Abstract
To characterize an outbreak of pandemic H1N1 2009 among healthcare personnel (HCP), we conducted a cross-sectional survey of HCP who had worked in four general hospitals during the outbreak. More than one-quarter of responding HCP (27.6%) had influenza-like illness (ILI) during the outbreak. The estimated infection rate of pandemic H1N1 2009 was 9.1% in the study of HCP. Independent risk factors for ILI were female gender, <40 years of age, the presence of chronic diseases associated with influenza complications, having family members with ILI or pandemic H1N1 2009, and working in influenza outpatient, influenza inpatient, non-influenza outpatient or emergency departments. During the outbreak of pandemic H1N1 2009, HCP frequently had ILI or the influenza infection. The development of the influenza infection in HCP was associated with some of their baseline characteristics, occupational risk factors, and non-occupational ones during the outbreak.
Keywords: Influenza A virus, H1N1 subtype, Disease outbreak, Healthcare personnel, Infectious disease transmission
Introduction
During an outbreak of pandemic H1N1 2009, healthcare personnel (HCP) were thought to be at substantial risk for acquiring influenza due to their frequent and close interactions with infected patients.1, 2, 3, 4, 5, 6, 7 Influenza infection in HCP may cause an HCP shortage and the spread of influenza to their patients and colleagues. It may also disrupt appropriate healthcare services during the outbreak and result in significant morbidity and mortality among patients with chronic cardiopulmonary disease, immunosuppression or those belonging to either age extreme.8, 9, 10 To engineer effective means of controlling influenza transmission in a healthcare setting, we need more information regarding the characteristics of influenza outbreaks among HCP. Recently, there have been several investigations describing clinical characteristics of HCP with pandemic H1N1 2009.1, 2, 3, 4, 5, 6, 7 However, the majority of these studies were conducted in single centers or included only HCP who developed pandemic H1N1 2009. Consequently, they may have a limited ability to explain the general features of the outbreaks or to investigate risk factors for the influenza infection among HCP. Therefore, we performed a multicenter survey of all HCP who had been on duty during the outbreak.
Patients and methods
A multicenter survey was performed in four general hospitals in the Republic of Korea between July and August 2010. The study hospitals included three >500-bed tertiary care centers and a single 350-bed secondary care center. All centers were assigned as local influenza centers by the Korean government during the outbreak.
During the outbreak, all of these centers established isolated influenza facilities using tents or separate warehouses outside of the main hospital buildings for daytime outpatient visits. Emergency departments undertook some outpatient care during holidays or weekday nights. Several inpatient rooms were designated for admitted patients with pandemic H1N1 2009. However, these rooms were located in the general ward due to the absence of specialized isolation wards among the study hospitals.
HCP who had been on duty during the outbreak were invited to participate in the survey. The anonymous, self-administered questionnaires were distributed and collected by infection control personnel of the study hospitals. The questionnaire was developed to assess the following characteristics of HCP: age, gender, job type, facility type in which they principally worked during the outbreak, presence of underlying diseases associated with influenza complications, adherence to isolation precautions, presence of influenza-like illness (ILI), the receipt of diagnostic tests for pandemic H1N1 2009, the results of the tests, the number of household members, the presence of ILI or pandemic H1N1 2009 among household members, the associations of the family member illnesses with those of HCP within one week, and the order of illness onset in HCP families.
Job types were classified as physician, nurse, nursing assistant, technician, or jobs not directly related to patient care. Facility types in which HCP had principally worked were classified as influenza outpatient department, influenza inpatient department, non-influenza outpatient department, non-influenza inpatient department, emergency department, or facilities not directly related to patient care. The levels of adherence to isolation precautions were rated according to a four-point scale (always-often-rarely-never). Optimal adherence was determined for responses of “always” ILI was considered to be present if HCP experienced an acute episode including at least one respiratory symptom, such as cough, sputum production, rhinorrhea or nasal obstruction, with documented or subjective fever. The presence of pandemic H1N1 2009 was noted if HCP had a positive result in either the rapid influenza antigen detection test (RIDT) for type A influenza or in the reverse transcriptase polymerase chain reaction (RT-PCR).
Statistical analyses were performed using SPSS software (version 12.0; SPSS, Chicago, IL). For univariate analysis, a χ 2 test or Fisher’s exact test was used. Logistic regression analysis was performed to investigate risk factors for the development of ILI among HCP. All p-values were two-tailed, and p < 0.05 was considered statistically significant.
