Abstract
Parasites are either dedicated to a narrow host range, or capable of exploiting a wide host range. Understanding how host ranges are determined is very important for public health, as well as wildlife, plant, livestock and agricultural diseases. Our current understanding of host–parasite associations hinges on co-evolution, which assumes evolved host preferences (host specialization) of the parasite. Despite the explanatory power of this framework, we have only a vague understanding of why many parasites routinely cross the host species’ barrier. Here we introduce a simple model demonstrating how superinfection (in a heterogeneous community) can promote host–parasite association. Strikingly, the model illustrates that strong host–parasite association occurs in the absence of host specialization, while still permitting cross-species transmission. For decades, host specialization has been foundational in explaining the maintenance of distinct parasites/strains in host species. We argue that host specializations may be exaggerated, and can occur as a byproduct (not necessarily the cause) of host–parasite associations.
Keywords: Superinfection, Host–parasite association, Host specialization, Cross-species transmission, Intrinsic generalists
Highlights
-
•
Many parasites appear to exhibit host specificity.
-
•
Many parasites are also efficient in cross-species transmissions.
-
•
The above two phenomenon are largely incompatible without adaptive mutations.
-
•
Superinfection facilitates apparent host specificity and cross-species transmission.
1. Introduction
Many parasites in nature are associated with a host species, or a group of related species. Examples of host–parasite association can be found in a range of disease systems including HIV/SIV, rabies, malaria, and Lyme borreliosis (Garamszegi, 2006, Hahn et al., 2000, Kurtenbach et al., 2002, Streicker et al., 2010). In certain cases there are natural barriers to the exploitation of multiple host species, e.g. sexually transmitted diseases. Yet other disease systems relying on direct, vectored, or environmental transmission allow for a potentially wide host range. In these systems, the factors that determine whether parasites focus on a narrow range of species or adopt a more generalist strategy are typically not known, yet the mechanisms at play have important consequences for public health and beyond. For instance, zoonotic parasites cause significant human disease burden worldwide (Jones et al., 2008), and any practical disease intervention strategy requires some knowledge of the associated host species. Further, these parasites may transmit through multiple wildlife species. Such complex transmission cycles are robust in the sense that blocking transmission from one host species may only partially control human disease risk—as demonstrated in North American Lyme disease (Tsao et al., 2004). Recently, an urgent hunt for the reservoir host(s) of Ebola virus, Henipavirus, and SARS-coronavirus has implicated bats (Dobson, 2005). Given our limited understanding of the transmission competency of alternative hosts, and of bat-virus dynamics in general, it is unclear if targeting any number of bat species would be effective in reducing human disease risk.
Arguably, one of the worst-case scenarios for public health is a host shifting event, defined as a parasite/strain that was previously zoonotic and now circulates exclusively among humans; HIV/AIDS is a prime example (Hahn et al., 2000). Additional examples are drawn from studies on primate malarias, which have identified multiple host shifts from non-human primates to humans (Krief et al., 2010, Mu et al., 2005), which include the malaria parasites Plasmodium falciparum and P. vivax. All of these examples illustrate that understanding parasite host ranges is crucial for global disease management.
The prevailing data on host–parasite systems suggest there are at least two influential factors in determining a parasite’s host range (Woolhouse et al., 2001): (1) the community-level contact structure, which determines opportunities for cross-species transmission; and (2) the standing genetic diversity of the parasite. Essentially, the combination of host species availability and the potential for adaptive evolution is thought to be the dominant force in shaping a parasite’s host range. A realized host range that is less than all contactable hosts (few infections in some species, despite them being accessible to the parasite) reflects a degree of host association by the parasite or strain. A central concept is that host–parasite association results from host specialization; the adaptive evolution of the parasite, leading to a specific host preference (Levene, 1953). The difficulty with host specialization is that it does not easily explain how many parasites routinely cross the species’ barrier, unless we invoke recurrent adaptation by the parasite. For example, a parasite that evolves a preference towards one host species is unlikely to cause an outbreak in an alternate species, unless a mutation occurs that either enhances cross-species transmission, or improves within-species transmission in the alternative host population. While this condition is plausible for rapidly-mutating viruses, it is cumbersome for relatively slow-evolving bacteria and protist parasites, and, we argue, not the only mechanism that can explain variable patterns of host–parasite association in nature.
