Abstract
The diagnosis of pregnancy in the domestic dog (Canis familiaris) often employs specialized equipment, experienced staff, and the cooperation of the bitch. These procedures can be challenging when the subject is a wild canid, particularly in a field setting. In addition, reproductive hormone assays are unreliable as a diagnostic tool because the estrous profiles of pregnant and pseudopregnant canines are similar. However, research has demonstrated that the hormone relaxin can be detected in maternal blood after embryonic implantation, but remains negligible in non-pregnant females. We investigated the use of relaxin as a diagnostic marker of pregnancy in the coyote (C. latrans). A commercially available canine relaxin enzyme immunoassay (ReproCHEK™) was used to test plasma collected from 124 female coyotes over four consecutive breeding seasons. Mating activities of the captive females were observed; then peripheral blood samples were collected at intervals throughout pregnancy, as well as after parturition. Results demonstrated that relaxin could be detected in the plasma of pregnant coyotes after 28 days of gestation, and in some cases as early as 23 days, while non-pregnant females and male coyotes consistently tested negative. Relaxin also remained detectable in the plasma of the majority of females tested 10–12 weeks after parturition. This qualitative assay for relaxin proved to be a reliable diagnostic tool for pregnancy in the coyote. In addition, blood sampling was relatively easy, could be accomplished with minimal handling, and did not require sedation or anesthesia.
Keywords: Canis latrans, Coyote, Pregnancy, Pseudopregnancy, Relaxin
1. Introduction
The coyote (Canis latrans) is a medium-sized wild canid, native to North America and closely related to the gray wolf (C. lupus), red wolf (C. rufus), and domestic dog (C. familiaris) (Roy et al., 1994). Coyotes are considered seasonally monestrous (Hamlett, 1938, Gier, 1968) and socially monogamous (Andelt, 1985, Bekoff and Wells, 1986, Gese, 2001). While there is some regional variation in the actual breeding season, coyotes generally mate in mid- to late-winter, have a gestation period of 60–63 days, and deliver an average litter of three to seven pups in the spring (Hamlett, 1938, Gier, 1968, Knowlton, 1972, Gese et al., 1989). Typically, only the dominant male and female within a coyote social group produce a litter (Gese et al., 1989, Gese et al., 1996), although subordinate associates will help defend the pups and territory (Andelt, 1985, Bekoff and Wells, 1986, Gese, 2001).
The reproductive hormone profile of the coyote's estrous cycle has been studied (Stellflug et al., 1981, Hodges, 1990) and appears to share certain features with the patterns described for the wolf (Seal et al., 1979, Kreeger et al., 1991, Walker et al., 2002) and domestic dog (reviewed in: Concannon et al., 1989, Concannon et al., 2001). Like the wolf and dog, differentiating a pregnant coyote from a non-pregnant female can be difficult if based on serological assessment alone. Pregnant and pseudopregnant coyotes have similar patterns of serum progesterone secretion, and the absolute concentration levels vary widely among individuals (Carlson, 2006). Also, while mid-gestation prolactin levels in coyotes rise significantly above those observed in non-gravid diestrous females, the absolute values still overlap (Carlson, 2006). Thus, single blood sampling for progesterone or prolactin seems unreliable as a method of determining reproductive status in coyotes. The hormone relaxin, however, has not yet been explored in this species.
Relaxin is a polypeptide shown to affect the reproductive tissues of mammals, most commonly “cervical extensibility and uterine contractibility” (Sherwood, 1994). The source of relaxin synthesis varies among species, but the predominant sites are the corpus luteum, placenta, and uterus (Sherwood, 1994). Depending on the species, detection of relaxin in peripheral blood is not always restricted to pregnant females; however in the domestic dog it has been established as a pregnancy-specific hormone (Steinetz et al., 1987, Steinetz et al., 1989). The site of synthesis in the bitch has been elucidated (Tsutsui and Stewart, 1991, Klonisch et al., 1999) and primarily ascribed to the placenta, although the hormone can also be traced in the ovary and uterus. These latter tissues may be areas influenced by the paracrine deposition of relaxin. A clinical study of domestic dogs using the commercially available canine relaxin enzyme-linked immunoassay (ELISA) ReproCHEK™ (Synbiotics Corporation, San Diego, CA, USA) reported detection of the hormone in maternal peripheral blood as early as 25 days after ovulation (Buff et al., 2001).
