Abstract
Objective
To evaluate the efficacy and safety of caffeine citrate in the treatment of apnea in bronchiolitis.
Study design
Eligible infants aged ≤4 months presenting to the main pediatric emergency service with apnea associated bronchiolitis were stratified by gestational age (<34 weeks or longer) and randomized to receive a single dose of intravenous 25 mg/kg caffeine citrate or saline placebo. The primary efficacy outcome was a 24-hour apnea-free period beginning after completion of the blinded study drug infusion. Secondary outcomes were frequency of apnea by 24, 48, and 72 hours after study medication, need for noninvasive/invasive ventilation, and length of stay in the hospital's pediatric intensive care/step-down unit.
Results
A total of 90 infants diagnosed with viral bronchiolitis associated with apnea (median age, 38 days) were enrolled. The rate of respiratory virus panel positivity was similar in the 2 groups (78% for the placebo group vs 84% for the caffeine group). The geometric mean duration to a 24-hour apnea-free period was 28.1 hours (95% CI, 25.6-32.3 hours) for the caffeine group and 29.1 hours (95% CI, 25.7-32.9 hours) for the placebo group (P = .88; OR, 0.99; 95% CI, 0.83-1.17). The frequency of apnea at 24 hours, 24-48 hours, and 48-72 hours after enrollment and the need for noninvasive and invasive ventilation were similar in the 2 groups. No safety issues were reported.
Conclusions
A single dose of caffeine citrate did not significantly reduce apnea episodes associated with bronchiolitis.
Trial registration
Keywords: respiratory syncytial virus, caffeine citrate, apnea, bronchiolitis
Abbreviation: PICU, Pediatric intensive care unit
See editorial, p 11
Viral bronchiolitis is the most common lower respiratory tract infection in infants, leading to 15 hospitalizations per 1000 person-years. Of these, 1.6% to 4% are admitted with apnea.1, 2, 3, 4 Bronchiolitis-associated apnea, a subset of apnea associated with respiratory viruses, appears to be a mixed central and obstructive apnea resulting from a complex interplay of respiratory drive suppression and airway secretions driven by a hyperactive laryngochemoreflex, somnogenic cytokines, and in some cases by virus-specific respiratory suppressant surface proteins.5, 6, 7
Caffeine is a standard treatment for apnea of prematurity8, 9 and postextubation apnea in preterm infants,10 and has been used to ameliorate apnea complicating bronchiolitis on the basis of case reports and observational studies.11, 12, 13, 14, 15, 16, 17 Caffeine increases central respiratory drive and chemoreceptor sensitivity to CO2 and improves skeletal muscle contractions, reducing diaphragm fatigue and leading to better ventilation.18 Increased metabolic demand, diuresis, tachycardia, dysrhythmias, feeding intolerance, reduced weight gain, and seizures are the reported short-term side effect of caffeine.19 Although evidence from prospective studies for caffeine used to treat apnea in bronchiolitis is lacking, the drug is commonly used in an attempt to avoid intubation and is considered a standard of care in some institutions.11, 17, 20 Thus, we compared blinded intravenous caffeine citrate with placebo for shortening the time to resolution of acute bronchiolitis-associated apnea.
Methods
This study involved a double-blind, randomized, parallel-group clinical trial of single-dose intravenous caffeine vs normal saline for the treatment of apnea in acute bronchiolitis (Clinicaltrials.gov: NCT01435486). The study was conducted during 3 bronchiolitis seasons in the infirmary/observation unit of the Pediatric Emergency Center of Hamad General Hospital, the sole pediatric emergency facility in the State of Qatar. The center serves an average of 280 000 outpatients annually and manages 45 beds in the infirmary/observation unit. All inpatient services are provided except intensive care. Patients with apnea and bronchiolitis are usually admitted to the pediatric intensive care unit (PICU) or a step-down unit for further observation and treatment. Patients who require invasive respiratory support, continuous positive airway pressure, and/or biphasic positive airway pressure are always admitted to the PICU, and other patients can be admitted to either of the 2 areas based on bed availability.
Infants aged ≤4 months presenting with a provisional diagnosis of viral bronchiolitis associated with apnea were eligible for this study. This provisional diagnosis required apnea preceded by a prodromal history consistent with viral upper respiratory tract infection, with physical findings of bilateral chest crackles possibly associated with cough, rapid breathing, wheezing, and/or intercostal retractions. Out-of-hospital apnea was defined as witnessed sudden cessation of breathing associated with cyanosis and hypotonia within 6 hours before presentation, as reported by the caregiver. Apnea in the pediatric emergency center was defined as witnessed sudden cessation of breathing for >20 seconds or prolonged respiratory pause associated with cyanosis and/or bradycardia, as observed by medical staff.21, 22 Bradycardia was defined as heart rate <90 beats/minute. Patients were excluded who had 1 or more of the following characteristics: previous diagnosis of seizure disorder, gastroesophageal reflux disorder, suspected sepsis, previous history of renal or liver disease, known inborn error of metabolism, congenital heart disease or major congenital anomaly of the upper or lower respiratory tract, hypoglycemia or electrolyte abnormality on presentation, or receipt of caffeine treatment at home. Written informed consent was sought from a parent or legal guardian for each consecutive eligible patient as soon as the patient was admitted to the resuscitation room or short-stay unit. The study was approved by the hospital's Institutional Review Board.
Potentially eligible patients were examined on presentation in the emergency center, and those with an outpatient history of apnea were admitted to the infirmary/observation unit. All patients with observed apnea were admitted to the critical care areas for treatment. Patients were assessed for study eligibility within 30 minutes of the initial physician assessment, and after stabilization and enrollment were transferred to either the step-down unit or the PICU, based on bed availability and the need for invasive/noninvasive respiratory support.
