Abstract
Angiotensin-converting enzyme, a member of the M2 metalloprotease family, and endothelin-converting enzyme, a member of the M13 family, are key components in the regulation of blood pressure and electrolyte balance in mammals. From this point of view, they serve as important drug targets. Recently, the involvement of these enzymes in the development of Alzheimer's disease was discovered. The existence of homologs of these enzymes in invertebrates indicates that these enzyme systems are highly conserved during evolution. Most invertebrates lack a closed circulatory system, which excludes the need for blood pressure regulators. Therefore, these organisms represent excellent targets for gaining new insights and revealing additional physiological roles of these important enzymes. This chapter reviews the structural and functional aspects of ACE and ECE and will particularly focus on these enzyme homologues in invertebrates.
Keywords: Endothelin-converting enzyme, Invertebrates, angiotensin-converting enzyme, Metalloproteases, ACE, ECE
I. Introduction
Protease (peptidase, proteinase) is the general name for an enzyme that cleaves peptide bonds in peptides and proteins causing an irreversible modification or destruction to their substrate. Proteases are, based on their nature of catalysis, classified into five major groups: serine proteases, cysteine proteases, aspartic proteases, metalloproteases, and proteases with an unknown mechanism (Barret et al., 1998). From these groups, the metalloproteases, with more than 30 families identified to date, are most diverse. In mammalian physiology, the high interest in metalloproteases originates from the fact that they are attractive targets for drug design.
In insects, regulatory peptides are the major and most diverse class of signaling molecules. They act as transmitters and modulators in the nervous system and as hormones controlling key physiological processes, behavior, and development. Peptidases are recognized as the key components of this peptidergic system, functioning in biosynthesis of active peptides from their precursors and in peptide inactivation.
Structural analysis of metalloproteases from invertebrate and vertebrate species indicates that these enzymes are very well conserved and arose early during evolution. The evolutionary conservation of universal peptide-signaling mechanisms has led to the general acceptance of the idea that invertebrates provide excellent models for the study of genetics and physiology. The information and knowledge gained by studying invertebrate peptidases will provide novel insights in the process of evolution and may reveal conserved functions of vertebrate peptidases that have remained unknown until now.
II. Angiotensin-Converting Enzyme Mammals
Angiotensin-converting enzyme (ACE, dipeptidyl carboxypeptidase I [DCP I], peptidase P, carboxycathepsin, peptidyl-dipeptidase A, peptidyl-dipeptidase I, kininase II, EC 3.4.15.1) was originally isolated in 1956 as a “hypertensin-converting enzyme” (Skeggs et al., 1956). Classified as a member of the M2 gluzincin family, ACE acts as a zinc-metalloprotease. The general reaction mechanism is identical for all zinc-metalloproteases. Coordination of the zinc ion within the catalytic site of metalloproteases is typically affected by an HEXXH motif, a third zinc ligand (E) located carboxyl to this motif, and a water molecule. The histidine residues within the active site motif are known to directly coordinate the active site zinc ion, whereas the HExxH glutamate has been shown to coordinate weakly to zinc via the activated molecule, thus facilitating the acid–base catalytic mechanism.
The renin-angiotensin system (RAS) plays a key role in the regulation of blood pressure and of fluid and electrolyte balance in mammals (Inagami, 1994). Being part of the renin–angiotensin system, ACE converts the decapeptide angiotensin I (AngI) into the potent vasopressor angiotensin II (AngII). ACE also inactivates the vasodilatory peptide bradykinin (BK). Hence, inhibition of ACE results in lowering the blood pressure by preventing formation of the hypertensive AngII and by inactivation of the hypotensive BK (Fig. 1 ).
FIG. 1.
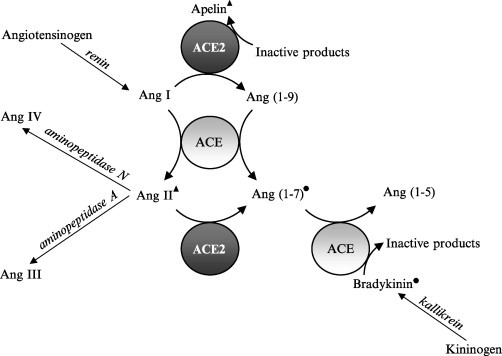
Components of the renin–angiotensin system with the principal enzymes ACE and ACE2 involved in the generation of biologically active peptides. ▴; vasoconstrincting effect; ●; vasodilating effect.
ACE is one of the most studied mammalian peptidases, and in particular for medical applications. ACE inhibitors are widely used in treatment of hypertension, diabetic nephropathy, and heart failure (Dell'Italia et al., 2002). The production of captopril, the first clinically used ACE inhibitor, represents a classical example of drug design (Menard and Patchett, 2001).
A. Structure and Isoforms
Vertebrate ACE is classified as a type I ecto-enzyme, with the major part of the protein, including the active site, located on the external surface of cells. ACE is anchored in the plasma membrane by the C-terminal part of the protein. There is an N-terminal signal peptide present, required for the passage through the endoplasmatic reticulum. Two distinct forms of ACE are known: a somatic form (sACE), which is found in many tissues, and a smaller iso-enzyme, germinal ACE (gACE, also called testicular ACE [tACE]), which is found exclusively in testes, where it has a crucial role in fertility (Fig. 2 ). The soluble, circulating form of the sACE protein is produced by a “secretase,” probably a zinc-metalloprotease, which releases ACE from the membrane by cleaving off the extracellular part (Turner and Hooper, 2002).
FIG. 2.
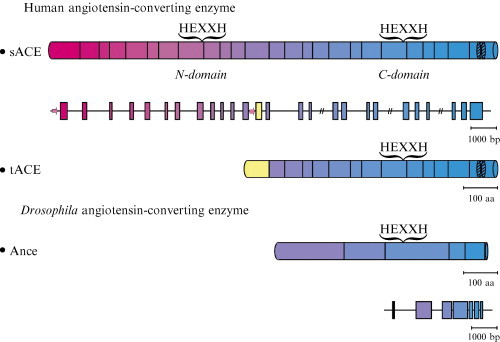
Schematic representation of human and Drosophila ACE protein and gene structure. Exons are indicated by boxes. Exons and corresponding amino acid regions in the protein are colored correspondingly. Zinc-binding sites (HExxH) are indicated. Human somatic and testicular promoters are indicated by arrows. One-kilobase intronic sequence was omitted at the ⧸⧸ signs.
The two forms differ in that the somatic isoform contains two active sites, whereas the germinal form contains only one. sACE is composed of two very similar domains (C and N domain), which are both catalytically active (Wei et al., 1991). tACE is, apart from its N-terminal sequence, identical to the C-domain of sACE.
The close similarity of the N and C domains of mammalian sACE and the fact that exons 4–11 and 17–24 of the human ACE gene, which code for the N and C domain, respectively, are very similar in size and have similar codon phases at exon–intron boundaries, strongly indicates that the coding gene arose from duplication of an ancestral ACE gene coding for a single domain enzyme (Cornell et al., 1995). The ancestral copy is predicted to be the C-domain, equivalent to the germinal isoform (Lattion et al., 1989). The two isoforms are transcribed from a single gene, under the control of two distinct promoters. sACE is transcribed from a promoter region upstream of the duplication, and tACE from a promoter within intron 12 (Langford et al., 1991).
The two domains of sACE have relatively broad substrate specificities and share some enzymatic features, but there are several biochemical properties that differentiate the two active sites (Corvol et al., 1995). For example, the hematopoietic stem cell regulator, N-acetyl-Ser-Asp-Lys-Pro (AcSDKP), is an in vivo substrate for the N-domain, but not for the C domain of human ACE (Azizi et al., 1996). The N and C domain can also be differentiated by their response to chloride ions, with the C domain's specific activity being dependent on chloride ion concentration (Wei et al., 1991). Their inhibitory affinity profiles also differ, illustrated by the existence of an N-domain-specific inhibitor (RXP 407) (Dive et al., 1999).
B. Biological Role
ACE acts as a peptidyl-dipeptidase, removing the C-terminal dipeptide from its substrate, but it can also act as an aminopeptidase and as an endopeptidase on peptides that are amidated at the C terminus. To date, only angiotensin I, bradykinin (Yang, 1970), and the haemoregulatory peptide Ac-DSKP (Azizi et al., 2001) have been confirmed as in vivo substrates for mammalian ACE.
The conversion of AngI into the vasoconstrictor AngII and the degradation of the vasodilator bradykinin reflect the key role of ACE as a component of the mammalian RAS. In recent years, the RAS turned out to be a far more complex regulatory system that involves more angiotensin-derived mediators (AngI, AngII, AngIII, AngIV, Ang(1–9)), Ang(1–7)) then initially recognized (Fig. 1). To this day, it includes four identified receptors (Turner and Hooper, 2002).
In vivo models using ACE-deficient mice have greatly advanced the knowledge of the RAS and confirmed the role of ACE in blood pressure regulation and fertility. Knock-out mice all show reduced blood pressure, altered kidney morphology, impaired somatic growth, and male sterility (Esther 1996, Eriksson 2002). These mice produce normal numbers of sperm that are indistinguishable from wild-type sperm in assays of viability, motility, capacitation, and induction of the acrosome reaction. Absence of ACE, however, does cause defects in sperm transport in the oviducts and in binding to zonae pellucidae (Hagaman et al., 1998). Knockout of tACE, leaving the sACE intact, has proven that tACE expression is both necessary and sufficient for fulfilling the role of ACE in male fertility (Ramaraj et al., 1998). Recently, immunocytochemical studies have shown that tACE is completely shed from the sperm membrane before ejaculation. This would imply that the defects in sperm transport in the oviducts and in binding to zonae pellucidae after tACE knockout are not directly related with tACE activity (Metayer et al. 2002), so the exact role of tACE remains to be uncovered. The fact that to date no in vivo substrate has been found for tACE is the main bottleneck in tACE research.
Expression of sACE, either in renal proximal tubes or in vasculature, is sufficient for maintaining normal kidney functions. However, for maintaining blood pressure, sACE must be expressed in vascular endothelial cells (Kessler et al., 2003). Expression of tACE alone is sufficient to restore male fertility in mutant mice without curing other problems of Ace −⧸− mice (Ramaraj et al., 1998). However, sACE cannot substitute for tACE, demonstrating that the two isozymes are not interchangeable for fertility functions (Kessler et al., 2000). Yet tACE expression in serum of Ace−⧸− mice can substitute for sACE in maintaining normal renal structure and functions without restoring blood pressure (Kessler et al., 2002).
All this indicates that the two isoforms of ACE are indispensable, as suggested by their high degree of evolutionary conservation. Also, the specific physiological function of ACE requires its expression in the correct tissue.
The peptide AcSDKP reversibly prevents the recruitment of pluripotent haematopoietic stem cells into the S phase of the cellular cycle by maintaining them into the Go phase. Because this peptide has been found to be an in vivo substrate specific for the N domain of sACE, it is suggested that ACE is implicated in the process of hematopoietic stem cell regulation by permanently degrading this natural circulating inhibitor (Azizi 2001, Rousseau 1995). However, in a very recent study, mutation of the N domain zinc-binding site in mice, rendering this N domain inactive indeed, resulted in accumulation of AcDSKp, but no physiological effect was seen (Fuchs et al., 2004).
ACE acts as an endopeptidase on the multifunctional neuropeptides substance P and cholecystokinin and may degrade the luteinizing hormone releasing hormone (LH–RH) (Skidgel and Erdos, 1985). Its biological significance in this context is not known, and the possibility of functional cross talk between the systems has not been excluded (Turner and Hooper, 2002).
The broad in vitro specificity (Table I ) and the presence of ACE in species that lack an identifiable RAS shows that the relevance of ACE to the animal must be more complex than just its role in the RAS.
TABLE I.
Primary Sequences of Peptides Hydrolyzed by ACE and ACE2
| ACE |
ACE2 |
||
|---|---|---|---|
| Substrate | Sequence | Substrate | Sequence |
| Ang I | DRVYIHPF↓HL | Ang I | DRVYIHPFH↓L |
| Ang (1-9) | DRVYIHP↓FH | Ang II | DRVYIHP↓F |
| Ang (1-7) | DRVYI↓HP | Apelin 18 (36) | (…)QRPRLSHKGPMP↓F |
| Bradykinin | RPPGF↓SP↓FR | Des-Arg9 bradykinin | RPPGFSP↓F |
| Enkephalins | YGG↓FM(L)-NH2 | β-Casamorphin | YPFVEP↓I |
| Substance P | RPLPQQFF↓G↓LM-NH2 | Neocasamorphin | YPVEP↓I |
| LH-RH | EHW↓SGLRPG | Dynorphin A 1-13 | YGGFLRRIRPKL↓K |
| Haemoregulatory peptide | NAcSD↓KP | Neurotensin 1-8 | Pe-LYENKP↓R |
| CCK-8 | NY↓(SO3H)MGWM↓DF-NH2 | ||
Cleavage sites are indicated by an arrow.
C. ACE2
Up until now, only one other member of the M2 family other than ACE has been identified. Almost 50 years after the discovery of ACE itself, two groups (Donoghue 2000, Tipnis 2000) introduced the first homolog of mammalian ACE. This protein and its homolog were named ACE2 and ACEH, respectively, and were shown to be an essential regulator of heart function (Crackower et al., 2002). Human ACE2 is an 805–amino acid–long type I integral membrane protein, including a 17–amino acid N-terminal signal peptide and a putative C-terminal membrane anchor. The extracellular domain shares a 42% sequence identity with the catalytic domain of sACE. It is 33% identical to human testicular (germinal) ACE and also contains a single catalytic site. ACE2 contains six putative N-linked glycosylation sites on the extracellular part (Tipnis et al., 2000). The C-terminal domain of ACE2 shares a 48% sequence identity with collectrin (Vickers 2002, Zhang 2001), a developmentally regulated renal transmembrane glycoprotein. The many similarities in the genomic sequence of ACE2 and ACE indicate that there is an evolutionary relationship between these two genes (Turner and Hooper, 2002). ACE2 transcripts are detected in heart, lung, kidney, the gastrointestinal tract, and testis (Harmer 2002, Komatsu 2002). ACE2 is located at the endothelium of coronary and intrarenal vessels and in the renal tubular epithelium. Like ACE, ACE2 also appears to be susceptible to cleavage and secretion from the cell surface (Donoghue et al., 2000).
However, the sequence identity of the juxtamembrane regions of ACE and ACE2 is different. The cleavage site present in ACE (–Ala-Arg Ser-Glu–) is absent in ACE2. Hence, a different secretase may be involved in the shedding of ACE2. ACE2 does not have the same substrate specificity as ACE, nor is it inhibited by the typical ACE inhibitors such as enalapril, lisinopril, and captropril. ACE2 hydrolyzes AngI and AngII but cleaves neither bradykinin nor the typical ACE substrate Hip-His-Leu (Fig. 1). To hydrolyze AngI and AngII, ACE2 acts as a carboxypeptidase, removing one amino acid from the C terminus of the substrate. This is also the case for kinetensin, des-Arg9 bradykinin, and neurotensin (Donoghue 2000, Turner 2002). Several other regulatory peptides, including the enkephalins, are not substrates of ACE2. It seems that ACE2 prefers to cleave a single C-terminal hydrophobic residue from its substrates (Table I) and consequently does not hydrolyze bradykinin that contains an arginine at its C terminus. ACE2 is the first mammalian carboxypeptidase identified that contains the HExxH active site. ACE2 also acts on apelin-13 and apelin-36 peptides, of which the role is not yet fully understood but involves an effect on hypertension and vasoconstriction (Lee et al., 2000). Two opioid peptides, dynorphin A(1–13) and β-casamorphin, are also ACE2 substrates (Eriksson 2002, Vickers 2002). These peptides activate G protein-coupled opioid receptors that regulate pain perception and are suggested to negatively affect cardiomyocyte contractility (Pugsley 2002, Ventura 1992, Wenzlaff 1998).
The differences in substrate specificity between ACE and ACE2 have important physiological consequences. Whereas ACE activity leads to the formation of the vasoconstrictor AngII, ACE2 is involved in the formation of vasodilator mediators (Danilczyk et al., 2003). The targeted disruption of ACE2 in mice results in increased AngII levels, impaired cardiac contractility, and up-regulation of hypoxia-induced genes in the heart (Crackower et al., 2002). Renal ACE is implicated in diabetic nephropathy. In the diabetic kidney, levels of ACE2 transcription and protein expression are down-regulated. It is suggested that this regulation occurs through the actions of ACE2 in the RAS (Tikellis et al., 2003).
Another, rather unexpected function of ACE2 was recently discovered. It was found to act as a functional receptor for the Severe Acute Respiratory Syndrome (SARS) coronavirus (CoV) (Dimitrov 2003, Li 2003, Xiao 2003). SARS is the abbreviation for the CoV-induced disease that causes SARS, with a mortality rate of approximately 10% (Ksiazek 2003, Kuiken 2003). The virus associates with cellular receptors to mediate infection of the target cells (Gallagher 2001, Holmes 2003). ACE2, acting as a cellular receptor, efficiently binds to the S1 domain of SARS-CoV, efficiently supporting the replication of SARS-CoV. Adding a soluble form of ACE2, but not that of ACE, blocks the association of SARS-CoV and its target cell. Replication of the virus in cells and the formation of syncitia can be blocked by antibodies against ACE2, which indicates the importance of ACE2 in the replication of SARS-CoV (Li et al., 2003).
Several attempts to reveal the three-dimensional structure of ACE2 have been made very recently. This provides an insight into the structural variations underlying the differences in substrate specificity between ACE and ACE2. It seems that these differences occur in the ligand binding cavity, influencing the binding of the peptide carboxyterminus (Guy et al., 2003). The crystal structure of ACE2, native and inhibitor bound, revealed a large inhibitor-dependent bending movement of the two enzyme domains to one another, positioning important catalytic residues (Towler et al., 2004). It has been suggested that ridges surrounding the cavity at the top of the molecule serve as a binding region for SARS (Prabakaran et al., 2004). The discovery of this SARS-related function of ACE2, together with the knowledge of its structure, may contribute substantially to the possible treatment of SARS patients.