In support of a longitudinal investigation in the reproductive biology of the coyote (Carlson, 2006), a diagnostic tool was needed to easily distinguish between pregnant and pseudopregnant females, while minimizing research induced disturbances. Although there are behavioral and physiological differences between the coyote and domestic dog, there are many common reproductive features (Gier, 1968, Silver and Silver, 1969, Kennelly and Johns, 1976, Kennelly, 1978). Therefore, we tested the use of relaxin as a serological marker of pregnancy in the coyotes with the hypothesis it would be as successful in a wild canid as it has been in its domestic congener.
2. Materials and methods
2.1. Animals
During 4 consecutive years (2000–2003), 124 intact female coyotes were tested for the presence or absence of relaxin at variable times following copulation and/or parturition. The coyotes were captive born and reared at the National Wildlife Research Center (NWRC) facility near Millville, UT, USA. All animals were housed in outdoor enclosures with natural lighting. Male–female pairs resided in 0.1 ha outdoor pens with access to den boxes sheltered within observation buildings. Multiple pens were within visual and audible range of each other but separated by fencing and concrete barriers. Some individual animals were sequestered from their mates during the breeding season and served as unmated controls. In these cases, the females were housed in sheltered outdoor kennels which also included den boxes for privacy.
The subjects ranged from 2 to 12 years of age and known weights ranged from 7.6 to 13.8 kg. They were fed a commercially prepared carnivore diet (Fur Breeders Agricultural Cooperative, Sandy, UT, USA) once daily, and fasted 1 day per week. Water was provided ad libitum. Vaccinations were given annually against canine distemper, hepatitis, leptospirosis, parvovirus, parainfluenza, type 2 coronavirus, adenovirus, and rabies. Routine parasite control was administered as indicated. All protocols were approved by the NWRC (QA799, QA944 and QA987) and Utah State University (IACUC#1114) Institutional Animal Care and Use Committees.
2.2. Specimen collection and handling
During the breeding season (January–March), mated pairs were observed in their pens throughout the day. Mating behavior (Golani and Mendelssohn, 1971, Bekoff and Diamond, 1976) was recorded including mounting attempts and copulatory ties. Peripheral blood specimens were initially collected 2–3 weeks after the first observed copulatory tie. These initial samples were presumed to be before embryonic implantation or placental development (Tsutsui, 1989, Concannon et al., 2001) and were therefore expected to test negative for relaxin. Subsequent samples were then periodically collected from the females until a positive result was obtained.
Anti-coagulated blood specimens were collected by venipuncture or an indwelling venous catheter, into evacuated tubes containing either sodium heparin or lithium heparin. Samples were collected before the animals were fed, and without sedation or anesthesia. The plasma was separated from the whole blood as soon as possible and stored at ≤−20 °C until testing. Due to behavioral differences between coyote pairs, the earliest samples were ultimately collected at an estimated 11 days of gestation with additional samples randomly obtained throughout diestrus. In addition, 44 females were randomly sampled for 20 weeks after parturition to assess how long relaxin remained detectable by this assay.
2.3. Relaxin assay
Presence or absence of canine relaxin was determined with a qualitative ELISA, ReproCHEK™ (Synbiotics Corporation, San Diego, CA, USA). The assay utilizes polyclonal anti-relaxin antibodies in solid phase (microtiter wells), and canine specific anti-relaxin monoclonal antibodies conjugated to horseradish peroxidase (HRP). Testing was performed on thawed samples (50 μl) according to the manufacturer's instructions using the optical density (OD) measurement option (Carlson, 2006).
