Abstract
Disinfection guidelines exist for pathogen inactivation in potable water and recycled water, but wastewater with high numbers of particles can be more difficult to disinfect, making compliance with the guidelines problematic. Disinfection guidelines specify that drinking water with turbidity ≥1 Nephelometric Turbidity Units (NTU) is not suitable for disinfection and therefore not fit for purpose. Treated wastewater typically has higher concentrations of particles (1–10 NTU for secondary treated effluent). Two processes widely used for disinfecting wastewater are chlorination and ultraviolet radiation. In both cases, particles in wastewater can interfere with disinfection and can significantly increase treatment costs by increasing operational expenditure (chemical demand, power consumption) or infrastructure costs by requiring additional treatment processes to achieve the required levels of pathogen inactivation. Many microorganisms (viruses, bacteria, protozoans) associate with particles, which can allow them to survive disinfection processes and cause a health hazard. Improved understanding of this association will enable development of cost-effective treatment, which will become increasingly important as indirect and direct potable reuse of wastewater becomes more widespread in both developed and developing countries. This review provides an overview of wastewater and associated treatment processes, the pathogens in wastewater, the nature of particles in wastewater and how they interact with pathogens, and how particles can impact disinfection processes.
Keywords: Disinfection, Particles, Pathogens, Reuse, Treatment, Wastewater
1. Introduction
Although water is abundant and covers 75% of the Earth's surface, most of the freshwater “is available at the wrong place, at the wrong time, or with the wrong quality” (Falkenmark & Lindh, 1974). The availability of freshwater is greatly impacted by global climatic changes and increases in human population, urbanization, and pollution (Vörösmarty et al., 2010), to the extent that the United Nations predicts that by 2050 more than half of the world's population will be living in water-deficient countries (Pigram, 2007).
Australia is considered to be the driest continent after Antarctica, with less than 1% of the world's available freshwater (Pigram, 2007). Increasing population growth and demand, combined with reductions in available freshwater due to climate change and drought (specifically the Millennium drought from 1995 to 2009), have driven developments in wastewater recycling and water management within Australia (Chiew et al., 2011, Greenway, 2005, Lazarova et al., 2001, Moe and Rheingans, 2006, Pigram, 2007). Similar challenges have affected other countries, including the United States, Western Europe, and Israel, leading to an increased focus on wastewater reuse internationally (Wade Miller, 2006). The State of California has been impacted particularly by water shortages, and an extreme 5-year drought prompted local water resource authorities to make regulatory provisions for direct and indirect potable reuse of wastewater (ORDER-WQ-2016-0068-DDW, 2016). In Australia the percentage of wastewater reuse varies in different states, but overall has increased from 2001 to 2015 (Table 1 ). The nonpotable reuse of treated wastewater includes irrigation of crops and parklands, dual reticulation within domestic and commercial buildings (Moe & Rheingans, 2006), and recreation and mining (Dillon, 2000). Wastewater can also be treated to high standards for direct or indirect potable reuse applications, such as the supplementation of surface or groundwaters for drinking (Moe & Rheingans, 2006).
Table 1.
Comparison of the rates of wastewater recycling (expressed as a percentage of total wastewater produced) in major Australian cities during 2001–15
| Capital city | Recycling (%) |
|||||
|---|---|---|---|---|---|---|
| 2001–02 | 2005–06 | 2007–08 | 2009–10 | 2012–13 | 2014–15 | |
| Adelaide | 11.1 | 18.1 | 30.6 | 28.7 | 31.3 | 33.7 |
| Melbourne | 2.0 | 14.3 | 23.2 | 22.8 | 16.1 | 15.8 |
| Perth | 3.3 | 5.3 | 6.4 | 6.1 | 8 | NA |
| Sydney | 2.3 | 3.5 | 4.4 | 7.3 | 8 | NAa |
| Total | 3.3 | 8.4 | 11.3 | 16.8 | 17 | 17 |
Information is not available or could not be calculated from available data.
Irrespective of the intended use, wastewater must be treated sufficiently so that it is fit for purpose and will not adversely affect human health or the environment. The Australian Guidelines for Wastewater Recycling (AGWR, 2006) use a risk management framework incorporating hazard analysis and critical control point principles to identify and manage human or environmental health hazards in wastewater. Hazards can be managed by limiting entry into wastewater destined for reuse (e.g., selective harvesting of wastewater sources to avoid high-risk contaminants), by the use of treatment processes to remove or reduce microbial or chemical hazards, and by preventative measures at the point of use to limit exposure to any microbial or chemical hazards.
The AGWR (2006) place particular emphasis on the control of microbial contaminants to protect human health and use the measure of disability adjusted life years (DALYs), in combination with end use and exposure scenarios, as the basis for setting health-based treatment targets for wastewater. In Australia regulatory authorities have deemed that the tolerable risk from any given pathogen in reuse water is no more than 1 micro-DALY per person per year. The removal or inactivation of pathogens by various treatment and disinfection processes can be impaired by particles in the wastewater, leading to noncompliance with health standards and increased risk to end users. It is therefore critical to understand the interactions between particles, pathogens, and wastewater treatment processes to enable optimal removal of pathogens and the production of safe reuse water. This paper provides an overview of wastewater, the pathogens and indicator organisms of interest in wastewater, the treatment processes commonly used for the production of reuse water, the interactions between wastewater particles and pathogens, and how these can affect treatment processes and impact upon wastewater reuse.
2. Wastewater
Today's rapidly growing societies generate wastes that enter water bodies (Parr, Smith, & Shaw, 2002). Different types of wastewater (Fig. 1 ) include those derived from domestic, commercial, industrial and agricultural sectors, as well as surface runoff (storm water) from urban areas (Abdel-Raouf et al., 2012, Metcalf and Eddy, 2003). Domestic wastes are derived from human communities and contain human wastes (feces and urine) as well as water from laundry, kitchen, bathing, and other household chores (Mara, 2004).
Figure 1.
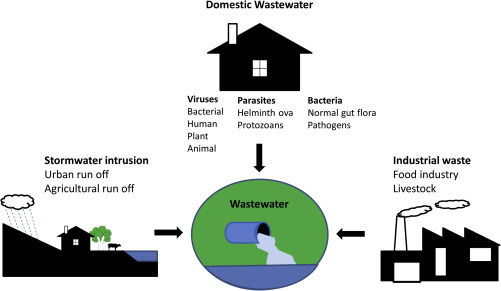
Major sources of wastewater contamination.
Water usage adds many natural organic, inorganic, and artificial compounds to the wastewater, such as grit, dirt, oil, nutrients, chemicals, metals, plant and animal wastes (Abdel-Raouf et al., 2012). Inorganic solids present in wastewater include salts, metals, and surface sediments (Templeton & Butler, 2011). Organic compounds are generally biodegradable and comprise body and food wastes that can be metabolized by microorganisms in a process which reduces the oxygen available for other life forms (Templeton & Butler, 2011). Hence organics in wastewater can be quantified by measuring biological oxygen demand (BOD) and chemical oxygen demand (COD) (Henze, van Loosdrecht, Ekama, & Brdjanovic, 2008).
The wastewater environment is an ideal medium for both pathogenic and nonpathogenic microorganisms (Abdel-Raouf et al., 2012). Dangerous pathogens include enteric bacteria, viruses, protozoa, parasitic worms, and their eggs (Abdel-Raouf et al., 2012). Fecal matter is a major component of domestic sewage and the source of the majority of human pathogens in wastewater (Symonds & Breitbart, 2014). Industrial waste from food production, particularly from animal processing, can also be a source of pathogenic microorganisms.
Although solid materials constitute only 0.1% of the total volume of wastewater (Middleton, 1977), suspended solids can alter the light penetrance and temperature of water bodies, impact benthic plants and clog waterways (Bilotta and Brazier, 2008, Templeton and Butler, 2011). Excesses of some nutrients, such as nitrogen and phosphorous, can be toxic for fish and other animals including humans, and also cause eutrophication of receiving waters, thus contributing to the formation of algal blooms that can present further human or environmental health hazards (Templeton & Butler, 2011). If untreated, wastewater will go septic and the decomposition of matter will create unhygienic and hazardous conditions. On the other hand, municipal wastewaters are also a rich source of nutrients that can be directly recovered or provide additional benefits if present in reuse water for agriculture, horticulture, forestry, and domestic gardening applications (Greenway, 2005).
Hence there are compelling reasons to treat wastewater to reduce the risk of transmitted diseases and environmental pollution (Mara, 2004), and to retrieve valuable nutrients and freshwater that would otherwise be lost in the waste stream.
3. Pathogens in Wastewater
Wastewater streams contain many different types of pathogens that present a major health risk (Fig. 1). Human pathogens include bacteria, viruses, parasitic protozoans, and helminths (Cai & Zhang, 2013). Pathogens can enter wastewaters from many sources. Enteric pathogens enter wastewater from human and animal fecal wastes or from fecally contaminated water from other household uses such as bathing or laundry (Gerardi & Zimmerman, 2004). Livestock and poultry can be infected with zoonotic enteric pathogens and so wastewater from food processing also represents a human health risk (Gerardi and Zimmerman, 2004, Hill, 2003). The major pathogens and diseases or illnesses they cause are shown in Table 2 (Ashbolt, 2004, Gerba and Smith, 2005). For a summary of pathogens and representative indicator organisms in wastewater, including their geographical distribution, numbers in primary and secondary treated wastewater and summary information of detection methods, readers are directed to the report by Keegan, Monis, Jagals, Toze, and Blackbeard (2010).
Table 2.
The major pathogens of concern in municipal wastewater and diseases or illness associated with them
| Name of pathogen | Major disease or symptoms | |
|---|---|---|
| Bacteria | Campylobacter jejuni | Gastroenteritis |
| Escherichia coli | Gastroenteritis | |
| Salmonella spp. | Salmonellosis, typhoid, paratyphoid | |
| Shigella spp. | Bacillary dysentery | |
| Vibrio cholerae | Cholera | |
| Yersinia spp. | Gastroenteritis | |
| Viruses | Adenovirus | Upper respiratory infection and gastroenteritis |
| Astrovirus | Gastroenteritis | |
| Coxsackie virus | Meningitis, pneumonia, fever | |
| Echovirus | Meningitis, paralysis, encephalitis, fever | |
| Hepatitis A virus | Infectious hepatitis | |
| Hepatitis E virus | Infectious hepatitis, miscarriage, and death | |
| Human calicivirus | Epidemic gastroenteritis with severe diarrhea | |
| Polio virus | Poliomyelitis | |
| Reovirus | Respiratory infections, gastroenteritis | |
| Rotavirus | Acute gastroenteritis with severe diarrhea | |
| TT hepatitis | Hepatitis | |
| Protozoa | Balantidium coli | Balantidiasis |
| Cryptosporidium spp. | Cryptosporidiosis | |
| Entamoeba histolytica | Acute amoebic dysentery | |
| Giardia duodenalis | Giardiasis | |
| Toxoplasma gondii | Toxoplasmosis | |
| Helminths | Ascaris lumbricoides | Ascariosis |
| Ascaris suum | Coughing and chest pain | |
| Hymenolepis nana | Hymenolepiasis | |
| Necator americanus | Hookworm disease | |
| Taenia saginata | Insomnia, anorexia | |
| Taenia solium | Insomnia, anorexia | |
| Toxocara canis | Fever, abdominal pain, muscle ache | |
| Trichuris trichiura | Diarrhea, anemia, weight loss |
3.1. Bacteria
Bacteria constitute the most diverse group of human pathogens in wastewater. Many types of bacteria colonize the human intestine and are shed in feces. While many of these bacteria are commensal and beneficial to their hosts, some are pathogenic and these enteric bacterial pathogens constitute the majority of bacterial pathogens in wastewater (Varela & Manaia, 2013). Major human bacterial pathogens in wastewater include Salmonella spp., Escherichia spp., Shigella spp., Yersinia spp., Klebsiella spp., Leptospira spp., Vibrio cholerae, Aeromonas hydrophila, Legionella pneumophila, Mycobacterium spp., and Pseudomonas (Cai & Zhang, 2013; Kristian Stevik et al., 2004, Maynard et al., 2005). Enteric bacterial pathogens such as Salmonella spp., Escherichia spp., Shigella spp., Yersinia spp., and V. cholerae typically cause gastrointestinal infections such as diarrhea, dysentery, and gastroenteritis (Anastasi et al., 2010; Okoh et al., 2007, Varela and Manaia, 2013). Helicobacter pylori, which causes gastric ulcers and is linked to some cancers, might also be waterborne but transmission pathways for this pathogen have not been conclusively demonstrated (Anastasi et al., 2010). Other diseases caused by bacteria in wastewater include wound infections (Pseudomonas aeruginosa), respiratory infections (L. pneumophila, Mycobacterium avium) and leptospirosis (Leptospira) (Gerardi & Zimmerman, 2004; Levy, Fine, & Bar-Tal, 2010). Some bacteria, such as L. pneumophila, M. avium, P. aeruginosa, and A. hydrophila, are environmental and are opportunistic rather than frank pathogens, since they cause disease in a host with a predisposing factor, such as reduced immunity or with reduced physical barriers to infection due to burns or wounds (Gerardi & Zimmerman, 2004).
Escherichia coli are genetically diverse and predominantly harmless bacteria that are part of the normal gut flora of warm-blooded animals, including humans. This species has been widely used as an indicator of fecal contamination and is found in densities of 105–1010 colony-forming units per liter of raw sewage (Matthews, Stratton, Schreoder, & Toze, 2010). Some strains of E. coli possess additional genes, encoding virulence determinants such as adhesion factors and toxins, which allow them to be pathogenic and cause intestinal or extraintestinal diseases. These pathogenic strains can cause gastroenteritis, diarrhea, urinary tract infections hemolytic uremic syndrome, and meningitis (Anastasi et al., 2010).
The increased production or use of antibiotics in countries such as the United States, India, China, and Greece has led to an increased awareness of the presence of antibiotics and antibiotic-resistant bacteria (ARB) in wastewater (Bitton, 2005; Bouki, Venieri, & Diamadopoulos, 2013). Many reports have described the persistence of ARB through the wastewater treatment train and there is currently much interest in the environmental fate of both ARB and the genetic elements encoding antibiotic resistance, particularly if these genes are passed to and persist in environmental bacteria (Bouki et al., 2013).
3.2. Viruses
Viruses are another diverse group of waterborne human pathogens. Untreated wastewater can have as many as 103–107 virus particles per liter of wastewater (Keegan et al., 2010, Okoh et al., 2007). However, the density of viruses in treated wastewater depends on various factors such as type of treatment process, season, and geographical area (Kitajima, Iker, Pepper, & Gerba, 2014). Many of the viruses are poorly removed by the secondary treatment processes used to remove bacterial pathogens (Ottoson, Hansen, Björlenius, Norder, & Stenström, 2006). The major viral pathogens in wastewater are enteric viruses such as hepatitis A, norovirus, rotavirus, adenoviruses, astroviruses, and the various enteroviruses (Ashbolt, 2004, Cai and Zhang, 2013). The site of virus multiplication in the host is generally related to the type of disease caused, with most enteric viruses multiplying in the host's intestine, although in some cases other tissues can be infected (Wyn-Jones et al., 2011). Consequently, enteric viruses can cause a variety of diseases in humans, such as gastroenteritis, meningitis, hepatitis, and myocarditis (Ashbolt, 2004). These enteric viruses are shed in high quantities, 105–1011 virus particles/gram of feces, by infected individuals (Fong & Lipp, 2005; Okoh, Sibanda, & Gusha, 2010). Several emerging viruses, such as severe acute respiratory syndrome coronavirus and human parechovirus, can also be excreted in feces and found in wastewater (Wyn-Jones et al., 2011). Industrial waste from slaughterhouses can add zoonotic viruses to wastewater, such as animal adenoviruses, sopaviruses, and hepatitis E (Wyn-Jones et al., 2011). A variety of pathogenic plant viruses, such as pepper mild mottle virus and tobacco mosaic virus, have been identified in human feces and wastewater (Symonds & Breitbart, 2014).
