Abstract
During an outbreak of severe pneumonia among new army recruits, an epidemiological investigation combined with repeated nasopharyngeal/oropharyngeal cultures from sick and healthy contacts subjects was conducted. Fifteen pneumonia cases and 19 influenza-like illness cases occurred among 596 recruits over a 4-week period in December 2005. Pneumonia attack rates reached up to 5.5%. A single pneumococcus serotype 5 clone was isolated from blood or sputum cultures in 4 patients and 30/124 (24.1%) contacts. Immunization with 23-valent polysaccharide vaccine supplemented with a 2-dose azithromycin mass treatment rapidly terminated the outbreak. Carriage rates dropped to <1%, 24 and 45 days after intervention.
Keywords: Pneumonia, Streptococcus pneumoniae, Outbreak, Vaccine, Young adults, Carriage
1. Introduction
Outbreaks of severe respiratory illness among healthy young adults are uncommon, and have a wide range of differential diagnoses. Such outbreaks caused by Streptococcus pneumoniae have rarely been described in non-elderly healthy adults [1], [2], [3], [4], and detailed description of such outbreaks and the interventions that took place are scarce. In the available reports, unique environmental settings such as weakened immune competence and extreme crowding were prevalent. S. pneumoniae is transmitted through carriage in the upper respiratory tract, but no detailed descriptions of carriage of epidemic strains during such outbreaks are available, in particular beyond day-care center settings.
During December 2005 and January 2006, an epidemic of 15 cases of radiologically proven pneumonia and 19 cases of influenza-like illness (ILI) occurred in a training unit of the Israel Defense Force (IDF) in southern Israel. An epidemiological investigation was consequently initiated, which included culture to detect pneumococcal carriage, followed by antibiotic prophylaxis administration and vaccination. The objectives of the present report are: (1) to describe the epidemiologic characteristics of the outbreak, including carriage among contacts; and (2) to document the effect of the preventive measures on disease rate and carriage of the epidemic strain.
2. Methods
2.1. Population and setting
Beyond the recruit unit, the military compound included several training units (∼2000 soldiers) with very limited mixing with the recruits. Recruits were 18–20-year old (median, 18), healthy men and women undergoing screening for field unit service. The training unit consisted of 6 platoons comprising each 64–142 recruits, all drafted on the same week in the month preceding the outbreak and immediately stationed at the training base. All were vaccinated against influenza upon recruitment, none was vaccinated against S. pneumoniae.
Platoons A–D lived in rooms while platoons E and H lived in tents. Platoon H was more crowded than the other platoons.
2.2. Case definition and epidemiological investigation
ILI was defined as an episode with respiratory symptoms (cough, coryza or sore throat) and fever (temperature >37.5 °C). Pneumonia was defined as an episode with acute respiratory signs and symptoms and evidence of a new infiltrate in chest radiography. Laboratory confirmed pneumococcal pneumonia was defined as pneumonia with isolation of S. pneumoniae from blood or endotracheal tube.
The first ILI case during the three weeks preceding the outbreak occurred on December 12, 2005. This day is denoted as ‘outbreak day 1’. The first pneumonia case was diagnosed on outbreak day 7. Active case finding (including retrospective medical records review for ILI symptoms) was started on outbreak day 13.
All ILI and pneumonia cases occurring after outbreak day 12 were interviewed for demographic, personal and military service-related common features with the cases. In addition, all underwent physical examination, and had a chest radiography. Peripheral white blood cell count, serum transaminase and creatinin levels and coagulation panel were obtained. Altogether, 12/15 pneumonia cases and 2/19 ILI cases had blood or sputum culture before initiation of antibiotic treatment. Two pneumonia cases were intubated, and respiratory secretions from endotracheal tubes were cultured.
A convenience sample of 252 recruits of the affected platoons responded to a structured questionnaire distributed on outbreak day 17 with regard to respiratory symptoms during the preceding month in the recruit himself and his family members, as well as demographic and personal details (gender, home town, transportation used to get to the base, clinic visits, smoking status). Recruits reporting respiratory symptoms in family members were further interviewed to determine the severity of the symptoms.
On outbreak day 12, oropharyngeal and nasopharyngeal cultures were sampled from 124 available recruits, mainly in companies in which a pneumonia case had already been recorded at the time. Eighteen staff members in direct contact with the recruits were also sampled. Additional oropharyngeal and nasopharyngeal cultures were sampled from 144 to 105 available contacts recruits of the two most inflicted platoon, 30 and 51 days following this first sample (outbreak days 47 and 68, respectively). Sixty-two recruits were sampled on all 3 occasions.
