Abstract
Assuming a synergistic or additive effect of Chlamydiaceae in coexistence with other enteropathogenic agents, the viral/bacterial interaction between a cell culture adapted porcine epidemic diarrhea virus (ca-PEDV) and different Chlamydiaceae strains was studied in vitro. Vero cells were dually infected with ca-PEDV and one of the three chlamydial strains Chlamydia trachomatis S45, Chlamydophila abortus S26/3 or Chlamydophila pecorum 1710S. Three experimental protocols were designed varying the inoculation sequence. Cell layers were first inoculated with Chlamydiaceae and 20 h later with ca-PEDV in protocol one. In protocol two, both agents were administered concurrently, whereas in protocol three, ca-PEDV was applied 20 h in advance of the Chlamydiaceae. Immunofluorescence techniques, immunohistochemical (IH) staining and electron microscopy were subsequently employed to investigate the cell layers. Using indirect immunofluorescence (IF) labeling, all mixed infections revealed dually infected cells, however, only incidentally and in low numbers. Characteristically, ca-PEDV syncytia with one or more chlamydial inclusions were detected but dually infected single cells were absent. Some syncytial cells contained enlarged C. abortus or C. pecorum inclusions with abnormally large developmental forms. In comparison with simultaneously conducted monoinfections, larger chlamydial inclusions were observed in dually infected cell layers. Experiments with C. trachomatis showed significantly increased numbers of chlamydial inclusions in dually infected cell layers compared to monoinfected ones. These findings indicate an influence of ca-PEDV on the chlamydial developmental cycle and in the case of C. trachomatis, a positive effect on chlamydial colonization in mixed infections.
Keywords: Chlamydia trachomatis, Chlamydophila abortus, Chlamydophila pecorum, PEDV, Mixed infections, Cell culture
1. Introduction
Porcine epidemic diarrhea virus (PEDV), a coronavirus, is known to cause diarrhea in pigs, whereas Chlamydiaceae, obligate intracellular bacteria, are highly prevalent in porcine intestines but only occasionally cause enteritis. They have been found in the intestine of diarrheic and healthy pigs (Nietfeld et al., 1997, Szeredi et al., 1996, Zahn et al., 1995) and could be demonstrated in mixed enteric infections. In 1987, Pospischil and Wood (Pospischil and Wood, 1987) first reported of an association between Chlamydiaceae and lesions in the intestinal tract of pigs and assumed a synergistic effect in coexistence with Salmonella typhimurium. Chlamydiaceae have also been detected in enterocytes already infected with Eimeria scabra (Koudela et al., 1990). It was suggested that enterocytes containing developmental stages of Eimeria scabra were more susceptible to Chlamydiaceae, other bacteria and cryptosporidia.
Following those studies, the question arose whether Chlamydiaceae might play a role in the course of PEDV infections and vice versa. Until now, little in vivo and no in vitro data on dual infections with Chlamydiaceae and PEDV have been available. However, based on the work of Bernasconi et al. (1995), where gnotobiotic piglets were monoinfected with a cell culture adapted PEDV (ca-PEDV), and of Guscetti et al., 1996, Guscetti et al., 1998b, Guscetti et al., 2000, where gnotobiotic piglets were monoinfected with Chlamydiaceae, some new experiments were designed. Ca-PEDV and Chlamydophila pecorum 1710S were administered to four groups of gnotobiotic piglets varying the inoculation sequence as follows: (1) ca-PEDV inoculated at 30 h post partum (hpp), C. pecorum at 48 hpp; (2) C. pecorum at 48 hpp, ca-PEDV at 66 hpp; (3) C. pecorum and ca-PEDV at 48 hpp and (4) C. pecorum at 48 hpp, ca-PEDV at 144 hpp. An exacerbation of the clinical course by the mixed infections compared with monoinfected piglets was not clearly evident. However, in group 4, clinical signs seemed slightly more severe than in monoinfections with C. pecorum. Immunohistochemical (IH) labeling of enterocytes with a genus-specific monoclonal antibody revealed a mild infection with C. pecorum and a higher amount of chlamydial antigen in the groups 2 and 4 than in the groups 1 and 3. The amount of viral antigen detected by a ca-PEDV-specific monoclonal antibody was in the same range in the groups 1–3 and slightly lower in group 4, whereas first detection was significantly delayed in group 4. Thus, an influence of the inoculation sequence on both the course of disease as well as on the replication of each agent was suggested (Grest et al., 2000).
In this study, an in vitro model was established to investigate the interaction of ca-PEDV and Chlamydiaceae in mixed infections and to detect possible synergistic or additive effects using indirect immunofluorescence (IF), IH staining and electron microscopy.
2. Materials and methods
2.1. Media and cells
Growth medium (GM) for cell propagation was Earle's minimal essential medium (MEM) (Life Technologies, Basel, Switzerland) buffered with 20 mM HEPES (BioConcept, Allschwil, Switzerland) and supplemented with 10% fetal calf serum (FCS) (BioConcept), 600 μg ml−1 l-glutamine (Life Technologies) and 20 μg ml−1 gentamycin (BioConcept). Maintenance medium (MM) I consisted of Earle's MEM with 5% FCS, 600 μg ml−1 l-glutamine, 20 μg ml−1 gentamycin and 55 μg ml−1 glucose (Fluka, Buchs SG, Switzerland). MM II was MM I supplemented with 1 μg ml−1 cycloheximide (Sigma, Buchs SG, Switzerland).
Vero cells (African green monkey kidney cells, CRL 1587 American Type Culture Collection) were seeded on round plastic coverslips (13 mm diameter, Bibby Sterilin, Stone, UK) and cultured in GM at 37 °C until they reached confluence. Before inoculation, the cells were washed twice with phosphate buffered saline (PBS).
