Abstract
Background. – The objective of this study was to evaluate, within the Italian National Influenza Epidemiological and Virological Surveillance, the rate of vaccination coverage, the incidence of Influenza Like-Illness (ILI), the incidence of Acute Respiratory Illness (ARI), and to identify the virus strains circulating in Apulia from 1999 to 2003.
Methods. – Vaccination coverage rates were calculated based on the number of doses administered to individuals >65 years of age.
Every week, sentinel physicians reported ILI and ARI cases having occurred among their patients. Voluntary general practitioners (GPs) and paediatricians (Ps) collected oropharyngeal swab samples from patients suspected with ILI. Influenza viruses were isolated and identified by cell culture (MDCK cells) and RT-PCR. Virological surveillance was carried out by the ISS, in collaboration with a network of peripheral laboratories.
Results. – In Apulia, vaccination coverage progressively increased to 68.6% during the 2002–2003 season. The analysis of ILI cases showed higher incidence rates during the 1999–2000 and 2002–2003 seasons. ARI rates appeared to have a more constant trend. ILI and ARI incidence rates were higher in the 0–14 year age group.
Conclusion. – The increase in vaccination coverage rates and implementation of the network of clinical, and epidemiological and virological surveillance are fundamental for the control and prevention of influenza.
Keywords: Epidemiological surveillance, Virological surveillance, Influenza, Vaccination
Keywords: Surveillance epidémiologique, Grippe, Vaccination
Résumé
Objectif. – Le but du travail a été d'évaluer, dans le cadre du Programme National de Surveillance épidémiologique et virologique, le taux de couverture des vaccins, l'incidence des cas de grippe “Like-Illness (ILI)”, l'incidence de “Acute Respiratory Illness (ARI)” et de répertorier les souches virales qui circulaient dans les Pouilles pendant la saison 1999-2003.
Méthodes. – Les taux de couverture des vaccins/doses de vaccin administrés aux patients de plus de 65 ans ont été mesurés. Les médecins sentinelles ont notifié, chaque semaine, les cas d'ILI et d'ARI parmi leurs patients.
Des médecins de Médecine Générale et des Pédiatres de Libre Choix ont recueilli des prélèvements pharyngés chez les patients soupçonnés d'ILI. L'isolement et l'identification des virus de la grippe ont été réalisés par cultures cellulaires (cellules MDCK) et RT-PC. La surveillance virologique a été réalisée par l'ISS (Centre National de la Grippe de l'OMS), en collaboration avec un réseau de laboratoires périphériques.
Résultats. – Dans les Pouilles on a assisté à une progressive augmentation de la couverture des vaccins jusqu'à 68.6% pendant la saison 2002–2003.
L'analyse des cas d'ILI a mis en évidence une incidence plus élevée de l'infection pendant les saisons 1999–2000 et 2002–2003. Les ARI ont souligné un cours plus constant. L'incidence d'ILI et ARI est plus élevée entre 0 et 14 ans.
Conclusion. – L'augmentation des taux de couverture des vaccins et du réseau de surveillance clinique, épidémiologique et virologique, jouent un rôle fondamental envers le contrôle et la prévention de la grippe.
1. Introduction
Influenza is a highly contagious viral infection responsible for seasonal epidemics. The virological basis of the frequent epidemics is related to the fact that the influenza viruses can quickly mutate within their own antigenic structure. Changes can occur in haemagglutinin and/or neuraminidase, producing new virus strains against which the population has no immunity. Point mutations (antigenic drift) are responsible for annual epidemics and can occur in both influenza viruses A and B. Major changes and reassortment between human and animal influenza viruses, resulting a substitution of one or both the surface proteins (antigenic shift), only occur in influenza A viruses and can be responsible for pandemics [1].
The incidence of the illness depends on the immunity acquired by previous exposure (infection or vaccination) to the circulating strain. Each year, 10% to 20% of the population is infected. In clusters (schools, healthcare facilities, etc.), incidence can reach 40–50% [2]. At present, the infection mainly affects the young population ≤14 years and adults in the 15–64 year old age group, while influenza-related complications occur most frequently in the elderly [3].