All patients were connected to a cardiorespiratory monitor (MP70; Philips Healthcare, Andover, Massachusetts) in the emergency center and throughout their PICU/step-down unit stay, and to a portable cardiorespiratory monitor (MP30; Philips Healthcare) during transfer, set up to alarm outside the following values: respiratory rate, 20 to 60 breaths/minute, with a default apnea alarm delay of 20 seconds; pulse rate, 90 to 180 beats/minute; oxygen saturation, 90% to 100%. Caregivers did not wait for a monitor alarm, which served a safety backup function in most instances, before intervening with patients. Recording each episode of apnea witnessed with or without monitor alarm was required immediately after patient stabilization, on the data collection sheet and PICU/step-down diary of apnea record. Data on the occurrence of apnea were collected from patient records and data collection sheets daily.
Patients for whom consent was obtained underwent plain chest radiography and nasopharyngeal swabs for a rapid respiratory virus panel capable of identifying 20 respiratory viruses (multiplex real-time PCR assay on an ABI 7500 analyzer; Thermo Fisher Scientific, Waltham, Massachusetts). Randomization was stratified into 2 groups, patients born at <34 weeks gestation and those born at ≥34 weeks gestation.
The unblinded study pharmacist used a computer-generated randomization list23 to prepare identical-looking numbered syringes containing either 15 mL of 0.9% sodium chloride or 25 mg/kg caffeine citrate diluted in D5W to make 15 mL of solution, to be infused intravenously by a syringe pump over 30 minutes. Inhaled therapies, supplemental oxygen, respiratory support, hydration, and other interventions were provided at the discretion of the treating physician. In 72 patients, venous blood gas analysis was requested by the treating physician, and samples were obtained within 30 minutes of arrival. All venous blood gas samples were obtained from an inserted intravenous infusion cannula in 1 of the 4 limbs from a free-flowing vein, collected in a microtainer tube at 500 μL. Samples were placed on ice immediately after extraction and sent to the department satellite laboratory for processing within 10 to 15 minutes.
A patient was transferred from the PICU/step-down unit to an ordinary inpatient pediatric bed when the treating physician determined that she or he was clinically stable, had no apnea in the preceding 24 hours, and was tolerating the start of feeding. The actual time of transfer could be delayed owing to lack of bed availability.
Study Outcomes
The primary efficacy outcome was time until a 24-hour apnea-free period beginning after completion of blinded study drug infusion. This is expressed as “time until last apnea episode” in our trial registration. An earlier proposed outcome, length of stay in the PICU, was changed before the start of study enrollment because it can be dependent on social factors and bed availability, unlike time until last apnea episode, which is measured objectively. Patients with a major protocol violation, such as receipt of caffeine in the placebo group or receipt of a second dose, were excluded in this proof-of-concept efficacy trial. Secondary outcomes were the frequency and duration of apnea in the first 24, 48, and 72 hours after study drug administration; invasive and noninvasive respiratory support administered; duration of oxygen therapy; time until feeding was tolerated; length of PICU/step-down unit stay; and overall length of hospital stay. As a safety measure, spot heart rate was compared every 4 hours from enrollment for up to 3 days.
Statistical Analyses
To estimate sample size, we performed a retrospective chart review of all patients admitted with apnea-associated bronchiolitis in 2010. A total of 87 patients who had not received caffeine were identified, of whom 52 (60%) were apnea-free at 12 hours after admission. We extrapolated these results to 24 hours because we did not have data for the proportion apnea-free at 24 hours. To enable detection of a 50% relative improvement (to 90%) in resolution of apnea with 90% power and 2-sided α value of 0.05, we estimated that 42 patients per group were required. To compensate for dropouts, we planned to recruit a total of 90 patients. However, the accuracy of this estimate is compromised because we used a dichotomous outcome for the calculation and the continuous outcome described above for our actual study outcome.
In 2013, we reported that “in 2011, we saw 8718 infants and young children in 10 666 visits for bronchiolitis,”24 of whom 30% were aged 4 months or younger. Between 1% and 2% of our patients with bronchiolitis present with apnea, a similar proportion as reported in the current literature.25, 26 We estimated that we would need 3 bronchiolitis seasons to complete this trial if we enrolled 50% of patients presenting with bronchiolitis-associated apnea.
Categorical and continuous data values are expressed as frequency (percentage) and mean ± SD or median and IQR, as appropriate. Descriptive statistics were used to summarize demographic, laboratory, and clinical characteristics of the patients. The Kolmogorov-Smirnov test or Q-Q plot as appropriate was used to test for normality.
Baseline participant characteristics in the 2 treatment groups (caffeine and placebo) were compared using an unpaired t test for continuous variables and the χ2 test for categorical variables. Accelerated failure time analysis27 was applied to compare geometric mean times for the treatment groups reaching the primary outcome, a 24-hour apnea-free interval beginning after the completion of study drug infusion. The proportion of patients with apnea resolution at different time points was compared using the χ2 test and Fisher exact test, as appropriate. Quantitative variable means between the 2 independent groups were compared using an unpaired t test or the Mann-Whitney U test, as appropriate, depending on the results of the normality test. Associations between 2 or more qualitative and categorical variables were assessed by using the χ2 test and Fisher exact test, as appropriate. The relationship between 2 continuous variables was examined using the Pearson correlation coefficients. The log-rank test was applied to determine any statistical difference in actual time of discharge from critical care unit between the 2 treatment groups. An exploratory subgroup analysis for the primary and secondary outcomes in patients with corrected age ≤28 days, in whom caffeine is reported to have a prolonged half-life, was performed post hoc.
Potential risk factors for further apnea and confounders were examined with univariate and multivariable logistic regression models using further apnea episode (yes or no) as an outcome variable and birth weight, respiratory syncytial virus positive/negative status, gestational age at birth, staff-observed apnea before study entry, nonobserved apnea before study entry, 2 or more apneic episodes at home, any positive pressure including high-flow oxygen, body temperature on arrival, chest radiography findings, and venous blood gas analysis results obtained within 30 minutes of arrival (the lattermost variable available for 72 patients) as independent variables or covariates. For multivariable regression models, variables were considered if statistically significant at the P < .10 level in the univariate analysis or if deemed clinically important a priori. Logistic regression results are presented as OR along with a corresponding 95% CI. All tests performed were 2-sided, and a P value <.05 considered statistically significant. Statistical analyses were performed using SPSS 21.0 (IBM, Armonk, New York). Data were transferred from the SPSS package to Stata SE 13.1 (StataCorp, College Station, Texas) for accelerated failure time model analysis.