2.2. Chlamydial strains
Three different strains of Chlamydiaceae were used in this study: Chlamydia trachomatis S45, Chlamydophila pecorum 1710S (both kindly provided by Prof. J. Storz, Baton Rouge, Louisiana, USA) and Chlamydophila abortus S26/3 (kindly donated by Dr. G.E. Jones, Moredun Research Institute, Edinburgh, GB). They were grown in embryonated chicken eggs, and yolk sac material was harvested, diluted 1:2 in sucrose–phosphate–glutamate (SPG) medium and stored at −70 °C. The titer was determined as inclusion forming units (IFU) per ml in Vero cell cultures (Guscetti et al., 1998a, Guscetti et al., 1998b).
2.3. PEDV
Ca-PEDV strain CV777 (kindly provided by Prof. Dr. M. Ackermann, Institute of Veterinary Virology, Zurich, Switzerland) was propagated as previously described (Hofmann and Wyler, 1988). The virus (105,5 TCID50 ml−1) was diluted in MM I to obtain an infective dose of 3 × 104,5 TCID ml−1.
2.4. Inoculation of cell cultures
An overview of the experimental design is given in Fig. 1 . For each strain of Chlamydiaceae, three different experimental protocols were used. Experiments were done in duplicate, repeated once and evaluated by indirect IF 24 and 48 h after dual infection. Monoinfections with ca-PEDV and Chlamydiaceae were performed simultaneously.
Fig. 1.
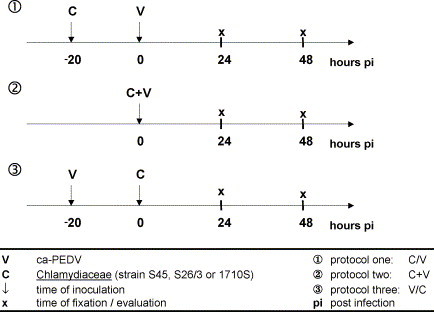
Experimental design.
2.4.1. Protocol one (C//V)
For dual infections, cell monolayers were infected with Chlamydiaceae suspension (2 × 102 IFU). For ca-PEDV-monoinfections and negative controls, a suspension of uninfected yolk sac material (YSM) and MM I was used. All coverslips were centrifuged at 2000 × g for 1 h at 37 °C and incubated for 2 h at 37 °C. The supernatant was then replaced by MM II and the cells were incubated for another 17 h at 37 °C. After a two-fold washing step with PBS, cell layers for dual infections were infected with ca-PEDV suspension (3 × 104,5 TCID50), whereas for chlamydial monoinfections, only MM I was applied. All cells were centrifuged again and incubated for 23 h. Half of the monolayers were fixed in absolute methanol (−20 °C) for 20 min, the other half was incubated again for 24 h at 37 °C after the MM I had been replaced by MM II. Fixation followed as described above.
2.4.2. Protocol two (C + V)
For dual infections, both Chlamydiaceae (2 × 102 IFU) and ca-PEDV (3 × 104,5 TCID50) were administered simultaneously; for monoinfections, cells were infected with either Chlamydiaceae suspension or ca-PEDV supplemented with uninfected YSM. All cells were centrifuged at 2000 × g for 1 h at 37 °C and incubated for 23 h at 37 °C. Half of the monolayers were fixed in absolute methanol (−20 °C) and the remaining half was treated as described in protocol one.
2.4.3. Protocol three (V//C)
For dual infections, cells were inoculated with ca-PEDV (3 × 104,5 TCID50) and for monoinfections, MM I was administered. All coverslips were centrifuged at 2000 × g for 1 h at 37 °C and incubated for 19 h. After a two-fold washing step with PBS, coverslips for dual infections were inoculated with Chlamydiaceae suspension (3 × 104,5 TCID50), whereas for ca-PEDV monoinfections, a suspension of uninfected YSM and MM I was applied. All cells were centrifuged again for 1 h and incubated for 2 h. Then the supernatant was replaced by MM II, and the coverslips were incubated for another 21 h. Fixation and further incubation followed as described in protocol one.
2.5. Indirect immunofluorescence
Indirect IF labeling of cell cultures was performed immediately after fixation. For viral antigen detection, a mouse monoclonal antibody against the M protein of PEDV (mcAb 204, kindly provided by Prof. Dr. M. Ackermann, Institute of Veterinary Virology, Zurich, Switzerland), diluted 1:4 in PBS supplemented with BSA and TBS, and an Alexa Fluor 488-conjugated secondary antibody (rabbit anti-mouse, 1:500, Molecular Probes, Eugene, USA) were used. C. trachomatis S45 inclusions were labeled by a polyclonal goat anti-C. trachomatis-MOMP antibody (1:100, Chemicon International, Temecula, USA) and a Cy3-conjugated rabbit anti-goat secondary antibody (1:500, Zymed Laboratories, San Francisco, USA). For detection of C. abortus S26/10 and C. pecorum 1710S antigen, a primary rabbit anti-chlamydial lipopolysaccharide antibody (1:50, Biodesign International, Kennebunk, USA) and a secondary Cy3-conjugated goat anti-rabbit antibody (1:50, Zymed Laboratories) were used. All staining procedures were conducted at room temperature. Antibody incubations were carried out for 1 h (primary antibodies) or 45 min (secondary antibodies), respectively, with three washes of PBS following fixation, between and after applications of different antisera. Dually infected cell layers were stained using sequential double immunofluorescence labeling. Coverslips were mounted in Immu-Mount (Shandon, Pittsburgh, USA) on glass slides and viewed with a Leica fluorescence microscope (DMBL). Photographs were taken using a 40× PL Fluotar oil objective, an Olympus OM-2N camera and Fuji Velvia ASA 50 film.