The nasopharyngeal and oropharyngeal samples for S. pneumoniae were transported within 6 h to the Soroka University Medical Center laboratories, and were processed as previously described [5]. Pulsed field gel electrophoresis (PFGE) using chromosomal DNA fragments, generated by SmaI digestion was performed as described elsewhere [5]. Since all S. pneumoniae serotype 5 (Sp5) had identical PFGE patterns, multilocus sequence typing (MLST) as described by Enright and Spratt [6] was performed on one Sp5 isolate. The sequences (alleles) at each locus were compared to those at the MLST website (www.mlst.net), to assign the sequence type (ST).
The list of tests for various viruses and bacteria is detailed in Table 1 . Nasopharyngeal specimens were tested for Chlamydophila pneumoniae DNA according to Black et al. [7]. Mycoplasma pneumoniae IgA and IgM were tested with a commercial ELISA assay (SeroMP™ ELISA kits, Savyon Diagnostics, Ashdod, Israel). Seroconversion was calculated as previously described [8]. Urine samples were tested for the presence of Legionella pneumophila serogroup 1 antigen (Binax NOW Legionella Urinary Immunochromatographic Antigen Test, Portland, ME). Nasopharyngeal swabs were studied for the presence of RSV, influenza A and B viruses, parainfluenza viruses, and adenovirus by direct immunofluorescence assay (IFA), using commercial monoclonal antibodies (Chemicon International), and by tissue culture. Residual aliquots were stored at −80 °C and later analyzed for the presence of adenovirus, influenza viruses, and human metapneumovirus by real-time PCR and reverse transcription PCR as previously described [9], [10], [11].
Table 1.
Summary of laboratory testing performed in pneumonia cases, ILI cases and healthy recruits.
| Pathogen (identification method) | Specimen tested | ILI and Pneumonia cases (n = 34)a | Healthy recruits (n = 562)a |
|---|---|---|---|
| Streptococcus pneumoniae (culture) | Respiratory secretion/blood | 5/14b | –/– |
| Oropharyngeal/nasopharyngeal swabs | –/– | 55/124 | |
| Mycoplasma pneumoniae (PCR) | Pharyngeal swabs | 1/4 | 0/14 |
| M. pneumoniae (serology) | Serum | 1/16 | 0/4 |
| Legionella pneumophila type 1 (urine antigen) | Urine | 0/8 | 0/14 |
| L. pneumophila (serology) | Serum | 0/14 | 0/4 |
| RSV, influenza A and B, parainfluenza, adenovirus (IFA) | Nasal swabs/sputum | 0/15 | 0/14 |
| Human Metapneumovirus (PCR) | Nasal swabs | 0/4 | 1/14 |
| Chlamydophila pneumoniae (PCR) | Nasal swabs | 0/9 | 0/14 |
| C. pneumoniae (serology) | Serum | 2/12 | – |
| Q fever (serology) | Serum | 0/7 | – |
| Streptococcus pyogenes (culture) | Pharyngeal swabs | 1/15 | 2/124 |
No. positive/no. tested.
Two patients with positive blood cultures, two with positive respiratory secretion cultures and one partially treated patient with gram-positive diplococci in sputum gram staining—all among the 15 pneumonia cases.
2.3. Risk factor assessment
The incidence of pneumococcal carriage, pneumonia or ILI was compared among strata of each of the potential risk factors studied, including gender, smoking, recent history of respiratory illness or clinic visits prior to the outbreak. Student's t-test was used to test statistical significance of the differences in each outcome variable between the two strata of each risk factor.
3. Results
3.1. Outbreak details
3.1.1. The outbreak
Fifteen pneumonia cases were identified among 596 new recruits during the 6-week outbreak period (attack rate = 2.5%) (Fig. 1 ). Cases occurred in all six platoons (A, B, C, D, E, H). The highest pneumonia attack rates were 5.5% and 5.2% in the two most severely affected platoons (Table 2 ), with an attack rate of 12.9% (4/31) in the most affected company in platoon H. Additional 19 ILI cases were identified during the outbreak period. At least 8 of the ILI patients received early antibiotic treatment. The combined ILI + pneumonia attack rates reached 9.3% and 7.7% in platoons A and H, respectively. No pneumonia cases occurred in the training unit in the 3 months preceding the outbreak
Fig. 1.
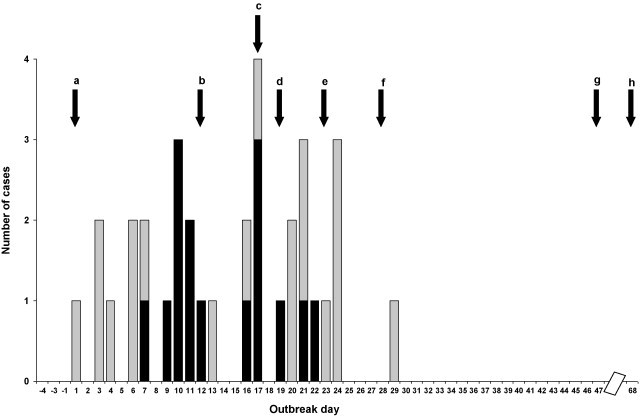
Pneumonia (black bars) and influenza-like illness (white bars) cases by date of fever onset (marked as ‘outbreak days’) (pneumonia follow-up was maintained until outbreak day 47. ILI follow-up was halted on outbreak day 37). a: First recorded ILI case (outbreak day 1); b: general practitioner alert of suspected outbreak (outbreak day 12); c: S. pneumoniae mass sampling among control recruits in the base (outbreak day 17); d: recruits sent home (outbreak day 19); e: antibiotic prophylaxis (first dose) and mass vaccination (outbreak day 23); f: recruits return to base, second dose of antibiotic prophylaxis administered (outbreak day 30); g: first follow-up cultures (outbreak day 47); h: second follow-up cultures (outbreak day 68).