3.3. Protozoa
Protozoans are another important group of microorganisms in wastewater and can be up to 10 times larger than bacteria (Boztoprak & Özbay, 2013). Protozoan parasites, such as Cryptosporidium parvum, Cryptosporidium hominis, and Giardia duodenalis are commonly detected in sewage (Li, Craik, Smith, & Belosevic, 2009), although their abundance may be seasonal, depending on country and climate. Cryptosporidium and Giardia are intestinal parasites that infect humans and animals. In the case of Cryptosporidium, there is no effective drug treatment that directly kills this parasite in the host and so infection can be fatal in patients lacking a functional immune system (Abrahamsen et al., 2004). The transmissive stage of Cryptosporidium is a nonreproductive and dormant oocyst (Searcy, Packman, Atwill, & Harter, 2005), which is shed in feces and is highly infective. Cryptosporidium infects host enterocytes, causing diarrhea, nausea, and abdominal pain, a condition commonly referred to as cryptosporidiosis (Fletcher, Stark, Harkness, & Ellis, 2012). G. duodenalis is a unicellular flagellate eukaryote that is another major waterborne pathogen causing intestinal infection (Adam, 2001). The transmissive stage of G. duodenalis is a dormant cyst (Adam, 2001) and infection, known as giardiasis, can cause acute diarrhea, abdominal pain, nausea, malabsorption, and weight loss (Fletcher et al., 2012). Other pathogenic protozoa, including Cyclospora, Entamoeba histolytica (which causes amoebic dysentery), Isospora belli, and Enterocytozoon bieneusi, have also been reported in outbreaks of waterborne illness (Khanum et al., 2013).
3.4. Pathogen Surrogates for Measuring Treatment Process Performance
Wastewater is a reservoir for pathogens and poses a major health risk, particularly when discharges enter recreational waters on in the case of reuse. Culture-based methods are traditionally used for detecting microorganisms, but their usefulness is limited due to the occurrence and prevalence of pathogens and because many are not easy to culture, cannot be cultured, or are otherwise expensive to isolate and enumerate (Gilbride et al., 2006, Keegan et al., 2010). Since it is not practical (or in some cases possible) to detect and monitor all known pathogens, indicator organisms are employed as surrogates for the presence of fecal contamination and hence possible presence of pathogens (Harwood et al., 2005). A good indicator should be present in the pathogen source and absent from unpolluted areas. It should be present in abundance, nonpathogenic, easy to culture, and show similar behavior as the pathogen (Bosch, 2010).
The most widely used indicator organisms are enteric bacteria, primarily due to the ease and low cost of the relevant culture detection methods. E. coli, in particular, is considered a mandatory fecal indicator by the United States Environmental Protection Agency and European Union for risk characterization of drinking water sources, compliance monitoring of drinking water, and monitoring of recycled water and wastewater discharges (Stevens, Ashbolt, & Cunliffe, 2003). Other bacteria, such as enterococci, have also been used as fecal indicators (Stevens et al., 2003). However, given differences in size and structure, it is unlikely that all groups of pathogens (bacteria, viruses, protozoa, and helminths) will behave in the same way, therefore it is not ideal to have a single, universal microbial indicator (Ashbolt, Grabow, & Snozzi, 2001).
Bacterial fecal indicators have been shown to be poor surrogates for viruses and protozoans, highlighting the need for different indicators for different groups of pathogens (Duran et al., 2003). Bacteriophage, such as somatic coliphage, F-specific RNA coliphage, and Bacteroides fragilis bacteriophage have been suggested as potential viral fecal indicators, as well as indicators for monitoring the effectiveness of treatment processes (Duran et al., 2003, Monis et al., 2015). Fecal indicator organisms are only linked to the presence or absence of fecal contamination (and hence risk of pathogen presence), but they do not necessarily give any information about the movement, removal, or inactivation of pathogens. Therefore, there is a need for process indicators or model organisms, which are defined as groups of organisms that are indicative of pathogen behavior in similar environments (Ashbolt et al., 2001). Process indicator organisms include E. coli and F-RNA phage (Monis et al., 2015). Alternative targets, such as particles or measuring particle size distributions (PSDs), are also potential process indicators. Analysis using PSD was found to be a useful surrogate method for helminth ova detected in wastewater influents and effluents (Chavez, Jimenez, & Maya, 2004) and was also predictive of pathogen removal in a pilot scale–activated sludge reactor (Keegan et al., 2010).
3.4.1. Bacteria
Shigella, Campylobacter, and Salmonella are the most common bacterial causes of gastroenteritis in Australia and industrialized countries but these organisms are present in low numbers in wastewater (Matthews et al., 2010). Therefore, indicator organisms such as E. coli have been used as an indicator for other enteric bacteria. The majority of enteric pathogenic bacteria respond to water treatment in a similar fashion to E. coli (Keegan et al., 2010) and hence it was used as a model organism for assessing ultraviolet (UV) inactivation (McElmurry, Ingram, Khalaf, & Pillai, 2011). E. coli is a Gram negative, rod-shaped bacterium approximately 1.8 μm long and 0.8 μm in diameter, which lives in the gut of warm-blooded animals (Berg, 2004, McElmurry et al., 2011). It is safe and easy to culture, inexpensive to analyze, and does not generally regrow in wastewater, making it an ideal process indicator for bacterial pathogens when monitoring the efficacy of wastewater treatment processes.
3.4.2. Viruses
Adenoviruses and noroviruses have been suggested as representative viruses due to their abundance in wastewaters (Hewitt et al., 2011, Keegan et al., 2010). However, these viruses are pathogenic and norovirus is not readily cultured, requiring highly specialized three-dimensional cell culture methods to produce the fully differentiated enterocytes needed to support infection and virus propagation. It has been suggested that fecal bacteriophage (viruses infecting enteric bacteria), such as somatic coliphage and F-specific coliphage, can be indicators for pathogenic viruses (Monis et al., 2015; Skraber, Gassilloud, Schwartzbrod, & Gantzer, 2004). However, there is not a universally accepted enteric virus indicator, in part because there is such diversity in virus size, shape, and genome type. Bacteriophage have been used as model organisms because they show similar resistance to disinfection processes as most of the enteric viruses and they also have similar or higher abundance compared to enteric viruses in natural water and wastewater (Ashbolt et al., 2001, Duran et al., 2003, Grabow, 2004). Phages have been used as models to examine raw and treated drinking water supplies (Grabow, 2004). F-RNA coliphage, which are viruses that infect coliform bacteria that possess an F plasmid and are actively expressing conjugative F pili, are the most attractive surrogates because their structure, morphology, and composition resembles that of human enteric viruses (Grabow, 2004). In addition, they are unable to multiply in natural water environments in the absence of actively growing host cells and have similar responses toward disinfectants as human enteric viruses (Grabow, 2004). MS2 is an F-RNA coliphage that has been used as a model organism for norovirus (Dawson, Paish, Staffell, Seymour, & Appleton, 2005). E. coli is the host for MS2, which bypasses the need for complex mammalian cell culture for enumeration of MS2 (Dawson et al., 2005). In addition, MS2 is relatively easy to propagate using E. coli cultures and so can be used for challenge testing if higher numbers of virus are required to validate the performance of treatment processes.
3.4.3. Protozoa
Cryptosporidium and Giardia are important pathogens that are problematic due to their resistance to chlorine (Cryptosporidium in particular), environmental persistence, low infectious dose, and relatively high cost for detection and measurement of infectivity. The bacterium Clostridium perfringens, a spore-forming obligate anaerobe, has been considered as an indicator for pathogenic protozoa (Ashbolt et al., 2001). Clostridium is found in abundance in sewage as it is associated with the feces of warm-blooded animals (Ashbolt et al., 2001). The small (1 μm) spores, which are 4–10 times smaller than protozoan oocysts or cysts, do not interact with soil grains and in some ways behave like colloids, making them highly resistant to degradation and inactivation (Schijven, De Bruin, Hassanizadeh, & de Roda Husman, 2003). These spores have been associated with the occurrence of Cryptosporidium oocysts and Giardia cysts in wastewater (Cheng, Broaders, Lucy, Mastitsky, & Graczyk, 2012) and have similar partitioning behavior to Cryptosporidium and Giardia in storm water (Cizek et al., 2008). It has been suggested that they are conservative indicators for the removal of Cryptosporidium and Giardia by wastewater treatment processes (Keegan et al., 2010).
3.5. Pathogen Detection Methods
Increases in population, habitat encroachment, international travel, and the globalization of world trade have all contributed to the emergence of new pathogens or reemergence of known pathogens of human health significance (Gilbride et al., 2006). There are many techniques for isolating and detecting pathogenic microorganisms in wastewater, ranging from simple culture-based techniques to next generation sequencing (NGS). Some of these are standard methods and their use may be mandated in different countries for regulatory compliance. It is beyond the scope of this review to discuss these techniques in any detail, but there are many useful review papers describing or evaluating molecular techniques (Gilbride et al., 2006; Monis et al., 2005, Ramirez-Castillo et al., 2015, Yergeau et al., 2016) and research reports are also a good source of information for both conventional and molecular detection protocols for pathogens or surrogates in wastewater (Francy et al., 2011, Keegan et al., 2010, Monis et al., 2015).
All techniques have advantages and limitations and a list of some traditional and modern techniques is shown in Table 3 . Emerging techniques include NGS, which has been used to detect pathogenic bacteria in wastewater (Cai and Zhang, 2013, Ye and Zhang, 2011, Yergeau et al., 2016). Next generation 454 pyrosequencing has also been used successfully to characterize microbial communities from different wastewater samples (Ye & Zhang, 2013). Caution needs to be used when interpreting NGS data, particularly when identification is based on the sequencing of relatively small amplicons, which makes misidentification possible, particularly for closely related species. The technique is also very sensitive and a thorough understanding of the level of background contamination from the laboratory environment or between samples is required to determine if a result is the detection of a rare taxon or an artifact.
Table 3.
List of detection methods used to study different pathogens found in wastewater
| Technique | Benefits | Limitations |
|---|---|---|
| Culture-based methods | Easy to perform Low cost |
Majority of bacterial species cannot be artificially cultured Not a direct measurement if using indicator organisms |
| Microscopy | Fast Direct observation |
Limited options for species identification Requires expertise |
| Fluorescent in situ hybridization (FISH) | Quantitative Direct visual resolution of cells including non culturable bacteria |
Labor intensive Limited ability to identify multiple target species |
| FISH and confocal scanning laser microscope | Direct visual resolution of cells including slow growing and non culturable bacteria | Expensive |
| Polymerase chain reaction (PCR) | Culture independent Rapid Highly sensitive Accurate |
False positive results Inhibition by contamination Requires knowledge of target organism sequences for assay design |
| Multiplex PCR | Rapid and simultaneous detection of target microorganisms | Primer dimers may function as single reaction |
| Amplified ribosomal DNA restriction analysis | Culture independent Suitable for wide range of microorganisms |
DNA extraction and PCR biases Not quantitative |
| Terminal restriction fragment length polymorphism | Fast and semiquantitative | DNA extraction and PCR biases |
| Denaturing gradient gel electrophoresis | Use of r-RNA gene sequence heterogeneity | Specificity can be an issue due to short target sequences |
| Ribosomal RNA intergenic spacer analysis | Heterogeneity in length and sequence among bacteria | DNA extraction and PCR biases Not quantitative |
| Nucleic acid microarray | High throughput design Various applications |
Low sensitivity for environmental samples Sample processing complexity |
| On chip technology | PCR and hybridization on a single chip Less interference between parallel reactions |
Integration and packaging |
| Next generation sequencing | Culture independent Rapid community analysis Versatile (community function or composition) |
DNA extraction and PCR biases Not quantitative Expertise for bioinformatic analysis Expensive equipment |
Adapted from Gilbride, K., Lee, D.-Y., & Beaudette, L. (2006). Molecular techniques in wastewater: understanding microbial communities, detecting pathogens, and real-time process control. Journal of Microbiological Methods, 66, 1–20.
4. Wastewater Treatment
It is important to treat wastewater cost effectively while ensuring the quality is sufficient to enable safe disposal or reuse. The majority of countries utilize conventional wastewater treatment processes in which physical processes and chemical and biological reactions remove suspended solids, biodegradable organics and pathogenic microorganisms (Metcalf and Eddy, 2003, Middleton, 1977). These processes are grouped into preliminary, primary, secondary, and tertiary stages and form a treatment train (Fig. 2 ).
Figure 2.
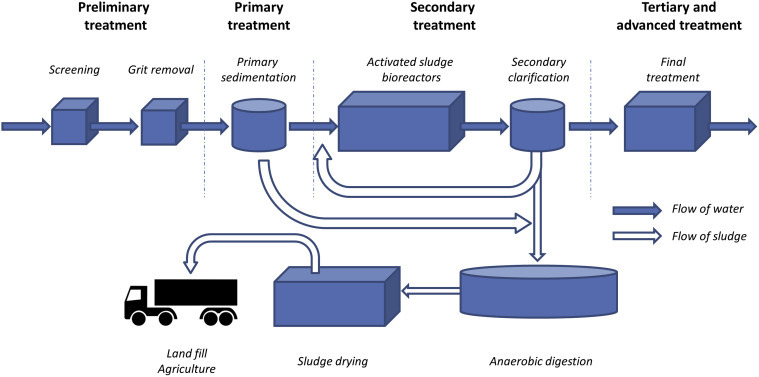
Schematic of a typical wastewater treatment.
4.1. Preliminary Treatment
The first wastewater treatment stage is designed to remove large objects such as bottles, cans, and plastics which can clog and block downstream processes (Okoh et al., 2007). Preliminary treatment typically consists of screening and grit removal and can use bar, drum, cutting, or band screens that are inclined toward the inflowing water and trap objects as the sewage water flows thorugh them (Templeton & Butler, 2011). The captured debris can be manually or mechanically removed and fibrous materials can be further dewatered. Grit removal involves removing abrasive inorganic materials such as sand, gravels, and other heavy particulate matter, and is necessary to avoid clogging and abrasive damage to the equipment and sewage pipes downstream (Templeton & Butler, 2011). There are different types of grit channels; velocity channels or aerated channels, which reduce the velocity of influent and allow the heavy abrasives to settle to the bottom before removal.
4.2. Primary Treatment
Primary treatment processes are designed to remove suspended solid wastes and reduce particulate forms of BOD. It is generally described as the first level of treatment and removes approximately 50–70% of total suspended solids (TSSs), 65% of oil and grease, and 25–50% of BOD (Sonune & Ghate, 2004). Major physical modes for separating solids from wastewater are flocculation and sedimentation, which involves settling solids under the influence of gravity (Templeton & Butler, 2011). The most common sedimentation tanks (also known as clarifiers) are rectangular or circular (Fig. 3 ) in shape. In rectangular tanks, water enters from one end and leaves from the other end (Fig. 3A) whereas in circular tanks water enters from the center and moves outward radially (Fig. 3B). An important feature of these tanks is a weir. In sedimentation, the speed of water affects settling of solids. Therefore, weirs are carefully designed physical barriers which determine the flow rate (Templeton & Butler, 2011). Dissolved and colloidal substances are not removed at this stage (Sonune & Ghate, 2004).
Figure 3.
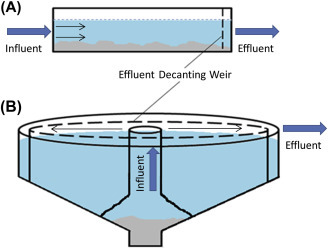
Illustration showing the most common designs of wastewater sedimentation tanks (clarifiers): (A) rectangular or horizontal flow clarifier and (B) circular or radial flow clarifier.
As well as basic primary treatment methods, advanced methods separate dissolved organic matter by the addition of coagulants or flocculants (Odegaard, 2000). The flocculent is a metal salt which aggregates the suspended colloids and facilitates separation by settling or filtration (Odegaard, 2000). The outflow water is known as primary effluent and it contains mainly dissolved organic and inorganic solids. Once clarified, the primary effluent enters secondary treatment.
4.3. Secondary Treatment
Secondary treatment processes remove nutrients and dissolved organic and inorganic solids from the primary effluent by the application of various biological treatment processes (Sonune and Ghate, 2004, Spellman, 2013). The different functional operations that occur during secondary treatment are carbon oxidation and nutrient removal. Carbon oxidation is mediated by microorganisms and involves the oxidation or metabolism of organic matter into carbon dioxide, water, and cellular biomass (Grady, Daigger, Love, & Filipe, 2011). The energy produced is utilized by microorganisms for growth and reproduction (Davies, 2005).
The two key nutrients that must be removed prior to discharge of wastewater to the environment are nitrogen and phosphorous. The biological processes of nitrification and denitrification remove inorganic nitrogen (Gerardi, 2010). Nitrification converts ammonia to nitrate, while denitrification converts the nitrate to nitrogen gas. Incomplete nitrification/denitrification can lead to the production of nitrous oxide, which is a potent greenhouse gas. A combination of biological and physiochemical processes can be used to remove phosphorus. Biological processes include enhanced biological phosphorus removal plants, which are designed to selectively support the growth of phosphate accumulating organisms that are capable of storing orthophosphate (Gerardi, 2010). There are a number of different types of secondary treatments that can be used to mediate biological nutrient removal processes (Table 4 ).
Table 4.