III. ACE in Invertebrates
Lamango and Isaac (1993) reported the metabolization of [D-Ala2, Leu5] enkephalin by head membranes of the housefly, Musca domestica. This hydrolysis was only partially inhibited by phosphoramidon, an inhibitor of endopeptidase-24.11. The combination of phosphoramidon and captopril, however, fully inhibited the cleavage of enkephalin, suggesting the presence in this fly of a second enkephalin-metabolizing enzyme in addition to endopeptidase-24.11. It turned out to be zn 87-kDa-enzyme that was activated by ZnCl2 and inhibited by EDTA as well as the mammalian ACE inhibitors captopril, fosinoprilat, and enalaprilat. This represented the discovery of the first invertebrate ACE homolog (Lamango and Isaac, 1994). Following the report on this ACE-like activity, the first results of putative Drosophila melanogaster ACE cDNA clones were published (Cornell et al., 1993).
Since then, ACE homologs have been detected in many invertebrate species. Cloning or purification of ACE from the fruit fly D. melanogaster (Cornell 1995, Tatei 1995), the cattle tick Boophilus microplus (Jarmey et al., 1995), the leech Theromyzon tessalutum (Laurent and Salzet, 1996), the housefly M. domestica (Lamango et al., 1996), the buffalo fly Haematobia irritans exigua (Wijffels et al., 1996), the mosquito Anopheles stephensi (Ekbote et al., 1999), the silk moth Bombyx mori (Quan et al., 2001), the grey fleshfly Neobellieria bullata (Vandingenen et al., 2002b), and the locust Locusta migratoria (Macours et al., 2003a) have contributed to the molecular, biochemical, and evolutionary characterization of invertebrate ACEs. The first D. melanogaster ACE homolog that was discovered is abbreviated as Ance, because ace was already used for acetylcholine esterase in the fruit fly. Of the invertebrate ACEs known today, the two D. melanogaster ACE homolog (Ance and Acer) have been studied in most detail. Ance is also known under the name Race, as the enzyme was discovered, sequenced, and named by two groups simultaneously (Cornell 1995, Tatei 1995). Nowadays, the focus on ACE research is beginning to shift from purification-characterization to a more physiological point of view. Considering the fact that most invertebrates have an open circulatory system, there is probably no need for a renin–angiotensin system analogous to that found in vertebrates, at least not for the regulation of blood pressure. Hence, understanding the biology of ACE homologs in invertebrates may, in due time, provide substantial information on additional roles for ACE.
A. Structure
In contrast to the occurrence of both a double and a single domain ACE in vertebrates, characterization of ACE homologs from different invertebrates showed that the overall size of these proteins was comparable to the nonglycosylated testicular form of mammalian ACE. Cloning of the corresponding cDNAs confirmed the existence of this single-domain form in insects. Until now, there has been no evidence for a functional two-domain form of insect ACE. In addition to the absence of a two-domain form, a structural difference exists between insect ACE and the mammalian enzyme, located in the N- and C-terminal ends. Cloning of the D. melanogaster ACE homologue (Ance) revealed that the insect form of ACE lacks both the heavily glycosylated N-terminal extension and the C-terminal transmembrane and cytoplasmic regions that are present in mammalian testicular ACE. The enzyme contains an N-terminal secretory signal peptide, which indicates that the mature enzyme is being secreted. Hence, Ance was predicted to code for a soluble secreted protein (Fig. 2). Confirmation of this prediction came when 90% of recombinantly expressed Ance activity was found in the cell culture medium (Cornell et al., 1995).
Although insect ACE activity was primarily detected in a membrane preparation from heads of the housefly, M. domestica (Lamango and Isaac, 1994), this ACE protein was later found not to behave as an integral membrane protein. The membrane-associated activity was probably the result of a contamination of the membrane fraction with soluble ACE (Lamango et al., 1996). Therefore, the general form of insect ACE seems to be a secreted single-domain protein with no recognizable C-terminal membrane anchor. The molecular identification of the ACE enzyme in B. microplus, however (Jarmey et al., 1995), which does posses a C-terminal hydrophobic region, showed that the membrane-bound form of ACE is not restricted to vertebrate organisms. Also, in the cockroach Leucophaea maderae and the noctoid moth Lacanobia oleracea, a substantial amount of ACE activity was detected in head membranes (Isaac et al., 2002). The nature of this membrane-associated ACE activity in these two organisms is, as yet, unidentified.
1. Sequence Conservation
Sequence comparison between human testicular ACE and several invertebrate ACE homologs showed that the overall sequence identity is approximately 40% and exceeds 70% in the regions surrounding the active site. In human ACE, 17 and 8 potential N-linked glycosylation sites (Asn-Xaa-Ser⧸Thr motifs) are present in the somatic isoform and in the germinal isoform, respectively. This contrasts to the situation in invertebrates, where ACE homologs have only three or fewer potential glycosylation sites. In D. melanogaster, it was shown that glycosylation of ACE is not required for the enzymatic properties. Expression of an unglycosylated mutant of Ance resulted in the secretion of a catalytically active enzyme, with the same substrate and inhibitor affinity as the wild-type enzyme (Williams et al., 1996).
2. Three-Dimensional Structure
Kim et al. (2003) determined the crystal structure of the D. melanogaster ACE homolog Ance. Ance is composed of 21 α-helices and three antiparallel β-strands. The substrate-binding channel exists as a large continuous internal path that comprises the entire protein molecule. The composition of this substrate-binding site is unusual in that it is composed of two unequal-sized “chambers” analogous to a peanut shell. The large chamber, referred to as the N chamber, contains the binding site of the N-terminal sequence of the substrate angiotensin I. The smaller chamber, called the C chamber, will bind the carboxy-dipeptide of the substrate. The size of these chambers is sufficiently large for binding short peptide substrates.
The bottleneck region connecting these two chambers encloses the zinc ion critical for catalysis and locates the inhibitor binding sites. The opening of these chambers to the exterior appears to be relatively small for the passage of peptide substrates, so flexibility and a so-called “breathing motion” are probably required for efficient catalysis.
B. Evolutionary Aspects
The presence of significant ACE activity was reported in extracts of the horse anemone, Actinia equine, a member of the cnidarians (Coates et al., 2000b). To this day, no ACE peptidase activity has been detected in nematode tissues. However, there is one copy of an ACE-like gene (CD42D8.5) with a recorded expressed sequence tag (EST) present in the genome of the nematode Caenorhabditis elegans. Yet taking a closer look at this ACE-like gene reveals that the amino acids crucial for enzyme activity are absent. The sequence lacks all three zinc coordinating His residues, which means that the C. elegans ACE homolog does not have a functional zinc-binding domain. Therefore, it cannot be classified as a metallopeptidase homolog. Studies on this ACE-like nonmetallopeptidase, termed ACN-1, demonstrated that the protein plays an essential role in larval molting and adult morphogenesis. If ACN-1 is indeed evolutionarily related to the ancestral ACE, it means that during evolution it lost its peptidase activity but acquired sites for new molecular interactions that have a direct or indirect effect on the molting process in nematodes (Brooks et al., 2003). This phenomenon can be further expanded to other organisms and enzymes, as in the genome of D. melanogaster and Anopheles gambiae, 15–25% of the protease-like genes lack one or more catalytic residues. Some of these proteins have probably lost the peptidase activity of their ancestor while acquiring new functions.
The analysis of the human ACE gene at the structural level forwarded the view that the vertebrate somatic form was transcribed from a tandem duplication (Lattion et al., 1989). In all vertebrate species examined, from humans at the top down to the electric fish Torpedo marmorata (Turner et al., 1987), the somatic ACE enzyme exists as the two-domain form. The analysis of the ACE gene of the housefly M. domestica revealed that this organism comprises a smaller form of the enzyme, indicating that this species does not contain the duplicated form of ACE. The existence of this single-domain isoform was confirmed when the Ance cDNA of D. melanogaster was sequenced (Cornell et al., 1995). All other invertebrate ACE enzymes that were discovered later on also consisted of the single-domain isoform. This leads to the view that invertebrate ACE represents the ancestral copy of the vertebrate two-domain enzyme and that the duplication event occurred before vertebrate radiation. However, this course of ACE evolution started to be questioned when the characterization of D. melanogaster ACE homologs began. The high level of homology between Ance and Acer points toward the existence of an ancestral ACE gene.
In addition, studying the relationship of Ance and Acer to the N and C domains of human somatic ACE showed that Ance was more enzymatically related to the C domain than to the N domain of human ACE (Williams et al., 1996). Acer, however, has much less in common with this C domain. The active sites of the N domain of sACE and Acer share structural features that permit the binding of the RXP407 inhibitor, which does not bind the C domain of sACE. An analysis of the genomic region around the Ance gene in D. melanogaster has led to the identification of four additional ACE-like genes (Isaac et al., 2000). From these four, Ance 3, 4, and 5 seem to be transcribed, because ESTs were reported for these genes. With the information available today, no EST seems to be present for Ance 2. The predicted protein sequence corresponding the Ance 3 gene includes a C-terminal transmembrane anchor, as is seen in vertebrate ACE. Ance 2 codes for an ACE-like protein with a putative signal peptide, but without a C-terminal membrane region. Both genes lack crucial active site residues. These two gene predictions are interesting because alternative splicing of the predicted transcripts would lead to a two-domain, membrane- bound ACE-like product, as is seen in the vertebrate somatic form (Coates et al., 2000a). Taken together, this information indicates that the duplication event that gave rise to the vertebrate tandem protein predates the divergence of the Ecdysozoa and deuterostomes, and that the differing activities on the N and C domains have been maintained over a long period of time.
C. Substrate Specificity
Insect ACE is capable of hydrolyzing the known in vivo substrates of mammalian ACE, angiotensin I, bradykinin, and the hemoregulatory peptide Ac-DSKP. However, there are no reports of insect homologs of these known in vivo substrates, and none of the many insect peptides isolated to date shows any structural resemblance to them. The search for angiotensin I–like peptides in the brain of several insect species could not prove otherwise (Isaac 1998b, Schoofs 1998). In addition, a search in the D. melanogaster genome did not reveal any potential homologs of the precursor proteins of angiotensin I and bradykinin (Isaac et al., 2000). This is in contrast to the situation in leeches, where several reports on the presence of members of the renin–angiotensin system have been published (Laurent 1996, Salzet 2001). Cleavage experiments with ACE from M. domestica showed that insect ACE exhibits a broad in vitro substrate specificity. The in vitro substrates of mammalian ACE, cholecystokinin, enkephalins, substance P, and LH-RH, are successfully cleaved by the endopeptidase activity of Musca ACE. This shows that insect ACE can hydrolyze C-terminally amidated peptides in vitro, functioning as an endopeptidase (Isaac 1997a, Lamango 1997). M. domestica ACE is unable to hydrolyze either CCAP (crustacean cardioactive peptide) or proctolin. Apparently, the penultimate C-terminal prolyl residue of proctolin makes this peptide resistant to ACE hydrolysis. The lack of hydrolysis of CCAP is likely a result of the presence of a disulfide bridge, which results in a secondary structure that prevents access to the active site.
A novel activity of insect ACE, namely, prohormone processing, was suggested when it was found to be capable of successfully removing basic dipeptides from locustamyotropin oligopeptides possessing a C-terminal Gly-Lys-Arg and Gly-Arg-Arg extension (Isaac et al., 1998b). Many insect peptides are synthesized as inactive prohormones and need posttranslational processing to acquire their biological activity. In several prohormones, the sequence -Gly-Arg⧸Lys-Arg- is an internal consensus recognition sequence for endoproteolysis by prohomone convertases, generating peptide intermediates with a C-terminal –Gly-Arg⧸Lys-Arg– extension. These are substrates for carboxypeptidase E (CPE) that sequentially removes the two basic amino acids from the C terminus, which is followed by amidation of the C terminus (Isaac et al., 1997b). The fact that insect ACE is capable of hydrolyzing these partially processed prohormone-like peptides, together with the intracellular colocalization of the enzyme with locustamyotropin peptides, implies a role for ACE in the biosynthesis of peptide hormones and neuropeptides (Schoofs et al., 1998).
Zhu et al. (2001) and coworkers demonstrated that the hexapeptide trypsin modulating oostatic factor from N. bullata (Neb-TMOF) (Bylemans et al., 1994) is selectively hydrolyzed by ACE that is present in substantial amounts in the fly's haemolymph (Zhu et al., 2001). Neb-TMOF is capable of regulating vitellogenesis and is thought to be released by the ovaries and transported to the midgut, where it terminates the protein meal–induced trypsin biosynthesis. Captopril feeding experiments suggest that Neb-TMOF represents a prime candidate for being an in vivo substrate for circulating insect ACE (Vandingenen et al., 2002a). TMOF was also detected in Aedes aegypti, probably synthesized by the brain (Borovsky and Meola, 2004). However, this Aea-TMOF, with six prolines at its C terminus, is not cleaved by Neb-ACE (K. Hens, personal communication).
Recently, new substrates were purified from the ovaries of N. bullata. Their primary structures were identified as NKLKPSWQWISL (Neb-ODAIF), NKLKPSQWISLSD (Neb-ODAIF-11–13), NKLKPSQWI (Neb-ODAIF- 11–9), NKLKPSQ (Neb-ODAIF- 11–7), SLKPSNWLTPSE (Neb-ODAIF-2), and LEQIYHL. These peptides show significant homology to the N-terminal part of fly yolk proteins (Hens 2002, Vandingenen 2002b). Kinetic analysis of these peptides with insect ACE and human ACE shows that the N. bullata ACE shares enzymatical properties with the C-domain sACE, in addition to features that seem unique to invertebrate ACE.
Characterization of the Ance protein demonstrated that this enzyme efficiently hydrolyzes angiotensin I and bradykinin (Cornell 1995, Williams 1996). Ance can operate as an endopeptidase, for instance on tachykinins, substance P, and leucokinins. Still, the nonamidated form of a peptide is always preferentially cleaved over the amidated form. Furthermore, insect ACE preferentially acts as a dipeptidase and shows reduced tripeptidase activity. Of the insect peptides tested, locustatachykinin (Lom TK-1) proved to be the best substrate for Ance. However, the D. melanogaster tachykinins turned out to be poor Ance substrates (Siviter et al., 2002). Acer is not capable of cleaving angiotensin I (Houard et al., 1998), and most peptides tested were hardly degraded by Acer (Siviter et al., 2002). To this day, there are no clues concerning the identity of the endogenous Acer substrates.
D. Biological Role
The structural and biochemical similarities between mammalian and invertebrate ACE do not necessarily imply a functional conservation as well. Although the presence of an invertebrate RAS has been reported in a leech that feeds on ducks (Laurent 1995, Laurent 1995a, Laurent 1995b, Laurent 1996), no other components of the RAS other than ACE have been described in insects or other invertebrates. It is therefore highly improbable that this invertebrate enzyme would physiologically act as a converting enzyme for an angiotensin-like peptide. As already mentioned, functionality of mammalian ACE is, however, not restricted to the RAS. Both its broad substrate specificity and widespread tissue distribution indicate its involvement in several other (but, unfortunately, still poorly described) physiological functions. To fully unravel the in vivo role of ACE in insects, information about substrate specificity and tissue and cellular localization of the enzyme, as well as its putative colocalization with its substrates, has to be combined. These data, together with the studies of ACE mutants and the effects of ACE inhibitors on insect physiology, will eventually lead to the elucidation of the roles of insect ACE. Today's concept on insect ACE functionality focuses on its possible role in reproduction, development, and defense.
1. Reproduction
The discovery of ACE in testes of several insect species, including H. irritans exigua (Wijffels et al., 1996), Lymantria dispar (Loeb et al., 1998), N. bullata, the Colorado potato beetle Leptinotarsa decemlineata, and the locust L. migratoria (Isaac 1999, Schoofs 1998), implies that the function of tACE may have been conserved during the course of evolution. In L. decemlineata, ACE was detected in the germ cells, more particularly in developing spermatids and mature spermatozoa, whereas in L. migratoria, ACE was found in peripheral somatic cell bodies of the apical compartment of the testicular follicles and not in the germ cells. In the haematophagus fly H. irritans, expression of testicular ACE is induced after a blood meal, indicating a specific role in the maturation of spermatozoa (Wijffels et al., 1996).
The possible importance of testicular ACE in reproduction became apparent by the observation that male D. melanogaster transheterozygotes of two mutant Ance alleles were found to be sterile (Tatei et al., 1995), with the infertility linked to the failure of the spermatocytes to develop into active mature spermatozoa. Spermatogenesis proceeds normally in the mutants up to the spermatid stage, but these spermatids do not succeed in differentiating into spermatozoa. This change is accompanied by nuclear scattering and an abnormal morphology of the spermatids. Together with the observed expression pattern of Ance, the enzyme is believed to be required for spermatid differentiation through the processing of a regulatory peptide synthesized within the developing cyst (Hurst et al., 2003).
The involvement of ACE in the control of spermatogenesis was also demonstrated by studies of the effects of AII and bovine ACE on the ability of the testes of L. dispar to synthesize and release ecdysteroids in response to testis ecdysiotropin (TE). The effect of TE on the initiation and up-regulation of ecdysteroid synthesis could be imitated by both AII and bovine ACE, raising the possibility that ACE is involved in the production of an AII-like peptide that modulates ecdysteroid synthesis in the testes of insects (Loeb et al., 1998).
In female adults of the mosquito A. stephensi, ACE activity increases by a factor 2.5 after a blood meal. It is not known where this induced ACE is synthesized, but it accumulates in developing ovaries and in mosquito eggs (Ekbote et al., 1999). Adding ACE inhibitors (captopril and lisinopril) to the blood meal of the mosquito leads to a size reduction of the batch of eggs laid in a dose-dependent manner. The ACE inhibitors reduce fecundity by interfering with the transfer of the oocytes along the oviducts (Ekbote et al., 2003a).
In the reproductive tissue of the tomato moth Lacanobia aleracea, almost all of the ACE activity is concentrated in the accessory glands of the male and in the spermatheca and bursa copulatrix of the (virgin) female. These high activities may imply the importance of ACE for peptide metabolism in the male and female reproductive tract. Changes in the levels of ACE activity in the reproductive tissues of males and females during mating, point toward a transfer of ACE from male to female during time of copulation (Ekbote et al., 2003b). This male ACE donated in the spermatophore might form a hitherto unknown male component of an energy-producing metabolic pathway, as is seen in the serine endopeptidase-dependent proteolytic cascade in the spermatophore of B. mori (Aigaki 1987, Aigaki 1988).