In this study we use mathematical modeling to examine whether general parasite transmission processes can lead to variability in host association patterns (along a spectrum of restricted to unrestricted host ranges) while still allowing for frequent cases of cross-species transmission. Notably, we explore this in the absence of recurrent adaptation to isolate the potential effects of ecology and transmission. Specifically, we focus on the role of superinfection in driving host associations and cross-species transmissions—two common, empirical phenomena that appear to be at odds with each other. Although earlier studies have investigated parasite transmission in heterogeneous populations (Gandon, 2004, Woolhouse et al., 2001), the effects of superinfection in such populations are rarely invoked.
2. System of equations
2.1. The deterministic model
The model depicted in Fig. 1 and the system of equations in Section 2 represents a host–parasite system of two host species , denoted by subscripts 1 and 2, and two parasites: and . Infected hosts are classified as either , , , . Host populations recover from losses (natural mortality and disease-induced mortality, also called virulence) via a density-dependent birth rate, , where is the density-independent birth rate and is a density-dependent factor. Both strains have a higher transmission rate between hosts of the same species compared to cross-species transmission, reflecting a degree of ecological separation between host types, controlled by parameter . Parasite transmits within each host species at rate , where is the transmission rate. Parasite transmits at rate in both host species, and additionally is capable of superinfection (infecting an individual currently infected by parasite ). The superinfection rates are for and for . This assumption articulates that we regard as an aggressive mutant, which superinfects preferentially (that is, parameter ensures the superinfection rate of type 2 hosts is lower than that of type 1 hosts). The reasoning for this assumption is that the ability to superinfect a host is jointly dependent on the aggression of the superinfecting strain (to outcompete the inhost resident strain) and host–specific immunity (to permit secondary infection), i.e. a combination of host and parasite effects. It is therefore conservative to assume that distinct host species differ in their degree to permit superinfection . Potential specific mechanisms include differences in the cost or quality of immune activation, which may be initiated (or exacerbated) from immune priming by an unrelated parasite (Telfer et al., 2010), or by species-specific energy expenditures, such as migration (Altizer et al., 2011, Weber and Stilianakis, 2007). The parameter allows a range of superinfection disparity to be explored. The outcome of superinfection is immediate takeover of the individual by strain , yielding an individual. Rates of primary infections of both hosts by both strains are equal; there is no intrinsic host preference. As a consequence of its aggression, strain carries a greater virulence cost (reflected by ) in and subpopulations than strain (where virulence is modeled by with ). We examine a range of differences in virulence costs between strains. We set equal population sizes and growth dynamics of and in order to distinguish the effects of superinfection in isolation, otherwise a larger (or more fecund) host group may confound the advantage or cost of superinfection; we show in Supplementary materials that relaxing these assumptions does not change the general outcome of our model. Initial conditions and parameters are listed in Table 1 . All deterministic simulations began with strain at equilibrium followed by an introduction of strain and an evolutionary period of 500 years. We arbitrarily define a host-associated parasite as having 80% of its infections in one host species.
Fig. 1.
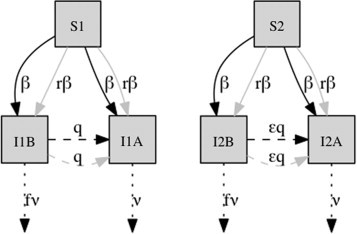
Host association model diagram.
Note: Model schematic: and represent susceptible hosts from distinct species. and are infected hosts of type 1 infected by or . and are infected hosts of type 2 infected by or . Transitions in the system of equations Section 2: Regular infection (solid arrows) can occur via direct transmission (DT, within host species, black arrows) and via cross-species transmission (CST, gray arrows, at a rate reduced by factor ). Superinfection (dashed arrows) can occur directly (DSI, within host species, black arrows) and between host species (CSI, gray arrows). Host species 2 experiences superinfection at a rate reduced by factor . Both parasites A and B cause disease-induced mortality, though the rate associated with parasite B is reduced by a factor .
Table 1.
