Abstract
We evaluated whether a bovine intestinal epithelial (BIE) cell line could serve as a useful in vitro model system for studying antiviral immune responses in bovine intestinal epithelial cells (IECs) and for the primary screening of immunobiotic microorganisms with antiviral protective capabilities. Immunofluorescent analyses revealed that toll-like receptor 3 (TLR3) was expressed in BIE cells, and the results of real-time quantitative PCR showed that these cells respond to stimulation with poly(I:C) by up-regulating pro-inflammatory cytokines and type I interferons. In addition, we demonstrated that BIE cells are useful for the primary screening of immunobiotic lactic acid bacteria strains which are able to beneficially modulate antiviral immune responses triggered by TLR3 activation in bovine IECs. The characterization of BIE cells performed in the present study represents an important step towards the establishment of a valuable bovine in vitro system that could be used for the development of immunomodulatory feed for bovine hosts.
Keywords: Antiviral immune response, Bovine intestinal epithelial cells, Immunobiotic, Toll-like receptor 3 (TLR3)
1. Introduction
Diarrhea is an important cause of morbidity and mortality in young calves, resulting in significant financial losses to cattle producers. In particular, bovine neonatal gastroenteritis is a multifactorial disease that can be caused by a number of pathogens, including bovine rotavirus (BRV), bovine coronavirus (BCV), and bovine viral diarrhea viruses (BVDVs) (Aich et al., 2007, Lee et al., 2008). Although these viruses belong to different families and have distinct physical characteristics, they are all able to infect intestinal epithelial cells (IECs), and induce villous atrophy and diarrhea.
IECs are able to distinguish between the diverse elements present in the intestinal environment through the expression of pattern recognition receptors, such as toll-like receptors (TLRs) (Westendorf et al., 2010). Among TLRs, TLR3 recognizes dsRNA and is therefore important for defense against viral infections. Upon recognition of viral dsRNA, TLR3 transmits signals that activate transcription factors responsible for the induction of type I interferon (IFN; IFN-α/β) and IFN-inducible genes, which play critical roles in antiviral host defense (Matsumoto et al., 2002). Thus, activation of TLR3 signaling is of great importance for defense against BRV, a dsRNA virus, and BCV and BVDV, which replicate via intermediary dsRNA.
Recently, significant progress has been made in understanding the role of TLR3 in innate and adaptive immunity. The majority of studies aimed at dissecting the mechanisms of TLR3 function have been performed principally in mouse and human cell lines (Gauzzi et al., 2010). However, few studies have been conducted on cattle, despite growing interest in the bovine immune system due to the economic importance of cattle as livestock. Therefore, investigating how TLR3 mediates antiviral defenses in bovine IECs is important for understanding the activation and regulation of the intestinal immune system of cattle. Moreover, determining the underlying mechanisms of TLR3 activation and regulation in bovine IECs may aid in the development of effective therapies for the prevention and treatment of viral diseases, such as oral vaccines and functional feed, which specifically target anti-viral immune responses in the bovine gut.
We have recently established a bovine intestinal epithelial (BIE) cell line originally derived from fetal bovine intestinal epitheliocytes (Miyazawa et al., 2010). In the present study, we aimed to characterize the immune response triggered by TLR3 activation in BIE cells in order to evaluate whether this cell line could serve as an in vitro model of bovine IECs for the study of TLR3-mediated antiviral responses. In addition, we attempted to determine whether BIE cells are suitable for the primary screening of immunomodulatory lactic acid bacteria (LAB) with antiviral protective capabilities in cattle.
2. Materials and methods
2.1. BIE cells
The BIE cell line used in this study is a non-transformed intestinal cell line that was previously derived from fetal bovine intestinal epitheliocytes (Miyazawa et al., 2010). BIE cells were routinely maintained in Dulbecco’s Modified Eagle medium (DMEM; Gibco, Grand Island, NY) containing 10% heat-inactivated fetal bovine serum (FBS) and penicillin–streptomycin. For passaging, BIE cells were treated with a sucrose/EDTA buffer (0.1 M Na2HPO4/12H2O, 0.45 M sucrose, 0.36% EDTA/4Na, and bovine serum albumin [BSA]) for 4 min and then detached using 0.04% trypsin in phosphate-buffered saline (PBS) (Moue et al., 2008). BIE cells were then plated in type I collagen-coated culture dishes (Sumilon, Tokyo, Japan) at a density of 1.5 × 104 cells/cm2 and cultured at 37 °C in an atmosphere of 5% CO2 in DMEM (10% FBS, 1% streptomycin/penicillin, 100 U/ml streptomycin, high glucose, l-glutamine, and 0.11 mg/ml sodium pyruvate; Gibco).
