worms was a change in consistency of their excreta from solid to liquid form, presumably due to the production of a glycol by-product. While glycol production was clearly associated with the microbiome, future studies are needed to deduce the exact chemical composition of this compound. As research related to plastic polymer biodegradation continues to accumulate, one of the most fascinating aspects of these investigations is the speed at which this process is achieved in vivo (inside an insect host) compared to in vitro (environmental isolates of microorganisms). This seemingly implies that the insect is not simply a vessel for LDPE-degrading microorganisms; rather, there may be a functional relationship between the host gastrointestinal physiology and the microbiome. Our studies focused primarily on the short-term degradation of LDPE by a population of G. mellonella, however, the ubiquity of this plastic-degrading ability should certainly be tested across diverse populations and using different polymer formulations. Moreover, future work is clearly needed to explore plastic breakdown as an in vivo process—specifically how the caterpillar's physiology, fitness and genetic underpinnings are directly affected by polyethylene breakdown.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Charlotte Smith and Patrick Gohl for assistance with the gut microbiome collections.
Data accessibility
The raw microbial sequence data are available from the NCBI short sequence read archive (SRA) under accession number PRJNA552585, and the qPCR and biochemical datasets are readily available as part of the electronic supplementary material. The rest of the data are available on demand.
Authors' contributions
B.J.C. and C.M.R.L.M., designed research; H.C.G., S.M.P.V., B.J.C and C.M.R.L.M. performed research; B.J.C, C.M.R.L.M., O.E. and H.C.G. analysed data; B.J.C, C.M.R.L.M. and H.C.G wrote the paper. All authors read and approved the final version of the paper.
Competing interests
We declare we have no competing interests
Funding
This work was supported by individual NSERC Discovery Grants to B.J.C and C.M.R.L.M., and a collaborative Canadian Foundation for Innovation (CFI) Grant.
References
- 1.Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. 2015. Plastic waste inputs from land into the ocean. Science 347, 768–771. ( 10.1126/science.1260352) [DOI] [PubMed] [Google Scholar]
- 2.PlasticsEurope , 2018. Plastics-the Facts 2018 An analysis of European plastics production, demand and waste data https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf (accessed 10/11/2019).
- 3.Sangale MK, Shahnawaz M, Ade AB. 2012. A review on biodegradation of polythene: the microbial approach. J. Bioremed. Biodeg. 3, 164 ( 10.4172/2155-6199.1000164) [DOI] [Google Scholar]
- 4.Yang J, Yang Y, Wu WM, Zhao J, Jiang L. 2014. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 48, 13 776–13 784. ( 10.1021/es504038a) [DOI] [PubMed] [Google Scholar]
- 5.Danso D, Chow J, Streit WR. 2019. Plastics: microbial degradation, environmental and biotechnological perspectives. Appl. Environ. Microbiol. 85, e01095-19 ( 10.1128/AEM.01095-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urbanek AK, Rymowicz W, Mirończuk AM. 2018. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl. Microbiol. Biotechnol. 102, 7669–7678. ( 10.1007/s00253-018-9195-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morohoshi T, Oi T, Aiso H, Suzuki T, Okura T, Sato S. 2018. Biofilm formation and degradation of commercially available biodegradable plastic films by bacterial consortiums in freshwater environments. Microbes Environ. 33, 332–335. ( 10.1264/jsme2.ME18033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raddadi N, Fava F. 2019. Biodegradation of oil-based plastics in the environment: existing knowledge and needs of research and innovation. Sci. Total Environ. 679, 148–158. ( 10.1016/j.scitotenv.2019.04.419) [DOI] [PubMed] [Google Scholar]
- 9.Restrepo-Flórez JM, Bassi A, Thompson MR. 2014. Microbial degradation and deterioration of polyethylene — a review. Int. Biodeterior. Biodegrad. 88, 83–90. ( 10.1016/j.ibiod.2013.12.014) [DOI] [Google Scholar]
