Abstract
Viruses are real menace to human safety that cause devastating viral disease. The high prevalence of these diseases is due to improper detecting tools. Therefore, there is a remarkable demand to identify viruses in a fast, selective and accurate way. Several biosensors have been designed and commercialized for detection of pathogenic viruses. However, they present many challenges. Nanotechnology overcomes these challenges and performs direct detection of molecular targets in real time. In this overview, studies concerning nanotechnology-based biosensors for pathogenic virus detection have been summarized, paying special attention to biosensors based on graphene oxide, silica, carbon nanotubes, gold, silver, zinc oxide and magnetic nanoparticles, which could pave the way to detect viral diseases and provide healthy life for infected patients.
Keywords: Virus, Biosensor, Nanomaterial, Electrochemistry, Optical detection
Highlights
-
•
Merits and demerits of conventional virus sensing methods have been compared.
-
•
Principles of bio-nano sensors and immune sensors have been defined.
-
•
The role of developing nanotechnology and nanostructured materials in virus detection is undeniable.
-
•
Illustrating various nano biosensors, coating with nanoparticles was our priority.
-
•
Virus bio- and immune-sensors have been classified based on nanoparticles.
1. Introduction
Viruses are the smallest infectious agents that can cause many diseases such as influenza, chicken pox, flu, acquired immune deficiency syndrome (AIDS), severe acute respiratory syndrome (SARS), Ebola and etc. Viruses are intracellular parasites unlike other microorganisms. These agents have only a few number of responsible genes for the synthesis of new viruses and most of them used different enzymes which created by the host cell [1]. So, fast and well-timed diagnosis of these diseases is very important due to the limited treatment options. Virus detection usually requires specific methodologies and equipment as: cell culturing, antigen or antibody detection (ELISA, immunofluorescence, immunoperoxidase) hemagglutination assay, nucleic acid detection (Polymerase chain reaction) and gene sequencing [2], [3]. Most of these methods are time consuming, labor intensive and often expensive. Table 1 offers a comparison of advantages and limitations of some of the most commonly used methods for virus detection. Therefore, we need rapid, simple, sensitive, and accurate methods for detection of pathogenic agents [4]. So in order to find alternative methodologies without the mentioned limitations, recently there has been growing attention for using biosensors in biomedical applications based on the advantages of these methods such as high speed results, excellent sensitivity and suitable selectivity [5]. Moreover, since viral and bacterial diseases are real menaces to human life, there is a growing demand to detect them meticulously by advanced methods [6]. Biosensors are highly accurate, sensitive and specific measurement systems that can determine very low analyte concentrations in biological samples. A biosensor is an analytical device which made up biological elements; such as microorganisms, organelles, cell receptor, enzymes, antibodies, nucleic acids, etc. that produces signals (electrical, optical or thermal) based on the interaction with tested element and a transducer that converts these signals into a measurable electrical parameter [7]. Due to the use of specific biological elements, these measurement systems have unique properties for the identification and measurement of target analytes. The importance of biosensor components is to assure a high level of specificity based on particular binding sites. Different medical and clinical applications are expected for biosensors such as: i) fast diagnosis and treatment of illness like cancer or diabetes, ii) detection of pathogens, iii) measurement of drugs and their metabolites, iv) discovery of new drugs and v) evaluation of drug activity, vi) assessment and measurement of analytes in biological samples and early detection of diseases using rapid tests [7], [8], [9].
Table 1.
Comparison of common virus detection methods.
| Technique | Detection principle | Time | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| Cell culture | Infectivity assay | Days to weeks | Broad spectrum; inexpensive | Difficulty in maintaining cell cultures; Contamination problems. | [10] |
| Electron microscopy | Viral particle | Hours | Broad spectrum; rapid method | Necessity for presence of around 106 virus particles/mL for detection; similarity of morphologies | [11] |
| Hemagglutination assay | Viral protein | Hours | Easy; inexpensive | Poor sensitivity; necessity for fresh reagents | [12] |
| ELISA | Viral protein | Hours | Only one incubation step; no hook effect at high analyte concentrations | Limited concentration range in which the analyte can be quantified without sample dilution; and that the antigen or antibody produce the same response and not distinguishable in a one step | [13] |
| PCR | Viral nucleic acid | Hours | Extremely high sensitivity; Easy to set up | Extremely liable to contamination; Not easy to quantitate results; High degree of operator skill required | [14] |
Considering special properties (physical, chemical, mechanical, magnetic, etc.), various kinds of nanomaterials, such as gold nanoparticles, carbon nanotubes (CNTs), magnetic nanoparticles and quantum dots (QDs), are being merged to biosensors.
2. Nano-material based biosensors
Recent developments in nanoscience and nanotechnology and the possibility of making electrodes on a very small scale, nanoscale sensors make possible and resulted in a new set of diagnostic biosensors called Nano-biosensors. Continuous decrease in material dimension, from large scale to small one in the range between 1 and 100 nm, does not change biosensor properties but significantly improves their applicability. The surface interaction of the sensors with analyte becomes highly efficient due to the extremely large surface to volume ratios in Nano-size devices [15]. Therefore, nanoscale materials show unique features, functionality, and effects. Nowadays, nanotechnology is focused on the elimination of disadvantages of existing methods for virus detection to minimize costs and time consuming. Furthermore, the use of nanomaterials in the construction of biosensors resulted in increasing of efficiency and sensitivity of these systems [16]. By designing the interaction between biological element and nanomaterial-based transducer, we can have biosensors by widely usage for the detection of biomolecules and disease diagnostics. These devices can detect patients physiological status at the moment and safety analyze the food and environmental samples for pesticides and water pollution quickly [17]. Several nanomaterials; such as nanorods, nanotubes, nanowires, thin films, and nanoparticles have been explored for biomedical applications due to their functional electrical and mechanical features for biomedical applications [18].
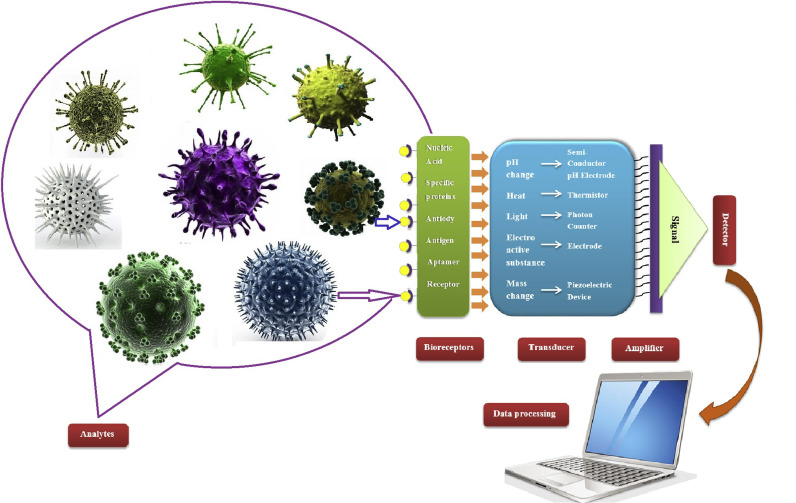
In this review, we will focus on the use of different nano-materials such as Quantum dots, Carbon nanotubes, Graphene oxide, silica and metal nanoparticles for the fabrication of pathogenic virus biosensors (Scheme 1 ).
Scheme 1.
Schematic diagram of biosensor for detection of pathogenic viruses (analyte).
3. Quantum dots (QDs)
QDs are nanoscale semiconductor crystals (with a diameter of 2–10 nm) with unique optical and electrical properties. QDs have high versatility because of their small size. QDs includes: 1) a core that made up of group II–VI atoms, e.g. cadmium selenide (CdSe), or group III–V atoms of the periodic table, like indium phosphide, 2) a shell made of another semiconductor that covers the core, in most cases zinc sulfide (ZnS), to improve optical properties and increase stability and reduce cytotoxicity and 3) an organic coating applied to change nanoparticle into hydrophilic compound to become a place to connect of biomolecules; such as, oligonucleotides, proteins, peptides and also small molecules [19].
By using ultraviolet radiation to QDs, different wavelengths of visible light are emitted from them. The emitted wavelength depends on the size of the QDs, the distance between energy bands in small QDs being higher than in larger ones. So, by illuminating small QDs with ultraviolet radiation, electrons move to high energy bar and loss additional energy by return to a steady state. Finally, visible light emitted from them has high energy and tends to blue. Also by using ultraviolet radiation to large QDs, visible light is emitted from them, in the reddish range, due to the loss of excess energy and return to a steady state, because of the small energy gap and less energy losing. So, by enlarging the size of the QDs, the light spectrum tends toward red from blue [20].
Growing interest for chemical and biological detection by Quantum dot (QD)-based sensors, leads to development of several ways to prepare QDs such as colloidal synthesis, plasma synthesis, viral assembly, electrochemical assembly, etc. Since the QDs have unique properties when compared to organic fluorescent dyes, such as wide absorption range, symmetric size photoluminescence, in addition to broad excitation range, multiplexed staining, long florescent lifetime and high resistance to photo bleaching, Therefore, bioconjugation of these nano-particles to sensor structure has an increasing interest.
QDs light emission is a useful property for a lot of medical labeling, imaging or sensing applications such as to distinguish between normal and tumor cells [21] gene therapy studies [22], proteomics [23], etc. From the perspective of virology, QDs are useful tools for providing rapid and sensitive virus detection to facilitate early treatment and monitoring of viral disease. Additionally, the combination of QDs and platforms with immobilized antibodies results in high sensitivity and selectivity [6].