Every year, influenza and influenza-related complications rouse increasing numbers of hospitalizations as well as mortality. It is estimated that about 90% of the excess mortality for influenza and pneumonia registered during epidemic periods occur in subjects aged over 65 years [4].
Influenza has an elevated social cost due to complications, hospitalization, increased mortality, high number of working and school days lost, increased use of drugs [5].
Although anti-viral drugs are available for therapy and prevention, vaccination is still today the most effective preventive measure to control influenza and its complications. Vaccination is specially recommended to the elderly as well as all people at risk to develop secondary complications due to age and underlying pathologies. Moreover, it is recommended to children and healthcare workers who care for people at high risk as they are more susceptible to becoming infected and spreading the epidemic [6].
The vaccines commercially available are composed of inactivated influenza viruses and contain two A virus strains and one B virus strain. Among healthy adults the vaccine-induced immune response is very high (70–90%), whereas the antibody response significantly decreases (30–40%) with aging and in particular in subjects >65 years; such individuals are more susceptible to infections of the upper respiratory tract [7], [8], [9].
In hospital settings or in confined spaces, where infections can more easily be transmitted, vaccination reduces the risk of epidemics [10].
Early identification of the influenza virus in circulation allows for the development of adequate measures and timely strategies to control influenza epidemics. Therefore, the influenza surveillance program established by the World Health Organization (WHO), which consists of 110 countries from all over the world including Italy, enables the identification of the virus strains in circulation and the annual change of the vaccine in order to match it with new antigenic variants [11].
The objective of this study was to evaluate, within the Italian influenza surveillance program, the rate of vaccination coverage (VC) in the region of Apulia, the incidence of influenza like-illness (ILI), the incidence of acute respiratory illness (ARI), and finally to characterize the virus strains circulating in Apulia throughout 1999–2003.
2. Materials and methods
2.1. Vaccination coverage
In Italy, one of the priority healthcare objectives of the 1998–2000 National Health Plan (NHP) was to reach an influenza vaccination coverage rate of 75% in the population aged >65 years [12].
The Apulia population amounts to 4,086,608 inhabitants (of which 629,649 over 65 years of age) and is divided into five provinces and 12 local health units (LHUs).
The influenza vaccination is provided free to subjects over 65 years and to people at high risk (children and adults with chronic diseases of the cardiovascular, pulmonary or renal systems, metabolic and haematological diseases, immunosuppression) according to the parameters provided by the Ministerial Memos issued annually prior to the beginning of each influenza season [13], [14], [15], [16]. The vaccine is administered both through the network of the Department of Prevention's vaccination centers and directly by general practitioners (GPs). The regional VC level was determined based on the number of vaccine doses administered annually by all the LHUs.
2.2. Clinical and epidemiological surveillance
In Italy, influenza is a disease subjected to notification only if confirmed by viral isolation. As influenza syndrome is in the majority of cases clinically diagnosed and rarely laboratory confirmed, notifications are a small part of all the ILI cases occurring each year and do not provide a real estimate of the incidence of the illness and the characteristics of the people affected.
In Italy, reliable estimates of the incidence of the illness in the whole population were not available until the 1998–1999 influenza season as influenza surveillance was only conducted in certain areas.
Since 1999–2000-influenza season, an Italian Influenza Surveillance Network (InfluNet) was created, coordinated by the Istituto Superiore di Sanità (ISS) and the Interuniversity Center for Influenza Research (CIRI).
During the surveillance period, influenza activity data (ILI or ARI cases) are reported weekly by the sentinel physicians to the national coordinators, detailing if subjects with influenza syndrome received vaccination. Rates for total incidence and those by age group (0–14, 15–64 and ≥65 years) are calculated using as a denominator the total number of patients assisted by physician participating in the study. The data are nationally computed by ISS and published weekly on the Ministry of Health Website: www.ministerosalute.it/promozione/malattie/influenza.jsp?lista=0.