Results
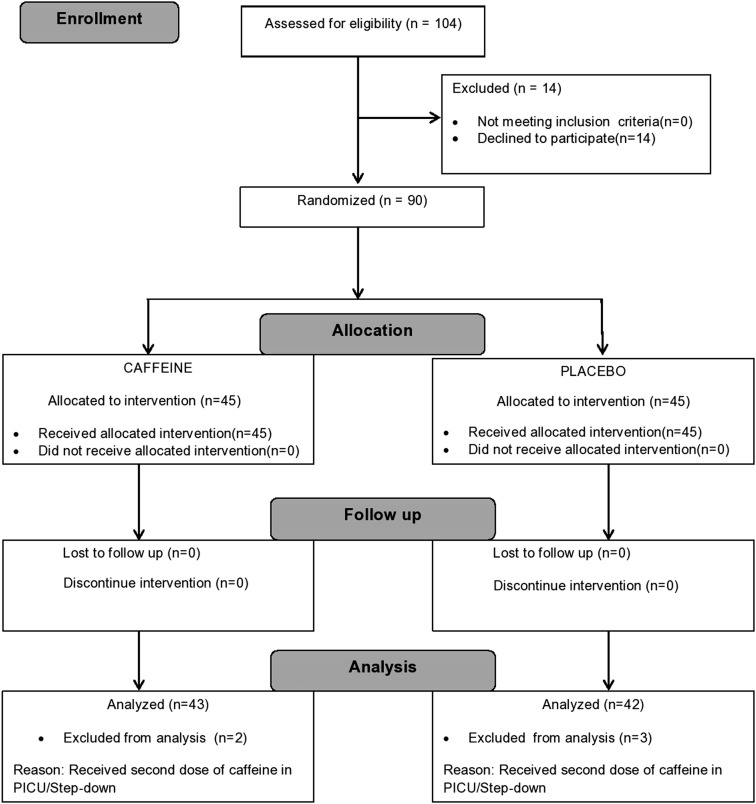
A total of 104 infants were assessed for enrollment between November 2011 and May 2014; of these, 90 infants diagnosed with viral bronchiolitis-associated apnea (median age, 38 days; IQR, 3 to 152 days) were enrolled in the study. This was an explanatory proof-of principle randomized trial rather than a pragmatic test of caffeine effectiveness in usual practice; thus, 5 infants (2 in the caffeine group and 3 in the placebo group) were excluded from analysis because each was given an extra dose of caffeine after admission to the PICU (Figure 1; available at www.jpeds.com). Of the remaining 85 infants, 43 were randomized to receive caffeine citrate and 42 were randomized to receive placebo. The subjects' baseline characteristics were similar in the 2 treatment arms, except for a significantly different mean duration of upper respiratory symptoms before enrollment (caffeine group, 3 ± 2 days; placebo group, 5 ± 4 days) (Table I ). Among these 85 patients, 81 had a discharge diagnosis of bronchiolitis. In the placebo group, 2 patients were diagnosed with gastroesophageal reflux disease. In the caffeine group, 1 patient was diagnosed with sepsis and 1 patient was diagnosed with bronchiolitis with myopathy.
Figure 1.
Study flow chart of infants presenting with bronchiolitis and apnea.
Table I.
Baseline characteristics of enrolled infants
| Characteristics | Caffeine group (n = 45) | Placebo group (n = 45) |
|---|---|---|
| Postnatal age, d, mean (SD) | 50 (35) | 52 (34) |
| Gestational age at birth, wk, mean (SD) | 35 (4) | 36 (3) |
| Birth at <37 wk gestation, n (%) | 21 (47) | 23 (51) |
| Gestational age at birth, in preterm infants, wk, mean (SD) | 32 (4) | 33 (3) |
| Gestational age at birth <34 wk, n (%) | 13 (29) | 11 (24) |
| Males/females, n | 25/20 | 21/24 |
| Birth weight, kg, mean (SD) | 2 (0.1) | 3 (0.1) |
| Weight at presentation, kg, mean (SD) | 4 (1) | 4 (1) |
| Baseline oxygen saturation, %, mean (SD) | 96 (5) | 97 (3) |
| Upper respiratory symptoms at presentation, n (%) | 45 (100) | 45 (100) |
| Duration of upper respiratory symptoms before enrollment, d, mean (SD) | 3 (2) | 5 (4) |
| Fever at presentation, n (%) | 9 (20) | 11 (24) |
| Difficulty breathing at presentation, n (%) | 32 (71) | 37 (82) |
| Physical findings, n (%) | ||
| Wheezing | 23 (51) | 27 (60) |
| Crackles | 37 (82) | 38 (84) |
| Retractions | 25 (56) | 30 (67) |
| Grunting | 14 (31) | 13 (29) |
| Apnea at home before study therapy, n (%) | 31 (69) | 35 (78) |
| Frequency of apnea episodes at home, mean (SD) | 2.5 (3) | 2 (1) |
| Duration of apnea episodes at home, s, mean (SD) | 105 (92) | 103 (95) |
| Action taken at home before study therapy, n % | ||
| No action required | 15 (33) | 12 (27) |
| Physical stimulation | 29 (64) | 31 (69) |
| Mouth-to-mouth breathing | 1 (2) | 2 (4) |
| Apneic episodes in ED before study therapy, n (%) | 28 (62) | 30 (67) |
| Frequency of apneic episodes in ED before study therapy, mean (SD) | 2 (1) | 2 (1) |
| Cyanosis in ED before study therapy, n (%) | 26 (58) | 28 (62) |
| Bradycardia in ED before study therapy, n (%) | 15 (33) | 20 (44) |
| Duration of apnea episodes in ED before study therapy, s, mean (SD) | 41 (27) | 33 (20) |
| Action taken in ED before study therapy, n (%) | ||
| Oxygen | 21 (47) | 21 (47) |
| Physical stimulation | 5 (11) | 2 (4) |
| Bag-mask ventilation | 2 (4) | 7 (16) |
| Positive PCR in nasopharyngeal swab, n (%) | 38 (84) | 35 (78) |
| Adenovirus | 0 (0) | 1 (2) |
| Bocavirus | 6 (13) | 0 (0) |
| Coronavirus | 8 (17) | 5 (11) |
| Human metpneumovirus | 1 (2) | 2 (4) |
| Influenza | 2 (4) | 1 (2) |
| Parainfluenza virus | 2 (4) | 0 (0) |
| Rhinovirus | 10 (22) | 12 (27) |
| Respiratory syncytial virus | 11 (24) | 14 (31) |
| Multiple viruses | 11 (24) | 12 (27) |
| Chest radiography findings, n (%) | ||
| Normal | 12 (27) | 14 (31) |
| Lobar consolidation/collapse | 16 (36) | 15 (33) |
| Increased bronchovascular marking | 17 (38) | 16 (36) |
| Need for crystalloid bolus on presentation for hypoperfusion, n (%) | 8 (18) | 13 (29) |
ED, emergency department; PCR, polymerase chain reaction.