In the fleshfly N. bullata, feeding of captopril results in an increase of vitellogenin titers in the fly hemolymph. It has been suggested that this effect is mediated through the trypsin-modulating oostatic factor TMOF. As already discussed, Neb-TMOF is an in vitro substrate of insect ACE. However, if TMOF were to be inactivated by ACE, inhibition of ACE would lead to an increase of TMOF levels in the hemolymph, resulting in a reduced vitellogenin synthesis. Therefore, an activating effect of ACE on TMOF is postulated (Vandingenen et al., 2001). The discovery of ACE interactive peptides in the ovaries of the fleshfly (Vandingenen et al., 2002a), and the discovery that these peptide substrates originate from yolk gene products, strengthen the idea that ACE is a regulator of vitellogenic and developmental processes (Hens 2002, Vandingenen 2002b).
These physiological observations, in combination with the discovery of ACE protein in the gonads of numerous insects, undoubtedly show that ACE is a vital enzyme in insect reproduction. The further elucidation of the full role of ACE in this process necessitates including the description of all endogenous substrates.
2. Development and Metamorphosis
The observation that some mutations in the D. melanogaster Ance gene result in death during larval⧸pupal stages (Tatei et al., 1995) indicates that insect ACE is important for normal growth and development. Many reports from different and independent laboratories have confirmed the role of insect ACE in development and metamorphosis. Along with the cloning of the first D. melanogaster ACE homolog (Race, Ance), the gene coding for this protein was shown to be a target of the homeobox regulatory gene zerknullt (zen) and decapentaplegic (dpp) (Tatei et al., 1995). A 533-bp-long enhancer located in the Ance promotor region covers three zen protein binding sites and was shown to mediate selective expression in the amnioserosa. During early development, Ance is activated by zen and becomes associated with the differentiating amnioserosa and with the anterior and posterior midgut, where it persists throughout embryogenesis. In late embryos, Ance expression is detected in epithelial cells of the midgut and in the pericardial cells of the heart. Ance expression is lost from the presumptive amnioserosa in zen − and dpp − mutants; the expression in the gut stays unaffected.
In D. melanogaster larvae, high expression of Ance is found before and peaks during pupal development, indicating a physiological role for the enzyme during metamorphosis (Wilson et al., 2002). Also, in the tomato moth, L. oleracea, ACE activity increases approximately fourfold in the last larval instar and in the early pupal stage (Ekbote et al., 2003b).
In insects, each larval molting is preceded by high ecdysteroid titers, and for pupal and adult development, multiple pulses of ecdysteroid hormones are essential. In wing discs of B. mori, the ACE gene (BmAcer) was found to be directly 30-hydroxyecdysone (20E) inducible in a dose-dependent manner. Because this ecdysteroid-dependency was not found in other tissues examined, transcription of BmAcer seems to be organ specific (Quan et al., 2001). During the transition from larva to pupa in D. melanogaster, the expression of Ance in imaginal disc cells was found to be induced by ecdysteroids. These data point toward a fundamental role for ACE during metamorphosis of holometabolous insects. Several relationships between ACE and metamorphosis are possible: the conversion of precursors into biologically active peptides necessary for metamorphosis, inactivation of signaling peptides, or recycling of amino acids from larval proteins for the synthesis of pupal and adult cuticle⧸tissues.
In A. stephensi, ACE expression is induced by a blood meal and ACE accumulates in developing ovaries, from which it passes into the mosquito eggs (Ekbote et al., 1999). This indicates a role for ACE-generating peptides that are essential for oocyte development and embryogenesis. The characterization of ACN-1 in the nematode C. elegans shows that it is not necessarily the proteolytic function of ACE that is involved in developmental processes. The hypodermal expression of acn-1 is under the control of nuclear hormone receptors that regulate molting in C. elegans. Functional acn-1 knockout by RNAi causes morphological defects in larvae and adults. More specifically, ACN-1 was found to be required for larval molting, male tail development, and formation of adult alae (Brooks et al., 2003).
3. ACE in the Central Nervous System: A Role in Prohormone Processing?
In N. bullata, L. migratoria, L. decemlineata, L. maderae, the walking stick insect Carausius morosus, B. mori, and the caterpillar Mamestra brassica, ACE immunoreactivity is seen in neuropil regions of the brain. In L. Migratoria, L. maderae, B. mori, and M. brassica, ACE immunoreactivity is also present in neurosecretory cells of the brain (Schoofs et al., 1998). In L. migratoria, immunoreactive staining can also be traced in the controlateral nervus corporis cardiaca I (NCC I), corpora cardiaca (CC), and suboesophageal ganglion (SOG), and in C. morosus, L. decemlineata, and L. maderae, ACE immunoreactivity was also detected in the corpora cardiaca (Veelaert et al., 1999).
The presence of ACE in neurosecretory cells and neuropil regions of the insect brain points toward a dual role for ACE in the insect nervous system (Schoofs et al., 1998). The localization of ACE in the neuropil indicates the metabolic inactivation of peptidic neurotransmitters. The presence of peptides from the adipokinetic hormone (AKH), kinin, and locustatachykinin family in the central nervous system (CNS), as well as the knowledge that these peptides are hydrolyzed by ACE (Lamango et al., 1997), indicates that they might be potential in vivo substrates for insect neuronal ACE. In neurosecretory cells, ACE is likely to be involved in the conversion of prohormones to active peptide hormones that are secreted into the hemolymph by the corpora cardiaca or corpora allata (Isaac et al., 1998b). Because insect ACE has been shown to exert in vitro prohormone processing activities against the locustamyotropins, and locustamyotropin-containing cells of the locust brain and suboesophageal ganglion also contain ACE, this insect enzyme most probably plays a role in the biosynthesis of these hormones (Veelaert et al., 1999).
4. ACE and the Defense System
In contrast to the situation in vertebrates, which have a dual system of immune reaction, namely, an innate and an acquired one, invertebrates only rely on their innate immune responses. This system comprises both humoral and cellular defense responses. Well-known humoral responses include the synthesis of a broad spectrum of antimicrobial peptides⧸proteins (Bulet et al., 1999), as well as two proteolytic cascades: the blood coagulation system identified in the horseshoe crab (Iwanaga 1993, Iwanaga 2002) and the prophenoloxidase-activating system (pro-PO-AS) in insects and crustaceans (Söderhäll and Cerenius, 1998). Cellular defenses cover hemocyte-mediated responses like phagocytosis, nodulation, and encapsulation. In insects, a large number of peptides is released into the hemolymph, where they are known to regulate a large diversity of physiological functions. The half-life of these peptides can be regulated by proteases and peptidases present in the hemolymph.
The discovery of ACE activity in the hemolymph of insects (Isaac and Lamango, 1994), together with the knowledge of putative prohormone converting activity of ACE, indicated the possible involvement of ACE in the proteolytic processing of insect antibacterial peptides from their precursors (Isaac et al., 1998a). Supportive data concerning this hypothesis came from expression studies in larvae from the sheep blowfly Lucilia cuprina and the old screwworm Chrysomya bezziana, which are known to secrete or excrete chymotrypsins and trypsins in larval cultures and wound sites. The knowledge that an ACE homolog is also secreted or excreted from these larvae indicated a dual role in wound formation. It may degrade chymotryptic and tryptic digestion products from host tissue proteins and may influence the host vascular and inflammatory response by processing or degrading host regulatory peptides (Wijffels et al., 1997).
In the endoparasitic wasp, Pimpla hypochondriaca, the female envenomates and oviposits into pupae of some lepidopteran species. This venom was shown to suppress the hemocyte-mediated immune responses of the host by impairing encapsulation and phagocytosis (Parkinson 2002, Richards 2000). The presence of ACE in this venom, together with the presence of antibacterial activity, implies that ACE could be one of the enzymes involved in the processing of antibacterial peptides from the venom sac (Dani et al., 2003). All the mentioned data indeed point toward an involvement of ACE in the defense system, but they are still only hypothetical. The first physiological evidence for the existence of a connection between ACE and defense came from studies in the locust L. migratoria. Locusts that are subjected to an immune challenge through injection of lipopolysaccharides (LPS) into the hemocoel showed an increase in ACE mRNA-expression. Comparing the level of ACE transcription from LPS-treated animals with control animals revealed an approximately 10-fold increase in ACE transcription 6 hours after the LPS-injection (Macours et al., 2003b). The exact nature of the ACE-defense connection remains to be clarified.
In mammals, all blood cells derive from hematopoietic stem cells that differentiate into different lineages. The involvement of ACE here resides in the inactivation of Ac-SDKP (N-acetyl-seryl-aspartyl-lysyl-proline), a peptide that prevents the recruitment of hematopoietic stem cells (Azizi 1996, Baudin 2002). Insects also continue to produce hemocytes via a division of stem cells or by continued division of hemocytes in circulation (Jones 1970, Ratcliffe 1985). On the basis of this information, a possible connection between ACE and the immune system can be suggested in the replenishment of the hemocytes that are involved in the insect immune response. The possibility of ACE being involved in the processing of antimicrobial peptides in locust hemolymph cannot be excluded, but so far, except for lysozyme and coagulogen, no major antibacterial activity could be detected in locust hemolymph (Van Sambeek and Weisner, 1999). The rise in ACE expression that follows LPS injection is not likely to result from an involvement of ACE in the prophenoloxidase cascade, as this pathway is not activated by injection of LPS. However, the immune system of the locust responds to LPS injection through the formation of nodules (Goldsworthy 2002, Hoffman 1974). Therefore ACE may be required in the process of nodule formation. This speculation is in agreement with the recent findings of Goldsworthy and colleagues (2003), who investigated the effect of LPS on the prophenoloxidase cascade and nodule formation in the locust. Activation of prophenoloxidase by LPS could only be achieved by coinjection with AKH, not by the injection of LPS alone. When captopril was coinjected, the activation of the PPO cascade remained unaffected (with⧸without AKH). However, captopril did inhibit the nodule formation induced by LPS (Goldsworthy et al., 2003). Considering these data, a possible role for ACE in the immune system of insects may reside in the regulation of nodulation.
E. ACE in Leeches
To date, leeches are the only group of invertebrates in which the existence of a renin–angiotensin system has been reported. In Theromyzon tessulatum, a parasite of ducks, an angiotensin II (AII)-like peptide was isolated (Salzet et al., 1993). The full sequencing of such AII-like peptide was realized in another leech species, namely Erpobdella octoculata (Salzet et al., 1995). This leech AII peptide differs from the vertebrate AII by a c-terminal amidation. Injection of this AII into the leech results in a decrease in mass, reflicting a diuretic effect. Also in T. tessulatum, a renin-like peptide was isolated (Laurent and Salzet, 1995a) and partial peptidic sequences were identified. This enzyme was found capable of releasing AI from an AI-like precursor, Angiotensinogen (Laurent et al., 1995), by cleavage of the Leu10–Leu11 bond. The mass of this invertebrate renin (ca. 32 kDa) was lower than that of vertebrate active renin (ca. 44 kDa) but displayed a comparable activity.
The leech renin is localized in the excretory and nervous system, which implies the involvement of the leech RAS in osmoregulation. The angiotensin-converting enzyme of this leech-RAS is a glycosylated membrane-bound enzyme of 120 kDa that is released into the hemolymph in a hydrophilic form (100 kDa) (Laurent 1996, Vandenbulcke 1997). The presence of this RAS in coelomocytes of leeches and the involvement of each part of this system in the immune response inhibition indicates the implication of the RAS in host–parasite cross-talk (Salzet and Verger-Bocquet, 2001).
IV. Endothelin-Converting Enzyme: State of the Art in Mammals
A. Introduction
Endothelin-converting enzymes (ECEs) belong to the class of type II integral membrane zinc metalloproteases named for their ability to hydrolyze big endothelins (big ETs) into the smaller vasoactive endothelins. Yanagisawa and colleagues (1988) were the first to predict the existence of ECEs based on the cloning of the prepro-endothelin gene. ECE is classified into the neutral endopeptidase or M13 group of proteins, which contains type II membrane glycoproteins with zinc peptidase catalytic activity. At present mammalian M13 family of zinc proteases consists of seven known members: neutral endopeptidase (NEP); the endothelin-converting enzymes ECE-1, ECE-2, and ECE-3; the Kell blood group antigen (Kell); the phosphate regulating gene (PEX); X-converting enzyme (XCE); and secreted endopeptidase (SEP).
Each member of this family shows homology to the others, mainly in the C-terminal part of the sequence that is responsible for catalytic activity. In addition, there are structural similarities in that all M13 members have short intracellular domains. Where identified, these enzymes have roles in the processing or metabolism of regulatory peptides and therefore represent potential therapeutic targets.
B. Structure and Isoforms
ECE-1 is a membrane-bound protein with a short N-terminal cytoplasmatic tail, a transmembrane hydrophobic domain, and a large extracellular domain, which shares high sequence homology to NEP and other members of the metallopeptidase family. Ten cysteine residues in the ECE sequence are conserved among the other members of the family and are probably involved in disulfide bridges. The ECE protein has 10 predicted sites for N glycosylation in the extracellular domain. This is consistent with the observation that ECE is a highly glycosylated protein and explains the difference between the predicted molecular mass and the mass shown on SDS-PAGE. Purification and cloning studies of rat ECE-1 have shown that it consists as a disulphide-linked dimer, in which Cys412 is solely responsible for the formation of the intermolecular disulphide bond (Shimada et al., 1996). The large extracellular domain contains the zinc-binding consensus sequence HEXXH, in which the glutamic acid (E) is the most important residue for catalytic activity. The two flanking histidine (H) residues act as zinc coordinating amino acids. The third ligand for the zinc atom is E651, whereas H716 is involved in the stabilization of the tetrahedral intermediate during the transition state (Shimada et al., 1995). Four ECE-1 isoforms have been identified in humans and were named ECE-1a (758 amino acids), ECE-1b (770 amino acids), ECE-1c (754 amino acids) and ECE-1d (767 amino acids) (Jafri 2003, Valdenaire 1995, Valdenaire 1999a). The four isoforms are transcribed from a single gene and result from the use of alternate promoters located upstream of specific exons. They only differ in the first half (approximately) of their cytoplasmatic tail and are identical over their remaining sequence, including the enzymatic catalytic site. As a logical consequence, the isoforms display comparable converting activity.
The specificity of the isoforms thus lies in their cytosolic tails, which results in a different subcellular distribution. Whereas ECE-1a and ECE-1c are present in the plasma membrane, ECE-1b and ECE-1d are retained inside the cell. Recently it was shown that ECE-1 isoforms can heterodimerize. It was suggested that heterodimerization of ECE-1 isoforms could constitute a means for regulating their subcellular distribution and, subsequently, their extracellular activity (Muller et al., 2003).
Considering the importance of the biosynthesis of both the intracellular and extracellular endothelins, the subcellular distribution of ECE isoforms may play a central role in the regulation of the endothelin system.
C. Biological Role
The endothelin system is composed of endothelin (ET) peptides and their receptors (Fig. 3a ), which together affect a wide variety of physiological functions and pathological conditions. The discovery of this system started in the mid-1980s, when an increasing awareness of the role of endothelial cells as active components of the vascular system led to the description of an endothelial cell–derived constricting factor (Hickey et al., 1985). In 1988, a 21–amino acid vasoconstricting factor termed “endothelin” was isolated from cultured porcine aortic endothelial cells (Yanagisawa et al., 1988).
FIG. 3.
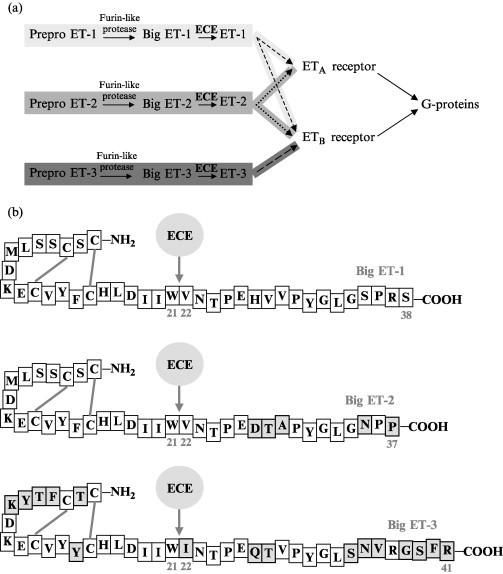
(a) The endothelin pathway. (b) Human big ET1–3. Cleavage by ECE leads to the generation of ET1–3 at the N-terminal and an inactive C-terminal fragment. Nonconserved amino acids are in grey boxes.
The expression of ET is associated with many pathological processes and with tumor growth. To fulfill their wide spectrum of physiological functions, ETs act through autocrine and paracrine mechanisms. Their biosynthesis thus requires tight local control. Endothelin-1 (ET-1) is a 21–amino acid peptide with a hydrophobic C terminus. Within 1 year of its discovery, two structurally related peptides were identified and termed ET-2 and ET-3, respectively. The three isoforms are encoded by three different genes and show highly conserved sequences in several species. All isoforms are composed of 21 amino acids with two intrachain disulfide bridges. ET-2 exhibits the closest structural similarity to ET-1, differing by only three amino acid residues, whereas ET-3 differs by six amino acids. ET isoforms are widely distributed among various cells and tissues. Of the three isoforms, the most widely distributed, best studied, and most potent is ET-1. ET-1 shares great structural homology to sarafotoxin, a snake (Atractapsis engaddensis) venom that induces myocardial infarction by exaggerated contraction of the cardiac vessels and interruption of the blood supply to the heart.
ETs are produced by a variety of organ and body tissues such as the lung and kidney as well as the brain, pituitary, peripheral endocrine tissues, and placenta. Most abundantly, endothelial cells of the vascular endothelium produce ET. ET binds to two types of receptors (ETA and ETB) (Arai 1990, Sakurai 1990). Both contain seven transmembrane domains and activate an overlapping set of G proteins, producing an array of different physiological responses (Douglas and Ohlstein, 1997). Although structurally highly similar, these receptors possess different affinities for the three isoforms of ET. Like many other regulatory peptides, the ETs are maturated through proteolytic processing from larger precursor polypeptides, termed “prepro-ETs.” This proteolytic cleavage generates an essentially inactive intermediate referred to as big ET. The subsequent hydrolysis of big ET into the final active product ET, and a presumed inactive C-terminal fragment, is catalyzed by ECE (Fig. 3a). In the case of big ET-1 and ET-2, proteolysis occurs at the Trp21-Val22 bond. ET-3 is formed through cleavage of the Trp21-Ile22 bond of big ET-3 (Inoue et al., 1989) (Fig. 3b). The conversion of big ET to ET can occur in the extracellular medium and in the secretory pathway; cells thus secrete big ETs either alone or together with endothelins (Parnot et al., 1997). The physiological importance of the cleavage of big ET is indicated by a 140-fold increase in vasoconstrictor potency on cleavage to ET. This makes ECE an important activating protease in the biosynthetic pathway of ET. ECE-1 null mice exhibit a phenotype similar to that of ET-1- or ETA-deficient mice, demonstrating the significance of ECE-1 in generating bio-available ET-1 (Yanagisawa et al., 1998).