In each Italian region, volunteer GPs and Pediatricians (Ps), called Sentinel Physicians, were recruited to monitor a sample of at least 1% of the population. The four-year surveillance program in Apulia has involved an increasing number of Sentinel Physicians, which grew from 32 in the 1999–2000 season to 154 in the 2002–2003 season (122 GPs and 32 Ps), which allowed 5.7% of the population to be monitored [17].
Moreover, a clinical definition of ILI cases was provided nationwide [acute respiratory affection characterized by abrupt onset with fever higher than 38 °C accompanied by at least one respiratory symptom (cough, pharyngodinia, stuffy nose) and by at least one general symptom (headache, malaise, perspiration, chills and extreme fatigue)], and for ARI cases (acute pharyngitis, bronchitis and pneumonia with or without fever) [13].
During the four influenza seasons studied, data for ILI and ARI cases were collected beginning from week 44 of one year and ending week 15 of the following year.
In the absence of mathematical models, which allow for the identification of the threshold value of the epidemic phenomenon, the epidemic is defined as a persistent and continuous increase in morbidity; this value therefore changes every year.
2.3. Virological surveillance
The virological surveillance was carried out by a smaller number of GPs and Ps, not necessarily belonging to the InfluNet network, and is coordinated by ISS as WHO National Influenza Center. These physicians collected nasopharyngeal specimens from patients with suspect influenza syndrome during the acute phase of the illness. Subsequently, influenza viruses were identified and/or isolated and characterized. The virological surveillance activity was conducted from week 46 of one year to week 15 of the following year.
Each swab was detailed with the week of collection, age, gender and location of patients in accordance with privacy laws. The specimens were then resuspended in Hank's (Gibco) and the supernatants were aliquoted in criotubes and stored at –80 °C.
For each swab, virus isolation was performed by cell culture (MDCK cells) in collaboration with the Department of Health Sciences, the Section of Hygiene and Preventive Medicine of the University of Genoa, Italy. The sub typing of the main influenza virus strains was performed by reverse transcriptase polymerase chain reaction (RT-PCR).
The isolation of viral RNA was conducted on 250 μl of clinical sample aliquoted by “Rneasy Mini Kit” (Qiagen, Hilden, Germany) and the “nested” RT-PCR typing of the viral genome was performed with a commercial kit (Poiesys Research S.a.S, Trieste, Italy), according to the manufacturer's instructions. For the first amplification, pairs of complementary primers were selected from highly conserved regions of the matrix protein of influenza viruses A and B, whereas primers that amplify specific regions of the HA gene (types 1 and 2) were used for the sub typing of A virus. The amplification products were then electrophoresed in a 1.5% agar gel stained with 0.25 μg/ml ethidium bromure. Electrophoretic migration was performed in TBE buffer at 120 V for 45 min. The electrophoretic migration pattern was visualized and photographed with GEL DOC 2000.
3. Results
3.1. Vaccination coverage
Influenza vaccination campaigns conducted among people over 65 years during the last few years in Italy and in Apulia have brought about a considerable increase in the VC rates throughout the years studied, reaching 54.9% on a national level in 2001–2002 (last data available) and 68.6% in Apulia in 2002–2003 (Fig. 1 ).
Fig. 1.
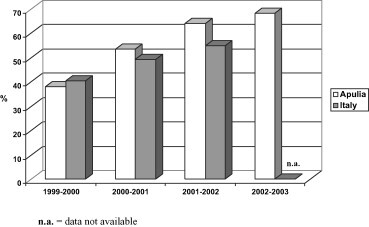
Vaccination coverage rate among persons aged 65 years of age or more in Apulia and Italy from 1999 to 2003.
Fig. 1. Taux de couverture des vaccins chez les patients âgés de 65 ans ou plus dans les Pouilles et l'Italie pendant la période 1999–2003.
In Apulia, even taking into account variations within the region, the trend of VC levels constantly increased throughout the first three seasons studied and then slightly decreased in particular LHUs throughout the 2002–2003 season.
The objective of 75% of VC indicated in the NHP was reached in the last season studied by 2 LHUs (BA/4 and FG/3) (Table 1 ).
Table 1.