2.6. Immunohistochemistry
Indirect single and double IH staining was performed on dually infected cell pellets as a control for successful infection before they were prepared for transmission electron microscopy. The formalin fixed pellets were processed using the Cytoblock cell block preparation system (Shandon, Pittsburgh, USA) and embedded in paraffin.
The presence of chlamydial lipopolysaccharide (LPS) in paraffin sections was investigated by using a genus-specific monoclonal antibody (clone AC-I, Progen, Heidelberg, Germany), which had been previously tested and described (Bernasconi et al., 1995, Guscetti et al., 1998a, Guscetti et al., 1998b), and an indirect streptavidin-biotin method (ChemMate Detection Kit [Peroxidase/AEC], DAKO Diagnostics AG, Zug, Switzerland). Primary antibodies were diluted 1:200 in ChemMate Antibody Diluent (DAKO Diagnostics AG). Microwave pretreatment (900 W) of paraffin slides was performed in buffer solution (ChemMate Buffer for Antigen Retrieval, DAKO Diagnostics AG) for 10 min.
For detection of the PEDV M-protein, a monoclonal antibody (mcAb204, Veterinary Virology, Zurich, Switzerland) and a two-step visualization system (EnVision + [HRP/DAB], DAKO Diagnostics AG) were used. Primary antibodies were diluted 1:10 in ChemMate Antibody Diluent (DAKO Diagnostics AG). Microwave pretreatment (900 W) of paraffin slides was performed in buffer solution (ChemMate Buffer for Antigen Retrieval, DAKO Diagnostics AG) for 10 min.
For simultaneous detection of Chlamydiaceae and PEDV antigen, the previously described monoclonal antibodies and a double immunoenzymatic staining kit (EnVision Doublestain System [HRP/DAB+; AP/Fast Red], DAKO Diagnostics AG) were used. Microwave pretreatment (900 W) of paraffin slides was performed in buffer solution (ChemMate Buffer for Antigen Retrieval, DAKO Diagnostics AG) for 10 min.
2.7. Transmission electron microscopy
Cell pellets from dually infected Vero cells were fixed in glutaraldehyde (Electron Microscopy Sciences, Ft. Washington, USA) for 1–2 h, centrifuged for 10 min at 700 × g and processed by routine methods for embedding in epoxy resin (Fluka). Appropriate areas for ultrastructural investigation were selected using semithin sections (1 μm) stained with toluidine blue (Fluka). Ultrathin sections (80 nm) were mounted on copper grids (Merck Eurolab AG, Dietlikon, Switzerland), contrasted with uranyl acetate dihydrate (Fluka) and lead citrate (lead nitrate and tri-natrium dihydrate; Merck Eurolab AG) and investigated in an electron microscope (Philips CM10).
2.8. Statistical analysis
To determine whether numbers of chlamydial inclusions or ca-PEDV syncytia differed between mono- and dually infected cell layers, the chi-square test and the Wilcoxon signed-rank test were used. In the chi-square one group test, for each experiment, numbers of chlamydial inclusions, respectively ca-PEDV syncytia, in monoinfected cell layers and their corresponding numbers in dually infected cell layers at 24 h pi, respectively, 48 h pi, were compared assuming equal proportions. For the Wilcoxon signed-rank test, pairs were formed by numbers of chlamydial inclusions, respectively ca-PEDV syncytia, in monoinfected cell layers and their corresponding numbers in dually infected cell layers at 24 h pi, respectively, 48 h pi. Pairs of all three protocols (24 and 48 h pi) were regarded as one dataset.
3. Results
3.1. Indirect immunofluorescence
IF labeling was used to address the question of whether host cells could be dually infected with Chlamydiaceae and ca-PEDV and to investigate whether significant differences in morphology and number of the two agents between monoinfected and dually infected monolayers were present.
3.1.1. C. trachomatis S45
IF microscopy revealed intracytoplasmic, mainly round to ovoid, sharply outlined inclusions with brilliant, red fluorescence, which corresponded well with previous descriptions (Mårdh et al., 1989). In all three protocols, inclusions grew larger in dually infected cell layers than in monoinfected ones. The largest inclusions were found in protocol one (C//V) in dually infected cell layers at 48 h pi, where an absolute incubation period of 68 h for C. trachomatis has to be considered. Inclusion numbers (Fig. 2 ) varied from 174 to 304 for protocol one (C//V), from 32 to 407 for protocol two (C + V) and from 49 to 70 for protocol three (V//C). Using the chi-square test, significantly higher numbers of chlamydial inclusions in dually infected cell layers compared with monoinfected ones were seen in both experiments of protocol two (C + V) at 24 and 48 h pi (p = 0.001). Given the experimental conditions of that protocol, C. trachomatis S45 inclusions were still very small in monoinfected cell layers after an absolute incubation time of 24 h. As a fact, inclusions smaller than a fifth of the host cell nucleus often were not clearly distinguishable from unspecific staining reactions. Therefore, counting of inclusions was difficult and, to a certain extent, inaccurate in monoinfected cell layers at 24 h pi. Consequently, these results were excluded from the Wilcoxon signed-rank test. Nevertheless, a p-value of 0.0051 was found. Variations in inclusion numbers also occurred between the different protocols. Inclusions were seen in much lower numbers in protocol three (V//C) than in the other two protocols.
Fig. 2.