The endothelin system plays a variety of roles in both normal physiology and pathological conditions in a number of tissues⧸organs of the body. In blood vessels, ET maintains a basal level of vasoconstriction and is involved in the development of hypertension, atherosclerosis, and vasospasm after subarachnoid haemorrhage. In the heart, the endothelin system affects force and rate of contraction and mediates hypertrophy and remodeling in congestive heart failure. In lungs, ET regulates the tone of both airways and blood vessels and is involved in the development of pulmonary hypertension. Kidney-ET regulates water and sodium excretion and acid–base balance, and it participates in the pathophysiology of acute and chronic renal failure. In the brain, the ET system modulates cardiorespiratory centers and hormone release (Kedzierski and Yanagisawa, 2001). In ovaries, ET is shown to play a role in regulating the female reproductive cycle, where it functions as an important component of the luteolytic cascade (Levy et al., 2003). Egidy and colleagues (2000) suggested that ET, as a powerful vasoconstrictor and mitogenic peptide that is produced by many cell lines, might play a role in cancer progression of the colon.
Studies by Johnson and colleagues (1999) revealed that ECE-1 possesses a broad in vitro substrate specificity and is potentially involved in the metabolism of peptides distinct from ET. They found that neurotensin, substance P, bradykinin, and the oxidized insulin β chain are in vitro substrates for ECE-1. Given this in vitro substrate specificity, it is likely that ECE-1 is involved in the degradation and processing of many biologically active peptides, both at the cell surface and in the secretory pathway (Johnson et al., 1999).
D. ECE-2 and ECE-3
A second form of ECE, ECE-2, was cloned from bovine adrenal cortex and was reported to possess a 59% identity with ECE-1. The main structural features of ECE-1 are conserved in ECE-2 (Emoto and Yanagisawa 1995). Both are type II integral membrane proteins with the HEXXH consensus sequence. The 10 cysteine residues conserved between ECE-1, NEP, and Kell, are also conserved in ECE-2, as is the cysteine residue responsible for dimerization of ECE-1. ECE-2 is also heavily glycosylated, with 10 possible glycosylation signals. Although ECE-2 shows specific activity to produce mature ET-1 from big ET-1, like ECE-1, the striking difference between ECE-2 and ECE-1 is the acidic pH optimum of 5.5 of ECE-2, in contrast to the neutral pH optimum of ECE-1. This indicates that ECE-2 is involved in ET synthesis in the intracellular compartments in which the pH is acidic (Emoto and Yanagisawa, 1995). ECE-2 has been reported to be expressed in various organs and tissues including cultured human vascular endothelial cells, uterus, ovary, heart, lung, and liver, indicating various functions for the enzyme.
Recent targeting studies with mouse ECE-2 revealed that it plays crucial roles in the formation of cardiac outflow structures (Yanagisawa et al., 2000). Four subisoforms of ECE-2 that differ only in their N-terminal cytoplasmatic tails were termed ECE-2a-1, ECE-2a-2, ECE-2b-1 and ECE-2b-2, ECE-2a-1 and ECE-2a-2 are expressed in a variety of tissues including liver, kidney, adrenal gland, testis, and endothelial cells. ECE-2b-1 and ECE-2b-2 are abundantly expressed in the brain and adrenal gland (Ikeda et al., 2002). ECE-3 was purified from bovine microsomes. This enzyme was found to be specific for the conversion of big ET-3 to ET-3 and was inhibited by phosphoramidon (Hasegawa et al., 1998). To the best of our knowledge, this is the only report on an ECE-3 enzyme.
E. Other Members of the M13 Metallopeptidase Family
1. Neutral Endopeptidase
Neutral endopeptidase (neprilysin, NEP), a 90–110-kDa plasma membrane protein, is the prototype and best characterized member of the M13 zinc metallopeptidase family. NEP is identical to the neutrophil and cluster-differentiation antigen CD10 and is also known as the common acute lymphoblastic leukemia antigen (CALLA), which is mainly associated with the precursors of B lymphocytes (Letarte et al., 1988). Purification and subsequent cloning of NEP revealed it to be a type II integral membrane protein of approximately 700 residues. Neprylisin's extracellular domain, which includes the active site, also contains 12 cysteine residues, 10 of which are involved in highly conserved disulfide bridges that are important in the maintenance of structure and activity of the enzyme (Oefner et al., 2000). NEP comprises three subisoforms that result from alternative splicing in the 5′ untranslated region, indicating that these spliceforms do not exhibit functional differences (Ishimaru and Shipp, 1995).
Regarding its catalytic activity, NEP shares some similarity with the bacterial zinc metalloproteinase thermolysin, and in particular in its substrate specificity (Malfroy et al., 1988). NEP exists as an ectoenzyme that preferentially hydrolyzes extracellular oligopeptides (<5 kDa) on the amino side of hydrophobic residues. NEP is the only enzyme in this family of which the x-ray crystallographic structure has been determined. The structure revealed a restricted active site cleft preventing access of large peptides and proteins, explaining its oligopeptidase character (Oefner et al., 2000).
NEP is primarily expressed in the kidney, where it has the capacity to cleave and inactivate the natriuretic and vasodilatory peptide (ANP), which controls arterial pressure (Beaulieu et al., 1999). The first physiological function of NEP was found in the brain, where NEP is located in neuronal cells. There, it controls the half-life of enkephalins and substance P (two neuropeptides involved in pain control) through inactivation (Malfroy et al., 1978). NEP also plays a pivotal role in various cancers, such as prostate cancer and leukemia (Letarte 1988, Papandreou 1998). A variety of other physiological substrates has subsequently been revealed for NEP (Turner and Tanzawa, 1997), although most attention has been focused on the vasodilator ANP. The development of selective NEP inhibitors, of which the best studied are thiorphan and phosphoramidon, has primarily been driven by their potential as novel cardiovascular agents (Roques and Beaumont, 1993). The medicinal treatment of various afflictions with NEP inhibitors has become so prevalent that they now constitute a class of drugs used in both gastroenterology and cardiology (Tanja et al., 2000). There is, however, a potential downside to the clinical use of NEP inhibitors, as downregulation of NEP levels is seen in various cancers and in the lung, where it potentiates neurogenic inflammatory responses. Recent reports that a NEP-like enzyme can degrade the neurotoxic β-amyloid peptide involved in Alzheimer's disease raises new concern about chronic blockade of NEP-like activity in the CNS (Carson and Turner, 2002).
2. The Kell Blood Group Antigen
The Kell blood group system is one of the major antigenic systems in human erythrocytes. Over 23 different blood group antigens have been assigned to this system, and the molecular basis for this polymorphic variation has been shown to be the result of single-base mutations in the Kell gene, resulting in the different Kell phenotypes (Lee, 1997). The protein is of clinical importance because incompatibility involving Kell antigens can cause severe hemolytic reactions to blood transfusions and leads to fetal and neonatal anemia.
Kell is a 93-kDa type II erythrocyte membrane glycoprotein that is attached to the cytoskeleton and that comprises a short N-terminal intracellular segment, a single transmembrane sequences and a large, 665–amino acid extracellular domain. In the C-terminal third of the molecule, Kell shares a 33–36% amino acid identity with human NEP, ECE-1, PEX, and XCE (Lee et al., 1991). Kell has 16 cysteine residues from which the 10 cysteine residues that are preserved in all members of the M13 family are conserved. One of the cysteine residues, Cys72, forms a disulfide band with the XK protein, which spans the membrane 10 times (Russo et al., 1998). The absence of XK, which occurs in the McLeod patients, is correlated with acanthocytic red blood cells and a late-onset form of nerve and muscle disorder (Redman et al., 1999).
Studies using a recombinant, truncated form of Kell, lacking the intracellular and transmembrane domain (sKell), have shown that Kell possesses converting enzyme activity for the substrate big ET-3. Conversion of big ET-1 and big ET-2 is much less efficient. The known substrates for NEP are not hydrolyzed by sKell. Individuals lacking Kell do have a normal ET-3 biosynthesis, so the protein probably has other substrates than big ET-3 and other biological functions (Lee et al., 1999).
3. Phosphate-Regulating Gene
The “phosphate-regulating gene with homology to endopeptidases on the X chromosome,” PEX, was identified from studies of patients with X-linked hypophosphataemic rickets (HYP Consortium, 1995). HYP is the most common inherited disorder of renal phosphate wasting characterized by hypophosphatemia and defective bone mineralization (Scriver and Tenenhouse, 1992).
It is postulated that the substrate of PEX is an unidentified peptide hormone (termed “phosphatin”) that modulates renal tubular phosphate handling. Such an activity could involve either the processing of a phosphate-reabsorbing hormone precursor to its active form or the inactivation of a circulating phosphaturetic factor. In situ hybridization revealed the presence of PEX in osteoblasts and odontoblasts, implicating the enzyme in the development of bones and teeth (Ruchon et al., 1998). In addition to these speculations, the physiological function of the PEX protein in bone development and its relation to the mechanisms that lead to renal and skeletal abnormalities remain to be defined.
Cloning of the full-length PEX cDNA revealed it to be a type II integral membrane protein with a 20-residue N-terminal cytoplasmatic tail and a C terminus of 700 amino acid residues in the extracellular compartment. Recombinant PEX functions as an endopeptidase by hydrolyzing parathyroid-hormone-derived peptides, although it is unlikely that these are physiological substrates, as they do not have the properties of the sought-after phosphatin (Lipman et al., 1998).
4. Soluble Secreted Endopeptidase
A novel member of the metalloprotease family was isolated from mouse embryos in search for an alternative ECE enzyme (Ikeda et al., 1999). This enzyme, called SEP (soluble secreted endopeptidase) shares higher structural (55% identity, 74% similarity) and functional similarities with NEP than with ECEs or other members of this family, indicating that SEP and NEP may constitute a subfamily within this group of metalloproteases. Similar enzymes have subsequently been cloned and characterized from mouse testis (referred to as neprilysin-like 1; NL 1) (Ghaddar et al., 2000) and rat brain (NEP II) (Tanja et al., 2000).
In vitro alternative splicing of SEP yields two isoforms, one membrane-bound isoform (SEPΔ) and another isoform that is secreted into the culture medium (SEP). Cellular expression of the cDNA showed that most of the enzyme is secreted into the culture medium. After translation, both isoforms are inserted into the ER as membrane type II proteins. SEPΔ then becomes an ER resident, whereas SEP is proteolytically cleaved and transported to the extracellular compartment (Raharjo et al., 2001). The presence of a furin cleavage site in the NL1 sequence indicated that a member of the substilin-like family of convertases is responsible for this secreted form (Ghaddar et al., 2000).
In vitro enzymological analysis shows that SEP can efficiently hydrolyze circulating vasoactive peptides, including ET-1, angiotensin-I, ANP, bradykinin, and substance P. The physiological relevance of SEP mainly remains to be determined. Its presence in testis and brain, however, indicates roles in fertility and neuropeptide inactivation, respectively, as it appears to localize in specific neuronal populations in the CNS (Tanja et al., 2000).
5. X-Converting Enzyme
X-converting enzyme (XCE) got its name from the fact that it is an orphan peptidase for which no substrate has as yet been identified. It was originally identified by homology cloning from a human brain cDNA library. It is most abundant in the CNS (Valdenaire et al., 1999b). The 775–amino acid–long XCE is more homologous to ECE-1 (42% identity in the last 500 residues) than to NEP or any other member of this family. A rat neuronal membrane-bound endopeptidase termed DINE (damage-induced neuronal endopeptidase), which is upregulated in response to neuronal injury and that was identified by differential display PCR, appears to be the rat homolog of human XCE (Kiryu-Seo et al., 2000). Therefore, it has been suggested that this enzyme may play a role in protecting injured neurons against neuronal death.
Although the conservation in the XCE sequence clearly indicates a peptidase function for XCE, its substrate remains to be identified. Candidate substrates, for which no cleavage by recombinant XCE could be detected, include ET-1, big ET-1, calcitonin, bradykinin, enkephalins, and galanin. Inactivation of the XCE gene in mice resulted in neonatal lethality caused by respiratory failure (Schweizer et al., 1999), indicating a role in respiration control. Involvement of XCE in defects in synaptic connections or transmission is a particularly attractive hypothesis because of the specific neuronal expression pattern of XCE (in the medulla oblongata), together with its putative metaiopeptidase nature, indicating that one or more neuropeptide transmitters can be considered as potential candidate substrates for this enzyme.
F. M13 Family in Invertebrates
For many years, knowledge about the M1 family of metalloproteases was limited to vertebrates. Nowadays, the number of research groups reporting on the presence of these enzymes in nonvertebrate organisms is increasing. This increase began with the notification of an endopeptidase-like activity in several invertebrates and has recently evolved into the direction of molecular identification. Early reports described NEP-like activity⧸homologs in invertebrates, but in recent years it has become clear that the presence of true ECE activity⧸homologues in invertebrates also can no longer be denied.
The distinction between a NEP and ECE homolog is not always easily made, as both enzymes share high sequence similarity and similar enzymatic properties. However, putative differences between the two enzymes have come forward. In vertebrates, NEP activity is discriminated from ECE activity on the basis of their difference in susceptibility to the inhibitors thiorphan and phosphoramidon. However, most of the data available on endopeptidase-activity in invertebrates reveal that these inhibitors are not always as efficient as in vertebrates. Furthermore, the distinction between these inhibitory profiles does not always seem to be straightforward. Because no expression studies with an ECE-like protein in invertebrates have as yet been done, at present there is no clarity in this matter. On the molecular level, the distinction between NEP and ECE is made in the ET-binding domain. This sequence, NAYY in vertebrate ECE, is replaced by a NAFY sequence in NEP. This knowledge came from mutational studies with vertebrate ECE that have shown that the replacement of the first tyrosine by a phenylalanine in ECE leads to an 18-fold reduction of the V max⧸K m for the conversion of big ET-1 to ET-1 (Sansom et al., 1998).
To date, it is not clear whether this big ET substrate of ECE is present in invertebrates. The analysis of ETs in nonvertebrates is mainly limited to immunocytochemical studies. A small number of studies have indicated the presence of endothelin in invertebrates, using antibodies to vertebrate endothelins (Hasegawa 1991, Kohidai 1995, Montuenga 1994).
1. Endopeptidase Activity in Invertebrates
Neuropeptide-degrading endopeptidase activity was found in synaptic membranes of the locust, Schistocerca gregaria. The locust adipokinetic hormone (AKH-1) was used as a substrate. It was inactivated by cleavage at the Asn-Phe bond, resembling a cleavage pattern typical for mammalian endopeptidases. Phosphoramidon partially inhibited this endopeptidase activity. This was the first time that the presence of an endopeptidase-like activity in insects was reported. The activity found in this insect species was less susceptible to inhibition by phosphoramidon and thiorpan compared to its mammalian counterparts. This may reflect a difference in inhibitor binding (Isaac, 1988). The enzyme responsible for the inactivation of the neurohormone AKH is located on the surfaces of tissues; this degradative activity could not be detected in hemolymph (O'Shea and Rayne, 1992).
In the mollusc Aplysia californica, endopeptidase activity was detected with the use of the substrate Leu-enkephalin. This activity could be inhibited by thiorphan and phosphoramidon for approximately 40% (Bawab 1993, Zappulla 1999). The presence of an identical endopeptidase-like activity was confirmed by studies in D. melanogaster (Isaac 2002, Wilson 2002), M. domestica (Lamango and Isaac, 1993), L. dispar (Masler et al., 1996), L. maderae, and L. migratoria (Isaac et al., 2002). These researchers studied the cleavage of either AKH or locustatachykinin (LomTK)-degradation.
In D. melanogaster, the degradation of LomTK-1 by head membrane fractions yields two main fragments, FYGVRamide and GPSGFYG. The use of the NEP inhibitor phosphoramidon led to 80% inhibition of the generation of the first fragment, whereas the formation of the second fragment was inhibited by only 50%. This points toward the presence of at least two distinct NEP-like enzymes that are responsible for the degradation of LomTK-1, the first resembling NEP and the second one resembling ECE, which is less susceptible to phosphoramidon (Isaac et al., 2002). Surprisingly, the effect of the inhibitor thiorphan, which allows differentiation between mammalian NEP and ECE, was not examined in this study on D. melanogaster endopeptidases.
The first evidence of an ECE-like activity in insects came from studies in neuronal membranes from the locust L. migratoria, where the conversion of big ET-1 to mature ET-1 was found to be less susceptible to inhibition with thiorphan when compared to phosphoramidon inhibition (Macours et al., 2003c). A second reason for not subscribing this activity to a typical NEP enzyme comes from the fact that NEP is known to rapidly degrade mature ET-1 at multiple cleavage sites (Vijayaraghavan et al., 1990). Also, the use of a fluorescent substrate highly selective for ECE (Luciani et al., 2001) confirmed the presence of ECE-activity in the locust. This ECE-like activity was found to be very abundant in the reproductive system (ovaries and testes) of the locust, indicating a role in reproduction and development. A lower but substantial amount of activity was found in midgut, brain, and fat body, so multiple functions of this enzyme in insects seem likely.
2. ECE in Hydra vulgaris and C. elegans
The first invertebrate putative endothelin-converting enzyme homologue was cloned from the Coelenterate Hydra vulgaris. The Coelentrates represent one of the oldest phyla of the animal kingdom. To the best of our knowledge, this is the only invertebrate organism, other than insects, from which an ECE homolog has been cloned and reported. This sequence shows the highest percentage of identity (37%) to human ECE-1 and a substantial amount of identity to the other members of the human M13 family. On the basis of this large sequence homology and the presence of the NAYY motif, this molecule was classified as an ECE homolog (Fig. 4 ). The cystein that is responsible for the dimerization of the protein in vertebrates is absent in this invertebrate ECE homolog, indicating that early forms of this enzyme existed in monomeric form. Expression patterns of H. vulgaris ECE during head and foot regeneration point toward a role for this enzyme in development. Homogenates from this organism also contained immunodetectable levels of big ET-1 and ET-1.