Results
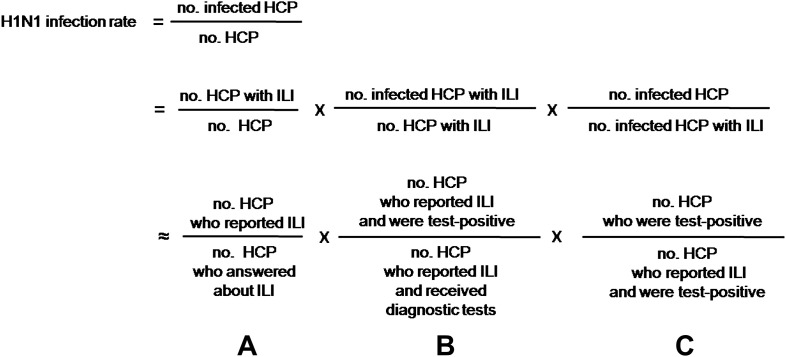
The questionnaires were distributed to 4555 HCP, 3431 (75.3%; range, 72.5–85.5%) of whom responded during the study period. After excluding the questionnaires of 66 who did not work in the study hospitals during the outbreak, the results of 3365 (73.9%) were analyzed. The presence or absence of ILI was reported in 2681 (79.7% of 3365). Of these, ILI was observed in 739 (27.6% of 2681). In each study hospital, the rate of ILI was as follows: hospital A, 35.0%; hospital B, 30.0%; hospital C, 21.8%; hospital D, 28.2%. Of the study HCP, 538 (16.0% of 3365) received diagnostic tests for pandemic H1N1 2009 and 141 had positive results (26.2% of 538). In each study hospital, the rate of positive test result was as follows: hospital A, 27.3%; hospital B, 25.6%; hospital C, 24.6%; hospital D, 19.3%. Of the 538, 411 (76.4%) had ILI. Of these, 115 (28.0% of 411) had positive test results. Of the remaining 127 who did not have ILI, 21 (16.5%) had positive results. The estimation of pandemic H1N1 2009 infection rate among the study HCP was performed as described in Fig. 1 . A (739/2681) was multiplied by B (115/411) and C (136/115), resulting in the pandemic H1N1 2009 infection rate of 9.1%.
Figure 1.
Estimation of true infection rate of pandemic H1N1 2009 among healthcare personnel. NOTES. H1N1, pandemic H1N1 2009; HCP, healthcare personnel; ILI, influenza-like illness.
The majority of the 141 infected HCP were female (74.4%) and <40 years of age (76.9%). Their job types were as follows: nurse (45.4%), physician (17.0%), nursing assistant (13.5%), technician (5.0%), and jobs not directly related to patient care (19.1%). They worked principally at the following facilities: non-influenza inpatient (23.0%), non-influenza outpatient (13.5%), influenza outpatient (8.5%), emergency (7.8%) and influenza inpatient departments (5.0%), and facilities not directly related to patient care (33.6%). The possible source of transmission was as follows: patients of the outpatient (45.7%) and inpatient departments (19.7%), those of the community (11.0%), HCP’s colleagues (3.1%), and unknown sources (20.5%).
Among all responding HCP, the rates of adherence to each part of isolation precautions were as follows, according to a four-point scale: wearing a mask always (32.2%), often (38.8%), rarely (24.4%) and never (4.6%); wearing a gown always (6.9%), often (9.2%), rarely (14.1%) and never (69.8%); wearing gloves always (15.4%), often (22.4%), rarely (25.6%) and never (36.6%); wearing goggles always (0.5%), often (2.2%), rarely (4.3%) and never (93.0%); washing hands always (54.2%), often (35.5%), rarely (7.9%) and never (2.4%). During the outbreak, 82.1% of 3183 HCP were vaccinated for seasonal influenza and 86.6% of 3235 for pandemic H1N1 2009.