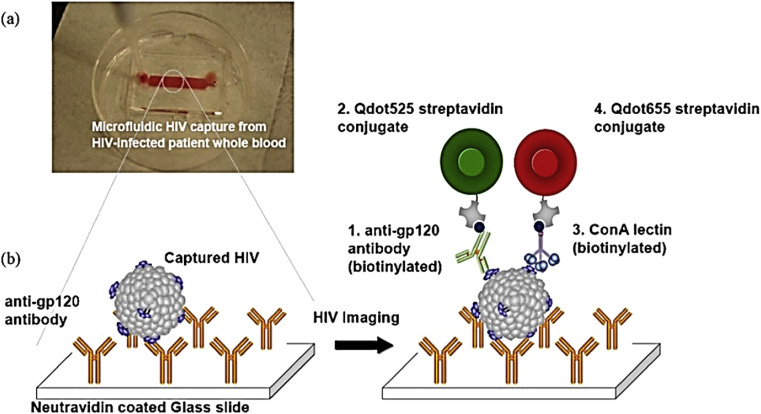
As an example for HIV, a type of virus that gradually attacks the immune system and makes it harder to fight off infections and diseases in infected body, a QDs-based rapid capture and imaging system was developed by Kim et al. This method is a type of dual-stain imaging technique, based on streptavidin conjugated Qdot525 and Qdot655, for HIV1 gp120 envelope glycoprotein and its high-mannose glycan capturing by an anti-gp120 antibody, immobilized on a microfluidic chip (see Fig. 1 ). The capabilities of this system are not only selective for capturing and detecting HIV virus in whole blood but also makes possible to obtain countable imaging. Considering the analytical features, it provides high detection speed, due to the lack of blood sample pre-processing (<10 min), even with 10 μL of blood sample, cost effectively, and portability, thus providing a new and effective tool for valid identification of HIV [24].
Fig. 1.
Schematic illustration of HIV capturing/imaging strategy using dual-stain imaging technique based on streptavidin conjugated Qdot525 and Qdot655 for detection of HIV1 gp120 antigen.
This figure was obtained with permission from Ref. [24].
Wang et al. developed a FRET-based QDs-DNA system for rapid, easy and sensitive detection of HBV-DNA and single-base mutation of this virus that presents increased benefits when virus try to become resistance to drug treatment. Bioconjugation of DNA to CdSe/ZnS QDs accrued by carbodiimide cross-linker chemistry in this method, and after addition of Cy5-modified signal DNAs and complementary DNA targets into the QDs-DNA conjugates. A sandwiched hybrids with Cy5 fluorophore, as the acceptor, and QDs, as the donor of the fluorescence resonance energy transfer system, were performed. The Oligonucleotide ligation assay was used for detection of single base mutation in this system which ligase recognize a mismatch base. Therefore, the ligation will not make and, subsequently, there is no Cy5 emission. The validity of this system was examined by synthetic 30-mer oligonucleotide targets of HBV DNA with a sensitivity of 4.0 nM by a multilabel counter [25].
Krejcova and coworkers [29] proposed an influenza virus detection sensor as a pandemic threat by using a 3D microfluidic chip, applied for influenza hemagglutinin. This method was based on two different steps, (i) specific isolation, after labeling HA with CdS-QDs, and conjugate them on a surface of glycan-modified MPs and (ii) voltammetry based detection step. Considering unique properties of QDs, these nanoparticles are a key part of this system that makes it a rapid sensitive and specific way for detection of isolated influenza virus. Table 2 summarizes some of biosensing methods for detection of viruses based on the use of various types of QDs.
Table 2.
QDs-based biosensors employed for detection of viruses.
| Virus | Impact on health | Biosensor type | Interaction cite | Labeling site | QDs type | Labeling propose | Reference |
|---|---|---|---|---|---|---|---|
| HBV | Incapable of infecting mammalians | Optical | Virus nucleocapsid | RBV | SA-QDs | Single-virus tracking | [26] |
| EBV | Infectious mononucleosis; malignancies | Optical | Carcinoma cells membrane | Anti-EBV capsid antigen IgA | CdTe@dBSA-QDs | Early screening and diagnosing EBV-associated cancers | [27] |
| ALVs-J | Neoplastic diseases | Electrochemical | Envelope | Ati- ALVs-J-Ab2 | GQDs | Virus detection | [28] |
| Influenza Vaxigrip® | Inactivated trivalent influenza vaccine | Electrochemical | Envelope | HA vaxi | CdTe –QDs | Virus vaccine isolation and detection | [29] |
In short, according to their unique optical properties, which covered a wide range of light absorbance, QDs are one of the best choices for the design of sensing instruments, especially optical ones. Another noticeable aspect about these nanoparticles is their biocompatibility, which make them suitable for sensing samples in biological environments. Also, in some cases, the detection process could be very rapid.
4. Carbon based nanomaterials
4.1. Graphene oxide (GO) and reduced graphene oxide (RGO)
In conclusion, between carbon based nanostructures, graphene oxide is the best choice which introduced to detecting systems due to especial variety of properties like having natural source, biocompatible and cost effective. One of the eye catching advantages of this carbon-based nanostructure is the ability of surface treatment to be a good hostage for immobilizing of ligands, nanoparticles and single-strand DNA in aptasensors.
Graphene is a well-known carbon based material that is made by mechanical exfoliation of graphite [30]. GO, which includes carboxylic, phenol hydroxyl and epoxide groups, can be produced from graphite oxide. Beside unique electronic, mechanical and thermal properties of GO, some noticeable applications in nanosensors and nanomedicine, make these materials in the center of attention [31]. Size controllability of GO nano sheets and changes in oxidation level are other interesting properties of these carbon based materials [32]. Recently a research has been conducted by Hu et al. that shows the antibacterial activity of graphene oxide towards Escherichia coli [33]. These compounds have been used in sensors, especially for the detection of biomolecules. QDs fluorescence quenching has been employed, together with GO, in an optical biosensing platform [34]. This property of GO leads to use of them in identifying some especial viruses.
Nowadays there is a high demand of novel methods of diagnosing and disinfecting of viruses, due to the low specificity and sensitivity of traditional diagnostic methods [35]. One of the novel tools for virus diagnostics is based on the use of bio and immune sensors which employed nanotechnology in their structure [36].
The World health organization (WHO) reported that H5N1 influenza virus has caused infection of 650 people and death of 386 since 2003 [37] and it is a good example on the need of sensitive and selective methods for detection of viruses. As an example, Xie and colleagues proposed an immunosensor for detection of viruses based on GO [38]. According to this report, this immunosensor improved virus sensing based on immobilization of H5-polychonal antibody (PAb) on GO. The nanocomposite leaded to amplify the signals. In this electrochemical immunosensor, for the loading of GO-PAb-BSA nanocomposite on gold electrode, thiourea, gold nanoparticles, H5 antigens and H5-monoclonal antibodies (MAb) were used as linkers (Fig. 2 ). The limit of detection (LOD) and linear range were respectively 2−15 HA unit/50 μL and 2−15 to 2−8 HA unit/50 μL.
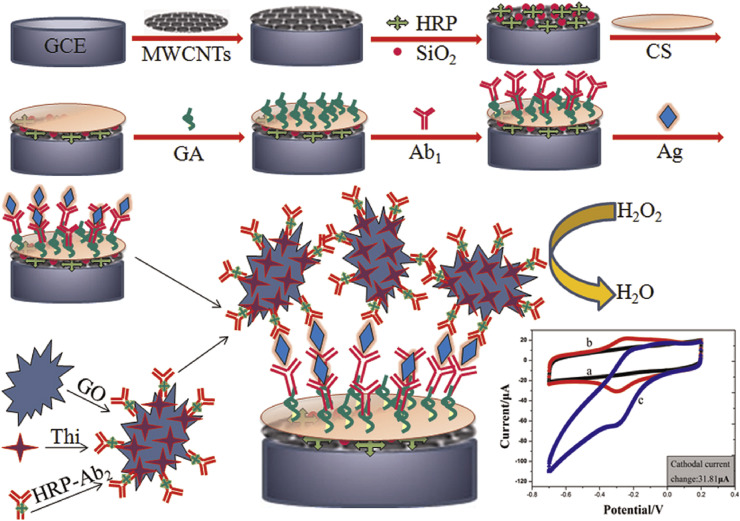
Fig. 2.
Schematic process of preparing modified electrode by encapsulation of enzyme in silica matrix.
This figure was obtained with permission from Ref. [39].
Another procedure was used to enhance the sensitivity of HIV virus detection. Wang et al. synthesized an electrochemical biosensor based on a nanocomposite which contained GO and carbon nanotubes to employ it in electrochemical biosensor [39]. One of the positive point in this modified electrode was the encapsulation of horseradish peroxidase enzyme by silica-carbon nanotubes, which grafted to GO (Fig. 2), which enhanced the sensitivity, the recoverability and stability of the system to be used to made measurements in human plasma. The LOD was 0.15 pg mL−1, that showed a progress in comparing with traditional and common electrochemical procedures, and the linear range was 0.5 pg mL−1 to 8.5 ng mL−1.
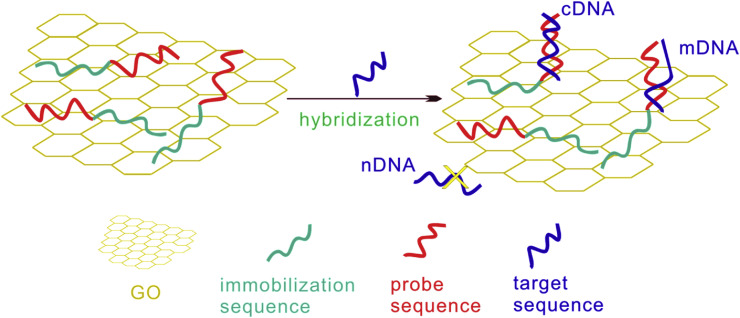
One of the unique properties of GO is the ability of being host of stranded DNA to make aptasensors. A research conducted by Bao et al. [40] about immobilizing DNA strand on GO provided the way to make a label free aptasensor for detection of viruses This novel procedure for the designing of sensors had some significant points; (i) There was no need to decoration of GO towards immobilization, (ii) Comparing with other apatasensors, it was unnecessary to label DNA. (iii) The device was also used for the biosensing of nanostructures. Two parts of DNA were used in this study, probe part and immobilization part. In the presence of human immune-deficiency virus (HIV), the probe part was linked to double helix DNA. Therefore, negative charge was formed on GO which was detected by electrochemical impedance spectroscopy (Fig. 3 ). In this assay, the linear range and LOD were 10−12 to 10−6 M and 1.1 × 10−13, respectively.