Table 2.
Pneumonia and ILI incidence rates and SP carriage rates by platoon.
| Platoon (# of companies included)a | Living setting | Pneumonia attack rateb | Combined ILI + pneumonia attack ratec | Pneumococcal carriage among controlsd | Sp5 carriage among controlse | Ratio of pneumonia attack rates and Sp5 carriage rates | Other serotypes (non-Sp5) carried |
|---|---|---|---|---|---|---|---|
| A (3/3) | 5–8 per room | 4/86 (4.7%) | 8/86 (9.3%) | 5/14 (35.7%) | 3/14 (21.4%) | 1:4.6 | 23F (1), 9V (1), 6B (1) |
| C (3/4) | 7–8 per room | 2/97 (2.1%) | 6/97 (6.2%) | 10/17 (58.8%) | 2/17 (11.8%) | 1:5.7 | 10B (3), 15B/C (2), 3 (2), non-typable (1) |
| D (2/3) | 8 per room | 1/49 (2%) | 4/49 (8.1%) | 4/22 (18.2%) | 1/22 (4.5%) | 1:2.2 | 16F (1), non-typable (2) |
| H (3/3) | 10–12 per tent | 5/91 (5.5%) | 7/91 (7.7%) | 34/63 (54%) | 22/63 (36.5%) | 1:6.6 | 3 (2), 15B/C (1), 15C (1), 17F (1), 18C (1), 23F(3), 35B (2) |
| 4 platoons Summary | 12/323 (3.7%) | 13/323 (7.7%) | 53/116 (45.7%) | 29/116 (25%) | 1:6.7 | ||
| Overall-all recruits | 15/596 (2.5%) | 34/596 (5.7%) | 55/124 (44.4%) | 30/124 (24.2%) | 1:7.8 |
Selected 4 of 6 platoon in which 5 or more recruits were sampled, and selected companies with at least 1 cases of pneumonia or ILI.
Number of pneumonia cases/total platoon recruits (%).
Number of ILI and pneumonia cases/total platoon recruits (%).
Number of pneumococcal carriers/total controls tested (%).
Number of Sp5 carriers/total controls tested (%).
The pneumonia cases occurred in 14 males and 1 female. All were previously healthy. Four of twelve pneumonia cases for which smoking data was available (33.3%) were smokers, a rate similar to that reported in controls. None of the pneumonia and ILI patients reported any respiratory illness that required clinic or hospital visit among household members.
All trainees were sent home on outbreak day 19. On outbreak day 23, when results suggested that S. pneumoniae was the causative agent, all trainees were vaccinated with a 23-valent pneumococcal polysaccharide vaccine (Pneumovax 23, MSD, USA). All base residents, including the recruits who were receiving antibiotic treatment, received a synchronized regimen of a two-dose 500 mg oral azithromycin (doses 1 and 2 on outbreak days 23 and 30, respectively). Oropharyngeal and nasopharyngeal cultures obtained 24 and 45 days following vaccination and first azithromycin dose (outbreak days 47 and 68) yielded only 1/144 and 1/105 positive S. pneumoniae samples, respectively, and none was Sp5.
None of the potential risk factor investigated (including gender, smoking, recent history of respiratory illness, clinic visits) was significantly associated with pneumococcal carriage, pneumonia or ILI.
During the outbreak period, the monthly incidence rate of pneumonia in the largest neighboring military training unit was 0.3 cases per 1000 soldiers, and 0–1 pneumonia cases per 1000 recruits per month were recorded in three other adjacent bases during the same period (data not shown).
