Abstract
Objective
Peptide hormones synthesized in gastrointestinal and adipose tissues in addition to neuropeptides regulate appetite and body weight. Previously, autoantibodies directed against melanocortin peptides were found in patients with eating disorders; however, it remains unknown whether autoantibodies directed against other appetite-regulating peptides are present in human sera and whether their levels are influenced by gut-related antigens.
Methods
Healthy women were studied for the presence of immunoglobulin (Ig) G and IgA autoantibodies directed against 14 key appetite-regulating peptides. The concept of molecular mimicry was applied to search in silico whether bacteria, viruses, or fungi contain proteins with amino acid sequences identical to appetite-regulating peptides. In addition, autoantibodies serum levels were studied in germ-free and specific pathogen-free rats.
Results
We found these IgG and IgA autoantibodies directed against leptin, ghrelin, peptide YY, neuropeptide Y, and other appetite-regulating peptides are present in human sera at levels of 100–900 ng/mL. Numerous cases of sequence homology with these peptides were identified among commensal and pathogenic micro-organisms including Lactobacilli, bacteroides, Helicobacter pylori, Escherichia coli, and Candida species. Decreased levels of IgA autoantibodies directed against several appetite-regulating peptides and increased levels of antighrelin IgG were found in germ-free rats compared with specific pathogen-free rats.
Conclusion
Healthy humans and rats display autoantibodies directed against appetite-regulating peptide hormones and neuropeptides, suggesting that these autoantibodies may have physiologic implications in hunger and satiety pathways. Gut-related antigens including the intestinal microflora may influence production of theses autoantibodies, suggesting a new link between the gut and appetite control.
Keywords: Neuroimmunology, Autoimmunity, Microbiota, Eating disorders, Gut–brain axis
Introduction
Peripheral and central regulatory peptides play important roles in mechanisms of appetite and body weight control. For instance, gastrointestinal or adipose tissue–derived peptide hormones such as ghrelin, insulin, peptide tyrosine-tyrosine (PYY), and leptin signal to the brain the state of hunger or satiety or energy storage [1], [2], [3], [4], [5], [6]. In the brain, these peptides interact with neuronal circuitries expressing orexigenic neuropeptides such as neuropeptide Y (NPY), agouti-related protein (AgRP), galanin, melanin-concentrating hormone (MCH), and orexin or anorexigenic neuropeptides such as α-melanocyte–stimulating hormone (α-MSH), corticotropin-releasing hormone (CRH), oxytocin, and vasopressin [7], [8], [9], [10], [11], [12]. In addition to appetite regulation, orexigenic and anorexigenic neuropeptides are involved in mechanisms related to stress, sleep/wakefulness, and reproductive, defensive/aggressive, and social behaviors, thereby integrating appetite, emotions, and other homeostatic functions [13], [14], [15]. Moreover, there is evidence that neuropeptides known for their central sites of action, such as NPY, also contribute to the mechanisms of energy homeostasis via peripheral effects [16].
Recently, autoantibodies (autoAbs) directed against two melanocortin (MC) peptides, α-MSH and adrenocorticotropic hormone (ACTH), have been detected in subjects with eating disorders, suggesting that the immune system may interfere with peptidergic systems involved in appetite and emotional control [17]. In fact, levels of α-MSH autoAbs correlated with the core psychopathologic traits in patients with eating disorders [18]. Moreover, a recent study implicated T cells in the cholecystokinin-mediated control of food intake [19]. Other studies have revealed the existence of autoAbs directed against several other regulatory peptides including vasoactive intestinal peptide, bradykinin, and hypocretin [20], [21], [22]. In addition, since the initial reports of insulin autoAbs before insulin treatment [23], [24], abundant data on autoAbs directed against insulin in diabetes have been accumulated [25]. These studies have reported that autoAbs directed against regulatory peptides can be also present in apparently healthy subjects, a finding that might signify physiologic roles of these autoAbs, such as modulation of the binding of regulatory peptides to their receptors [18] or post-transcriptional modification [20]. However, because self-reacting Abs are normally associated with autoimmune diseases [26], the presence of autoAbs directed against regulatory peptides in healthy subjects remains largely unexplored.
It is also unknown if autoAbs exist against appetite-regulating peptide hormones including ghrelin, PYY, or leptin or neuropeptides including NPY, AgRP, galanin, and CRH. Thus, in the present work, we investigated the presence in physiologic conditions of autoAbs directed against these peptides and studied gut-related factors that might influence production of these autoAbs. First, we examined healthy subjects for the presence of immunoglobulin (Ig) G and IgA autoAbs able to bind any of 14 regulatory peptides chosen for their key roles in mechanisms of appetite and body weight. The presence of the IgA class of autoAbs may point to a luminal, most commonly intestinal origin of the antigenic stimulation, including food-derived antigens and gut microflora [27].
In fact, the gut-associated lymphoid tissue is one of the main sources of IgA, produced by the class switch from IgM stimulated by cytokines such as transforming growth factor-β [28]. The IgA is the predominant immunoglobulin class in mucosal secretions and plays an important role by binding bacterial and viral antigens. In serum IgA accounts for 6–15% of total immunoglobulins. Although the systemic role of IgA has not been fully clarified, human peripheral blood neutrophils express Fcα immunoglobulin receptors that may trigger IgA-mediated functional responses including phagocytosis or release of interleukin-6 and tumor necrosis factor-α cytokines [29], [30]. In this study, we focused on the possible role of intestinal microflora in relation to neuropeptide autoAb response. However, the role of nutrients, as direct sources of antigens or as prebiotics, may also be important in this process. For instance, serum IgA antigliadin antibodies induced by a wheat protein comprise a well-established marker of celiac disease, but this antibody can also bind to neurons [31]. In contrast, IgG is the predominant class of immunoglobulins found in serum and it is the main molecule of adaptive immunity by activating complement and phagocytosis pathways. The presence of IgG reflects previous exposure to antigens.
The microflora of the gastrointestinal system is a major antigenic source with approximately 1014 micro-organisms [32]. Based on the concept of molecular mimicry [33], such intestinal antigens could potentially trigger the production of autoAbs cross-reacting with regulatory peptides. Hence, the second goal of this study was to determine the presence of amino acid (aa) sequence homologies between appetite-regulating peptides and microbial proteins of bacterial, viral, or fungal origin. To verify the relevance of intestinal microflora to the presence of these autoAbs, their serum levels were measured in germ-free and specific pathogen-free rats.
Materials and methods
Study subjects
Healthy female volunteers (n = 15, mean ± SD 30 ± 5 y of age, body mass index 21.5 ± 1 kg/m2) served as study subjects. They were recruited by the Department of Nutrition at Rouen University Hospital, France, and gave informed consent for involvement in the study approved by the Rouen University Hospital ethical committee. Routine venous blood samples were taken; serum was immediately separated by centrifugation and frozen at −20°C.