FIG. 4.
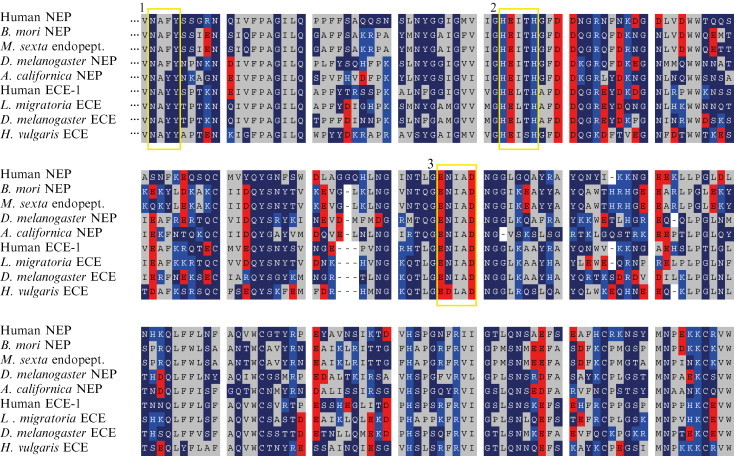
Partial sequence comparison of the C-terminal region from Human NEP (P08473), Bombyx mori NEP (BAB33300), endopeptidase sequence from Manduca sexta (AAO21504), Drosophila melanogaster NEP (NP_511056), Aplysia californica NEP (AAD51382), Human ECE-1 (NP_001388), Locusta migratoria ECE (AAN73018), Drosophila melanogaster ECE (NP_523417), Hydra vulgaris ECE (AAD46624). 1, ET-binding site; 2, active site HExxH; 3, zinc-binding consensus site ExxxD. The colors indicate the nature of the amino acids (red: acid; light-blue: base; dark-blue: hydrophilic; grey: hydrophobic.)
Human ETs are able to induce contraction in H. vulgaris, indicating the involvement of a H. vulgaris endothelin-like system in contraction of the cytoskeleton (Zhang et al., 2001). However, human big ET could not exert this effect on contraction, indicating that H. vulgaris ECE does not properly convert human big ETs or that H. vulgaris ECE works intracellularly (Sarras et al., 2002). The unambiguous confirmation of the presence of a functional endothelin-like system in H. vulgaris will have to result from the molecular identification of H. vulgaris ET and its precursor big ET.
Sequencing of the entire genome of C. elegans in 1998 (The C. elegans Sequencing Consortium) revealed 22 possible NEP⧸ECE–like genes in this organism. Intriguingly, one of the C. elegans genes codes for a two-domain product (cf. ACE in vertebrates). The temporal and spatial expression of five C. elegans NEP⧸ECE–like genes has been studied, revealing all five genes to be expressed in a tissue specific manner, indicating that these genes control different physiological processes (Turner et al., 2001).
3. ECE in Insects
Simultaneously with the detection of ECE-like activity in the locust, the first insect ECE homolog was cloned (Macours et al., 2003c). The L. migratoria ECE (LomECE) sequence reveals a putative type II transmembrane protein of 727 amino acid residues, with a calculated mass of 83 kDa deduced from its longest open reading frame. The sequence contains a short N-terminal cytoplasmatic tail of nine amino acids, a hydrophobic stretch representing the putative membrane-spanning domain, and a large extracellular stretch of 695 amino acids containing the characterizing active site HExxH. Hence, the overall structure of this insect ECE resembles that of mammalian ECE. Ten potential glycosylation sites are present in LomECE, indicating that like vertebrate ECE, the insect form is also highly glycosylated. Among these glycosylation signals, Asn 589 and Asn 608, which are known to be important for the bioactivity of mammalian ECE-1 are conserved (Nelboeck et al., 1998).
The putative zinc-coordinating residue Glu 624, which is located 61 residues downstream from the active site, compares with a 61–amino acid separation in mammalian ECE (Shimada et al., 1995) in contrast to mammalian NEP, which possesses a 64-residue separation (Hooper et al., 1997). The C-terminal CEVW sequence, critical for proper enzyme maturation and activity (MacLeod et al., 2001), is present in LomECE, as is the endothelin-binding site NAYY (Fig. 4). The cysteine residue responsible for dimerization of the protein in mammalian organisms (Shimada et al., 1996) is absent in LomECE, supporting the hypothesis that early divergent forms of this enzyme may have existed in monomeric form (Zhang et al., 2001). The need for a dimeric form of ECE for enzyme functionality was refuted by studies with solubilized recombinant ECE-1 in which dimerization did not appear to be essential or preferential for full ECE activity (Korth et al., 1997). Studies with a monomeric and dimeric from of bovine ECE demonstrated that these two forms posses a comparable biochemical profile. The major difference between the two forms is that the dimeric form is found to be more efficient in catalyzing the conversion of big ET-1 into ET-1 (Kulathila et al., 2002), sustaining the possibility of other in vivo substrates for the invertebrate ECE form. The fact that ECE mRNA is found in a variety of tissues in the locust is indicative of a major, and most probably diversified, biological role for the enzyme.
In the D. melanogaster genome (Adams 2000, FlyBase Consortium 2003), predictions of approximately 24 members of the M13 family can be made (Coates et al., 2000b). The identification and classification of these peptidase genes was performed with gene prediction strategies, sequence comparison with known genes, and searches for active site regions by use of the MEROPS database (Rawlings et al., 2002). Sequence comparison of these genes with members of the human metalloproteases family identified the CG9565 sequence to posses the highest homology with human ECE-1 (Fig. 4).
4. NEP in Invertebrates
An M13 homolog that contained all the essential characteristic amino acids necessary for enzymatic activity was cloned from the CNS of the slug Aplysia californica. This molecule was assigned NEP. The sequence contains the NAYY sequence in combination with a 63–amino acid separation (64 in vertebrate NEP) between the active site and the third zinc-binding ligand, thus combining both ECE and NEP characteristics (Fig. 4). The presence of NEP in the central and peripheral nervous system may indicate that it could be a major player in the modulation of synaptic transmission in A. californica and in the metabolism of neuropeptides (Zappulla et al., 1999).
In B. mori, an ecdysteroid-inducible neutral endopeptidase-like gene was cloned from the wing discs (BmNEP). Its sequence showed 36% identity with rat-NEP. It contained the same zinc-binding motif (HEITH) as a mammalian NEP and contained a 63-residue separation between the putative third zinc-coordinating ligand Glu and the active site (Fig. 4). Hence, it was classified as a NEP homolog. The expression of BmNEP is induced by 20-hydroxyecdysone (20E) and is suggested to be a secondary response gene, following the expression of BmAcer, which starts within 2 hours after 20E stimulation.
From this point of view, it is suggested that this peptidase is involved in the activation or inactivation of peptides in wing discs, or peptide hormones secreted from different organs in the course of metamorphosis (Zhao et al., 2001).
In the D. melanogaster genome, the CG5894 and CG9761 sequences display the highest homology to human NEP.
V. Other Roles of ACE and ECE
A. ACE and ECE in Prokaryotes
The M2 and M13 family of metallopeptidases contain both prokaryotic and eukaryotic members. In the genome of the bacteria, Gloeobacter violaceus (accession BAC91084), Shewanella oneidensis (accession NP_71808), and Xanthomonas axonopodis (accession NP_64155), the predicted protein sequences of ACE homolog resemble the single-domain form of invertebrate ACE, with a possible signal sequence and the absence of a transmembrane domain at the C terminus of the enzyme.
An invertebrate NEP⧸ECE homolog was discovered in the gram-negative bacterium, Porphyromonas gingivalis. This is an important pathogen in the manifestation of acute periodontitis. This chronic inflammatory disease is the major cause of tooth loss in adults (Haffajee 1994, Socransky 1992). The proteases of these bacteria, in addition to their primary function in providing peptides for growth, are thought to be directly involved in tissue invasion and modulation of the host immune system (Lamont and Jenkinson, 1998). The Porphyromonas gingivalis pepO sequence (PgPepO) shows 31% sequence identity with human ECE-1 and 29.9% with human NEP. Instead of a membrane-bound domain, it contains a putative signal sequence of 25 amino acids at the N-terminal end of the sequence, indicating that the protease could be secreted across the plasma membrane, possibly with pathological consequences as a result. All activity-essential amino acids are present in the PgPepO sequence, but the 10 cystein residues that are conserved between all members of the vertebrate NEP⧸ECE family are absent. Because this also includes the absence of the cystein responsible for dimerization of the ECE protein, the bacterial ECE homolog exists in monomer form (Awano et al., 1999).
The PgPepO protein displays a substrate specificity similar to mammalian ECE. It converts big ET-1, -2, and -3 into their respective mature endothelin peptides and cleaves substance P, angiotensin I and II, and neurotensin at multiple sites. Urotensin II, angiotensin III, and oxytocin are not hydrolyzed by PgPepO (Carson et al., 2002). The P. gingivalis bacteria were shown to succeed in invading and multiplication within aortic endothelial cells and are localized in atherosclerotic plaques (Deshpande et al., 1998–1999). Because several associations between periodontal and coronary artery diseases have been made (Beck et al., 1996), invasion of these bacteria into cells was suggested to cause a distortion of the finely balanced equilibrium involved in ET production. This may provide a possible link between periodontitis and cardiovascular diseases (Carson et al., 2002).
Experiments using a mutant form of bacteria, showing PepO-gene deficiency, showed a decreased invasion of such bacteria in mammalian cells. This indicates that PepO gene is at least involved in the first step of the bacterial cycle; namely, the invasion and lysis of the mammalian cell membrane (Ansai et al., 2003). In the MEROPS database for peptidases (Rawlings et al., 2002), a large number of bacteria can be found that all contain at least one M13 homolog in their genome. Also in Archeae, M13 homolog are reported, but the majority of the Archeae do not contain an M13 homolog in their genome. The presence of homolog of these enzymes in prokaryotes could perhaps have resulted from a horizontal exchange of genes between prokaryotes and eukaryotes (Froeliger et al., 1999). However, because members of the M13 family are present in Eubacteria and some Archeabacteria, as well as in eukaryotes, the possibility that an ultimate ancestor of the M13 family may have been present in the organism that preceded both modern bacteria and eukaryotes cannot be excluded. Although the exact evolutionary relationship between eukaryotic and prokaryotic metalloproteases cannot yet be determined, the high level of sequence conservation indicates that these enzymes are under strong evolutionary constraints, thereby protecting structural features essential for enzyme activity.
B. ACE, ECE, and NEP in Alzheimer's Disease: Recent Data
The amyloidogenic pathway of AβPP (amyloid beta precursor protein) processing leads to the production of the neurotoxic amyloid β-peptide (Aβ40 or Aβ42). The formation of the Aβ peptides occurs through the initial cleavage by a β secretase, followed by the action of a γ secretase (Fig. 5 ). The pathological accumulation of Aβ in the brain (formation of plaques) is the primary cause of neuronal cell death and dementia in patients with Alzheimer's disease (Hardy and Higgins, 1992). The degree of Aβ accumulation in the brain is dependent not only on its production but also on its removal. The three zinc-metalloproteases NEP, ECE, and possibly ACE have been put forward as Aβ degrading enzymes, thus limiting the amount of Aβ accumulation in the brain.
FIG. 5.
Schematic representation of the synthesis of Aβ from its precursor. The shaded area represents the membrane-bound region. The cleavage of Aβ by NEP, ECE, and ACE is shown by arrows.
In vitro assays revealed that ACE is capable of preventing the aggregation of Aβ and prevents the deposition of Aβ onto a synthetic matrix. In addition, it inhibits Aβ fibril formation and Aβ-induced cytotoxicity (Hu et al., 2001). The catabolism of Aβ by ACE occurs through a rather unusual cleavage, between the Asp7–Ser8 bond of Aβ. However, infusion of ACE inhibitors in the brain of AβPP transgenic mice had no effect on the level of Aβ present in the brain. This raises some questions concerning the significance of ACE in the Aβ catabolism in vivo. This is in contrast to the M13 metalloproteases, NEP and ECE, for which the in vivo involvement has been confirmed.
In vitro models have characterized ECE-1 as an Aβ-degrading enzyme that appears to act intracellularly, thereby limiting the amount of Aβ available for secretion. Overexpression of ECE-1 in cells that lack endogenous ECE activity results in a pronounced decrease in Aβ accumulation in the conditioned media (Eckman et al., 2001). The physiological relevance of ECE activity on Aβ catabolism was supported by the analysis of Aβ levels in the brain of mice deficient for ECE-1. Here, significant increases in the levels of Aβ were detected (Eckman et al., 2003).
NEP cleaves the Aβ peptide at five possible positions, with Gly9–Tyr10 being the initial cleavage site (Howell 1995, Shirotani 2001). Chronic injection of thiorphan in the rat brain leads to an accumulation of Aβ (Iwata et al., 2000). In NEP-deficient mice, increased levels of Aβ are detected (Iwata et al., 2001) because of a reduction in the level of exogenous Aβ catabolism. In addition, areas in the brain of patients with Alzheimer's that are enriched in plaques display reduced levels of NEP (Yasojima 2001a, Yasojima 2001b). All this indicates that NEP and ECE are rate-limiting peptidases participating in Aβ catabolism (Fig. 5). Future research may include the up-regulation of these metalloproteases as a promising strategy for the treatment and prevention of Alzheimer's disease.
VI. Concluding Remarks
For many years, the study of M2 and M13 metalloproteases in invertebrates lagged behind that in mammals. The first reason was that it was not logical to start searching for peptidic regulators of blood pressure in animals with an open circulatory system. Second, many invertebrates are small, and harvesting enough tissue for the purification of enzymes is difficult and time consuming. Molecular studies in invertebrates have expanded rapidly with the development of microtechniques that make it much easier to work with these small quantities. Many of the identified invertebrate genes are also known to be in vertebrate species, where they posses similar characteristics and functions. These observations support the fact that invertebrates provide excellent model systems for the study of genetics. By comparing the human genome with those of other organisms, regions of similarity and divergence can be identified, thereby providing critical clues about the structure and function of human genes. This information may be beneficial for developing better strategies for treating and preventing some human diseases. With each molecule that is sequenced, this approach becomes more powerful.
The identification of ACE and ECE as key components in the regulation of the mammalian blood pressure system made them interesting targets for the development of efficient antihypertensive drugs. However, the large in vitro substrate specificity and the recent discovery of their involvement in Alzheimer's disease asks for caution in the use of ACE and ECE inhibitors as therapeutic drugs. The large cellular and tissue distribution of ACE and ECE in the human body also points toward the possibility of as-yet-unknown physiological roles of these enzymes. The idea that invertebrate counterparts of these enzymes function as regulators of blood pressure is very unlikely. Hence, these organisms can be useful in the clarification of these extra enzymatic functions. Future research concerning the roles of these enzymes will have to involve the identification of new substrates and inhibitors. Possible substrates can be tested on their in vivo characteristics by colocalization studies and knockout experiments.
From the above listed data, it is clear that our knowledge about the occurrence and physiology of the M2 peptidase family in invertebrates is beginning to take shape, whereas research on the M13 peptidase family members is still in its infancy. Available information on these enzyme families in invertebrates is restricted to ACE, NEP, and ECE. However, the exploration of invertebrate genomes that have already been published indicates that a large number of M2 and M13 family members is present. Further data mining of invertebrate genomes in combination with the recombinant expression of isolated sequences might lead to the identification of homologs to ACE2, Kell, SEP, XCE, ECE-2, and ECE-3 in invertebrates.