Fig. 3.
Process of DNA hybridization on GO to build a modified electrode.
This figure was obtained with permission from Ref. [40].
Recently functionalized GO has been used in biosensors for enhancement of sensitivity. Seo and coworkers [41] used GO, decorated by AuNPs, to make the GO sheets suitable for being a photoluminescence quencher. The target in this biosensor was rotavirus and the amine groups were the linkage of Go and antibody. Due to the unique structure of modified electrode in this biosensor, selectivity and sensitivity were impressive. LOD of this biosensor was 105 pfu mL−1. To sum up, GO based biosensors decorated with nanoparticles, like AuNPs, are more precise to detect viruses than conventional methods.
Between carbon based nanostructures, graphene oxide seems to be the best choice to be employed in detection systems due to their especial variety of properties as their natural source, biocompatibility and cost effective relationship. One of the eye catching advantages of this carbon-based nanostructures is the ability of surface treatment to provide a good hostage for immobilizing of ligands, nanoparticles and single-strand DNA in aptasensors (see Table 3 ).
Table 3.
GO based biosensors for detection of viruses.
| Virus | Impact on health | Biosensor type | Bio receptor | LOD | Linear range | Reference |
|---|---|---|---|---|---|---|
| HBV | Incapable of infecting mammals and plants; | Electrochemical | Aptamer | 2.02 μM | 20–160 μg mL−1 | [42] |
| HIV | AIDS | Optical | Aptamer | 0.1 ng mL−1 | 0.01 μg mL−1 to 10 ng mL−1 | [43] |
| HIV-1 | AIDS | Optical | Aptamer | 0.1 pM | 0.1 pM to 10 nM | [44] |
| Rotavirus | Diarrhoeal disease | Electrochemical | Antibody | 103 pfu mL−1 | – | [45] |
| HIV | AIDS | Optical | Antibody | 2 nM | 5–150 nM | [46] |
4.2. Carbon nanotubes (CNTs)
According to specific properties and activities; such as thermal, electrical, chemical and mechanical behavior, CNTs provided a great area of scientific research. The biomedical utility of these carbon based nanomaterials is of special interest in the field of biosensors. CNTs are playing an important role in preparing biosensors which can detect target molecules in trace amounts. This powerful aspect of CNTs is sourced from transduction of physical/chemical interactions and high surface area-to-volume ratio [47].
In the case of virus detection, Pu et al. [48] used the ability of surface decorating of CNTs for making them ready to immobilize on gold sheets to synthesize a modified electrode. Horseradish peroxidase enzyme was set on carbon nanotubes which contain capture probes, and then joined to the gold layers by Au nanoparticles. This biosensor was used for the detection of HIV virus effects in human body by investigation of Nuclear paraspeckle assembly transcript 1 (NEAT1). Because of unique structure designing in this biosensor, the target capturing was very high due to the great surface area of CNTs. The LOD was considerably low (0.8863 fM mL−1). Also the linear calibration ranged from 1 fM mL−1 to 100 nM mL−1.
In another study which has been done recently by Yang et al. [49] DNA enzyme of hemin/G-quadruplex was produced which trigger oxidation of the 3,3′,5,5′-tetramethylbenzidine (TMB). This biosensor was based on detection of hepatitis B virus (HBV) DNA portion. The result of setting AuNPs in the structure was responsible for linking hairpin capture probes to the carbon based nanocomposite (GO–carboxyl multi-walled carbon nanotube), and provided the enhancement in amplification effects with a linear range from 10 pM to 10 nM. Due to selective identifying between DNA of target molecules from those which were incompatible in one or two-base, the specificity and selectivity of this sensor was strongly high. Also the LOD achieved for the target DNA was about 0.5 pM.
To prepare a modified CNT-based electrode containing AuNPs, electrochemical impedance was used by Wang et al. [50] to deposit AuNPs on single walled carbon nanotube (SWCNTs) previously prepared by in situ procedure. The target molecules, which were the DNA of hepatitis B and papilloma virus, were captured by probe DNA (single stranded (ssDNA)) and immobilized on SWCNTs/Au. The experimental LOD for the hepatitis B and papilloma virus were respectively 0.1 pmol and 1 amol. Early diagnosis of diseases related to gene and high sensitivity and specificity are some of the advantages of combination of AuNPs and SWCNTs. The LOD of SWCNTs/Au/ssDNA biosensor for detection of hepatitis B and papilloma virus were 0.1 pM and 1 aM, respectively. So, it can be seen that carbon nanoparticles provide a simple, rapid and precise sensing method to recognize viruses.
CNT-based systems have introduced a new generation of biosensors which resulted in high sensitivity and selectivity according to their high surface area, the ability of easy functionalization and the fact that they provide a good hostage for immobilizing nanoparticles.
5. Silica nanoparticles (SiNPs)
Silica nanoparticles have wide surface area, stability in critical thermal and chemical conditions, good compatibility with biomolecules, like proteins, being green about environmental challenges [55]. There are many biomolecules which could be linked to silica nanoparticles, including antigen-antibodies, peptides and DNA and that, subsequently, make these nanomaterials as unique elements to be incorporated to bio- and immunosensors. In addition to their biocompatibility, some considerable properties about optoelectronic aspects such as visible luminescence activities [56], make them important in bioanalytical research.
The new generation of biosensors, called microcantilevers, have shown outstanding applications in viruses detection. The research group of Kim et al. used microcantilevers for the detection of Hepatitis B Virus (HBV) [57]. In this research, a high sensitivity was obtained being improved the limit of detection from picomolar (pM) HBV target DNA in the absence of SiNPs till femtomolar (fM) level in the presence of nanoparticles.
Due to harmful effect of viruses in our body, it is necessary to detect some species of them before being late. For example Epstein–Barr virus-derived latent membrane protein 1 (LMP-1) make disturbances in morphology and growing of cells which causes irreparable effects in human body [58]. A research was done by Liu et al. about detection of this virus using a multi nanolayer structure to enhance the sensitivity of the detection [59]. Using silica nanospheres together with QDs of cadmium telluride (CdTe/QDs) and the possibility of bringing these nanoparticles on gold layer it was prepared a biosensor to amplify the signals in order to obtain a limit of detection of 1 pg mL−1 and a linear range of 0.001–10 ng mL−1.
Hybridization of organic and inorganic compounds is another solution for improving biosensors applications. Enrichi et al. [60] combined SiNPs with commercial QDs and organic dyes in the structure of a biosensor. Result was significant about sensitivity in order to achieve a sensor to detect traces of targets. A DNA microarray was added to this structure for improving selectivity and increasing optical signal. They compared fluorescence activity of QDs, silica NPs and free dyes separately, the best LOD was related to the dye doped silica nanoparticles. The detection limit for free dyes, QDs and dye doped SiNPs were 150, 250 and 20 pM, respectively. Table 4 summarizes applications of SiNPs on the construction of different biosensors for viruses detection.
Table 4.
CNTs based biosensors for detection of viruses.
| Virus | Impact on health | Biosensor type | Bio receptor | LOD | Linear range | Reference |
|---|---|---|---|---|---|---|
| HBV | Incapable of infecting mammals and plants | Electrochemical | DNA | 1.1 × 10−8 M | 7.94 × 10−8 M and 1.58 × 10−6 M | [51] |
| Influenza | Causes an acute respiratory disease and fever, lethargy, nasal, discharge, coughing, and dyspnea | Electrochemical | DNA aptamer | 4.3 × 10−13 | 5.0 × 10−12 to 1.0 × 10−9 M | [52] |
| HIV | AIDS | Electrochemical | lncRNA NEAT1 | 0.8863 fM mL−1 | 1 fM mL−1 to 100 nM mL−1 | [48] |
| HBV | Incapable of infecting mammals and plants | Electrochemical | DNA | 0.5 pM | 10 pM to 10 nM | [49] |
| Influenza | Causes an acute respiratory disease and fever, lethargy, nasal, discharge, coughing, and dyspnea | Electrochemical | DNA | 0.5 nM | – | [53] |
| Cauliflower mosaic virus | Incapable of infecting plants | Electrochemical | DNA | 8.5 × 10−14 M | 1.0 × 10−13 to 5 × 10−10 M | [54] |
The noticeable advantages of silica nanoparticles concern their capability for designing unique nanolayer structures including SiNPs compatible with organic compounds in biological conditions. It favors their use in different types of bio and immunosensors.
6. Metal and metal oxide nanoparticles
6.1. Silver nanoparticles (AgNPs)
The size of silver nanoparticles is in the range from 1 to 100 nm at one dimension which make these NPs suitable for bio applications. By decreasing the size of AgNPs, the ratio of surface area to volume surprisingly increases considerably which results to noticeable changes in biological, physical and chemical activities [61]. Recently, AgNPs have been tested in novel and high-tech diagnostic instruments such as bio and immunosensors.
The compatibility of mixing Ag nanoparticles with other nano metal patterns, like gold nanoarray, permitted to design a new generation of biosensors using surface-enhanced Raman scattering (SERS). Wu et al. [62] prepared an optical biosensor for detection of hepatitis B viruse. The outcome of this coupling was to produce a wide plasmonic hot spots, which increased accessibility of Raman labels to produce great electromagnetic field. The LOD and linear range of this biosensor for the detection of hepatitis B virus DNA were 50 aM and 0.5–100 fM, respectively.
The fluorescence characteristics of AgNPs provided optical biosensors with high sensitivity. A biosensor has been prepared by Wang et al. based on detection of target DNA sequence of (HIV), (HBV) and (HTLV-I) gene by fluorescence activity of silver nanoclusters (AgCNs) [63]. Before conjugation of hairpin probe with mentioned viruses DNA, as target molecules, the fluorescence activity of AgCNs are high and bright, but after binding between probe strand and DNA of target molecules, the structure of hairpin probe was disordered thus, decreasing the fluorescence intensity of nanoparticles. The advantages of this optical-based biosensor were the high sensitivity and low LODs for the detection of HIV, HBV and HTLV-I, which were 4.4 nM, 6.8 nM and 8.5 nM, respectively.
