Highlights
-
•
Vaccination represents one of the most cost-effective public health interventions.
-
•
The demands for next-generation and novel vaccines are steadily growing.
-
•
Development can be enhanced by stronger collaboration and technological innovation.
-
•
Sustainable development requires a balanced portfolio and procurement strategy.
Abstract
Vaccination remains the most cost-effective public health intervention after clean water, and the benefits impressively outweigh the costs. The efforts needed to fulfill the steadily growing demands for next-generation and novel vaccines designed for emerging pathogens and new indications are only realizable in a sustainable business model. Vaccine development can be fast-tracked through strengthening international collaborations, and the continuous innovation of technologies to accelerate their design, development, and manufacturing. However, these processes should be supported by a balanced project portfolio, and by managing sustainable vaccine procurement strategies for different types of markets. Collectively this will allow a gradual shift to a more streamlined and profitable vaccine production, which can significantly contribute to the worldwide effort to shape global health.
Current Opinion in Immunology 2018, 53:111–118
This review comes from a themed issue on Vaccines
Edited by Patrick C Wilson and Florian Krammer
For a complete overview see the Issue and the Editorial
Available online 8th May 2018
https://doi.org/10.1016/j.coi.2018.04.019
0952-7915/© 2018 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
Vaccination remains one of the most cost-effective public health interventions to address the world-wide health economic (HE) burden associated with infectious diseases. Indeed, for every US$ 1 spent on vaccination against diseases associated with 10 antigens in low-income and middle-income countries (LMICs), the estimated return on investment for society is US$16 due to direct savings on healthcare and increased productivity, and nearly three times higher (US$44) when broader economic and social benefits are considered [1••]. From 2001 to 2020, the broader benefits could amount to a staggering US$ 820 billion in the 73 countries supported by the Global Alliance for Vaccines and Immunization (GAVI) [2], a public–private partnership (PPP) involving amongst others the UN, the vaccine industry and the Bill and Melinda Gates Foundation (BMGF). Due to the higher disease burden and more limited medical infrastructure, the HE gain from introducing a vaccine in LMICs will be greater than in higher-income countries (HICs), where the gain will largely be determined by competition between the different health options on offer [3].
The global demand for vaccines is growing due to a host of factors, such as global population growth, future implementation of newly licensed or advanced-stage vaccines into health-care systems, and ongoing global immunization campaigns. The latter is illustrated by GAVI's aim to reach an additional 300 million children for routine childhood vaccination by 2020. Also, the pressure is mounting to deliver improved or new vaccines against challenging infectious diseases (e.g. tuberculosis, HIV/AIDS), new zoonotic pathogens, and therapeutic vaccines against non-communicable chronic diseases such as cancer and neurodegenerative diseases. They are also needed to address the scourge of antibiotic-resistant bacteria [4] and the varying vaccine needs across a person's life-span (vaccine ‘life-cycle management to support life-course immunization’ [5]).
To meet the increasing demands, there is a continuous quest for innovation of vaccine design and manufacturing technologies. Traditionally, the multiphase vaccine development process, which typically progresses over a 10–15-year period from vaccine discovery to advanced clinical development in Phase 3 efficacy trials, can require investments of US$ 0.5–1 billion [6, 7, 8]. This, combined with the slim (<10%) probability of candidates to enter the market, has negatively impacted the number of investing vaccine manufacturers and has contributed to the current productivity gap in vaccine development. The selection criteria supporting prioritization of a vaccine project must therefore be increasingly stringent. Here we discuss the key considerations in this decision-making process from a vaccine manufacturer's perspective.
Toward sustainable vaccine development
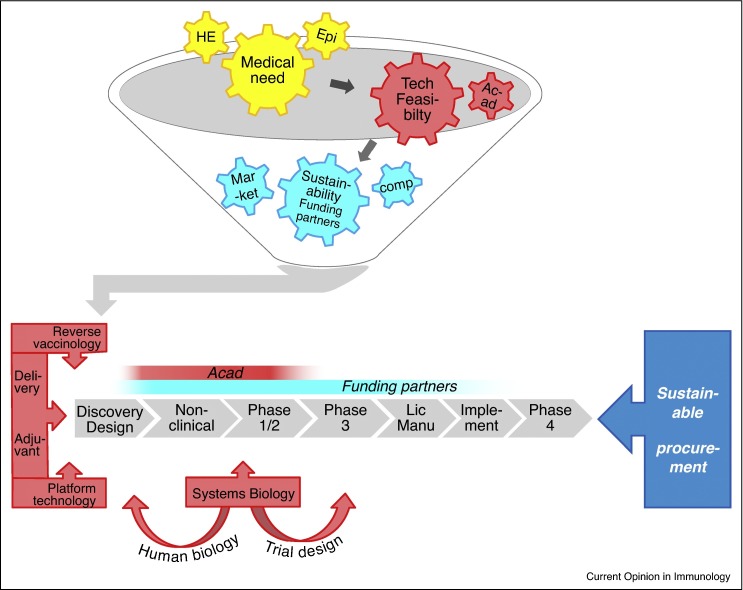
Medical need is a key factor for project prioritization, as illustrated by the spurred development of vaccines against the globally emerging threat posed by Clostridium difficile infections, or by the accelerated clinical development periods during the devastating Ebola crisis in West Africa in 2014, which for some vaccines could be shortened to less than a year. In the prevailing business model, other key guiding criteria are the technical feasibility, as well as the expected return on investment. The latter is largely determined by competitive landscape analyses, and is dependent of the economic development status of the market in question (Figure 1 ). To nurture and sustain the R&D processes, manufacturers’ business strategies will strive to maintain a project portfolio that is balanced between projects offering a solid business case, and higher-risk, longer-term and/or lower-feasibility projects. The considerable financial risks inherent to the latter category, combined with a pressing immediate need or expected future medical need, has been prompting industry to seek strategic funding partners such as governments and/or non-profit international vaccination foundations. Indeed, nearly every vaccine available in resource-poor settings today has been developed through combinations of public and private efforts. Underpinning the criterion of technical feasibility is a solid understanding of human immunopathology, and it is at this point that academic partnerships have been delivering critical know-how and contributions, particularly in the early clinical development stages [9]. Ultimately, vaccine development will also be driven by strategies to accomplish a sustainable procurement of these vaccines, such as tiered pricing policies. We next examine the importance of collaborative development in different market scenarios, and the key technological advances in vaccinology supporting it.