2.2. Immunocytochemistry
BIE cells were cultured in collagen type I-coated culture dishes (12-well plate; Sumilon) at a cell density of 3 × 104 cells/well for 3 days (37 °C, 5% CO2), washed once with cold PBS (2% FCS), and then treated with FACS permeabilization solution (4 °C, 10 min). Following three washes with PBS, the cells were incubated with 5% normal goat serum (Sigma, St. Louis, MO) for 10 min at 4 °C. Cells were again washed with PBS three times and then incubated with anti-TLR3 polyclonal antibody (sc-28999; Santa Cruz, CA, USA) for 16 h at 4 °C. Following three washes with PBS, cells were treated with secondary Alexa Fluor 488-conjugated goat anti-rabbit polyclonal IgG (Invitrogen, Tokyo, Japan) for 1 h at room temperature. BIE cells incubated with rabbit IgG isotype control antibody (20304E; Imgenex, San Diego, CA) and the identical secondary antibody as above were used as controls. After reaction with secondary antibody, cells were washed three times with PBS and then counterstained with SYTOX orange (Invitrogen) for 5 min at room temperature. Cells were washed three times with PBS, rinsed in distilled water, and then mounted on glass slides with PermaFluor (Thermo Fisher, Pittsburgh, PA). Immunofluorescence microscopy was performed using a confocal laser microscope (MRC-1024; Bio-Rad, Richmond, CA).
2.3. Microorganisms
The following LAB strains were used in this study: Lactobacillus gasseri TMC0356, Lactobacillus rhamnosus LGG, L. rhamnosus LA-2, Lactobacillus casei TMC0409, Streptococcus thermophilus TMC1543, Bifidobacterium bifidum 2-2, and B. bifidum 3-9. The lactobacilli and bifidobacteria strains were grown in MRS medium (Difco, Detroit, MI, USA) for 16 h at 37 °C. S. thermophilus cells were grown in Elliker medium (Difco) for 16 h at 37 °C.
2.4. Anti-inflammatory assay in BIE cells
Bacteria were re-suspended in DMEM (10% FBS and 1% SP), enumerated microscopically using a Petroff-Hausser counting chamber, and stored at −80 °C until use. BIE cells were plated at a density of 1.5 × 104 cells/well in 24-well type I collagen-coated plates (Sumilon) containing 1 ml DMEM, and were then cultured for 3 days. After changing the medium, lactobacilli or bifidobacteria (5 × 107 cells/ml) were added to 1 ml DMEM in each well. After 24 and 48 h of incubation, each well was washed vigorously with medium at least three times to eliminate all of the stimulants. For comparative treatment, BIE cells were stimulated with 200 ng/ml Pam3CSK4 for 24 or 48 h. After the elimination of lactobacilli, bifidobacteria, or Pam3CSK4, BIE cells were stimulated with poly(I:C) at concentrations of 12.5 and 60 ng/ml for 3, 6, 12, and 24 h.
2.5. Quantitative expression analysis by real-time polymerase chain reactions (PCR) in BIE cells
We performed a two-step real-time quantitative PCR to study the expression of mRNAs in BIE cells. Total RNA was isolated from BIE cells using TRIzol reagent (Invitrogen). All cDNAs were synthesized using a Quantitect Reverse Transcription kit (Qiagen, Tokyo, Japan) according to the manufacturer’s recommendations. Real-time quantitative PCR was performed using a 7300 Real-time PCR System (Applied Biosystems, Warrington, UK) using Platinum SYBR Green qPCR SuperMix UDG with ROX (Invitrogen). The primers used in this study were: β-actin forward (TGG ATT GGC GGC TCC AT) and reverse (GCT GAT CCA CAT CTG CTG GAA); IFN-α forward (GGT GGC AGC CAG TTA CAG AAG) and reverse (TGC TGG GTC ACC TCA TGG A), IFN-β forward (CGA TGG TTC TCC TGC TGT GTT) and reverse (GAG CAA GCT GTA GCT CCT GGAA); IFN-γ forward (GGA GGA CTT CAA AAA GCT GAT TCA) and reverse (GGC TTT GCG CTG GAT CTG); IL-6 forward (CCA CCC CAG GCA GAC TAC TTC) and reverse (CCA TGC GCT TAA TGA GAG CTT); IL-8 forward (TGC TCT CTT GGC AGC TTT CC) and reverse (TCT TGA CAG AAC TGC AGC TTC AC); and MCP-1 forward (CAC CAG CAG CAA GTG TCC TAA A) and reverse (CAC ATA ACT CCT TGC CCA GGA T). The PCR cycling conditions consisted of 2 min at 50 °C, followed by 2 min at 95 °C, and then 40 cycles of 15 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C. The reaction mixture contained 5 ml of sample cDNA and 15 ml of master mix, which included the sense and antisense primers. Cytokines, chemokines, and β-actin cDNAs for real-time PCR analysis were amplified using the primers listed above. The PCR products were inserted into the vector pGEM-T Easy DNA (Promega, Madison, WI, USA) using standard techniques. We confirmed the sequence of each insert with the dideoxy chain-termination method using an ABI PRISM 310 Genetic Analyzer (Applied Biosystems). The constructed plasmid vectors were applied to calculate the relative expression levels of each cDNA in samples by the relative standard curve method. The expression levels of β-actin were used to normalize individual cytokine and chemokine cDNA expression levels in the samples. The relative index of cytokine mRNA in BIE cells stimulated with LAB was calculated by first averaging the target cytokine expression levels from a minimum of three samples stimulated with poly(I:C) and without pre-treatment with LAB. After setting this value as 1, the relative expression of LAB pre-stimulated samples following poly(I:C) stimulation was then calculated.