Secondary wastewater treatment options and their key features (ESCWA, 2003, Liu et al., 2003, Parr et al., 2002)
| Treatment process | Description | Key features |
|---|---|---|
| Activated sludge process (ASP) | Aerobic digestion of organic matter by bacteria, can also include anaerobic, anoxic, and aerobic zones for N and P removal |
|
| Aerated lagoons | Mechanically aerated ponds 1–4 m deep |
|
| Land treatment | Sewage is applied in controlled conditions to soil |
|
| Oxidation pond | Modified ASP with long retention times |
|
| Constructed wetlands | Sewage flows through artificial vegetated pond systems |
|
| Rotating biological contactor | Attached growth biological process with vertical rotating discs partially submerged in wastewater |
|
| Trickling filters | Attached growth biological process. Sewage flows through a fixed bed of filter media covered with biofilm |
|
| Up-flow anaerobic sludge blanket | Anaerobic process uses a blanket of bacteria to absorb sewage load |
|
| Waste stabilization ponds | Large surface area ponds use mixed biological processes |
|
4.3.1. Activated Sludge Process
The activated sludge process (ASP) is commonly used for biological removal of nutrients from wastewater. An ASP involves two major stages. The first stage is the decomposition of pollutants by a heterogeneous and highly diverse culture of microorganisms, which metabolizes organic matter and inorganic nutrients to more simplified and environmentally benign end products such as carbon dioxide and nitrogen gas (Tong, Beck, & Latten, 1980). The heterogeneous microbial culture is termed “activated sludge” (Okoh et al., 2007) and the biomass is normally arranged in microbial aggregates called flocs, which are kept in suspension by aeration and mechanical mixing (Seviour and Nielsen, 2010, Sustarsic, 2009). The most basic ASP set-up for this first stage comprises an aeration tank and an aeration source. However, there are many modifications to this basic design (Fig. 4 ) to include anoxic and/or anaerobic zones to improve total nitrogen removal by nitrification/denitrification and phosphorous uptake (Fux and Siegrist, 2004, Okoh et al., 2007; Seviour et al., 2003, Sustarsic, 2009; Vaiopoulou, Melidis, & Aivasidis, 2007).
Figure 4.
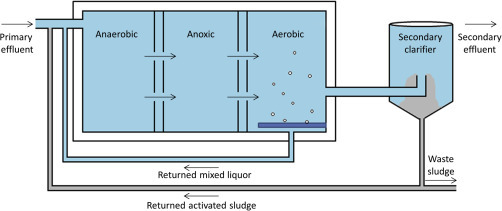
Schematic of a modified activated sludge process that promotes biological removal of nitrogen and phosphorous.
The second stage of the ASP is separation of the biomass from the treated water in a secondary clarifier, which uses gravity sedimentation (Seviour and Nielsen, 2010, Sustarsic, 2009). The clarified supernatant is sent for tertiary treatment, while a large portion of the settled biomass (termed return activated sludge or RAS) is recycled back to the head of the ASP. While the main purpose of an ASP is biological removal and stabilization of nutrients, it is also recognized to be an effective treatment barrier against pathogens via predation (by higher organisms) and by attachment, adsorption or entrapment to or within the biological floc (Bitton, 2005, Keegan et al., 2010, Okoh et al., 2007). Reports describing the efficacy ASP for pathogen removal vary and this may be related to operational differences between wastewater treatment plants (WWTPs) and also the effect of season on pathogen density and treatment performance. Removal of E. coli has been reported to be between 1.5 and 2.5 log10, while Cryptosporidium removal was reported to be between 1 and 3 log10 (Keegan et al., 2010, King et al., 2015). In contrast, removal of viruses varied between different WWTPs and also appeared to be different for some viral species (Keegan et al., 2010). For example, removal of rotavirus was 7–8 log10, whereas norovirus removal ranged from 1 to 6 log10 (Keegan et al., 2010).
4.3.2. Waste Stabilization Ponds
Waste stabilization ponds (WSPs) are large shallow basins in which wastewater is stored for extended periods of time to enable biological treatment by communities of bacteria and algae species (Alexiou & Mara, 2003). WSPs provide a green treatment technology with the advantages of low energy demand and low operational costs combined with highly efficient removal of organic matter and pathogens (Faleschini & Esteves, 2011). WSPs are often used in small rural communities as the sole treatment option for sewage, or as a polishing step after ASP, or other secondary treatments prior to discharge or reuse.
Three major mechanisms contribute to the elimination of pathogens from WSPs: (1) adverse conditions in the ponds (e.g., temperature, sunlight, and predation), (2) long residence times for microorganisms in ponds leading to natural death, and (3) adsorption to particles and sedimentation (Campos et al., 2002, Greenway, 2005; Karim, Manshadi, Karpiscak, & Gerba, 2004). The removal of pathogens and the final effluent density of pathogens is also related to pond depth, detention time, number of ponds, and pond geometry (Von Sperling, 2005). WSPs remove fecal coliforms, E. coli and other pathogenic microorganisms through photooxidative DNA damage arising from sunlight, as well as through other physicochemical factors such as temperature and pH (Davis-Colley, Donnison, & Speed, 2000). Sunlight and temperature have also been found to inactivate Cryptosporidium suspended in a WSP (King et al., 2015). Predation by other microorganisms or zooplankton can also contribute to removal of pathogens, especially bacteria and protozoan parasites (King et al., 2015; Stott, May, Matsushita, & Warren, 2001). WSPs have shown removals of 2–4 log10 for viruses, 3–6 log10 for bacteria, 1–2 log10 for protozoan cysts (Templeton, Andrews, & Hofmann, 2005) and up to a 3 log10 for helminth eggs (Jiménez, Mara, Carr, & Brissaud, 2010). Protozoan removal in WSPs can be highly seasonal, with higher removal in the summer/autumn months (2.5–3 log10) and lower removal in the winter/spring months (0.5–1.2 log10) reported for an Australian pond system (King et al., 2015).
There are many types of pond designs (Symonds et al., 2014), with the most common configuration being a sequence of facultative and maturation ponds (Shilton, 2005). In a relatively simple configuration (Fig. 5 A) there is no pretreatment and only one primary facultative pond is connected to the maturation ponds. However, more advanced facilities include an anaerobic pretreatment step before the facultative pond (Fig. 5B). The different types of pond systems are described in the following sections.
Figure 5.
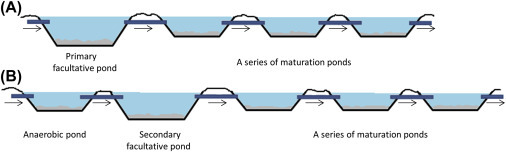
Schematics of two common variations of standard pond systems. (A) One primary facultative pond with no pretreatment and (B) pretreatment using an additional anaerobic pond.
4.3.2.1. Anaerobic Ponds
Anaerobic ponds operate without oxygen and function to remove organic bulk. They have short retention times and can remove 40–70% of the organic load in wastewater (Shilton, 2005). Sedimentation is a major mechanism of pathogen elimination in these ponds. In general, the density and hence settling velocity of microorganisms is low [e.g., 30 mm/day for Cryptosporidium oocysts (Medema, Schets, Teunis, & Havelaar, 1998)], so attachment of pathogens to denser particles is required for sedimentation to occur. Helminth eggs, which are large and relatively dense, readily settle under gravity and are removed in these ponds (Campos et al., 2002).
4.3.2.2. Facultative Ponds
Facultative ponds operate under both aerobic and anaerobic conditions. Pathogen removal in these ponds is a complex process involving factors such as sedimentation, sunlight-mediated inactivation, high pH, low carbon dioxide, and high oxygen concentrations (Campos et al., 2002). These ponds consist of different functional layers or zones, namely anaerobic, heterotrophic and photic zones (Bitton, 2010). The lowest zone is anaerobic and removes sedimented organic matter in the absence of oxygen while producing gases such as methane and carbon dioxide (Faleschini & Esteves, 2011). In the heterotrophic zone, carbon dioxide stimulates algal growth, which provides oxygen for heterotrophic aerobes to decompose organic matter (Bitton, 2010). The top or surface zone, also known as the photic zone, is characterized by high rates of algal photosynthesis causing the water to become highly oxygenated. This oxygen is utilized in the aerobic decomposition of organic matter by heterotrophic bacteria (Von Sperling, 2007). Facultative ponds are generally shallow and range from 1 to 2.5 m in depth, with detention times that range from 5 to 30 days (Bitton, 2010, Shilton, 2005)
4.3.2.3. Maturation Ponds
Maturation ponds are 1–2 m deep with a detention time of approximately 20 days (Bitton, 2010). Their major function is pathogen removal but they also serve to remove nutrients (Shilton, 2005, Von Sperling, 2007). A series of small maturation ponds is usually used instead of a single maturation pond (Shilton, 2005) because it easier to prevent short circuiting. Maturation ponds tend to be shallower than other ponds since this allows the efficient removal of pathogens by solar radiation (UV penetration), high pH, high dissolved oxygen, and low nutrient content (Symonds et al., 2014, Von Sperling, 2007). Maturation ponds can achieve 100% removal of protozoans and helminth eggs (Amahmid, Asmama, & Bouhoum, 2002) and 99% removal of coliforms (Von Sperling, 2007).
WSPs are commonly used in developing countries such as India and Bolivia, as well as in developed nations such as Australia (Phuntsho et al., 2016), but mechanisms for the removal of enteric viruses are not well understood and require further study (Symonds et al., 2014).
4.3.3. High Rate Algal Ponds
A less commonly used pond format for treating primary effluent is the high rate algal pond (HRAP). These are generally shallow ponds that are well mixed to promote the growth of green microalgae (Craggs, Park, Heubeck, & Sutherland, 2014), which provides reductions in the organic load and pathogen numbers (Araki, Martin-Gomez, Becares, De Luis-Calabuig, & Rojo-Vazquez, 2001). An added benefit of HRAP is it can also cause pathogen inactivation, with one study measuring a 97% reduction in Cryptosporidium infectivity (Araki et al., 2001). In addition to secondary treatment, HRAP provides some tertiary treatment, with the algae removing contaminants such as heavy metals (Ramanan, Kim, Cho, Oh, & Kim, 2016). While HRAP has a higher energy demand compared with other pond systems, it is relatively low energy and cost-effective compared with other secondary treatment options, especially if energy-efficient paddle mixers are used. HRAP can be used either directly with primary effluent or with wastewater that has been pretreated by anaerobic ponds or clarifiers to remove solids (Craggs et al., 2014). If carbon is limiting in the wastewater then the performance of HRAP can be enhanced by aeration with CO2 (Craggs et al., 2014). There has been increased interest in HRAP as an option for culturing algae for biofuel production, as well as a treatment option for limiting blooms of cyanobacteria since HRAP allows better control of the bacterial/microalgal community compared to WSPs (Ramanan et al., 2016). The smaller footprint of HRAP systems (compared with WSPs) makes them an attractive option for urban or semiurban regions that are rapidly expanding and require a decentralized sewage treatment option that is low cost with minimal land use.
4.4. Tertiary Treatment and Disinfection
Tertiary treatment is the final polishing step required to achieve the desired quality of reclaimed water (Guardabassi, Wong, & Dalsgaard, 2002) and is mediated by a variety of chemical, biological, and physical processes. The selection of treatment processes is dependent upon the desired end use. In the case of applications such as woodlot or subsurface irrigation, where human contact with the reuse water is unlikely, secondary treated effluent might be suitable without the need for further treatment. However, the tertiary treatment requirements for reuse water increase as the likelihood of human exposure to the reuse water increases. In general, the effluent needs to be treated and/or disinfected sufficiently to reduce pathogen numbers to levels that meet public health safety requirements. These target numbers are determined by risk assessments that consider exposure routes, exposure amounts, infectious doses, and disease outcomes (AGWR, 2006). Nutrients such as phosphorous can be precipitated out by the addition of lime or alum (Templeton & Butler, 2011) and, less commonly, microalgae (e.g., using HRAP) have also been reported to effectively remove nitrogen and phosphorous (Aslan & Kapdan, 2006). Pathogens can be physically removed by filtration methods, such as dissolved air flotation filtration or microfiltration for bacteria and protozoans and ultrafiltration for virus removal. Membrane filtration methods are highly effective for the removal of pathogens, especially larger organisms such as protozoa and bacteria (Ottoson et al., 2006). Filtration has the added benefit of removing particulates to improve downstream disinfection processes that are required to inactivate remaining pathogens.
The final and possibly most important step in tertiary treatment (in terms of microbial safety at least) is the disinfection of the wastewater prior to reuse. UV radiation and chlorination are widely used and well characterized disinfection processes. Chlorine is added to treated wastewater for predetermined periods of time designed to optimize microorganism exposure and inactivation (described in more detail in Section 7.1), following which any residual chlorine is neutralized prior to discharge to the environment or aquifer storage (Templeton & Butler, 2011). By convention, chlorine disinfection targets are set by contact time, or CT, which is measured as the product of the chlorine dose (in mg/L) and time (in minutes). It is therefore possible to achieve the same CT using a high dose/short time or low dose/long time. The CT is affected by the level of free available chlorine, which is determined by temperature and pH. This is an important consideration since the required CTs for pathogen inactivation are much higher in cold water [e.g., a CT of 8 mg min/L for viruses 5°C, (EPA, 2003)] than in warmer water [e.g., a CT of 3 mg min/L for viruses 20°C, (EPA, 2003)]. The CTs for chlorine disinfection of drinking water or wastewater have been determined for the major enteric pathogens and these are defined in many guidelines (AGWR, 2006, EPA, 2003, WHO, 2006). Achieving the desired CT in reuse water can be more difficult compared with drinking water on account of higher chlorine demand and also due to the formation of chloramine in cases when ammonia is present, both of which make the CT calculation more complex (Keegan, Wati, & Robinson, 2012). Chloramine is a far less potent oxidant compared to chlorine and requires orders of magnitude higher CTs to achieve the same level of disinfection as chlorine (Keegan et al., 2012). Common enteric bacterial pathogens, such as Salmonella, Campylobacter, and E. coli, have relatively low chlorine CTs of 1 mg min/L or less (WHO, 2006). Viruses are also effectively inactivated by chlorine, although they are slightly more resistant compared with enteric bacteria. Chorine is ineffective against some protozoan parasites, particularly Cryptosporidium [CT 15,300 mg min/L for 3 log10 inactivation, (WHO, 2006)] and Toxoplasma [CT >144,000 mg min/L, (Wainwright et al., 2007)].
UV radiation is often preferred to chlorination because it requires fewer steps, is safer (compared with handling chlorine gas or other methods of generating chlorine), and avoids the production of disinfection by-products (Templeton & Butler, 2011). However, UV disinfection also tends to be more expensive than chlorination, especially for building the required infrastructure. UV treatment involves exposure of wastewater to a UV-C light source (described in more detail in Section 7.2), usually a UV lamp enclosed in a quartz sleeve within a stainless steel pipe or suspended in a concrete channel (Gadgil, Kazakevicius, & Drescher, 2002). UV is particularly effective against bacteria and enteric protozoans (Giardia and Cryptosporidium) but some viruses, in particular adenovirus, have high UV resistance (Hijnen, Beerendonk, & Medema, 2006). A combination of UV and chlorination can be particularly effective, using suitable doses of chlorine for virus inactivation and UV for Cryptosporidium inactivation, allowing the most cost-effective use of each treatment technology. Other disinfection methods have also been developed and tested. Ozone has been proven to be effective against viruses, protozoan cysts, and helminth eggs (Paraskeva & Graham, 2002) and peracetic acid has also been considered a strong disinfectant because of its effective bactericidal, fungicidal, sporicidal, and virucidal properties (Kitis, 2004). Conductive-diamond electrochemical oxidation (CDEO) has proven effective in disinfecting wastewater and a combination of CDEO with ultrasound technology increased the disinfection efficacy by reducing the agglomeration of E. coli cells (Llanos, Cotillas, Cañizares, & Rodrigo, 2015). However, this technology does not appear to have been adopted for large-scale commercial use.
5. Turbidity and Particles in Wastewater
Turbidity has been considered an important indicator of water quality for many years (Mccoy & Olson, 1986) and is related to other water quality parameters such as TSSs and microbial load, although the relationships are variable (Hannouche et al., 2011; Joannis, Ruban, Gromaire, Bertrand-Krajewski, & Chebbo, 2008). It is always essential to monitor turbidity when treating drinking water or wastewater because it interferes with disinfection processes, such as chlorination and UV radiation (Berman, Rice, & Hoff, 1988; Dietrich, 2003, Madge and Jensen, 2006, Rowe and Abdel-Magid, 1995). Turbidity can be defined as the optical property of water that causes light shone through the water sample to be scattered rather than absorbed or transmitted (Hannouche et al., 2011). Turbidity also defines the clarity or cloudiness of water (Madhavi & Rajkumar, 2013). In wastewater, the suspended matter (particles) contributing to turbidity includes clay, grit, organic or inorganic matter, and algae or other microorganisms (Madhavi & Rajkumar, 2013). In the effluent of a WWTP after different treatment steps, turbidity provides a measure of the remaining suspended solids or particulate matter in the treated effluent and can provide a measure of treatment performance. High turbidity is associated with the availability of a support medium for the transport of attached pathogenic microorganisms and with increased disinfection demand (Mccoy & Olson, 1986). Turbidity is an indicator of particulate pollution and can be used to measure and control effluent quality, which is particularly important for effluent discharge since suspended solids can have adverse environmental impacts (Hannouche et al., 2011). Increases in wastewater turbidity have been associated with increased densities of bacteria, Cryptosporidium oocysts, and Giardia cysts (Crittenden, Trussell, Hand, Howe, & Tchobanoglous, 2012).