Figure 1.
Sustainable vaccine development and collaborations. Funnel: Guiding criteria for vaccine project prioritization are, first, the unmet medical need, as supported by health-economical (‘HE’) and epidemiological (‘Epi’) evaluations; second, technical feasibility, often benefitting from partnerships with academia (‘Acad’), and third, the sustainability of its development, which depends on the availability of funding partners for collaborative development, as well as on the competitive landscape (‘comp’) and the economic development status of the market for which the vaccine is intended (‘Market’). While the development of vaccines with a market that includes high-income countries is often predominantly industry-funded, trials evaluating vaccines for predominantly low-to-middle-income markets, or prepandemic vaccines, are typically co-funded by public–private partnerships including industry, governments and international non-governmental organizations. Bars: Red and blue bars indicate the development stages typically benefitting from involvement/support by academia and international funding organizations, respectively. Academic partners mostly contribute by providing immunological insights in late preclinical and Phase 1/2 clinical phases. Funding partners can provide support throughout the whole process, including the licensing (‘Lic’) phase, and the post-licensing phases comprising vaccine manufacturing (‘Manu’) and implementation (‘Implement’), for example the supply chain management support provided by the public–private partnership (mVacciNation). Post-marketing Phase 4 studies monitoring vaccine usage, adverse effects (pharmacovigilance), and long-term immunity are typically industry-funded. Red arrowed bars indicate the technologies used to guide antigen discovery and/or vaccine design (reverse vaccinology, delivery, adjuvants and platform technologies), while systems biology data, often generated in industry-academic partnerships, can guide during the discovery phase, as well as in later clinical phases, by supporting adaptive trial designs to expedite progression to Phase 3 clinical evaluations. Finally, strategies to manage the sustainable procurement of new vaccines, such as tiered pricing policies, will also majorly drive the vaccine development process.
Collaboration in different market scenarios
The first market scenario involves the development of vaccines with a high technical feasibility targeting an unmet medical need that is at least partially identified in the developed portions of the world. Such vaccines are generally expected to have a large market potential and a favorable return on investment, and are often developed with only a minimum of external funding. Prime examples are the quadrivalent influenza vaccines (QIVs), recommended by the WHO since 2013/2014, the meningococcus B vaccines, and the recently licensed subunit herpes zoster vaccine (HZ/su) indicated for the prevention of shingles in persons aged ≥50 years. These vaccines are expected to translate into considerable HE benefits for society [10•, 11, 12, 13].
Considering their considerable HE burden worldwide, for example, US$1 trillion/year for Alzheimer's disease [14], development of therapeutic vaccines against chronic disorders such as neurodegenerative diseases or cancer would be commercially appealing. Yet, given their low technical feasibility and the lack of knowledge surrounding what constitutes a protective response, their development has been deprioritized by major manufacturers, but is prioritized by several biotech companies, which can afford to take higher risks. The groundwork laid by international PPPs such as the Human Vaccines Project, or the European Innovative Medicines Initiative (IMI) consortium ‘BIOVACSAFE’, which aim to provide insight in the human immunity required for a successful vaccine, may break the technical impasse at least in some areas.
In a second scenario, a major public health threat is present primarily in limited commercial markets, as is the case for HIV/AIDS, tuberculosis and malaria infections. For the majority of these diseases, incomplete knowledge of the immunopathogenesis and mechanisms of natural or acquired immunity amount to a weak business case for vaccine development [8], and all important vaccines targeting these diseases have been developed in collaborative frameworks. Prequalification by supranational organizations, such as for the four-dose vial presentation of a pneumococcal vaccine recently awarded by the WHO, makes the vaccines eligible for procurement by UN agencies, and provides an early return on investments. Tiered pricing can also support the development of new vaccines using the profits made from the higher prices in HIC markets, an approach followed for many childhood vaccines [15, 16].
The world's first malaria vaccine, RTS,S/AS01, was developed through PPPs that have included the Walter Reed Army Institute for Research, and subsequently PATH Malaria Vaccine Initiative (MVI) and BMGF. Supported by vaccine donations from industry and MVI, the vaccine will enter a pilot implementation in Sub-Saharan Africa in 2018, which will be coordinated by the WHO and funded by multiple supranational organizations. In theory, post-marketing access to this vaccine is enhanced by setting prices affordable for LMICs, which are expected to cover manufacturing costs as well as a small return, to be reinvested in R&D for next-generation malaria vaccines or vaccines against other tropical diseases. In practice however, realization of a sustainable post-marketing phase and procurement of RTS,S/AS01 is still challenging due to the absence of a sustainable market for this vaccine. Furthermore, an AERAS-funded collaboration, with academic support for in-depth cellular immunity assessments [17, 18], yielded the tuberculosis candidate vaccine M72/AS01, for which the first results of the Phase 2b efficacy trial are expected in 2018 [NCT01755598]. Collaborative development also brought about the only licensed dengue vaccine (though recent safety concerns have posed a major setback in its roll-outs [19]), as well as several Shigella candidate vaccines, particularly important to combat the high shigellosis burden in infants in LMIC settings. Though no established correlates of protection exist, Shigella vaccine development is facilitated by generally accepted immunological associations with protection. Two promising approaches already evaluated in Phase 2 studies include the bioconjugate S. flexneri 2a vaccine (Flexyn2a) supported by The Wellcome Trust, and a monovalent S. sonnei vaccine (1790GAHB) based on genetically-derived outer membrane vesicles of Shigella, generated using the Generalized Modules for Membrane Antigens (GMMA) technology ([20, 21] and NCT02646371). The latter vaccine is being developed with funding from the European Commission's FP7 program. In addition, several multivalent Shigella vaccines are in early development.