2.6. Statistical analysis
All data were adjusted to give relative values against the appropriate controls. Statistical analyses were performed using the GLM procedure of SAS statistical software (SAS, 1994). Comparisons among mean values (N = 4) for relative mRNA expression levels of target cytokines in cells were performed using one-way ANOVA and Fisher’s least significant difference (LSD) test against the controls. For the analyses, P values of <0.05 and <0.01 were considered significant. Comparisons (N = 4) among relative mRNA expression levels of cytokines in BIE cells stimulated with LAB strains for 48 h are shown using two broken lines for P values of <0.05 and <0.01, following one-way ANOVA and LSD multi-comparison test. All presented data represents the results of four independent experiments.
3. Results
3.1. Functional expression of TLR3 in BIE cells
To evaluate the expression of TLR3 protein in BIE cells, we first performed immunohistochemical analysis. As shown in Fig. 1 A, BIE cells strongly expressed TLR3 in the cytoplasm, whereas no expression of TLR3 was detected at the cell surface (data not shown).
Fig. 1.
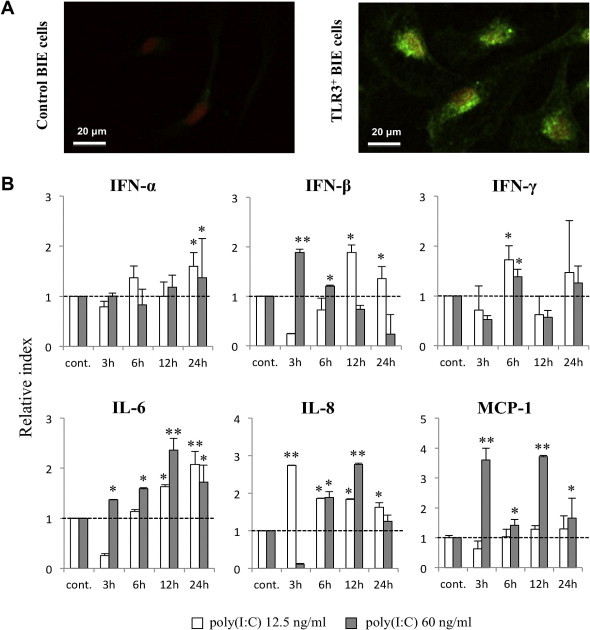
Functional expression of toll-like receptor 3 (TLR3) in bovine intestinal epithelial (BIE) cells. (A) Immunofluorescent localization of TLR3 in BIE cells. Green fluorescence indicates bovine TLR3-positive BIE cells, while nuclei were stained orange (red) with SYTOX. Control experiments were performed by omitting the primary antibody. The images represent typical results of four independent experiments. (B) Expression of IFN-α, IFN-β, IFN-γ, IL-6, IL-8, and MCP-1 mRNAs in BIE cells after stimulation with two concentrations (12.5 or 60 ng/ml) of poly(I:C). Cytokine levels were evaluated at the indicated times post-stimulation. All values are presented as the mean ± standard deviation (error bars). The results represent four independent experiments. ∗∗P < 0.01 and ∗P < 0.05 vs. cells cultured in the absence of poly(I:C). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As delineating cytokine and chemokine responses to TLR3 stimulation is important to understand TLR3-mediated immune responses and pathogenicity in IECs, we next evaluated the response of BIE cells to stimulation with poly(I:C). For these experiments, we treated BIE cells with two doses of TLR3 agonist to establish the most appropriate dose to study TLR3-mediated immune responses. Challenge of BIE cells with both low (12.5 ng/ml) and high (60 ng/ml) concentrations of poly(I:C) significantly increased the expression of type I IFN, IFN-γ, and pro-inflammatory cytokines and chemokines (Fig. 1B). The most significant changes were observed in the expression of IFN-β and the pro-inflammatory cytokines IL-6, IL-8, and MCP-1. We also observed that treatment of cells at the higher dose of poly(I:C) induced earlier up-regulation of IFN-β, as well as IL-6, IL-8, and MCP-1, when compared with the lower dose. Thus, poly(I:C) at a concentration of 60 ng/ml was selected for the following experiments.