Detection of autoantibodies against appetite-regulating peptides
Serum levels of IgG or IgA autoAbs reacting with 14 regulatory peptides, including human leptin (amino acids 22–56), insulin (Sigma, St. Louis, MO, USA), PYY (amino acids 29–64), ghrelin (amino acids 24–51), NPY, AgRP (amino acids 83–132; Phoenix Pharmaceutical Inc., Belmont, CA, USA), galanin (amino acids 33–62), orexin A, MCH, α-MSH, ACTH, CRH, oxytocin, and vasopressin, were measured using an enzyme-linked immunosorbent assay (ELISA) technique. The peptides (all purchased from Bachem AG, Bubendorf, Switzerland, unless specified) were coated on Maxisorp plates (Nunc, Rochester, NY, USA) using 100 μL and a concentration of 2 μg/mL in 100 mM NaHCO3 buffer, pH 9.6, for 24 h at 4°C. Plates were washed (5 min × 3) in phosphate buffered saline (PBS) with 0.05% Tween 200, pH 7.4, and then incubated overnight at 4°C with 100 μL of serum from subjects diluted 1:100 in PBS. The plates were washed (×3) and incubated with 100 μL of alkaline phosphatase-conjugated rabbit anti-human IgG or IgA (1:2000; Sigma) for 3 h at room temperature. After washing (×3), 100 μL of p-nitrophenyl phosphate solution (Sigma) was added as an alkaline phosphatase substrate. After 40 min of incubation at room temperature, the reaction was stopped by adding 3 N NaOH. The optical density (OD) was determined at 405 nm using a microplate reader. Blank OD values resulting from the reading of plates without addition of human sera were subtracted from the sample OD values. The optimal dilutions of human sera (1:100) were determined by dilution curves (1:50, 1:100, 1:200). Each determination was done in duplicate. The coefficient of variation between duplicate values was <5%. To estimate the concentrations of measured autoAbs, standard linear curves (R 2 = 0.99) were obtained by sandwich ELISA on serial dilutions of total human IgG and IgA (Sigma) with known concentrations.
Briefly, ELISA plates were coated overnight with 100 μL of anti-human immunoglobulin polyvalent (Caltag Laboratories, Burlingame, CA, USA; 1:1000 in coating buffer). Plates were washed in PBS, incubated with 100 μL of 3% bovine serum albumin, washed again, and incubated overnight at 4°C with 24 serial dilutions (each 1:2) of IgG or IgA standards starting from 1 μg/mL. Plates were washed (×3) and incubated with 100 μL of alkaline phosphatase-conjugated rabbit anti-human IgG or IgA (1:2000; Sigma) for 3 h at room temperature. After washing (×3), the chromogenic reaction and OD reading was performed as described above. This ELISA test showed detection limits of 0.2 ng/mL for IgG and 1.0 ng/mL for IgA.
The specificity of IgA and IgG binding to each regulatory peptide was determined by ELISA after preadsorption of sera with decreasing concentrations of the corresponding synthetic peptide. In addition to whole serum (1:100 in PBS), sera from several subjects were purified from IgG using protein G agarose (Sigma, P7700) and from IgM using dialysis against distilled water via a membrane (MWCO 6-8000, Spectrum Laboratory, Inc. Interchim, France). The remaining serum (diluted 1:20 in PBS) containing IgA, but not IgG or IgM, was incubated overnight in decreasing concentrations of each peptide (0.2, 0.04, and 0.02 μg/μL) in a volume of 300 μL. Controls consisted of overnight incubation of the IgA-containing sera in PBS only. The ELISA was performed as described above with peptide coating (0.2 μg/mL). As an additional specificity control, the purified IgG fraction was tested on ELISA for the presence of IgA autoAbs.
Molecular mimicry with appetite-regulating peptides
To test the concept of molecular mimicry (for details, see discussion) in relation to the origin of autoAbs reacting with all 14 regulatory peptides, the aa sequence of these peptides was “blasted” in the protein databases of bacteria, viruses, fungi, or archaea at the National Center for Biotechnology Information (NCBI; Bethesda, MD, USA; www.ncbi.nlm.nih.gov). The identical (at least five aa consecutive sequences) were recorded and aligned with the sequence of the corresponding regulatory peptide (displaying the name of the source micro-organism). In some cases longer sequences of six or more identical amino acids were also included, even if the alignment was interrupted by one or two non-matching amino acids.
Autoantibodies against appetite-regulating peptides in rats
Germ-free and specific pathogen-free (SPF) 12-wk-old male Sprague-Dawley rats (n = 6/group) were purchased from Charles River Laboratories (L’Arbresle, France). The germ-free status of the rats was routinely verified before delivery by analyzing feces for aerobic and anaerobic bacteria and yeast growth. Upon arrival (germ-free rats in sterile boxes), all rats were immediately anesthetized by sodium thiopental (1 mL/kg, intraperitoneally) and 4-mL blood samples were taken from the right atrium. Body weight (mean ± SD) did not differ significantly between groups of germ-free and SPF rats (402.8 ± 43.3 versus 401.2 ± 3.5 g, respectively), and all animals were of good physical appearance. Serum was separated by centrifugation and the detection of autoAbs directed against appetite-regulating peptides was performed by ELISA as described above, with the exception of serum dilution (1:25) and the use of anti-rat IgG or anti-rat IgA goat antibodies conjugated with alkaline phosphatase at 1:500 (Rockland, Gilbertsville, PA, USA). Data were analyzed in the GraphPad inStat program (GraphPad Software Inc., San Diego, CA, USA) using Student’s t test or Mann-Whitney test according to the normality test.
Results
Detection of autoantibodies against appetite-regulating peptides in human subjects
Clearly detectable IgG and IgA autoAbs serum levels with signal intensity exceeding ≥4× background of the detection system (range 4–20×) reacting with leptin, insulin, PYY, ghrelin, NPY, AgRP, galanin, orexin A, MCH, α-MSH, ACTH, CRH, oxytocin, or vasopressin were found in all subjects. Signal intensity (OD 0.4–2.0) was in the linear range and was transformed into concentrations according the standard curve formula. Considering sample dilutions (1:100), plasma concentrations of these autoAbs were found to be in the range of 100–900 ng/mL (Fig. 1).
Fig. 1.
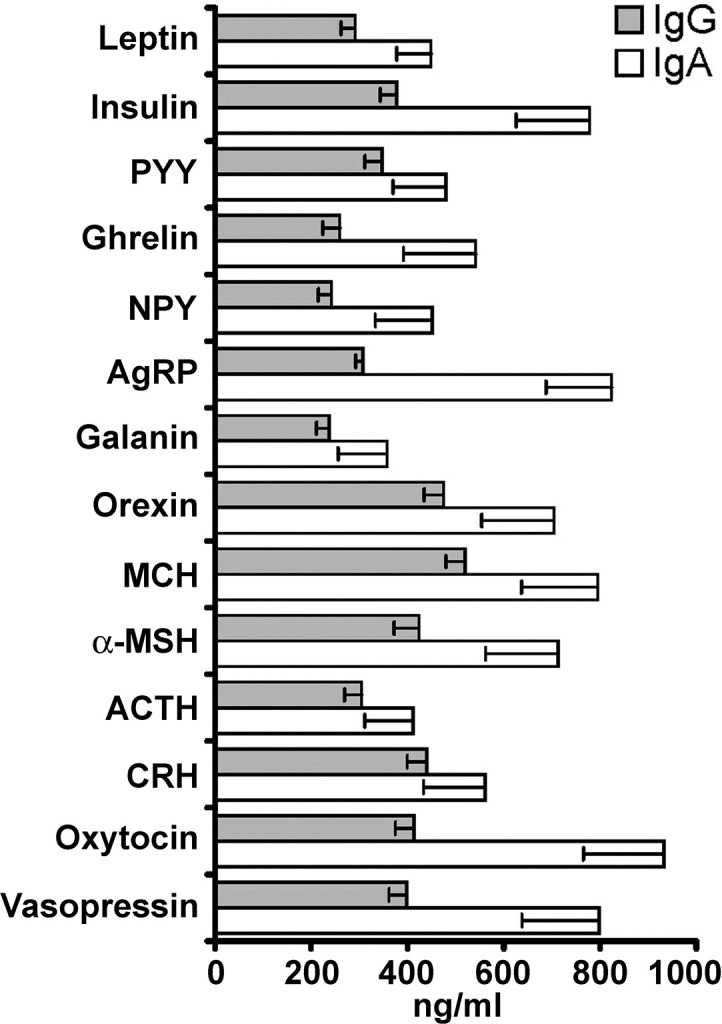
Serum concentration (mean ± SE) of IgG and IgA autoantibodies directed against appetite-regulating peptides in healthy women (n = 15). α-MSH, α-melanocyte–stimulating hormone; ACTH, adrenocorticotropic hormone; AgRP, agouti-related protein; CRH, corticotropin-releasing hormone; Ig, immunoglobulin; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; PYY, peptide tyrosine-tyrosine.