5.1. Turbidity Measurement
Turbidity is measured using a nephelometer and expressed in Nephelometric Turbidity Units (Davies-Colley and Smith, 2001, Rowe and Abdel-Magid, 1995). The basic principle involves passing a light beam through a sample and measuring any scattered light at a 90 degree angle from the incident light path (Fig. 6 ). The amount of light measured reflects the number of particulates scattering the light beam (Joannis et al., 2008).
Figure 6.
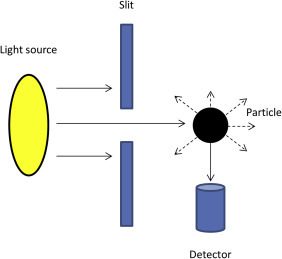
Simplified representation of the operating principles of a nephelometer. Light is directed from a light source to the sample through a narrow slit and the reflected light is collected by a detector and analyzed.
5.2. Particles in Wastewater
Wastewater is made up of wastes from municipal, industrial and, in some cases, agricultural sources. All of these sources can contribute particulate matter that is either inert (inorganic) or of biological origin (organic) and can be different sizes, shapes, and densities (Madge & Jensen, 2006). Particles can be categorized based on their size (Fig. 7 ) and are either dissolved (<0.001 μm), colloidal (0.001–1 μm), supracolloidal (1–100 μm), or settleable (>100 μm) (Azema et al., 2002, Pallarès et al., 2011). Particles in water can be of various shapes such as spherical, semispherical, ellipsoid, rod-shaped, strings, or random coils (Crittenden et al., 2012).
Figure 7.
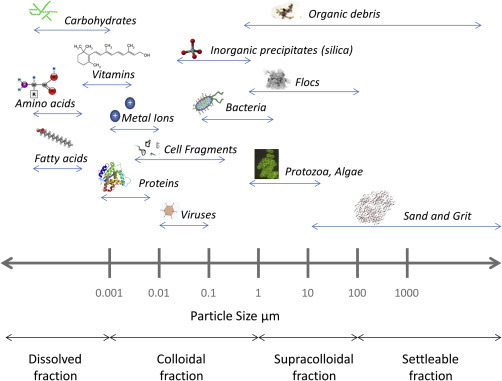
Comparison of the size distribution of different types of particles in wastewater.
The organic matter in wastewater is of high molecular weight, up to 103 Da, and can be classified into different fractions, such as carbohydrates, fatty acids, and proteins (Sophonsiri & Morgenroth, 2004). Depending on their composition, organic particles can be readily biodegradable, slowly biodegradable, soluble nonbiodegradable, or particulate nonbiodegradable (Sophonsiri & Morgenroth, 2004). Municipal wastes are the source of many of these organic fractions and contain small colloids to large particles of up to 63 μm (Sophonsiri & Morgenroth, 2004). Industrial and agricultural wastes mainly comprise soluble organic matter and large particles of >1.2 μm and >10 μm, respectively (Sophonsiri & Morgenroth, 2004). Wastewater contains many inorganic constituents such as metals, salts, and surface sediments. Toxic heavy metals such as copper (Cu), zinc (Zn), cadmium (Cd), nickle (Ni), and chromium (Cr) mostly enter wastewater via industrial wastes (Barakat, 2011). Domestic wastewater also includes contaminants such as soaps, detergents, and other household chemicals.
5.3. Effect of Wastewater Treatment on Particles
Particles in wastewater are affected by physical, chemical, and biological processes during treatment (Garcia-Mesa, Delgado-Ramos, Muñio, Hontoria, & Poyatos, 2012). The PSD is different for primary, secondary, and tertiary treated water (Neis & Tiehm, 1997). Dense, readily settleable inorganic particles, such as sand and grit larger than 0.01 mm, are removed in preliminary treatment stages and less dense organic and inorganic particles in the size range of 0.1 mm–35 μm are removed in primary sedimentation tanks (Shon, Vigneswaran, Kandasamy, & Cho, 2007). Measurement of the PSD for three different municipal primary effluents using a combination of differently sized membrane filters or sieves showed that primary effluent is dominated by small particles <8 μm, which comprise 70–88% of the particles by mass (Neis & Tiehm, 1997). Parallel analysis of primary effluent PSDs using laser scanning was shown to compare favorably with physical sizing by straining (Neis & Tiehm, 1997).
Traditional primary treatment applies sedimentation under gravity and can remove particles of <50 μm, whereas smaller particles are more efficiently removed by chemically enhanced primary treatment processes (CEPT) and chemical–biological flocculation (CBF) (Zhang, Zhao, Xia, Liu, & Kang, 2007). CEPT has been shown to be effective in removing particles in the size range of 20–80 μm; however, it was not effective in removing particles <10 μm, whereas CBF was highly effective at removing particles >5 μm (Zhang et al., 2007). The majority of organic particles in wastewater are colloidal and supracolloidal; however, after biological treatment (CBF), the remaining organic matter is in the soluble fraction (García-Mesa et al., 2010). Particles can transform during biological treatment, with most of the settleable and suspended organic matter metabolized and incorporated into sludge mass or active organisms suspended in the bulk water. This biotransformation of particles also occurs in subsequent secondary or tertiary treatment steps. Most of the settleable and suspended inorganic particles are entangled in the sludge mass, while nonsettleable, nonbiodegradable, or dissolved organic and inorganic particles pass out in the primary effluent (Henze et al., 2008).
During secondary treatment (e.g., in activated sludge plants), fine particulates, colloidal particles, and large molecules become entangled to form flocs (Davies, 2005). Flocs are made up of a diverse community of microorganisms and nonliving organic matter, such as extracellular polymeric substances (EPS), which are secreted by microorganisms and play an important role in floc formation (Fig. 8 ). Bacteria, fungi, and protozoans attach to the internal and external surfaces of the flocs, which are typically in the size range of 10–1000 μm (Davies, 2005). Secondary clarifiers remove most of the flocs and the clarified water can be disinfected and discharged to the environment or subjected to tertiary treatment. Particle size analysis of effluent from a conventional secondary clarifier identified with a size range of 1–10 μm (Wu, Jiang, & Wheatley, 2009). Particle size and load within clarified effluent is influenced by the settling performance of the activated sludge biomass, as poor settling biomass (termed sludge bulking) results in the carryover of high concentrations of suspended solids and attached pathogens, which can also impact negatively on downstream tertiary treatment processes such as filtration and disinfection. Tertiary treatment, depending on the process, further reduces the loads of particulates and organic contaminants, and disinfection is used as the final step to reduce pathogen numbers to below guideline levels for reuse (Shon et al., 2007).
Figure 8.
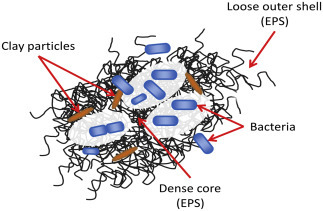
Bacterial Floc: a typical structure of a bacterial floc held together by extracellular polymeric substances (EPS) associated with inorganic clay particles.
Reproduced from Farnood, R. (2014). New insights into the ultraviolet disinfection of wastewater. In D. Santoro (Ed.), Wastewater and biosolids treatment and reuse: Bridging modeling and experimental studies. ECI Symposium series, Otranto, Italy.
5.4. Particle Characterization Techniques
Organic matter in wastewater is characterized by its BOD, COD, total organic carbon, and volatile suspended solids (Sophonsiri & Morgenroth, 2004). The major issue with studying the size distribution of particles in wastewater is that they are diverse, ranging from a 1000 Da in the case of organic molecules to hundreds of microns in the case of biological flocs. Therefore, multiple methods are required in combination, such as filtration and chromatography (Sophonsiri & Morgenroth, 2004). Different techniques for measuring the size of particles include microscopy, electrical sensing, light scattering, light obstruction, and membrane filtration (Aguilar, Saez, Llorens, Soler, & Ortuno, 2003). Analysis by microscopy allows direct visualization of the particles, with measurements typically made using computer-aided image analysis, which can automate both particle sizing and counting from the captured images (Emerick, Loge, Thompson, & Darby, 1999). The only issue with microscopy is that the sample needs to be dispersed in such a way that large and small particles are uniformly distributed on the slide (Aguilar et al., 2003). The electrical sensing technique applies voltage across a small orifice through which the particles are passed. The changes in electric potential are directly related to the volume of particles passing (Aguilar et al., 2003). Coulter counters and multisizers are such instruments, which have been used previously for measuring PSD in raw untreated wastewater samples (Chavez et al., 2004).
Particle interactions with light can also be used to estimate particle size. In the case of light obstruction, the amount of light blocked by a particle is measured as it passes through a sensing zone. A particle analyzer uses this principle and measures the size and quantity of particles that pass through the diode (Jolis, Lam, & Pitt, 2001). The light scattering method uses a similar principle to flow cytometry, with the magnitude of light deflection around the particle (equivalent to forward scatter) used to estimate particle size (Aguilar et al., 2003). Instruments such as the Coulter laser granulometer and Sequoia LISST (laser in situ scattering and transmissometry) use this method and have been used to measure PSD in wastewater (Azema et al., 2002, Keegan et al., 2010). A limitation of the light-based methods is that the optical properties of the particles can affect sizing. Apart from size, the refractive index of a particle will also influence how the light scatters, and some diffraction-based particle analyzers use specific calibration factors for different compounds to improve the accuracy of particle sizing. As a consequence, when measuring the PSD of a water or wastewater sample using light diffraction, it is important to understand the nature of the particles being studied (e.g., are they organic or inorganic, if inorganic what type of material?) to enable the selection of appropriate calibration factors.
Particles can be analyzed using methods that separate them on the basis of size or density, such as membrane filtration, sieving, or centrifugation (Characklis et al., 2005, Dietrich, 2003). These methods are cheaper and simpler than using PSD analyzers and have the added advantage of collecting the particles for further characterization. This approach is useful for studying the partitioning behavior of chemical or microbial contaminants, which can be measured in the different fractions. For example, particles in a water sample can be stained with a colored dye and filtered using a series of membranes with different pore sizes. Particles captured on the filters can then be observed under a microscope and analyzed by an image analyzer (Dietrich, Loge, Ginn, & Başaǧaoǧlu, 2007). When using flat-bed membranes for size exclusion, caution must be employed to avoid overloading the filter and blocking the membrane pores because this results in the capture of particles smaller than the nominal pore size of the membrane, which would lead to erroneous results.
Particle structure is another important parameter to study. Scanning electron microscopy has been used to study the structure of mixed liquor particles (Fig. 9 ). There are various compartments and complexities within these particles (Fig. 9) and characterizing such structural aspects can help to elucidate the nature of particle–pathogen associations.
Figure 9.

An environmental scanning electron microscope image of a mixed liquor particle in the size range of 90–106 μm highlighting its structure. Arrows indicate different compartments outlined by fibrils.
Reproduced from Gibson, J. H., Hon, H., Farnood, R., Droppo, I. G., & Seto, P. (2009). Effects of ultrasound on suspended particles in municipal wastewater. Water Research, 43, 2251–2259.
6. Pathogen–Particle Associations
Bacteria, viruses, and protozoans, from a diverse range of water types (wastewater, freshwater, marine, estuarine), can be free in suspension or associated with particles (Characklis et al., 2005, Dietrich et al., 2007, Malham et al., 2014). There are two different types of particle–microorganism interactions; particles physically associated with microorganisms as clumps, and particles not physically associated with microorganisms but providing protection by shielding them from UV light or by contributing to disinfectant demand (Sophonsiri & Morgenroth, 2004). It has been found that the shielding effect of particles increases with increasing particle size (Madge & Jensen, 2006). The binding of microorganisms to particles can be through electrostatic attractions, hydrophobic interactions or physical entrapment (Templeton et al., 2005). The association between microorganisms and particles can change with time, as the formation and disaggregation of biological flocs is a continual process in environmental waters (Malham et al., 2014). Microorganisms associated with denser particles settle quickly, whilst microorganisms associated with lighter particles tend to stay suspended in water and survive for longer (Characklis et al., 2005). Fig. 10 shows bacteria attached to different types of wastewater particles (Ben van den Akker, unpublished data).
Figure 10.
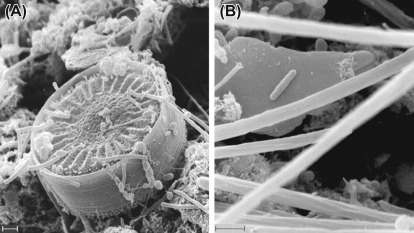
Attachment of Escherichia coli to organic and inorganic particles: Scanning electron microscopic image of (A) growth of E. coli attached to a diatom in a biofilm (B) E. coli attached to a clay particle. Scale bars indicate 1 μm.
The binding of pathogens to particles has been studied using either direct visualization or physical separation techniques. High resolution visualization using confocal microscopy or fluorescent microscopy has been used to image the attachment of protozoan oocysts to inorganic particles and river sediments (Li et al., 2009, Searcy et al., 2005). The same techniques used to characterize particles (described above in Section 5.4) can be used to study pathogen–particle binding. Both size exclusion and centrifugation have been used to determine the partitioning behavior of protozoans (Cizek et al., 2008). Centrifugation has the advantage over filtration because it separates microorganisms and particles using both size and density (Cizek et al., 2008). Centrifugation has been used to analyze the partitioning behavior of E. coli, enterococci, C. perfringens, Cryptosporidium, Giardia, and coliphage in storm water (Characklis et al., 2005, Cizek et al., 2008, Krometis et al., 2007). There are few reports characterizing the nature of the wastewater particles with attached pathogens and this is a knowledge gap that requires further investigation.
6.1. Bacterial Associations With Particles
There can be different types of associations between bacteria and particles. Nutrients released from the surface of different types of particles may attract bacteria, which can migrate to the particles, attach and colonize the particle surface (Winkelmann & Harder, 2009). Bacteria have an affinity for inorganic particles and can be adsorbed onto the surface of these particles (Kristian Stevik et al., 2004). Various factors influence bacterial association with particles (summarized in Table 4), for example, particle size, particle composition, and the age or growth status of the bacteria (Kristian Stevik et al., 2004, Madge and Jensen, 2006). The adsorption of bacteria to the surface of a particle can be explained using the Derjaguin, Landau, Verwey, and Overbeck (DLVO) double layer theory (Hipsey et al., 2006, Kristian Stevik et al., 2004). According to the DLVO theory, bacterial attraction occurs at two zones around a particle, the first (“primary energy”) is within 1 nm of the particle surface and the second (“secondary energy”) is within 5–10 nm of the particle surface (Hipsey et al., 2006, Kristian Stevik et al., 2004). There are consequently two steps involved in the adsorption of bacteria to a particle surface. The first step occurs within the secondary energy zone and is weak and can be reversed (Hipsey et al., 2006, Kristian Stevik et al., 2004). In this step, the bacterial cell overcomes any repulsive electrostatic forces and adsorbs to the particle's surface. Weak Van der Waal and electrostatic forces contribute to this adsorption and can be easily overcome by other physical forces such as a change in the ionic composition of the medium or hydraulic shear forces (Hipsey et al., 2006, Kristian Stevik et al., 2004). The second adsorption step, also known as adhesion, occurs within the primary energy zone and is stronger and irreversible. It occurs when the bacterial cell forms a permanent bond with the surface and involves a large amount of energy (Hipsey et al., 2006, Kristian Stevik et al., 2004). The adhesion can be mediated by extracellular polymers such as EPS, via the formation of by dipole–dipole interactions or hydrogen bonding (Kristian Stevik et al., 2004). Apart from direct adsorption to a particle surface, bacteria can associate with particles by either harboring in the cracks of particles or by adhering to biofilms (LeChevallier et al., 1984, Winkelmann and Harder, 2009).
The formation of biofilm requires actively growing bacteria. Considering that pathogenic bacteria are unlikely to replicate under the nutrient and temperature conditions typical of wastewater treatment systems (Keegan et al., 2010), it is more likely that heterotrophic bacteria will mediate the formation of biofilm or production of EPS and bacterial aggregates, which form a substrate for the binding of pathogenic bacteria, viruses, or protozoans.