3.1.2. Clinical characteristics
Symptoms recorded on admission among the 15 pneumonia cases included fever (100%); cough (100%); sputum production (93.3%); chest pain (46.7%); dyspnea (33.3%); and hemoptysis (13.3%). Admission temperature ranged from 37.2 to 40.6 °C (median, 39.1 °C). Crackles were present in 53.3%. Radiographic evidence for a lobar infiltrate was found in 14/15 pneumonia cases. Two patients had enlargement of the heart, one of whom also had a lung abscess. Peripheral WBC count ranged from 7300 to 34,700 cells/mm3 (median, 17,730). Five pneumonia cases required intensive care unit treatment, one for respiratory failure and renal dysfunction, one for abnormal coagulation profile and pleural effusion, one for hypotensive shock, one due to a large pleural effusion and one for septic shock and multiorgan failure. The latter patient (the only female patient) was the most severely affected case requiring prolonged mechanical ventilation; she also developed a secondary infection with brain abscess. All other patients required hospital care for 3–17 days (median, 3), and recovered
Active case finding, once initiated, reduced the mean time from initial fever to antibiotic treatment in pneumonia cases from a mean of 2.3 days (first 7 cases) to a mean of 0.5 day (8 cases identified during the active case finding).
3.1.3. Microbiologic test results
Of all samples tested for non-pneumococcal organisms, only 1 paired sera (from a patient with pneumonia and positive nasopharyngeal culture for Sp5) was positive for M. pneumoniae (Table 1). Seroconversion to C. pneumoniae occurred in 1/11 paired sera, and another sample taken two weeks following the illness (from a patient with pneumonia and positive nasopharyngeal culture for Sp5 for whom an early sera testing was not available) yielded a high antibody titer for C. pneumoniae. S. pneumoniae was isolated from 4 of the pneumonia cases (two blood cultures, two endotracheal cultures). All were Sp5. One additional, partially treated pneumonia case, had gram-positive diplococci in sputum gram staining, but the organism did not grow in culture.
S. pneumoniae was isolated from 55/124 (44.35%) sampled controls (Table 1). An additional 3/18 staff members tested were positive for S. pneumoniae. Of the 58 positive samples, 49 (84.5%) were positive in nasopharyngeal cultures. In 9 cases, only oropharyngeal culture was positive. All 58 S. pneumoniae isolates from controls were serotyped: 35 (60.3%) were Sp5. The other 19 isolates belonged to 10 different serotypes: 23F (4), 15B/C (4), 10B (3), 35B (2), and one of each 3, 6B, 9V, 16F, 17F and 18F. The remaining 4 isolates were non-typable. Of the 52 isolates recovered from contacts, 27 (51.9%) were Sp5.
All Sp5 isolates were susceptible to penicillin, erythromycin, clindamycin, tetracycline and chloramphenicol; 4 Sp5 isolates from carriers were nonsusceptible to TMP/SMX. Pulsed field gel electrophoresis was performed on all Sp5 isolates and all showed an identical pattern (Fig. 2 ). We compared PFGE of Sp5 from the current outbreak to those among Sp5 from children in southern Israel during 2004–2006. A total of 24 isolates from nasopharynx (n = 9), middle ear fluid (n = 9) and blood (n = 6) were available. All 24 isolates belonged to the same clonal type as the outbreak strain, but only 5/24 had a pattern fully identical to the outbreak strain (Fig. 3 ). Multilocus sequence typing of one representative blood Sp5 isolate from a pneumonia case, showed an allelic profile identical to the Colombia5-19 clone (ST-289, according to http://www.mlst.net).
Fig. 2.
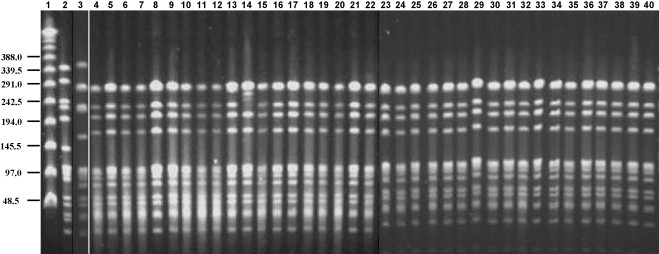
PFGE patterns generated by SmaI digestion of S. pneumoniae serotype 5 isolates recovered from military recruits. Lane 1 contains a lambda ladder; lane 2 contains a reference strain R6 used as a molecular weight marker; lane 3 contains the Columbia5-19 international clone. Numbers on the left show molecular weight sizes in kilobases. Lanes 4–34 contain nasopharyngeal isolates; lanes 35–36 contain oropharyngeal isolates; lanes 37–38 contain blood isolates; lanes 39–40 contains respiratory secretion isolate.
Fig. 3.
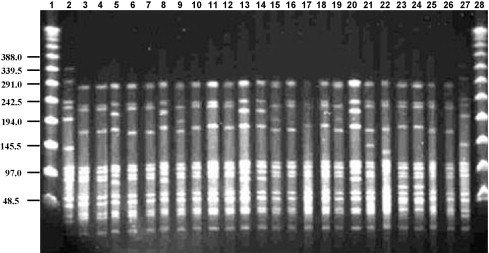
PFGE patterns generated by SmaI digestion of S. pneumoniae serotype 5 isolates recovered from children in Southern Israel during 2004–2006. Lanes 1 and 28 contain a lambda ladder; lanes 2 and 27 contain a reference strain R6 used as a molecular weight marker. Numbers on the left show molecular weight sizes in kilobases. Lanes 3–11 contain nasopharyngeal isolates; lanes 12–20 contain isolates recovered from middle ear fluids; lanes 21–26 contain blood isolates.