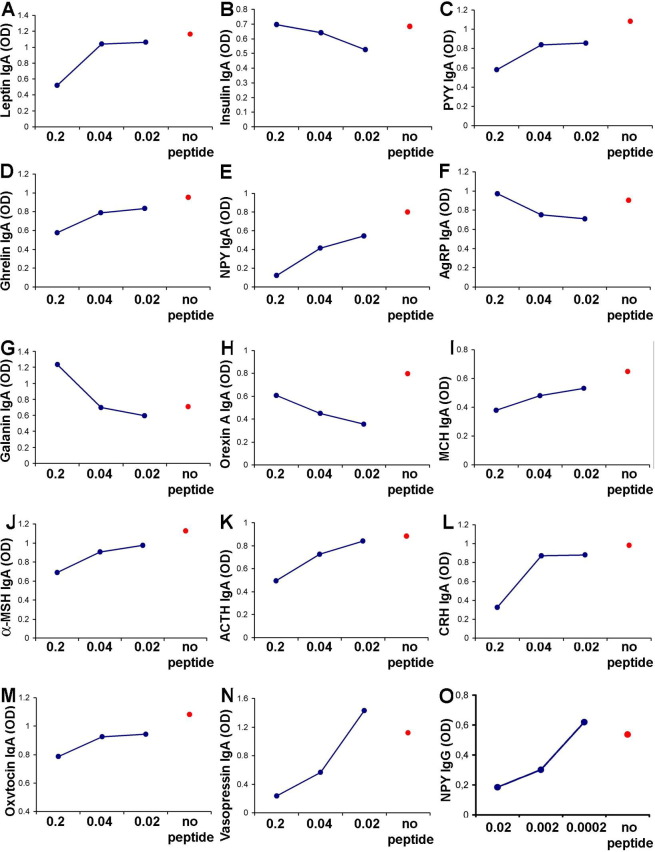
The specificity tests showed that preadsorption of the IgA-containing sera after extraction of IgG and IgM or of whole serum with increasing concentrations of each appetite-regulating synthetic peptide was able to dose-dependently modify the IgA levels (Fig. 2). Similar results were obtained in IgG preadsorption tests. In a majority of cases, increasing concentrations of preadsorbing peptide were associated with reduced IgA and IgG autoAb binding, but several reverse responses such as with insulin were also observed. Such a reverse response was seen previously in immunohistochemical studies [17] and is most likely related to the kinetics of interactions of preadsorbing peptide with the same peptide-containing immune complexes. In fact, insulin-containing immune complexes have been found in human subjects [34]. ELISA for IgA detection using the IgG fraction purified from the same serum did not result in any detectable signal.
Fig. 2.
Specificity control test (representative human serum) for IgA autoantibodies to bind appetite-regulating peptides. IgA-containing serum (after extraction of IgG and IgM) was preadsorbed with decreasing concentrations (0.2, 0.04, and 0.02 μg/μL) of corresponding synthetic peptides: (A) leptin, (B) insulin, (C) PYY, (D) ghrelin, (E) NPY, (F) AgRP, (G) galanin, (H) orexin A, (I) MCH, (J) α-MSH, (K) ACTH, (L) CRH, (M) oxytocin, and (N) vasopressin or no peptide was added (red dot). (O) Effect of preadsorption of an IgG-containing human serum with decreasing concentrations of NPY on binding to enzyme-linked immunosorbent assay plates coated with NPY. α-MSH, α-melanocyte–stimulating hormone; ACTH, adrenocorticotropic hormone; AgRP, agouti-related protein; CRH, corticotropin-releasing hormone; Ig, immunoglobulin; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; OD, optical density in enzyme-linked immunosorbent assay; PYY, peptide tyrosine-tyrosine.
Molecular mimicry with regulatory peptides
The NCBI database search for exact matches between aa sequences of appetite-regulating peptides and proteins from various micro-organisms showed that matches of at least five aa fragments within each of the 14 peptides exist among proteins from bacteria, viruses, fungi, and archaea (Fig. 3 and supplementary Fig. 1). For instance, within the α-MSH sequence (Fig. 3) six five-aa fragments and three six-aa fragments were identical to protein fragments of bacterial origin, four five-aa fragments to protein fragments from viruses, four five-aa fragments and two six-aa fragments to protein fragments from fungi, and six five-aa fragments and one six-aa fragment to protein fragments from archaea. In addition, many regulatory peptides exhibited alignments of more than six consecutive amino acids, such as leptin, insulin, PYY, ghrelin, NPY, AgRP, galanin, orexin, ACTH, and CRH.
Fig. 3.
Amino acid sequence alignment between α-melanocyte-stimulating hormone (α-MSH) and protein fragments from bacterial, viral fungal or archeal origin. Identical fragments of at least five amino acids are shown and referenced to the source micro-organism.
To facilitate the analysis of these data (summarized in Table 1, Table 2, Table 3, Table 4), the micro-organisms displaying sequence homology with regulatory peptides were grouped into commensal, intestinal pathogens, other pathogens, or pathogenic and environmental. Although some micro-organisms could play commensal and pathogenic roles, for simplicity they are presented only in one of these groups.
Table 1.