Baseline characteristics were similar in the 2 treatment groups except for a longer duration of upper respiratory symptoms in the placebo group compared with the caffeine group (mean, 5 ± 4 days vs 3 ± 2 days).
Efficacy
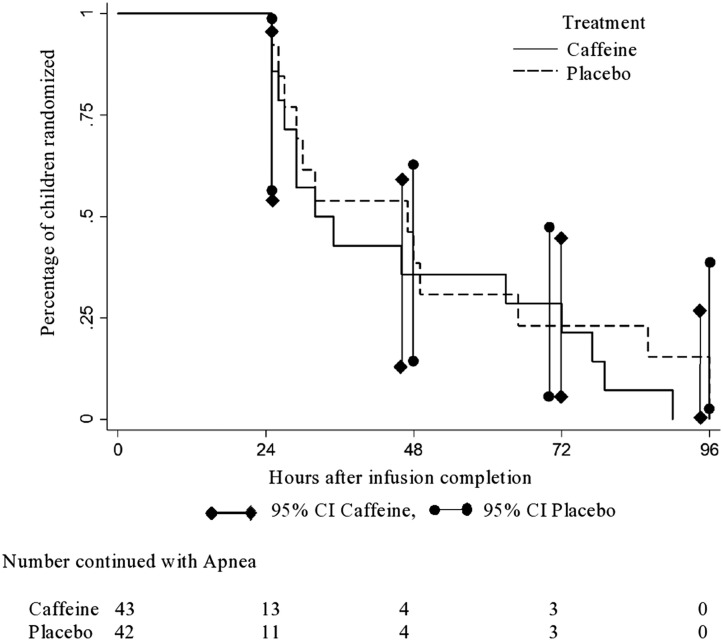
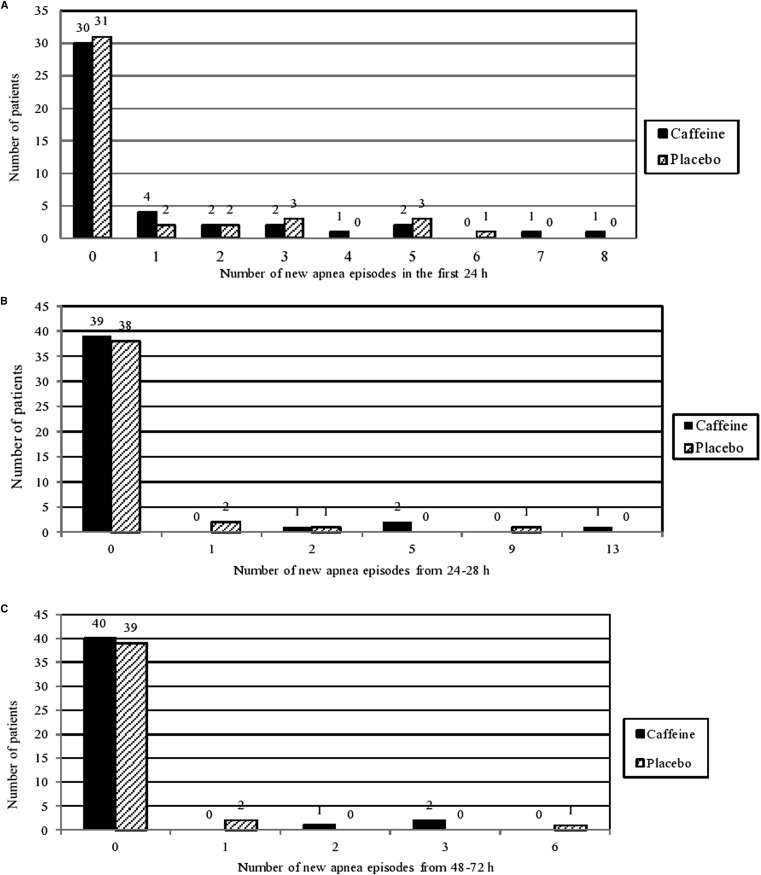
The interval from study drug infusion to the end of a 24-hour apnea-free period was similar in the 2 groups, with a geometric mean duration of 28.7 hours (95% CI, 25.6-32.3 hours) for the caffeine group and 29.1 hours (95% CI, 25.7-32.9 hours) for the placebo group (OR, 0.99; 95% CI, 0.83-1.17; P = .88) (Figure 2 ). The frequency of apnea in the first 24, 48, and 72 hours was similar in the 2 groups (Figure 3; available at www.jpeds.com). The caffeine group had significantly longer mean and median length of stay in PICU/step-down units (mean difference, 68.2 hours [P = .04]; median difference, 17.0 hours), but, as noted above, bed availability and social issues may impact those durations. The mean duration of apnea episodes was significantly shorter in the caffeine group, by 5.7 seconds (P = .003), a difference of limited clinical importance. Other secondary outcomes did not differ between the 2 groups (Table II ).