3.2. Effect of LAB on cytokine expression by BIE cells
We evaluated changes in the expression of IFN-β, IL-6, IL-8, and MCP-1 in BIE cells after treatment with different LAB strains or the TLR2 agonist Pam3CSK4 for 24 and 48 h (Fig. 2 ). We observed that L. rhamnosus GG was able to significantly increase IFN-β levels after 48 h, whereas the other tested strains did not induce marked differences in IFN-β levels when compared with untreated BIE cells. In addition, L. rhamnosus LA-2 was the only strain able to increase MCP-1 levels in BIE cells after 48 h of treatment (Fig. 2). Levels of IL-6 mRNA were up-regulated within 24 and 48 h of treatment with Pam3CSK4, while the only LAB strain able to increase IL-6 expression was S. thermophilus TMC1543 after 24 h of stimulation (Fig. 2). Our analysis also revealed that Pam3CSK4, L. rhamnosus LA-2, L. casei TMC0409, and both B. bifidum strains were able to up-regulate IL-8 after 24 h.
Fig. 2.
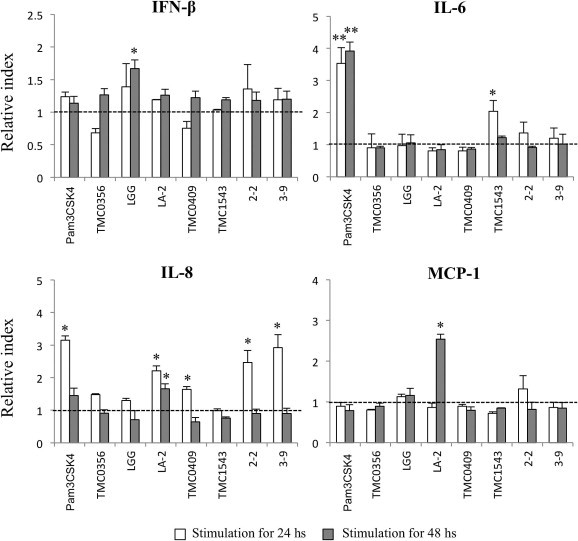
Effect of lactic acid bacteria (LAB) on cytokine expression in bovine intestinal epithelial (BIE) cells. Expression of IFN-β, IL-6, IL-8 and MCP-1 mRNA in BIE cells after stimulation with the bacteria Lactobacillus gasseri TMC0356, Lactobacillus rhamnosus LGG, L. rhamnosus LA-2, Lactobacillus casei TMC0409, Streptococcus thermophilus TMC1543, Bifidobacteriumbifidum 2-2, and B.bifidum 3-9, or the toll-like receptor 2 agonist Pam3CSK4. Cytokines were studied at the indicated times post-stimulation. All values are presented as the mean ± standard deviation (error bars). The results represent four independent experiments. ∗∗P < 0.05 vs. cells cultured in the absence of bacterial stimulation (broken lines).
3.3. Effect of LAB on the response of BIE cells to poly(I:C) stimulation
We next evaluated the ability of BIE cells to serve as an in vitro system for the selection of LAB strains with the capacity to modulate the response of bovine IECs to poly(I:C) challenge. As IFN-β is an important cytokine for protection against viral infections, we aimed to identify LAB strains with the ability to augment the production of IFN-β by BIE cells stimulated with TLR3 agonist. BIE cells were stimulated with the different LAB strains for 48 h and then challenged with poly(I:C) (Fig. 3 ). The determination of IFN-β mRNA expression levels at 3 h (Fig. 3A) and 12 h (Fig. 3B) post-stimulation with poly(I:C) revealed that only L. rhamnosus LA-2 and S. thermophilus TMC1543 were able to increase IFN-β levels after 12 h when compared to controls. Notably, the effect of L. rhamnosus LA-2 exposure was superior to that of S. thermophilus TMC1543. In addition, we observed that L. rhamnosus LA-2 induced the down-regulation of IL-6 after 3 h (Fig. 3A), while S. thermophilus TMC1543, L. casei TMC0409, and both B. bifidum strains had reduced the expression of IL-6 by 12 h (Fig. 3B). In addition, L. gasseri TMC0356 down-regulated the expression of IL-8, while L. casei TMC0409 reduced IL-8 and MCP-1 mRNAs levels after 12 h. These results were confirmed by comparing the relative mRNA levels of cytokines in BIE cells stimulated with each LAB strain and challenged with poly(I:C) for 12 h using multi-comparison tests (Fig. 3C).