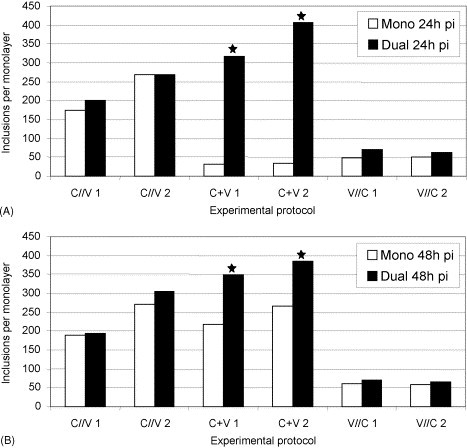
Number of C. trachomatis S45 inclusions (mono- and mixed infections [ca-PEDV]) at 24 h pi (A) and at 48 h pi (B). Statistically significant differences between mono- and dual infections are indicated (*): (A) and (B) p = 0.001; chi-square test.
Ca-PEDV positive single cells showed brilliant, distinct, green–yellow, cytoplasmic fluorescence. Cytopathogenic effects caused by PEDV in Vero cells are characterized by fusion of cell membranes and syncytium formation (Hofmann and Wyler, 1988). Syncytia showed an accumulation of nuclei in the center or the periphery and moderate to bright, fine-granular, cytoplasmic staining. Over an incubation period of 24–48 h (and 68 h in protocol three [V//C]), syncytia formed, grew and detached from cell layers in all three protocols. Morphologically, no differences were seen between mono- and dually infected cell layers or between the protocols. Numbers of ca-PEDV syncytia varied from 30 to 89 for protocol one (C//V), from 33 to 73 for protocol two (C + V) and from 23 to 73 for protocol three (V//C; Fig. 3 ). The chi-square test revealed a significantly increased number of ca-PEDV syncytia in dually infected cell layers in protocol one (C//V; p = 0.031). However, at 24 h pi, this only applied to experiment two, whereas at 48 h pi, both experiments showed significantly higher numbers of ca-PEDV syncytia in dually infected monolayers (p 1 = 0.035, p 2 = 0.02).
Fig. 3.
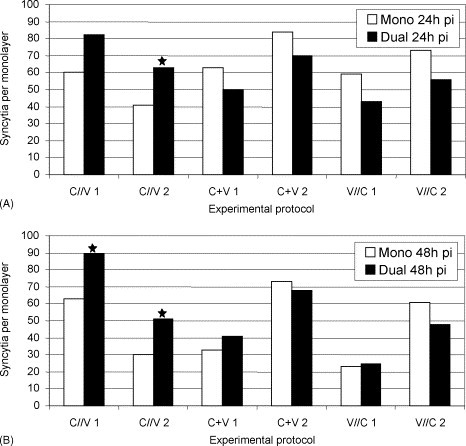
Number of ca-PEDV syncytia (mono- and mixed infections [C. trachomatis S45]) at 24 h pi (A) and at 48 h pi (B). Statistically significant differences between mono- and dual infections are indicated (*): (A) p = 0.031 and (B) p1 = 0.035, p2 = 0.02; chi-square test.
Syncytial cells with C. trachomatis S45 inclusions were found in all three protocols. Numbers were very low (0–6) and no pattern was detectable.
3.1.2. C. abortus S26/3
Inclusions formed by C. abortus S26/3 were round–oval to pleomorphic as previously described (Spears and Storz, 1979, Schiller and Pospischil, 1998). In all the three protocols, inclusions grew larger in dually infected than in monoinfected cell layers, and the largest inclusions were observed in protocol one (C//V) in dually infected cell layers at 48 h pi. Inclusion numbers varied from 185 to 322 for protocol one (C//V), from 63 to 509 for protocol two (C + V) and from 167 to 484 for protocol three (V//C; Fig. 4 ). In the chi-square test, significantly increased inclusion numbers in dually infected cell layers compared with monoinfected ones were seen in protocol two (C + V; p = 0.001). At 48 h pi, the p-value was only slightly below 0.05 in experiment one, and no significant difference was seen in experiment two.
Fig. 4.
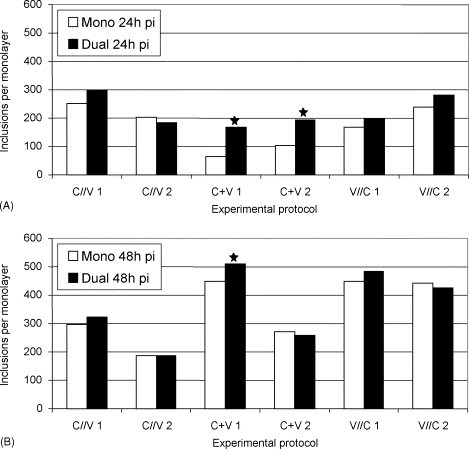
Number of C. abortus S26/3 inclusions (mono- and mixed infections [ca-PEDV]) at 24 h pi (A) and at 48 h pi (B). Statistically significant differences between mono- and dual infections are indicated (*): (A) p = 0.001 and (B) p = 0.049; chi-square test.
Ca-PEDV positive single cells, formation of ca-PEDV syncytia, fusion of syncytial cells and detachment from monolayers were seen in all the three protocols. No morphological differences were observed between mono- and dually infected cell layers or between the protocols. Numbers of ca-PEDV syncytia varied from 35 to 349 for protocol one (C//V), from 163 to 309 for protocol two (C + V) and from 55 to 180 for protocol three (V//C; Fig. 5 ). There were no significant quantitative differences between mono- and dually infected cell layers. In experiment one of the protocols two (C + V) and three (V//C), as well as in experiment two of the protocols one (C//V) and two (C + V), numbers of syncytial cells at 48 h pi were lower than at 24 h pi.