- 10.Bombelli P, Howe CJ, Bertocchini F. 2017. Polyethylene bio-degradation by caterpillars of the wax moth Galleria mellonella. Curr. Biol. 27, 292–293. ( 10.1016/j.cub.2017.02.060) [DOI] [PubMed] [Google Scholar]
- 11.(Orr) IG, Hadar Y, Sivan A. 2004. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 65, 97–104. ( 10.1007/s00253-004-1584-8) [DOI] [PubMed] [Google Scholar]
- 12.Nauendorf A, Krause S, Bigalke NK, Gorb EV, Gorb SN, Haeckel M, Wahl M, Treude T. 2016. Microbial colonization and degradation of polyethylene and biodegradable plastic bags in temperate fine-grained organic-rich marine sediments. Mar. Pollut. Bull. 103, 168–178. ( 10.1016/j.marpolbul.2015.12.024) [DOI] [PubMed] [Google Scholar]
- 13.Satlewal A, Soni R, Zaidi MGH, Shouche Y, Goel R. 2008. Comparative biodegradation of HDPE and LDPE using an indigenously developed microbial consortium. J. Microbiol. Biotechnol. 18, 477–482. [PubMed] [Google Scholar]
- 14.Chalup A, Ayup MM, Monmany Garzia AC, Malizia A, Martin E, De Cristóbal R, Galindo-Cardona A. 2018. First report of the lesser wax moth Achroia grisella F. (Lepidoptera: Pyralidae) consuming polyethylene (silo-bag) in northwestern Argentina. J. Apic. Res. 57, 569–571. ( 10.1080/00218839.2018.1484614) [DOI] [Google Scholar]
- 15.Yang Y, Yang J, Wu W-M, Zhao J, Song Y, Gao L, Yang R, Jiang L. 2015. Biodegradation and mineralization of polystyrene by plastic-eating mealworms: part 2. Role of gut microorganisms. Environ. Sci. Technol. 49, 12 087–12 093. ( 10.1021/acs.est.5b02663) [DOI] [PubMed] [Google Scholar]
- 16.Yang S-S, et al. 2018. Ubiquity of polystyrene digestion and biodegradation within yellow mealworms, larvae of Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Chemosphere 212, 262–271. ( 10.1016/j.chemosphere.2018.08.078) [DOI] [PubMed] [Google Scholar]
- 17.Kundungal H, Gangarapu M, Sarangapani S, Patchaiyappan A, Devipriya SP. 2019. Efficient biodegradation of polyethylene (HDPE) waste by the plastic-eating lesser waxworm (Achroia grisella). Environ. Sci. Pollut. Res. 26, 18 509–18 519. ( 10.1007/s11356-019-05038-9) [DOI] [PubMed] [Google Scholar]
- 18.Kundungal H, Gangarapu M, Sarangapani S, Patchaiyappan A, Devipriya SP. 2019. Role of pretreatment and evidence for the enhanced biodegradation and mineralization of low-density polyethylene films by greater waxworm. Environ. Technol. ( 10.1080/09593330.2019.1643925) [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Yang J, Wu WM, Zhao J, Song Y, Gao L, Yang R, Jiang L. 2015. Biodegradation and mineralization of polystyrene by plastic-eating mealworms: part 1. Chemical and physical characterization and isotopic tests. Environ. Sci. Technol. 49, 12 080–12 086. ( 10.1021/acs.est.5b02661) [DOI] [PubMed] [Google Scholar]
- 20.Brandon AM, Gao S-H, Tian R, Ning D, Yang S-S, Zhou J, Wu W-M, Criddle CS. 2018. Biodegradation of polyethylene and plastic mixtures in mealworms (larvae of Tenebrio molitor) and effects on the gut microbiome. Environ. Sci. Technol. 52, 6526–6533. ( 10.1021/acs.est.8b02301) [DOI] [PubMed] [Google Scholar]
- 21.Kwadha CA, Ong'amo GO, Ndegwa PN, Raina SK, Fombong AT. 2017. The biology and control of the greater wax moth, Galleria mellonella. Insects 8, 61 ( 10.3390/insects8020061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dadd RH. 1966. Beeswax in the nutrition of the wax moth, Galleria mellonella (L.). J. Insect Physiol. 12, 1479–1492. ( 10.1016/0022-1910(66)90038-2) [DOI] [Google Scholar]
- 23.Kong HG, et al. 2019. The Galleria mellonella hologenome supports microbiota-independent metabolism of long-chain hydrocarbon beeswax. Cell Rep. 26, 2451–2464. ( 10.1016/j.celrep.2019.02.018) [DOI] [PubMed] [Google Scholar]
- 24.Yang S-S, et al. 2018. Biodegradation of polystyrene wastes in yellow mealworms (larvae of Tenebrio molitor Linnaeus): factors affecting biodegradation rates and the ability of polystyrene-fed larvae to complete their life cycle. Chemosphere 191, 979–989. ( 10.1016/j.chemosphere.2017.10.117) [DOI] [PubMed] [Google Scholar]
- 25.Peng B-Y, Su Y, Chen Z, Chen J, Zhou X, Benbow ME, Criddle CS, Wu W-M, Zhang Y. 2019. Biodegradation of polystyrene by dark (Tenebrio obscurus) and yellow (Tenebrio molitor) mealworms (Coleoptera: Tenebrionidae). Environ. Sci. Technol. 53, 5256–5265. ( 10.1021/acs.est.8b06963) [DOI] [PubMed] [Google Scholar]