A third category of vaccines are those targeting zoonotic pathogens with pandemic potential but which do not pose an immediate threat. Such vaccines generally lack market incentives. For the purpose of pandemic preparedness, these pathogens necessitate not only continuous surveillance in humans and animals, but also intensified development of prepandemic vaccines in the inter-outbreak periods. Beyond recent outbreaks of pandemic influenza, Ebola and Zika viruses, the WHO lists 70 diseases with outbreak potential. Yet with the possible exception of pandemic influenza vaccines [8], industry alone cannot deliver the resources needed for the world's vaccine supplies. Therefore, new globally-funded PPPs have been tasked with prepandemic vaccine development, such as CEPI (founded by industry, the Wellcome Trust, BMGF and several national governments), which will initially target three viruses from the WHO priority list (i.e., Middle East respiratory syndrome coronavirus and Lassa and Nipah viruses). An additional initiative, the proposed BioPreparedness Organization, will focus on development and utilization of vaccine platform technologies, but the sustainable supply, pricing and procurement of these vaccines have not been addressed yet. Platform technologies are critical in overcoming the bottlenecks in manufacturing, as further discussed below.
Technical feasibility: fast-tracking design, development and manufacturing
Multiple innovative tools and technologies originating from advances in virology, genetics, structural biology and biotechnology are available, to support prioritization based on technical feasibility throughout the whole development spectrum.
Antigen design
Antigen discovery and design has been transformed by novel bioinformatics technologies, and by the whole-genome and proteome data currently available for many pathogens. For example the identification of new antigens has been revolutionized by ‘reverse vaccinology’, the genome-wide sequencing of pathogens to scour for conserved surface-expressed proteins, which is then complemented by confirmation of their surface location in vitro and subsequent preclinical immunogenicity evaluation. The combination of such immunogens led to the development of several broadly protective bacterial and viral (candidate) vaccines, such as a recent Shigella candidate vaccine, and a licensed vaccine against serogroup B meningococcus [22•, 23].
Structural biology has delivered the insights in pathogen entry processes leading to the design of the DS-Cav1 RSV antigen (NIAID). DS-Cav1 comprises the fusion glycoprotein stabilized in its pre-fusion trimeric conformation (PreF), the only conformation containing a highly neutralization-sensitive epitope (antigenic site Ø [24]). Preclinical evaluation of DS-Cav1 showed that the vaccine can boost pre-existing RSV neutralizing responses, and that adjuvants can enhance its immunogenicity [25, 26•], which may be particularly relevant for vaccines targeting the elderly [27]. Adjuvanted and non-adjuvanted DS-Cav1 vaccines are currently in Phase 1 trials (NCT03049488), and different antigen versions such as a ‘head-only’ version have been pre-clinically evaluated [28, 29, 30].
The need for such ‘universal’ broadly protective vaccines generating immunity toward highly conserved epitopes is particularly evident for vaccines against the seasonal influenza virus, for which the frequent changes of its surface proteins have been necessitating annual vaccine re-formulations, and for pandemic strains as part of pandemic preparedness strategies. Facilitated by innovations in structure-based design, decade-long efforts have resulted in influenza vaccine candidates targeting the extracellular domain of the M2 protein, conserved regions in the HA1 domain, or the conserved HA2 stalk domain, of which particularly the latter approach appears promising for both seasonal and pandemic purposes [31]. Given that upon exposure to new strains, immune responses will be preferably directed to conserved epitopes on the stalk [32, 33], several avenues are being explored. One of them, which takes advantage of the current manufacturing processes, entails repeated vaccination with constructs expressing chimeric HA subunits with the same conserved stalk domain, but different exotic HA heads that are never encountered by humans under natural conditions ([34•, 35] and NCT03275389). When combined with the Adjuvant System 03 (AS03), such regimens were shown in pre-clinical models to induce anti-stalk IgG antibodies, as well as anti-NA antibodies which may also contribute to protection. Another approach includes vaccines based on ‘headless’ HA immunogens constructed through removal or glycan-masking of the HA head domain, which are still in preclinical phases [32, 33].
Adjuvants
Adjuvants are essential components of many vaccines. They are used to enhance immunity to the antigen, which is particularly important for populations with reduced immune responses, including for instance those with hyporesponsiveness to vaccines, and/or for populations that are naïve to the pathogen [36, 37]. This ability can also serve to increase the viability of potentially promising vaccine approaches such as recombinant protein vaccines, and to allow antigen dose-sparing, which can streamline production by overcoming limitations in manufacturing capability. For instance, while the use of a higher antigen dose improved the efficacy of seasonal influenza vaccines in the elderly [38], seasonal vaccines containing oil-in-water adjuvants such as MF59 and AS03 have shown to be efficacious in this age-group at the standard dose, at least for certain strains [39, 40]. By broadening the B-cell repertoire, these adjuvants can also extend the protective coverage of monovalent pandemic influenza vaccines [41, 42].