Vaccination coverage rate among persons 65 years of age or more, by LHU in Apulia from 1999 to 2003
Table 1
Taux de couverture des vaccins parmi les habitants âgés de 65 ans ou plus, rapporté par LHU dans le Pouilles pendant la période 1999–2003
| LHU | 1999–2000 | 2000–2001 | 2001–2002 | 2002–2003 |
|---|---|---|---|---|
| BA/1 | 23.8 | 77.9 | 86 | 68.1 |
| BA/2 | 26.5 | 73.0 | 80.0 | 70.3 |
| BA/3 | 66.5 | 56.3 | 60 | 91.6 |
| BA/4 | 45.2 | 41.7 | 52.9 | 70.9 |
| BA/5 | 39.9 | 45.5 | 51.8 | 55.9 |
| BR/1 | 33.4 | 48.6 | 74.1 | 65.3 |
| FG/1 | 43.1 | 42.8 | 61.5 | 58.2 |
| FG/2 | 22.6 | 69.3 | 74 | 67.7 |
| FG/3 | 67.1 | 64.9 | 75 | 79 |
| LE/1 | 62.5 | 54.5 | 72 | 64.1 |
| LE/2 | 35.2 | 43.4 | 49.9 | 63.6 |
| TA/1 | 33.9 | 50.8 | 80.3 | 73.0 |
| Total |
41.6 |
55.7 |
64.1 |
68.6 |
3.2. Clinical and epidemiological surveillance
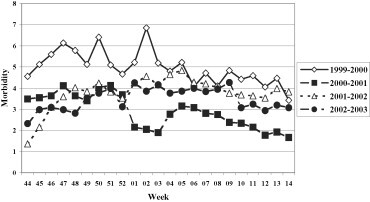
The clinical and epidemiological surveillance evidenced a different circulation of the influenza viruses throughout the various seasons studied, in terms of both incidence and weekly distribution (Fig. 2 ). In fact, the highest levels of incidence were registered in the 1999–2000 and 2002–2003 seasons, with peaks respectively of 10 and 12 cases per 1000 subjects, significantly higher than those found in the other two periods studied (4/1000 in 2000–2001 and 7/1000 in 2001–2002).
Fig. 2.
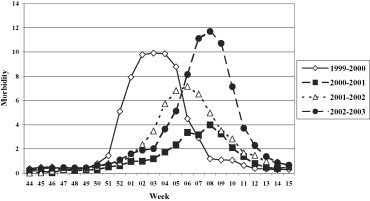
Morbidity (per 1000 inhabitants) for ILI in the 1999–00, 2000–01, 2001–02, and 2002–03 influenza seasons.
Fig. 2. Morbidité (parmi 1000 habitants) due à l'ILI pendant les saisons de grippe 1999–2000, 2000–2002 et 2002–2003.
In general, the epidemic period occurred between late December and January/February. In the 1999/2000 season, the circulation of the influenza virus was slightly early with an epidemic period between week 52 of 1999 and week 7 of 2000. In the 2002–2003 season, the epidemic period occurred between week 4 and week 11 of 2003.
It is estimated that the last influenza season in Apulia affected about 270,000 patients; during the other three seasons (1999–2000, 2000–2001 and 2001–2002) the infection affected, respectively, about 235,000, 90,000 and 183,000 patients.
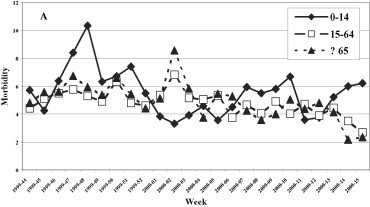
A uniformed distribution among the three age groups was evidenced in the 1999–2000 season (Fig. 3 ), while in the last three seasons the group most affected was that of 0–14 years, with a maximum incidence of 12.7, 20.4 and 27.96 cases per 1000 patients in the 2000–2001, 2001–2002 and 2002–2003 seasons, respectively.
Fig. 3.