In unchlorinated drinking water, culture independent methods (cell counts by flow cytometry and estimates of cell numbers using ATP measurement) were used to determine that there were 25–50 bacterial cells associated with each particle (Liu et al., 2013). However, this study did not determine the particle size, capturing particle-associated bacteria using 1.2 μm membranes. Analysis of rainfall runoff flowing into a drinking water reservoir identified a relationship between turbidity and coliform/E. coli counts, with the strongest correlation (R2 = 0.8) being with particles in the size range of 3.2–17 μm (Hipsey et al., 2006). While the authors speculated that this strong correlation was due to association between the particles and bacteria, analyses (such as size fractionation or microscopy) were not conducted to verify the association. Analysis of bacteria in estuary water demonstrated that the numbers of bacteria associated with particles (measured using 3 μm filters) increased with increased turbidity (Bidle & Fletcher, 1995). Few equivalent studies have been conducted for wastewater particles.
Coliform bacteria, which fall in the size range of 1–10 μm, have been shown to be associated with particles greater than 10 μm in diameter (Templeton et al., 2005). Fluorescent in situ hybridization (FISH) has been used to detect particle-associated microorganisms in wastewater and allows the examination of organisms while they are in contact with the particles, providing a better understanding of their associations (Örmeci & Linden, 2008). Particle-associated coliform bacteria in wastewater have been enumerated using 1.2 μm membrane filtration and in situ hybridization (Loge, Emerick, Ginn, & Darby, 2002). The association of coliforms with particles in activated sludge appeared to decrease with increased mean cell residence time through the ASP (Loge et al., 2002), although it was not clear if the residence time affected coliform-particle binding, or if the decrease in coliforms reflected natural die-off in the ASP as a function of time.
6.2. Viral Associations With Particles
Enteric viruses (15–80 μm) are much smaller than bacteria (1–10 μm) and are generally associated with much smaller particles, less than 10 μm in size (Madge & Jensen, 2006). The association of viruses with particles (Fig. 11 ) depends upon the surface charge of the particle and virus charge, morphology, and size (Madge & Jensen, 2006). Different virus species have different proteins that protect their genome (called a capsid or virus coat), these differences are likely responsible for differences in charge and removal through treatment processes and mediate adsorption to particles. Viruses, depending on species, can be readily adsorbed onto sand particles, clay particles, suspended colloids, transparent extracellular polymer, and fecal matter via electrostatic and hydrophobic interactions (Mari, Kerros, & Weinbauer, 2007; Templeton, Andrews, & Hofmann, 2007). These associations increase their survival rates and render them more resistant to disinfection processes (Templeton et al., 2007).
Figure 11.
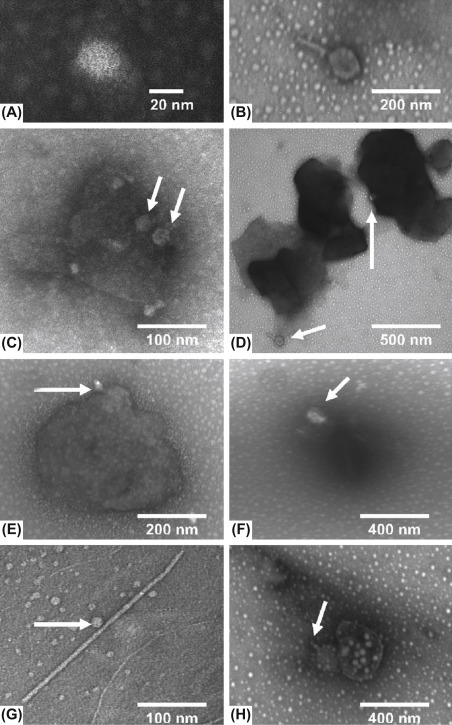
Particle associated viruses: Transmission electron microscopy images of MS2 (left panels) and T4 bacteriophage (right panels). (A and B) Phage free in suspension; (C and D) phage associated with kaolin clay particles; (E and F) phage associated with humic acid flocs; (G) MS associated with a bacterial flagellum; (H) T4 associated with a sludge particle. Arrows indicate the bacteriophage.
Reproduced from Templeton, M. R., Andrews, R. C., & Hofmann, R. (2005). Inactivation of particle-associated viral surrogates by ultraviolet light. Water Research, 39, 3487–3500.
Although there have been many studies of the occurrence of viruses in wastewater (reviewed by Keegan et al. (2010)), there have been few studies of the association of viruses with particles in wastewater. The association of norovirus with wastewater particles has been successfully demonstrated using cascade filtration of water from a WSP (Silva et al., 2008). In this report, sets of different-sized filters were used to capture particle-associated norovirus. The filters were then analyzed for the presence of virus to determine the size of particles to which the virus was attached (Silva et al., 2008). Norovirus, detected using reverse-transcription PCR, were found free in solution and on a wide range of particle sizes, including large settleable particles >180 μm, smaller particles captured on a 0.45-μm filter, and colloidal particles. This finding contrasts with a study of virus–particle associations in wastewater from an ASP, which used continuous flow centrifugation of filtration to size separate particles and detected enteroviruses using cell culture of buffalo green monkey kidney cells (Hejkal, Wellings, Lewis, & LaRock, 1981). This study reported minimal association between viruses and large particles, with 72% of virus particles associated with particles <0.3 μm in primary effluent, and 96.6% of virus particles associated with particles <0.3 μm in clarified secondary effluent. As discussed earlier, a possible reason for the differences between the two studies could be the physical differences between the different virus species (norovirus vs enterovirus), although it is also possible that the differences could be due to differences in the particles in activated sludge and pond systems.
6.3. Protozoan Associations With Particles
Parasites such as Cryptosporidium and Giardia exist as oocysts and cysts in wastewater streams. There are few studies about the association of either oocysts or cysts with suspended wastewater particles. With the exception of bacterial specific characteristics such as the production of EPS and the presence of pili/fimbriae, many of the factors that influence attachment of bacteria to surfaces (Table 5 ) might also play a role in (oo)cyst attachment to particles. The attachment of oocysts to inorganic particles in soil is variable and greatly influenced by any organic molecules present, with oocysts showing significant attachment to clay loam in the presence of manure (Kuczynska, Shelton, & Pachepsky, 2005). Oocysts have a negative surface charge and both steric and electrostatic forces can contribute to association with particles (Searcy et al., 2005). In contrast Giardia cysts are hydrophobic and may consequently interact with particles in different ways compared with C. parvum oocysts (Dai, Boll, Hayes, & Aston, 2004). Oocysts or cysts can interact with surface attached microbial communities (biofilms) and such attachment can influence the transport of (oo)cysts during water or wastewater treatment, contributing to increased sedimentation and removal (Helmi et al., 2008; Searcy, Packman, Atwill, & Harter, 2006). Considering this and other studies of the factors influencing oocyst and cyst attachment to suspended particles in surface waters (Medema et al., 1998, Searcy et al., 2005), it is likely that there is some level of association between (oo)cysts and wastewater particles.
Table 5.
Summary of the factors affecting the association of bacteria with particles
| Particle type | Physical |
|---|---|
| DLVO | Derjaguin, Landau, Verwey and Overbeck double layer theory There are attractive and repulsive electrostatic forces between bacteria and particle surfaces that are stratified into three zones; a near zone (“primary minimum”) within 1 nm of the particles surface, in which bacteria are attracted to the particle, then an electrostatic repulsion zone, then a “secondary minimum” attractive zone 5–10 nm from the particles surface. The strength of attraction and repulsion between the bacterium and particle is affected by the ionic strength of the matrix such that increased ionic strength increases the repulsion. |
| Temperature | Decreased temperature decreases the energy available for adsorption and increases the viscosity of the bacterial cell wall or capsular polymers which also decreases adhesion. |
| Water flow | Higher flow/velocity reduces the contact time between bacteria and particles and also increases hydraulic shear which can disrupt the first stage of binding under DLVO. |
| DOM | Dissolved organic matter (DOM) Organic matter attached to particle surfaces may increase bacterial adsorption if positively charged, but organic material in the water may compete with bacteria for adsorption sites. The type and concentration of DOM might also influence bacterial chemotaxis/biofilm formation. |
| Composition and size of particle(s) | The size, surface area, volume, and surface roughness can all influence the number of adsorption sites and rate of disassociation. |
| Particle type | Chemical |
|---|---|
| pH | The effect of pH on bacterial adsorption is related to the characteristics of the adsorbing surfaces (e.g., carboxyl and amino groups on bacterial surfaces) and the ionic strength of the matrix. Particle surfaces commonly have a negative electrostatic charge, as do bacteria at neutral pH. Bacterial adsorption to inorganic particles increases as their zeta potential decreases. |
| Hydrophobicity | Bacterial hydrophobicity and charge increase during exponential growth, and this promotes adhesion to particles. |
| Ions | The ionic strength of the matrix affects electrostatic interactions between pathogens and particles. Divalent cations (e.g., Ca2+, Mg2+, Cu2+, Zn2+) promote adsorption by acting as a bridge between negatively charged particles/bacteria, more so than monovalent cations (e.g., Na+). Anions do not affect adsorption. |
| Gouy–Chapman | The charge on the surfaces of particles or bacteria is neutralized by oppositely charged ions in the water. This causes formation of a Gouy–Chapman diffuse electric double layer. Bacterial-particle adsorption is affected by the thickness of this layer, which is a function of ionic strength. |
| Particle type | Microbiological |
|---|---|
| Cell surface | Flagella, fimbriae, and pili have the effect of increasing diameter and promote the breach of electrostatic barriers. Motility increases the likelihood of bacterial–particle contact, and can overcome electrostatic repulsion. |
| Bacterial size and shape | Smaller bacteria more likely to be lodged in crevices in particles. |
| Extracellular polymeric substances (EPS) and biofilm | Extracellular polymeric secretions are often polysaccharides with the potential for hydrogen bonding and dipole–dipole-type interactions, and these characteristics promote irreversible adhesion even in the absence of favorable DVLO association conditions. Rarely applicable to pathogenic bacteria in the environment which do not actively grow and produce EPS. The presence of EPS may affect the adsorption of pathogenic bacteria to particles. |
| Chemotaxis | Bacteria are attracted to many chemicals, and this may play a role in particle adsorption. |
| Bacterial concentration | Particle adsorption may be proportional to cell concentration. The numbers of pathogenic bacteria are in turn related to factors that affect survival, such as pH, temperature, nutrient availability, and predators. |
In one study, the association of oocysts with particles in surface water was thought to aid in the recovery efficiency of oocysts from the water sample, but this was dependent on particle size and concentration method (Feng et al., 2003). Oocysts and cysts are not thought to attach to inorganic particles in the water column (Dai & Boll, 2003); however, considering the effect of organics on oocyst binding in soil it is possible that the organics in wastewater could similarly facilitate binding of oocysts to inorganic particles. The surface charge characteristics of particles and microorganisms can alter during the wastewater treatment processes (Medema et al., 1998) and certain surface macromolecules can hinder the attachment of oocysts to surfaces (Kuznar & Elimelech, 2006). It is therefore possible that the nature of the interactions can change depending on the stage of treatment. Oocyst age or integrity might also play a role in particle associations. Characterization of oocysts in raw sewage and clarified secondary effluent suggested that damaged, noninfective oocysts were preferentially removed during ASP treatment (King et al., 2015). In this study, the total number of oocysts decreased following ASP treatment, but the proportion of infectious oocysts in the clarified effluent (31%) increased compared with the proportion of infectious oocysts in the raw sewage (10%), suggesting selective removal of noninfectious oocysts (King et al., 2015). However, this study did assess if particle binding was responsible for the oocyst removal. While it is possible for oocyst–particle association, a PCR-based detection study suggested that oocysts in secondary effluent were not particle associated (Tsuchihashi, Loge, & Darby, 2003). The association of protozoan parasites with wastewater particles still requires further investigation to determine if this occurs at different treatment stages and how this might impact oocyst removal and inactivation.
7. Impact of Pathogen–Particle Associations on Disinfection Processes
7.1. Chlorination
Chlorination has been used for many decades and is the leading technology for disinfection of recycled water. Chlorine has high oxidizing capacity and is mostly used in high concentrations to kill pathogens, although high dosages can cause the formation of harmful by-products (Virto, Manas, Alvarez, Condon, & Raso, 2005). Excessive use of chemicals beyond that required to achieve target levels of disinfection is also not cost effective and increases the cost of producing recycled water. Chlorine reacts with the cell membrane and alters or damages vital cell functions (Venkobachar, Iyengar, & Prabhakara Rao, 1977). Exposure to chlorine causes stress to microorganisms via irreversible cell injuries and in some cases it causes bacteria (e.g., Salmonella typhimurium) to enter a viable nonculturable state if the dose is not high enough to cause outright cell death (Oliver, Dagher, & Linden, 2005). A major disadvantage of chlorination is that the majority of protozoans with cyst forms (Toxoplasma, C. parvum, and G. duodenalis), helminths, and certain strains of bacteria are highly or moderately resistant to chlorine (Liberti, Notarnicola, & Petruzzelli, 2003). After disinfection, dechlorination is generally carried out to remove residual chlorine, which increases the overall cost of the process (Lazarova, Savoye, Janex, Blatchley, & Pommepuy, 1999). This step is critical to protect the environment that receives any wastewater discharges because chlorine and derivatives (e.g., chloramines) are toxic to many aquatic organisms.
Microorganisms (bacteria and viruses) associated with particles are more resistant to chlorine compared with microorganisms free in suspension (Winward, Avery, Stephenson, & Jefferson, 2008). The protective effect is related to the nature of the particle, with organic particles providing more protection compared with inorganic particles (Berman et al., 1988). Chlorine is able to penetrate particles by radial diffusion (Dietrich, 2003, Winward et al., 2008) in a two-step process in which it passes through different boundary layers of the wastewater particle (Fig. 12 ). Chlorine penetration of particles is therefore controlled by the initial chlorine concentration, which influences the diffusion rate (Winward et al., 2008). The presence of organic matter increases the chlorine demand of wastewater; the residual-free chlorine reduces with an increase in the amount of organic matter and reduces the availability of free chlorine for disinfection (Winward et al., 2008). The presence of organic matter can also stabilize the cell membrane and reduce the sensitivity of bacteria to chlorine by reducing the access of chlorine to the cell membrane (Virto et al., 2005).
Figure 12.
Wastewater particle structural pathways: various interstitial diffusive layers of a wastewater particle.
Modified from Dietrich, J. P., Başaǧaoǧlu, H., Loge, F. J., & Ginn, T. R. (2003). Preliminary assessment of transport processes influencing the penetration of chlorine into wastewater particles and the subsequent inactivation of particle-associated organisms. Water Research, 37, 139–149.
Pathogens embedded in a particle are further protected from chlorine due to the presence of the extracellular materials that surround it (Templeton et al., 2005). The protective effects of particles in terms of chlorine (or other oxidants) therefore can be linked to chlorine demand, membrane stabilization, or the incomplete penetration of the chemical into the particle in the case of embedded pathogens (Dietrich et al., 2007).
The presence of particle-associated bacteria has been linked to a phenomenon known as tailing (Fig. 13 ), which is the deviation of any disinfection process from first-order kinetics at relatively high doses of disinfectant (Dietrich, 2003, Liang et al., 2010, Loge et al., 2002, Winward et al., 2008). Tailing is characterized by no further increase in the inactivation of microorganisms even though increased amounts of disinfectant are applied (Liang et al., 2010). This phenomenon is problematic for the production of reuse water, since the survival of any pathogens or key process indicators will mean that the water is not fit for use and will require additional treatment to make it safe. Therefore, it is important to tailor the treatment processes so that disinfection processes can work with maximum efficiency, minimizing the chlorine dose required and any the residual chlorine, as well as minimizing the number of surviving cells.
Figure 13.
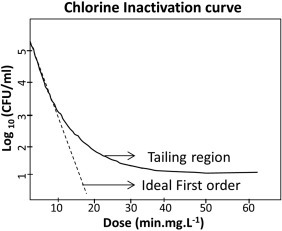
Graph of chlorine inactivation of microorganisms illustrating a first-order disinfection curve (dashed line) and disinfection with tailing (solid line).
7.2. Ultraviolet Radiation
An effective alternative to chemical disinfection is UV radiation, which is a physical process that involves exposing water to a UV light source. UV radiation is preferred to chlorine because it does not cause the formation of toxic by-products (Hassen et al., 2000, Lazarova et al., 1999). The UV light spectrum can be split into UV-C (200–280 nm), UV-B (280–320 nm), and UV-A (320–400 nm), with only UV-C used for disinfection (King, Hoefel, Daminato, Fanok, & Monis, 2008). The other components of UV (UV-B and UV-A) occur in natural sunlight. Key biological components required by microorganisms are DNA and RNA, these molecules absorb light at 260–280 nm and so can be damaged by UV. The germicidal wavelength of UV light is 254 nm, which is the wavelength that causes maximum DNA damage by inducing DNA adducts called thymine dimers, which hinder normal transcriptional and DNA replication processes and prevent cell division (Gehr, Wagner, Veerasubramanian, & Payment, 2003). Other wavelengths of UV across the spectrum cause cell death by damaging critical proteins that are required for cell function (King et al., 2008). There are two types of UV lamps used for disinfection, low-pressure UV lamps, which produce UV light around 254 nm, and medium-pressure lamps, which produce UV light of range 200–300 nm (Chen, Craik, & Bolton, 2009; Craik, Weldon, Finch, Bolton, & Belosevic, 2001).