Examples of commensal, intestinal, or other pathogenic and environmental bacteria that display identical (at least five amino acids) proteins sequences with appetite-regulating peptides
| Bacteria | Peptides |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leptin | Insulin | PYY | Ghrelin | NPY | AgRP | Galanin | Orexin | MCH | α-MSH | ACTH | CRH | OT | VP | |
| Commensal | ||||||||||||||
| Lactococcus lactis | + | |||||||||||||
| Lactobacilli | + | + | + | |||||||||||
| Bifidobacterium longum | + | + | ||||||||||||
| Bacteroides | + | + | + | + | ||||||||||
| Bacillus cereus | + | + | ||||||||||||
| Escherichia coli strains | + | + | + | |||||||||||
| Intestinal pathogens | ||||||||||||||
| Escherichia coli strains | + | + | + | + | + | + | ||||||||
| Enterococcus faecalis | + | |||||||||||||
| Salmonella enterica | + | + | + | |||||||||||
| Helicobacter | + | + | + | + | ||||||||||
| Campylobacter | + | + | + | |||||||||||
| Staphylococcus aureus | + | |||||||||||||
| Enteropathogenic shigella | + | + | + | |||||||||||
| Clostridium perfringens | + | |||||||||||||
| Listeria monocytogenes | + | + | ||||||||||||
| Other pathogens | ||||||||||||||
| Yersinia pestis | + | + | + | |||||||||||
| Yersinia pseudotuberculosis | + | + | ||||||||||||
| Mycobacterium tuberculosis | + | |||||||||||||
| Clostridium tetani | + | + | ||||||||||||
| Burkholderia cepacia cmpx | + | + | + | + | + | + | + | + | ||||||
| Bordetella bronchiseptica | + | + | + | + | ||||||||||
| Neisseria gonorrhaeae | + | |||||||||||||
| Brucella suis | + | |||||||||||||
| Streptococcus agalactiae | + | |||||||||||||
| Treponema denticola | + | + | ||||||||||||
| Environmental | ||||||||||||||
| Cyanobacteria (soil, water) | + | + | + | + | ||||||||||
| Erwinia carotovorum (food) | + | + | ||||||||||||
| Corynebacterium (food) | + | + | ||||||||||||
| Pseudomonas (food) | + | + | ||||||||||||
| Xanthomonas (food) | + | + | + | + | ||||||||||
| Frankia (soil) | + | + | + | |||||||||||
| Streptomyces (soil) | + | + | + | + | + | + | ||||||||
α-MSH, α-melanocyte–stimulating hormone; ACTH, adrenocorticotropic hormone; AgRP, agouti-related protein; CRH, corticotropin-releasing hormone; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; OT, oxytocin; PYY, peptide tyrosine-tyrosine; VP, vasopressin
Table 2.
Examples of commensal, pathogenic, and environmental viruses that display identical (at least five amino acids) proteins sequences with appetite-regulating peptides
| Viruses | Peptides |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leptin | Insulin | PYY | Ghrelin | NPY | AgRP | Galanin | Orexin | MCH | α-MSH | ACTH | CRH | OT | VP | |
| Commensal | ||||||||||||||
| Lactobacilli bacteriophage | + | + | + | + | ||||||||||
| Enterobacteria phage | + | + | ||||||||||||
| Salmonella bacteriophage | + | |||||||||||||
| Mycobacteriophage | + | + | ||||||||||||
| Pathogenic | ||||||||||||||
| Hepatitis C virus | + | + | ||||||||||||
| Hepatitis B virus | + | |||||||||||||
| Influenza A virus | + | |||||||||||||
| Gastroenteritis virus | + | |||||||||||||
| HIV-1 | + | + | + | + | ||||||||||
| HIV-2 | + | |||||||||||||
| Orf virus | + | + | + | |||||||||||
| Human foamy virus | + | |||||||||||||
| Human coronavirus | + | |||||||||||||
| Human herpesvirus 4 | + | |||||||||||||
| Mayaro virus | + | |||||||||||||
| Rabies virus | + | |||||||||||||
| Environmental | ||||||||||||||
| Cowpox virus | + | |||||||||||||
| Porcine respiratory syndrome v. | + | + | ||||||||||||
| Duck adenovirus | + | |||||||||||||
| Suid herpes virus | + | |||||||||||||
| Gallid herpesvirus | + | |||||||||||||
| Lymphocystic disease v. (fish) | + | |||||||||||||
| Equine arteritis virus | + | |||||||||||||
α-MSH, α-melanocyte–stimulating hormone; ACTH, adrenocorticotropic hormone; AgRP, agouti-related protein; CRH, corticotropin-releasing hormone; HIV, human immunodeficiency virus; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; OT, oxytocin; PYY, peptide tyrosine-tyrosine; VP, vasopressin; v., virus
Table 3.
Examples of commensal or food-associated fungi which may become pathogenic in susceptible individuals as well as some environmental fungi which display identical (at least 5 a. a.) proteins sequences with appetite-regulating peptides
| Fungi | Peptides |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leptin | Insulin | PYY | Ghrelin | NPY | AgRP | Galanin | Orexin | MCH | α-MSH | ACTH | CRH | OT | VP | |
| Commensal (pathogenic) | ||||||||||||||
| Yarrowia lipolytica (food) | + | + | + | + | + | + | + | + | ||||||
| Saccharomyces cerevisiae (food) | + | + | + | + | + | + | + | |||||||
| Kluyveromyces lactis (food) | + | + | + | + | ||||||||||
| Debaryomyces hansenii (food) | + | + | + | |||||||||||
| Candida albicans | + | + | + | + | + | + | + | + | + | + | + | |||
| Candida glabrata | + | + | + | + | + | |||||||||
| Aspergillus fumigatus | + | + | + | + | + | + | + | + | + | + | ||||
| Aspergillus nidulans | + | + | + | + | + | + | + | + | ||||||
| Cryptococcus neoformans | + | + | + | + | + | + | + | + | ||||||
| Encephalitozoon cuniculi | + | + | ||||||||||||
| Environmental | ||||||||||||||
| Gibberella zeae (plants) | + | + | + | + | + | + | + | + | + | |||||
| Neurospora crassa (plants) | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Magnaporthe grisea (plants) | + | + | + | + | + | + | + | + | + | + | + | |||
| Ashbya gossypii (plants) | + | + | + | + | + | + | + | + | ||||||
| Ustilago maydis (plants) | + | + | + | + | ||||||||||
| Schizosaccharomyces pombe (plants) | + | + | ||||||||||||
| Blumeria graminis (plants) | + | + | ||||||||||||
α-MSH, α-melanocyte–stimulating hormone; ACTH, adrenocorticotropic hormone; AgRP, agouti-related protein; CRH, corticotropin-releasing hormone; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; OT, oxytocin; PYY, peptide tyrosine-tyrosine; VP, vasopressin
Table 4.
Examples of environmental archea which display identical (at least 5 a. a.) proteins sequences with appetite-regulating peptides
| Archaea | Peptides |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leptin | Insulin | PYY | Ghrelin | NPY | AgRP | Galanin | Orexin | MCH | α-MSH | ACTH | CRH | OT | VP | |
| Environmental | ||||||||||||||
| Methanosarcina (ruminants) | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Methanococcoides (ruminants) | + | + | + | |||||||||||
| Methanopyrus kandleri (hot sources) | + | + | + | + | + | + | + | |||||||
| Methanococcus (hot sources) | + | + | + | |||||||||||
| Methanothermobacter (hot sources) | + | + | + | |||||||||||
| Aeropyrum pernix (hot sources) | + | + | + | + | + | |||||||||
| Sulfolobus (hot sources) | + | + | + | + | + | + | + | + | + | |||||
| Pyrococcus (hot sources) | + | + | + | + | + | + | + | |||||||
| Halobacteria (salt-rich sources) | + | + | + | + | + | + | ||||||||
| Thermococcus (hot sources) | + | + | + | + | ||||||||||
α-MSH, α-melanocyte–stimulating hormone; ACTH, adrenocorticotropic hormone; AgRP, agouti-related protein; CRH, corticotropin-releasing hormone; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; OT, oxytocin; PYY, peptide tyrosine-tyrosine; VP, vasopressin
Autoantibodies against appetite-regulating peptides in rats
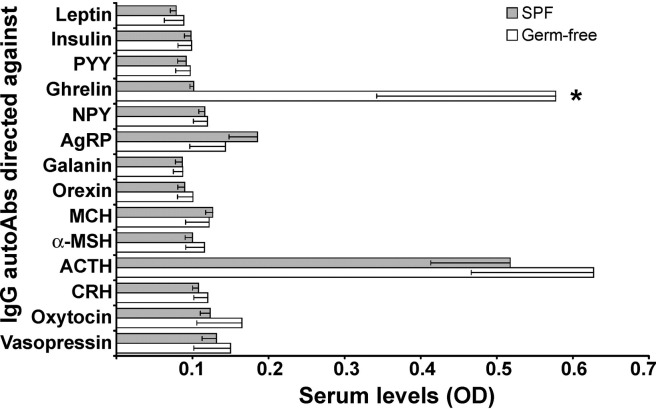
For many of the regulatory peptides studied, the levels of corresponding IgG autoAbs in germ-free and SPF rats displayed comparatively low OD values (2–3× of background OD; Fig. 4). However, the levels of IgG autoAbs directed against ghrelin in germ-free rats and against ACTH in germ-free and SPF rats were found in a range of around 10× background OD (Fig. 4). The levels of ghrelin IgG autoAbs in germ-free rats were significantly higher than in SPF rats (t test, P < 0.05).