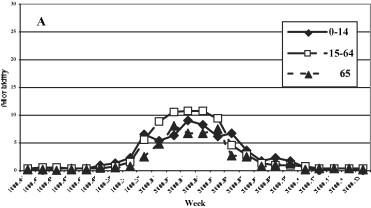
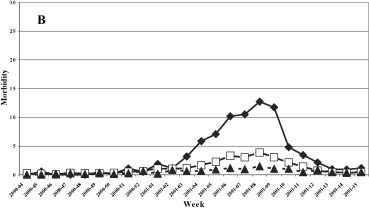
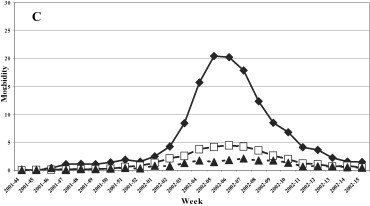
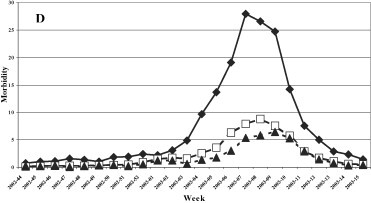
Morbidity (per 1000 inhabitants) for ILI by age groups. (A) 1999–2000, (B) 2000–2001, (C) 2001–2002, and (D) 2002–2003 influenza seasons.
Fig. 3. Morbidité (parmi 1000 habitants) due à l'ILI, triés par âge. Saisons de grippe 1999–2000 (A), 2000–2001 (B), 2001–2002 (C) et 2002–2003 (D).
The trend of the ARI in the four years studied was constant during the whole surveillance period with a slight decrease during the period of maximum circulation of the influenza viruses (Fig. 4 ). The highest incidence of ARI was registered in the 1999–2000 influenza season with an average of about five cases per 1,000 patients and a peak in week 2 of 2000 (about 7/1000 patients). The lowest incidence of ARI was registered during the 2000–2001 season with an average of about 3 cases/1000. The trend of ARI by age group (Fig. 5 ) was similar in all age groups in the 1999–2000 and 2000–2001 seasons, while in the following two seasons (2001–2002 and 2002–2003) a higher incidence of cases was found in the young population (0–14 years).
Fig. 4.
Morbidity (per 1000 inhabitants) for ARI in the 1999–2000, 2000–2001, 2001–2002, and 2002–2003 seasons.
Fig. 4. Morbidité (parmi 1000 habitants) due à l'ARI pendant les saisons 1999–2000, 2000–2001, 2001–2002, et 2002–2003.
Fig. 5.
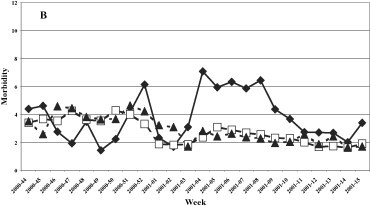
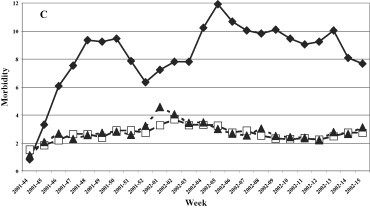
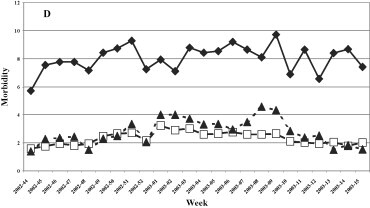
Morbidity (per 1000 inhabitants) for ARI by age groups. 1999–2000, 2000–2001, 2001–2002, and 2002–2003 influenza seasons.
Fig. 5. Morbidité (parmi 1000 habitants) due à l'ARI rassemblées par âge, saisons de grippe: 1999–2000, 2000–2001, 2001–2002, et 2002–2003.
3.3. Virological surveillance
During the virological surveillance conducted in Apulia in the last 4 years (Table 2 ), 18 out of 70 specimens tested were found PCR-positive in the 1999–2000 season, 10/30, 6/75 and 34/64 in the 2000–2001, 2001–2002 and 2002–2003 influenza seasons, respectively.
Table 2.