UV radiation provides effective inactivation of bacteria, protozoa, and some viruses (Chen et al., 2009, Craik et al., 2001; Sangsanont, Oguma, & Katayama, 2012). There are two main formats for UV reactors, open channels, where UV lamps encased in quartz sleeves are suspended in the channel as water flows through it, and closed pipe systems, which are normally constructed of stainless steel with the lamps enclosed in a quartz sleeve and sited in the middle of the pipe (Hassen et al., 2000, Lazarova et al., 1999, Templeton and Butler, 2011). UV disinfection has been shown to be very effective for the inactivation of pathogenic protozoans such as C. parvum and G. duodenalis, with UV doses of 25 mJ/cm2 resulting in 3 log10 reduction of Cryptosporidium oocysts and doses of 40 mJ/cm2 have shown 4 log10 reduction of G. duodenalis (Craik et al., 2001; Linden, Shin, Faubert, Cairns, & Sobsey, 2002). Many studies have shown that low pressure UV doses of 30–40 mJ/cm2 can cause 4 log10 inactivation of pathogenic viruses; however, a high dose of 200 mJ/cm2 is required to inactivate (4 log10) adenoviruses (Eischeid, Meyer, & Linden, 2009). In the case of adenovirus, medium pressure UV is more effective, with a lower wavelength around 220 nm associated with the inactivation (Chen et al., 2009). UV has also been shown to cause 0–1.5 log10 reduction of Ascaris lumbricoides eggs, which are one of the most resistant pathogens to other disinfection processes (Brownell & Nelson, 2006).
There are some limitations with UV disinfection. The first is that it does not provide any residual disinfection, which means that any surviving microorganisms can regrow post disinfection and also that if there is any subsequent contamination of the water (e.g., due to a pipe break) then there is no disinfectant to inactivate any introduced contaminants. Another is that many microorganisms have systems for the repair of UV-induced DNA damage, which means that they can regain the capacity to grow or cause infection if the level of UV damage is not enough to overwhelm the capacity of these repair systems (Hassen et al., 2000).
Factors that affect the efficiency of UV include turbidity, suspended solids, dissolved organic carbon, lamp sleeve fouling, and lamp aging (Hassen et al., 2000). The presence of organics causes attenuation of the light, which can be overcome by the use of sufficient lamp power. However, as with chlorine, particles (or cell aggregation) can also cause tailing. An example of a UV dose–response curve is shown in Fig. 14 , with the initial steep slope indicating the inactivation of free in suspension microorganisms, followed by a plateau in inactivation representing tailing that is caused by particles (Farnood, 2014, Gehr et al., 2003).
Figure 14.
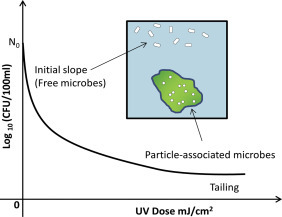
Typical UV inactivation curve for microorganisms comparing log inactivation versus UV dose, highlighting the steep inactivation slope representing inactivation of free microorganisms and a shallow slope representing tailing.
Reproduced from Farnood, R. (2014). New insights into the ultraviolet disinfection of wastewater. In D. Santoro (Ed.), Wastewater and biosolids treatment and reuse: Bridging modeling and experimental studies. ECI Symposium series, Otranto, Italy.
Particles can shield microorganisms in different ways, by providing shading or partial absorption of the UV energy to reduce the effective dose, or by scattering the light (Fig. 15 ). Large particles have been shown to affect the disinfection process more than smaller particles (Jolis et al., 2001) and particles of 50 μm or greater can completely shade pathogens from UV (Blume, Martinez, & Neis, 2002). Particles around 10 μm affect disinfection because they are capable of shielding embedded bacteria from UV radiation. In addition, some smaller particles (such as inorganic silica) can scatter the UV light and limit light penetration through the reactor, protecting microorganisms. Larger particles can be easily removed by filtration prior to disinfection in drinking water treatment facilities (Templeton et al., 2005). Therefore, if filtration can be applied upstream at wastewater treatment process, then large particles can be easily removed and the effectiveness of disinfection can be improved for both chlorination and UV.
Figure 15.
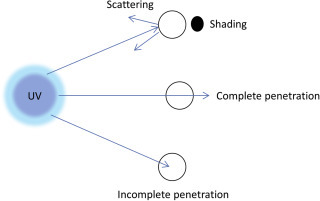
Limitations of UV radiation: different protective effects of particles on inactivation of pathogens by UV radiation.
Smaller particles can easily pass through filters, and viruses are associated with these smaller particles (Hejkal et al., 1981). However, these smaller particles tend to provide less shielding, although this is dependent on whether the particle is organic or inorganic (Jolis et al., 2001, Templeton et al., 2005). Bacteria can contribute to the formation of particles by forming aggregates, a natural phenomenon known as bioflocculation. This is often mediated by EPS, which not only holds the bacterial floc together but also provides protection to the enmeshed bacteria by absorbing UV radiation. Consequently, bacterial flocs are also a cause of tailing (Farnood, 2014). The formation of flocs can be related to the density of microorganisms, and similar aggregation can also occur in the absence of EPS when there are high densities of virus particles or protozoan (oo)cysts. This aggregation of microorganisms presents a challenge for measuring UV dose–responses—if the number of organisms used in experimental systems is too high then tailing caused by aggregation occurs and the dose response will not be correctly determined. In addition, the aggregation of microorganisms confounds culture-based enumeration and affects the accuracy of direct counting methods such as microscopy of flow cytometry.
8. Concluding Remarks
Wastewater is becoming increasingly important to society, not only because it can be used to augment dwindling freshwater supplies, but also because it can be used for energy production and the recovery of nutrients and other resources. To realize these benefits, wastewater needs to be treated sufficiently to ensure that it is affordable while still protecting public health. This is an important consideration because without public confidence in recycled water it will not be accepted, but at the same time if it is too expensive then consumers will use the cheapest water available, which is often surface water or groundwater.
One of the major costs associated with the production of reuse water is treatment for pathogen removal or inactivation. While chemical contaminants are also important, health regulators tend to focus on contaminants that cause acute disease, especially in the context of nonpotable reuse of wastewater when chronic human exposure is unlikely. With this in mind, an understanding of the fate of pathogens through wastewater treatment and disinfection processes, as well as knowledge of the factors that influence these processes, is required to ensure optimal treatment for managing the risk from pathogens in wastewater. One of the major influencing factors on the fate of pathogens in water is association with particles. It is therefore important to understand the nature of pathogen–particle associations, the factors influencing formation and stability of the association and how the association affects treatment disinfection processes.
Much of the knowledge regarding pathogen–particle interactions has been gathered from studies of freshwater or storm water systems and we know that various particle characteristics such as shape, size, composition, and structure all play important roles in the association process. However, there have been few studies characterizing particles in wastewater and the nature of the associations between pathogens and particles in wastewater remains a knowledge gap. Similarly, there have been studies on the impact of particles on the disinfection of some pathogens or pathogen indicators in wastewater, but these have not examined the nature of the pathogen–particle interactions and how these affect disinfection. Future studies need to characterize particles and pathogen–particle interactions along different stages of the wastewater treatment train, to provide a better understanding of how the different treatment steps influence the nature of the particles present and their interaction with pathogens. A better understanding of this behavior may identify ways to modify processes to alter pathogen partitioning or identify other treatment strategies for dealing with particle-associated pathogens. Controlling pathogen–particle associations provides an opportunity to enhance wastewater treatment and reduce treatment costs; increased levels of association can enhance removal by sedimentation processes, reduced levels of association can enhance disinfection.
References
- Abdel-Raouf N., Al-Homaidan A., Ibraheem I. Microalgae and wastewater treatment. Saudi Journal of Biological Sciences. 2012;19:257–275. doi: 10.1016/j.sjbs.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsen M.S., Templeton T.J., Enomoto S., Abrahante J.E., Zhu G., Lancto C.A.…Tzipori S. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304:441–445. doi: 10.1126/science.1094786. [DOI] [PubMed] [Google Scholar]
- Adam R.D. Biology of Giardia lamblia. Clinical Microbiology Reviews. 2001;14:447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar M., Saez J., Llorens M., Soler A., Ortuno J. Microscopic observation of particle reduction in slaughterhouse wastewater by coagulation–flocculation using ferric sulphate as coagulant and different coagulant aids. Water Research. 2003;37:2233–2241. doi: 10.1016/S0043-1354(02)00525-0. [DOI] [PubMed] [Google Scholar]
- AGWR . In: Australian guidelines for water recycling: Managing health and environmental risks (Phase 1). Environment Protection and Heritage Council, the Natural Resource Management Ministerial Council and the Australian Health Ministers' Conference, Australia. Resources, A.G.D.o.A.a.W., editor. 2006. Australian guidelines for water recycling: managing health and environmental risks (Phase 1) [Google Scholar]
- Alexiou G., Mara D. Anaerobic waste stabilization ponds. Applied Biochemistry and Biotechnology. 2003;109:241–252. doi: 10.1385/abab:109:1-3:241. [DOI] [PubMed] [Google Scholar]
- Amahmid O., Asmama S., Bouhoum K. Urban wastewater treatment in stabilization ponds: occurrence and removal of pathogens. Urban Water. 2002;4:255–262. [Google Scholar]
- Anastasi E., Matthews B., Gundogdu A., Vollmerhausen T., Ramos N., Stratton H.…Katouli M. Prevalence and persistence of Escherichia coli strains with uropathogenic virulence characteristics in sewage treatment plants. Applied and Environmental Microbiology. 2010;76:5882–5886. doi: 10.1128/AEM.00141-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S., Martin-Gomez S., Becares E., De Luis-Calabuig E., Rojo-Vazquez F. Effect of high-rate algal ponds on viability of Cryptosporidium parvum oocysts. Applied and Environmental Microbiology. 2001;67:3322–3324. doi: 10.1128/AEM.67.7.3322-3324.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbolt N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology. 2004;198:229–238. doi: 10.1016/j.tox.2004.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbolt N.J., Grabow W., Snozzi M. In: Water quality: Guidelines, standards and health. Assessment of risk and risk management for water-related infectious disease. Fewtrell L., Bartram J., editors. IWA Publishing; London: 2001. Indicators of microbial water quality; pp. 289–316. [Google Scholar]
- Aslan S., Kapdan I.K. Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecological Engineering. 2006;28:64–70. [Google Scholar]
- Azema N., Pouet M.-F., Berho C., Thomas O. Wastewater suspended solids study by optical methods. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2002;204:131–140. [Google Scholar]
- Barakat M. New trends in removing heavy metals from industrial wastewater. Arabian Journal of Chemistry. 2011;4:361–377. [Google Scholar]
- Berg H.C. Springer; Cambridge, MA, USA: 2004. E. coli in motion. [Google Scholar]
- Berman D., Rice E.W., Hoff J.C. Inactivation of particle-associated coliforms by chlorine and monochloramine. Applied and Environmental Microbiology. 1988;54:507–512. doi: 10.1128/aem.54.2.507-512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidle K.D., Fletcher M. Comparison of free-living and particle-associated bacterial communities in the Chesapeake Bay by stable low-molecular-weight RNA analysis. Applied and Environmental Microbiology. 1995;61:944–952. doi: 10.1128/aem.61.3.944-952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilotta G.S., Brazier R.E. Understanding the influence of suspended solids on water quality and aquatic biota. Water Research. 2008;42:2849–2861. doi: 10.1016/j.watres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Bitton G. 3rd ed. John Wiley & Sons; Hoboken, New Jersey: 2005. Wastewater microbiology. [Google Scholar]
- Bitton G. 4th ed. John Wiley & Sons; Hoboken, New Jersey: 2010. Waste stabilization ponds, wastewater microbiology; pp. 307–319. [Google Scholar]
- Blume T., Martinez I., Neis U. Vol. 35. 2002. pp. 117–128. (Wastewater disinfection using ultrasound and UV light). TU Hamburg–Harburg Reports on Sanitary Engineering. [Google Scholar]
- Bosch A. Human enteric viruses in the water environment: a minireview. International Microbiology. 2010;1:191–196. [PubMed] [Google Scholar]
- Bouki C., Venieri D., Diamadopoulos E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: a review. Ecotoxicology and Environmental Safety. 2013;91:1–9. doi: 10.1016/j.ecoenv.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Boztoprak H., Özbay Y. Detection of protozoa in wastewater using ANN and active contour in image processing. IU–Journal of Electrical & Electronics Engineering. 2013;13:1661–1666. [Google Scholar]
- Brownell S.A., Nelson K.L. Inactivation of single-celled Ascaris suum eggs by low-pressure UV radiation. Applied and Environmental Microbiology. 2006;72:2178–2184. doi: 10.1128/AEM.72.3.2178-2184.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L., Zhang T. Detecting human bacterial pathogens in wastewater treatment plants by a high-throughput shotgun sequencing technique. Environmental Science & Technology. 2013;47:5433–5441. doi: 10.1021/es400275r. [DOI] [PubMed] [Google Scholar]
- Campos C., Guerrero A., Crdenas M. Removal of bacterial and viral faecal indicator organisms in a waste stabilization pond system in Choconta, Cundinamarca (Colombia) Water Science & Technology. 2002;45:61–66. [PubMed] [Google Scholar]
- Characklis G.W., Dilts M.J., Simmons O.D., III, Likirdopulos C.A., Krometis L.-A.H., Sobsey M.D. Microbial partitioning to settleable particles in stormwater. Water Research. 2005;39:1773–1782. doi: 10.1016/j.watres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Chavez A., Jimenez B., Maya C. Particle size distribution as a useful tool for microbial detection. Water Science & Technology. 2004;50:179–186. [PubMed] [Google Scholar]
- Chen R.Z., Craik S.A., Bolton J.R. Comparison of the action spectra and relative DNA absorbance spectra of microorganisms: information important for the determination of germicidal fluence (UV dose) in an ultraviolet disinfection of water. Water Research. 2009;43:5087–5096. doi: 10.1016/j.watres.2009.08.032. [DOI] [PubMed] [Google Scholar]
- Cheng H., Broaders M., Lucy F., Mastitsky S., Graczyk T. Determining potential indicators of Cryptosporidium oocysts throughout the wastewater treatment process. Water Science & Technology. 2012;65:875–882. doi: 10.2166/wst.2012.918. [DOI] [PubMed] [Google Scholar]
- Chiew F., Young W., Cai W., Teng J. Current drought and future hydroclimate projections in southeast Australia and implications for water resources management. Stochastic Environmental Research and Risk Assessment. 2011;25:601–612. [Google Scholar]
- Cizek A.R., Characklis G.W., Krometis L.-A., Hayes J.A., Simmons O.D., III, Di Lonardo S.…Sobsey M.D. Comparing the partitioning behavior of Giardia and Cryptosporidium with that of indicator organisms in stormwater runoff. Water Research. 2008;42:4421–4438. doi: 10.1016/j.watres.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Craggs R., Park J., Heubeck S., Sutherland D. High rate algal pond systems for low-energy wastewater treatment, nutrient recovery and energy production. New Zealand Journal of Botany. 2014;52:60–73. [Google Scholar]