Continuous efforts are being made to unravel the modes of action of different adjuvants, and thus the opportunities to maximize their potential for use in new vaccines. Evaluation of the innate immunity induced by several adjuvants (AS01, AS03, AS04, or Alum) has highlighted the role of the IFN-signaling pathway activation in the enhancement of adaptive immunity induced by AS03 and AS01 [43•, 44], and further research using systems biology approaches has been performed [45••, 46]. AS01 is used in candidate malaria, tuberculosis and HIV vaccines, as well as in the licensed HZ/su vaccine.
Platform technologies
While effective, the egg-based vaccine production currently used for the majority of seasonal (TIV/QIV) and pandemic influenza vaccines [47] has the drawback of being less amenable for the response to surging vaccine needs during a pandemic. Cell-culture manufacturing, as used for a licensed MF59-adjuvanted pandemic influenza vaccine, is a generally faster and more controlled process, though some obstacles such as the cell-line scale-up still need to be addressed. Furthermore, the need for new, high-performance vaccine platforms has prompted the development of nucleic acid-based technologies (viral vectors, plasmid DNA, conventional RNA, or self-amplifying RNA [SAM]), which are able to elicit broadly protective and robust cell-mediated and humoral immune responses, since expression of the vaccine antigens occurs in situ. Generally, RNA and SAM vaccines have proven more potent than plasmid DNA vaccines, and their production and purification is cost-effective, relatively versatile and readily transferable between different viral targets [48, 49]. Besides influenza vaccines, RNA and SAM vaccines targeting several other pathogens such as HIV and RSV have been evaluated, and were shown to confer protection in animal models [50, 51]. Some of these vaccines, including those against influenza and rabies, have also been tested in clinical trials, with promising but not yet satisfactory results [52, 53]. The underlying mechanism of action of SAM-induced immunity, particularly with respect to early innate immunity, is not fully known [54, 55] but may be further elucidated in the first clinical evaluation of a SAM vaccine, which could occur in 2019.
While taking more time for vaccine generation as compared to the SAM technology, viral vectors such as VSV, MVA or simian adenovirus vectors represent a technically more established vehicle for vaccine delivery. Prime examples are the replication-incompetent species C chimpanzee-derived adenovirus (ChAd) vectors, which have been used for an Ebola vaccine (ChAd3-EBO [56]) and, more recently, an RSV PreF vaccine (ChAd155-RSV; currently in pediatric Phase 1/2 trials [NCT02927873]). The attractiveness of the ChAd technology platform lies in the ability of these vectors to generate potent antigen-specific T-cell and humoral responses, with a reduced risk of being neutralized by human sera, which is due to their negligible seroprevalence in humans.
Fast-tracking clinical development
Systems biology has the potential to change vaccine development by providing a more informative characterization of the transcriptional and cellular signatures of vaccine-induced responses, which has been used to increase our knowledge of the mechanisms of protection and pathogenesis of challenging diseases such as HIV/AIDS and tuberculosis [57, 58]. By integrating molecular pathway data generated by one or several ‘omics’ technologies, molecular correlates of early immunogenicity or protection have been identified for important vaccines including the YF-17D yellow fever vaccine, adjuvanted influenza vaccines and the RTS,S malaria vaccine [45••, 46, 59, 60•, 61, 62••]. Omics approaches also allowed identification of age-related or inter-subject differences in post-vaccination reactogenicity events and immune responses, such as a molecular signature of adverse events associated with a common B-cell phenotype, or the networks of antibody characteristics linking with protective immunity (‘systems serology’) [63••, 64].
Integration of common signatures observed for different populations, adjuvants and antigens aids in the identification of novel biomarkers for vaccine efficacy and safety, and, when supported by mechanistic data in animal models, can guide the antigen/adjuvant selections for a given target population, as well as the development of novel adjuvants [65]. The latter can be relevant in the context of the accelerated development of vaccines for LMICs, amongst others. Since ‘omics’ technologies are used to monitor the occurrence of common signatures, they allow for smaller-scale clinical trials. Such data could also help in introducing a more adaptive clinical trial design, in which multiple vaccine formulations and regimens and several hypotheses are screened, and of which the most promising arm is then expanded in a Phase 3 efficacy study [66, 67]. Moreover, the application of such technologies to human challenge studies presents a potential powerful method to identify or elucidate correlates and/or immunological pathways associated with protection. This approach has been followed in the context of vaccines against malaria, influenza and RSV [45••, 46, 68], and is currently being developed for pertussis by the IMI consortium ‘PERISCOPE’.
Finally, multiplexed, high-throughput affinity separation of cells and proteins are interesting as they enable fast isolation of the most effective specific antibodies, or rare B cells or plasma cells from vaccinees [69]. This may aid the identification of biomarkers for certain antibody-directed effector functions, which could then guide early-phase strategies or support the design of later-phase trials.
Conclusions
The efforts needed to fulfill the growing demands for next-generation vaccines, and for novel vaccines designed for emerging pathogens and new indications, are only realizable in a sustainable business model supported by a balanced portfolio. The strengthening of international collaborations and continuous innovation of technologies to accelerate design, development and manufacturing, will allow a gradual shift to a more streamlined and profitable vaccine production. This will significantly contribute to the global efforts to prevent infectious diseases, protect vulnerable populations, and obtain a more rapid response to future outbreaks, shaping global health.
Author contributions
Both authors contributed to the development of this manuscript, gave final approval before submission and agree to be accountable for all aspects of the work.