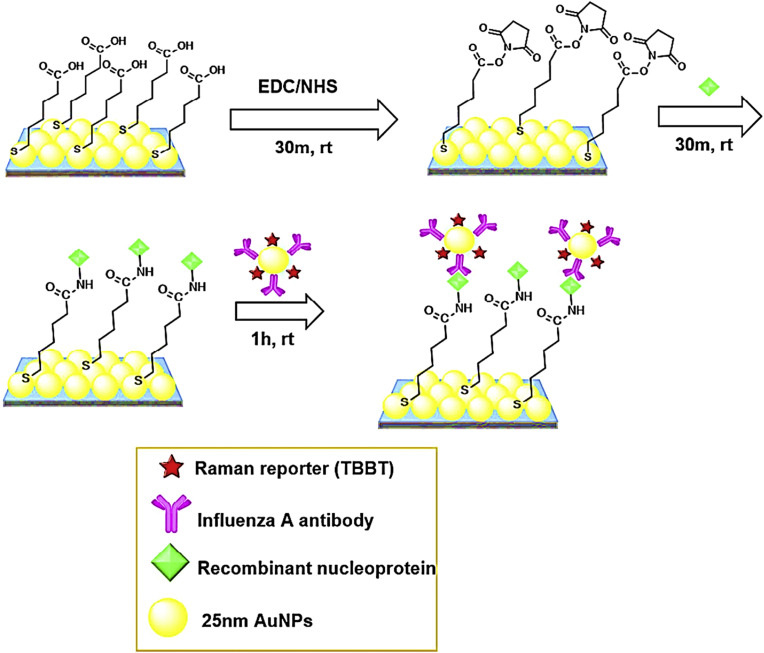
In another study two-dimensional (2D) core–shell structure of (Au@Ag) NPs arrays was used as substrate for SERS based biosensor [64]. In this report, the target molecule was influenza A virus, being obtained a LOD of 6 TCID50/mL and a linear range between 5 and 56 TCID50/mL using a unique SERS substrate (well-tuned Au@Ag 2D array) (Fig. 4 ).
Fig. 4.
Design of a modified electrode with two-dimensional (2D) core–shell structure of (Au@Ag) NPs array as substrate.
This figure was obtained with permission from Ref. [64].
Table 5 summarizes the main figures of merit of several optical and electrochemical AgNPs-based biosensors proposed in the literature for detection of viruses using different bio-receptors.
Table 5.
AgNPs based biosensors for detection of viruses.
| Virus | Impact on health | Biosensor type | Bio receptor | LOD | Linear range | Reference |
|---|---|---|---|---|---|---|
| HBV | Incapable of infecting mammals and plants | Optical | Aptamer | 0.65 nM | 1–800 nM | [22] |
| WNV | Neurological disease | Optical | Antibody | 5 fg mL−1 | – | [65] |
| HBV | Incapable of infecting mammals and plants | Electrochemical | Aptamer | 6.46 × 10−13 M | 3.23 × 10−12 to 5.31 × 10−9 M | [66] |
| Influenza (H5N1) | Breathing problems and pneumonia | Optical | Aptamer | 2 ng mL−1 in buffer and 3.5 ng mL−1 in human serum | 2–100 ng mL−1 in buffer and 3.5–100 in human serum | [67] |
6.2. Gold nanoparticles (AuNPs)
AuNPs have been extensively used in the field of virus detection owing to their unique optical/electrical properties [68]. For instance, Darbha et al. [69] detected HIV-1 viral DNA sequence with a sensitivity of about 100 pmol L−1 by taking advantage of second-order nonlinear optical properties of gold nanorods (AuNRs). This method can be applied to in-vitro selected aptamers for detecting a wide range of analytes such as small organic molecules and divalent cations. Lu et al. [70] proposed a FRET system, containing AuNRs and fluorescein (FAM), for the detection of hepatitis B virus DNA sequences. In this method AuNRs were synthesized and the surface of the AuNRs wrapped with a thin layer of cetyltrimethylammonium bromide (CTAB), leading to the positive charge of AuNRs. Their designed, structure led to a fluorescence resonance energy transfer (FRET) process from FAM to AuNRs. The fluorescence intensity of FAM was consequently quenched. The decline of the fluorescence intensity of FAM (DF) was linear with the concentration of the complementary DNA from 0.045 to 6.0 nmol L−1 and the LOD was as low as 15 pmol L−1 (signal/noise ratio of 3).
A highly sensitive and selective hepatitis B DNA biosensor using AuNPs has been developed by Mashhadizadeh and Talemi [71]. In this work, mercapto-benzaldehyde was used for the enhanced detection of a short DNA sequence of HBV virus. The fabrication process offered a very simple and convenient methodology for the preparation of a self-assembled monolayer and covalent immobilization of NH2-HBV-ss-DNA. A detection limit of 7.6(±0.1) × 10−12 mol L−1 was estimated for target HBV-DNA. The successful discrimination between the complementary HBV DNA, three-base mismatched, and non-complementary ss-DNA displayed good selectivity for the biosensor.
A sandwich immunoassay with AuNPs was developed by Escosura-Muñiz et al [72], that allowed to distinguish 3 mIU mL−1, α_HBs Ag IgG antibodies in human serum samples. The platform of assay was made of magnetic beads. The α_HBs Ag IgG antibodies were captured from human sera and the further signaling with AuNPs tags was made. 3 mIU mL−1 of α-HBsAg IgG antibodies in human serum can be detected by this biosensor. In comparison with MEIA method this method provided a deviation of 6.5%.
In another report, an immune assay based on label-free electrochemical method was proposed by Ma et al. for detecting the core antigen of the hepatitis C virus [73]. In this work, using synergetic effect of AuNPs, zirconia NPs and chitosan, an electrochemical immunosensor was built. This immunosensor exhibited board liner range between 2 and 512 ng mL−1, a detection limit of 0.17 ng mL−1, high consistence and cost-effective way to detect HCV core antigen, which pave the way to early diagnosis of HCV infection in clinical.
Although, immunoassays have a very good selectivity, they require long time of analysis to get results going from 2 h to 4–26 days [74]. In order to overcome immunoassays limitations and enhance sensitivity, sophisticated assays based on DNA detection were exploited for virus detection. Therefore, improved sensitive and specific HBV genomic DNA assay, utilizing rolling circle amplification (RCA)-based quartz crystal microbalance (QCM) assay has been proposed for direct DNA detection of HBV in clinical samples. During the assay the covalent bonding between the capture probes and the gold electrode surface maintains the connection between probes and amplified RCA products, being possible to determine less than 104 copies/mL concentration of HBV genomic DNA [75].
6.3. Magnetic nanoparticle (MNPs)
MNP includes a wide variety of materials and properties, including magnetic fluids, catalysis activity and magnetic resonance imaging [76] that made them useful in the field of biosensing. Because of being controllable by external magnet, these MNPs, are widely used in hybrid catalysts, drug delivery systems and reusable biosensor platforms [77]. Kamikawa et al. took advantage of MNPs to detect surface glycoprotein hemagglutinin (HA) from the Influenza A virus (FLUAV) H5N1. Electrically active polyaniline coated magnetic nanoparticles (EAPM) are the basis of this biosensors [78]. Compared to other nanoparticles employed in the field, this system provided increased assay kinetics due to the close proximity to targets, easy magnetic manipulation of the nanoparticles, and minimized matrix interference from complex samples as food and clinical specimens [79]. This assay is able to distinguish recombinant H5 HA at 1.4 μM in 10% mouse serum, in which specificity for H5 is highly than for H1. Novel EAM nanoparticles provide delicate, specific, inexpensive, and easy-to-use biosensor with applications in disease monitoring and biosecurity [78].
Since viruses are considered a greet menace to human life it is necessary to develop sophisticated diagnostic technique to effectively control pandemic strains. Krejcova et al. utilized paramagnetic particles to detect viral A/H5N1/Vietnam/1203/2004 protein-labeled QDs. The use of these particles being able to detect a wide panel of influenza virus strains. The LOD of viral protein was estimated as 0.1 μg mL−1 [80]. Krejcova et al. used MNPs, with covalently Boundoligooligo (dT25) for isolation of complementary H5N1 chains, in order to do Point Mutation Detection of H5N1 in Neuraminidase Gene. Oligonucleotide chains lengths were 12 (+5 adenine) or 28 (+5 adenine) bp that were labeled with QDs (136). The merit of this assay is its capability to multi-target diagnosis of NAIs-resistant influenza sub-types with QDs. These point mutations are resistant to NAIs, which is of vital importance for accurately treating severe human influenza cases, and to avoid the occurrence of severe influenza diseases, especially based on HPAI H5N1 [81].
Recently, amino functional carbon coated MNPs have been used to distinguish hybridization of HBV Nucleic Acids. Altay et al., in 2016 made possible to transfer immobilized molecules from one solution into the appropriate environment with magnetic separation. The omitted signal would be useful in highly selective label-free HBV DNA hybridization. In this assay pre-treatment was not required before separation which reduced the cost and time required [82]. The limit of detection of 1.15 μg mL−1 (20 pmol in 110 μL solution) was obtained with a linear target DNA concentration range of 5–25 μg mL−1.