References
References
- Adams M.D., Celniker S.E., Holt R.A., Evans C.A., Gocayne J.D. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Aigaki T., Kasuga H., Osanai M. A specific endopeptidase BAEE esterase, in the glandula prostatica of the male reproductive system of the silkworm, Bombyx mori. Insect Biochem. 1987;17:323–328. [Google Scholar]
- Aigaki T., Osanai M., Kasuga H. Auginine carboxypeptidase activity in the male reproductive glands of the silkworm, Bombyx mori. Insect Biochem. 1988;18:295–298. [Google Scholar]
- Ansai T., Yu W., Urnowey S., Barik S., Takehara T. Construction of a pepO gene-deficient mutant of Porphyromonas gingivalis: Potential role of endopeptidase O in the invasion of host cells. Oral Microbiol. Immunol. 2003;18:398–400. doi: 10.1046/j.0902-0055.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- Arai H., Hori S., Aramori I., Okhubo H., Nakanishi S. Cloning and expression of a cDNA encoding an endothelin a receptor. Nature. 1990;348:732–735. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Awano S., Ansai T., Mochizuki H., Yu W., Tanzawa K., Turner A.J., Takehara T. Sequencing, expression and biochemical characterization of the Porphyromonas gingivalis pepO gene encoding a protein homologous to human endothelin-converting enzyme. FEBS Lett. 1999;460:139–144. doi: 10.1016/s0014-5793(99)01326-5. [DOI] [PubMed] [Google Scholar]
- Azizi M., Junot C., Ezan E., Menard J. Angiotensin I-converting enzyme and metabolism of the haematological peptide N-acetyl-seryl-aspartyl-lysyl-proline. Clin. Exp. Pharmacol. Physiol. 2001;28:1066–1069. doi: 10.1046/j.1440-1681.2001.03560.x. [DOI] [PubMed] [Google Scholar]
- Azizi M., Rousseau A., Ezan E., Guyene T.T., Michelet S., Grognet J.M., Lenfant M., Corvol P., Ménard J. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J. Clin. Invest. 1996;97:839–844. doi: 10.1172/JCI118484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barret A.J., Rawlings N.D., Woessner J.F. “Handbook of Proteolytic Enzymes”. Academic Press; London: 1998. [Google Scholar]
- Baudin B. New aspects on angiotensin-converting enzyme: From gene to disease. Clin. Chem. Lab. Med. 2002;40:256–265. doi: 10.1515/CCLM.2002.042. [DOI] [PubMed] [Google Scholar]
- Bawab W., Aloyz R.S., Crine P., Roques B.P., DesGroseillers L. Identification and characterization of a neutral endopeptidase activity in Aplysia californica. Biochem. J. 1993;296:459–465. doi: 10.1042/bj2960459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu H., Elagoz A., Crine P., Rokeach L.A. Interaction of mammalian neprilysin with binding protein and calnexin in Schizosaccharomyces pombe. Biochem. J. 1999;340:813–819. [PMC free article] [PubMed] [Google Scholar]
- Beck J., Garcia R., Heiss G., Vokonas P.S., Offenbacher S. Periodontal disease and cardiovascular disease. J. Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- Borovsky D., Meola S.M. Biochemical and cytoimmunological evidence for the control of Aedes aegypti larval trypsin with Aea-TMOF. Arch. Insect Biochem. Physiol. 2004;55:124–139. doi: 10.1002/arch.10132. [DOI] [PubMed] [Google Scholar]
- Brooks D.R., Appleford P.J., Murray L., Isaac R.E. An essential role in molting and morphogenesis of Caenorhabditis elegans for ACN-1, a novel member of the angiotensin-converting enzyme family that lacks a metallopeptidase active site. J. Biol. Chem. 2003;278:52340–52346. doi: 10.1074/jbc.M308858200. [DOI] [PubMed] [Google Scholar]
- Bulet P., Hetru C., Dimarcq J.L., Hoffman D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999;23:329–344. doi: 10.1016/s0145-305x(99)00015-4. [DOI] [PubMed] [Google Scholar]
- Bylemans D., Borovsky D., Hunt D.F., Shabanowitz H., Grauwels L., De Loof A. Sequencing and characterisation of trypsin modulating oostatic factor (TMOF) from the ovaries of the grey fleshfly, Neobellieria (Sarcophaga) bullata. Reg. Pept. 1994;50:61–72. doi: 10.1016/0167-0115(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Carson J.A., Turner A.J. β-Amyloid catabolism: Roles for neprilysin (NEP) and other metallopeptidases? J. Neurochem. 2002;81:1–8. doi: 10.1046/j.1471-4159.2002.00855.x. [DOI] [PubMed] [Google Scholar]
- Carson J.A., Ansai T., Awano S., Yu W., Takehara T., Turner A. Characterization of PgPepO, a bacterial homologue of endothelin-converting enzyme-1. Clin. Sci. 2002;103:90S–93S. doi: 10.1042/CS103S090S. [DOI] [PubMed] [Google Scholar]
- Coates D., Isaac R.E., Cotton J., Siviter R., Williams T.A., Shirras A., Corvol P., Dive V. Functional conservation of the active sites of human and Drosophila angiotensin I-converting enzyme. Biochem. 2000;39:8963–8969. doi: 10.1021/bi000593q. [DOI] [PubMed] [Google Scholar]
- Coates D., Siviter R., Isaac R.E. Exploring the Caenorhabditis elegans and Drosophila melanogaster genomes to understand neuropeptide and peptidase function. Biochem. Soc. Trans. 2000;28:464–469. [PubMed] [Google Scholar]
- Cornell M.J., Coates D., Isaac R.E. Characterization of putative Drosophila angiotensin converting enzyme cDNA clones. Biochem. Soc. Trans. 1993;21:243S. doi: 10.1042/bst021243s. [DOI] [PubMed] [Google Scholar]
- Cornell M.J., Williams T.A., Lamango N.S., Coates D., Corvol P., Soubrier F., Hoheisel J., Lehrach H., Isaac R.E. Cloning and expression of an evolutionary conserved single-domain angiotensin converting enzyme from Drosophila melanogaster. J. Biol. Chem. 1995;270:13613–13619. doi: 10.1074/jbc.270.23.13613. [DOI] [PubMed] [Google Scholar]
- Corvol P., Williams T.A., Soubrier F. Peptidyl dipeptidase A: Angiotensin I-converting enzyme. Methods Enzymol. 1995;248:283–305. doi: 10.1016/0076-6879(95)48020-x. [DOI] [PubMed] [Google Scholar]
- Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-Dos-Santos A.J., Da Costa J., Zhang L., Pei Y., Scholey J., Ferrario C.M., Manoukian A.S., Chappell M.C., Backx P.H., Yagil Y., Penninger J.M. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- Dani M.P., Richards E.H., Isaac R.E., Edwards J.P. Antibacterial and proteolytic activity in venom from the endoparasitic wasp Pimpla hypochondriaca (Hymenoptera: Ichneumonidae) J. Insect Physiol. 2003;49:945–954. doi: 10.1016/s0022-1910(03)00163-x. [DOI] [PubMed] [Google Scholar]
- Danilczyk U., Eriksson U., Crackower M.A., Penninger J.M. A story of two ACEs. J. Mol. Med. 2003;81:224–234. doi: 10.1007/s00109-003-0419-x. [DOI] [PubMed] [Google Scholar]
- Dell'Italia L.J., Rocic P., Lucchesi P.A. Use of angiotensin-converting enzyme inhibitors in patients with diabetes and coronary artery disease. Curr. Probl. Cardiol. 2002;27:6–36. doi: 10.1067/mcd.2002.121580. [DOI] [PubMed] [Google Scholar]
- Deshpande R.G., Khan M., Genco C.A. Invasion strategies of the oral pathogen porphyromonas gingivalis: Implications for cardiovascular disease. Invasion Metastasis. 1998–1999;18:57–69. doi: 10.1159/000024499. [DOI] [PubMed] [Google Scholar]
- Dimitrov D.S. The secret life of ACE2 as a receptor for the SARS virus. Cell. 2003;115:652–653. doi: 10.1016/S0092-8674(03)00976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dive V., Cotton J., Yiotakis A., Michaud A., Vassiliaou S., Jiracek J., Vazuex G., Chauvet M.T., Cuniasse P., Corvol P. RXP 407, a phosphinic peptide, is a potent inhibitor of angiotensin I converting enzyme able to differentiate between its two active sites. Biochemistry. 1999;96:4330–4335. doi: 10.1073/pnas.96.8.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Douglas S.A., Ohlstein E.H. Signal transduction mechanisms mediating vascular actions of endothelin. J. Vasc. Res. 1997;34:152–164. doi: 10.1159/000159219. [DOI] [PubMed] [Google Scholar]
- Eckman E.A., Reed D.K., Ekman C.B. Degrading of the Alzheimer's amyloid β peptide by endothelin converting enzyme. J. Biol. Chem. 2001;276:24520–24548. doi: 10.1074/jbc.M007579200. [DOI] [PubMed] [Google Scholar]
- Eckman E.A., Watson M., Marlow L., Sambamurti K., Ekman C.B. Alzheimer's disease β-amyloid peptide is increased in mice deficient in endothelin converting enzyme. J. Biol. Chem. 2003;278:2081–2084. doi: 10.1074/jbc.C200642200. [DOI] [PubMed] [Google Scholar]
- Egidy G., Juillerat-Jeanneret L., Jeannin J.F., Korth P., Bosman F.T., Pinet F. Modulation of human colon tumor-stromal interactions by the endothelin system. Am. J. Pathol. 2000;157:1863–1874. doi: 10.1016/S0002-9440(10)64825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekbote U., Coates D., Isaac R.E. A mosquito (Anopheles stephensi) angiotensin I-converting enzyme (ACE) is induced by a blood meal and accumulates in the developing ovary. FEBS Lett. 1999;455:219–222. doi: 10.1016/s0014-5793(99)00870-4. [DOI] [PubMed] [Google Scholar]
- Ekbote U., Looker M., Isaac R.E. ACE inhibitors reduce fecundity in the mosquito, Anopheles stephensi. Comp. Biochem. Physiol. B. 2003;134:593–598. doi: 10.1016/s1096-4959(03)00019-8. [DOI] [PubMed] [Google Scholar]
- Ekbote U.V., Weaver R.J., Isaac R.E. Angiotensin I-converting enzyme (ACE) activity of the tomato moth, Lacanobia oleracea: Changes in levels of activity during development and after copulation suggest roles during metamorphosis and reproduction. Insect Biochem. Mol. Biol. 2003;33:989–998. doi: 10.1016/s0965-1748(03)00105-x. [DOI] [PubMed] [Google Scholar]
- Emoto N., Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J. Biol. Chem. 1995;270:15262–15268. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- Eriksson U., Danilczyk U., Penninger J.M. Just the beginning: Novel functions for angiotensin-converting enzymes. Curr. Biol. 2002;12:745–752. doi: 10.1016/s0960-9822(02)01255-1. [DOI] [PubMed] [Google Scholar]
- Esther C.R., Howard T.E., Marino E.M., Goddard J.M., Capecchi M.R., Bernstein K.E. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab. Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- FlyBase Consortium The FlyBase database of the Drosophila genome projects and community literature. Nucleic Acids Res. 2003;31:172–175. doi: 10.1093/nar/gkg094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeliger E.H., Oetjen J., Bond J.P., Fives-Taylor P. Streptococcus parasanguis pepO encodes an endopeptidase with structure and activity similar to those of enzymes that modulate peptide receptor signaling in eukaryotic cells. Infect. Immun. 1999;67:5206–5214. doi: 10.1128/iai.67.10.5206-5214.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S., Frenzel K., Xiao H.D., Adams J.W., Zhao H., Keshelava G., Teng L., Bernstein K.E. Newly recognized physiologic and pathophysiologic actions of the Angiotensin-converting enzyme. Curr. Hypertens. Rep. 2004;6:124–128. doi: 10.1007/s11906-004-0087-4. [DOI] [PubMed] [Google Scholar]
- Gallagher T.M., Buchmeier M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaddar G., Ruchon A.F., Carpentier M., Marcinkiewicz M., Seidah N.G., Crine P., Desgroseillers L., Boileau G. Molecular cloning and biochemical characterization of a new mouse testis soluble zinc metallopeptidase of the neprilysin family. Biochem. J. 2000;347:419–429. doi: 10.1042/0264-6021:3470419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy G., Chandrakant S. The endocrinology of nodule formation in locusts in response to injections of microbial products. Comp. Biochem. Physiol. 2002;132A:565–566. [Google Scholar]
- Goldsworthy G., Mullen L., Kwaku O.W., Chandrakant S. Interactions between the endocrine and immune system in locusts. Physiol. Entomol. 2003;28:54–61. [Google Scholar]
- Guy J.L., Jackson R.M., Acharya K.R., Sturrock E.D., Hooper N.M., Turner A.J. Angiotensin-converting enzyme-2 (ACE2): Comparative modeling of the active site, specificity requirements, and chloride dependence. Biochemistry. 2003;42:13185–13192. doi: 10.1021/bi035268s. [DOI] [PubMed] [Google Scholar]
- Haffajee A.D., Socransky S.S. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Hagaman J.R., Moyer J.S., Bachman E.S., Sibony M., Magyar P.L., Welch J.E., Smithies O., Krege J.H., O'Brien D.A. Angiotensin-converting enzyme and male fertility. Proc. Natl. Acad. Sci. USA. 1998;95:2552–2557. doi: 10.1073/pnas.95.5.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J.A., Higgins G.A. Alzheimer's disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Hiki K., Sawamura T., Aoyama T., Okamoto Y., Miwa S., Shimohama S., Kimura J., Masaki T. Purification of a novel endothelin-converting enzyme specific for big endothelin-3. FEBS Lett. 1998;428:304–308. doi: 10.1016/s0014-5793(98)00554-7. [DOI] [PubMed] [Google Scholar]
- Hasegawa K., Kobayashi H. Immunohistochemical localization of endothelin-1 in the nervous system of the earthworm Eisenia foetida. Gen. Comp. Endocrinol. 1991;81:433–441. doi: 10.1016/0016-6480(91)90171-2. [DOI] [PubMed] [Google Scholar]
- Hens K., Vandingenen A., Macours N., Baggerman G., Karaoglanovic A.C., Schoofs L., De Loof A., Huybrechts R. Characterization of four substrates emphasizes kinetic similarity between insect and human C-domain angiotensin-converting enzyme. Eur. J. Biochem. 2002;269:3522–3530. doi: 10.1046/j.1432-1033.2002.03043.x. [DOI] [PubMed] [Google Scholar]
- Hickey K.A., Rubanyi G., Paul R.J., Highsmith R.F. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am. J. Physiol. Cell Physiol. 1985;248:C550–C556. doi: 10.1152/ajpcell.1985.248.5.C550. [DOI] [PubMed] [Google Scholar]
- Hoffman D., Brehelin M., Hoffman J.A. Modifications of the hemogram and of haematopoietic tissue of male adults of Locusta migratoria (Orthoptera) after injection of Bacillus thuringiensis. J. Invertebr. Pathol. 1974;24:238–247. doi: 10.1016/0022-2011(74)90017-2. [DOI] [PubMed] [Google Scholar]
- Holmes K.V. SARS coronavirus: a new challenge for prevention and therapy. J. Clin. Invest. 2003;111:1605–1609. doi: 10.1172/JCI18819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N.M., Karran E.H., Turner A.J. Membrane protein secretase. Biochem. J. 1997;321:265–279. doi: 10.1042/bj3210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houard X., Williams T.A., Michaud A., Dani P., Isaac R.E., Shirras A.D., Coates D., Corvol P. The Drosophila melanogaster-related angiotensin-I-converting enzymes Acer and Ance. Distinct enzymic characteristics and alternative expression during pupal development. Eur. J. Biochem. 1998;257:599–606. doi: 10.1046/j.1432-1327.1998.2570599.x. [DOI] [PubMed] [Google Scholar]
- Howell S., Nalbantoglu J., Crine P. Neutral endopeptidase can hydrolyze beta-amyloid(1–40) but shows no effect on beta-amyloid precursor protein metabolism. Peptides. 1995;16:647–652. doi: 10.1016/0196-9781(95)00021-b. [DOI] [PubMed] [Google Scholar]
- Hu J., Igarashi A., Kamata M., Nakagawa H. Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. Biol. Chem. 2001;276:47863–47868. doi: 10.1074/jbc.M104068200. [DOI] [PubMed] [Google Scholar]
- Hurst D., Rylett C.M., Isaac R.E., Shirras A.D. The Drosophila angiotensin-converting enzyme homologue Ance is required for spermiogenesis. Dev. Biol. 2003;254:238–247. doi: 10.1016/s0012-1606(02)00082-9. [DOI] [PubMed] [Google Scholar]
- HYP Consortium A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat. Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Emoto N., Raharjo S.B., Nurhantari Y., Saiki K., Yokoyama M., Matsuo M. Molecular identification and characterization of novel membrane-bound metalloprotease, the soluble secreted form of which hydrolyzes a variety of vasoactive peptides. J. Biol. Chem. 1999;274:32469–32477. doi: 10.1074/jbc.274.45.32469. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Emoto N., Alimsardjono H., Yokoyama M., Matsuo M. Molecular isolation and characterization of novel four isoforms of ECE-2. Biochem. Biophys. Res. Commun. 2002;293:421–426. doi: 10.1016/S0006-291X(02)00252-8. [DOI] [PubMed] [Google Scholar]
- Inagami T. The renin-angiotensin system. Essays Biochem. 1994;28:147–164. [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Takuwa Y., Mitsui Y., Kobayashi M., Masaki T. The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. Biol. Chem. 1989;264:14954–14959. [PubMed] [Google Scholar]
- Isaac R.E. Neuropeptide degrading activity of locust (Schistocerca gregaria) synaptic membranes. Biochem. J. 1988;255:843–847. doi: 10.1042/bj2550843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac R.E., Lamango N.S. Peptidyl dipeptidase activity in the haemolymph of insects. Biochem. Soc. Trans. 1994;22:292S. doi: 10.1042/bst022292s. [DOI] [PubMed] [Google Scholar]
- Isaac R.E., Coates D., Williams T.A., Schoofs L. Insect angiotensin-converting enzyme: Comparative biochemistry and evolution. In: Coast G.M., Webster S.G., editors. “Recent Advances in Arthropod Endocrinology”. 1998. pp. 357–378. Soc. Exp. Biol., Seminar series 65(Part IV); [Google Scholar]
- Isaac R.E., Ekbote U., Coates D., Shirras A.D. Insect angiotensin-converting enzyme. A processing enzyme with broad substrate specificity and a role in reproduction. Ann. N. Y. Acad. Sci. 1999;897:342–347. doi: 10.1111/j.1749-6632.1999.tb07904.x. [DOI] [PubMed] [Google Scholar]
- Isaac R.E., Lamango N.S., Nachman R.J., Strey A., Hayes T.K. Angiotensin-converting enzyme and the metabolism of regulatory peptides in insects. Ann. N. Y. Acad. Sci. 1997;814:339–341. doi: 10.1111/j.1749-6632.1997.tb46179.x. [DOI] [PubMed] [Google Scholar]