Conflicts of interest
The authors declare the following conflicts: RR and EH are employees and shareholders of the GSK group of companies.
Acknowledgments
The authors thank Ellen Oe (GSK) for scientific writing services and Ulrike Krause (GSK) for publication management and coordination.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
References
- 1••.Ozawa S., Clark S., Portnoy A., Grewal S., Brenzel L., Walker D.G. Return on investment from childhood immunization in low- and middle-income countries, 2011–20. Health Aff (Millwood) 2016;35:199–207. doi: 10.1377/hlthaff.2015.1086. [DOI] [PubMed] [Google Scholar]; By quantifying the value that people place on living longer and healthier lives, the authors found that net returns of vaccination amounted to 44 times the costs, consolidating the merit of vaccination as an effective health intervention.
- 2.Ozawa S., Clark S., Portnoy A., Grewal S., Stack M.L., Sinha A., Mirelman A., Franklin H., Friberg I.K., Tam Y. Estimated economic impact of vaccinations in 73 low- and middle-income countries, 2001–2020. Bull World Health Organ. 2017;95:629–638. doi: 10.2471/BLT.16.178475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Standaert B., Rappuoli R. 3. How comprehensive can we be in the economic assessment of vaccines? J Market Access Health Policy. 2017;5:1336044. doi: 10.1080/20016689.2017.1336044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rappuoli R., Bloom D.E., Black S. Deploy vaccines to fight superbugs. Nature. 2017;552:165–167. doi: 10.1038/d41586-017-08323-0. [DOI] [PubMed] [Google Scholar]
- 5.Rappuoli R., Mandl C.W., Black S., De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11:865–872. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plotkin S.A., Mahmoud A.A., Farrar J. Establishing a global vaccine-development fund. N Engl J Med. 2015;373:297–300. doi: 10.1056/NEJMp1506820. [DOI] [PubMed] [Google Scholar]
- 7.Pronker E.S., Weenen T.C., Commandeur H.R., Osterhaus A.D., Claassen H.J. The gold industry standard for risk and cost of drug and vaccine development revisited. Vaccine. 2011;29:5846–5849. doi: 10.1016/j.vaccine.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 8.Pronker E.S., Weenen T.C., Commandeur H., Claassen E.H., Osterhaus A.D. Risk in vaccine research and development quantified. PLoS ONE. 2013;8:e57755. doi: 10.1371/journal.pone.0057755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rappuoli R., De Gregorio E. Editorial overview: vaccines: novel technologies for vaccine development. Curr Opin Immunol. 2016;41:v–vii. doi: 10.1016/j.coi.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 10•.Le P., Rothberg M.B. Cost-effectiveness of the adjuvanted herpes zoster subunit vaccine in older adults. JAMA Intern Med. 2018;178:248–258. doi: 10.1001/jamainternmed.2017.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]; Based on an analysis of total costs and quality-adjusted life-years, the authors show that the introduction of the adjuvanted subunit herpes zoster vaccine HZ/su in older adults would cost less that the live zoster vaccine and will therefore likely be cost-effective.
- 11.Tsuzuki S., Schwehm M., Eichner M. Simulation studies to assess the long-term effects of Japan's change from trivalent to quadrivalent influenza vaccination. Vaccine. 2017;36:624–630. doi: 10.1016/j.vaccine.2017.12.058. [DOI] [PubMed] [Google Scholar]
- 12.Tisa V., Barberis I., Faccio V., Paganino C., Trucchi C., Martini M., Ansaldi F. Quadrivalent influenza vaccine: a new opportunity to reduce the influenza burden. J Prev Med Hyg. 2016;57:E28–E33. [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen H., Hickman M., Edmunds W.J., Trotter C.L. Introducing vaccination against serogroup B meningococcal disease: an economic and mathematical modelling study of potential impact. Vaccine. 2013;31:2638–2646. doi: 10.1016/j.vaccine.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ADI . Aug 2015. World Alzheimer Report 2015 Reveals Global Cost of Dementia Set to Reach US $1 Trillion by 2018. https://www.alz.co.uk/news/world-alzheimer-report-2015-reveals-global-cost-of-dementia-set-to-reach-usd-1-trillion-by-2018. Accessed 27/02/2018. [Google Scholar]
- 15.Plahte J. Tiered pricing of vaccines: a win-win-win situation, not a subsidy. Lancet Infect Dis. 2005;5:58–63. doi: 10.1016/S1473-3099(04)01255-1. [DOI] [PubMed] [Google Scholar]
- 16.Outterson K. Pharmaceutical arbitrage: balancing access and innovation in international prescription drug markets. Yale J Health Policy Law Ethics. 2005;5:193–291. [PubMed] [Google Scholar]
- 17.Day C.L., Tameris M., Mansoor N., van Rooyen M., de Kock M., Geldenhuys H., Erasmus M., Makhethe L., Hughes E.J., Gelderbloem S. Induction and regulation of T-cell immunity by the novel tuberculosis vaccine M72/AS01 in South African adults. Am J Respir Crit Care Med. 2013;188:492–502. doi: 10.1164/rccm.201208-1385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penn-Nicholson A., Geldenhuys H., Burny W., van der Most R., Day C.L., Jongert E., Moris P., Hatherill M., Ofori-Anyinam O., Hanekom W. Safety and immunogenicity of candidate vaccine M72/AS01E in adolescents in a TB endemic setting. Vaccine. 2015;33:4025–4034. doi: 10.1016/j.vaccine.2015.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO . July 2017. GACVS Statement on Dengvaxia® (CYD-TDV) http://www.who.int/vaccine_safety/committee/GACVS-StatementonDengvaxia-CYD-TDV/en/. Accessed 27/02/2018. [Google Scholar]
- 20.Obiero C.W., Ndiaye A.G.W., Sciré A.S., Kaunyangi B.M., Marchetti E., Gone A.M., Schütte L.D., Riccucci D., Auerbach J., Saul A. A Phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against Shigella sonnei administered intramuscularly to adults from a shigellosis-endemic country. Front Immunol. 2017;8:1884. doi: 10.3389/fimmu.2017.01884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riddle M.S., Kaminski R.W., Di Paolo C., Porter C.K., Gutierrez R.L., Clarkson K.A., Weerts H.E., Duplessis C., Castellano A., Alaimo C. Safety and Immunogenicity of a candidate bioconjugate vaccine against Shigella flexneri 2a administered to healthy adults: a single-Blind, randomized phase I study. Clin Vaccine Immunol. 2016;23:908–917. doi: 10.1128/CVI.00224-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Pahil S., Taneja N., Ansari H.R., Raghava G.P.S. In silico analysis to identify vaccine candidates common to multiple serotypes of Shigella and evaluation of their immunogenicity. PLoS ONE. 2017;12:e0180505. doi: 10.1371/journal.pone.0180505. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a bio-informatics approach including reverse vaccinology, the authors were able to identify novel cross-protective antigens that are conserved between multiple serotypes of Shigella species, and represent potential vaccine candidates.