Nine intact females that were not bred or who had been paired with castrated males, and eight intact males were included as internal controls. Because the absorbance values of these control animals sometimes exceeded the manufacturer's negative control (intra-run comparison), a range of values (<0.030 OD) representing a negative result was established. Mated females that initially tested negative were resampled 1–2 weeks later to either confirm the negative test for relaxin or obtain a positive result.
3. Results
During the study, 83 female coyotes tested positive for relaxin prior to parturition and appearance of pups (Table 1 ). Concurrently, 26 females repeatedly tested negative and no pups were seen. Four females tested positive for relaxin although pups were subsequently never found, and because neither ultrasonography nor radiography was performed it remains inconclusive whether these four females were actually pregnant. In contrast, two females tested positive and were known to have experienced spontaneous abortions, confirmed by recovery of the expelled fetuses.
Table 1.
Number of coyotes within each cohort that tested positive or negative for relaxin during 2000–2003 breeding seasons; NWRC facility, Millville, UT, USA
| Mated females |
Control animals |
|||||
|---|---|---|---|---|---|---|
| With pups | No pups | Aborted | Males | Unmated females | Females with castrated mate | |
| Positive | 83 | 4 | 2a | 0 | 0 | 0 |
| Negative | 0 | 26 | 0 | 8 | 7 | 2 |
Confirmed via recovery of fetuses.
In the first 2 years of the study, eight males, seven unmated females, and two females with castrated mates were also sampled (Table 1). The OD measurements of these animals provided some guidelines as to how this assay would perform against plasma from non-pregnant coyotes. The measurements of these negative internal controls fell within the range of the manufacturer's negative controls throughout the study (Table 2 ).
Table 2.
Optical density measurements of ReproCHEK™ reagent (Synbiotics Corporation, San Diego, CA, USA) and internal (coyote) controls
| Positive relaxin control | Negative relaxin control | Female coyotes not-bred | Male coyotes | |
|---|---|---|---|---|
| Minimum | 0.051 | −0.001 | 0.004 | 0.001 |
| Maximum | 0.487 | 0.037 | 0.032 | 0.018 |
| Mean | 0.249 | 0.009 | 0.015 | 0.009 |
| Median | 0.252 | 0.006 | 0.014 | 0.009 |
| n | 63 | 63 | 13 | 8 |
Reagent positive and negative controls were included in each assay run; internal controls were randomly assigned. Values represent the parameters observed during 2000–2003 breeding seasons; NWRC facility, Millville, UT, USA. n: number of manufacturer's controls or non-pregnant coyote samples tested.
Absorbance values were recorded for 82 full term pregnant females. A gestation length of 62 days was assumed (Gier, 1968, Kennelly, 1978) and the OD readings were aligned using the day of parturition as the reference point. Partitioning of the gestation period into weekly intervals showed optical density increasing by week 4 (Table 3 ). When more than one sample was collected, it was possible to estimate the day of gestation based upon when a coyote first showed a positive relaxin test result (Table 4 ). Under these conditions, the earliest positive result was obtained at an estimated 23 days gestation; on days 24–27 results either were negative, indeterminate, or positive. However, from day 28 on, all results were positive and had an OD ≥ 0.100 (Fig. 1 ). We also note that while some negative and indeterminate results were recorded, at least 90% of the samples collected on days 25–27 were interpreted as positive (Table 4).
Table 3.
Range of weekly relaxin optical density readings measured during gestation (standardized to 62 days) for female coyotes during the 2000–2003 breeding seasons; NWRC facility, Millville, UT, USA
| Week of gestation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Minimum | n.a. | 0.010 | 0.000 | 0.009 | 0.125 | 0.855 | 1.066 | 1.060 | 0.825 |
| Maximum | n.a. | 0.039 | 0.042 | 0.653 | 2.387 | 2.309 | 2.123 | 1.886 | 2.004 |
| Median | n.a. | 0.034 | 0.008 | 0.154 | 0.750 | 1.627 | 1.735 | 1.581 | 1.594 |
| n | 0 | 4 | 25 | 42 | 42 | 21 | 14 | 13 | 11 |
n: number of coyote samples included in each weekly dataset; n.a.: samples were not collected in the first week.