Virological surveillance in Apulia, 1999–2000, 2000–2001, 2001–2002, and 2002–2003 influenza seasons
Table 2
Surveillance virologique dans les Pouilles. Saisons de grippe: 1999–2000, 2000–2001, 2001–2002, et 2002–2003
| Season | Age group (year) | Subjects (N) | RT-PCR (P/N) | Culture (P/N)* | Vaccination (P/N) |
|---|---|---|---|---|---|
| 1999–2000 | <15 | 5 | 0/5 | 0/5 | 0/0 |
| 15–64 | 54 | 14/40 | 5/38 | 0/0 | |
| ≥65 | 10 | 3/7 | 1/3 | 1/1 | |
| n.i. | 1 | 1/0 | 0/1 | 0/0 | |
| 2000–2001 | <15 | 11 | 4/7 | 0/1 | 0/0 |
| 15–64 | 19 | 6/13 | 0/7 | 1/0 | |
| ≥65 | 0 | 0/0 | 0/0 | 0/0 | |
| 2001–2002 | <15 | 26 | 4/22 | 0/14 | 0/0 |
| 15–64 | 44 | 2/42 | 0/13 | 0/0 | |
| ≥65 | 5 | 0/5 | 0/1 | 0/3 | |
| 2002–2003 | <15 | 23 | 16/7 | 1/4 | 2/2 |
| 15–64 | 39 | 17/22 | 0/20 | 2/1 | |
| ≥65 |
2 |
1/1 |
0/0 |
1/1 |
N, number; P = positive; N = negative; * = Culture done; n.i. = not identified.
During the first three seasons, no influenza virus was identified in vaccinated patients (with the exception of one single subject in the 1999–2000 season), while in the 2002–2003 season 7.8% (5/64) of the specimens were identified in vaccinated subjects.
During the four-year virological surveillance, the typing of viruses conducted during the epidemic period on nasopharyngeal secretions from patients with suspect influenza syndrome evidenced the circulation of different virus strains.
In particular, during the 1999–2000 influenza season, all the viruses identified (apart from one non-subtyped) belonged to the A/H3N2 subtype. During the 2000–2001 season a A/H1N1 subtype virus was identified in all the samples tested. In the following season (2001–2002), all viruses belonged to type B. Finally, in the 2002–2003 season, the characterization of the positive samples showed a substantial prevalence of A/H3N2 influenza viruses (97%). Only one virus belonged to type B.
4. Discussion
Influenza is an important worldwide public health issue due to the fact that it is ubiquitous, contagious and antigenically unstable. The circulation of new antigenic variants cause annual epidemics characterized by high morbidity, increased hospitalization and mortality [3]. The majority of influenza-related deaths occur in subjects over 65 years of age and are mainly registered during the circulation of A/H3N2 influenza viruses [4]; in children the incidence rate reaches 30% and causes mainly a significant increase in medical treatment and hospitalization [18]. Moreover, the high level of influenza virus diffusion and the short period of incubation make it difficult to develop timely strategies to control and limit the spread of the illness. Consequently, vaccination remains the best measure for the prevention and control of the illness. In order to limit the circulation of the influenza virus and protect the subjects at high risk, the objective established by the NHP for 1998–2000 in Italy was 75% of vaccination coverage in the population older than 65 years [12]. In Italy, vaccine immunoprophylaxis, conducted yearly through specific vaccination campaigns promoted by the Ministry of Health and ISS, is free administered to subjects over 65 years or at high risk of complications through the network of vaccination centers of the Departments of Prevention or through regional GPs.
As stated in the Ministerial Memo no. 5, 22/07/03, a particular attention was given to the prevention and control during the 2003–2004 influenza season because: “vaccination assumes an even more important role due to the clinical similarities, at least in the early stages, between influenza and Severe Acute Respiratory Syndrome (SARS).” [19].