- 23.Kelly D.F., Rappuoli R. Reverse vaccinology and vaccines for serogroup B Neisseria meningitidis. Adv Exp Med Biol. 2005;568:217–223. doi: 10.1007/0-387-25342-4_15. [DOI] [PubMed] [Google Scholar]
- 24.Ngwuta J.O., Chen M., Modjarrad K., Joyce M.G., Kanekiyo M., Kumar A., Yassine H.M., Moin S.M., Killikelly A.M., Chuang G.Y. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med. 2015;7:309ra162. doi: 10.1126/scitranslmed.aac4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sastry M., Zhang B., Chen M., Joyce M.G., Kong W.P., Chuang G.Y., Ko K., Kumar A., Silacci C., Thom M. Adjuvants and the vaccine response to the DS-Cav1-stabilized fusion glycoprotein of respiratory syncytial virus. PLoS ONE. 2017;12:e0186854. doi: 10.1371/journal.pone.0186854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Steff A.M., Monroe J., Friedrich K., Chandramouli S., Nguyen T.L., Tian S., Vandepaer S., Toussaint J.F., Carfi A. Pre-fusion RSV F strongly boosts pre-fusion specific neutralizing responses in cattle pre-exposed to bovine RSV. Nat Commun. 2017;8:1085. doi: 10.1038/s41467-017-01092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate that even a single immunization of a non-adjuvanted prefusion RSV F antigen, but not of a postfusion F antigen, is able to boost RSV neutralizing responses in the relevant bovine cattle model.
- 27.Cayatte C., Snell Bennett A., Rajani G.M., Hostetler L., Maynard S.K., Lazzaro M., McTamney P., Ren K., O’Day T., McCarthy M.P., Schneider-Ohrum K. Inferior immunogenicity and efficacy of respiratory syncytial virus fusion protein-based subunit vaccine candidates in aged versus young mice. PLoS ONE. 2017;12:e0188708. doi: 10.1371/journal.pone.0188708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joyce M.G., Zhang B., Ou L., Chen M., Chuang G.Y., Druz A., Kong W.P., Lai Y.T., Rundlet E.J., Tsybovsky Y. Iterative structure-based improvement of a fusion-glycoprotein vaccine against RSV. Nat Struct Mol Biol. 2016;23:811–820. doi: 10.1038/nsmb.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart-Jones G.B., Thomas P.V., Chen M., Druz A., Joyce M.G., Kong W.P., Sastry M., Soto C., Yang Y., Zhang B. A cysteine zipper stabilizes a pre-fusion F glycoprotein vaccine for respiratory syncytial virus. PLoS ONE. 2015;10:e0128779. doi: 10.1371/journal.pone.0128779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyington J.C., Joyce M.G., Sastry M., Stewart-Jones G.B., Chen M., Kong W.P., Ngwuta J.O., Thomas P.V., Tsybovsky Y., Yang Y. Structure-based design of head-only fusion glycoprotein immunogens for respiratory syncytial virus. PLoS ONE. 2016;11:e0159709. doi: 10.1371/journal.pone.0159709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krammer F., Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol. 2013;3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krammer F. Strategies to induce broadly protective antibody responses to viral glycoproteins. Expert Rev Vaccines. 2017;16:503–513. doi: 10.1080/14760584.2017.1299576. [DOI] [PubMed] [Google Scholar]
- 33.Krammer F., García-Sastre A., Palese P. Is it possible to develop a “universal” influenza virus vaccine?. Toward a universal influenza virus vaccine: potential target antigens and critical aspects for vaccine development. Cold Spring Harb Perspect Biol. 2017 doi: 10.1101/cshperspect.a028845. pii: a028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Nachbagauer R., Kinzler D., Choi A., Hirsh A., Beaulieu E., Lecrenier N., Innis B.L., Palese P., Mallett C.P., Krammer F. A chimeric haemagglutinin-based influenza split virion vaccine adjuvanted with AS03 induces protective stalk-reactive antibodies in mice. NPJ Vaccines. 2016;1 doi: 10.1038/npjvaccines.2016.15. pii: 16015. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a primed mouse model, the authors show that a monovalent chimeric HA-based universal influenza virus split virion vaccine induced protective, persistent and cross-reactive stalk antibody responses, supporting development of universal influenza vaccines based on chimeric HA technology platform.