Among 2180 HCP who answered the major parts of the questionnaires and had data for ILI, the crude and adjusted odds of clinical factors for the development of ILI were calculated (Table 1, Table 2 ). Female gender, <40 years of age, the presence of chronic diseases associated with influenza complications, the presence of ILI or pandemic H1N1 2009 among household members of HCP, and the history of contact with infected patients were more commonly observed in HCP with ILI than in those without. Physicians, nurses and nursing assistants were at a higher risk for ILI than were HCP who had jobs not directly related to patient care. Regarding facility types, HCP who worked in influenza outpatient, influenza inpatient, non-influenza outpatient and emergency departments were at a higher risk for ILI than were those in facilities not directly related to patient care. The risk of developing ILI was lower in HCP who always wore a gown and gloves than in those who did not. In the multivariate analysis, independent risk factors for ILI were female gender, age <40 years, the presence of chronic diseases associated with influenza complications, and the presence of ILI or pandemic H1N1 2009 among household members of HCP. HCP who worked in influenza outpatient, influenza inpatient, non-influenza outpatient and emergency departments had higher adjusted odds for ILI than did those in facilities not directly related to patient care.
Table 1.
Univariate analysis of risk factors for influenza-like illness (ILI) among healthcare personnel during the outbreak of pandemic H1N1 2009.
| Risk factors for ILI | Number of HCP | Number (%) of HCP with ILI | Crude odds ratio (95% CI) | p Value |
|---|---|---|---|---|
| Gendera | ||||
| Male | 507 | 113 (22.3) | ||
| Female | 1673 | 484 (28.9) | 1.42 (1.12–1.79) | 0.003 |
| Agea | ||||
| ≥40 years | 463 | 87 (18.8) | ||
| <40 years | 1717 | 510 (29.7) | 1.83 (1.41–2.35) | <0.001 |
| Presence of chronic diseases associated with influenza complicationsa | ||||
| No | 2007 | 530 (26.4) | ||
| Yes | 173 | 67 (38.7) | 1.76 (1.28–2.43) | <0.001 |
| Presence of ILI or pandemic H1N1 2009 among household members of HCPb | ||||
| No | 1686 | 389 (23.1) | ||
| Yes | 390 | 181 (46.4) | 2.89 (2.30–3.63) | <0.001 |
| History of contact with possible source patient with pandemic H1N1 2009c | ||||
| No | 385 | 268 (69.6) | ||
| Yes | 381 | 297 (78.0) | 1.54 (1.12–2.14) | 0.01 |
| Colleagues with pandemic H1N1 2009c | ||||
| No | 730 | 540 (74.0) | ||
| Yes | 36 | 25 (69.4) | 0.80 (0.39–1.66) | 0.55 |
| Sources outside hospitalsc | ||||
| No | 720 | 526 (73.1) | ||
| Yes | 46 | 39 (84.8) | 2.01 (0.90–4.67) | 0.08 |
| Job typea | ||||
| Jobs not directly related to patient care | 420 | 82 (19.5) | Reference | |
| Physicians | 330 | 104 (31.5) | 1.90 (1.36–2.65) | <0.001 |
| Nurses | 1072 | 312 (29.1) | 1.69 (1.28–2.23) | <0.001 |
| Nursing assistants | 140 | 46 (32.9) | 2.02 (1.32–3.09) | 0.001 |
| Technicians | 218 | 53 (24.3) | 1.32 (0.89–1.96) | 0.16 |
| Facility typea | ||||
| Facilities not directly related to patient care | 820 | 173 (21.1) | Reference | |
| Influenza outpatient department | 104 | 51 (49.0) | 3.60 (2.37–5.47) | <0.001 |
| Influenza inpatient department | 227 | 77 (33.9) | 1.92 (1.39–2.65) | <0.001 |
| Emergency department | 130 | 69 (53.1) | 4.23 (2.88–6.20) | <0.001 |
| Non-influenza outpatient department | 245 | 80 (32.7) | 1.81 (1.32–2.49) | <0.001 |
| Non-influenza inpatient department | 654 | 147 (22.5) | 1.08 (0.85–1.39) | 0.52 |
| Adherence to isolation precautions | ||||
| Wearing a maskd, not always | 1260 | 381 (30.2) | ||
| Always | 602 | 168 (27.9) | 0.89 (0.72–1.11) | 0.30 |
| Wearing a gowne, not always | 1702 | 515 (30.3) | ||
| Always | 135 | 28 (20.7) | 0.60 (0.39–0.93) | 0.02 |
| Wearing glovesf, not always | 1578 | 487 (30.9) | ||
| Always | 275 | 58 (21.1) | 0.60 (0.44–0.81) | 0.001 |
| Wearing gogglesg, not always | 1835 | 545 (29.7) | ||
| Always | 12 | 1 (8.3) | 0.21 (0.03–1.67) | 0.12 |
| Hand washingh, not always | 870 | 255 (29.3) | ||
| Always | 989 | 292 (29.5) | 1.01 (0.83–1.23) | 0.92 |
| Receipt of influenza vaccination seasonal influenza vaccinationi | ||||
| No | 403 | 119 (29.5) | ||
| Yes | 1666 | 452 (27.1) | 0.89 (0.70–1.13) | 0.33 |
| Pandemic H1N1 2009 vaccinationj | ||||
| No | 296 | 109 (36.8) | ||
| Yes | 1821 | 475 (26.1) | 0.60 (0.47–0.78) | <0.001 |
NOTES. ILI, influenza-like illness; HCP, healthcare personnel; total numbers of healthcare personnel were: a2180, b2076, c766, d1862, e1837, f1853, g1847, h1859, i2069 and j2117.