Table 4.
Range of daily relaxin optical density readings for female coyotes, days 23–28 of gestation during the 2000–2003 breeding seasons; NWRC facility, Millville, UT, USA
| Day of gestation |
||||||
|---|---|---|---|---|---|---|
| 23a | 24 | 25 | 26 | 27 | 28b | |
| Minimum | 0.020 | 0.009 | 0.049 | 0.097 | 0.024 | 0.100 |
| Maximum | 0.138 | 0.424 | 0.188 | 0.242 | 0.502 | 0.653 |
| Median | 0.043 | 0.056 | 0.107 | 0.140 | 0.275 | 0.497 |
| % Pos | 25 | 50 | 91 | 100 | 90 | 100 |
| n | 4 | 4 | 11 | 4 | 10 | 7 |
Days are aligned from day of parturition and assume a common 62-day gestation for all females. % Pos: percentage of samples (n) testing positive per day.
Earliest day of gestation when a positive sample was observed.
All samples from day 28 forward tested positive.
Fig. 1.
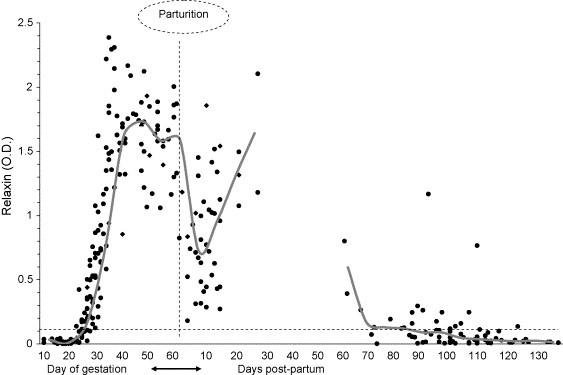
Individual optical density measurements of relaxin in female coyotes sampled during 2000–2003 breeding seasons, NWRC facility, Millville, UT, USA. Pre-partum plasma samples (n = 214) were collected from 82 females between day 10 of gestation and parturition. Post-partum sampling continued after the 2000–2002 breeding seasons with 44 females (n = 114) spanning 140 days, although sampling was suspended between days 28 and 60. Data were aligned by day of parturition (indicated by the vertical dashed line) and assumed a full term gestation of 62 days. During this study, the optical density measurements for non-pregnant females consistently remained below the 0.100 OD threshold as indicated by the horizontal dashed line. Solid line represents the median observed OD measurement.
An indeterminate threshold range of 0.030–0.050 OD was established based upon the absorbance values of the internal coyote controls and those of the manufacturer's reagents; and reinforced by the absorbance measurements recorded for the mated females with and without pups. All internal (coyote) controls were ≤0.032, and the difference between the maximum ReproCHEK™ negative control (0.037) and the minimum ReproCHEK™ positive control (0.051) was in the range of 0.038–0.050 (Table 2). Samples (n = 69) from the 26 mated females without pups fell in the range of −0.005 to 0.072 (median 0.009 OD). The singular female with the OD measurement of 0.072 retested 2 weeks later at 0.033; and while 5 other samples in this group were also between 0.030–0.050, 62 samples (90%) were <0.030.
Serial blood sampling from five subjects revealed that within 48–72 h a pregnant female coyote could display a rapid increase in OD measurements representing a plasma-conversion from negative to positive. For example, one female changed from 0.015 to 0.117 in 2 days, while another from 0.012 to 0.160 in 3 days. This latter data suggested that the absorbance readings of females who were pregnant (true-positives) would quickly increase in intensity (Fig. 1), thus differentiating them from non-pregnant cohorts within a short period of time. However, the timing of a sample collection might produce an indeterminate result (0.030–0.050 OD), and such results need to be rechecked.