Model parameters and initial conditions.
| Name | Description | Value |
|---|---|---|
| Susceptible hosts of species 1 | 20 | |
| Susceptible hosts of species 2 | 20 | |
| Infected species 1 with type | 5 | |
| Infected species 1 with type a | 75 | |
| Infected species 2 with type | 5 | |
| Infected species 2 with type a | 75 | |
| Density-independent birth rateb | 0.02 | |
| Slope of per-capita birth rate with population sizeb | 0.00014 | |
| Transmission rate | 0.0005/0.0008/0.0002 | |
| Mortality ratec | 0.006 | |
| Scaling factor for between species transmission relative to within species transmission | 0.01 | |
| Scaling factor for superinfection in species 1 | – | |
| Virulence | 0–0.005 | |
| Scaling factor for superinfection in species 2 (used as a multiplier of ) | 0–0.5 | |
| Scaling factor for virulence of strain (used as a multiplier of ) | 0–0.5 |
Equilibrated values of resident parasite.
Per capita birth rate is density-dependent: , with a carrying capacity of 100 individuals.
Mortality is constant and broadly consistent with a variety of rodent and avian hosts.
2.2. The stochastic model
We extended our analysis with stochastic simulations of the superinfection model and compared these with results from a stochastic host-specialization model, both implemented by the adaptive tau-leap method (Cao et al., 2007) in a Gillespie framework (Gillespie, 1977). Our chief aim was to examine whether appreciable cross-species transmission occurred in the absence of adaptive evolution. Modeling host specializations typically includes some form of explicit tradeoff in parasite transmission (Anderson and May, 1979, Gudelj et al., 2004, Regoes et al., 2000). The essential idea is that a parasite that increases its transmission to one host species does so at the cost of transmission to alternative host types. The cost to the specialist parasite is that by increasing its exploitation of one host, it consequently reduces its exploitation and transmission in alternative hosts (Frank, 1996, Regoes et al., 2000). We model this phenomenon by having two values, and , which represent a low and high transmission rate, respectively. We then compare this asymmetrical transmission model with the superinfection/virulence model in a stochastic framework. Aside from the absence of a single transmission parameter and the inclusion of two transmission parameters , the asymmetric transmission model shares an identical structure with the other models.
3. Results and discussion
Our modeling approach builds on previous work (Gudelj et al., 2004, Nowak and May, 1994, Regoes et al., 2000) with several novel extensions: (1) we incorporate superinfection in a heterogeneous host environment, rather than a single host population; (2) we assume a parasite strain superinfects distinct host species differentially, rather than equally—since primary infections are interactions between a parasite and host that can either mitigate or facilitate superinfection (Sadd and Schmid-Hempel, 2009, Telfer et al., 2010, Ulrich and Schmid-Hempel, 2012); and (3) strains do not have a transmission advantage in either host species. At low levels of virulence, the superinfection advantage of outweighs its virulence cost, leading to the complete dominance of in both host species (Fig. 2 A—Type I). At moderate virulence, we observe strong host association of the two strains, yielding and subpopulations. This phenomenon is indicated by the ‘windows’ outlined in dashed boxes in Fig. 2—Type III, which is defined by an 80/20 rule where at least 80% of all hosts infected by a strain belong to one species. Note that a low relative prevalence of in host species 2 represents a high prevalence of in host species 2. At higher virulence, the cost of outweighs its superinfection advantage, which leads to the dominance of in both host species (Fig. 2A—Type V). Lastly, at intermediate ranges of virulence we find an overlap in the host ranges of either strain (Fig. 2A—Types II and IV), which results from less dramatic changes in the cost–benefit balance of virulence and superinfection. Details on equilibria can be found in supplemental Figs. S1–S4. In contrast, if a mutant strain were to arise that had only one of the characteristics assumed here (superinfection or virulence) then it would either drive the resident strain extinct (superinfection alone) or go extinct itself (virulence alone) as demonstrated in supplemental Fig. S5. The variable patterns of parasite coexistence (Fig. 2, regimes I–V) requires both superinfection and virulence in the mutant strain .
Fig. 2.