6.4. Zinc oxide nanoparticles
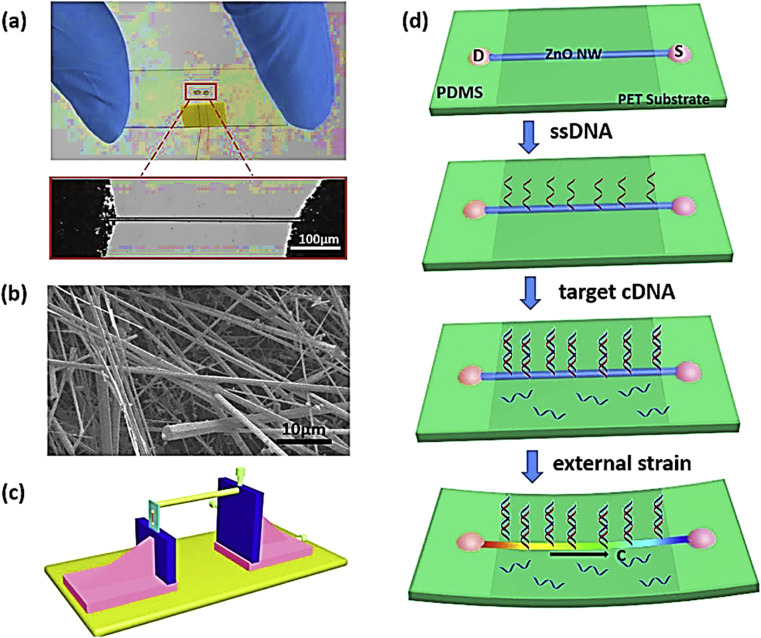
Zinc oxide (ZnO) is the main zinc species used widely in nanostructures. ZnO has been employed in different types of transducers, instruments of surface acoustic wave, gas sensors and also photonic devices. Having piezoelectric properties, ZnO plays an important role in some special sensors called mechanochemicals [83]. Additionally, being ZnO an innocuous material, biocompatible and environmentally friendly, it can be used without special requirements in the clinical field. As it is summarized in Fig. 5 , Cao and coworkers used the piezotronic effect of ZnO NPs is nanowire template to design a sensitive biosensor for detection of human immunodeficiency virus (HIV). The ZnO nanowire provided an in situ selective DNA detection of HIV virus.
Fig. 5.
ZnO nanowire-based biosensor for detection of human immunodeficiency virus (HIV). (a) Digital and microscopy image of ZnO nanowire DNA sensor; (b) SEM image of ZnO nanowires; (c) schematic explanation of biosensor design; (d) performance of ZnO nanowire DNA sensor regarding external strains.
This figure was obtained with permission from Ref. [84].
Composing ZnO nanoparticles with a natural and carbon based polymer, like graphene, could provide a new way to design a novel modified electrode or platform to achieve a sensitive biosensor for measuring target molecules at trace levels.
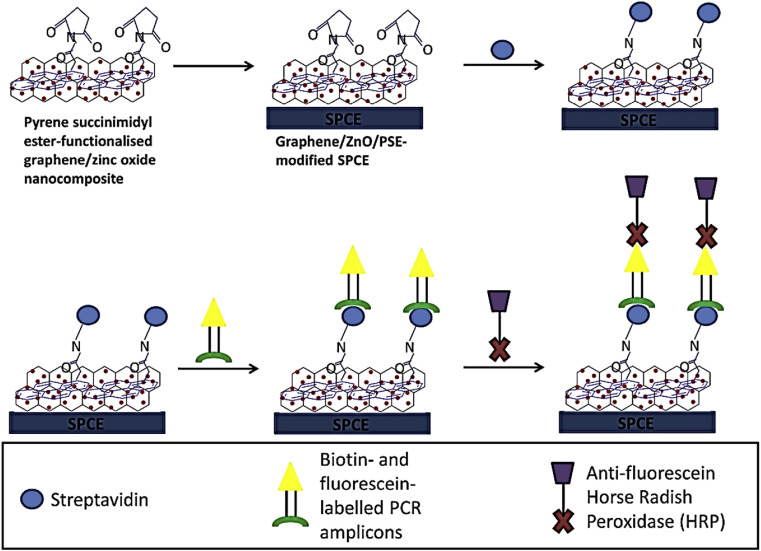
Tan et al. synthesized pure graphene and composing it with zinc oxide nanoparticles produced by hydrothermal process [85]. Because of its unique structure design (graphene/zinc oxide nanocomposite) and using hydrogen peroxide, with noticeable electrocatalytic activity, the sensitivity of the biosensor made was very high in comparing with conventional methods (P < 0.05) for Influenza H5 gene detection: the LOD being 7.4357 μM (Fig. 6 ).
Fig. 6.
Preparation of a biosensor based on graphene/zinc oxide nanocomposite and its application in detection of Influenza H5 gene.
This figure was obtained with permission from Ref. [85].
6.5. Aluminum nanoparticles (Al NPs)
From different species of Al NPs, nanoporous morphology is the most famous and attractive one for the scientists involved in biosensing. Some considerable chemical, optical and physical properties like chemical and thermal stability, being compatible in bioconditions like human body and having high surface area make this nanostructure suitable to be used it in analytical methods [86]. One of the advantages of having porous structure is the increasing in surface to volume ratio which resulted in an increased number of target molecules inside nanopores. Therefore, alumina nanoporous structures could be a rational option to be employed as platforms in modified electrodes for biosensing.
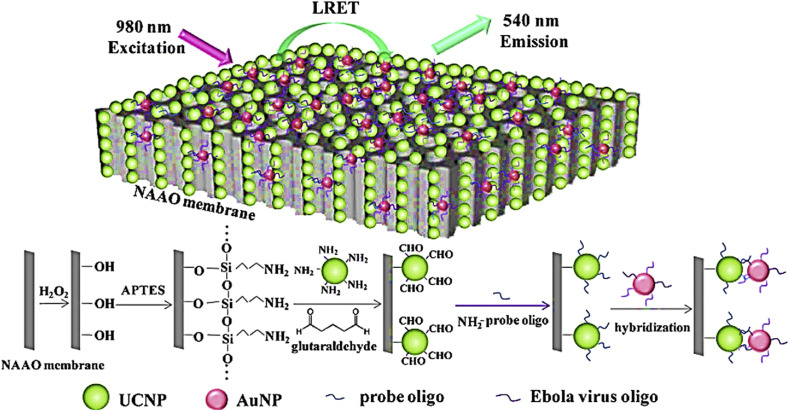
From pathogenic viruses, Ebola is a mortal species which is spread quickly by fatality results. Recently, Yang and coworkers [87] designed a high sensitive biosensor for detection of Ebola oligonucleotide. In this luminescence-based biosensor, nanoparticles which are up conversion and contains BaGdF5:Yb/Er, were used. Nanoparticles were connected with oligonucleotide probe and the oligonucleotide of Ebola virus as a target linked to AuNPs. A homogeneous system was prepared with an increased specificity and sensitivity. The novelty of this research was adding the nanoporous alumina in the structure of prepared biosensor which was resulted in considerable LOD enhancing from pM to fM level and forming a heterogeneous system. The reason was the efficient light interaction by means of nanoporous alumina walls (Fig. 7 ). In this report, before adding alumina nanoporous to the biosensor structure, the LOD was about 7 pM. But after employing this nanoparticle in the proposed biosensor, the LOD was improved to fM.
Fig. 7.
Nanoprobe/nanoporous membrane system for detection ebola virus oligonucleotide.
This figure was obtained with permission from Ref. [87].
In another study, thin film of anodic aluminum oxide (AAO) was used as a substrate of electrode by Wu et al. [88]. In this work, AuNPs were immobilized by an electrochemical method to provide a suitable hostage for deposition of HBV genome probe. Polymerase chain reaction (PCR) was the process of detection in this biosensor by using two linear ranges, 102 to 103 and 103 to 105.1 copies mL−1 and the LOD of the method was 111 copies mL−1.
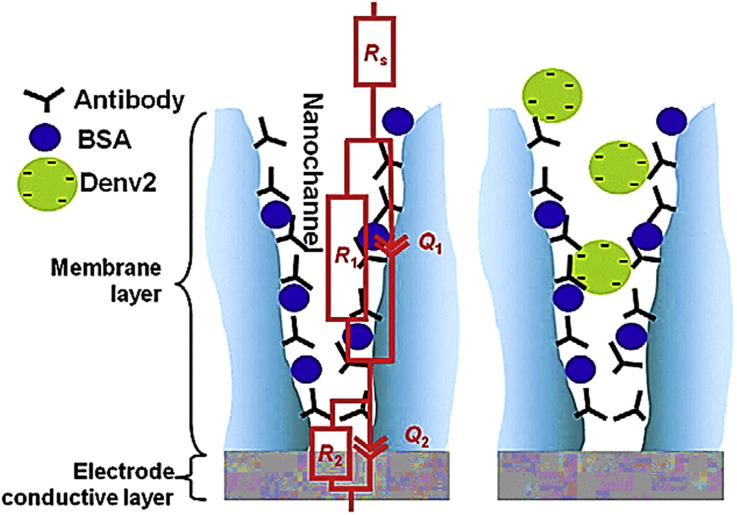
Because of the nanoporous unique structures, with a wide surface area, it leads to make a sensitive biosensor by providing opportunities to capture a great amount of target molecules. Toh and coworkers used nanoporous alumina and designed a biosensor for detection of dengue virus [89]. They immobilized considerable amount of antibodies on nanoporous alumina to make impedance changing in mentioned nanostructure by binding antibodies to dengue virus (Fig. 8 ). By existence of channel capacitance in this system, the biosensor provided a special specificity by responding to just Dengue virus in the presence of other virus like West Nile and Chikungunya viruses. A concentration of 1–900 pfu mL−1 Dengue virus was distinguished by this system, thus, producing a sensitive anodic aluminum biosensing application based on simple, cost-effective device.
Fig. 8.
Alumina nanobiosensor for the to precise detection of Dengue 2 virus using immunoglobulin G.
This figure was obtained with permission from Ref. [89].