After parturition, 44 females were sampled initially during the first 4 weeks post-partum, then randomly sampled from weeks 9 to 20 (Table 5 ). All females continued to test positive (OD measurement >0.050) until day 72 when one female had an OD reading of 0.001. All other post-partum subjects remained positive until the next female tested negative (0.005) at day 87. Gradually all females reverted to negative levels (Fig. 1) and the last OD measurement that was >0.030 was recorded on day 127.
Table 5.
Range of post-partum relaxin optical density readings for female coyotes shown in 2 week increments; 2000–2003 breeding season, NWRC facility, Millville, UT, USA
| Weeks post-partum |
||||||||
|---|---|---|---|---|---|---|---|---|
| 1–2 | 3–4a | 9–10 | 11–12 | 13–14 | 15–16 | 17–18 | 19–20 | |
| Minimum | 0.180 | 1.076 | 0.072 | 0.001 | 0.004 | 0.004 | 0.007 | 0.009 |
| Maximum | 1.858 | 2.103 | 0.801 | 0.193 | 1.167 | 0.766 | 0.136 | 0.036 |
| Median | 0.773 | 1.315 | 0.328 | 0.123 | 0.090 | 0.049 | 0.028 | 0.015 |
| % Neg | 0 | 0 | 0 | 17 | 26 | 28 | 62 | 80 |
| n | 37 | 5 | 4 | 6 | 19 | 25 | 13 | 5 |
The females were not sampled between weeks 4 and 9 after parturition. % Neg: percentage of samples (n) testing negative in each 2-week dataset.
4. Discussion
We found the hormone relaxin to be an acceptable diagnostic marker of pregnancy in the coyote. Through four consecutive breeding seasons all female coyotes with young tested positive prior to parturition (including spontaneous abortions). Under these conditions, a true-positive was a female that had pups and tested positive for relaxin. Once these subjects converted from negative to positive (between 23 and 28 days gestation) the intensity of the OD measurements increased rapidly (Fig. 1). This marked increase in OD made a true-positive distinguishable from cohorts who were never seen with pups. Because more traditional diagnostic tools (e.g., ultrasonography or radiography) were not routinely used, the negative cohorts could not be confirmed as being non-pregnant (true-negatives); however 26/30 (86.6%) of the females without pups and 17/17 (100%) of the control coyotes tested negative for relaxin.
Another useful aspect of relaxin in the determination of a coyote's reproductive status was that it remained detectable throughout gestation, and well into the post-partum period (Fig. 1). Thus, collection of a meaningful specimen became less dependent on critical timing. All samples collected after day 28 of gestation were >0.100 OD until the end of week 10 post-partum (Fig. 1), and this level of color development (a visible blue color) is easily discernible from the ReproCHEK™ negative control (no color).
We observed a general trend of increased color intensity as the females progressed through their pregnancies (Fig. 1). Color intensified between weeks 4 and 5, maintained a maximum intensity then appeared to weaken after week 7 (Table 3). There also appeared to be a slight peri-partum decrement in relaxin followed by a short rebound in the first month of lactation (Fig. 1). For technical reasons the manufacturer makes no attempt to correlate color intensity or absorbance with circulating levels of relaxin, and this study did not provide any evidence that these measurements could predict either reproductive success or litter size. Follow up testing of the two females who experienced spontaneous abortions (neither the etiology nor the day of fetal death is known) produced very different results. Both females were positive 3 or 5 days before the fetuses were expelled; but while the first female tested negative 4 and 10 days after, the second female remained positive up to 34 days later.