- 26.Rahman A, Bharali P, Borah L, Bathari M, Taye RR. 2017. Post embryonic development of Galleria mellonella L. and its management strategy. J. Entomol. Zool. Stud. 5, 1523–1526. [Google Scholar]
- 27.Cook SM, McArthur JD. 2013. Developing Galleria mellonella as a model host for human pathogens. Virulence 4, 350–353. ( 10.4161/viru.25240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston PR, Rolff J. 2015. Host and symbiont jointly control gut microbiota during complete metamorphosis. PLoS Pathog. 11, e1005246 ( 10.1371/journal.ppat.1005246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansson P, Masson P. 1989. Simple enzymatic screening assay for ethylene glycol (ethane-1,2-diol) in serum. Clin. Chim. Acta 182, 95–101. ( 10.1016/0009-8981(89)90153-8) [DOI] [PubMed] [Google Scholar]
- 30.Rogers DR, Casciotti KL. 2010. Abundance and diversity of archaeal ammonia oxidizers in a coastal groundwater system. Appl. Environ. Microbiol. 76, 7938–7948. ( 10.1128/AEM.02056-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108. ( 10.1038/nprot.2008.73) [DOI] [PubMed] [Google Scholar]
- 32.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 ( 10.1093/nar/29.9.e45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl Acad. Sci. USA 108(Suppl. 1), 4516–4522. ( 10.1073/pnas.1000080107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caporaso JG, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. ( 10.1038/nmeth.f.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. ( 10.1093/bioinformatics/btq461) [DOI] [PubMed] [Google Scholar]
- 36.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. 2010. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26, 266–267. ( 10.1093/bioinformatics/btp636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeSantis TZ, et al. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072. ( 10.1128/AEM.03006-05) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas BJ, et al. 2011. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21, 494–504. ( 10.1101/gr.112730.110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, 590–596. ( 10.1093/nar/gks1219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lozupone CA, Knight R. 2008. Species divergence and the measurement of microbial diversity. FEMS Microbiol. Rev. 32, 557–578. ( 10.1111/j.1574-6976.2008.00111.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnes DKA, Galgani F, Thompson RC, Barlaz M. 2009. Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B 364, 1985–1998. ( 10.1098/rstb.2008.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahalakshmi V, Siddiq A, Andrew SN. 2012. Analysis of polyethylene degrading potentials of microorganisms isolated from compost soil. Int. J. Pharm. Biol. Arch. 3, 1190–1196. [Google Scholar]
- 43.Geyer R, Jambeck JR, Law KL. 2017. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 ( 10.1126/sciadv.1700782) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pathak VM, Navneet. 2017. Review on the current status of polymer degradation: a microbial approach. Bioresour. Bioprocess. 4, 15 ( 10.1186/s40643-017-0145-9) [DOI] [Google Scholar]
- 45.Weber C, Pusch S, Opatz T. 2017. Polyethylene bio-degradation by caterpillars? Curr. Biol. 27, 744–745. ( 10.1016/j.cub.2017.07.004) [DOI] [PubMed] [Google Scholar]
- 46.Yamada H, Nagao A, Nishise H, Tani Y. 1982. Glycerol dehydrogenase from Cellulomonas sp. NT3060: purification and characterization. Agric. Biol. Chem. 46, 2333–2339. ( 10.1271/bbb1961.46.2333) [DOI] [Google Scholar]