Fig. 4.
Serum levels of IgG autoAbs directed against appetite-regulating peptides in germ-free and SPF male Sprague-Dawley rats (mean ± SE). *T test, P < 0.05. α-MSH, α-melanocyte–stimulating hormone; ACTH, adrenocorticotropic hormone; AgRP, agouti-related protein; autoAbs, autoantibodies; CRH, corticotropin-releasing hormone; IgA, immunoglobulin A; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; OD, optical density in enzyme-linked immunosorbent assay; PYY, peptide tyrosine-tyrosine; SPF, specific pathogen-free.
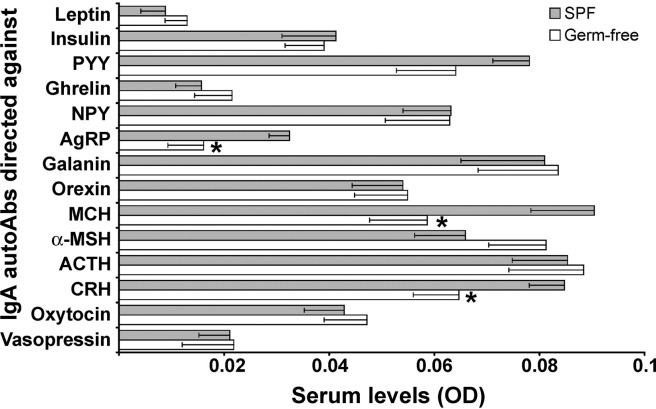
The levels of IgA autoAbs directed against all studied regulatory peptides in germ-free and SPF rats were at the limit of detection (OD values 1–2× background OD; Fig. 5). Germ-free rats displayed significantly lower levels of autoAbs directed against three neuropeptides including AgRP and MCH (both t test, P < 0.05) and CRH (Mann-Whitney test, P < 0.05) compared with SPF rats.
Fig. 5.
Serum levels of IgA autoAbs directed against appetite-regulating peptides in germ-free and SPF male Sprague-Dawley rats (mean ± SE). *T test, P < 0.05; *Mann-Whitney test, P < 0.05, for CRH. α-MSH, α-melanocyte–stimulating hormone; ACTH, adrenocorticotropic hormone; AgRP, agouti-related protein; autoAbs, autoantibodies; CRH, corticotropin-releasing hormone; IgA, immunoglobulin A; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; OD, optical density in enzyme-linked immunosorbent assay; PYY, peptide tyrosine-tyrosine; SPF, specific pathogen-free.
Discussion
Finding of autoantibodies directed against appetite-regulating peptides
This study revealed, for the first time, in healthy human subjects, the presence of autoAbs of IgG and IgA classes reacting specifically with several appetite-regulating peptides, including leptin, ghrelin, PYY, NPY, AgRP, galanin, or CRH. The levels of these autoAbs were in the range of 100–900 ng/mL, probably sufficient to influence functions of corresponding regulatory peptides normally present in plasma in the range of picograms to nanograms per milliliter [35].
Autoantibodies directed against all studied appetite-regulating peptides were found at similar levels with levels of insulin autoAbs, which have been extensively studied as a marker of type 1 diabetes, but their levels alone show low predictive value [25]. Although insulin autoAbs were repeatedly reported in healthy subjects, their physiologic levels (mean ±3 SDs) were used merely to define the detection limit for autoAbs in diabetic patients, with around 49% displaying elevated values compared with 3% of the general population [36]. The limited interest in insulin autoAbs in healthy subjects is probably related to the view that production of insulin autoAbs must be triggered by “immunogenic” insulin derived from pancreatic β-cells destroyed by an autoimmune process, and the assumption that these autoAbs should reflect the disease [37]. Thus, a potential physiologic origin and function of insulin autoAbs has thus far not been considered. The present data showing that autoAbs directed against insulin and against other regulatory peptides are constantly present in healthy subjects suggest that these autoAbs represent a general phenomenon of possible physiologic significance.
Origin of autoantibodies directed against appetite-regulating peptides
The presence of the IgA class of autoAbs directed against regulatory peptides found in human subjects suggests that at least some of these autoAbs might be triggered by luminal antigens that can include food-related antigens and gut microflora. In fact, the major role of commensal and pathogenic microflora in inducing IgA responses has been previously shown [38], [39], [40]. However, our results obtained from the germ-free rats show that commensal microflora are not required for the presence of IgA or IgG autoAbs directed against regulatory peptides but can selectively influence the levels of at least some of these autoAbs. For instance, the relatively lower levels of IgA autoAbs directed against AgRP, MCH, and CRH found in germ-free rats might be related to the absence of intestinal antigens. However, it appears that the presence of SPF rat microflora would inhibit the production of IgG autoAbs directed against ghrelin, suggesting a complex mechanism of interaction between microbial antigens and levels of IgG autoAbs. The potential ability of the microflora to selectively modulate the levels of some regulatory peptide autoAbs could also be related to the concept of molecular mimicry.
Concept of molecular mimicry
The presence of fragments with identical sequences between microbial proteins and regulatory peptides suggests that such microbial proteins presenting these sequences in the Payer’s patches or other lymphoid organs may stimulate the production of immunoglobulins capable of binding to the identical region present in endogenous regulatory peptides. According to the concept of molecular mimicry, the functional implications of such sequence similarities are possible only if the protein fragment targeted by self-reacting antibodies possesses biological activity [33]. For example, it has been shown that six consecutive amino acids shared between hepatitis B virus and myelin basic protein is sufficient to induce experimental autoimmune encephalomyelitis [41]. In the case of regulatory peptides, which are released and function as messenger molecules, any part of their entire sequence targeted by autoAbs could result in modification of their biological activity. Radioimmunoassay and immunohistochemical studies have shown that antibodies generated by immunization are able to selectively recognize regulatory peptides as small as the three-aa peptide thyrotropin-releasing hormone [42], [43]. In the present study, we recorded the presence of at least five identical consecutive aa fragments in regulatory peptides and microbial proteins qualifying as candidates for molecular mimicry [44]. However, as indicated above, it is important to note that sequences shorter than five amino acids could be implicated, in addition to discontinuous fragment homology. Of note, only a limited number of microbial protein sequences are currently available in the NCBI database, and molecular mimicry between regulatory peptides and microbial proteins may thus be more extensive.