In conclusion, we found this assay easy to perform; and although a spectrophotometer was used in this study, it was not a requirement. Color development of unknown samples can be compared to the ReproCHEK™ negative control (per the manufacturer's instructions), but our experience suggests that in coyotes a weak-positive result should be interpreted carefully. We recommend that sampling not begin until 4 weeks (28 days) after the most active mating behavior is observed, and that initial negative results be confirmed with a new specimen 1–2 weeks later. With these considerations in mind, this assay should be a reliable tool in this species and possibly other wild congeners of Canis.
Acknowledgements
The authors thank the following for their review of this manuscript: Drs. Thomas D. Bunch, Frederick F. Knowlton, Ramona T. Skirpstunas, and Michael L. Wolfe. Thanks also to D. Wannemacher. We appreciate Thomas J. DeLiberto and NWRC staff, and the following undergraduate and graduate students at Utah State University for assistance in the handling and care of the study animals, behavior observations, and specimen collections: K. Anderson, R. Bartel, S. Brummer, K. Casper, P. Darrow, R. Harrison, J. Hedelius, D. Jones, R. Kikkert, S. Kirshner, L. Minter, H. Phillips, J. Robinson, A. Seglund, H. Smith, J. Tegt, K. Wenning, M. Wollbrink, and D. Zemlicka. Funding and logistical support provided by USDA/APHIS/WS/National Wildlife Research Center, Logan Field Station, Utah State University, Logan, UT.
References
- Andelt W.F. Behavioral ecology of coyotes in south Texas. Wild. Monogr. 1985;94:1–45. [Google Scholar]
- Bekoff M., Diamond J. Precopulatory and copulatory behavior in coyotes. J. Mamm. 1976;57:372–375. [Google Scholar]
- Bekoff M., Wells M.C. Social ecology and behavior of coyotes. Adv. Stud. Behav. 1986;16:251–338. [Google Scholar]
- Buff S., Fontbonne A., Lopez P., Rauer M., Crevat D. Circulating relaxin concentrations in pregnant and nonpregnant bitches: evaluation of a new enzymeimmunoassay for determination of pregnancy. J. Reprod. Fertil. Suppl. 2001;57:187–191. [PubMed] [Google Scholar]
- Carlson, D.A., 2006. Investigation in the reproduction of the coyote (Canis latrans). PhD Dissertation. Utah State University, Logan, UT, USA.
- Concannon P.W., McCann J.P., Temple M. Biology and endocrinology of ovulation, pregnancy and parturition in the dog. J. Reprod. Fertil. Suppl. 1989;39:3–25. [PubMed] [Google Scholar]
- Concannon P., Tsutsui T., Shille V. Embryo development, hormonal requirements and maternal responses during canine pregnancy. J. Reprod. Fertil. Suppl. 2001;57:170–179. [PubMed] [Google Scholar]
- Gese E.M. Territorial defense by coyotes (Canis latrans) in Yellowstone National Park, Wyoming: who, how, where, when, and why. Can. J. Zool. 2001;79:980–987. [Google Scholar]
- Gese E.M., Rongstad O.J., Mytton W.R. Population dynamics of coyotes in southeastern Colorado. J. Wildl. Manage. 1989;53:174–181. [Google Scholar]
- Gese E.M., Ruff R.L., Crabtree R.L. Social and nutritional factors influencing the dispersal of resident coyotes. Anim. Behav. 1996;52:1025–1043. [Google Scholar]
- Gier H.T. Agri. Exper. Stat, Kan. State Univ., Agri. App. Sci.; Manhattan, KS, USA: 1968. Coyotes in Kansas. [Google Scholar]
- Golani I., Mendelssohn H. Sequences of precopulatory behavior of the jackal (Canis aureus L.) Behaviour. 1971;38:169–192. [Google Scholar]
- Hamlett G.W.D. The reproductive cycle of the coyote. USDA Tech. Bull. 1938;616:1–11. [Google Scholar]
- Hodges, C.M., 1990. The reproductive biology of the coyote (Canis latrans). PhD Dissertation. Texas A&M University, College Station, TX, USA.