- 47.Staudacher H, Kaltenpoth M, Breeuwer JAJ, Menken SBJ, Heckel DG, Groot AT. 2016. Variability of bacterial communities in the moth Heliothis virescens indicates transient association with the host. PLoS ONE 11, e0154514 ( 10.1371/journal.pone.0154514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen B, Teh B-S, Sun C, Hu S, Lu X, Boland W, Shao Y. 2016. Biodiversity and activity of the gut microbiota across the life history of the insect herbivore Spodoptera littoralis. Sci. Rep. 6, 29505 ( 10.1038/srep29505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia X, et al. 2017. Metagenomic sequencing of diamondback moth gut microbiome unveils key holobiont adaptations for herbivory. Front. Microbiol. 8, 663 ( 10.3389/fmicb.2017.00663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammer TJ, Janzen DH, Hallwachs W, Jaffe SP, Fierer N. 2017. Caterpillars lack a resident gut microbiome. Proc. Natl Acad. Sci. USA 114, 9641–9646. ( 10.1073/pnas.1707186114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voirol LRP, Frago E, Kaltenpoth M, Hilker M, Fatouros NE. 2018. Bacterial symbionts in lepidoptera: their diversity, transmission, and impact on the host. Front. Microbiol. 9, 556 ( 10.3389/fmicb.2018.00556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiang H, Wei G-F, Jia S, Huang J, Miao X-X, Zhou Z, Zhao L-P, Huang Y-P. 2006. Microbial communities in the larval midgut of laboratory and field populations of cotton bollworm (Helicoverpa armigera). Can. J. Microbiol. 52, 1085–1092. ( 10.1139/w06-064) [DOI] [PubMed] [Google Scholar]
- 53.Robinson CJ, Bohannan BJM, Young VB. 2010. From structure to function: the ecology of host-associated microbial communities. Microbiol. Mol. Biol. Rev. 74, 453–476. ( 10.1128/MMBR.00014-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia F, Chen J, Fung WK, Li H. 2013. A logistic normal multinomial regression model for microbiome compositional data analysis. Biometrics 69, 1053–1063. ( 10.1111/biom.12079) [DOI] [PubMed] [Google Scholar]
- 55.Przemieniecki SW, Kosewska A, Ciesielski S, Kosewska O. 2019. Changes in the gut microbiome and enzymatic profile of Tenebrio molitor larvae biodegrading cellulose, polyethylene and polystyrene waste. Environ. Poll. 256, 113265 ( 10.1016/j.envpol.2019.113265) [DOI] [PubMed] [Google Scholar]
- 56.Wynants E, Crauwels S, Lievens B, Luca S, Claes J, Borremans A, Bruyninckx L, Van Campenhout L. 2017. Effect of post-harvest starvation and rinsing on the microbial numbers and the bacterial community composition of mealworm larvae (Tenebrio molitor). Innov. Food Sci. Emerg. Technol. 42, 8–15. ( 10.1016/j.ifset.2017.06.004) [DOI] [Google Scholar]
- 57.Dillon RJ, Webster G, Weightman AJ, Keith Charnley A. 2010. Diversity of gut microbiota increases with aging and starvation in the desert locust. Antonie Van Leeuwenhoek 97, 69–77. ( 10.1007/s10482-009-9389-5) [DOI] [PubMed] [Google Scholar]
- 58.Malhotra J, Anand S, Jindal S, Rajagopal R, Correspondence RL, Lal R. 2012. Acinetobacter indicus sp. nov., isolated from a hexachlorocyclohexane dump site. Int. J. Syst. Evol. Microbiol. 62, 2883–2890. ( 10.1099/ijs.0.037721-0) [DOI] [PubMed] [Google Scholar]
- 59.Pramila R, Padmavathy K, Ramesh KV, Mahalakshmi K. 2012. Brevibacillus parabrevis, Acinetobacter baumannii and Pseudomonas citronellolis—potential candidates for biodegradation of low density polyethylene (LDPE). J. Bacteriol. Res. 4, 9–14. ( 10.5897/JBR12.003) [DOI] [Google Scholar]
- 60.Engel P, Moran NA. 2013. The gut microbiota of insects—diversity in structure and function. FEMS Microbiol. Rev. 37, 699–735. ( 10.1111/1574-6976.12025) [DOI] [PubMed] [Google Scholar]
- 61.Pramila R, Vijaya Ramesh K. 2015. Potential biodegradation of low density polyethylene (LDPE) by Acinetobacter baumannii. Afr. J. Bacteriol. Res. 7, 24–28. ( 10.5897/jbr12.003) [DOI] [Google Scholar]
- 62.Montazer Z, Habibi Najafi MB, Levin DB. 2018. Microbial degradation of low-density polyethylene and synthesis of polyhydroxyalkanoate polymers. Can. J. Microbiol. 65, 224–234. ( 10.1139/cjm-2018-0335) [DOI] [PubMed] [Google Scholar]
- 63.Montazer Z, Habibi-Najafi MB, Mohebbi M, Oromiehei A. 2018. Microbial degradation of UV-pretreated low-density polyethylene films by novel polyethylene-degrading bacteria isolated from plastic-dump soil. J. Polym. Environ. 26, 3613–3625. ( 10.1007/s10924-018-1245-0) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw microbial sequence data are available from the NCBI short sequence read archive (SRA) under accession number PRJNA552585, and the qPCR and biochemical datasets are readily available as part of the electronic supplementary material. The rest of the data are available on demand.