- Isaac R.E., Parkin E.T., Keen J.N., Nassel D.R., Siviter R.J., Shirras A.D. Inactivation of a tachykinin-related peptide: identification of four neuropeptide-degrading enzymes in neuronal membranes of insects from four different orders. Peptides. 2002;23:725–733. doi: 10.1016/s0196-9781(01)00653-2. [DOI] [PubMed] [Google Scholar]
- Isaac R.E., Schoofs L., Williams T.A., Veelaert D., Sajid M., Corvol P., Coates D. A novel activity of insect peptidyl-dipeptidase A (angiotensin I-converting enzyme): the hydrolysis of lysyl-arginine and arginyl-arginine from the C-terminus of an insect prohormone peptide. Biochem. J. 1998;330:61–65. doi: 10.1042/bj3300061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac R.E., Siviter R.J., Stancombe P., Coates D., Shirras A.D. Conserved roles for peptidases in the processing of invertebrate neuropeptides. Biochem. Soc. Trans. 2000;28:460–464. [PubMed] [Google Scholar]
- Isaac R.E., Williams T.A., Sajid M., Corvol P., Coates D. Cleavage of arginyl-arginine and lysyl-arginine from the C-terminus of pro-hormone peptides by human germinal angiotensin I-converting enzyme (ACE) and the C-domain of human somatic ACE. Biochem. J. 1997;328:587–591. doi: 10.1042/bj3280587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru F., Shipp M.A. Analysis of the human CD10⧸neutral endopeptidase 24.11 promotor region: Two separate regulatory elements. Blood. 1995;85:3199–3207. [PubMed] [Google Scholar]
- Iwanaga S. The Limulus clotting reaction. Curr. Opin. Immunol. 1993;5:74–82. doi: 10.1016/0952-7915(93)90084-6. [DOI] [PubMed] [Google Scholar]
- Iwanaga S. The molecular basis of innate immunity in the horseshoe crab. Curr. Opin. Immunol. 2002;14:87–95. doi: 10.1016/s0952-7915(01)00302-8. [DOI] [PubMed] [Google Scholar]
- Iwata N., Tsubuki S., Takaki Y., Shirotani K., Lu B., Gerard N.P., Gerard C., Hama E., Lee H.J., Saido T.C. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- Iwata N., Tsubuki S., Takaki Y., Watanabe K., Sekiguchi M., Hosoki E., Kawashima-Morishima M., Lee H.J., Hama E., Sekine-Aizawa Y., Saido T.C. Identification of the major Abetal-42-degrading catabolic pathway in brain parenchyma: Suppression leads to biochemical and pathological deposition. Nat. Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- Jafri F., Ergul A. Nuclear localization of endothelin-converting enzyme-1: Subisoform specificity. Arterioscler Thromb. Vasc. Biol. 2003;23:2192–2196. doi: 10.1161/01.ATV.0000099787.21778.55. [DOI] [PubMed] [Google Scholar]
- Jarmey J.M., Riding G.A., Pearson R.D., McKenna R.V., Willadsen P. Carboxypeptidase from Boophilus microplus: A “concealed” antigen with similarity to angiotensin-converting enzyme. Insect Biochem. Mol. Biol. 1995;25:969–974. doi: 10.1016/0965-1748(95)00038-w. [DOI] [PubMed] [Google Scholar]
- Jones J.C. Hemacytopoiesis in insects. In: Gordon A.S., editor. “Regulation of Hematopoiesis”. Appleton Press; New York: 1970. pp. 7–65. [Google Scholar]
- Johnson G.D., Stevenson T., Ahn K. Hydrolysis of peptide hormones by endothelin-converting enzyme-1. A comparison with neprilysin. J. Biol. Chem. 1999;274:4053–4058. doi: 10.1074/jbc.274.7.4053. [DOI] [PubMed] [Google Scholar]
- Kedzierski R.M., Yanagisawa M. Endothelin system: The double-edged sword in health and disease. Annu. Rev. Pharmacol. Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- Kessler S.P., Gomos J.B., Scheidemantel T.S., Rowe T.M., Smith H.L., Sen G.C. The germinal isozyme of angiotensin-converting enzyme can substitute for the somatic isozyme in maintaining normal renal structure and functions. J. Biol. Chem. 2002;277:4271–4276. doi: 10.1074/jbc.M109474200. [DOI] [PubMed] [Google Scholar]
- Kessler S.P., Rowe T.M., Gomos J.E., Kessler P.M., Sen G.C. Physiological non-equivalence of the two isoforms of angiotensin-converting enzyme. J. Biol. Chem. 2000;275:26259–26264. doi: 10.1074/jbc.M004006200. [DOI] [PubMed] [Google Scholar]
- Kessler S.P., Senanayake Pd.P., Scheidernantel T.S., Gomos J.B., Rowe T.M., Sen G.C. Maintenance of normal blood pressure and renal functions are independent effects of angiotensin-converting enzyme. J. Biol. Chem. 2003;278:21105–21112. doi: 10.1074/jbc.M3023472000. [DOI] [PubMed] [Google Scholar]
- Kim H.M., Shin D.R., Yoo O.J., Lee H., Lee J.O. Crystal structure of Drosophila angiotensin I-converting enzyme bound to captopril and lisinopril. FEBS Lett. 2003;538:65–70. doi: 10.1016/s0014-5793(03)00128-5. [DOI] [PubMed] [Google Scholar]
- Kiryu-Seo S., Sasaki M., Yokohama H., Nakagomi S., Hirayama T., Aoki S., Wada K., Kiyama H. Damage-induced neuronal endopeptidase (DINE) is a unique metallopeptidase expressed in response to neuronal damage and activates superoxide scavengers. Proc. Natl. Acad. Sci. USA. 2000;97:4345–4350. doi: 10.1073/pnas.070509897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohidai L., Csaba G. Effects of the mammalian vasoconstrictor peptide, endothelin-1, on Tetrahymena pyriformis GL, and the immunocytological detection of endogenous endothelin-like activity. Comp. Biochem. Physiol. C. Pharmacol. Toxicol. Endocrinol. 1995;111:311–316. doi: 10.1016/0742-8413(95)00055-s. [DOI] [PubMed] [Google Scholar]
- Komatsu T., Suzuki Y., Imai J., Sugano S., Hida M., Tanigami A., Muroi S., Yamada Y., Hanaoka K. Molecular cloning, mRNA expression and chromosomal localization of mouse angiotensin-converting enzyme-related carboxypeptidase (mACE2) DNA Seq. 2002;13:217–220. doi: 10.1080/1042517021000021608. [DOI] [PubMed] [Google Scholar]
- Korth P., Egidy G., Parnot C., LeMoullec J.M., Corvol P., Pinet F. Construction, expression and characterization of a soluble form of human endothelin-converting-enzyme-1. FEBS Lett. 1997;417:365–370. doi: 10.1016/s0014-5793(97)01323-9. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J., SARS Working Group A novel coronavirus associated with severe acute respiratory syndrome. N Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., van Amerongen G., van Riel D., Laman J.D., de Jong T., van Doornum G., Lim W., Ling A.E., Chan P.K., Tam J.S., Zambon M.C., Gopal R., Drosten C., van der Werf S., Escriou N., Manuguerra J.C., Stohr K., Peiris J.S., Osterhaus A.D. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:63–70. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulathila R., Clark K., Savage P., Bowen B.R., Emoto N., Yanagisawa M., Jeng A.Y. Expression, purification and characterization of the monomeric and dimeric forms of soluble bovine endothelin converting enzyme-1a. Clin. Sci. 2002;103:94–97. doi: 10.1042/CS103S094S. [DOI] [PubMed] [Google Scholar]
- Lamango N.S., Isaac R.E. Metabolism of insect neuropeptides: Properties of a membrane-bound endopeptidase from heads of Musca domestica. Insect Biochem. Mol. Biol. 1993;23:801–808. doi: 10.1016/0965-1748(93)90068-4. [DOI] [PubMed] [Google Scholar]
- Lamango N.S., Isaac R.E. Identification and properties of a peptidyl dipeptidase in the housefly, Musca domestica. that resembles mammalian angiotensin-converting enzyme. Biochem. J. 1994;299:651–657. doi: 10.1042/bj2990651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamango N.S., Nachman R.J., Hayes T.K., Smey A., Isaac R.E. Hydrolysis of insect neuropeptides by an angiotensin-converting enzyme from the housefly, Musca domestica. Peptides. 1997;18:47–52. doi: 10.1016/s0196-9781(96)00232-x. [DOI] [PubMed] [Google Scholar]
- Lamango N.S., Sajid M., Isaac R.E. The endopeptidase activity and the activation by Cl- of angiotensin-converting enzyme is evolutionarily conserved: Purification and properties of an angiotensin-converting enzyme from the housefly, Musca domestica. Biochem. J. 1996;314:639–646. doi: 10.1042/bj3140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont R.J., Jenkinson H.F. Life below the gum line: Pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford K.G., Shai S.Y., Howard T.E., Kovac M.J., Overbeek P.A., Bernstein K.E. Transgenic mice demonstrate a testis-specific promoter for angiotensin converting enzyme. J. Biol. Chem. 1991;266:15559–15562. [PubMed] [Google Scholar]
- Lattion A.L., Soubrier F., Allegrini J., Hubert C., Corvol P., Alhenc-Gelas F. The testicular transcript of the angiotensin-I converting enzyme encodes for the ancestral, non-duplicated form of the enzyme. FEBS Lett. 1989;252:99–104. doi: 10.1016/0014-5793(89)80897-x. [DOI] [PubMed] [Google Scholar]
- Laurent V., Salzet M. Isolation of a renin-like enzyme from the leech Theromyzon tessulatum. Peptides. 1995;16:1351–1358. doi: 10.1016/0196-9781(95)02038-1. [DOI] [PubMed] [Google Scholar]
- Laurent V., Salzet M. A comparison of the N-terminal sequence of the leech Theromyzon tessulatum angiotensin converting-like enzyme with forms of vertebrate angiotensin converting enzymes. Neurosci. Lett. 1995;198:60–64. doi: 10.1016/0304-3940(95)11954-u. [DOI] [PubMed] [Google Scholar]
- Laurent V., Salzet M. Biochemical properties of the angiotensin-converting-like enzyme from the leech Theromyzon tessulatum. Peptides. 1996;17:737–745. doi: 10.1016/0196-9781(96)00074-5. [DOI] [PubMed] [Google Scholar]
- Laurent V., Bulet P., Salzet M. A comparison of the leech Theromyzon tessulatum angiotensin I-like molecule with forms of vertebrate angiotensinogens: A hormonal system conserved in the course of evolution. Neurosci. Lett. 1995;190:175–178. doi: 10.1016/0304-3940(95)11533-3. [DOI] [PubMed] [Google Scholar]
- Lee D.K., Cheng R., Nguyen T., Fan T., Kariyawasam A.P., Liu Y., Osmond D.H., George S.R., O'Dowd B.F. Characterization of apelin, the ligand for the APJ receptor. J. Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- Lee S. Molecular basis of Kell blood group phenotypes. Vox Sanguinis. 1997;73:1–11. doi: 10.1046/j.1423-0410.1997.7310001.x. [DOI] [PubMed] [Google Scholar]
- Lee S., Lin M., Mele A., Cao Y., Farmar J., Russo D., Redman C. Proteolytic processing of big endothelin-3 by the kell blood group protein. Blood. 1999;94:1440–1450. [PubMed] [Google Scholar]
- Lee S., Zambas E.D., Marsh W.L., Redman C.M. Molecular cloning and primary structure of Kell blood group protein. Proc. Natl. Acad. Sci. USA. 1991;88:6353–6357. doi: 10.1073/pnas.88.14.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letarte M., Vera S., Tran R., Addis J.B.L., Onizuka R.J., Quackenbush E.J., Jongeneel C.V., McInnes R.R. Common acute lymphocytic leukemia antigen is identical to neutral endopeptidase. J. Exp. Med. 1988;168:1247–1253. doi: 10.1084/jem.168.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N., Gordin M., Smith M.F., Bolden-Tiller O.U., Meidan R. Hormonal regulation and cell-specific expression of endothelin converting enzyme 1 isoforms in bovine ovarian endothelial and steroidogenic cells. Biol. Reprod. 2003;68:1361–1368. doi: 10.1095/biolreprod.102.009134. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman M.L., Panda P., Bennett H.P.J., Henderson J.E., Shane E., Shen Y., Goltzman D., Karaplis A.C. Cloning of human PEX cDNA: Expression, subcellular localization and endopeptidase activity. J. Biol. Chem. 1998;273:13729–13737. doi: 10.1074/jbc.273.22.13729. [DOI] [PubMed] [Google Scholar]
- Loeb M.J., De Loof A., Schoofs L., Isaac R.E. Angiotensin II and angiotensin-converting enzyme as candidate compounds modulating the effects of testis ecdysiotropin in testes of the gypsy moth, Lymantria dispar. Gen. Comp. Endocrinol. 1998;112:232–239. doi: 10.1006/gcen.1998.7169. [DOI] [PubMed] [Google Scholar]
- Luciani N., De Rocquigny H., Turcaud S., Romieu A., Roques P. Highly sensitive and selective fluorescence assays for rapid screening of endothelin-converting enzyme inhibitors. Biochem. J. 2001;356:813–819. doi: 10.1042/0264-6021:3560813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod K.J., Fuller R.S., Scholten J.D., Ahn K. Conserved cysteine and tryptophan residues of the endothelin-converting enzyme-1 CXAW motif are critical for protein maturation and enzyme activity. J. Biol. Chem. 2001;276:30608–30614. doi: 10.1074/jbc.M103928200. [DOI] [PubMed] [Google Scholar]
- Macours N., Vandingenen A., Gielens C., Hens K., Baggerman G., Schoofs L., Huybrechts R. Isolation of angiotensin converting enzyme from testes of Locusta migratoria. Eur. J. Entomol. 2003;100:467–474. [Google Scholar]
- Macours N., Hens K., Francis C., De Loof A., Huybrechts R. Molecular evidence for the expression of angiotensin converting enzyme in hemocytes of Locusta migratoria: Stimulation by bacterial lipopolysaccharide challenge. J. Insect Physiol. 2003;49:739–746. doi: 10.1016/s0022-1910(03)00110-0. [DOI] [PubMed] [Google Scholar]
- Macours N., Poels J., Hens K., Luciani N., De Loof A., Huybrechts R. An endothelin-converting enzyme homologue in the locust, Locusta migratoria: Functional activity, molecular cloning and tissue distribution. Insect Mol. Biol. 2003;12:233–240. doi: 10.1046/j.1365-2583.2003.00406.x. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Kuang W.J., Seeburg P.H., Mason A.J., Schofield P.R. Molecular cloning and amino acid sequence of human enkephalinase (neutral endopeptidase) FEBS Lett. 1988;229:206–210. doi: 10.1016/0014-5793(88)80828-7. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Swerts J.P., Guyon A., Roques B.P. High affinity enkephalin-degrading peptidase in brain is increased after morphine. Nature. 1978;276:523–526. doi: 10.1038/276523a0. [DOI] [PubMed] [Google Scholar]
- Masler E.P., Wagner R.M., Kovaleva E.S. In vitro metabolism of an insect neuropeptide by neural membrane preparations from Lymantria dispar. Peptides. 1996;17:321–326. doi: 10.1016/0196-9781(95)02098-5. [DOI] [PubMed] [Google Scholar]
- Menard J., Patchett A.A. Design of angiotensin converting enzyme inhibitors. Nat. Mcd. 2001;5:1110–1112. [Google Scholar]
- Metayer S., Dacheux F., Dacheux J.L., Gatti J.L. Germinal angiotensin I-converting enzyme is totally shed from the rodent sperm membrane during epididymal maturation. Biol Reprod. 2002;67:1763–1767. doi: 10.1095/biolreprod.102.006684. [DOI] [PubMed] [Google Scholar]
- Montuenga L.M., Prado M.A., Springall D.R., Polak J.M., Sesma P. Endothelin-like immunoreactivity in midgut endocrine cells of the desert locust, Locusta migratoria. Gen. Comp. Endocrinol. 1994;93:9–20. doi: 10.1006/gcen.1994.1002. [DOI] [PubMed] [Google Scholar]
- Muller L., Barret A., Etienne E., Meidans R., Valdenaire O., Corvol P., Tougard C. Heterodimerization of endothelin-converting enzyme-1 isoforms regulates the subcellular distribution of this metalloprotease. J. Biol. Chem. 2003;278:545–555. doi: 10.1074/jbc.M208949200. [DOI] [PubMed] [Google Scholar]
- Nelboeck P., Fuchs M., Bur D., Loffler B.M. Glycosylation of Asn-632 and Asn-651 is important for functional expression of endothelin-converting enzyme-1. J. Cardiovasc. Pharmacol. 1998;31:S4–S6. doi: 10.1097/00005344-199800001-00003. [DOI] [PubMed] [Google Scholar]
- Oefner C., D'Arcy A., Hennig M., Winkler F.K., Dale G.E. Structure of human neutral endopeptidase (neprilysin) complexed with phosphoramidon. J. Mol. Biol. 2000;269:341–349. doi: 10.1006/jmbi.1999.3492. [DOI] [PubMed] [Google Scholar]
- O'Shea M., Rayne R.C. Adipokinetic hormones: Cell and molecular biology. Experientia. 1992;48:430–438. doi: 10.1007/BF01928161. [DOI] [PubMed] [Google Scholar]
- Papandreou C.N., Usmani B.A., Geng Y., Bogenrieder T., Freeman R., Wilk S., Finstad C.L., Reuter V.E., Powell C.T., Scheinberg D., Magill C., Scher H.I., Albino A.P., Nanus D.M. Neutral endopeptidase 24.11 loss in metastatic human prostate cancer contributes to androgen-independent progression. Nat. Med. 1998;168:1247–1253. doi: 10.1038/nm0198-050. [DOI] [PubMed] [Google Scholar]
- Parkinson N., Richards E.H., Conyers C., Smith I., Edwards J.P. Analysis of venom constituents from the parasitoid wasp Pimpla hypochondriaca and cloning of a cDNA encoding a venom protein. Insect Biochem. Mol. Biol. 2002;32:729–735. doi: 10.1016/s0965-1748(01)00155-2. [DOI] [PubMed] [Google Scholar]
- Parnot C., Le Moullec J.M., Cousin M.A., Guedin D., Corvol P., Pinet F. A live-cell assay for studying extracellular and intracellular endothelin-converting enzyme activity. Hypertension. 1997;30:837–844. doi: 10.1161/01.hyp.30.4.837. [DOI] [PubMed] [Google Scholar]
- Prabakaran P., Xiao X., Dimitrov D.S. A model of the ACE2 structure and function as a SARS-CoV receptor. Biochem. Biophys. Res. Commun. 2004;314:235–241. doi: 10.1016/j.bbrc.2003.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley M.K. The diverse molecular mechanisms responsible for the actions of opioids on the cardiovascular system. Pharmacol Ther. 2002;93:51–75. doi: 10.1016/s0163-7258(02)00165-1. [DOI] [PubMed] [Google Scholar]
- Quan G.X., Mita K., Okano K., Shimada T., Ugajin N., Xia Z., Goto N., Kanke E., Kawasaki H. Isolation and expression of the ecdysteroid-inducible angiotensin-converting enzyme-related gene in wing discs of Bombyx mori. Insect Biochem. Mol. Biol. 2001;31:97–103. doi: 10.1016/s0965-1748(00)00112-0. [DOI] [PubMed] [Google Scholar]