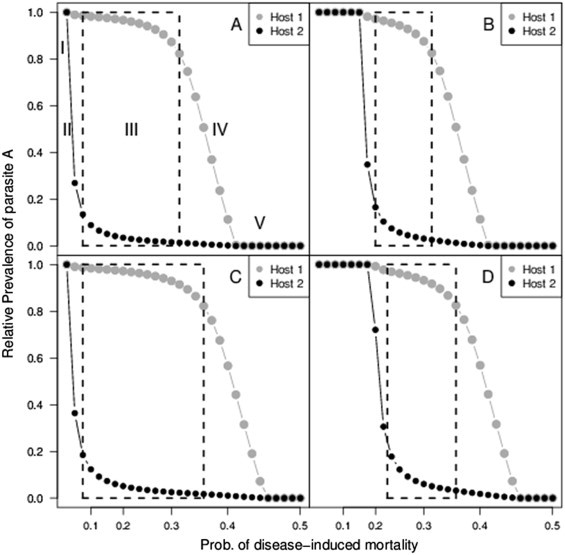
Host association patterns under varied assumptions.
Note: The four panels show the (equilibrium) relative prevalence of parasite in both host species on the -axis and the probability of disease-induced mortality on the -axis. Dashed boxes represent parameter space leading to reciprocal host association. Roman numerals (panel A) indicate types of host association patterns described in the main text. Panel A illustrates no superinfection of and no virulence cost for parasite . Panel B depicts and . Panel C depicts and . And panel D depicts and . The basic superinfection rate is set to 0.1 for all panels. Note the relative prevalences indicate that high reflects low , and low reflects high . The -axes show the probability of disease-induced mortality, defined as .
The four panels of Fig. 2 represent strict and relaxed parameterizations of the model. Fig. 2A represents the strict case where the difference between the mutant strain in host species 1 and 2 is most extreme (no superinfection in ), and no virulence cost for . We relax the assumptions of superinfection and virulence by allowing to additionally superinfect individuals in at a reduced rate (Fig. 2—panels B, D), and by setting a virulence cost for in both host species (see Fig. 2—panels C, D). The corresponding windows are altered as follows: (1) allowing superinfection in both hosts reduces the parameter space for host association; and (2) including a virulence cost for both parasites increases this parameter space.
Superinfection can explain host–parasite association in a unique manner: intrinsic generalists become host-associated due to outcomes of within-host competition. Conventional thinking dictates that adaptation creates a specialist parasite, and subsequent adaptation enables that parasite to cross the species’ barrier. Superinfection relaxes these conditions of adaptation, and can promote cross-species transmission while maintaining host-parasite associations (Fig. 3 and Table 2 ). Fig. 3 illustrates a comparison of superinfection versus host specialization and how each scenario influences cross-species transmission; indicated by relative prevalence approaching in both host species. Both mechanisms can produce similar, mean levels of host association. However, in the superinfection model, stochastic transmission can allow relative prevalences to transiently deviate from our 80/20 definition of host-parasite association, typifying outbreak dynamics without recurrent adaptive mutations. This is not the case under host specialization, which depicts strains and transmitting through preferred hosts with biased transmission efficiencies. In order for host specialization to present stochastic dynamics similar to superinfection, recurrent adaptation is required. Discerning the mechanism responsible for observed host-parasite association patterns from data is not trivial, especially since many time series are data-sparse and cover a short time interval. This finding raises questions on whether surveyed host associations (Brisson and Dykhuizen, 2004, Fallon et al., 2005, Garamszegi, 2006, Sasal et al., 1998, Streicker et al., 2010) are actually examples of host specialization. Host-parasite associations have been observed in distinct host orders (Blanquart and Gascuel, 2011, Fallon et al., 2005, Hanincová et al., 2006); equally, diverse parasite groups have been observed to form structured hosts associations, including macroparasites (Abollo et al., 1998), ectoparasites (Wells et al., 2013), viruses (Streicker et al., 2010), and bacteria (Brisson and Dykhuizen, 2004). Moreover, when host specialization does occur, it can be a consequence of host-parasite association, rather than a cause. For example, relaxed selection (Lahti et al., 2009, Remold et al., 2008) can account for specialization through erosion of (under-utilized) genetic determinants that facilitate transmission to alternative hosts. Another possibility is the fixation of specializing alleles by genetic drift, and not by natural selection. Consequently, the role of host specialization in driving host-parasite associations in nature is potentially exaggerated. A basic test for genuine host specialization is a two-host, two-parasite infection study that demonstrates a host preference either through greater transmissibility or a longer duration of infection. If surveyed host-association data supports host specialization while experimental infections do not, then we can be certain that an alternative mechanism is at play. Superinfection may be operating if there is evidence of a competitive advantage within a host type, and if the competitive parasite carries a high virulence cost relative to a sub-competitive parasite. The most immediate concern raised by our work relates to the study and prevention of zoonoses. Essentially, detecting a majority of parasite infections in a narrow group of wildlife species suggests, but does not confirm, host specialization. Therefore, targeting putative reservoir species through culling or vaccination may have unpredictable, or even undesirable, effects on disease dynamics (Bolzoni and De Leo, 2013, Choisy and Rohani, 2006).