Figure 2.
Time from the end of study drug infusion to a 24-hour apnea-free period. Accelerated failure time analysis shows a geometric mean time for the caffeine group 28.7 hours (95% CI, 25.6-32.3 hours) and 29.1 hours (95% CI, 25.7-32.9 hours) for the placebo group (OR, 0.99; 95% CI, 0.83-1.17; P = .88).
Figure 3.
Number of new apnea episodes after blinded treatment. A, First 24 hours. B, 24-48 hours. C, 48-72 hours.
Table II.
Secondary outcomes
| Outcomes | Caffeine group (n = 43) | Placebo group (n = 42) | Mean difference | 95% CI | P value |
|---|---|---|---|---|---|
| Apnea episodes after enrollment, n (%) | 14 (32.6) | 13 (31.0) | - | - | .87 |
| Frequency of apnea episodes after enrollment, mean (SD) | 7 (5) | 6 (7) | 1.0 | −3.8 to 6.0 | .65 |
| Duration of apnea episodes after enrollment, s, mean (SD) | 19.6 (11.3) | 25.3 (13.8) | 5.7 | 1.9 to 9.6 | .003 |
| Need for oxygen support after enrollment, n (%) | 38 (88.4) | 33 (78.6) | - | - | .22 |
| Duration of oxygen support, h, mean (SD) | 127.2 (121.0) | 85.7 (62.1) | 41.4 | −5.1 to 88.1 | .08 |
| Use of high-flow oxygen after enrollment, n (%) | 15 (34.9) | 13 (31.0) | - | - | .70 |
| Duration of high-flow oxygen, h, mean (SD) | 18.5 (13.7) | 24.7 (16.2) | −6.1 | −17.8 to 5.4 | .28 |
| Need for noninvasive respiratory support besides high-flow oxygen, n (%) | 6 (13.9) | 3 (7.1) | - | - | .31 |
| Noninvasive respiratory support other than high-flow oxygen, n (%) | |||||
| Nasopharyngeal tube | 1 (2.3) | 0 (0) | |||
| Continuous positive airway pressure | 2 (4.7) | 3 (7.1) | - | - | .54 |
| Duration of noninvasive respiratory support besides high-flow oxygen, h, mean (SD) | 47.6 (20.5) | 44.8 (19.9) | 2.8 | −10.8 to 16.4 | .67 |
| Need for invasive respiratory support (intubation) after enrollment, n (%) | 7 (16.3) | 3 (7.2) | - | - | .30 |
| Duration of invasive respiratory support, h, mean (SD) | 46.8 (23.3) | 44.5 (19.0) | 2.3 | −46.2 to 50.8 | .9 |
| Time until feeding was tolerated, h, mean (SD) [median (IQR)] | 41.3 (66.4)[13.2 (0.1-303)] | 22.7 (29.9) [12 (0.1-142)] | 18.6 | −3.7 to 40.9 | .46 |
| Bronchiolitis treatment received after enrollment, n (%) | |||||
| Salbutamol nebulization | 23 (53.5) | 32 (76.2) | |||
| Epinephrine nebulization | 5 (11.6) | 4 (9.5) | - | - | .56 |
| 3% hypertonic saline | 5 (11.6) | 10 (23.8) | |||
| Other medications, n (%) | |||||
| Antibiotics | 32 (74.4) | 30 (71.4) | .75 | ||
| Length of stay in critical care unit, h, mean (SD) [median (IQR)]∗ | 126.4 (143.1) [64.1 (4.3-593.5)] | 58.2 (50.6) [47.1 (3.3-265.4)] | 68.2 | 21.6 to 114.7 | .04 |
| Total length of hospital stay, h, mean (SD) [median (IQR)] | 205.2 (158.9) [165.5 (5-710)] | 140.4 (101.7) [112.7 (15.5-442.3)] | 64.7 | 7.1 to 122.2 | .07 |
Range from minimum length of stay in critical care unit to the maximum length of stay.