6.6. Copper nanoparticles (CuNPs)
CuNPs have attracted much consideration because of its massive potential for replacing other expensive nanoparticles. Novel nanotechnology reveals the new opportunities for exploring the viral effect of CuNPs. Small size and high surface to volume ratio of copper nanoparticles, are able to interact closely with virus and detect their easily. A major drawback of this particles is their inherent tendency to oxidize in ambient conditions. However, the properties of copper nanoparticles are well-established and base on their use, several biosensors for virus detection have been developed [90]. Also, from the chemical point of view, the process of producing CuNPs from its various salts is cost effective and simple such as hydrothermal procedures. Chen et al. fabricated an ultrasensitive electrochemical biosensor for distinguishing influenza A virus utilizing copper nanoparticles. This biosensor is able to detect single stranded DNA (ss-DNA) of influenza A virus with a detection limit down to fM levels. They utilized CuHCF nanoparticles on the electrode surface through introducing GOx-A into the system via biotin–avidin interaction to amplify the DNA hybridization. A detection limit of ∼103 copies (1 fM in a sample volume of 2 μL) was successfully achieved by this system. Meanwhile, the previous reported limits of detection were around 105–107 copies mL−1 [91].
Mao et al. took advantage of copper nanoclusters to develope a colorimetric biosensing method. In this biosensor, the DNA of Hepatitis B virus can be distinguished by naked eyes. This method has great potential in comparison to conventional methods; such as the detection of three base-pair mismatches target DNA, high sensitivity and selectivity, precise diagnosis of genetic disease and a favorable cost effective. Moreover, the detection limit of this assay is 12 × 109 molecules. To sum up, this assay is a highly attractive candidate in DNA analysis which do not requires the use of sophisticate and expensive solvents [92].
7. Concluding remarks and future perspectives
Viruses are real menace to human safety since they infect host cells and cause various acute and chronic diseases. AIDs, smallpox, polio, influenza, diarrhea, and hepatitis are examples of the devastating effects of viral diseases that can affect the safety of people. Therefore, an initial warning system for proper detection and recognition of virus is required for eradicating viruses. Extensive investigation has been conducted for this purpose over decades. Meanwhile, the potential of nanotechnology in onset recognition of viruses is in the eye caching. These particles enhance mechanical, electrochemical, optical and magnetic properties of biosensors, and pave the way for an increased precision and selectivity of their detection. Herein we have critically discussed, the use of nanomaterials; such as nanoparticles and nanotubes in fabrication of different biosensors to detect pathogenic viruses. Several immobilization procedures, immunosensing schemes and their applications in various virus detection have been also mentioned. Although, biosensor technologies is highly promising, they present many challenges in order to move from the bench to their use in the point of care. Nanotechnologies offer new tools to overcome these challenges and perform direct detection of molecular targets in real time. Emerging graphene oxide, silica, carbon, gold, magnetic nanoparticles and nanotubes provide delicate and accurate platform in this field. Apart from mentioned advantages, to guarantee the profits of biosensors, further efforts should be considered concerning the appropriate selection of nanomaterials. The immobilization method of the concerned nanomaterials and biological elements is critical in order to minimize the risk of mistakes and errors in virus detection. Another important issue is lifetime of the assay, which is sometimes considerable. Indeed, great effort is required to provide portable and reusable devices capable for discriminating of viruses with high selectivity and sensitivity levels. To sum up, advances in nanotechnology will be in a near future a great breakthrough in medicine to halt viral diseases and provide a healthy life to potentially infected patients.
References
- 1.Katayama Y., Ohgi T., Mitoma Y., Hifumi E., Egashira N. Detection of influenza virus by a biosensor based on the method combining electrochemiluminescence on binary SAMs modified Au electrode with an immunoliposome encapsulating Ru (II) complex. Anal. Bioanal. Chem. 2016 doi: 10.1007/s00216-016-9587-8. [DOI] [PubMed] [Google Scholar]
- 2.Laurence J.C. Hepatitis A and B immunizations of individuals infected with human immunodeficiency virus. Am. J. Med. 2005;118(Suppl. 10A):75S–83S. doi: 10.1016/j.amjmed.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Mokhtarzadeh A., Bouzari M. Detection of the frequency of the novel TT virus by PCR and its role in the induction of hepatic injuries in blood donors in West Azarbaijan, Iran. IJBC. 2009;5:25–31. [Google Scholar]
- 4.Vidic J., Manzano M., Chang C.-M., Jaffrezic-Renault N. Advanced biosensors for detection of pathogens related to livestock and poultry. Vet. Res. 2017;48:11. doi: 10.1186/s13567-017-0418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iqbal S.S., Mayo M.W., Bruno J.G., Bronk B.V., Batt C.A., Chambers J.P. A review of molecular recognition technologies for detection of biological threat agents. Biosens. Bioelectron. 2000;15:549–578. doi: 10.1016/s0956-5663(00)00108-1. [DOI] [PubMed] [Google Scholar]
- 6.Cecchini F., Fajs L., Cosnier S., Marks R.S. Vibrio cholerae detection: traditional assays, novel diagnostic techniques and biosensors. TrAC Trends Anal. Chem. 2016;79:199–209. [Google Scholar]
- 7.Pashazadeh P., Mokhtarzadeh A., Hasanzadeh M., Hejazi M., Hashemi M., de la Guardia M. Nano-materials for use in sensing of salmonella infections: recent advances. Biosens. Bioelectron. 2017;87:1050–1064. doi: 10.1016/j.bios.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Hasanzadeh M., Sadeghi S., Bageri L., Mokhtarzadeh A., Shadjou N., Mahboob S. Poly-dopamine-beta-cyclodextrin: a novel nanobiopolymer towards sensing of some amino acids at physiological pH. Mater. Sci. Eng. C. 2016;69:343–357. doi: 10.1016/j.msec.2016.06.081. [DOI] [PubMed] [Google Scholar]
- 9.Hasanzadeh M., Mokhtari F., Shadjou N., Eftekhari A., Mokhtarzadeh A., Jouyban-Gharamaleki V., Mahboob S. Poly arginine–graphene quantum dots as a biocompatible and non-toxic nanocomposite layer-by-layer electrochemical preparation, characterization and non-invasive malondialdehyde sensory application in exhaled breath condensate. Mater. Sci. Eng. C. 2017;75:247–258. doi: 10.1016/j.msec.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Hematian A., Sadeghifard N., Mohebi R., Taherikalani M., Nasrolahi A., Amraei M., Ghafourian S. Traditional and modern cell culture in virus diagnosis. Osong Public Health Res. Perspect. 2016;7:77–82. doi: 10.1016/j.phrp.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sridhar S., To K.K., Chan J.F., Lau S.K., Woo P.C., Yuen K.Y. A systematic approach to novel virus discovery in emerging infectious disease outbreaks. J. Mol. Diagn. 2015;17:230–241. doi: 10.1016/j.jmoldx.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soler M. [Laboratory diagnosis to dengue virus infections] Acta Sci. Venezolana. 1998;49(Suppl. 1):25–32. [PubMed] [Google Scholar]
- 13.Diel D.G., Lawson S., Okda F., Singrey A., Clement T., Fernandes M.H., Christopher-Hennings J., Nelson E.A. Porcine epidemic diarrhea virus: an overview of current virological and serological diagnostic methods. Virus Res. 2016 doi: 10.1016/j.virusres.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balasuriya U.B., Crossley B.M., Timoney P.J. A review of traditional and contemporary assays for direct and indirect detection of Equid herpesvirus 1 in clinical samples. J. Vet. Diagn. Invest. 2015;27:673–687. doi: 10.1177/1040638715605558. [DOI] [PubMed] [Google Scholar]
- 15.Bellan L.M., Wu D., Langer R.S. Current trends in nanobiosensor technology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011;3:229–246. doi: 10.1002/wnan.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen B.T., Koh G., Lim H.S., Chua A.J., Ng M.M., Toh C.S. Membrane-based electrochemical nanobiosensor for the detection of virus. Anal. Chem. 2009;81:7226–7234. doi: 10.1021/ac900761a. [DOI] [PubMed] [Google Scholar]
- 17.Yockell-Lelievre H., Bukar N., Toulouse J.L., Pelletier J.N., Masson J.F. Naked-eye nanobiosensor for therapeutic drug monitoring of methotrexate. Analyst. 2016;141:697–703. doi: 10.1039/c5an00996k. [DOI] [PubMed] [Google Scholar]
- 18.Jianrong C., Yuqing M., Nongyue H., Xiaohua W., Sijiao L. Nanotechnology and biosensors. Biotechnol. Adv. 2004;22:505–518. doi: 10.1016/j.biotechadv.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Vannoy C.H., Tavares A.J., Noor M.O., Uddayasankar U., Krull U.J. Biosensing with quantum dots: a microfluidic approach. Sensors (Basel) 2011;11:9732–9763. doi: 10.3390/s111009732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramamoorthy A., Akis R., Bird J.P., Maemoto T., Ferry D.K., Inoue M. Signatures of dynamical tunneling in semiclassical quantum dots. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2003;68:026221. doi: 10.1103/PhysRevE.68.026221. [DOI] [PubMed] [Google Scholar]