Molecular mimicry with commensal micro-organisms
Our screening revealed that molecular mimicry for each studied regulatory peptide is present at least in one micro-organism and usually in several representatives of commensal or relatively pathogenic micro-organisms. For instance, peptide fragments identical to leptin were found in proteins belonging to Lactococcus lactis, Escherichia coli, Lactobacilli bacteriophage, Yarrowia lipolytica, Candida, and Aspergillus species. Notably, the most frequent molecular mimicry (7–12 of the examined regulatory peptides) occurs with the class of fungi (Table 3). They include the commensal or relatively pathogenic yeasts Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans, the food-associated yeasts Y. lipolytica and Saccharomyces, and several plant fungi such as Neurospora crassa. Anorexigenic and orexigenic peptides displayed molecular mimicry with the same fungus, thus not allowing an association of a single fungus species with a potential specific effect on food intake. Among the commensal bacteria and viruses, sequence homology was often seen in Lactobacilli and their phages, bacteroides, and commensal strains of E. coli, which displayed molecular mimicry with leptin, insulin, ghrelin, PYY, NPY, AgRP, orexin, α-MSH, ACTH, oxytocin, or vasopressin. These results suggest that commensal microflora could trigger production of many autoAbs reactive with these regulatory peptides, and that eventually, under normal conditions, not a single regulatory peptide may escape this microbial-derived immune-mediated control.
We found that commensal or relatively pathogenic Enterococcus faecalis displayed molecular mimicry only with ghrelin. The fact that E. faecalis is present in the original microflora of SPF rats bred in Charles River Laboratories is relevant to our finding that the levels of ghrelin-reactive IgG was higher in germ-free than in SPF rats. These data support the idea that a negative link could exist between production of some IgG autoAbs and selective micro-organisms [45]. Moreover, a recent finding that active immunization with ghrelin fragments reduces adiposity in mice [46] supports our conclusions that ghrelin autoAbs may participate in physiologic regulation of appetite and body weight.
Molecular mimicry with pathogenic micro-organisms
We previously reported that levels of α-MSH–reactive IgG autoAbs are associated with core psychopathologic traits in anorexia and bulimia nervosa [18]. In the present study, we demonstrated that various fragments of the α-MSH sequence exists in several pathogenic micro-organisms including enteropathogenic E. coli strains, Helicobacter pylori, Clostridium tetani, human immunodeficiency virus type 1, C. albicans, A. fumigatus, and C. neoformans. Some of these micro-organisms may therefore trigger the production of α-MSH–reactive autoAbs relevant to the pathophysiology of eating disorders.
Interestingly, molecular mimicry related to proteins belonging to H. pylori was found only among the anorexigenic peptides (Table 1), e.g., leptin, insulin, α-MSH, and ACTH, suggesting that H. pylori–triggered autoAbs reactive with these regulatory peptides could selectively alter satiety pathways resulting in appetite change. In fact, the presence of H. pylori has been associated with decreased adiposity [47], high levels of stomach leptin [48], and insulin resistance [49].
The sequence homology of insulin with pathogenic micro-organisms such as H. pylori, Burkholderia cepacia, Yersinia pseudotuberculosis, hepatitis C virus, and gastroenteritis virus may be responsible for the production of some insulin-reactive autoAbs. These autoAbs, in our view, would not be markers of pancreatic β-cell lysis, but could be mechanistically relevant to altered insulin signaling in diabetes. In fact, it has been shown that sera of some diabetic patients can inhibit insulin binding to its receptor [50]. Recent data corroborate our conclusions showing that some pathologic properties of insulin autoAbs [51] may be related to their epitopes [52], whereas changes in intestinal microflora were associated with the development of diabetes and obesity [53], [54].
Physiologic versus pathologic role of autoantibodies
The presence of various sequence homologies for the same regulatory peptide found in commensal and pathogenic micro-organisms suggests that they could be responsible for the appearance of cross-reacting autoAbs. However, the functional implication of binding of such autoAbs to the regulatory peptide could be different depending on what part of the molecule was targeted. We found that sequence homologies were often different between commensal and pathogenic micro-organisms. For instance, commensal bacteroides have mimicry close to the carboxyl-terminal side of α-MSH, whereas pathogenic H. pylori displays homology with the larger part of its carboxyl-terminal and pathogenic E. coli strains with various regions of the peptide (Fig. 3A). It is well known that different parts of the α-MSH peptide have specific functions [55]. For example, the central core sequence HFRW is responsible for the binding of α-MSH to the appetite-related melanocortin receptor MC4; the amino terminal SYS is important for binding at MC3 and MC5 receptors, thus influencing autonomic functions; and the amino terminal KPV sequence may trigger the anti-inflammatory effects at MC1 receptors [56], [57]. Thus, autoAbs binding to different regions of α-MSH peptide could differentially influence its biological activities.
It is likely that infections or changes in the composition of intestinal microflora could result in an appearance of pathologic autoAbs able to alter peptidergic transmission with corresponding metabolic and behavioral consequences. The factors triggering such a switch may be related directly to alterations of intestinal microflora, such as special diets, starvation, antibiotic or antifungal treatment, and gastrointestinal infections, or to changes in the mechanisms of antigen presentation to the immune system, including alterations of the intestinal barrier by inflammation or stress [58], and genetic vulnerability in downstream pathways of the immune system leading to autoAb production. In fact, many of these factors have previously been proposed to contribute to the etiology of eating disorders, one example being the association of anorexia nervosa with streptococcal infection [59]. Following this example, we have in this study revealed the existence of sequence homology between Streptococcus and leptin and in a previous study with gonadotropin-releasing hormone [60], presenting a potential link between micro-organisms and immune-mediated regulation of appetite-related peptides. Interestingly, increased levels of autoAbs against gonadotropin-releasing hormone were associated with intestinal pseudo-obstruction [61], suggesting that this link might also be relevant to the regulation of the gut propulsive activity considering important role of several neuropeptides including CRH in gut motor function [62].
Concluding remarks
Some gastrointestinal and neuropsychiatric disorders may have pathophysiologic mechanisms in common, involving the bidirectional brain–gut axis signaling through humoral and neural routes [63], [64], [65], [66]. The present work suggests an additional link between the gut and the brain, which could contribute to the elaborate interactions existing between the immune and nervous systems [67], [68], [69]. In fact, the results of this study show that the phenomenon of autoAbs directed against regulatory peptides is not restricted to pathologic conditions but may be involved in the regulation of energy and emotional homeostases in healthy subjects. Furthermore, our data provide evidence that production of these autoAbs can be influenced by intestinal microflora, suggesting that autoAbs directed against regulatory peptides may constitute a functional link between intestinal microflora and mechanisms of appetite and emotion.
Such a link may represent a fundamental evolutionary mechanism of adaptation, whereby intrinsic neuroendocrine mechanisms of homeostasis are tuned according to the prevailing microbial environment. Moreover, our data demonstrate multiple cases of molecular mimicry of regulatory peptides with microbial proteins, identifying micro-organisms as putative biological targets to be tested for their relevance to the normal or pathophysiologic mechanisms of appetite and emotion.
Acknowledgments
The authors thank Dr. Danièle Gilbert from the Experimental and Clinical Immunology Laboratory, INSERM U519, Rouen University, France for methodologic help.
Footnotes
This study was supported by the Rouen University Hospital (02-037HP), the Swedish Science Council, and the Torsten and Ragnar Söderberg’s Foundation.