The circulation of new antigenic variants of influenza virus requires constant updating in the composition of the vaccine, as highlighted in the World Influenza Surveillance Program, coordinated by WHO [20], in which Italy participates through the ISS [21]. The clinical, epidemiological and virological surveillance in Apulia is conducted according to this project. The network of sentinel physicians allowed for reliable data to be available in real time, regarding the beginning of the seasonal epidemic and its progression. The association of the virological, clinical and epidemiological surveillance permitted the integration of the incidence data with the identification of the viruses in circulation. The surveillance data, reported weekly to ISS, are published weekly on the website of the Ministry of Health and reported to the WHO in Geneva (www.oms2.b3e.jussieu.fr/flunet) and to the other European countries participating in the network (EuroGROG, www.grog.org; EISS, www.eiss.org) [22].
The VC data recorded in Italy and in Apulia showed a progressive increase in the vaccine doses administered to subjects over 65 years, even though the NHP levels have not yet been reached. Subjects 65 years of age or older represent an important sector of the Apulia population, which accounts for the effort of the Regional Health Office to reach the vaccination coverage objective established by NHP. The impact of this increase on the clinical-epidemiological data is difficult to demonstrate; however, at a national level the incidence rate showed a decreasing trend in elderly, in particular during the 2000–2001 season, when H1N1 virus spread. A more accurate estimate should take in account the type of virus circulating and evaluate how the rate of complications, hospitalizations and the extra-mortality in older people vaccinated changes as VC increases. However, it is important to note that quite few influenza viruses have been identified in vaccinated subjects.
The clinical, epidemiological and virological surveillance showed a higher incidence of the illness and a longer epidemic period (8 and 9 weeks, respectively) during the circulation of the type A/H3N2 influenza virus in seasons 1999–2000 and 2002–2003 with a peak incidence in the 2 years of about 10 and 12 cases per 1000 patients. The lowest incidence was registered when the type A/H1N1 influenza virus was in circulation (2000–2001 season) while the B virus caused an intermediate incidence (2001–2002 season). During the 1999–2000 season, A/H3N2 viruses spread in all age groups, whereas in the following three seasons a higher incidence was observed in the 0–14 year age group, as expected as this group consists of subjects who were not exposed to previous influenza epidemics nor were vaccinated. The data relative to the 1999/2000 season are attributable to the fact that during the first clinical and epidemiological surveillance season, Ps had not been involved as sentinel physicians, whereas their number progressively increased in the following years, improving the performance of the surveillance system.
The progression of the ILI cases registered in Apulia during the period studied is similar to the data recorded on a national level with a slight delay; in fact, the beginning of the epidemic period and the peak of the epidemic were registered always with a week of delay (6–7 days) in respect to the North of Italy [23].
The progression of ARI showed a constant incidence throughout the period studied and a higher diffusion in the 0–14 year age group. In fact, ARI represent the most common childhood infection and are the main cause of medical consultations for children [24].
The data obtained in these first four years of activity highlight the importance of the network of clinical, epidemiological and virological influenza surveillance as it permits to monitor the progression of the illness over time, rapidly identify potential epidemics, early isolate and characterize new virus strains in circulation, update the vaccine composition, and estimate the efficacy of vaccination campaigns as well as integrate and validate the national data.