- Craik S.A., Weldon D., Finch G.R., Bolton J.R., Belosevic M. Inactivation of Cryptosporidium parvum oocysts using medium-and low-pressure ultraviolet radiation. Water Research. 2001;35:1387–1398. doi: 10.1016/s0043-1354(00)00399-7. [DOI] [PubMed] [Google Scholar]
- Crittenden J.C., Trussell R.R., Hand D.W., Howe K.J., Tchobanoglous G. 3rd ed. John Wiley & Sons; Hoboken, New Jersey: 2012. MWH's water treatment: Principles and design. [Google Scholar]
- Dai X., Boll J. Evaluation of Cryptosporidium parvum and Giardia lamblia attachment to soil particles. Journal of Environmental Quality. 2003;32:296–304. doi: 10.2134/jeq2003.2960. [DOI] [PubMed] [Google Scholar]
- Dai X., Boll J., Hayes M., Aston D. Adhesion of Cryptosporidium parvum and Giardia lamblia to solid surfaces: the role of surface charge and hydrophobicity. Colloids and Surfaces B: Biointerfaces. 2004;34:259–263. doi: 10.1016/j.colsurfb.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Davies P.S. Strathkelvin Instruments Ltd; Glasgow, Scotland: 2005. The biological basis of wastewater treatment; pp. 3–11. [Google Scholar]
- Davies-Colley R., Smith D. Turbidity suspended sediment and water quality: a review. Journal of the American Water Resources Association. 2001;37:1085–1101. [Google Scholar]
- Davis-Colley R., Donnison A., Speed D. Towards a mechanistic understanding of pond disinfection. Water Science & Technology. 2000;42:149–158. [Google Scholar]
- Dawson D., Paish A., Staffell L., Seymour I., Appleton H. Survival of viruses on fresh produce, using MS2 as a surrogate for norovirus. Journal of Applied Microbiology. 2005;98:203–209. doi: 10.1111/j.1365-2672.2004.02439.x. [DOI] [PubMed] [Google Scholar]
- Dietrich J.P., Başaǧaoǧlu H., Loge F.J., Ginn T.R. Preliminary assessment of transport processes influencing the penetration of chlorine into wastewater particles and the subsequent inactivation of particle-associated organisms. Water Research. 2003;37:139–149. doi: 10.1016/s0043-1354(02)00239-7. [DOI] [PubMed] [Google Scholar]
- Dietrich J.P., Loge F.J., Ginn T.R., Başaǧaoǧlu H. Inactivation of particle-associated microorganisms in wastewater disinfection: modeling of ozone and chlorine reactive diffusive transport in polydispersed suspensions. Water Research. 2007;41:2189–2201. doi: 10.1016/j.watres.2007.01.038. [DOI] [PubMed] [Google Scholar]
- Dillon P. CSIRO Land and Water & Australian Water Association; Adelaide: 2000. Water reuse in Australia: Current status, projections and research, water recycling Australia 2000; pp. 99–104. [Google Scholar]
- Duran A., Muniesa M., Mocé-Llivina L., Campos C., Jofre J., Lucena F. Usefulness of different groups of bacteriophages as model micro-organisms for evaluating chlorination. Journal of Applied Microbiology. 2003;95:29–37. doi: 10.1046/j.1365-2672.2003.t01-1-01948.x. [DOI] [PubMed] [Google Scholar]
- Eischeid A.C., Meyer J.N., Linden K.G. UV disinfection of adenoviruses: molecular indications of DNA damage efficiency. Applied and Environmental Microbiology. 2009;75:23–28. doi: 10.1128/AEM.02199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerick R.W., Loge F.J., Thompson D., Darby J.L. Factors influencing ultraviolet disinfection performance part II: association of coliform bacteria with wastewater particles. Water Environment Research. 1999;71:1178–1187. [Google Scholar]
- EPA . Office of Water; USA: 2003. LT1ESWTR disinfection profiling and benchmarking, Appendix B CT tables. [Google Scholar]
- ESCWA . Social Commission for Western Asia; New York, USA: 2003. Wastewater treatment technologies: A general review, Social Commission for Western Asia. [Google Scholar]
- Faleschini M., Esteves J. Characterization and degradation process of sludge profiles inside a facultative pond (Patagonia, Argentina) Water Science & Technology. 2011;64:2239–2245. doi: 10.2166/wst.2011.788. [DOI] [PubMed] [Google Scholar]
- Falkenmark M., Lindh G. 1974. Impact of water resources on population, Swedish contribution to the UN World Population Conference; pp. 19–30. Bucharest, Romania. [Google Scholar]
- Farnood R. In: Wastewater and biosolids treatment and reuse: Bridging modeling and experimental studies. ECI Symposium series, Otranto, Italy. Santoro D., editor. 2014. New insights into the ultraviolet disinfection of wastewater. [Google Scholar]
- Feng Y.Y., Ong S.L., Hu J.Y., Song L.F., Tan X.L., Ng W.J. Effect of particles on the recovery of Cryptosporidium oocysts from source water samples of various turbidities. Applied and Environmental Microbiology. 2003;69:1898–1903. doi: 10.1128/AEM.69.4.1898-1903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher S.M., Stark D., Harkness J., Ellis J. Enteric protozoa in the developed world: a public health perspective. Clinical Microbiology Reviews. 2012;25:420–449. doi: 10.1128/CMR.05038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong T., Lipp E.K. Enteric viruses of humans and animals in aquatic environments: health risks, detection, and potential water quality assessment tools. Microbiology and Molecular Biology Reviews. 2005;69:357–371. doi: 10.1128/MMBR.69.2.357-371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francy D.S., Stelzer E.A., Bushon R.N., Brady A.M.G., Mailot B.E., Specncer S.K.…Gellner T.M. US Geological Survey; Reston, Virginia: 2011. Quantifying viruses and bacteria in wastewater—Results, interpretation methods, and quality control: Scientific investigations report 2011–5150; p. 44. [Google Scholar]
- Fux C., Siegrist H. Nitrogen removal from sludge digester liquids by nitrification/denitrification or partial nitritation/anammox: environmental and economical considerations. Water Science & Technology. 2004;50:19–26. [PubMed] [Google Scholar]
- Gadgil, A., Kazakevicius, E., Drescher, A. (2002). US6803587 B2: UV water disinfector. In USPTO (Ed.). WaterHealth International, USA.
- Garcia-Mesa J., Delgado-Ramos F., Muñio M., Hontoria E., Poyatos J. Comparison of activated sludge technologies by particle size analysis. Water, Air, and Soil Pollution. 2012;223:4319–4331. [Google Scholar]
- García-Mesa J.J., Poyatos J.M., Delgado-Ramos F., Muñio M.M., Osorio F., Hontoria E. Water quality characterization in real biofilm wastewater treatment systems by particle size distribution. Bioresource Technology. 2010;101:8038–8045. doi: 10.1016/j.biortech.2010.05.071. [DOI] [PubMed] [Google Scholar]
- Gehr R., Wagner M., Veerasubramanian P., Payment P. Disinfection efficiency of peracetic acid, UV and ozone after enhanced primary treatment of municipal wastewater. Water Research. 2003;37:4573–4586. doi: 10.1016/S0043-1354(03)00394-4. [DOI] [PubMed] [Google Scholar]
- Gerardi M.H. John Wiley & Sons; Hoboken, New Jersey: 2010. Troubleshooting the sequencing batch reactor. [Google Scholar]
- Gerardi M.H., Zimmerman M.C. John Wiley & Sons; Hoboken, New Jersey: 2004. Wastewater pathogens. [Google Scholar]
- Gerba C.P., Smith J.E. Sources of pathogenic microorganisms and their fate during land application of wastes. Journal of Environmental Quality. 2005;34:42–48. [PubMed] [Google Scholar]
- Gilbride K., Lee D.-Y., Beaudette L. Molecular techniques in wastewater: understanding microbial communities, detecting pathogens, and real-time process control. Journal of Microbiological Methods. 2006;66:1–20. doi: 10.1016/j.mimet.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Grabow W. Bacteriophages: update on application as models for viruses in water. Water SA. 2004;27:251–268. [Google Scholar]
- Grady C.L.J., Daigger G.T., Love N.G., Filipe C.D. 3rd ed. CRC Press; Boca Raton, Florida: 2011. Biological wastewater treatment. [Google Scholar]
- Greenway M. The role of constructed wetlands in secondary effluent treatment and water reuse in subtropical and arid Australia. Ecological Engineering. 2005;25:501–509. [Google Scholar]
- Guardabassi L., Wong D.M.L.F., Dalsgaard A. The effects of tertiary wastewater treatment on the prevalence of antimicrobial resistant bacteria. Water Research. 2002;36:1955–1964. doi: 10.1016/s0043-1354(01)00429-8. [DOI] [PubMed] [Google Scholar]
- Hannouche A., Ghassan C., Ruban G., Tassin B., Lemaire B.J., Joannis C. Relationship between turbidity and total suspended solids concentration within a combined sewer system. Water Science & Technology. 2011;64:2445–2452. doi: 10.2166/wst.2011.779. [DOI] [PubMed] [Google Scholar]
- Harwood V.J., Levine A.D., Scott T.M., Chivukula V., Lukasik J., Farrah S.R., Rose J.B. Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Applied and Environmental Microbiology. 2005;71:3163–3170. doi: 10.1128/AEM.71.6.3163-3170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassen A., Mahrouk M., Ouzari H., Cherif M., Boudabous A., Damelincourt J.J. UV disinfection of treated wastewater in a large-scale pilot plant and inactivation of selected bacteria in a laboratory UV device. Bioresource Technology. 2000;74:141–150. [Google Scholar]
- Hejkal T.W., Wellings F.M., Lewis A.L., LaRock P.A. Distribution of viruses associated with particles in wastewater. Applied and Environmental Microbiology. 1981;41:628–634. doi: 10.1128/aem.41.3.628-634.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmi K., Skraber S., Gantzer C., Willame R., Hoffmann L., Cauchie H.M. Interactions of Cryptosporidium parvum, Giardia lamblia, vaccinal poliovirus type 1, and bacteriophages phiX174 and MS2 with a drinking water biofilm and a wastewater biofilm. Applied and Environmental Microbiology. 2008;74:2079–2088. doi: 10.1128/AEM.02495-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze M., van Loosdrecht M., Ekama G., Brdjanovic D. IWA Publishing; London, UK: 2008. Biological wastewater treatment: Principles, modelling and design. [Google Scholar]
- Hewitt J., Leonard M., Greening G.E., Lewis G.D. Influence of wastewater treatment process and the population size on human virus profiles in wastewater. Water Research. 2011;45:6267–6276. doi: 10.1016/j.watres.2011.09.029. [DOI] [PubMed] [Google Scholar]
- Hijnen W.A.M., Beerendonk E.F., Medema G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Research. 2006;40:3–22. doi: 10.1016/j.watres.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Hill V.R. Prospects for pathogen reductions in livestock wastewaters: a review. Critical Reviews in Environmental Science and Technology. 2003;33:187–235. [Google Scholar]
- Hipsey M.R., Brookes J.D., Regel R.H., Antenucci J.P., Burch M.D. In situ evidence for the association of total coliforms and Escherichia coli with suspended inorganic particles in an Australian reservoir. Water, Air, and Soil Pollution. 2006;170:191–209. [Google Scholar]
- Jiménez B., Mara D., Carr R., Brissaud F. International Water Management Institute; Colombo, Sri Lanka: 2010. Wastewater treatment for pathogen removal and nutrient conservation: Suitable systems for use in developing countries. [Google Scholar]
- Joannis C., Ruban G., Gromaire M., Bertrand-Krajewski J., Chebbo G. Reproducibility and uncertainty of wastewater turbidity measurements. Water Science & Technology. 2008;57:1667–1673. doi: 10.2166/wst.2008.292. [DOI] [PubMed] [Google Scholar]
- Jolis D., Lam C., Pitt P. Particle effects on ultraviolet disinfection of coliform bacteria in recycled water. Water Environment Research. 2001;73:233–236. doi: 10.2175/106143001x139218. [DOI] [PubMed] [Google Scholar]
- Karim M.R., Manshadi F.D., Karpiscak M.M., Gerba C.P. The persistence and removal of enteric pathogens in constructed wetlands. Water Research. 2004;38:1831–1837. doi: 10.1016/j.watres.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Keegan A., Monis P., Jagals P., Toze S., Blackbeard J. Water Environment Research Foundation; USA: 2010. WERF report 03-HHE-2: Pathogen risk indicators for wastewater and biosolids. [Google Scholar]
- Keegan A., Wati S., Robinson B. Victorian Smartwater Fund; Melbourne, Victoria: 2012. Project SWF62M-2114: Chlor(am)ine disinfection of human pathogenic viruses in recycled waters. [Google Scholar]
- Khanum H., Khanam S.S., Sultana M., Uddin M., Dhar R.C., Islam M. Protozoan parasites in a wastewater treatment plant of Bangladesh. University Journal of Zoology, Rajshahi University. 2013;31:05–08. [Google Scholar]
- King B., Fanok R., Phillips R., Lau M., van den Akker B., Monis P.T. Victorian Smartwater Fund; Melbourne, VIC: 2015. Project 8OS – 8012: Inactivation of Cryptosporidium across the wastewater treatment train: Recycled water fit for purpose (Phase II) [Google Scholar]
- King B.J., Hoefel D., Daminato D.P., Fanok S., Monis P.T. Solar UV reduces Cryptosporidium parvum oocyst infectivity in environmental waters. Journal of Applied Microbiology. 2008;104:1311–1323. doi: 10.1111/j.1365-2672.2007.03658.x. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Iker B.C., Pepper I.L., Gerba C.P. Relative abundance and treatment reduction of viruses during wastewater treatment processes—identification of potential viral indicators. Science of the Total Environment. 2014;488:290–296. doi: 10.1016/j.scitotenv.2014.04.087. [DOI] [PubMed] [Google Scholar]
- Kitis M. Disinfection of wastewater with peracetic acid: a review. Environment International. 2004;30:47–55. doi: 10.1016/S0160-4120(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Kristian Stevik T., Aa K., Ausland G., Fredrik Hanssen J. Retention and removal of pathogenic bacteria in wastewater percolating through porous media: a review. Water Research. 2004;38:1355–1367. doi: 10.1016/j.watres.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Krometis L.-A.H., Characklis G.W., Simmons O.D., III, Dilts M.J., Likirdopulos C.A., Sobsey M.D. Intra-storm variability in microbial partitioning and microbial loading rates. Water Research. 2007;41:506–516. doi: 10.1016/j.watres.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Kuczynska E., Shelton D.R., Pachepsky Y. Effect of bovine manure on Cryptosporidium parvum oocyst attachment to soil. Applied and Environmental Microbiology. 2005;71:6394–6397. doi: 10.1128/AEM.71.10.6394-6397.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznar Z.A., Elimelech M. Cryptosporidium oocyst surface macromolecules significantly hinder oocyst attachment. Environmental Science & Technology. 2006;40:1837–1842. doi: 10.1021/es051859p. [DOI] [PubMed] [Google Scholar]
- Lazarova V., Levine B., Sack J., Cirelli G., Jeffrey P., Muntau H.…Brissaud F. Role of water reuse for enhancing integrated water management in Europe and Mediterranean countries. Water Science & Technology. 2001;43:25–33. [PubMed] [Google Scholar]
- Lazarova V., Savoye P., Janex M., Blatchley E., III, Pommepuy M. Advanced wastewater disinfection technologies: state of the art and perspectives. Water Science & Technology. 1999;40:203–213. [Google Scholar]
- LeChevallier M.W., Hassenauer T.S., Camper A.K., McFeters G.A. Disinfection of bacteria attached to granular activated carbon. Applied and Environmental Microbiology. 1984;48:918–923. doi: 10.1128/aem.48.5.918-923.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G., Fine P., Bar-Tal A. Wiley–Blackwell; Oxford, UK: 2010. Treated wastewater in agriculture: Use and impacts on the soil environments and crops. [Google Scholar]
- Liang Y.-M., Liu C.-B., Ma Z.-B., He J., Zhou Y.-L., Chen J.-C., Liu W. IEEE; 2010. UV enhanced chlorination of sewage against “tailing” phenomenon, 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE) pp. 1–3. [Google Scholar]
- Liberti L., Notarnicola M., Petruzzelli D. Advanced treatment for municipal wastewater reuse in agriculture. UV disinfection: parasite removal and by-product formation. Desalination. 2003;152:315–324. [Google Scholar]
- Li D., Craik S.A., Smith D.W., Belosevic M. The assessment of particle association and UV disinfection of wastewater using indigenous spore-forming bacteria. Water Research. 2009;43:481–489. doi: 10.1016/j.watres.2008.10.025. [DOI] [PubMed] [Google Scholar]
- Linden K.G., Shin G.-A., Faubert G., Cairns W., Sobsey M.D. UV disinfection of Giardia lamblia cysts in water. Environmental Science & Technology. 2002;36:2519–2522. doi: 10.1021/es0113403. [DOI] [PubMed] [Google Scholar]
- Liu G., Ling F.Q., Magic-Knezec A., Liu W.T., Verberk J.Q., van Dijk J.C. Quantification and identification of particle-associated bacteria in unchlorinated drinking water from three treatment plants by cultivation-independent methods. Water Research. 2013;47:3523–3533. doi: 10.1016/j.watres.2013.03.058. [DOI] [PubMed] [Google Scholar]
- Liu Y., Xu H.-L., Yang S.-F., Tay J.-H. Mechanisms and models for anaerobic granulation in upflow anaerobic sludge blanket reactor. Water Research. 2003;37:661–673. doi: 10.1016/s0043-1354(02)00351-2. [DOI] [PubMed] [Google Scholar]