4. Discussion
Outbreaks of pneumococcal pneumonia became relatively rare in the post-antibiotic era [12], [13], [14], although reports appear to be more frequent in recent years, mainly among the very young, the disabled and the elderly [13], as well as among young adults living in confined settings [12]. Pneumococcal outbreaks in non-elderly adults involve either a single [1], [3], [4], [12], [15], [16], [17], [18], [19] or multiple [2], [20], [21], [22] pneumococcal serotypes. One large outbreak of invasive pneumococcal disease caused by Sp5 was recently reported from Canada, in a non-elderly urban population living in crowded and poor conditions [23]. In military outbreaks, serotypes 1, 4, 7F, 8, and 9V have been previously implicated [2], and in the most recently reported outbreak in the military a mixture of serotypes was identified (serotype serogroups 3, 6, 9, 14, 19, 20, 22, and 23) [20]. To the best of our knowledge, this is the first described Sp5 outbreak in the military.
Several factors clearly implicate Sp5 as the causative agent in this outbreak. First, Sp5 is one of the most virulent and invasive S. pneumoniae serotypes, exhibiting a high disease-potential for invasive pneumococcal infection [24], [25], [26]. Second, all 4 identified isolates from the pneumonia cases were Sp5. Third, Sp5 carriage rates are usually very low in healthy young individuals [3], [27], [28], [29], [30], [31], yet, Sp5 was isolated from 24.1% of the cultured contacts during the outbreak. Furthermore, a near linear correlation between platoon-specific pneumonia incidence rates and Sp5 carriage rates was observed (Table 2). Fourth, PFGE analysis showed a complete homogeneity of all tested isolates from the current outbreak in both cases and controls, in contrast to a marked heterogeneity (although within one clone) among sporadic isolates from children living in the same region.
The low S. pneumoniae detection rate in our pneumonia cases (4/12, 33.3%) matches previously documented low isolation rates of S. pneumoniae during outbreaks (6.7–12%) [4], [20], as well as sporadic pneumonia cases (25%) [32]. Furthermore, some of these cases were sampled during antibiotic treatment, and indeed one sputum sample from a fifth patient obtained during antibiotic treatment demonstrated gram-positive diplococci, but was culture-negative.
The current outbreak was composed of two components: 19 cases of ILI and 15 cases of radiologically proven pneumonia. A considerable proportion of these patients received early antibiotic treatment that likely modified the outcome and prevented overt pneumonia. These are therefore likely different manifestations of the same outbreak, since both components overlapped. However, a more interesting question is whether these two components were caused both by new acquisition of Sp5, or was the ILI component caused by a virus and the pneumonia component was caused by a secondary infection Sp5. Viral pathogens are a well established predisposing factor for pneumococcal pneumonia outbreaks and sporadic cases [19], [22], [33], but were absent in other reports [2], [12], [20]. Recent analysis of the 1914–1918 influenza pandemic also showed a similar pattern where in military outbreaks, pneumonia severe morbidity and mortality did not occur before 5–7 days after initiation of the vital outbreak [34]. Indeed, the fact that no pneumonia cases were observed during the first 5 days of the outbreak (Fig. 1) suggests a primary viral ILI.
A primary bacterial infection in the present outbreak may be also plausible for three main reasons: (1) other than S. pneumoniae, tests for a wide variety of non-pneumococcal bacterial and viral agents in cases and controls were nearly uniformly negative. Still, one cannot rule out a role of other viruses that were not tested, such as rhinovirus or coronavirus; (2) S. pneumoniae type 5 is one of the most virulent S. pneumoniae serotypes, and thus may not necessarily require a preceding or concomitant viral infection, especially in the stressful military environment. New recruits are known to be highly susceptible to most S. pneumoniae serotypes, with only 15% of recruits having protective IgG antibody to common serotypes [2], [35]. In recruit bases, increased periodic introduction of naive subjects may interfere with herd immunity and increase carriage and morbidity. Physical and psychological stressors may further lead to immune depression in these populations, thus increasing the risk of infection [36], [37]; and (3) Both ILI and pneumococcal cases ceased abruptly and immediately after initiation of the mass antibiotic treatment. Thus, the ILI cases observed may represent mild or ‘aborted’ Sp5 cases that were resolved as a result of the early antibiotic treatment.