References
- 1.Cox N.J., Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 2.Klimov A., Simonsen L., Fukuda K., Cox N. Surveillance and impact of influenza in the United States. Vaccine. 1999;17:42–46. doi: 10.1016/s0264-410x(99)00104-8. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Prevention and control of influenza. MMWR Recomm Rep. 2002;51(RR-3):1–31. Recommendations of the Advisory Committee on Immunization Practices (ACIP) [PubMed] [Google Scholar]
- 4.Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine. 1999;17:3–10. doi: 10.1016/s0264-410x(99)00099-7. [DOI] [PubMed] [Google Scholar]
- 5.Schoenbaum S.C. Economic impact of influenza. Am J Med. 1987;82:26–30. doi: 10.1016/0002-9343(87)90557-2. The individual's perspective. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Surveillance for influenza –United States, 1997–98, 1998–99, and 1999–00 seasons. MMWR. 2002;51(SS-7):1–10. [PubMed] [Google Scholar]
- 7.Monto A.S. The clinical efficacy of influenza vaccination. Pharmacoeconomics. 1996;9:16–22. doi: 10.2165/00019053-199600093-00006. [DOI] [PubMed] [Google Scholar]
- 8.Dorrell L., Hassan I., Marshall S., Chakraverty P., Ong E. Clinical and serological responses to an inactivated influenza vaccine in adults with HIV infection, diabetes, obstructive airways disease, elderly adults and healthy volunteers. Int J STD AIDS. 1997;8:776–779. doi: 10.1258/0956462971919264. [DOI] [PubMed] [Google Scholar]
- 9.Bridges C.B., Thompson W.W., Meltzer M.I., Reeve G.R., Talamonti W.J., Cox N.J., et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. JAMA. 2000;284:1655–1663. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- 10.Patriarca P.A., Weber J.A., Parker R.A., Orenstein W.A., Hall W.N., Kendal A.P., et al. Risk factors for outbreaks of influenza in nursing homes. Am J Epidemiol. 1986;124:114–119. doi: 10.1093/oxfordjournals.aje.a114355. A case-control study. [DOI] [PubMed] [Google Scholar]
- 11.Ghendon Y. Influenza surveillance. Bull WHO. 1991;69:509–515. [PMC free article] [PubMed] [Google Scholar]
- 12.DPR 23, luglio 1998. Piano Sanitario Nazionale 1998–2000. Un patto di solidarietà per la salute. Gazzetta Ufficiale del 10/12/1998.
- 13.Ministero della Salute. Profilassi antinfluenzale. Raccomandazioni per la stagione 1999–2000. Circolare Ministeriale no 11 del 25 giugno 1999.
- 14.Ministero della Salute. Prevenzione e controllo dell'influenza: raccomandazioni per la stagione 2000–2001. Circolare Ministeriale no 9 del 13 luglio 2000.
- 15.Ministero della Salute. Prevenzione e controllo dell'influenza: raccomandazioni per la stagione 2001–2002. Circolare Ministeriale no 8 del 31 maggio 2001.
- 16.Ministero della Salute. Prevenzione e controllo dell'influenza: raccomandazioni per la stagione 2002–2003. Circolare Ministeriale no 1 del 1 luglio 2002.
- 17.Delibera Regionale no 24/19270/Coord.– 4. Sorveglianza epidemiologica e virologica dell'influenza (stagione 2002/2003) Assessorato alla Sanità e Servizi Sociali, Regione Puglia. 21/10/2002 [Google Scholar]
- 18.Glezen W.P. Emerging infections: pandemic influenza. Epidemiol Rev. 1996;18:64–76. doi: 10.1093/oxfordjournals.epirev.a017917. [DOI] [PubMed] [Google Scholar]
- 19.Ministero della Salute. Prevenzione e controllo dell'influenza: raccomandazioni per la stagione 2003-2004. Circolare Ministeriale no 5 del 22 luglio 2003.
- 20.Pereira M., Assaad F.A., Delon P.J. Influenza surveillance. Bull WHO. 1978;56:193–203. [PMC free article] [PubMed] [Google Scholar]
- 21.Istituto Superiore di Sanità FLU-ISS: Sistema di sorveglianza sentinella dell'influenza basata su medici di medicina generale e pediatri di libera scelta. Rapporti ISTISAN. 2000;21:1–75. [Google Scholar]
- 22.Istituto Superiore di Sanità FLU-ISS. Sistema di sorveglianza sentinella dell'influenza basata su medici di medicina generale e pediatri di libera scelta. Rapporto sulla stagione influenzale 2001–2002. Rapporti ISTISAN. 2002;19:1–87. [Google Scholar]
- 23.Istituto Superiore di Sanità FLU-ISS. Sorveglianza virologica dell'influenza in Italia (stagione 2002–2003). Rapporto preliminare. Rapporti ISTISAN. 2003;16:1–26. [Google Scholar]
- 24.Jacobs B., Young N.L., Dick P.T., Ipp M.M., Dutkowski R., Davies H.D., et al. Canadian acute respiratory illness and flu scale (CARIFS): development of a valid measure for childhood respiratory infections. J Clin Epidemiol. 2000;53:793–799. doi: 10.1016/s0895-4356(99)00238-3. [DOI] [PubMed] [Google Scholar]