The online version of this article contains supplementary data.
Supplementary data associated with this doi:10.1016/j.nut.2007.12.006.
Supplementary data
References
- 1.Friedman J.M., Halaas J.L. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 2.Niswender K.D., Baskin D.G., Schwartz M.W. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab. 2004;15:362–369. doi: 10.1016/j.tem.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Williams D.L., Cummings D.E. Regulation of ghrelin in physiologic and pathophysiologic states. J Nutr. 2005;135:1320–1325. doi: 10.1093/jn/135.5.1320. [DOI] [PubMed] [Google Scholar]
- 4.Strader A.D., Woods S.C. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–191. doi: 10.1053/j.gastro.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 5.Murphy K.G., Bloom S.R. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 6.Kojima M., Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 7.Leibowitz S.F., Wortley K.E. Hypothalamic control of energy balance: different peptides, different functions. Peptides. 2004;25:473–504. doi: 10.1016/j.peptides.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Ellacott K.L.J., Cone R.D. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Prog Horm Res. 2004;59:395–408. doi: 10.1210/rp.59.1.395. [DOI] [PubMed] [Google Scholar]
- 9.Meister B. Control of food intake via leptin receptors in the hypothalamus. Vitam Horm. 2000;59:265–304. doi: 10.1016/s0083-6729(00)59010-4. [DOI] [PubMed] [Google Scholar]
- 10.Pissios P., Maratos-Flier E. Melanin-concentrating hormone: from fish skin to skinny mammals. Trends Endocrinol Metab. 2003;14:243–248. doi: 10.1016/s1043-2760(03)00079-1. [DOI] [PubMed] [Google Scholar]
- 11.Sakurai T. Reverse pharmacology of orexin: from an orphan GPCR to integrative physiology. Regul Pept. 2005;126:3–10. doi: 10.1016/j.regpep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Winsky-Sommerer R., Yamanaka A., Diano S., Borok E., Roberts A.J., Sakurai T. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolaidis S. Metabolic mechanism of wakefulness (and hunger) and sleep (and satiety): role of adenosine triphosphate and hypocretin and other peptides. Metabolism. 2006;55:S24–S29. doi: 10.1016/j.metabol.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Kishi T., Elmquist J.K. Body weight is regulated by the brain: a link between feeding and emotion. Mol Psychiatry. 2005;10:132–146. doi: 10.1038/sj.mp.4001638. [DOI] [PubMed] [Google Scholar]
- 15.Kalra S.P., Kalra P.S. NPY—an endearing journey in search of a neurochemical on/off switch for appetite, sex and reproduction. Peptides. 2004;25:465–471. doi: 10.1016/j.peptides.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Kuo L.E., Kitlinska J.B., Tilan J.U., Li L., Baker S.B., Johnson M.D. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 17.Fetissov S.O., Hallman J., Oreland L., af Klinteberg B., Grenbäck E., Hulting A.L. Autoantibodies against α-MSH, ACTH, and LHRH in anorexia and bulimia nervosa patients. Proc Natl Acad Sci U S A. 2002;99:17155–17160. doi: 10.1073/pnas.222658699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fetissov S.O., Harro J., Jaanisk M., Järv A., Podar I., Allik J. Autoantibodies against neuropeptides are associated with psychological traits in eating disorders. Proc Natl Acad Sci U S A. 2005;102:14865–14870. doi: 10.1073/pnas.0507204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDermott J.R., Leslie F.C., D’Amato M., Thompson D.G., Grencis R.K., McLaughlin J.T. Immune control of food intake: enteroendocrine cells are regulated by CD4+ T lymphocytes during small intestinal inflammation. Gut. 2006;55:492–497. doi: 10.1136/gut.2005.081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul S., Volle D.J., Beach C.M., Johnson D.R., Powell M.J., Massey R.J. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science. 1989;244:1158–1162. doi: 10.1126/science.2727702. [DOI] [PubMed] [Google Scholar]
- 21.Myagkova M.A., Gavrilova S.I., Lermontova N.N., Kalyn Y.B., Selezneva N.D., Zharikov G.A. Content of autoantibodies to bradykinin and beta-amyloid(1-42) as a criterion for biochemical differences between Alzheimer’s dementias. Bull Exp Biol Med. 2003;136:49–52. doi: 10.1023/a:1026036829237. [DOI] [PubMed] [Google Scholar]
- 22.Black J.L., III, Silber M.H., Krahn L.E., Fredrickson P.A., Pankratz V.S., Avula R. Analysis of hypocretin (orexin) antibodies in patients with narcolepsy. Sleep. 2005;28:427–431. doi: 10.1093/sleep/28.4.427. [DOI] [PubMed] [Google Scholar]
- 23.Sebriakova M., Little J.A. A method for the determination of plasma insulin antibodies and its application in normal and diabetic subjects. Diabetes. 1973;22:30–40. doi: 10.2337/diab.22.1.30. [DOI] [PubMed] [Google Scholar]
- 24.Goldman J., Baldwin D., Rubenstein A.H., Klink D.D., Blackard W.G., Fisher L.K. Characterization of circulating insulin and proinsulin-binding antibodies in autoimmune hypoglycemia. J Clin Invest. 1979;63:1050–1059. doi: 10.1172/JCI109374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franke B., Galloway T.S., Wilkin T.J. Developments in the prediction of type 1 diabetes mellitus, with special reference to insulin autoantibodies. Diabetes Metab Res Rev. 2005;21:395–415. doi: 10.1002/dmrr.554. [DOI] [PubMed] [Google Scholar]
- 26.Palmer J.P., Asplin C.M., Clemons P., Lyen K., Tatpati O., Raghu P.K. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222:1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 27.Macpherson A.J., Hunziker L., McCoy K., Lamarre A. IgA responses in the intestinal mucosa against pathogenic and non-pathogenic microorganisms. Microbes Infect. 2001;3:1021–1035. doi: 10.1016/s1286-4579(01)01460-5. [DOI] [PubMed] [Google Scholar]
- 28.Tezuka H., Abe Y., Iwata M., Takeuchi H., Ishikawa H., Matsushita M. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 29.Weisbart R.H., Kacena A., Schuh A., Golde D.W. GM-CSF induces human neutrophil IgA-mediated phagocytosis by an IgA Fc receptor activation mechanism. Nature. 1988;332:647–648. doi: 10.1038/332647a0. [DOI] [PubMed] [Google Scholar]
- 30.Patry C., Herbelin A., Lehuen A., Bach J.F., Monteiro R.C. Fc alpha receptors mediate release of tumour necrosis factor-alpha and interleukin-6 by human monocytes following receptor aggregation. Immunology. 1995;86:1–5. [PMC free article] [PubMed] [Google Scholar]
- 31.Alaedini A., Okamoto H., Briani C., Wollenberg K., Shill H.A., Bushara K.O. Immune cross-reactivity in celiac disease: anti-gliadin antibodies bind to neuronal synapsin I. J Immunol. 2007;178:6590–6595. doi: 10.4049/jimmunol.178.10.6590. [DOI] [PubMed] [Google Scholar]
- 32.Bäckhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host–bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 33.Oldstone M.B. Molecular mimicry and immune-mediated diseases. FASEB J. 1998;12:1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiittanen M., Knip M., Vaarala O. Anti-insulin activity in IgG-fractions from children with newly-diagnosed type 1 diabetes and negative for insulin autoantibodies. Autoimmunity. 2004;37:45–49. doi: 10.1080/0887044032000158929. [DOI] [PubMed] [Google Scholar]