Fig. 3.
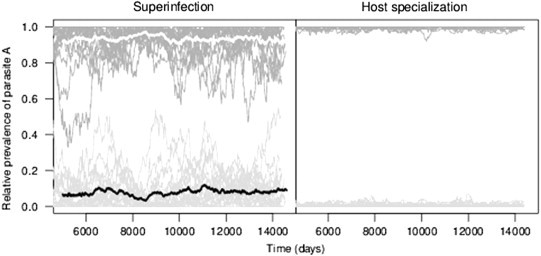
Superinfection versus host specialization.
Note: Shown above are results from stochastic formulations of the host association model (Fig. 1). The two panels show 25 stochastic projections of the relative prevalence of parasite in host 1 (dark gray) and host 2 (light gray) on the -axis against time on the -axis. The superinfection model is parameterized as shown in Fig. 2D; transmission is unbiased at and virulence is . White and black lines represent mean prevalences calculated from the stochastic projections. The host specialization model has no superinfection (), no virulence (), and a transmission bias ( or ) for the associated hosts ( and ). Trajectories that approach the interior of a plot indicate an increase of a parasite ( or ) in a host population from previously low levels; note that a decrease in the relative prevalence of indicates an increase in the relative prevalence of . Simulation time represents 40 years.
Table 2.
Parasite extinction rates under varied levels of virulence (stochastic superinfection model illustrated in Fig. 3).
| Type | Virulence | ||||||
|---|---|---|---|---|---|---|---|
| I | 0.0005 | 0.05 | 0.016 | 0.112 | 0.91 | 0.836 | 0.958 |
| II | 0.002 | 0.17 | 0.102 | 0.258 | 0.02 | 0.002 | 0.07 |
| III | 0.0025 | 0.29 | 0.203 | 0.389 | 0 | 0 | 0.036 |
| IV | 0.004 | 0.81 | 0.719 | 0.881 | 0 | 0 | 0.036 |
| V | 0.0045 | 0.97 | 0.914 | 0.993 | 0 | 0 | 0.03 |
Note: Extinction the number of simulations in which parasite or went extinct.
Lower lower bound binomial confidence limit .
Upper upper bound binomial confidence limit .
100 simulations were performed for each virulence level.
4. Conclusion
We conclude that host–parasite association and pervasive cross-species transmission can co-occur without recurrent adaptation, provided there are biases in superinfection between host species. Our study introduces a novel mechanism to the growing modeling literature in community epidemiology (Fenton and Pedersen, 2005, Roche et al., 2013), and provides new insight into the nature of host–parasite assemblages.
Acknowledgment
This work was supported by a complex systems grant from the James S. McDonnell Foundation.
Footnotes
Supplementary material related to this article can be found online at http://dx.doi.org/10.1016/j.tpb.2013.09.015.
Appendix A. Supplementary material
The following is the Supplementary material related to this article.
The electronic supplementary materials contain seven figures and associated text expanding on two important features of the deterministic model: (1) the contribution of model terms, e.g. superinfection and direct transmission, in determining model outcomes (Figs. S1–S4); and (2) a detailed exploration of parameter space and its effect on model outcomes (Figs. S5–S7).