Subgroup Analysis of Patients of Corrected Age ≤28 Days
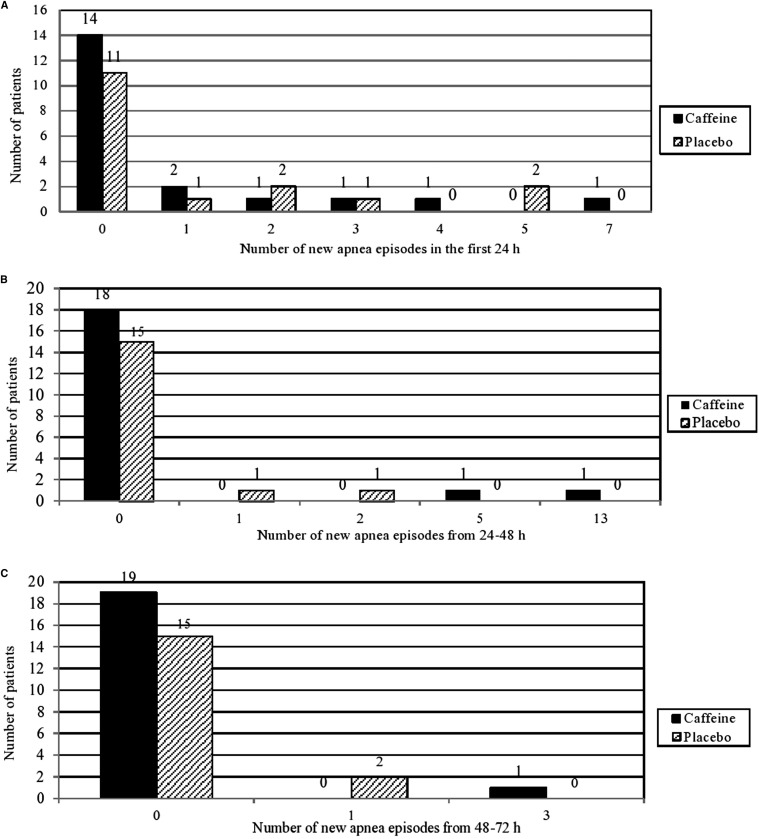
We examined the resolution of apnea in this subgroup of infants, in whom the half-life of caffeine is prolonged.28 Clear caffeine efficacy in this subgroup would support a hypothesis that repeated caffeine dosing (rather than the single large dose that we gave) might be efficacious. The subgroup comprised 37 patients, 17 from the placebo group and 20 from the caffeine group. Baseline characteristics were similar in the caffeine and placebo groups (data not shown). The geometric mean duration until a 24-hour apnea-free period was 28.0 hours (95% CI, 23.6-33.07 hours) for the caffeine group and 32.8 hours (95% CI, 25.5-42.2 hours) for the placebo group (OR, 0.85; 95% CI, 0.64-1.31; P = .26). Moreover, the frequency of apnea in the first 24, 24-48, and 48-72 hours was again similar in the 2 groups (Figure 4; available at www.jpeds.com).
Figure 4.
Number of new apnea episodes after blinded treatment in infants of corrected age ≤28 days. A, First 24 hours. B, 24-48 hours. C, 48-72 hours.
Risk Factors Associated with Continuing Apnea Symptoms
We analyzed risk factors associated with repeated apneas7, 26, 29, 30 in univariate and multivariable models (Table III ). In the multivariable analysis, the need for high-flow oxygen or positive pressure support (OR, 9.98; P = .016) and venous PO2 ≥40 mmHg within 30 minutes after presentation to the emergency center (OR, 29.44; P = .008) were the significant variables associated with a higher likelihood of further apnea after admission.
Table III.
Univariate and multivariate risk factor analysis for further apnea after randomized study treatment
| Characteristics | No further apnea (n = 58) | Further apnea (n = 27) | Univariate risk factor analysis |
Multivariate risk factor analysis |
||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |||
| Age, corrected for gestational age <37 wk | ||||||||
| ≤8 wk:>8 wk | 45:13 | 21:6 | 1.01 | 0.33-3.02 | .98 | |||
| >8 wk | 1.00 | Reference | ||||||
| Birth weight | ||||||||
| ≤2.0 kg:>2.0 kg | 12:46 | 12:15 | 3.07 | 1.14-8.25 | .02 | 1.82 | 0.21-15.55 | .58 |
| >2.0 kg | 1.00 | Reference | 1.00 | Reference | ||||
| Gestational age at birth | ||||||||
| <34 wk:≥34 wk | 9:49 | 12:15 | 4.35 | 1.54-12.32 | .006 | 0.44 | 0.05-3.59 | .44 |
| ≥34 wk | 1.00 | Reference | 1.00 | Reference | ||||
| Sex | ||||||||
| Female:male | 27:31 | 14:13 | 1.23 | 0.49-3.08 | .64 | |||
| Male | 1.00 | Reference | ||||||
| RSV-positive:RSV-negative | 14:44 | 5:22 | 0.71 | 0.22-2.23 | .56 | |||
| RSV-negative | 1.00 | Reference | ||||||
| ≥2 home apneas:1 home apnea:no home apnea | 19:27:12 | 13:4:10 | 0.82∗ | 0.27-2.45 | .72 | 0.95 | 0.19-4.62 | .95 |
| 1 home apnea | 0.17 | 0.04-0.68 | .012 | 0.28 | 0.03-2.21 | .23 | ||
| No home apnea | 1.00 | Reference | 1.00 | Reference | ||||
| Staff-observed apnea before study entry:no staff-observed apnea before study entry | 32:26 | 23:4 | 4.67 | 1.43-15.22 | .011 | 5.37 | 0.84-34.27 | .07 |
| Apnea not observed by staff before study entry | 1.00 | Reference | 1.00 | Reference | ||||
| Temperature | ||||||||
| <38°C:≥38°C | 50:8 | 25:2 | 0.50 | 0.09-2.53 | .40 | |||
| ≥38°C | 1.00 | Reference | ||||||
| Any positive pressure including high-flow oxygen (1-10 L):no positive pressure | 32:26 | 25:2 | 10.15 | 2.19-46.92 | .003 | 9.98 | 1.54-64.79 | .016 |
| No positive pressure | 1.00 | Reference | 1.00 | Reference | ||||
| Chest radiography | ||||||||
| No lobar consolidation/collapse:lobar consolidation/collapse |
38:20 | 18:9 | 0.95 | 0.36-2.49 | .91 | |||
| Lobar consolidation/collapse | 1.00 | Reference | ||||||
| pH | ||||||||
| <7.25:≥7.25 | 9:39 | 9:15 | 0.38 | 0.12-1.15 | .08 | 0.63 | 0.14-2.82 | .54 |
| ≥7.25 | 1.00 | Reference | 1.00 | Reference | ||||
| PCO2 | ||||||||
| <50:≥50 | 21:27 | 9:15 | 1.29 | 0.47-3.53 | .61 | |||
| ≥50 | 1.00 | Reference | ||||||
| PvO2 | ||||||||
| <40:≥40 | 13:35 | 1:23 | 8.54 | 1.04-69.8 | .04 | 29.44 | 2.43-356.04 | .008 |
| ≥40 | 1.00 | Reference | 1.00 | Reference | ||||
PvO2, venous PO2; RSV, respiratory syncytial virus.