- 35.Nachbagauer R., Liu W.C., Choi A., Wohlbold T.J., Atlas T., Rajendran M., Solorzano A., Berlanda-Scorza F., García-Sastre A., Palese P. A universal influenza virus vaccine candidate confers protection against pandemic H1N1 infection in preclinical ferret studies. NPJ Vaccines. 2017;2:26. doi: 10.1038/s41541-017-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Didierlaurent A.M., Laupèze B., Di Pasquale A., Hergli N., Collignon C., Garçon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16:55–63. doi: 10.1080/14760584.2016.1213632. [DOI] [PubMed] [Google Scholar]
- 37.Garçon N., Di Pasquale A. From discovery to licensure, the adjuvant system story. Hum Vaccin Immunother. 2017;13:19–33. doi: 10.1080/21645515.2016.1225635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson K., Wei Y., Szwajcer A., Rabbani R., Zarychanski R., Abou-Setta A.M., Mahmud S.M. Efficacy and safety of high-dose influenza vaccine in elderly adults: a systematic review and meta-analysis. Vaccine. 2017;35:2775–2780. doi: 10.1016/j.vaccine.2017.03.092. [DOI] [PubMed] [Google Scholar]
- 39.Domnich A., Arata L., Amicizia D., Puig-Barberà J., Gasparini R., Panatto D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: a systematic review and meta-analysis. Vaccine. 2017;35:513–520. doi: 10.1016/j.vaccine.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 40.McElhaney J.E., Beran J., Devaster J.M., Esen M., Launay O., Leroux-Roels G., Ruiz-Palacios G.M., van Essen G.A., Caplanusi A., Claeys C. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis. 2013;13:485–496. doi: 10.1016/S1473-3099(13)70046-X. [DOI] [PubMed] [Google Scholar]
- 41.van der Most R.G., Roman F.P., Innis B., Hanon E., Vaughn D.W., Gillard P., Walravens K., Wettendorff M. Seeking help: B cells adapting to flu variability. Sci Transl Med. 2014;6:246ps8. doi: 10.1126/scitranslmed.3008409. [DOI] [PubMed] [Google Scholar]
- 42.Khurana S., Chearwae W., Castellino F., Manischewitz J., King L.R., Honorkiewicz A., Rock M.T., Edwards K.M., Del Giudice G., Rappuoli R., Golding H. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci Transl Med. 2010;2:15ra5. doi: 10.1126/scitranslmed.3000624. [DOI] [PubMed] [Google Scholar]
- 43•.Burny W., Callegaro A., Bechtold V., Clement F., Delhaye S., Fissette L., Janssens M., Leroux-Roels G., Marchant A., van den Berg R.A. Different adjuvants induce common innate pathways that are associated with enhanced adaptive responses against a model antigen in humans. Front Immunol. 2017;8:943. doi: 10.3389/fimmu.2017.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors elucidate the role of innate responses in vaccine immunogenicity for different adjuvants and show that the ability of AS01 and AS03 to enhance adaptive responses is likely linked to their capacity to activate innate immunity, particularly the IFN-signaling pathway.
- 44.Coccia M., Collignon C., Hervé C., Chalon A., Welsby I., Detienne S., van Helden M.J., Dutta S., Genito C.J., Waters N.C. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. NPJ Vaccines. 2017;2:25. doi: 10.1038/s41541-017-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Kazmin D., Nakaya H.I., Lee E.K., Johnson M.J., van der Most R., van den Berg R.A., Ballou W.R., Jongert E., Wille-Reece U., Ockenhouse C. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc Natl Acad Sci U S A. 2017;114:2425–2430. doi: 10.1073/pnas.1621489114. [DOI] [PMC free article] [PubMed] [Google Scholar]; Utilizing a systems biological approach the authors provided molecular signatures of protective immunity elicited by the RTS,S malaria vaccine, and delivered insights in the role of NK cells.