References
- Abollo E., López A., Gestal C., Benavente P., Pascual S. Macroparasites in cetaceans stranded on the Northwestern Spanish Atlantic coast. Dis. Aquat. Org. 1998;32:227–231. doi: 10.3354/dao032227. [DOI] [PubMed] [Google Scholar]
- Altizer S., Bartel R., Han B.A. Animal migration and infectious disease risk. Science. 2011;331:296–302. doi: 10.1126/science.1194694. [DOI] [PubMed] [Google Scholar]
- Anderson R.M., May R.M. Population biology of infectious diseases: part I. Nature. 1979;280:361–367. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- Blanquart S., Gascuel O. Mitochondrial genes support a common origin of rodent malaria parasites and Plasmodium falciparum’s relatives infecting great apes. BMC Evol. Biol. 2011;11:70. doi: 10.1186/1471-2148-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzoni L., De Leo G.A. Unexpected consequences of culling on the eradication of wildlife diseases: the role of virulence evolution. Am. Nat. 2013;181:301–313. doi: 10.1086/669154. [DOI] [PubMed] [Google Scholar]
- Brisson D., Dykhuizen D.E. OSPC diversity in borrelia burgdorferi. Genetics. 2004;168:713–722. doi: 10.1534/genetics.104.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Gillespie D.T., Petzold L.R. Adaptive explicit–implicit tau-leaping method with automatic tau selection. J. Chem. Phys. 2007;126:224101. doi: 10.1063/1.2745299. [DOI] [PubMed] [Google Scholar]
- Choisy M., Rohani P. Harvesting can increase severity of wildlife disease epidemics. Proc. R. Soc. B. 2006;273:2025–2034. doi: 10.1098/rspb.2006.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A.P. What links bats to emerging infectious diseases? Science. 2005;310:628–629. doi: 10.1126/science.1120872. [DOI] [PubMed] [Google Scholar]
- Fallon S.M., Bermingham E., Ricklefs R.E. Host specialization and geographic localization of avian malaria parasites: a regional analysis in the lesser antilles. Am. Nat. 2005;165:466–480. doi: 10.1086/428430. [DOI] [PubMed] [Google Scholar]
- Fenton A., Pedersen A.B. Community epidemiology framework for classifying disease threats. Emerging Infect. Dis. 2005;11:1815–1821. doi: 10.3201/eid1112.050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S.A. Models of parasite virulence. Q. Rev. Biol. 1996;71:37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- Gandon S. Evolution of multihost parasites. Evolution. 2004;58:455–469. [PubMed] [Google Scholar]
- Garamszegi L.Z. The evolution of virulence and host specialization in malaria parasites of primates. Ecol. Lett. 2006;9:933–940. doi: 10.1111/j.1461-0248.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- Gillespie D.T. Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. 1977;81:2340–2361. [Google Scholar]
- Gudelj I., Fitt B.D.L., van den Bosch F. Evolution of sibling fungal plant pathogens in relation to host specialization. Phytopathology. 2004;94:789–795. doi: 10.1094/PHYTO.2004.94.7.789. [DOI] [PubMed] [Google Scholar]
- Hahn B.H., Shaw G.M., Cock De, K.M., Sharp P.M. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287:607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- Hanincová K., Kurtenbach K., Diuk-Wasser M., Brei B., Fish D. Epidemic spread of Lyme borreliosis, Northeastern United States. Emerging Infect. Dis. 2006;12:604–611. doi: 10.3201/eid1204.051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krief S., Escalante A.A., Pacheco M.A., Mugisha L., André C., Halbwax M., Fischer A., Krief J.-M., Kasenene J.M., Crandfield M., Cornejo O.E., Chavatte J.-M., Lin C., Letourneur F., Grüner A.C., McCutchan T.F., Rénia L., Snounou G. On the diversity of malaria parasites in African apes and the origin of Plasmodium falciparum from Bonobos. PLoS Pathog. 2010;6:e1000765. doi: 10.1371/journal.ppat.1000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtenbach K., De Michelis S., Etti S., Schäfer S.M., Sewell H.-S., Brade V., Kraiczy P. Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol. 2002;10:74–79. doi: 10.1016/s0966-842x(01)02298-3. [DOI] [PubMed] [Google Scholar]