Fig. 3.
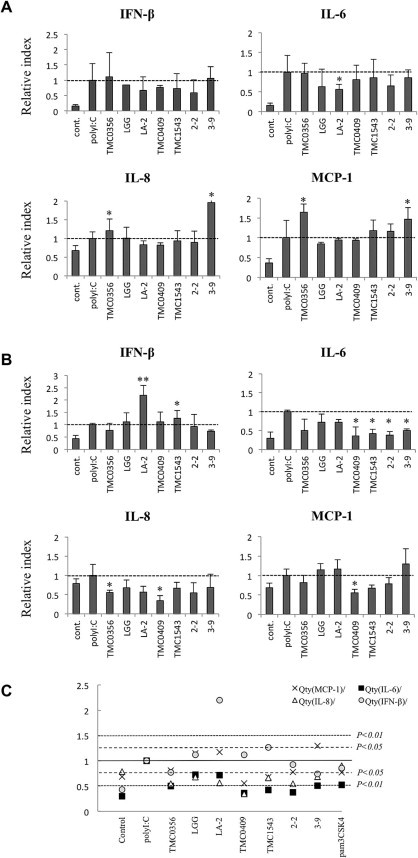
Effect of lactic acid bacteria (LAB) on the response of bovine intestinal epithelial (BIE) cells to poly(I:C) challenge. Expression of IFN-β, IL-6, IL-8, and MCP-1 mRNA in bovine intestinal epithelial (BIE) cells after stimulation with different bacterial strains and poly(I:C). BIE cells were stimulated with Lactobacillus gasseri TMC0356, Lactobacillus rhamnosus LGG, L. rhamnosus LA-2, Lactobacillus casei TMC0409, Streptococcus thermophilus TMC1543, Bifidobacteriumbifidum 2-2, or B.bifidum 3-9 for 48 h. Cells were then challenged with poly(I:C) (60 ng/ml) and cytokine levels were evaluated 3 h (A) and 12 h (B) post-challenge. All values are presented as the mean ± standard deviation (error bars). The results represent four independent experiments. ∗∗P < 0.01 and ∗P < 0.05 vs. cells cultured in the absence of bacterial stimulation and challenged with poly(I:C). (C) Comparisons of relative mRNA levels of cytokines in BIE cells stimulated with the indicated LAB strains for 48 h and challenged with poly(I:C) for 12 h. One-way ANOVA and Fisher’s least significant difference multi-comparison test were used. P values of < 0.05 and < 0.01 are indicated by the two types of broken lines.
4. Discussion
Epithelial TLR expression is thought to play a key role in host defenses against pathogens. Epithelial cells, more than any other cell type, express TLR3 in numerous organs, including the gastrointestinal tract (Cario and Podolsky, 2000). It was reported that human IECs express low levels of TLR2 and TLR4, whereas TLR3 appears to be abundantly expressed in the normal human small intestine and colon (Cario and Podolsky, 2000). In addition, our laboratory has previously shown that TLR3 is also strongly expressed in swine IECs (Moue et al., 2008). Moreover, TLR3 transcription in bovine colonic epithelium was recently described for the first time (Bridger et al., 2010). In the present study, immunohistochemical analyses revealed abundant expression of TLR3 in BIE cells. Therefore, BIE cells, in addition to displaying characteristics of epithelial cells, such as cobblestone morphology, microvilli-like structures, and strong expression of cell-to-cell junctional proteins and cytokeratin (Miyazawa et al., 2010), also express TLR3 and thus resemble the IECs of other species.
We also evaluated the response of BIE cells to stimulation with the TLR3 agonist poly(I:C) and found that the cells up-regulate the expression of type I IFN, IFN-γ, and pro-inflammatory cytokines and chemokines. The observed changes in the expression of cytokines induced by poly(I:C) correlate with the changes reported in various intestinal viral infections of cattle and other hosts. For example, increased gene expression of RANTES, IP-10, IL-8, and MCP-1 were observed in rotavirus-infected HT-29 cells (Rollo et al., 1999, Xu et al., 2009). In addition, in vitro studies with bovine intestinal tissues demonstrated that exposure to BRV activated TLR3-induced up-regulation of NF-kB and IL-6 production (Aich et al., 2007). These findings, together with our present results, indicate that BIE cells are valuable tools for the in vitro study of immune responses triggered by TLR3 expressed on bovine IECs.