Table 2.
Multivariate analysis of risk factors for influenza-like illness among healthcare personnel during the outbreak of pandemic H1N1 2009.
| Risk factor for ILI | Adjusted odds ratio | 95% CI | p Value |
|---|---|---|---|
| Female gender | 1.44 | 1.03–2.01 | 0.034 |
| Age ≥ 40 years | 0.52 | 0.38–0.71 | <0.001 |
| Presence of chronic diseases associated with influenza complications | 1.80 | 1.23–2.64 | 0.003 |
| Presence of ILI or pandemic H1N1 2009 among household members of HCP | 2.92 | 2.25–3.79 | <0.001 |
| Facility type | |||
| Facilities not directly related to patient care | Reference | ||
| Influenza outpatient department | 2.71 | 1.69–4.35 | <0.001 |
| Influenza inpatient department | 1.58 | 1.06–2.35 | 0.024 |
| Emergency department | 3.21 | 2.05–5.02 | <0.001 |
| Non-influenza outpatient department | 1.46 | 1.01–2.13 | 0.045 |
NOTES. ILI, influenza-like illness; HCP, healthcare personnel.
Of 3135 HCP who answered the questions of the presence of ILI or pandemic H1N1 2009 in their households, 641 (20.4%) reported that at least one of their household members had ILI or pandemic H1N1 2009. Among those HCP whose household members exhibited ILI or pandemic H1N1 2009, 77 (12.0% of 641) cases occurred within one week prior to or after their household members becoming ill. Among these, 57 (74.0%) reported that ILI or pandemic H1N1 2009 occurred first in the HCP and then in their household members. Physicians and nurses were more common in these 57 than in the remaining 20 (12.3% vs. 5.3%; 45.6% vs. 36.8%). The remaining 20 subjects had jobs not directly related to patient care or worked in facilities not directly related to patient care more frequently than the 57 (31.6% vs. 19.3%; 60.0% vs. 34.8%). Also, the former more frequently had affected household members ≤19 years of age compared to that of the latter (84.2% vs. 57.4%, p = 0.04) (Table 3 ).
Table 3.
Comparison of characteristics between HCP who transmitted pandemic H1N1 2009 or influenza-like illness from themselves to their household members and vice versa.
| Characteristic | HCP who had ILI or pandemic H1N1 among themselves and their household members within one week |
p Value | |
|---|---|---|---|
| Number (%) of HCP with transmission occurred from HCP to their household members (n = 57) | Number (%) of HCP with transmission occurred from their household members to HCP (n = 20) | ||
| Female gender | 39/51 (76.5) | 16/18 (88.9) | 0.33 |
| ≥40 years of age | 12/49 (24.5) | 6/17 (35.3) | 0.53 |
| Job type | 0.17 | ||
| Physicians | 7/57 (12.3) | 1/19 (5.3) | |
| Nurses | 26/57 (45.6) | 7/19 (36.8) | |
| Nursing assistants | 8/57 (14.0) | 3/19 (15.8) | |
| Technicians | 5/57 (8.8) | 2/19 (10.5) | |
| Jobs not directly related to patient care | 11/57 (19.3) | 6/19 (31.6) | |
| Facility type | 0.18 | ||
| Influenza outpatient department | 7/46 (15.2) | 2/15 (13.3) | |
| Influenza inpatient department | 3/46 (6.5) | 0 | |
| Emergency department | 3/46 (6.5) | 0 | |
| Non-influenza outpatient department | 9/46 (19.6) | 2/15 (13.3) | |
| Non-influenza inpatient department | 8/46 (17.4) | 2/15 (13.3) | |
| Facilities not directly related to patient care | 16/46 (34.8) | 9/15 (60.0) | |
| Presence of chronic diseases associated with influenza complications | 7/56 (12.5) | 1/20 (5.0) | 0.67 |
| Presence of affected household member who was ≤19 years of age | 27/47 (57.4) | 16/19 (84.2) | 0.04 |
| Median number of household members (range) | 4 (2–7) | 5 (2–7) | 0.29 |
| Gender of affected household members | |||
| Male | 27/44 (61.4) | 10/13 (76.9) | 0.35 |
| Female | 27/44 (61.4) | 6/13 (46.2) | 0.33 |
NOTES. Data are presented as number of healthcare personnel (percentage), unless otherwise indicated; HCP, healthcare personnel; ILI, influenza-like illness.