- 21.Liu X., Braun G.B., Zhong H., Hall D.J., Han W., Qin M., Zhao C., Wang M., She Z.G., Cao C., Sailor M.J., Stallcup W.B., Ruoslahti E., Sugahara K.N. Tumor-targeted multimodal optical imaging with versatile cadmium-free quantum dots. Adv. Funct. Mater. 2016;26:267–276. doi: 10.1002/adfm.201503453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao D., Li J., Pan Y., Zhang X., Zheng X., Wang Z., Zhang M., Zhang H., Chen L. Noninvasive theranostic imaging of HSV-TK/GCV suicide gene therapy in liver cancer by folate-targeted quantum dot-based liposomes. Biomater. Sci. 2015;3:833–841. doi: 10.1039/c5bm00077g. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z.Y., Abdelhamid H.N., Wu H.F. Effect of surface capping of quantum dots (CdTe) on proteomics. Rapid Commun. Mass Spectrom. 2016;30:1403–1412. doi: 10.1002/rcm.7575. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y.G., Moon S., Kuritzkes D.R., Demirci U. Quantum dot-based HIV capture and imaging in a microfluidic channel. Biosens. Bioelectron. 2009;25:253–258. doi: 10.1016/j.bios.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X., Lou X., Wang Y., Guo Q., Fang Z., Zhong X., Mao H., Jin Q., Wu L., Zhao H., Zhao J. QDs-DNA nanosensor for the detection of hepatitis B virus DNA and the single-base mutants. Biosens. Bioelectron. 2010;25:1934–1940. doi: 10.1016/j.bios.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Wen L., Lin Y., Zheng Z.H., Zhang Z.L., Zhang L.J., Wang L.Y., Wang H.Z., Pang D.W. Labeling the nucleocapsid of enveloped baculovirus with quantum dots for single-virus tracking. Biomaterials. 2014;35:2295–2301. doi: 10.1016/j.biomaterials.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 27.Li Yilin, Jing Lihong, Ding Ke, Gao Jing, Peng Zhi, Li Yanyan, Shen Lin, Gao Mingyuan. Detection of Epstein–Barr virus infection in cancer by using highly specific nanoprobe based on dBSA capped CdTe quantum dots. RSC Adv. 2014;4:22545–22550. [Google Scholar]
- 28.Wang S., Li L., Jin H., Yang T., Bao W., Huang S., Wang J. Electrochemical detection of hepatitis B and papilloma virus DNAs using SWCNT array coated with gold nanoparticles. Biosens. Bioelectron. 2013;41:205–210. doi: 10.1016/j.bios.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 29.Krejcova L., Nejdl L., Hynek D., Krizkova S., Kopel P., Adam V., Kizek R. Beads-based electrochemical assay for the detection of influenza hemagglutinin labeled with CdTe quantum dots. Molecules (Basel, Switzerland) 2013;18:15573–15586. doi: 10.3390/molecules181215573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasanzadeh M., Shadjou N., Mokhtarzadeh A., Ramezani M. Two dimension (2-D) graphene-based nanomaterials as signal amplification elements in electrochemical microfluidic immune-devices: recent advances. Mater. Sci. Eng. C. 2016;68:482–493. doi: 10.1016/j.msec.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 31.Yang K., Zhang S., Zhang G., Sun X., Lee S.-T., Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nanoletters. 2010;10:3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 32.Hasanzadeh M., Karimzadeh A., Sadeghi S., Mokhtarzadeh A., Shadjou N., Jouyban A. Graphene quantum dot as an electrically conductive material toward low potential detection: a new platform for interface science. J. Mater. Sci. Mater. Electron. 2016;27:6488–6495. [Google Scholar]
- 33.Yousefi M., Dadashpour M., Hejazi M., Hasanzadeh M., Behnam B., de la Guardia M., Shadjou N., Mokhtarzadeh A. Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater. Sci. Eng. C. 2017 doi: 10.1016/j.msec.2016.12.125. [DOI] [PubMed] [Google Scholar]
- 34.Morales-Narváez E., Merkoçi A. Graphene oxide as an optical biosensing platform. Adv. Mater. 2012;24:3298–3308. doi: 10.1002/adma.201200373. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y., Xu F., Fan X., Luo H., Ge S., Zheng Q., Xia N., Chen H., Guan Y., Zhang J. Evaluation of a rapid test for detection of H5N1 avian influenza virus. J. Virol. Methods. 2008;154:213–215. doi: 10.1016/j.jviromet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Eivazzadeh-Keihan R., Pashazadeh P., Hejazi M., de la Guardia M., Mokhtarzadeh A. Recent advances in nanomaterial-mediated bio and immune sensors for detection of aflatoxin in food products. TrAC Trends Anal. Chem. 2017;87:112–128. [Google Scholar]
- 37.Beigel J.H., Farrar J., Han A.M., Hayden F.G. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 2005;353:1374. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 38.Xie Z., Huang J., Luo S., Xie Z., Xie L., Liu J., Pang Y., Deng X., Fan Q. Ultrasensitive electrochemical immunoassay for avian influenza subtype H5 using nanocomposite. PLoS One. 2014;9:e94685. doi: 10.1371/journal.pone.0094685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang Y.-S., Huang X.-J., Wang L.-S., Wang J.-F. An enhanced sensitive electrochemical immunosensor based on efficient encapsulation of enzyme in silica matrix for the detection of human immunodeficiency virus p24. Biosens. Bioelectron. 2015;64:324–332. doi: 10.1016/j.bios.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 40.Hu Y., Li F., Han D., Wu T., Zhang Q., Niu L., Bao Y. Simple and label-free electrochemical assay for signal-on DNA hybridization directly at undecorated graphene oxide. Anal. Chim. Acta. 2012;753:82–89. doi: 10.1016/j.aca.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 41.Jung J.H., Cheon D.S., Liu F., Lee K.B., Seo T.S. A graphene oxide based immuno-biosensor for pathogen detection. Angew. Chem. Int. Ed. 2010;49:5708–5711. doi: 10.1002/anie.201001428. [DOI] [PubMed] [Google Scholar]
- 42.Muti M., Sharma S., Erdem A., Papakonstantinou P. Electrochemical monitoring of nucleic acid hybridization by single-use graphene oxide-based sensor. Electroanalysis. 2011;23:272–279. [Google Scholar]
- 43.Kim M.-G., Shon Y., Lee J., Byun Y., Choi B.-S., Kim Y.B., Oh Y.-K. Double stranded aptamer-anchored reduced graphene oxide as target-specific nano detector. Biomaterials. 2014;35:2999–3004. doi: 10.1016/j.biomaterials.2013.12.058. [DOI] [PubMed] [Google Scholar]
- 44.Bi S., Zhao T., Luo B. A graphene oxide platform for the assay of biomolecules based on chemiluminescence resonance energy transfer. Chem. Commun. 2012;48:106–108. doi: 10.1039/c1cc15443e. [DOI] [PubMed] [Google Scholar]
- 45.Liu F., Kim Y.H., Cheon D.S., Seo T.S. Micropatterned reduced graphene oxide based field-effect transistor for real-time virus detection. Sensors Actuators B Chem. 2013;186:252–257. [Google Scholar]
- 46.Wu Y.-M., Cen Y., Huang L.-J., Yu R.-Q., Chu X. Upconversion fluorescence resonance energy transfer biosensor for sensitive detection of human immunodeficiency virus antibodies in human serum. Chem. Commun. 2014;50:4759–4762. doi: 10.1039/c4cc00569d. [DOI] [PubMed] [Google Scholar]
- 47.Yang N., Chen X., Ren T., Zhang P., Yang D. Carbon nanotube based biosensors. Sensors Actuators B Chem. 2015;207:690–715. [Google Scholar]
- 48.Liu F., Xiang G., Zhang L., Jiang D., Liu L., Li Y., Liu C., Pu X. A novel label free long non-coding RNA electrochemical biosensor based on green L-cysteine electrodeposition and Au–Rh hollow nanospheres as tags. RSC Adv. 2015;5:51990–51999. [Google Scholar]
- 49.Shi L., Yu Y., Chen Z., Zhang L., He S., Shi Q., Yang H. A label-free hemin/G-quadruplex DNAzyme biosensor developed on electrochemically modified electrodes for detection of a HBV DNA segment. RSC Adv. 2015;5:11541–11548. [Google Scholar]
- 50.Wang X., Chen L., Su X., Ai S. Electrochemical immunosensor with graphene quantum dots and apoferritin-encapsulated Cu nanoparticles double-assisted signal amplification for detection of avian leukosis virus subgroup J. Biosens. Bioelectron. 2013;47:171–177. doi: 10.1016/j.bios.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 51.Li X.-M., Zhan Z.-M., Ju H.-Q., Zhang S.-S. Label-free electrochemical detection of short sequences related to the hepatitis B virus using 4, 4′-diaminoazobenzene based on multiwalled carbon nanotube-modified GCE. Oligonucleotides. 2008;18:321–328. doi: 10.1089/oli.2008.0143. [DOI] [PubMed] [Google Scholar]
- 52.Liu X., Cheng Z., Fan H., Ai S., Han R. Electrochemical detection of avian influenza virus H5N1 gene sequence using a DNA aptamer immobilized onto a hybrid nanomaterial-modified electrode. Electrochim. Acta. 2011;56:6266–6270. [Google Scholar]
- 53.Tam P.D., Van Hieu N., Chien N.D., Le A.-T., Tuan M.A. DNA sensor development based on multi-wall carbon nanotubes for label-free influenza virus (type A) detection. J. Immunol. Methods. 2009;350:118–124. doi: 10.1016/j.jim.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Wang Q., Zhang B., Lin X., Weng W. Hybridization biosensor based on the covalent immobilization of probe DNA on chitosan–mutiwalled carbon nanotubes nanocomposite by using glutaraldehyde as an arm linker. Sensors Actuators B Chem. 2011;156:599–605. [Google Scholar]
- 55.Tang D., Yuan R., Chai Y. Magnetic control of an electrochemical microfluidic device with an arrayed immunosensor for simultaneous multiple immunoassays. Clin. Chem. 2007;53:1323–1329. doi: 10.1373/clinchem.2006.085126. [DOI] [PubMed] [Google Scholar]
- 56.Cullis A., Canham L.T., Calcott P. The structural and luminescence properties of porous silicon. J. Appl. Phys. 1997;82:909–965. [Google Scholar]