- 46.van den Berg R.A., Coccia M., Ballou W.R., Kester K.E., Ockenhouse C.F., Vekemans J., Jongert E., Didierlaurent A.M., van der Most R.G. Predicting RTS,S vaccine-mediated protection from transcriptomes in a malaria-challenge clinical trial. Front Immunol. 2017;8:557. doi: 10.3389/fimmu.2017.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manini I., Trombetta C.M., Lazzeri G., Pozzi T., Rossi S., Montomoli E. Egg-independent influenza vaccines and vaccine candidates. Vaccines (Basel) 2017:5. doi: 10.3390/vaccines5030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iavarone C., O’Hagan D.T., Yu D., Delahaye N.F., Ulmer J.B. Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines. 2017;16:871–881. doi: 10.1080/14760584.2017.1355245. [DOI] [PubMed] [Google Scholar]
- 49.DeFrancesco L. The ‘anti-hype’ vaccine. Nat Biotechnol. 2017;35:193–197. doi: 10.1038/nbt.3812. [DOI] [PubMed] [Google Scholar]
- 50.Maruggi G., Chiarot E., Giovani C., Buccato S., Bonacci S., Frigimelica E., Margarit I., Geall A., Bensi G., Maione D. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine. 2017;35:361–368. doi: 10.1016/j.vaccine.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 51.Brazzoli M., Magini D., Bonci A., Buccato S., Giovani C., Kratzer R., Zurli V., Mangiavacchi S., Casini D., Brito L.M. Induction of broad-based immunity and protective efficacy by self-amplifying mRNA vaccines encoding influenza virus hemagglutinin. J Virol. 2015;90:332–344. doi: 10.1128/JVI.01786-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alberer M., Gnad-Vogt U., Hong H.S., Mehr K.T., Backert L., Finak G., Gottardo R., Bica M.A., Garofano A., Koch S.D. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511–1520. doi: 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- 53.Bahl K., Senn J.J., Yuzhakov O., Bulychev A., Brito L.A., Hassett K.J., Laska M.E., Smith M., Almarsson O., Thompson J. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther. 2017;25:1316–1327. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pepini T., Pulichino A.M., Carsillo T., Carlson A.L., Sari-Sarraf F., Ramsauer K., Debasitis J.C., Maruggi G., Otten G.R., Geall A.J. Induction of an IFN-mediated antiviral response by a self-amplifying RNA vaccine: implications for vaccine design. J Immunol. 2017;198:4012–4024. doi: 10.4049/jimmunol.1601877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ljungberg K., Liljeström P. Self-replicating alphavirus RNA vaccines. Expert Rev Vaccines. 2015;14:177–194. doi: 10.1586/14760584.2015.965690. [DOI] [PubMed] [Google Scholar]
- 56.Ledgerwood J.E., DeZure A.D., Stanley D.A., Coates E.E., Novik L., Enama M.E., Berkowitz N.M., Hu Z., Joshi G., Ploquin A. Chimpanzee adenovirus vector Ebola vaccine. N Engl J Med. 2017;376:928–938. doi: 10.1056/NEJMoa1410863. [DOI] [PubMed] [Google Scholar]
- 57.Chung A.W., Kumar M.P., Arnold K.B., Yu W.H., Schoen M.K., Dunphy L.J., Suscovich T.J., Frahm N., Linde C., Mahan A.E. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell. 2015;163:988–998. doi: 10.1016/j.cell.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu L.L., Chung A.W., Rosebrock T.R., Ghebremichael M., Yu W.H., Grace P.S., Schoen M.K., Tafesse F., Martin C., Leung V. A functional role for antibodies in tuberculosis. Cell. 2016;167:433–443. doi: 10.1016/j.cell.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Querec T.D., Akondy R.S., Lee E.K., Cao W., Nakaya H.I., Teuwen D., Pirani A., Gernert K., Deng J., Marzolf B. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60•.Nakaya H.I., Clutterbuck E., Kazmin D., Wang L., Cortese M., Bosinger S.E., Patel N.B., Zak D.E., Aderem A., Dong T. Systems biology of immunity to MF59-adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood. Proc Natl Acad Sci U S A. 2016;113:1853–1858. doi: 10.1073/pnas.1519690113. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a systems vaccinology approach, the authors identify potential molecular correlates of antibody responses to trivalent influenza vaccines in young children, and demonstrate the role of an oil-in-water adjuvant in enhancing these immune responses.
- 61.Howard L.M., Hoek K.L., Goll J.B., Samir P., Galassie A., Allos T.M., Niu X., Gordy L.E., Creech C.B., Prasad N. Cell-based systems biology analysis of human AS03-adjuvanted H5N1 avian influenza vaccine responses: a phase I randomized controlled trial. PLoS ONE. 2017;12:e0167488. doi: 10.1371/journal.pone.0167488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62••.Li S., Sullivan N.L., Rouphael N., Yu T., Banton S., Maddur M.S., McCausland M., Chiu C., Canniff J., Dubey S. Metabolic phenotypes of response to vaccination in humans. Cell. 2017;169:862–877. doi: 10.1016/j.cell.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors used an integrative analysis of classical immune response data combined with transcriptomics and metabolomics, to reveal an immune network of gene and metabolic pathways correlating with the adaptive response, furthering our understanding of human immune responses to a live virus vaccine.
- 63••.Sobolev O., Binda E., O’Farrell S., Lorenc A., Pradines J., Huang Y., Duffner J., Schulz R., Cason J., Zambon M. Adjuvanted influenza-H1N1 vaccination reveals lymphoid signatures of age-dependent early responses and of clinical adverse events. Nat Immunol. 2016;17:204–213. doi: 10.1038/ni.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]; This systems biology study for an oil-in-water-adjuvanted swine flu vaccine identified a molecular signature of adverse events that was associated with an existing B cell phenotype, and revealed age-related differences in the early (day 1 post-vaccination) response, which emerged at a younger age (∼35 years) than expected for age-related differences in immune responses.
- 64.Chung A.W., Alter G. Systems serology: profiling vaccine induced humoral immunity against HIV. Retrovirology. 2017;14:57. doi: 10.1186/s12977-017-0380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Hagan D.T., Friedland L.R., Hanon E., Didierlaurent A.M. Towards an evidence based approach for the development of adjuvanted vaccines. Curr Opin Immunol. 2017;47:93–102. doi: 10.1016/j.coi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 66.Hagan T., Pulendran B. Will systems biology deliver its promise and contribute to the development of new or improved vaccines?. From data to understanding through systems biology. Cold Spring Harb Perspect Biol. 2017 doi: 10.1101/cshperspect.a028894. pii: a028894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rappuoli R., Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473:463–469. doi: 10.1038/nature10124. [DOI] [PubMed] [Google Scholar]
- 68.Barton A.J., Hill J., Pollard A.J., Blohmke C.J. Transcriptomics in human challenge models. Front Immunol. 2017;8:1839. doi: 10.3389/fimmu.2017.01839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarkar A., Hou H.W., Mahan A.E., Han J., Alter G. Multiplexed affinity-based separation of proteins and cells using inertial microfluidics. Sci Rep. 2016;6:23589. doi: 10.1038/srep23589. [DOI] [PMC free article] [PubMed] [Google Scholar]