Discussion
During the outbreak, 27.6% of the study HCP reported ILI. The estimated infection rate of pandemic H1N1 2009 was 9.1%. Among the clinical variables, baseline characteristics such as female gender, age <40 years, and chronic underlying diseases were associated with pandemic H1N1 2009 infection. The type of facilities in which HCP had principally worked was the most important occupational risk factor for the infection. Non-occupational risk factor, such as having infected household members, was also associated with the development of the influenza infection among HCP.
ILIs have been reported to develop in 23–36% of HCP during the peak of the influenza season.11, 12 Our data showed that the frequency of ILI was 27.6% among the study HCP, in agreement with the results of the previous studies. Regarding the incidence of pandemic H1N1 2009 among HCP, there have been no reports except a few seroepidemiological studies.2, 3 One study from Taiwan reported a seropositive rate of H1N1 antibodies to be 20% among HCP.3 In one large Singapore center designated for outbreak management, seroconversion was observed in 7% of HCP.2 The direct comparison of the infection rates between the hospitals of the previous studies and our institutions may not be plausible due to the different study designs and case definitions. However, the estimated rate of this study (9.1%) simply suggest that influenza infection may be considerable among HCP during an influenza pandemic, with the data from several large centers rather than those of single centers. In addition, it suggests that a large number of infected HCP might be unrecognized or untested during the pandemic, because only 4.2% (141/3365) was reported to have the infection in the study hospitals. Therefore, these data show that there needs to be a higher level of suspicion for influenza infection and the easy availability of diagnostic tests during an influenza pandemic among HCP.
HCP of a younger age or who have underlying chronic diseases were more frequently associated with ILI than those without. This association may be due to the relative lack of preexisting immunity to this novel influenza virus among younger age groups,13 and the lack of local or general immunity associated with chronic diseases. However, the higher susceptibility in female HCP in this study may not be easily understood. One possible explanation is that this is due to women being caregivers in the household setting and, therefore, more likely to be infected from the household. Unfortunately, we did not make inquiries about the presence or absence of children for all female participants. Instead, we compared the proportion of ILI in female HCP with the number of their family members being ≤2, and with those with the number being >3. The latter were more likely to have children than the former, but there was insignificant difference in the development of ILI between them (29.8% vs. 27.7%, p = 0.38). In addition, pandemic H1N1 2009 transmission from household to hospital was not associated with the gender of HCP in Table 3. A recent review has reported a higher incidence of pandemic H1N1 2009 infection in young women than young men of compatible age, although the exact causes have not been suggested.14 More data may be needed for higher susceptibility to influenza infection in female HCP.
HCP who worked at influenza outpatient and emergency departments had the highest risk for ILI. It has been previously suggested that much of the risk for infection to HCP during an outbreak likely exists in an outpatient setting.1 Additionally, in Korean general hospitals, a huge number of outpatient visits took place only for the confirmatory diagnostic tests for pandemic H1N1 2009, maybe from the pervasive anxiety over fatality potential of the infection. At least in part, it may increase the risk for pandemic H1N1 2009 among HCP working in outpatient settings rather than those in inpatient ones. HCP working in the non-influenza outpatient department were also at risk for ILI. This may be due to the variable presentation of influenza infection.15 A substantial number of patients with atypical or mild symptoms, especially pediatric patients, frequently visited the non-influenza outpatient department.