- Raharjo S.B., Emoto N., Ikeda K., Sat R., Yokoyama M., Matsua M. Alternative splicing regulates the endoplasmatic reticulum localization or secretion of soluble secreted endopeptidase. J. Biol. Chem. 2001;276:25612–25620. doi: 10.1074/jbc.M101703200. [DOI] [PubMed] [Google Scholar]
- Ratcliffe N.A., Rowley A.F., Fitzgerald S.W., Rhodes C.P. Invertebrate immunity: Basic concepts and recent advances. Int. Rev. Cytol. 1985;97:186–350. [Google Scholar]
- Ramaraj P., Kessler S.P., Colmenares C., Sen G.C. Selective restoration of male fertility in mice lacking angiotensin-converting enzymes by sperm-specific expression of the testicular isozyme. J. Clin. Invest. 1998;102:371–378. doi: 10.1172/JCI3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings N.D., O'Brien E.A., Barrett A.J. MEROPS: The protease database. Nucleic Acids Res. 2002;30:343–346. doi: 10.1093/nar/30.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C.M., Russo D., Lee S. Kell, Kx and the McLeod syndrome. Baillieres Best Pract. Res. Clin. Haematol. 1999;12:621–635. doi: 10.1053/beha.1999.0045. [DOI] [PubMed] [Google Scholar]
- Richards E.H., Parkinson N.M. Venom from the endoparasitic wasp Pimpla hypochondriaca adversely affects the morphology, viability, and immune function of hemocytes from larvae of the tomato moth, Lacanobia oleracea. J. Invertebr. Pathol. 2000;76:33–42. doi: 10.1006/jipa.2000.4948. [DOI] [PubMed] [Google Scholar]
- Roques B.P., Beaumont A. Neutral endopeptidase 24.11 inhibitors: From analgesics to antihypertensives. Trends Pharmacol. Sci. 1993;11:245–249. doi: 10.1016/0165-6147(90)90252-4. [DOI] [PubMed] [Google Scholar]
- Rousseau A., Michaud A., Chauvet M.T., Lenfant M., Corvol P. The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J. Biol. Chem. 1995;270:3656–3661. doi: 10.1074/jbc.270.8.3656. [DOI] [PubMed] [Google Scholar]
- Ruchon A.F., Marcinkiewicz M., Siegfries G., Tenehouse H.S., DesGrosseilers L., Crine P., Boileau G. Pex mRNA is localized in developing mouse osteoblasts and odontoblasts. J. Histochem. Cytochem. 1998;46:459–468. doi: 10.1177/002215549804600405. [DOI] [PubMed] [Google Scholar]
- Russo D., Redman C., Lee S. Association of XK and kell blood group proteins. J. Biol. Chem. 1998;273:13950–13956. doi: 10.1074/jbc.273.22.13950. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Takuwa Y., Miyazaki H., Kimura S., Goto K., Masaki T. Cloning of a cDNA encoding a non-isopetide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Salzet M., Verger-Bocquet M. Elements of angiotensin system are involved in leeches and mollusks immune response modulation. Brain. Res. Mol. Brain. Res. 2001;94:137–147. doi: 10.1016/s0169-328x(01)00229-7. [DOI] [PubMed] [Google Scholar]
- Salzet M., Bulet P., Wattez C., Verger-Bocquet M., Malecha J. Structural characterization of a diuretic peptide from the central nervous system of the leech Erpobdella octoculata. Angiotensin II Amide. J. Biol. Chem. 1995;270:1575–1582. doi: 10.1074/jbc.270.4.1575. [DOI] [PubMed] [Google Scholar]
- Salzet M., Wattez C., Baert J.L., Malecha J. Biochemical evidence of angiotensin II-like peptides and proteins in the brain of the rhynchobdellid leech Theromyzon tessulatum. Brain Res. 1993;631:247–255. doi: 10.1016/0006-8993(93)91542-z. [DOI] [PubMed] [Google Scholar]
- Sansom C.E., Hoang M.V., Turner A.J. Molecular modelling and site-directed mutagenesis of the active site of endothelin-converting enzyme. Protein Eng. 1998;12:1235–1241. doi: 10.1093/protein/11.12.1235. [DOI] [PubMed] [Google Scholar]
- Sarras M.P., Jr., Yan L., Leontovich A., Zhang J.S. Structure, expression, and developmental function of early divergent forms of metalloproteinases in hydra. Cell Res. 2002;12:163–176. doi: 10.1038/sj.cr.7290123. [DOI] [PubMed] [Google Scholar]
- Schoofs L., Veelaert D., De Loof A., Huybrechts R., Isaac E. Immunocytochemical distribution of angiotensin I-converting enzyme-like immunoreactivity in the brain and testis of insects. Brain. Res. 1998;785:215–227. doi: 10.1016/s0006-8993(97)01398-x. [DOI] [PubMed] [Google Scholar]
- Schweizer A., Valdenaire O., Köster A., Lang Y., Schmitt G., Lenz B., Bluethmann H., Rohreh J. Neonatal lethality in mice deficient in XCE, a novel member of the endothelin-converting enzyme and neutral endopeptidase family. J. Biol. Chem. 1999;274:20450–20456. doi: 10.1074/jbc.274.29.20450. [DOI] [PubMed] [Google Scholar]
- Scriver C.R., Tenenhouse H.S. X-linked hypophosphataemia: A homologous phenotype in humans and mice with unusual organ-specific gene dosage. J. Inherited Metab. Dis. 1992;15:610–624. doi: 10.1007/BF01799618. [DOI] [PubMed] [Google Scholar]
- Shimada K., Matsushita Y., Wakabayashi K., Takahashi M., Matsubara A., Iijima Y., Tanzawa K. Cloning and functional expression of human endothelin-converting enzyme cDNA. Biochem. Biophys. Res. Commun. 1995;207:807–812. doi: 10.1006/bbrc.1995.1258. [DOI] [PubMed] [Google Scholar]
- Shimada K., Takahashi M., Turner A.J., Tanzawa K. Rat endothelin-converting enzyme-1 forms a dimer through Cys412 with a similar catalytic mechanism and a distinct substrate binding mechanism compared with neutral endopeptidase-24.11. Biochem. J. 1996;315:863–867. doi: 10.1042/bj3150863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirotani K., Tsubuki S., Iwata N., Takaki Y., Harigaya W., Maruyama K., Kiryu-Seo S., Kiyama H., Iwata H., Tomita T., Iwatsubo T., Saido T.C. Neprilysin degrades both amyloid beta peptides 1–40 and 1–42 most rapidly and efficiently among thiorphan- and phosphoramidon-sensitive endopeptidases. J. Biol. Chem. 2001;276:21895–21901. doi: 10.1074/jbc.M008511200. [DOI] [PubMed] [Google Scholar]
- Siviter R.J., Nachman R.J., Dani M.P., Keen J.N., Shirras A.D., Isaac R.E. Peptidyl dipeptidases (Ance and Acer) of Drosophila melanogaster: Major differences in the substrate specificity of two homologs of human angiotensin I-converting enzyme. Peptides. 2002;23:2025–2034. doi: 10.1016/s0196-9781(02)00190-0. [DOI] [PubMed] [Google Scholar]
- Skeggs L.T., Kahn J.R., Shumway N.P. The preparation and function of the hypertensin-converting enzyme. J. Exp. Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidgel R.A., Erdos E.G. Novel activity of human angiotensin I converting enzyme: Release of the NH2- and COOH-terminal tripeptides from the luteinizing hormone-releasing hormone. Proc. Natl. Acad. Sci. USA. 1985;82:1025–1029. doi: 10.1073/pnas.82.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S.S., Haffajee A.D. The bacterial etiology of destructive periodontal disease: Current concepts. J. Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- Söderhäll K., Cerenius L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998;10:23–28. doi: 10.1016/s0952-7915(98)80026-5. [DOI] [PubMed] [Google Scholar]
- Tanja O., Facchinetti P., Rose C., Bonhomme M.C., Gros C., Schwartz J.C. Neprilysin II: A putative novel metalloprotease and its isoforms in CNS and testis. Biochem. Biophys. Res. Commun. 2000;271:565–570. doi: 10.1006/bbrc.2000.2664. [DOI] [PubMed] [Google Scholar]
- Tatei K., Cai H., Ip Y.T., Levine M. Race: A Drosophila homolog of the angiotensin-converting enzyme. Mech. Dev. 1995;51:157–168. doi: 10.1016/0925-4773(95)00349-5. [DOI] [PubMed] [Google Scholar]
- The C. elegans Sequencing Consortium Genome sequence of the nematode C. elegans: A platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- Tikellis C., Johnston C.I., Forbes J.M., Burns W.C., Burrell L.M., Risvanis J., Cooper M.E. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension. 2003;41:392–397. doi: 10.1161/01.HYP.0000060689.38912.CB. [DOI] [PubMed] [Google Scholar]
- Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- Towler P., Staker B., Prasad S.G., Menon S., Tang J., Parsons T., Ryan D., Fisher M., Williams D., Dales N.A., Patane M.A., Pantoliano M.W. ACE2 structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 2004;279:17996–18007. doi: 10.1074/jbc.M311191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A.J., Hooper N.M. The angiotensin-converting enzyme gene family: Genomics and pharmacology. Trends Pharmacol. Sci. 2002;23:177–183. doi: 10.1016/s0165-6147(00)01994-5. [DOI] [PubMed] [Google Scholar]
- Turner A.J., Tanzawa K. Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. The Faseb Journal. 1997;11:355–364. doi: 10.1096/fasebj.11.5.9141502. [DOI] [PubMed] [Google Scholar]
- Turner A.J., Hryszko J., Hooper N.M., Dowdall M.J. Purification and characterization of a peptidyl dipeptidase resembling angiotensin converting enzyme from the electric organ of Torpedo marmorata. J. Neurochem. 1987;48:910–916. doi: 10.1111/j.1471-4159.1987.tb05603.x. [DOI] [PubMed] [Google Scholar]
- Turner A.J., Isaac R.E., Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: Genomics and function. Bioessays. 2001;23:261–269. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Turner A.J., Tipnis S.R., Guy J.L., Rice G., Hooper N.M. ACEH⧸ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors. Can. J. Physiol. Pharmacol. 2002;80:346–353. doi: 10.1139/y02-021. [DOI] [PubMed] [Google Scholar]
- Valdenaire O., Lepailleur-Enouf D., Egidy G., Thouard A., Barret A., Vranckx R., Tougard C., Michel J.B. A fourth isoform of endothelin-converting enzyme (ECE-1) is generated from an additional promoter molecular cloning and characterization. Eur. J. Biochem. 1999;264:341–349. doi: 10.1046/j.1432-1327.1999.00602.x. [DOI] [PubMed] [Google Scholar]
- Valdenaire O., Richards J.G., Faull R.L., Schweizer A. XCE, a new member of the endothelin-converting enzyme and neutral endopeptidase family, is preferentially expressed in the CNS. Brain Res. Mol. Brain Res. 1999;64:211–221. doi: 10.1016/s0169-328x(98)00321-0. [DOI] [PubMed] [Google Scholar]
- Valdenaire O., Rohrbacher E., Mattei M.G. Organization of the gene encoding the human endothelin-converting enzyme (ECE-1) J. Biol. Chem. 1995;270:29794–29798. doi: 10.1074/jbc.270.50.29794. [DOI] [PubMed] [Google Scholar]
- Vandenbulcke F., Laurent V., Verger-Bocquet M., Stefano G.B., Salzet M. Biochemical identification and ganglionic localization of leech angiotensin-converting enzymes. Brain. Res. Mol. Brain Res. 1997;49:229–237. doi: 10.1016/s0169-328x(97)00146-0. [DOI] [PubMed] [Google Scholar]
- Vandingenen A., Hens K., Baggerman G., Macours N., Schoofs L., De Loof A., Huybrechts R. Isolation and characterization of an angiotensin converting enzyme substrate from vittelogenic ovaries of Neobellieria bullata. Peptides. 2002;23:1853–1863. doi: 10.1016/s0196-9781(02)00144-4. [DOI] [PubMed] [Google Scholar]
- Vandingenen A., Hens K., Macours N., Schoofs L., De Loof A., Huybrechts R. Presence of an angiotensin converting enzyme (ACE) interactive factors in ovaries of the grey fleshfly Neobellieria bullata. Comp. Biochem. Phys. 2002;132:27–35. doi: 10.1016/s1096-4959(01)00529-2. [DOI] [PubMed] [Google Scholar]
- Vandingenen A., Hens K., Macours N., Zhu W., Janssen I., Breuer M., De Loof A., Huybrechts R. Captopril, a specific inhibitor of angiotensin converting enzyme, enhances both trypsin and vitellogenin titers in the grey fleshfley, Neobellieria bullata. Arch. Insect Biochem. Physiol. 2001;132:27–35. doi: 10.1002/arch.1047. [DOI] [PubMed] [Google Scholar]
- Van Sambeek J., Wiesner A. Successful parasitation of locusts by entomopathogenic nematodes is correlated with inhibition of insect phagocytes. J. Invertebr. Pathol. 1999;73:154–161. doi: 10.1006/jipa.1998.4823. [DOI] [PubMed] [Google Scholar]
- Veelaert D., Schoofs L., Macours N., Vandingenen A., De Loof A., Isaac E., Salzet M., Huybrechts R. Immonocytochemical distribution of angiotensin-I converting enzyme in the central nervous system of insects and speculations about its possible function. Eur. J. Entomol. 1999;96:323–326. [Google Scholar]
- Ventura C., Spurgeon H., Lakatta E.G., Guarnieri C., Capogrossi M.C. Kappa and delta opioid receptor stimulation affects cardiac myocyte function and Ca2+ release from an intracellular pool in myocytes and neurons. Circ. Res. 1992;70:66–81. doi: 10.1161/01.res.70.1.66. [DOI] [PubMed] [Google Scholar]
- Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J., Godbout K., Parsons T., Baronas E., Hsieh F., Acton S., Patane M., Nichols A., Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan J., Scicli A.G., Carretero O.A., Slaughter C., Moomaw C., Hersh L.B. The hydrolysis of endothelins by neutral endopeptidase 24.11 (enkephalinase) J. Biol. Chem. 1990;265:14150–14155. [PubMed] [Google Scholar]
- Wei L., Alhenc-Gelas F., Corvol P., Clauser E. The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J. Biol. Chem. 1991;266:9002–9008. [PubMed] [Google Scholar]
- Wenzlaff H., Stein B., Teschemacher H. Diminution of contractile response by kappa-opioid receptor agonists in isolated rat ventricular cardiomyocytes is mediated via a pertussis toxin-sensitive G protein. Naunyn Schmiedebergs Arch. Pharmacol. 1998;358:360–366. doi: 10.1007/pl00005265. [DOI] [PubMed] [Google Scholar]
- Wijffels G., Fitzgerald C., Gough J., Riding G., Elvin C., Kemp D., Willadsen P. Cloning and characterisation of angiotensin-converting enzyme from the dipteran species, Haematobia irritans exigua, and its expression in the maturing male reproductive system. Eur. J. Biochem. 1996;237:414–423. doi: 10.1111/j.1432-1033.1996.0414k.x. [DOI] [PubMed] [Google Scholar]
- Wijffels G., Gough J., Muharsini S., Donaldson A., Eisemann C. Expression of angiotensin-converting enzyme-related carboxydipeptidases in the larvae of four species of fly. Insect Biochem. Mol. Biol. 1997;27:451–460. doi: 10.1016/s0965-1748(97)00020-9. [DOI] [PubMed] [Google Scholar]
- Williams T.A., Michaud A., Houard X., Chauvet M.T., Soubrier F., Corvol P. Drosophila melanogaster angiotensin I-converting enzyme expressed in Pichia pastoris resembles the C domain of the mammalian homologue and does not require glycosylation for secretion and enzymic activity. Biochem. J. 1996;318:125–131. doi: 10.1042/bj3180125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C.L., Shirras A.D., Isaac R.E. Extracellular peptidases of imaginal discs of Drosophila melanogaster. Peptides. 2002;23:2007–20014. doi: 10.1016/s0196-9781(02)00188-2. [DOI] [PubMed] [Google Scholar]
- Xiao X., Chakraborti S., Dimitrov A.S., Gramatikoff K., Dimitrov D.S. The SARS-CoV S glycoprotein: Expression and functional characterization. Biochem. Biophys. Res. Commun. 2003;312:1159–1164. doi: 10.1016/j.bbrc.2003.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H., Hammer R.E., Richardson J.A., Emoto N., Williams S., Takeda D.E., Clouthier D.E., Yanagisawa M. Disruption of ECE-1 and ECE-2 reveals a new role for endothelin-converting enzyme 2 in murine cardiac development. J. Clin. Invest. 2000;105:1373–1382. doi: 10.1172/JCI7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H., Yanagisawa M., Kapur R.P., Richardson J.A., Williams S.C., Clouthier D.E., De Wit D., Emoto N., Hammer R.E. Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development. 1998;125:825–836. doi: 10.1242/dev.125.5.825. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yang H.Y.T. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim. Biophys. Acta. 1970;214:374–376. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]
- Yasojima K., Akiyama H., McGeer E.G., McGeer P.L. Reduced neprilysin in high plaque areas of Alzheimer brain: A possible relationship to deficient degradation of beta-amyloid peptide. Neurosci. Lett. 2001;297:97–100. doi: 10.1016/s0304-3940(00)01675-x. [DOI] [PubMed] [Google Scholar]
- Yasojima K., McGeer E.G., McGeer P.L. Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 2001;919:115–121. doi: 10.1016/s0006-8993(01)03008-6. [DOI] [PubMed] [Google Scholar]
- Zappulla J.P., Wickham L., Bawab W., Yang X.F., Storozhuk M.V., Castellucci V.F., DesGroseillers L. Cloning and characterization of Aplysia neutral endopeptidase, a metallo-endopeptidase involved in the extracellular metabolism of neuropeptides in Aplysia californica. J. Neurosci. 1999;19:4280–4292. doi: 10.1523/JNEUROSCI.19-11-04280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Mita K., Shimada T., Okano K., Quan G.X., Kanke E., Kawasaki H. Isolation and expression of an ecdysteroid-inducible neutral endopeptidase 24.11-like gene in wing discs of Bombyx mori. Insect Biochem. Mol. Biol. 2001;31:1213–1219. doi: 10.1016/s0965-1748(01)00069-8. [DOI] [PubMed] [Google Scholar]
- Zhang J., Leontovich A., Sarras M.P., Jr. Molecular and functional evidence for early divergence of an endothelin-like system during metazoan evolution: Analysis of the Cnidarian, Hydra. Development. 2001;128:1607–1615. doi: 10.1242/dev.128.9.1607. [DOI] [PubMed] [Google Scholar]
- Zhu W., Vandingenen A., Huybrechts R., Baggerman G., De Loof A., Poulos C.P., Velentza A., Breuer M. In vitro degradation of the Neb-Trypsin Modulating Oostatic Factor (Neb-TMOF) in gut luminal content and hemolymph of the grey fleshfly, Neobellieria bullata. Insect. Biochem. Mol. Biol. 2001;31:87–95. doi: 10.1016/s0965-1748(00)00111-9. [DOI] [PubMed] [Google Scholar]