This large outbreak serves as a reminder of the potential extent and severity of pneumococcal outbreaks even if occurring among young, generally healthy adults. Early detection and treatment of new ILI cases may have prevented their progression to pneumonia, while mass antibiotic prophylaxis combined with universal pneumococcal immunization resulted in interruption of the outbreak. Previous outbreaks were halted either using mass antibiotic prophylaxis [1], mass pneumococcal vaccination [3], [4], [15], [38], a combination of these two measures [2], [12], [20], [39] or without any intervention [16]. As is evident from the epidemiological curve (Fig. 1), dispersal of recruits home did not alter the course of the outbreak, but neither was it associated with morbidity among household contacts. We believe that the role of antibiotics in such an outbreak is important, since the response to vaccine takes at least 7–10 days to be successfully mounted, while in such outbreaks an immediate response is needed. On the other hand, logistically, the most effective approach would use long-acting drugs, since they are the easiest to administer, need less frequent dosing and can improve compliance. We therefore chose to combine antibiotic mass treatment and vaccination to achieve both immediate protection and prolonged reduction of spread of the implicated S. pneumoniae serotype. We chose the long-acting azithromycin with a known prolonged nasopharyngeal effect on carriage that can last for ≥28 days [40]. The relatively abrupt halt in the outbreak was likely due to the use of azithromycin prophylaxis (single dose of 500 mg repeated after 1 week) coupled with vaccination of recruits. Immediately following day 1 of the intervention, no new pneumonia cases occurred and overall pneumococcal carriage was reduced to <1% (a decrease that was sustained for over 1 month), confirming the effectiveness of azithromycin prophylaxis, as previously documented in some outbreaks [2], [12], [20] but not in others [18], [35]. However, when using azithromycin for mass treatment, one must remember that this long-acting drug is clearly associated with increased risk of selection of antibiotic resistance among respiratory pathogens in general and azithromycin in particular [41].
Previous studies with pneumococcal polysaccharide vaccine demonstrated that S. pneumoniae carriage rates are not affected by this vaccine [42], yet in at least one study [4], vaccination caused complete elimination of the outbreak strain (serotype 1) which like Sp5 is characterized by a short carriage episode and high virulence. Indeed in a recent pneumococcal carriage study conducted in an Israeli army training base there were no serotypes 1 and 5 among 216 pneumococcal isolates (unpublished data). Further studies on carriage and spread of pneumococci are being conducted to assess the potential need for vaccination in specific circumstances.
Conflict of interest and source of funding statements: Ron Dagan has had the following financial interests and/or arrangements with the corporate organizations listed here below in the past 2 years.
Grant/Research support: Berna/Crucell, Wyeth/Pfizer, MSD.
Scientific Consultancy: Berna/Crucell, GlaxoSmithKline, Novartis, Wyeth/Pfizer, Protea, MSD.
Speaker: Berna/Crucell, GlaxoSmithKline, Wyeth/Pfizer.
Shareholder: Protea.
References
- 1.Banerjee A., Kalghatgi A., Saiprasad G., Nagendra A., Panda B., Dham S. Outbreak of pneumococcal pneumonia among military recruits. Med J Armed Forces India. 2005;61:16–21. doi: 10.1016/S0377-1237(05)80111-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crum N.F., Wallace M.R., Lamb C.R., Conlin A.M., Amundson D.E., Olson P.E. Halting a pneumococcal pneumonia outbreak among United States Marine Corps trainees. Am J Prev Med. 2003;25:107–111. doi: 10.1016/s0749-3797(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 3.Dagan R., Gradstein S., Belmaker I., Porat N., Siton Y., Weber G. An outbreak of Streptococcus pneumoniae serotype 1 in a closed community in southern Israel. Clin Infect Dis. 2000;30:319–321. doi: 10.1086/313645. [DOI] [PubMed] [Google Scholar]
- 4.Proulx J.F., Dery S., Jette L.P., Ismael J., Libman M., De Wals P. Pneumonia epidemic caused by a virulent strain of Streptococcus pneumoniae serotype 1 in Nunavik, Quebec. Can Commun Dis Rep. 2002;28:129–131. [PubMed] [Google Scholar]
- 5.Dagan R., Givon-Lavi N., Greenberg D., Frizell B., Siegrist C. Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J Infect Dis. 2010;201:1570–1579. doi: 10.1086/652006. [DOI] [PubMed] [Google Scholar]