- 57.Cha B.H., Lee S.-M., Park J.C., Hwang K.S., Kim S.K., Lee Y.-S., Ju B.-K., Kim T.S. Detection of hepatitis B virus (HBV) DNA at femtomolar concentrations using a silica nanoparticle-enhanced microcantilever sensor. Biosens. Bioelectron. 2009;25:130–135. doi: 10.1016/j.bios.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 58.Wilson M.S., Nie W. Multiplex measurement of seven tumor markers using an electrochemical protein chip. Anal. Chem. 2006;78:6476–6483. doi: 10.1021/ac060843u. [DOI] [PubMed] [Google Scholar]
- 59.Chen L., Qi Z., Chen R., Li Y., Liu S. Sensitive detection of Epstein–Barr virus-derived latent membrane protein 1 based on CdTe quantum dots-capped silica nanoparticle labels. Clin. Chim. Acta. 2010;411:1969–1975. doi: 10.1016/j.cca.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 60.Riccò R., Meneghello A., Enrichi F. Signal enhancement in DNA microarray using dye doped silica nanoparticles: application to human papilloma virus (HPV) detection. Biosens. Bioelectron. 2011;26:2761–2765. doi: 10.1016/j.bios.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 61.Varner K., Sanford J., El-Badawy A., Feldhake D., Venkatapathy R. US Environmental Protection Agency; Washington DC: 2010. State of the Science Literature Review: Everything Nanosilver and More; p. 363. [Google Scholar]
- 62.Li M., Cushing S.K., Liang H., Suri S., Ma D., Wu N. Plasmonic nanorice antenna on triangle nanoarray for surface-enhanced Raman scattering detection of hepatitis B virus DNA. Anal. Chem. 2013;85:2072–2078. doi: 10.1021/ac303387a. [DOI] [PubMed] [Google Scholar]
- 63.Cao Q., Teng Y., Yang X., Wang J., Wang E. A label-free fluorescent molecular beacon based on DNA-Ag nanoclusters for the construction of versatile biosensors. Biosens. Bioelectron. 2015;74:318–321. doi: 10.1016/j.bios.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 64.Karn-orachai K., Sakamoto K., Laocharoensuk R., Bamrungsap S., Songsivilai S., Dharakul T., Miki K. Extrinsic surface-enhanced Raman scattering detection of influenza A virus enhanced by two-dimensional gold@ silver core–shell nanoparticle arrays. RSC Adv. 2016;6:97791–97799. [Google Scholar]
- 65.Neng J., Harpster M.H., Wilson W.C., Johnson P.A. Surface-enhanced Raman scattering (SERS) detection of multiple viral antigens using magnetic capture of SERS-active nanoparticles. Biosens. Bioelectron. 2013;41:316–321. doi: 10.1016/j.bios.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 66.Niu S., Han B., Cao W., Zhang S. Sensitive DNA biosensor improved by Luteolin copper (II) as indicator based on silver nanoparticles and carbon nanotubes modified electrode. Anal. Chim. Acta. 2009;651:42–47. doi: 10.1016/j.aca.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 67.Pang Y., Rong Z., Wang J., Xiao R., Wang S. A fluorescent aptasensor for H5N1 influenza virus detection based-on the core–shell nanoparticles metal-enhanced fluorescence (MEF) Biosens. Bioelectron. 2015;66:527–532. doi: 10.1016/j.bios.2014.10.052. [DOI] [PubMed] [Google Scholar]
- 68.Lee C., Wang P., Gaston M.A., Weiss A.A., Zhang P. 2017. Plasmonics-based detection of virus using sialic acid functionalized gold nanoparticles; pp. 109–116. Biodetection: Methods and Protocols Volume 1: Optical-Based Detectors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Darbha G.K., Rai U.S., Singh A.K., Ray P.C. Gold-nanorod-based sensing of sequence specific HIV-1 virus DNA by using hyper-Rayleigh scattering spectroscopy. Chem. Eur. J. 2008;14:3896–3903. doi: 10.1002/chem.200701850. [DOI] [PubMed] [Google Scholar]
- 70.Lu X., Dong X., Zhang K., Han X., Fang X., Zhang Y. A gold nanorods-based fluorescent biosensor for the detection of hepatitis B virus DNA based on fluorescence resonance energy transfer. Analyst. 2013;138:642–650. doi: 10.1039/c2an36099c. [DOI] [PubMed] [Google Scholar]
- 71.Mashhadizadeh M.H., Talemi R.P. A highly sensitive and selective hepatitis B DNA biosensor using gold nanoparticle electrodeposition on an Au electrode and mercaptobenzaldehyde. Anal. Methods. 2014;6:8956–8964. [Google Scholar]
- 72.de la Escosura-Muñiz A., Maltez-da Costa M., Sánchez-Espinel C., Díaz-Freitas B., Fernández-Suarez J., González-Fernández Á., Merkoçi A. Gold nanoparticle-based electrochemical magnetoimmunosensor for rapid detection of anti-hepatitis B virus antibodies in human serum. Biosens. Bioelectron. 2010;26:1710–1714. doi: 10.1016/j.bios.2010.07.069. [DOI] [PubMed] [Google Scholar]
- 73.Ma C., Xie G., Zhang W., Liang M., Liu B., Xiang H. Label-free sandwich type of immunosensor for hepatitis C virus core antigen based on the use of gold nanoparticles on a nanostructured metal oxide surface. Microchim. Acta. 2012;178:331–340. [Google Scholar]
- 74.Kwon J.-A., Yoon S.-Y., Lee C.-K., Lim C.S., Lee K.N., Sung H.J., Brennan C.A., Devare S.G. Performance evaluation of three automated human immunodeficiency virus antigen–antibody combination immunoassays. J. Virol. Methods. 2006;133:20–26. doi: 10.1016/j.jviromet.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 75.Yao C., Xiang Y., Deng K., Xia H., Fu W. Sensitive and specific HBV genomic DNA detection using RCA-based QCM biosensor. Sensors Actuators B Chem. 2013;181:382–387. [Google Scholar]
- 76.T. Hyeon, Y. Piao, Y.I. Park, Method of preparing iron oxide nanoparticles coated with hydrophilic material, and magnetic resonance imaging contrast agent using the same, U.S. Patent No. 9,352,058. 31 May 2016.
- 77.Templier V., Roux A., Roupioz Y., Livache T. Ligands for label-free detection of whole bacteria on biosensors: a review. TrAC Trends Anal. Chem. 2016;79:71–79. [Google Scholar]
- 78.Kamikawa T.L., Mikolajczyk M.G., Kennedy M., Zhang P., Wang W., Scott D.E., Alocilja E.C. Nanoparticle-based biosensor for the detection of emerging pandemic influenza strains. Biosens. Bioelectron. 2010;26:1346–1352. doi: 10.1016/j.bios.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 79.Pal S., Alocilja E.C. Electrically active polyaniline coated magnetic (EAPM) nanoparticle as novel transducer in biosensor for detection of Bacillus anthracis spores in food samples. Biosens. Bioelectron. 2009;24:1437–1444. doi: 10.1016/j.bios.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 80.Krejcova L., Dospivova D., Ryvolova M., Kopel P., Hynek D., Krizkova S., Hubalek J., Adam V., Kizek R. Paramagnetic particles coupled with an automated flow injection analysis as a tool for influenza viral protein detection. Electrophoresis. 2012;33:3195–3204. doi: 10.1002/elps.201200304. [DOI] [PubMed] [Google Scholar]
- 81.Krejcova L., Hynek D., Kopel P., Rodrigo M.A.M., Adam V., Hubalek J., Babula P., Trnkova L., Kizek R. Development of a magnetic electrochemical bar code array for point mutation detection in the H5N1 neuraminidase gene. Viruses. 2013;5:1719–1739. doi: 10.3390/v5071719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Altay C., Senay R.H., Eksin E., Congur G., Erdem A., Akgol S. Development of amino functionalized carbon coated magnetic nanoparticles and their application to electrochemical detection of hybridization of nucleic acids. Talanta. 2017;164:175–182. doi: 10.1016/j.talanta.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 83.Özgür Ü., Alivov Y.I., Liu C., Teke A., Reshchikov M., Doğan S., Avrutin V., Cho S.-J., Morkoc H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005;98:11. [Google Scholar]
- 84.Cao X., Cao X., Guo H., Li T., Jie Y., Wang N., Wang Z.L. Piezotronic effect enhanced label-free detection of DNA using a Schottky-contacted ZnO nanowire biosensor. ACS Nano. 2016;10:8038–8044. doi: 10.1021/acsnano.6b04121. [DOI] [PubMed] [Google Scholar]
- 85.Low S.S., Tan M.T., Loh H.-S., Khiew P.S., Chiu W.S. Facile hydrothermal growth graphene/ZnO nanocomposite for development of enhanced biosensor. Anal. Chim. Acta. 2016;903:131–141. doi: 10.1016/j.aca.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 86.Kumeria T., Santos A., Losic D. Nanoporous anodic alumina platforms: engineered surface chemistry and structure for optical sensing applications. Sensors. 2014;14:11878–11918. doi: 10.3390/s140711878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsang M.-K., Ye W., Wang G., Li J., Yang M., Hao J. Ultrasensitive detection of Ebola virus oligonucleotide based on upconversion nanoprobe/nanoporous membrane system. ACS Nano. 2016;10:598–605. doi: 10.1021/acsnano.5b05622. [DOI] [PubMed] [Google Scholar]
- 88.Chen C.-C., Lai Z.-L., Wang G.-J., Wu C.-Y. Polymerase chain reaction-free detection of hepatitis B virus DNA using a nanostructured impedance biosensor. Biosens. Bioelectron. 2016;77:603–608. doi: 10.1016/j.bios.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 89.Nguyen B.T.T., Peh A.E.K., Chee C.Y.L., Fink K., Chow V.T., Ng M.M., Toh C.-S. Electrochemical impedance spectroscopy characterization of nanoporous alumina dengue virus biosensor. Bioelectrochemistry. 2012;88:15–21. doi: 10.1016/j.bioelechem.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 90.Magdassi S., Grouchko M., Kamyshny A. Copper nanoparticles for printed electronics: routes towards achieving oxidation stability. Materials. 2010;3:4626–4638. doi: 10.3390/ma3094626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen X., Xie H., Seow Z.Y., Gao Z. An ultrasensitive DNA biosensor based on enzyme-catalyzed deposition of cupric hexacyanoferrate nanoparticles. Biosens. Bioelectron. 2010;25:1420–1426. doi: 10.1016/j.bios.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 92.Mao X., Liu S., Yang C., Liu F., Wang K., Chen G. Colorimetric detection of hepatitis B virus (HBV) DNA based on DNA-templated copper nanoclusters. Anal. Chim. Acta. 2016;909:101–108. doi: 10.1016/j.aca.2016.01.009. [DOI] [PubMed] [Google Scholar]