- 35.Hökfelt T., Bartfai T., Bloom F. Neuropeptides: opportunities for drug discovery. Lancet Neurol. 2003;2:463–472. doi: 10.1016/s1474-4422(03)00482-4. [DOI] [PubMed] [Google Scholar]
- 36.Strebelow M., Schlosser M., Ziegler B., Rjasanowski I., Ziegler M. Karlsburg type I diabetes risk study of a general population: frequencies and interactions of the four major type I diabetes-associated autoantibodies studied in 9419 schoolchildren. Diabetologia. 1999;42:661–670. doi: 10.1007/s001250051213. [DOI] [PubMed] [Google Scholar]
- 37.Leslie D., Lipsky P., Notkins A.L. Autoantibodies as predictors of disease. J Clin Invest. 2001;108:1417–1422. doi: 10.1172/JCI14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balish E., Yale C.E., Hong R. Serum proteins of gnotobiotic rats. Infect Immun. 1972;6:112–118. doi: 10.1128/iai.6.2.112-118.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shroff K., Meslin K., Cebra J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macpherson A.J., Gatto D., Sainsbury E., Harriman G.R., Hengartner H., Zinkernagel R.M. A primitive T cell–independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 41.Fujinami R.S., Oldstone M.B. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985;230:1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- 42.Oliver C., Eskay R.L., Ben-Janathan N., Porter J.C. Distribution and concentration of TRH in the rat brain. Endocrinology. 1974;95:540–546. doi: 10.1210/endo-95-2-540. [DOI] [PubMed] [Google Scholar]
- 43.Hökfelt T., Fuxe K., Johansson O., Jeffcoate S., White N. Distribution of thyrotropin-releasing hormone (TRH) in the central nervous system as revealed with immunohistochemistry. Eur J Pharmacol. 1975;34:389–392. doi: 10.1016/0014-2999(75)90269-1. [DOI] [PubMed] [Google Scholar]
- 44.Oldstone M.B. Molecular mimicry, microbial infection, and autoimmune disease: evolution of the concept. Curr Top Microbiol Immunol. 2005;296:1–17. doi: 10.1007/3-540-30791-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallberg M., Harris R.A. Co-infection with Trypanosoma brucei brucei prevents experimental autoimmune encephalomyelitis in DBA/1 mice through induction of suppressor APCs. Int Immunol. 2005;17:721–728. doi: 10.1093/intimm/dxh253. [DOI] [PubMed] [Google Scholar]
- 46.Zorrilla E.P., Iwasaki S., Moss J.A., Chang J., Otsuji J., Inoue K. From the cover: vaccination against weight gain. Proc Natl Acad Sci U S A. 2006;103:13226–13231. doi: 10.1073/pnas.0605376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamada T., Hata J., Kusunoki H., Ito M., Tanaka S., Kawamura Y. Eradication of Helicobacter pylori increases the incidence of hyperlipidaemia and obesity in peptic ulcer patients. Dig Liver Dis. 2005;37:39–43. doi: 10.1016/j.dld.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 48.Azuma T., Suto H., Ito Y., Ohtani M., Dojo M., Kuriyama M. Gastric leptin and Helicobacter pylori infection. Gut. 2001;49:324–329. doi: 10.1136/gut.49.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aydemir S., Bayraktaroglu T., Sert M., Sokmen C., Atmaca H., Mungan G. The effect of Helicobacter pylori on insulin resistance. Dig Dis Sci. 2005;50:2090–2093. doi: 10.1007/s10620-005-3012-z. [DOI] [PubMed] [Google Scholar]
- 50.Zick Y., Rees-Jones R., Taylor S., Gorden P., Roth J. The role of antireceptor antibodies in stimulating phosphorylation of the insulin receptor. J Biol Chem. 1984;259:4396–4400. [PubMed] [Google Scholar]
- 51.Achenbach P., Schlosser M., Williams A.J., Yu L., Mueller P.W., Bingley P.J. Combined testing of antibody titer and affinity improves insulin autoantibody measurement: Diabetes Antibody Standardization Program. Clin Immunol. 2007;122:85–90. doi: 10.1016/j.clim.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Padoa C.J., Crowther N.J., Thomas J.W., Hall T.R., Bekris L.M., Torn C. Epitope analysis of insulin autoantibodies using recombinant Fab. Clin Exp Immunol. 2005;140:564–571. doi: 10.1111/j.1365-2249.2005.02802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brugman S., Klatter F.A., Visser J.T., Wildeboer-Veloo A.C., Harmsen H.J., Rozing J. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat: Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49:2105–2108. doi: 10.1007/s00125-006-0334-0. [DOI] [PubMed] [Google Scholar]
- 54.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 55.Fung S., Hruby V.J. Design of cyclic and other templates for potent and selective peptide [alpha]-MSH analogues. Curr Opin Chem Biol. 2005;9:352–358. doi: 10.1016/j.cbpa.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Catania A., Gatti S., Colombo G., Lipton J.M. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 57.Schioth H.B., Mutulis F., Muceniece R., Prusis P., Wikberg J.E. Selective properties of C- and N-terminals and core residues of the melanocyte-stimulating hormone on binding to the human melanocortin receptor subtypes. Eur J Pharmacol. 1998;349:359–366. doi: 10.1016/s0014-2999(98)00212-x. [DOI] [PubMed] [Google Scholar]
- 58.Yang P.-C., Jury J., Soderholm J.D., Sherman P.M., McKay D.M., Perdue M.H. Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol. 2006;168:104–114. doi: 10.2353/ajpath.2006.050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sokol M.S., Gray N.S. Case study: an infection-triggered, autoimmune subtype of anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 1997;36:1128–1133. doi: 10.1097/00004583-199708000-00021. [DOI] [PubMed] [Google Scholar]
- 60.Fetissov S.O. Autoimmune component in anorexia and bulimia nervosa. In: Fatemi S.H., editor. Neuropsychiatric disorders and infection. Taylor & Francis Books Ltd; London: 2004. pp. 253–262. [Google Scholar]
- 61.Ohlsson B., Veress B., Janciauskiene S., Montgomery A., Haglund M., Wallmark A. Chronic intestinal pseudo-obstruction due to buserelin-induced formation of anti-GnRH antibodies. Gastroenterology. 2007;132:45–51. doi: 10.1053/j.gastro.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 62.Taché Y., Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwartz G.J. Neural-immune gut-brain communication in the anorexia of disease. Nutrition. 2002;18:528–533. doi: 10.1016/s0899-9007(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 64.Woods S.C., Lutz T.A., Geary N., Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Phil Trans R Soc Lond B Biol Sci. 2006;361:1219–1235. doi: 10.1098/rstb.2006.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stanley S., Wynne K., McGowan B., Bloom S. Hormonal regulation of food intake. Physiol Rev. 2005;85:1131–1158. doi: 10.1152/physrev.00015.2004. [DOI] [PubMed] [Google Scholar]
- 66.Dinan T.G., Quigley E.M., Ahmed S.M., Scully P., O’Brien S., O’Mahony L. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 67.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 68.Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 69.Guijarro A., Laviano A., Meguid M.M. Hypothalamic integration of immune function and metabolism. Prog Brain Res. 2006:367–405. doi: 10.1016/S0079-6123(06)53022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.