- 6.Enright M.C., Spratt B.G. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144:3049–3060. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 7.Black C.M., Fields P.I., Messmer T.O., Berdal B.P. Detection of Chlamydia pneumoniae in clinical specimens by polymerase chain reaction using nested primers. Eur J Clin Microbiol Infect Dis. 1994;13:752–756. doi: 10.1007/BF02276060. [DOI] [PubMed] [Google Scholar]
- 8.Klement E., Talkington D.F., Wasserzug O., Kayouf R., Davidovitch N., Dumke R. Identification of risk factors for infection in an outbreak of Mycoplasma pneumoniae respiratory tract disease. Clin Infect Dis. 2006;43:1239–1245. doi: 10.1086/508458. [DOI] [PubMed] [Google Scholar]
- 9.Heim A., Ebnet C., Harste G., Pring-Akerblom P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol. 2003;70:228–239. doi: 10.1002/jmv.10382. [DOI] [PubMed] [Google Scholar]
- 10.Poddar S.K. Influenza virus types and subtypes detection by single step single tube multiplex reverse transcription-polymerase chain reaction (RT-PCR) and agarose gel electrophoresis. J Virol Methods. 2002;99:63–70. doi: 10.1016/s0166-0934(01)00380-9. [DOI] [PubMed] [Google Scholar]
- 11.van den Hoogen B.G., van Doornum G.J., Fockens J.C., Cornelissen J.J., Beyer W.E., de Groot R. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188:1571–1577. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- 12.Hoge C.W., Reichler M.R., Dominguez E.A., Bremer J.C., Mastro T.D., Hendricks K.A. An epidemic of pneumococcal disease in an overcrowded, inadequately ventilated jail. N Engl J Med. 1994;331:643–648. doi: 10.1056/NEJM199409083311004. [DOI] [PubMed] [Google Scholar]
- 13.Gleich S., Morad Y., Echague R., Miller J.R., Kornblum J., Sampson J.S. Streptococcus pneumoniae serotype 4 outbreak in a home for the aged: report and review of recent outbreaks. Infect Control Hosp Epidemiol. 2000;21:711–717. doi: 10.1086/501717. [DOI] [PubMed] [Google Scholar]
- 14.Feikin D.R., Klugman K.P. Historical changes in pneumococcal serogroup distribution: implications for the era of pneumococcal conjugate vaccines. Clin Infect Dis. 2002;35:547–555. doi: 10.1086/341896. [DOI] [PubMed] [Google Scholar]
- 15.DeMaria A., Jr., Browne K., Berk S.L., Sherwood E.J., McCabe W.R. An outbreak of type 1 pneumococcal pneumonia in a men's shelter. JAMA. 1980;244:1446–1449. [PubMed] [Google Scholar]
- 16.Mercat A., Nguyen J., Dautzenberg B. An outbreak of pneumococcal pneumonia in two men's shelters. Chest. 1991;99:147–151. doi: 10.1378/chest.99.1.147. [DOI] [PubMed] [Google Scholar]
- 17.Gratten M., Morey F., Dixon J., Manning K., Torzillo P., Matters R. An outbreak of serotype 1 Streptococcus pneumoniae infection in central Australia. Med J Aust. 1993;158:340–342. doi: 10.5694/j.1326-5377.1993.tb121794.x. [DOI] [PubMed] [Google Scholar]
- 18.CDC Outbreak of Invasive Pneumococcal Disease—Alaska, 2003–2004. Morb Mortal Wkly Rep. 2005;54:72–75. [PubMed] [Google Scholar]
- 19.Reichler M., Reynolds R., Schwartz B., Musher D., Pratt D., Hohenhaus G. 31st interscience conference on antimicrobial agents and chemotherapy. American Society for Microbiology; Washington, DC: 1991. Epidemic of pneumococcal pneumonia in military training camp [abstract 107] [Google Scholar]
- 20.Sanchez J.L., Craig S.C., Kolavic S., Hastings D., Alsip B.J., Gray G.C. An outbreak of pneumococcal pneumonia among military personnel at high risk: control by low-dose azithromycin postexposure chemoprophylaxis. Mil Med. 2003;168:1–6. [PubMed] [Google Scholar]
- 21.Mufson M.A., Kruss D.M., Wasil R.E., Metzger W.I. Capsular types and outcome of bacteremic pneumococcal disease in the antibiotic era. Arch Intern Med. 1974;134:505–510. [PubMed] [Google Scholar]
- 22.Musher D.M., Groover J.E., Reichler M.R., Riedo F.X., Schwartz B., Watson D.A. Emergence of antibody to capsular polysaccharides of Streptococcus pneumoniae during outbreaks of pneumonia: association with nasopharyngeal colonization. Clin Infect Dis. 1997;24:441–446. doi: 10.1093/clinids/24.3.441. [DOI] [PubMed] [Google Scholar]
- 23.Romney M.G., Hull M.W., Gustafson R., Sandhu J., Champagne S., Wong T. Large community outbreak of Streptococcus pneumoniae serotype 5 invasive infection in an impoverished, urban population. Clin Infect Dis. 2008;47:768–774. doi: 10.1086/591128. [DOI] [PubMed] [Google Scholar]