- Llanos J., Cotillas S., Cañizares P., Rodrigo M.A. Conductive diamond sono-electrochemical disinfection (CDSED) for municipal wastewater reclamation. Ultrasonics Sonochemistry. 2015;22:493–498. doi: 10.1016/j.ultsonch.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Loge F.J., Emerick R.W., Ginn T.R., Darby J.L. Association of coliform bacteria with wastewater particles: impact of operational parameters of the activated sludge process. Water Research. 2002;36:41–48. doi: 10.1016/s0043-1354(01)00204-4. [DOI] [PubMed] [Google Scholar]
- Madge B.A., Jensen J.N. Ultraviolet disinfection of fecal coliform in municipal wastewater: effects of particle size. Water Environment Research. 2006;78:294–304. doi: 10.2175/106143005x94385. [DOI] [PubMed] [Google Scholar]
- Madhavi T.P., Rajkumar R. Utilisation of natural coagulant for reduction of turbidity from waste water. International Journal of ChemTech Research. 2013;5:1119–1123. [Google Scholar]
- Malham S.K., Rajko-Nenow P., Howlett E., Tuson K.E., Perkins T.L., Pallet D.W.…McDonald J.E. The interaction of human microbial pathogens, particulate material and nutrients in estuarine environments and their impacts on recreational and shellfish waters. Environmental Science: Processes & Impacts. 2014;16:2145–2155. doi: 10.1039/c4em00031e. [DOI] [PubMed] [Google Scholar]
- Mara D. Earthscan; London, UK: 2004. Domestic wastewater treatment in developing countries. [Google Scholar]
- Mari X., Kerros M., Weinbauer M.G. Virus attachment to transparent exopolymeric particles along trophic gradients in the southwestern lagoon of New Caledonia. Applied and Environmental Microbiology. 2007;73:5245–5252. doi: 10.1128/AEM.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B., Stratton H., Schreoder S., Toze S. The Urban Water Research Alliance; Brisbane, QLD: 2010. Pathogen detection methodologies for wastewater and reservoirs, urban water security research alliance technical report no. 32. [Google Scholar]
- Maynard C., Berthiaume F., Lemarchand K., Harel J., Payment P., Bayardelle P.…Brousseau R. Waterborne pathogen detection by use of oligonucleotide-based microarrays. Applied and Environmental Microbiology. 2005;71:8548–8557. doi: 10.1128/AEM.71.12.8548-8557.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccoy W.F., Olson B.H. Relationship among turbidity, particle counts and bacteriological quality within water distribution lines. Water Research. 1986;20:1023–1029. [Google Scholar]
- McElmurry S.P., Ingram S.M., Khalaf N., Pillai G. Fifth International Water Technology Conference. IWTC, Alexandria, Egypt. 2011. UV treatment efficiency for E. coli in stormwater containing different size fractions of suspended solids. [Google Scholar]
- Medema G., Schets F., Teunis P., Havelaar A. Sedimentation of free and attached Cryptosporidium oocysts and Giardia cysts in water. Applied and Environmental Microbiology. 1998;64:4460–4466. doi: 10.1128/aem.64.11.4460-4466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf I., Eddy H. McGraw-Hill Education; New York, NY: 2003. Wastewater engineering; treatment and reuse. [Google Scholar]
- Middleton F. In: Water renovation and reuse. Shuval H., editor. Academic Press; New York, NY: 1977. Advanced wastewater treatment technology in water reuse; p. 1. [Google Scholar]
- Moe C.L., Rheingans R.D. Global challenges in water, sanitation and health. Journal of Water and Health. 2006;4:41–57. [PubMed] [Google Scholar]
- Monis P.T., Giglio S., Keegan A.R., Andrew Thompson R.C. Emerging technologies for the detection and genetic characterization of protozoan parasites. Trends in Parasitology. 2005;21:340–346. doi: 10.1016/j.pt.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Monis P.T., Lau M., Reeves P., King P., Fanok S., Sidhu J.…Guruge S. Australian Water Recycling Centre of Excellence; Brisbane, QLD: 2015. National validation guidelines for water recycling: Methods for pathogen isolation, culture, detection and enumeration. [Google Scholar]
- Neis U., Tiehm A. Particle size analysis in primary and secondary waste water effluents. Water Science & Technology. 1997;36:151–158. [Google Scholar]
- Odegaard H. Advanced compact wastewater treatment based on coagulation and moving bed biofilm processes. Water Science & Technology. 2000;42:33–48. [Google Scholar]
- Okoh A.I., Odjadjare E.E., Igbinosa E.O., Osode A.N. Wastewater treatment plants as a source of microbial pathogens in receiving watersheds. African Journal of Biotechnology. 2007;6:2932–2944. [Google Scholar]
- Okoh A.I., Sibanda T., Gusha S.S. Inadequately treated wastewater as a source of human enteric viruses in the environment. International Journal of Environmental Research and Public Health. 2010;7:2620–2637. doi: 10.3390/ijerph7062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J., Dagher M., Linden K. Induction of Escherichia coli and Salmonella typhimurium into the viable but nonculturable state following chlorination of wastewater. Journal of Water and Health. 2005;3:249–257. doi: 10.2166/wh.2005.040. [DOI] [PubMed] [Google Scholar]
- ORDER-WQ-2016-0068-DDW . In: State Water Resources Control Board Order WQ 2016-0068-DDW water reclamation requirements for recycled water use. Agency, C.E.P., editor. California Environmental Protection Agency; California, USA: 2016. [Google Scholar]
- Örmeci B., Linden K.G. Development of a fluorescence in situ hybridization protocol for the identification of micro-organisms associated with wastewater particles and flocs. Journal of Environmental Science and Health Part A. 2008;43:1484–1488. doi: 10.1080/10934520802293586. [DOI] [PubMed] [Google Scholar]
- Ottoson J., Hansen A., Björlenius B., Norder H., Stenström T. Removal of viruses, parasitic protozoa and microbial indicators in conventional and membrane processes in a wastewater pilot plant. Water Research. 2006;40:1449–1457. doi: 10.1016/j.watres.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Pallarès A., François P., Pons M., Schmitt P. Suspended particles in wastewater: their optical, sedimentation and acoustical characterization and modeling. Water Science & Technology. 2011;63:240–247. doi: 10.2166/wst.2011.042. [DOI] [PubMed] [Google Scholar]
- Paraskeva P., Graham N.J. Ozonation of municipal wastewater effluents. Water Environment Research. 2002:569–581. doi: 10.2175/106143002x140387. [DOI] [PubMed] [Google Scholar]
- Parr J., Smith M., Shaw R. Wastewater treatment options: technical brief no. 64. Waterlines: Journal of Appropriate Water Supply and Sanitation Technologies. 2002;21:15–18. [Google Scholar]
- Phuntsho S., Shon H.K., Vigneswaran S., Ngo H.H., Kandasamy J., Dorji P. Performance of waste stabilization ponds: experience from cold climatic conditions of Bhutan. Journal of Water Sustainability. 2016;6:1–16. [Google Scholar]
- Pigram J. CSIRO Publishing; Collingwood, VIC: 2007. Australia's water resources: From use to management. [Google Scholar]
- Ramanan R., Kim B., Cho D., Oh H., Kim H. Algae–bacteria interactions: evolution, ecology and emerging applications. Biotechnology Advances. 2016;34:14–29. doi: 10.1016/j.biotechadv.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Ramirez-Castillo F.Y., Loera-Muro A., Jacques M., Garneau P., Avelar-Gonzalez F.J., Harel J., Guerrero A. Waterborne pathogens: detection methods and challenges. Pathogens. 2015;4:307–334. doi: 10.3390/pathogens4020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D.R., Abdel-Magid I.M. Lewis Publishers; Boca Raton, Florida: 1995. Handbook of wastewater reclamation and reuse. [Google Scholar]
- Sangsanont J., Oguma K., Katayama H. Relative effectiveness of ultraviolet light irradiation and chlorination against indigenous bacteriophage and bacteria in primary treated wastewater. Journal of Environmental Science and Engineering B. 2012;1:1003–1009. [Google Scholar]
- Schijven J., De Bruin H., Hassanizadeh S., de Roda Husman A. Bacteriophages and clostridium spores as indicator organisms for removal of pathogens by passage through saturated dune sand. Water Research. 2003;37:2186–2194. doi: 10.1016/S0043-1354(02)00627-9. [DOI] [PubMed] [Google Scholar]
- Searcy K.E., Packman A.I., Atwill E.R., Harter T. Association of Cryptosporidium parvum with suspended particles: impact on oocyst sedimentation. Applied and Environmental Microbiology. 2005;71:1072–1078. doi: 10.1128/AEM.71.2.1072-1078.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy K.E., Packman A.I., Atwill E.R., Harter T. Capture and retention of Cryptosporidium parvum oocysts by Pseudomonas aeruginosa biofilms. Applied and Environmental Microbiology. 2006;72:6242–6247. doi: 10.1128/AEM.00344-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seviour R.J., Mino T., Onuki M. The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiology Reviews. 2003;27:99–127. doi: 10.1016/S0168-6445(03)00021-4. [DOI] [PubMed] [Google Scholar]
- Seviour R.J., Nielsen P.H. IWA Publishing; London, UK: 2010. Microbial ecology of activated sludge. [Google Scholar]
- Shilton A. IWA Publishing; Cornwall, UK: 2005. Pond treatment technology. [Google Scholar]
- Shon H., Vigneswaran S., Kandasamy J., Cho J. Eolss Publishers; Paris, France: 2007. Characteristics of effluent organic matter in wastewater, water and wastewater treatment technologies; pp. 52–101. [Google Scholar]
- Silva A.K.D., Guyader F.S.L., Saux J.-C.L., Pommepuy M., Montgomery M.A., Elimelech M. Norovirus removal and particle association in a waste stabilization pond. Environmental Science & Technology. 2008;42:9151–9157. doi: 10.1021/es802787v. [DOI] [PubMed] [Google Scholar]
- Skraber S., Gassilloud B., Schwartzbrod L., Gantzer C. Survival of infectious Poliovirus-1 in river water compared to the persistence of somatic coliphages, thermotolerant coliforms and Poliovirus-1 genome. Water Research. 2004;38:2927–2933. doi: 10.1016/j.watres.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Sonune A., Ghate R. Developments in wastewater treatment methods. Desalination. 2004;167:55–63. [Google Scholar]
- Sophonsiri C., Morgenroth E. Chemical composition associated with different particle size fractions in municipal, industrial, and agricultural wastewaters. Chemosphere. 2004;55:691–703. doi: 10.1016/j.chemosphere.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Spellman F.R. CRC Press; Boca Raton, FL: 2013. Handbook of water and wastewater treatment plant operations. [Google Scholar]
- Stevens M., Ashbolt N., Cunliffe D. Australia Government National Health and Medical Research Council; Canberra, ACT: 2003. Recommendations to change the use of coliforms as microbial indicators of drinking water quality, review of coliforms as microbial indicators of drinking water quality. [Google Scholar]
- Stott R., May E., Matsushita E., Warren A. Protozoan predation as a mechanism for the removal of Cryptosporidium oocysts from wastewaters in constructed wetlands. Water Science & Technology. 2001;44:191–198. [PubMed] [Google Scholar]
- Sustarsic M. Wastewater treatment: understanding the activated sludge process. Chemical Engineering Progress. 2009;105:26–29. [Google Scholar]
- Symonds E.M., Breitbart M. Affordable enteric virus detection techniques are needed to support changing paradigms in water quality management. CLEAN – Soil, Air, Water. 2014;43:8–12. doi: 10.1002/clen.201400235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds E., Verbyla M., Lukasik J., Kafle R., Breitbart M., Mihelcic J. A case study of enteric virus removal and insights into the associated risk of water reuse for two wastewater treatment pond systems in Bolivia. Water Research. 2014;65:257–270. doi: 10.1016/j.watres.2014.07.032. [DOI] [PubMed] [Google Scholar]
- Templeton M.R., Andrews R.C., Hofmann R. Inactivation of particle-associated viral surrogates by ultraviolet light. Water Research. 2005;39:3487–3500. doi: 10.1016/j.watres.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Templeton M.R., Andrews R.C., Hofmann R. Removal of particle-associated bacteriophages by dual-media filtration at different filter cycle stages and impacts on subsequent UV disinfection. Water Research. 2007;41:2393–2406. doi: 10.1016/j.watres.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Templeton M.R., Butler D. Bookboon; London, UK: 2011. Introduction to wastewater treatment. [Google Scholar]
- Tong R., Beck M., Latten A. Fuzzy control of the activated sludge wastewater treatment process. Automatica. 1980;16:695–701. [Google Scholar]
- Tsuchihashi R., Loge F.J., Darby J.L. Detection of Cryptosporidium parvum in secondary effluents using a most probable number-polymerase chain reaction assay. Water Environment Research. 2003;75:292–299. [PubMed] [Google Scholar]
- Vaiopoulou E., Melidis P., Aivasidis A. An activated sludge treatment plant for integrated removal of carbon, nitrogen and phosphorus. Desalination. 2007;211:192–199. [Google Scholar]
- Varela A.R., Manaia C.M. Human health implications of clinically relevant bacteria in wastewater habitats. Environmental Science and Pollution Research. 2013;20:3550–3569. doi: 10.1007/s11356-013-1594-0. [DOI] [PubMed] [Google Scholar]
- Venkobachar C., Iyengar L., Prabhakara Rao A. Mechanism of disinfection: effect of chlorine on cell membrane functions. Water Research. 1977;11:727–729. [Google Scholar]
- Virto R., Manas P., Alvarez I., Condon S., Raso J. Membrane damage and microbial inactivation by chlorine in the absence and presence of a chlorine-demanding substrate. Applied and Environmental Microbiology. 2005;71:5022–5028. doi: 10.1128/AEM.71.9.5022-5028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Sperling M. Modelling of coliform removal in 186 facultative and maturation ponds around the world. Water Research. 2005;39:5261–5273. doi: 10.1016/j.watres.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Von Sperling M. IWA Pub.; London, UK: 2007. Waste stabilisation ponds. [Google Scholar]
- Vörösmarty C.J., McIntyre P., Gessner M.O., Dudgeon D., Prusevich A., Green P.…Liermann C.R. Global threats to human water security and river biodiversity. Nature. 2010;467:555–561. doi: 10.1038/nature09440. [DOI] [PubMed] [Google Scholar]
- Wade Miller G. Integrated concepts in water reuse: managing global water needs. Desalination. 2006;187:65–75. [Google Scholar]
- Wainwright K.E., Miller M.A., Barr B.C., Gardner I.A., Melli A.C., Essert T.…Conrad P.A. Chemical inactivation of Toxoplasma gondii oocysts in water. Journal of Parasitology. 2007;93:925–931. doi: 10.1645/GE-1063R.1. [DOI] [PubMed] [Google Scholar]
- WHO . 3rd ed. WHO Press; Geneva: 2006. Guidelines for drinking-water quality: Incorporating first addendum. [PubMed] [Google Scholar]
- Winkelmann N., Harder J. An improved isolation method for attached-living Planctomycetes of the genus Rhodopirellula. Journal of Microbiological Methods. 2009;77:276–284. doi: 10.1016/j.mimet.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Winward G.P., Avery L.M., Stephenson T., Jefferson B. Chlorine disinfection of grey water for reuse: effect of organics and particles. Water Research. 2008;42:483–491. doi: 10.1016/j.watres.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Wu J., Jiang X., Wheatley A. Characterizing activated sludge process effluent by particle size distribution, respirometry and modelling. Desalination. 2009;249:969–975. [Google Scholar]
- Wyn-Jones A.P., Carducci A., Cook N., D'Agostino M., Divizia M., Fleischer J.…Höller C. Surveillance of adenoviruses and noroviruses in European recreational waters. Water Research. 2011;45:1025–1038. doi: 10.1016/j.watres.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Zhang T. Pathogenic bacteria in sewage treatment plants as revealed by 454 pyrosequencing. Environmental Science & Technology. 2011;45:7173–7179. doi: 10.1021/es201045e. [DOI] [PubMed] [Google Scholar]
- Ye L., Zhang T. Bacterial communities in different sections of a municipal wastewater treatment plant revealed by 16S rDNA 454 pyrosequencing. Applied Microbiology and Biotechnology. 2013;97:2681–2690. doi: 10.1007/s00253-012-4082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yergeau E., Masson L., Elias M., Xiang S., Madey E., Juang H.…Beaudette L.A. Comparison of methods to identify pathogens and associated virulence functional genes in biosolids from two different wastewater treatment facilities in Canada. PLoS One. 2016;11:1–20. doi: 10.1371/journal.pone.0153554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.-B., Zhao J.-F., Xia S.-Q., Liu C.-Q., Kang X.-S. Particle size distribution and removal by a chemical-biological flocculation process. Journal of Environmental Sciences. 2007;19:559–563. doi: 10.1016/s1001-0742(07)60093-x. [DOI] [PubMed] [Google Scholar]