Multivariate risk factors were analyzed if univariate analysis showed P < .10 or were considered clinically important a priori.
0.82 refers to OR for further apnea in children with ≥2 home apneas.
Safety
Recorded spot heart rates for both groups were similar at all times except 52 and 56 hours, at which heart rate was significantly higher in the caffeine group compared with the placebo group (mean increase at both time points, 7; 95% CI, 0-15; P = .05 for both). No patient experienced rhabdomyolysis, arrhythmia, cardiovascular collapse, or death.
Discussion
Most of the patients with apnea-associated bronchiolitis had complete resolution of apnea within 24 hours, consistent with recently reported data.26 A single dose of intravenous caffeine citrate was not superior to normal saline in any of the outcomes measured in the overall treatment groups or the subgroups of patients aged ≤28 days, in whom caffeine would be expected to have a longer half-life.
The currently available evidence for caffeine use in bronchiolitis-related apnea is observational, from case reports including 7 patients and 3 retrospective studies including 60 patients. Three case reports suggested a decreased need for intubation and mechanical ventilation after caffeine administration, but 1 report found no benefit.13, 14, 15, 16 One retrospective study showed a decrease in apnea attacks at 2 hours after caffeine administration,17 another found a decreased need for intubation in a caffeine group compared with a non-caffeine group, and a third reported inconclusive findings and called for further research.17, 18, 31
Our results show no difference in the frequency of apneas reported for the 2 groups in the first 3 days. Our findings do not support the routine use of caffeine to treat apnea in bronchiolitis, especially in light of the reported serious side effects of the drug.19 We believe that our findings are robust because we applied strict enrollment criteria in a randomized blinded trial, with patients undergoing continuous cardiorespiratory monitoring in PICU/step-down units for a median length of stay of 55 hours with a ≤1:2 nurse:patient.
We also explored previously described risk factors for apnea7, 26, 29, 30 in addition to those that we deemed relevant to our study patients. In multivariable analysis, we found patients with staff-witnessed apnea on presentation were more likely to experience further apneic episodes compared with patients with apneas witnessed at home (OR, 5.37), as has been reported previously,29 but the difference was not significant (P = .07). Apnea was associated with a venous PO2 level ≥40 mmHg obtained within 30 minutes of arrival regardless of the use or nonuse of oxygen therapy, and with the need for high-flow oxygen and/or positive-pressure respiratory support.
Our study has some limitations. Our power to find a significant difference in the time from the end of study drug infusion to a 24-hour apnea-free period was limited because our sample size was calculated based on a retrospective survey in which a 12-hour rather than a 24-hour apnea-free period was used because the time to a 24-hour apnea-free period after admission was unknown. Moreover, our sample size estimate was based on a dichotomous outcome, apnea-free or not at 24 hours, whereas in this clinical trial we used a continuous outcome, time from the end of study drug infusion to a 24-hour apnea-free period. Although we found no benefit from caffeine, our sample size estimation method might have led to a study underpowered to identify any possible measurable benefit. Thus, a larger sample size might have shown a more pronounced difference between treatment groups in time to achieve the 24-hour apnea-free outcome.
Administration of a higher dose of caffeine than what we used in the present study has been described previously.31 Given the acute respiratory illness and its associated increase in metabolic rate in our study population, we chose instead to test more than double the recommended dose for apnea of prematurity (10 mg/kg caffeine citrate),28 which is higher than the dosage for apnea in bronchiolitis used in previous studies.15, 16, 17 Our study was designed to administer a single dose of caffeine rather than daily dosing, based on the practice of previous studies,11, 15, 16, 17 and our finding of a high proportion of apnea resolution in the retrospective data that we collected. Nonetheless, our post hoc subgroup analysis did not show any superiority of caffeine in younger infants, in whom the expected half-life of caffeine is 72-96 hours.28 Our randomization did not yield entirely similar groups, with a shorter duration of symptoms before presentation, and thus possibly more severe apnea syndromes, in the group treated with caffeine. Finally, 30% of the patients in each study arm required high-flow oxygen, which may provide nearly similar support as continuous positive airway pressure32 and might have masked further apnea attacks in these patients.
Acknowledgments
We thank the patients and their families, our study and clinical staff, and consulting biostatistician, Dr Rajvir Singh (funded by Hamad Medical Corporation), for his thoughtful guidance in biostatistical analysis.
Footnotes
Supported by Hamad Medical Corporation (11146/11). The authors declare no conflicts of interest.
Appendix.