- 24.Vieira A.C., Gomes M.C., Rolo Filho M., Eudes Filho J., Bello E.J., de Figueiredo R.B. Streptococcus pneumoniae: a study of strains isolated from cerebrospinal fluid. J Pediatr (Rio J) 2007;83:71–78. doi: 10.2223/JPED.1580. [DOI] [PubMed] [Google Scholar]
- 25.Shouval D.S., Greenberg D., Givon-Lavi N., Porat N., Dagan R. Site-specific disease potential of individual Streptococcus pneumoniae serotypes in pediatric invasive disease, acute otitis media and acute conjunctivitis. Pediatr Infect Dis J. 2006;25:602–607. doi: 10.1097/01.inf.0000220231.79968.f6. [DOI] [PubMed] [Google Scholar]
- 26.Brueggemann A.B., Peto T.E., Crook D.W., Butler J.C., Kristinsson K.G., Spratt B.G. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis. 2004;190:1203–1211. doi: 10.1086/423820. [DOI] [PubMed] [Google Scholar]
- 27.Jebaraj R., Cherian T., Raghupathy P., Brahmadathan K.N., Lalitha M.K., Thomas K. Nasopharyngeal colonization of infants in southern India with Streptococcus pneumoniae. Epidemiol Infect. 1999;123:383–388. doi: 10.1017/s0950268899003131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbelle N., Huebner R.E., Wasas A.D., Kimura A., Chang I., Klugman K.P. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis. 1999;180:1171–1176. doi: 10.1086/315009. [DOI] [PubMed] [Google Scholar]
- 29.Smith T., Lehmann D., Montgomery J., Gratten M., Riley I.D., Alpers M.P. Acquisition and invasiveness of different serotypes of Streptococcus pneumoniae in young children. Epidemiol Infect. 1993;111:27–39. doi: 10.1017/s0950268800056648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott J.A., Hall A.J., Hannington A., Edwards R., Mwarumba S., Lowe B. Serotype distribution and prevalence of resistance to benzylpenicillin in three representative populations of Streptococcus pneumoniae isolates from the coast of Kenya. Clin Infect Dis. 1998;27:1442–1450. doi: 10.1086/515013. [DOI] [PubMed] [Google Scholar]
- 31.Finland M., Barnes M.W. Changes in occurrence of capsular serotypes of Streptococcus pneumoniae at Boston City Hospital during selected years between 1935 and 1974. J Clin Microbiol. 1977;5:154–166. doi: 10.1128/jcm.5.2.154-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austrian R. Pneumococcal pneumonia. Diagnostic, epidemiologic, therapeutic and prophylactic considerations. Chest. 1986;90:738–743. doi: 10.1378/chest.90.5.738. [DOI] [PubMed] [Google Scholar]
- 33.Madhi S.A., Klugman K.P., The Vaccine Trialist G A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morens D.M., Taubenberger J.K., Fauci A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musher D.M., Groover J.E., Rowland J.M., Watson D.A., Struewing J.B., Baughn R.E. Antibody to capsular polysaccharides of Streptococcus pneumoniae: prevalence, persistence, and response to revaccination. Clin Infect Dis. 1993;17:66–73. doi: 10.1093/clinids/17.1.66. [DOI] [PubMed] [Google Scholar]
- 36.Gray G.C., Callahan J.D., Hawksworth A.W., Fisher C.A., Gaydos J.C. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis. 1999;5:379–385. doi: 10.3201/eid0503.990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieman D.C. Exercise and resistance to infection. Can J Physiol Pharmacol. 1998;76:573–580. doi: 10.1139/cjpp-76-5-573. [DOI] [PubMed] [Google Scholar]
- 38.Defense Medical Surveillance System. Washington DC: Army Medical Surveillance Activity, 2002. Updated March 2002 (Internet database). Available at http://amsa.army.mil/AMSA_home.htm [accessed 19.03.02].
- 39.Reido F., Schwartz B., Giono S., Hierholzer J., Ostroff S., Groover J. Pneumococcal pneumonia outbreak in a ranger training battalion, Georgia [abstract 106]. 31st interscience conference on antimicrobial agents and chemotherapy; Chicago, IL; 1991. [Google Scholar]
- 40.Dagan R., Lipsitch M., Dagan R., Lipsitch M. Changing the ecology of pneumococci with antibiotics and vaccines. In: Tuomanen E.I., Mitchell T.J., Morrison D.A., Spratt B.G., editors. The pneumococcus. ASM Press; Washington, DC: 2004. pp. 283–313. [Chapter 18] [Google Scholar]
- 41.Greenberg D., Givon-Lavi N., Sharf A.Z., Vardy D., Dagan R. The association between antibiotic use in the community and nasopharyngeal carriage of antibiotic-resistant Streptococcus pneumoniae in Bedouin children. Pediatr Infect Dis J. 2008;27:776–782. doi: 10.1097/INF.0b013e3181715184. [DOI] [PubMed] [Google Scholar]
- 42.Dagan R., Melamed R., Muallem M., Piglansky L., Greenberg D., Abramson O. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. 1996;174:1271–1278. doi: 10.1093/infdis/174.6.1271. [DOI] [PubMed] [Google Scholar]