To date, a few studies have evaluated the antiviral effects of probiotic LAB strains in animals. In-vivo studies using gnotobiotic pigs demonstrated that probiotic LAB administration has a significant influence on IFN-α, TGF-β, IL-4, and IFN-γ serum levels induced by rotavirus infection (Wen et al., 2009). The antiviral effects of immunobiotics have also been examined in a few animal cell lines. For example, Maragkoudakis et al. (2010) reported that probiotics are able to protect porcine and goat epithelial cells against rotavirus and transmissible gastroenteritis virus challenges; however, the immunological mechanisms involved in the protective effect were not determined. Recent studies have also demonstrated that probiotics significantly decrease IL-6 production by porcine IPEC-J2 cells infected with porcine rotavirus, suggesting that probiotics have immunoregulatory effects (Liu et al., 2010). To our knowledge, no prior studies have investigated the effects of probiotics in bovine IECs lines. Therefore, we evaluated whether our bovine in vitro system could be used for the selection of LAB strains with antiviral immune-enhancing activities.
We first examined if the stimulation of BIE cells with different LAB strains was able to induce changes in the expression of IFN-β, IL-6, IL-8, or MCP-1. We found that the different strains had distinct effects on cytokine production by BIE cells. Notably, L. rhamnosus GG and S. thermophilus TMC1543 were the only strains able to increase IFN-β and IL-6 mRNA, respectively, in BIE cells after 48 h of stimulation. In addition, only L. rhamnosus LA-2 increased MCP-1 mRNA, while this bacterium, L. casei TMC0409, and both B. bifidum strains up-regulated IL-8. It has been shown that many of the immunomodulatory effects of probiotic microorganisms are mediated by their ability to activate TLR2 (Tohno et al., 2005, Tohno et al., 2006, Tohno et al., 2007, Kitazawa et al., 2008, Alvarez et al., 2009, Fujie et al., 2011). In addition, TLR2 agonists are able to induce IFN-β transcription (Dietrich et al., 2010). Moreover, it was observed that certain lactobacilli trigger the expression of IFN-β genes in dendritic cells in a TLR2-dependent manner (Weiss et al., 2010). In our present analyses, we therefore expected to identify strains capable of increasing IFN-β levels in BIE cells; however, we only detected a slight increase of this cytokine in BIE cells treated with L. rhamnosus GG. In addition, we found that the TLR2 agonist Pam3CSK4 was not able to modify the expression of IFN-β in BIE cells. Therefore, the information obtained in these experiments did not allow us to draw any conclusions concerning the antiviral effects of LAB strains in bovine IECs.
We next studied the effects of LAB strains on the response of BIE cells to TLR3 stimulation and found that only L. rhamnosus LA-2 and S. thermophilus TMC1543 were able to increase IFN-β levels by 12 h post-stimulation. The increased production of IFN-β by BIE cells in response to TLR3 activation induced by L. rhamnosus LA-2 may have significant in vivo effects in the protection against enteric viruses. Global gene-expression analyses of bovine intestinal tissues following infection with BRV or BCV indicate that several IFN-regulatory and -stimulatory genes are down-regulated, supporting the conclusion that both viruses may have evolved mechanism(s) to inhibit IFN-mediated immune responses (Aich et al., 2007). Moreover, it was shown that BVDV significantly interferes with the induction of type I IFN, which impairs not only innate defenses, but also interferes with the establishment of adaptive immune responses (Peterhans et al., 2003, Lee et al., 2008). Based on these findings, L. rhamnosus LA-2, which enhances IFN-β production in BIE cells, may play an important role in the improvement of innate and specific immune responses against bovine intestinal virus. Thus, our studies with the BIE cell line have allowed us to identify a LAB strain that is a good candidate for future in vivo studies.
As the degree and duration of pro-inflammatory cytokine secretion after TLR3 recognition of dsRNA can become harmful to the host (Vercammen et al., 2008), we also evaluated the production of the inflammatory cytokines IL-6, IL-8, and MCP-1 in BIE cells. Our analyses demonstrated that BIE cells pretreated with L. casei TMC0409 produce lower levels of the three pro-inflammatory cytokines when compared with control cells 12 h post-stimulation with poly(I:C). It was reported that TLR3 mediates harmful inflammatory responses in the intestine, thus contributing to the pathogenesis of viral infections (Zhou et al., 2007). Therefore, the lower production of pro-inflammatory cytokines following exposure to L. casei TMC0409 may allow for the efficient regulation of inflammatory responses and avoidance of tissue injury, offering a different protection mechanism against bovine viral infection.