The isolation precautions were not shown to markedly reduce the transmission of pandemic H1N1 2009. Although a recall bias might be the cause of this finding, overall low levels of compliance were likely to be another problem. Whereas the compliance rates for hand washing and frequent mask wearing (often-always) were 89.7% and 71.0%, compliance rates for frequently wearing a gown, goggles and gloves were 16.1%, 2.7%, and 37.8% of HCP, respectively. The Center for Disease Control and Prevention recommended the use of standard and contact precautions with eye protection during the pandemic.1 Besides hand washing and mask wearing, use of other personal protective equipment should be taught and encouraged.
This study showed that household members may transmit pandemic H1N1 2009 to HCP or vice versa. HCP whose household members had ILI or pandemic H1N1 2009 were more frequently infected than were those whose household members were ILI or pandemic H1N1 2009 negative. A seroepidemiological study from Singapore reported that having a child with ILI was a non-occupational risk factor for HCP seroconversion. However, whether transmission of the infection occurred from the child to HCP or vice versa was not clear in the study.2 Our data suggest that HCP who are involved directly in patient care may transmit pandemic H1N1 2009 to their household members, whereas those who are not involved in patient care are more likely to transmit the infection from their homes to their hospitals. These findings may indicate that variable infection control measures should be implemented for HCP who are actively involved in influenza patient care in order to prevent transmission from hospital to household. Additionally, control of influenza among children or adolescents may lead to the reduction of influenza infection in hospitals as well as in the community.
This study has several important limitations. First, the proportion of HCP not reporting the receipt of diagnostic tests was substantial. Therefore, the total number of pandemic H1N1 2009 among HCP may be underreported. This, in turn, might limit the power of the data to identify meaningful risk factors. Instead, we used ILI to identify risk factors for influenza infection in HCP. However, the ILI case definition of self-reported fever with respiratory illness was not highly specific for the diagnosis of pandemic H1N1 2009. Any non-pandemic H1N1 illness classified as ILI might result in a bias in this study. The laboratory surveillance data from the Korean government reported that rhinovirus, adenovirus, coronavirus and respiratory syncytial virus co-circulated in the community during the influenza pandemic.16 However, even with this bias prone to nullify any remarkable differences in infected HCP, the identified risk factors for ILI of this study were similar to those for pandemic H1N1 2009 identified in other studies.2, 3, 5 Second, the impact of recall bias may be considerable due to the study design, especially on the source of infection, the compliance to the isolation precautions, and the direction of transmission between HCP and their family members. Third, working at pediatric wards or clinics may be an important risk factor for the infection, but the data could not be collected mainly due to unseparated wards or clinics in the study hospitals. Fourth, we presented the crude odds of seasonal or pandemic vaccination for the development of ILI in Table 1. However, influenza vaccination only started in late October 2009 (just before the peak of the pandemic) in Korea and we could not collect information on the onset of ILI and the date of vaccination among HCP from the study design. Consequently, we could not determine the effectiveness of influenza vaccination in this study. The receipt of vaccination was excluded in the multivariate analysis. Fifth, we did not present the variation in the characteristics of the outbeak by the study hospital. We calculated the infection rate of each hospital as described in Fig. 1. The infection rate was as follows: hospital A, 14.2%; hospital B, 9.9%; hospital C, 6.9%; hospital D, 3.4%. We compared the characteristics of HCP in the study hospitals with lower infection rates (hospital A and B), with those in the study hospitals with higher infection rates (hospital C and D). However, the comparison did not show any differences in the preventive measures or systems unique to each study hospital, except some differences in demographic data of participating HCP between the study hospitals. In addition, the estimated rate may be less reliable due to the quartered number of the study HCP. Therefore, these data were not presented here.
In conclusion, ILI or pandemic H1N1 2009 was frequently observed among HCP. The risk of the influenza infection was associated with variable characteristics of HCP, such as baseline demographics, occupational or non-occupational risk factors.
Conflicts of interest
No authors have any conflicts of interest to report.
Acknowledgment
We thank our colleagues in each study hospital who actively participated in the clinical and infection control practices during the outbreak and also in this study during the post-oubreak period, especially Mi Ae Choi, R.N., Department of Infection Control, Chung-Ang University Yongsan Hospital; Ji Youn Choi, R.N., and In Soon Choi, R.N., Department of Infection Control, Chung-Ang University Hospital; Hee Kyung Chun, R.N., and Mee La Kim, R.N., Department of Infection Control, Kyung Hee University Medical Center; Eun Ju Lee, R.N., and Sun Mi Park, R.N., Department of Infection Control, Soonchunhyang University Cheonan Hospital.