Fig. 5.
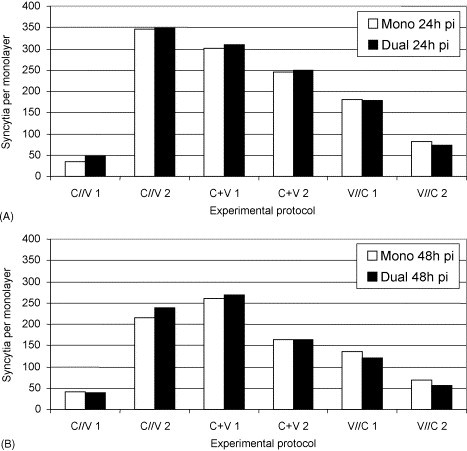
Number of ca-PEDV syncytia (mono- and mixed infections [C. abortus S26/3]) at 24 h pi (A) and at 48 h pi (B).
Syncytial cells with C. abortus S26/3 inclusions were found in all the three protocols. Numbers were low (0–11) and no pattern was detectable. Some remarkably large inclusions were observed in syncytial cells of protocol one (C//V). They revealed enlarged developmental bodies.
3.1.3. C. pecorum 1710S
Morphologically, inclusions formed by C. pecorum 1710S were pleomorphic and often showed a lobular structure as described by Kaltenboeck and Storz (1992). They were larger in dually than in monoinfected cell layers in all three protocols. A considerable amount of inclusions releasing elementary bodies (EBs) were seen, especially in the protocols one (C//V) and two (C + V). Inclusion numbers varied from 339 to 436 for protocol one (C//V), from 353 to 411 for protocol two (C + V) and from 407 to 568 for protocol three (V//C; Fig. 6 ). No significant quantitative differences between mono- and dually infected cell layers were found in any of the three protocols.
Fig. 6.
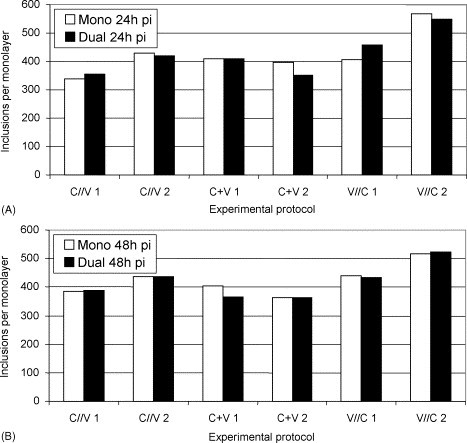
Number of C. pecorum 1710S inclusions (mono- and mixed infections [ca-PEDV]) at 24 h pi (A) and at 48 h pi (B).
Ca-PEDV positive single cells, formation of ca-PEDV syncytia, fusions of syncytial cells and detachment were seen in all the protocols without detecting morphological differences between mono- and dually infected cell layers or between the protocols. Numbers of ca-PEDV syncytia varied from 146 to 288 for protocol one (C//V), from 314 to 619 for protocol two (C + V) and from 113 to 601 for protocol three (V//C; Fig. 7 ). There were no significant quantitative differences between mono- and dually infected cell layers. However, in most experiments, numbers of syncytial cells at 48 h pi were lower than at 24 h pi.
Fig. 7.
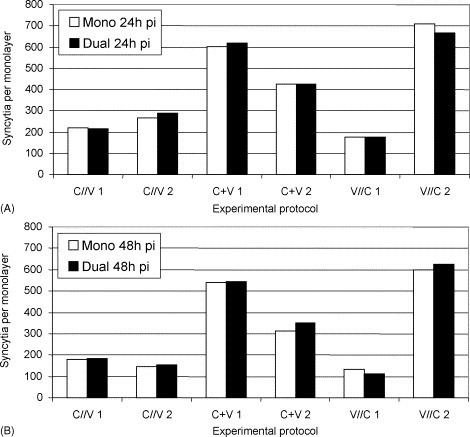
Number of ca-PEDV syncytia (mono- and mixed infections [C. pecorum 1710S]) at 24 h pi (A) and at 48 h pi (B).
Syncytial cells with C. pecorum 1710S inclusions were observed in low to moderate numbers (2–45; see Fig. 8 ) in all the three protocols. Furthermore, they revealed syncytial cells with several inclusions. Most of them were associated with chlamydial dispersion centers (e.g. inclusions that had been formed by EBs shortly released by the same primary inclusion (see Fig. 8).
Fig. 8.
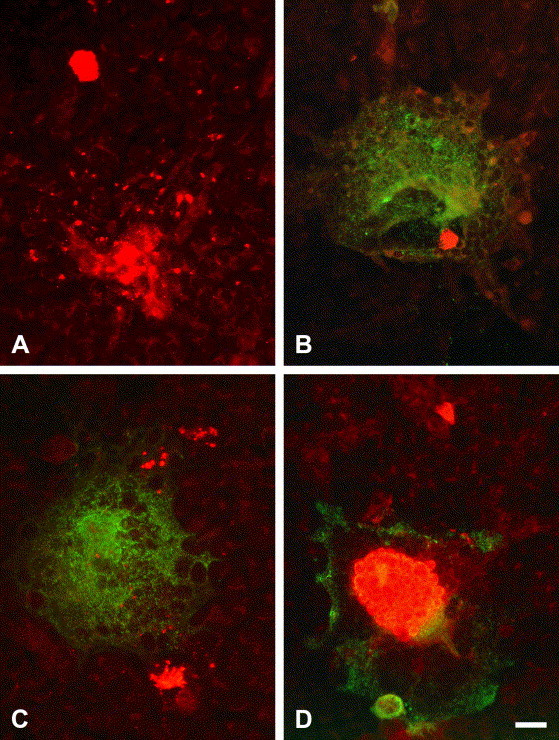
Fluorescence micrographs of chlamydial inclusions (red) releasing elementary bodies (A, C) and of viral syncytial cells (green) with chlamydial inclusions (B–D). Vero cells were monoinfected with C. pecorum 1710S (A) and dually infected with C. pecorum 1710S and ca-PEDV (B–D); 48 h pi. (A) Intact chlamydial inclusion (top) and inclusion releasing EBs (bottom). (B) Ca-PEDV syncytium with chlamydial inclusion. (C) Viral syncytial cell with two chlamydial inclusions releasing EBs nearby; infection of syncytial cell with C. pecorum 1710S in second round of chlamydial cycle. (D). Syncytial cell with large inclusion showing atypically enlarged developmental bodies. Magnification 40×. Bar 20 μm.