In conclusion, the in vitro system described in this study was useful for the primary screening of two types of immunomodulatory LAB strains that would be able to protect against viral intestinal diseases in cattle: strains capable of increasing antiviral defenses and strains with anti-inflammatory capacities. To define the characteristic immunomodulatory abilities of individual LAB strains, their influence on cytokine production after challenge of BIE cells with TLR3 agonist can be studied using statistical multi-comparison tests, as shown in Fig. 3C. We also demonstrated that TLR3 is expressed in BIE cells and that these cells respond to stimulation with the TLR3 agonist poly(I:C). Characterization of the inflammatory immune response triggered by TLR3 activation in BIE cells showed that this in vitro system can be used for the study of TLR3-mediated immune responses in bovine IECs. In addition, our findings indicate that BIE cells are useful for the primary screening of immunobiotic LAB strains which are able to beneficially modulate the antiviral immune response triggered by TLR3 activation in bovine IECs. Although it is difficult at present to predict if the observed in vitro changes in gene expression are biologically relevant in vivo, given the diverse factors capable of influencing intestinal immune responses, we propose that BIE cells can serve as a useful in vitro tool to identify a small number of potentially immunobiotic strains which can then be subjected to appropriate in vivo trials. In fact, the use of immunobiotic microorganisms in animal feeding is expected to be enhanced by preliminary host-specific in vitro screening tests. The present characterization of BIE cells represents an important step towards the establishment of a valuable bovine in vitro system that could be used for the development of immunomodulatory feed for bovine hosts.
5. Conflict of interest statement
The authors declare no financial or commercial conflicts of interest.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (B)(2) (No. 21380164) from the Japan Society for the Promotion of Science (JSPS), the Kieikai Research Foundation, and the Japan Racing Association to Dr. H. Kitazawa. Dr. Julio Villena was supported by JSPS (Postdoctoral Fellowship for Foreign Researchers, Program No. 21-09335).
References
- Aich P., Wilson H.L., Kaushik R.S., Potter A.A., Babiuk L.A., Griebel P. Comparative analysis of innate immune responses following infection of newborn calves with bovine rotavirus and bovine coronavirus. Journal of General Virology. 2007;88:2749–2761. doi: 10.1099/vir.0.82861-0. [DOI] [PubMed] [Google Scholar]
- Alvarez, S., Villena, J., Tohno, M., Salva, S., Kitazawa, H., 2009. Modulation of innate immunity by lactic acid bacteria: impact on host response to infections. Current Research in Immunology, Research Media (Ed). India, 3, 87–126.
- Bridger P.S., Mohr M., Stamm I., Fröhlich J., Föllmann W., Birkner S., Metcalfe H., Werling D., Baljer G., Menge C. Primary bovine colonic cells: a model to study strain-specific responses to Escherichia coli. Veterinary Immunology and Immunopathology. 2010;137:54–63. doi: 10.1016/j.vetimm.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Cario E., Podolsky D.K. Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infection and Immunity. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich N., Lienenklaus S., Weiss S., Gekara N.O. Murine toll-like receptor 2 activation induces type I interferon responses from endolysosomal compartments. PLoS One. 2010;5:e10250. doi: 10.1371/journal.pone.0010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujie H., Villena J., Tohno M., Morie K., Shimazu T., Aso H., Suda Y., Shimosato T., Iwabuchi N., Xiao J.Z., Yaeshima T., Iwatsuki K., Saito T., Numasaki M., Kitazawa H. Toll-like receptor-2 activating bifidobacteria strains differentially regulate inflammatory cytokines in porcine intestinal epithelial cell culture system: finding new anti-inflammatory immunobiotics. FEMS Immunology and Medical Microbiology. 2011;63:129–139. doi: 10.1111/j.1574-695X.2011.00837.x. [DOI] [PubMed] [Google Scholar]
- Gauzzi M.C., Del Cornò M., Gessani S. Dissecting TLR3 signalling in dendritic cells. Immunobiology. 2010;215:713–723. doi: 10.1016/j.imbio.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Kitazawa H., Tohno M., Shimosato T., Saito T. Development of molecular immunoassay system for probiotics via toll-like receptors based on food immunology. Animal Science Journal. 2008;79:11–21. [Google Scholar]