- Kennelly J.J. Coyote reproduction. In: Bekoff M., editor. Coyotes: Biology, Behavior, and Management. Academic Press; New York: 1978. [Google Scholar]
- Kennelly J.J., Johns B.E. The estrous cycle of coyotes. J. Wildl. Manage. 1976;40:272–277. [Google Scholar]
- Klonisch T., Hombach-Klonisch S., Froehlich C., Kauffold J., Steger K., Steinetz B.G., Fischer B. Canine preprorelaxin: nucleic acid sequence and localization within the canine placenta. Biol. Reprod. 1999;60:551–557. doi: 10.1095/biolreprod60.3.551. [DOI] [PubMed] [Google Scholar]
- Knowlton F.F. Preliminary interpretations of coyote population mechanics with some management implications. J. Wildl. Manage. 1972;36:369–382. [Google Scholar]
- Kreeger T.J., Seal U.S., Cohen Y., Plotka E.D., Asa C.S. Characterization of prolactin secretion in gray wolves (Canis lupus) Can. J. Zool. 1991;69:1366–1374. [Google Scholar]
- Roy M.S., Geffen E., Smith D., Ostrander E.A., Wayne R.K. Patterns of differentiation and hybridization in North American wolflike canids, revealed by analysis of microsatellite loci. Mol. Biol. Evol. 1994;11:553–570. doi: 10.1093/oxfordjournals.molbev.a040137. [DOI] [PubMed] [Google Scholar]
- Seal U.S., Plotka E.D., Packard J.M., Mech L.D. Endocrine correlates of reproduction in the wolf. I. Serum progesterone, estradiol and LH during the estrous cycle. Biol. Reprod. 1979;21:1057–1066. doi: 10.1095/biolreprod21.5.1057. [DOI] [PubMed] [Google Scholar]
- Sherwood O.D. Relaxin. In: Knobil E., Neil J.D., editors. The Physiology of Reproduction. 2nd ed. Raven Press; New York: 1994. [Google Scholar]
- Silver H., Silver W.T. Growth and behavior of the coyote-like canid of northern New England with observations on canid hybrids. Wildl. Monogr. 1969;17:1–41. [Google Scholar]
- Steinetz B.G., Goldsmith L.T., Lust G. Plasma relaxin levels in pregnant and lactating dogs. Biol. Reprod. 1987;37:719–725. doi: 10.1095/biolreprod37.3.719. [DOI] [PubMed] [Google Scholar]
- Steinetz B.G., Goldsmith L.T., Harvey H.J., Lust G. Serum relaxin and progesterone concentrations in pregnant, pseudopregnant, and ovariectomized, progestin-treated pregnant bitches: detection of relaxin as a marker of pregnancy. Am. J. Vet. Res. 1989;50:68–71. [PubMed] [Google Scholar]
- Stellflug J.N., Muse P.D., Everson D.O., Louis T.M. Changes in serum progesterone and estrogen of the nonpregnant coyote during the breeding season. Proc. Soc. Exp. Biol. Med. 1981;167:220–223. doi: 10.3181/00379727-167-41153. [DOI] [PubMed] [Google Scholar]
- Tsutsui T. Gamete physiology and timing of ovulation and fertilization in dogs. J. Reprod. Fertil. Suppl. 1989;39:269–275. [PubMed] [Google Scholar]
- Tsutsui T., Stewart D.R. Determination of the source of relaxin immunoreactivity during pregnancy in the dog. J. Vet. Med. Sci. 1991;53:1025–1029. doi: 10.1292/jvms.53.1025. [DOI] [PubMed] [Google Scholar]
- Walker S.L., Waddell W.T., Goodrowe K.L. Reproductive endocrine patterns in captive female and male red wolves (Canis rufus) assessed by fecal and serum hormone analysis. Zoo Biol. 2002;21:321–335. [Google Scholar]