Additionally, remarkably large inclusions within syncytial cells (see Fig. 8) were present in all the protocols. These inclusions showed abnormal, enlarged developmental forms resembling those described by Kaltenboeck and Storz (1992).
3.2. Immunohistochemistry
IH staining of paraffin sections from dually infected cell pellets was performed to detect the presence and intensity of chlamydial and viral infection before pellets were processed for electron microscopy (see Fig. 9 ). Cell layers were often disrupted into several patches and the largest ones (approximately 10–15 mm2) were evaluated. Single staining revealed red (AEC), finely granular, round to oval or pleomorphic, sharply delimited C. trachomatis S45 and C. pecorum 1710S inclusions which were variable in size. Ca-PEDV positive single and syncytial cells showed red–brown (DAB), intracytoplasmic granules of variable size.
Fig. 9.
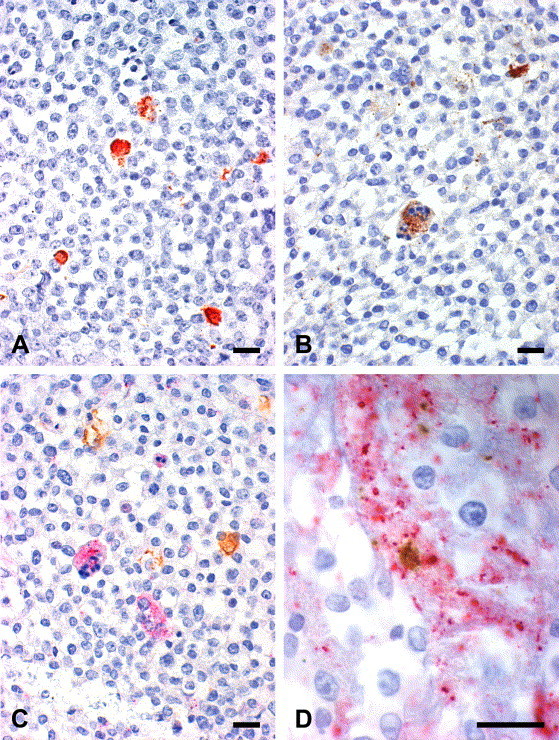
Immunohistochemical labeling of chlamydial inclusions (A, C), ca-PEDV single and syncytial cells (B) as well as viral syncytial cell with chlamydial inclusion (D). Vero cells were dually infected with C. trachomatis S45 and ca-PEDV and pelleted. Paraffin sections. Immunoperoxidase stain. (A) Chlamydial inclusions; red (AEC). (B) Ca-PEDV syncytial cell with apoptosis (bottom) and single cells (top); brown (DAB). (C) Chlamydial inclusions (brown, DAB+) and syncytial cells (left one showing apoptosis; pink, Fast Red). (D) Syncytial cell (pink) with chlamydial inclusion (brown). Magnifications: 40× (A, B, C), 100× (D). Bars 20 μm.
In double staining, labeled C. trachomatis S45 and C. pecorum 1710S inclusions were brown (DAB+), ca-PEDV single and syncytial cells bright pink (Fast Red). Ca-PEDV syncytial cells with chlamydial inclusions were detected in low numbers.
3.3. Transmission electron microscopy
Forty copper grids were examined. Intracytoplasmic C. trachomatis S45 and C. pecorum 1710S inclusions were found in small numbers (5–10 per grid). In general, they were lined by a membrane and contained various numbers of EBs, reticulate and intermediate bodies.
Small numbers of syncytial cells (2–5) were observed. Virus particles were seen in the lumina of vacuoles and occasionally in the cytoplasm of syncytial cells. They showed typical coronavirus morphology (Pensaert and Debouck, 1978, Cheville, 1994). They were round–oval to pleomorphic and measured between 50 and 130 nm. The inner structure consisted of an electron-dense core with a central electron-opaque halo and was surrounded by a corona. The individual spikes forming the corona were not visible.
Dually infected cells were not detectable in the ultrathin sections.
4. Discussion
Cell cultures provided an appropriate mean to establish dual infections under specific experimental conditions and allowed for direct comparison of monoinfections with dual infections. Although in vitro replication of PEDV has been described to be trypsin dependent (Hofmann and Wyler, 1988), in this study, all experiments were conducted without adding trypsin to the maintenance media due to its known effect to reduce or inhibit attachment of chlamydial EBs to host cells (Gutiérrez-Martin et al., 1997, Moulder, 1991, Su et al., 1990). Nevertheless, propagation of ca-PEDV was evident. Possibly, the ca-PEDV strain used had become better adapted after many cell passages and, therefore, did not require trypsin for propagation.