- Lee S.R., Pharr G.T., Boyd B.L., Pinchuk L.M. Bovine viral diarrhea viruses modulate toll-like receptors, cytokines and co-stimulatory molecules genes expression in bovine peripheral blood monocytes. Comparative Immunology, Microbiology and Infectious Disease. 2008;31:403–418. doi: 10.1016/j.cimid.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Liu F., Li G., Wen K., Bui T., Cao D., Zhang Y., Yuan L. Porcine small intestinal epithelial cell line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics. Viral Immunology. 2010;23:135–149. doi: 10.1089/vim.2009.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragkoudakis P.A., Chingwaru W., Gradisnik L., Tsakalidou E., Cencic A. Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection. International Journal of Food Microbiology. 2010;141:S91–S97. doi: 10.1016/j.ijfoodmicro.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Kikkawa S., Kohase M., Miyake K., Seya T. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochemical and Biophysical Research Communications. 2002;239:1364–1369. doi: 10.1016/S0006-291X(02)00380-7. [DOI] [PubMed] [Google Scholar]
- Miyazawa K., Hondo T., Kanaya T., Tanaka S., Takakura I., Itani W., Rose M.T., Kitazawa H., Yamaguchi T., Aso H. Characterization of newly established bovine intestinal epithelial cell line. Histochemistry and Cell Biology. 2010;133:125–134. doi: 10.1007/s00418-009-0648-3. [DOI] [PubMed] [Google Scholar]
- Moue M., Tohno M., Shimazu T., Kido T., Aso H., Saito T., Kitazawa H. Toll-like receptor 4 and cytokine expression involved in functional immune response in an originally established porcine intestinal epitheliocyte cell line. Biochimica et Biophysica Acta. 2008;1780:134–144. doi: 10.1016/j.bbagen.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Peterhans E., Jungi T.W., Schweizer M. BVDV and innate immunity. Biologicals. 2003;31:107–112. doi: 10.1016/s1045-1056(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Rollo E.E., Kumar K.P., Reich N.C., Cohen J., Angel J., Greenberg H.B., Sheth R., Anderson J., Oh B., Hempson S.J., Mackow E.R., Shaw R.D. The epithelial cell response to rotavirus infection. Journal of Immunology. 1999;163:4442–4452. [PubMed] [Google Scholar]
- Tohno M., Shimosato T., Kitazawa H., Katoh S., Iliev I.D., Kimura T., Kawai Y., Watanabe K., Aso H., Yamaguchi T., Saito T. Toll-like receptor 2 is expressed on the intestinal M cells in swine. Biochemical and Biophysical Research Communications. 2005;330:547–554. doi: 10.1016/j.bbrc.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Tohno M., Shimosato T., Moue M., Aso H., Watanabe K., Kawai Y., Yamaguchi T., Saito T., Kitazawa H. Toll-like receptor 2 and 9 are expressed and functional in gut-associated lymphoid tissues of presuckling newborn swine. Veterinary Research. 2006;37:791–812. doi: 10.1051/vetres:2006036. [DOI] [PubMed] [Google Scholar]
- Tohno M., Shimosato T., Kawai Y., Aso H., Ikegami S., Takemoto N., Saito T., Kitazawa H. Advanced molecular immunoassay system for immunobiotic lactic acid bacteria using a transfectant of Toll-like receptor 2. Animal Science Journal. 2007;78:195–205. [Google Scholar]
- Vercammen E., Staal J., Beyaert R. Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clinical Microbiology Review. 2008;21:13–25. doi: 10.1128/CMR.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G., Rasmussen S., Zeuthen L.H., Nielsen B.N., Jarmer H., Jespersen L., Frøkiaer H. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunology. 2010;13:268–281. doi: 10.1111/j.1365-2567.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen K., Azevedo M.S., Gonzalez A., Zhang W., Saif L.J., Li G., Yousef A., Yuan L. Toll-like receptor and innate cytokine responses induced by lactobacilli colonization and human rotavirus infection in gnotobiotic pigs. Veterinary Immunology and Immunophatology. 2009;127:304–315. doi: 10.1016/j.vetimm.2008.10.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf A.M., Fleissner D., Hansen W., Buer J. T cells, dendritic cells and epithelial cells in intestinal homeostasis. International Journal of Medical Microbiology. 2010;300:11–18. doi: 10.1016/j.ijmm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Xu J., Yang Y., Wang C., Jiang B. Rotavirus and coxsackievirus infection activated different profiles of toll-like receptors and chemokines in intestinal epithelial cells. Inflammation Research. 2009;58:585–592. doi: 10.1007/s00011-009-0022-x. [DOI] [PubMed] [Google Scholar]
- Zhou R., Wei H., Sun R., Tian Z. Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. Journal of Immunology. 2007;178:4548–4556. doi: 10.4049/jimmunol.178.7.4548. [DOI] [PubMed] [Google Scholar]