References
- 1.Center for Disease Control and Prevention Novel influenza A (H1N1) virus infections among health-care personnel – United States, April–May 2009. Morb Mortal Wkly Rep. 2009;58:641–645. [PubMed] [Google Scholar]
- 2.Chen M.I., Lee V.J., Barr I., Lin C., Goh R., Lee C. Risk factors for pandemic (H1N1) 2009 virus seroconversion among hospital staff, Singapore. Emerg Infect Dis. 2010;16:1554–1561. doi: 10.3201/eid1610.100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan Y.J., Lee C.L., Hwang S.J., Fung C.P., Wang F.D., Yen D.H. Seroprevalence of antibodies to pandemic (H1N1) 2009 influenza virus among hospital staff in a medical center in Taiwan. J Chin Med Assoc. 2010;73:62–66. doi: 10.1016/S1726-4901(10)70003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng V.C., Tai J.W., Wong L.M., Chan J.F., Li I.W., To K.K. Prevention of nosocomial transmission of swine-origin pandemic influenza virus A/H1N1 by infection control bundle. J Hosp Infect. 2010;74:271–277. doi: 10.1016/j.jhin.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos C.D., Bristow R.B., Vorenkamp J.V. Which health care workers were most affected during the spring 2009 H1N1 pandemic? Disaster Med Public Health Prep. 2010;4:47–54. doi: 10.1017/s193578930000241x. [DOI] [PubMed] [Google Scholar]
- 6.Kiertiburanakul S., Apivanich S., Muntajit T., Sukkara S., Sirinavin S., Leelaudomlipi S. H1N1 2009 influenza among healthcare workers in a tertiary care hospital in Thailand. J Hosp Infect. 2010;74:300–302. doi: 10.1016/j.jhin.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Wise M.E., Perio M.D., Halpin J., Jhung M., Magill S., Black S.R. Transmission of pandemic (H1N1) 2009 influenza to healthcare personnel in the United States. Clin Infect Dis. 2011;52:S198–S204. doi: 10.1093/cid/ciq038. [DOI] [PubMed] [Google Scholar]
- 8.Salgado C.D., Farr B.M., Hall K.K., Hayden F.G. Influenza in the acute hospital setting. Lancet Infect Dis. 2002;2:145–155. doi: 10.1016/s1473-3099(02)00221-9. [DOI] [PubMed] [Google Scholar]
- 9.Maltezou H.C. Novel (pandemic) influenza A H1N1 in healthcare facilities: implications for prevention and control. Scand J Infect Dis. 2010;42:412–420. doi: 10.3109/00365541003699649. [DOI] [PubMed] [Google Scholar]
- 10.Poalillo F.E., Geiling J., Jimenez E.J. Healthcare personnel and nosocomial transmission of pandemic 2009 influenza. Crit Care Med. 2010;38:e98–102. doi: 10.1097/CCM.0b013e3181d41d45. [DOI] [PubMed] [Google Scholar]
- 11.Ng T.C., Lee N., Hui S.C.D., Lai R., Ip M. Preventing healthcare workers from acquiring influenza. Infect Control Hosp Epidemiol. 2009;30:292–295. doi: 10.1086/595690. [DOI] [PubMed] [Google Scholar]
- 12.Lester R.T., McGeer A., Tomlinson G., Detsky A.S. Use of, effectiveness of, and attitudes regarding influenza vaccine among house staff. Infect Control Hosp Epidemiol. 2003;24:839–844. doi: 10.1086/502146. [DOI] [PubMed] [Google Scholar]
- 13.Center for Disease Control and Prevention Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccination. MMWR Morb Mortal Wkly Rep. 2009;58:521–524. [PubMed] [Google Scholar]
- 14.Klein S.L., Passaretti C., Anker M., Olukoya P., Pekosz A. The impact of sex, gender, and pregnancy on 2009 H1N1 disease. Biol Sex Differ. 2010;1:5. doi: 10.1186/2042-6410-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harper S.A., Bradley J.S., Englund J.A., File T.M., Gravenstein S., Hayden F.G. Seasonal influenza in adults and children-diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1003–1032. doi: 10.1086/604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korean Center for Disease Control and Prevention. Novel influenza A (H1N1) homepage. http://flu.cdc.go.kr.