We could demonstrate that Vero cells can be dually infected with Chlamydiaceae and ca-PEDV, whereas in previous in vivo experiments, no dually infected cells were present (Grest, personal communication). However, no single cells but only ca-PEDV syncytia with chlamydial inclusions were detectable. Dual infections appeared to be occasional and incidental events, which implies that Chlamydiaceae and ca-PEDV do not preferentially infect the same cell. Chlamydiaceae infected cells do not seem to be a main target of ca-PEDV or more susceptible than non-infected ones and vice versa. The lack of evidence of dually infected cells in electron microscopy reflected the results obtained by IF and immunohistochemistry.
A remarkable finding was that inclusions of all three chlamydial strains were larger in dually infected cell layers than in monoinfected ones in all protocols. This observation might suggest a viral influence in mixed infections with Chlamydiaceae. However, whether the assumed effect of ca-PEDV is a positive or negative one remains to be determined. Thus far, no correlation between the increased inclusion size and the number of inclusions has been evident. The enlarged chlamydial forms of C. abortus S26/3 and C. pecorum 1710S found only in syncytial cells further support the theory of viral interaction. A direct or indirect effect of ca-PEDV on the chlamydial developmental cycle seems possible, and a continued chlamydial growth without proper division and differentiation may be assumed. Kaltenboeck and Storz (1992) had suggested that strain 1710S was highly nutrient dependent and that this could elicit aberrant forms. Ca-PEDV might interfere with the host cell metabolism and either alter or inhibit production of essential nutrients for Chlamydiaceae or induce certain cytokines, such as interferon gamma, with inhibitory effects on chlamydial development (Moulder, 1991). Beatty et al., 1993, Beatty et al., 1994, Beatty et al., 1995 conducted several in vitro studies focused on interferon gamma-mediated persistent infections with Chlamydiaceae. Those studies also revealed enlarged, abnormal chlamydial forms. In ultrastructural analysis of enterocytes infected with PEDV, decreased energy-producing capacity and functional deficiencies as well as reduced numbers of cell organelles had been described (Ducatelle et al., 1982, Pospischil et al., 1981). However, it remains unclear which mechanisms were involved in the cell culture systems and what role experimental conditions might have played. Future investigations could include measurement of cell metabolites and cytokines as well as specific variation of physical and chemical cell culture conditions.
Lower numbers of ca-PEDV syncytia at 48 h pi than at 24 h pi can be explained by fusion of two or several syncytia as well as detachment from the cell layer after the syncytia had reached a certain size and were consequently washed away during the staining procedure.
Mixed infections with C. trachomatis S45 showed a tendency to higher inclusion numbers in dually infected cell layers than in monoinfected ones, whereas this was not evident with C. abortus S26/3 or C. pecorum 1710S. The three chlamydial strains possibly do not react alike in mixed infections as differences in their pathogenic potential had already been shown in monoinfections with gnotobiotic piglets (Guscetti, 1999). C. trachomatis S45 might have profited from the viral presence, especially in protocol two (C + V).
In contrast to mixed infections with C. pecorum and ca-PEDV in vivo (Grest et al., 2000), no dependency on the inoculation sequence was observed in vitro. Furthermore, the amount of C. pecorum antigen did not apparently vary between mono- and dually infected cell layers, whereas IH labeling of enterocytes generally revealed less chlamydial antigen in dually infected piglets compared with monoinfected ones (Grest, personal communication). A possible inhibition of C. pecorum by ca-PEDV assumed in vivo could not be demonstrated in cell cultures. However, in vivo and in vitro results were not entirely comparable as limitations of cell culture assays did not allow simulation of experimental conditions of group 4 in vivo. In that group, C. pecorum 1710S had been applied well in advance (96 h) of ca-PEDV, and the increased time span between the two agents seemed to slightly enhance chlamydial infection and to possibly have an inhibitory effect on ca-PEDV. IH detection of ca-PEDV in the groups 1–3 in vivo corresponded well with results of this study where no obvious differences were found between the protocols.
Further work in vitro will be needed to improve the understanding of interactions between Chlamydiaceae and PEDV and to develop a more complete picture of their pathogenic role in mixed infections.
Acknowledgements
The authors would like to express their appreciation to Ms. A. Schifferli and the laboratory staff at the Institute of Veterinary Pathology, Zurich, for technical assistance and to thank the Institute of Veterinary Virology, Zurich, for providing the virus and antibodies.