References
- 1.Koehoorn M., Karr C.J., Demers P.A., Lencar C., Tamburic L., Brauer M. Descriptive epidemiological features of bronchiolitis in a population-based cohort. Pediatrics. 2008;122:1196–1203. doi: 10.1542/peds.2007-2231. [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa K., Tsugawa Y., Brown D.F., Mansbach J.M., Camargo C.A., Jr. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132:28–36. doi: 10.1542/peds.2012-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jafri H.S., Wu X., Makari D., Henrickson K.J. Distribution of respiratory syncytial virus subtypes A and B among infants presenting to the emergency department with lower respiratory tract infection or apnea. Pediatr Infect Dis J. 2013;32:335–340. doi: 10.1097/INF.0b013e318282603a. [DOI] [PubMed] [Google Scholar]
- 4.Staat M.A., Henrickson K., Elhefni H., Groothuis J., Makari D. Prevalence of respiratory syncytial virus-associated lower respiratory infection and apnea in infants presenting to the emergency department. Pediatr Infect Dis J. 2013;32:911–914. doi: 10.1097/INF.0b013e31828df3e3. [DOI] [PubMed] [Google Scholar]
- 5.Pappano D.A., Bass E.S. Respiratory virus-associated apnea. Pediatr Emerg Med Rep. 2007;12:1–12. [Google Scholar]
- 6.Raza M.W., Blackwell C.C. Sudden infant death syndrome, virus infections and cytokines. FEMS Immunol Med Microbiol. 1999;25:85–96. doi: 10.1111/j.1574-695X.1999.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 7.Schiller O., Levy I., Pollak U., Kadmon G., Nahum E., Schonfeld T. Central apnoeas in infants with bronchiolitis admitted to the paediatric intensive care unit. Acta Paediatr. 2011;100:216–219. doi: 10.1111/j.1651-2227.2010.02004.x. [DOI] [PubMed] [Google Scholar]
- 8.Henderson-Smart D.J., De Paoli A.G. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst Rev. 2010;(12):CD000140. doi: 10.1002/14651858.CD000140.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Schoen K., Yu T., Stockmann C., Spigarelli M.G., Sherwin C.M. Use of methylxanthine therapies for the treatment and prevention of apnea of prematurity. Paediatr Drugs. 2014;16:169–177. doi: 10.1007/s40272-013-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson-Smart D.J., Davis P.G. Prophylactic methylxanthines for endotracheal extubation in preterm infants. Cochrane Database Syst Rev. 2010;(12):CD000139. doi: 10.1002/14651858.CD000139.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Sajit N.T., Steggall M., Padmakumar B. Apnoeas in bronchiolitis: is there a role for caffeine? Arch Dis Child. 2005;90:438. doi: 10.1136/adc.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston D.M., Kuzemko J.A. Virus-induced apnoea and theophylline. Lancet. 1992;340:1352. doi: 10.1016/0140-6736(92)92534-m. [DOI] [PubMed] [Google Scholar]
- 13.Pappano D.A. Failure of caffeine to ameliorate respiratory syncytial virus-induced apnea. Open Emerg Med J. 2010;3:1–2. [Google Scholar]
- 14.DeBuse P., Cartwright D. Respiratory syncytial virus with apnea treated with theophylline. Med J Aust. 1979;2:307–308. [PubMed] [Google Scholar]
- 15.Tobias J.D. Caffeine in the treatment of apnea associated with respiratory syncytial virus infection in neonates and infants. South Med J. 2000;93:294–296. [PubMed] [Google Scholar]
- 16.Duffett M., Liu D.M., Deshpande A., Choong K. Caffeine in bronchiolitis associated apnea: a retrospective cohort study. J Pediatr Intensive Care. 2012;2:95–98. doi: 10.3233/PIC-2012-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cesar K., Iolster T., White D., Latifi S. Caffeine as treatment for bronchiolitis-related apnoea. J Paediatr Child Health. 2012;48:619. doi: 10.1111/j.1440-1754.2012.02497.x. [DOI] [PubMed] [Google Scholar]
- 18.Natarajan G., Lulic-Botica M.L., Aranda J.V. Pharmacology review: clinical pharmacology of caffeine in the newborn. Neo Rev. 2007;8:e214–e221. [Google Scholar]
- 19.Atkinson E., Fenton A.C. Management of apnoea and bradycardia in neonates. Paediatr Child Health. 2009;19:550–554. [Google Scholar]
- 20.Al-balkhi A., Klonin H., Marinaki K., Southall D.P., Thomas D.A., Jones P. Review of treatment of bronchiolitis related apnoea in two centres. Arch Dis Child. 2005;90:288–291. doi: 10.1136/adc.2003.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institutes of Health Consensus development summaries. Infantile apnea and home monitoring. Conn Med. 1987;51:45–51. [PubMed] [Google Scholar]
- 22.Committee on Fetus and Newborn. American Academy of Pediatrics Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914–917. [PubMed] [Google Scholar]
- 23.Dean AG. Open source statistics for public health. http://www.openepi.com/Random/Random.htm. Accessed October 2011.
- 24.Alansari K., Sakran M., Davidson B.L., Ibrahim K., Alrefai M., Zakaria I. Oral dexamethasone for bronchiolitis: a randomized trial. Pediatrics. 2013;132:e810–e816. doi: 10.1542/peds.2012-3746. [DOI] [PubMed] [Google Scholar]
- 25.Mansbach J.M., Piedra P.A., Stevenson M.D., Sullivan A.F., Forgey T.F., Clark S. Prospective multicenter study of children with bronchiolitis requiring mechanical ventilation. Pediatrics. 2012;130:e492–e500. doi: 10.1542/peds.2012-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroeder A.R., Mansbach J.M., Stevenson M., Macias C.G., Fisher E.S., Barcega B. Apnea in children hospitalized with bronchiolitis. Pediatrics. 2013;132:e1194–e1201. doi: 10.1542/peds.2013-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kay R., Kinnersley N. On the use of the accelerated failure time model as an alternative to the proportional hazards model in the treatment of time to event data: a case study in influenza. Drug Inf J. 2002;36:571–579. [Google Scholar]
- 28.Carol K.T., Jane H.H., Donna M.K. 12th ed. Lexi-Comp; Hudson (OH): 2006. Pediatric dosage handbook; pp. 218–219. [Google Scholar]
- 29.Willwerth B.M., Harper M.B., Greenes D.S. Identifying hospitalized infants who have bronchiolitis and are at high risk for apnea. Ann Emerg Med. 2006;48:441–447. doi: 10.1016/j.annemergmed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Kneyber M.C., Brandenburg A.H., de Groot R., Joosten K.F., Rothbarth P.H., Ott A. Risk factors for respiratory syncytial virus associated apnoea. Eur J Pediatr. 1998;157:331–335. doi: 10.1007/s004310050822. [DOI] [PubMed] [Google Scholar]
- 31.Steer P.A., Flenady V.J., Shearman A., Lee T.C., Tudehope D.I., Charles B.G. Periextubation caffeine in preterm neonates: a randomised dose response trial. J Paediatr Child Health. 2003;39:511–515. doi: 10.1046/j.1440-1754.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 32.Sreenan C., Lemke R.P., Hudson-Mason A., Osiovich H. High-flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics. 2001;107:1081–1083. doi: 10.1542/peds.107.5.1081. [DOI] [PubMed] [Google Scholar]