- Lahti D.C., Johnson N.A., Ajie B.C., Otto S.P., Hendry A.P., Blumstein D.T., Coss R.G., Donohue K., Foster S.A. Relaxed selection in the wild. Trends Ecol. Evol. 2009;24:487–496. doi: 10.1016/j.tree.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Levene H. Genetic equilibrium when more than one ecological niche is available. Am. Nat. 1953;87:331–333. [Google Scholar]
- Mu J., Joy D.A., Duan J., Huang Y., Carlton J., Walker J., Barnwell J., Beerli P., Charleston M.A., Pybus O.G., Su X. Host switch leads to emergence of Plasmodium vivax malaria in humans. Mol. Biol. Evol. 2005;22:1686–1693. doi: 10.1093/molbev/msi160. [DOI] [PubMed] [Google Scholar]
- Nowak M.A., May R.M. Superinfection and the evolution of parasite virulence. Proc. Biol. Sci. 1994;255:81–89. doi: 10.1098/rspb.1994.0012. [DOI] [PubMed] [Google Scholar]
- Regoes R.R., Nowak M.A., Bonhoeffer S. Evolution of virulence in a heterogeneous host population. Evolution. 2000;54:64–71. doi: 10.1111/j.0014-3820.2000.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Remold S.K., Rambaut A., Turner P.E. Evolutionary genomics of host adaptation in vesicular stomatitis virus. Mol. Biol. Evol. 2008;25:1138–1147. doi: 10.1093/molbev/msn059. [DOI] [PubMed] [Google Scholar]
- Roche B., Rohani P., Dobson A.P., Guégan J.-F. The impact of community organization on vector-borne pathogens. Am. Nat. 2013;181:1–11. doi: 10.1086/668591. [DOI] [PubMed] [Google Scholar]
- Sadd B.M., Schmid-Hempel P. A distinct infection cost associated with trans-generational priming of antibacterial immunity in bumble-bees. Biol. Lett. 2009;5:798–801. doi: 10.1098/rsbl.2009.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasal P., Desdevises Y., Morand S. Host-specialization and species diversity in fish parasites: phylogenetic conservatism? Ecography. 1998;21:639–643. [Google Scholar]
- Streicker D.G., Turmelle A.S., Vonhof M.J., Kuzmin I.V., McCracken G.F., Rupprecht C.E. Host phylogeny constrains cross-species emergence and establishment of rabies virus in bats. Science. 2010;329:676–679. doi: 10.1126/science.1188836. [DOI] [PubMed] [Google Scholar]
- Telfer S., Lambin X., Birtles R., Beldomenico P., Burthe S., Paterson S., Begon M. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330:243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao J.I., Wootton J.T., Bunikis J., Luna M.G., Fish D., Barbour A.G. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl. Acad. Sci. USA. 2004;101:18159–18164. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich Y., Schmid-Hempel P. Host modulation of parasite competition in multiple infections. Proc. Biol. Sci. 2012;279:2982–2989. doi: 10.1098/rspb.2012.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T.P., Stilianakis N.I. Ecologic immunology of avian influenza (H5N1) in migratory birds. Emerg. Infect. Dis. 2007;13:1139–1143. doi: 10.3201/eid1308.070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells K., O’Hara R.B., Pfeiffer M., Lakim M.B., Petney T.N., Durden L.A. Inferring host specificity and network formation through agent-based models: tick-mammal interactions in Borneo. Oecologia. 2013:1–10. doi: 10.1007/s00442-012-2511-9. [DOI] [PubMed] [Google Scholar]
- Woolhouse M.E., Taylor L.H., Haydon D.T. Population biology of multihost pathogens. Science. 2001;292:1109–1112. doi: 10.1126/science.1059026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The electronic supplementary materials contain seven figures and associated text expanding on two important features of the deterministic model: (1) the contribution of model terms, e.g. superinfection and direct transmission, in determining model outcomes (Figs. S1–S4); and (2) a detailed exploration of parameter space and its effect on model outcomes (Figs. S5–S7).