References
- Bernasconi C., Guscetti F., Utiger A., Van Reeth K., Ackermann M., Pospischil A. Experimental infection of gnotobiotic piglets with a cell culture adapted porcine epidemic diarrhea virus: clinical, histopathological and immunohistological findings. Proceedings of the Third Congress of the European Society of Veterinary Virology, Interlaken; Switzerland; 1995. pp. 542–546. [Google Scholar]
- Beatty W.L., Byrne G.I., Morrison R.P. Morphologic and antigenic characterization on interferon γ-mediated persistent Chlamydia trachomatis infection in vitro. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty W.L., Morrison R.P., Byrne G.I. Persistent Chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol. Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty W.L., Morrison R.P., Byrne G.I. Reactivation of persistent Chlamydia trachomatis infection in cell culture. Infect. Immun. 1995;63:199–205. doi: 10.1128/iai.63.1.199-205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheville N.F. Ultrastructural Pathology: An Introduction to Interpretation. first ed. Iowa State University Press; Ames, Iowa: 1994. Cytopathology of viral diseases. pp. 545–548. [Google Scholar]
- Ducatelle R., Coussement W., Debouck P., Hoorens J. Pathology of experimental CV777 coronavirus enteritis in piglets Part II. Electron microscopy study. Vet. Pathol. 1982;19:57–66. doi: 10.1177/030098588201900109. [DOI] [PubMed] [Google Scholar]
- Grest P., Guscetti F., Sydler T., Pospischil A. Experimental mixed enteric infection of gnotobiotic piglets with Chlamydia and coronavirus: effect of inoculation sequence. Proceedings of the Fourth Meeting of the European Society for Chlamydia Research, Helsinki; Finland; 2000. p. 263. [Google Scholar]
- Guscetti F. Institute of Veterinary Pathology Zurich, University of Zurich; Switzerland: 1999. Chlamydia in Swine: Field Studies and Experimental Infections in Gnotobiotic Piglets, Habilitation. [Google Scholar]
- Guscetti F., Schiller I., Hoop R., Sydler T., Pospischil A. Experimental infection of gnotobiotic piglets with porcine Chlamydia: patterns of replication in the gut and of fecal excretion. Proceedings of the Third Meeting of the European Society for Chlamydia Research, Vienna; Austria; 1996. p. 123. [Google Scholar]
- Guscetti F., Bernasconi C., Tobler K., Van Reeth K., Pospischil A., Ackermann M. Immunohistochemical detection of porcine epidemic diarrhea virus compared to other methods. Clin. Diagn. Lab. Immunol. 1998;5:412–414. doi: 10.1128/cdli.5.3.412-414.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guscetti F., Schiller I., Sydler T., Corboz L., Pospischil A. Experimental Chlamydia psittaci serotype 1 enteric infection in gnotobiotic piglets: histopathological, immunohistochemical and microbiological findings. Vet. Microbiol. 1998;62:251–263. doi: 10.1016/s0378-1135(98)00221-1. [DOI] [PubMed] [Google Scholar]
- Guscetti F., Hoop R., Schiller I., Corboz L., Sydler T., Pospischil A. Experimental enteric infection of gnotobiotic piglets with a Chlamydia psittaci strain of avian origin. J. Vet. Med. B. 2000;47:561–572. doi: 10.1046/j.1439-0450.2000.00385.x. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Martin C.B., Ojcius D.M., Hsia R., Hellio R., Bavoil P.M., Dautry-Varsat A. Heparin-mediated inhibition of Chlamydia psittaci adherence to HeLa cells. Microb. Pathog. 1997;22:47–57. doi: 10.1006/mpat.1996.0090. [DOI] [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J. Clin. Microbiol. 1988;26:2235–2239. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenboeck B., Storz J. Biological properties and genetic analysis of the ompA locus in chlamydiae isolated from swine. Am. J. Vet. Res. 1992;53:1482–1487. [PubMed] [Google Scholar]
- Koudela B., Vitovec J., Sterba J. Concurrent infection of enterocytes with Eimeria scabra and other enteropathogens in swine. Vet. Parasitol. 1990;35:71–77. doi: 10.1016/0304-4017(90)90117-t. [DOI] [PubMed] [Google Scholar]
- Mårdh P.A., Paavonen J., Puolakkainen M. Diagnosis of Chlamydial infections. In: Mårdh P.A., Paavonen J., Puolakkainen M., editors. Chlamydia. Plenum Medical Book Co.; New York: 1989. pp. 71–99. [Google Scholar]
- Moulder J.W. Interaction of Chlamydiae and host cells in vitro. Microbiol. Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietfeld J.C., Leslie-Steen P., Zeman D.H., Nelson D. Prevalence of intestinal chlamydial infection in pigs in the midwest, as determined by immunoperoxidase staining. Am. J. Vet. Res. 1997;58:260–264. [PubMed] [Google Scholar]
- Pensaert M.B., Debouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospischil A., Hess R.G., Bachmann P.A. Light microscopy and ultrahistology of intestinal changes in pigs infected with epizootic diarrhoea virus (EVD): comparison with transmissible gastroenteritis (TGE) virus and porcine rotavirus infections. Zbl. Vet. Med. B. 1981;28:564–577. doi: 10.1111/j.1439-0450.1981.tb01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospischil A., Wood R.L. Intestinal Chlamydia in pigs. Vet. Pathol. 1987;24:568–570. doi: 10.1177/030098588702400617. [DOI] [PubMed] [Google Scholar]
- Schiller I., Pospischil A. Comparison of growth characteristics of porcine, ovine and avian chlamydial strains in different cell culture systems. Proceedings of the Sixteenth Meeting of the European Society of Veterinary Pathology, Lillehammer; Norway; 1998. p. 125. [Google Scholar]
- Spears P., Storz J. Biotyping of Chlamydia psittaci based on inclusion morphology and response to diethylaminoethyl-dextran and cycloheximide. Infect. Immun. 1979;24:224–232. doi: 10.1128/iai.24.1.224-232.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Watkins N.G., Zhang Y.X., Caldwell H.D. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect. Immun. 1990;58:1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeredi L., Schiller I., Sydler T., Guscetti F., Heinen E., Corboz L., Eggenberger E., Jones G.E., Pospischil A. Intestinal Chlamydia in finishing pigs. Vet. Pathol. 1996;33:369–374. doi: 10.1177/030098589603300401. [DOI] [PubMed] [Google Scholar]
- Zahn I., Szeredi L., Schiller I., Straumann Kunz U., Buergi E., Guscetti F., Heinen E., Corboz L., Sydler T., Pospischil A. Immunhistologischer Nachweis von Chlamydia psittaci/pecorum und C. trachomatis in Ferkel-Darm. J. Vet. Med. B. 1995;42:266–276. [PubMed] [Google Scholar]


