Abstract
A placebo-controlled field trial was conducted to compare the effectiveness of intranasal (IN) vaccines containing Bordetella bronchiseptica and canine-parainfluenza virus, with (IN-BPA) or without (IN-BP) canine-adenovirus type 2, for prevention of kennel cough at a humane shelter. Dogs were examined on admission to the shelter and those without respiratory signs of disease were assigned daily, on a rotating basis, to receive one of three vaccines. We enrolled 972 healthy dogs. Dogs were monitored for up to 30 days post-vaccination for coughing and other clinical signs of respiratory disease. Thirty-three (10.7%; 95% confidence interval (CI): 7.2, 14.2) dogs in the IN-BP group, 36 (10.2%; 95% CI: 7.0, 13.4) dogs in the IN-BPA group, and 42 (13.5%; 95% CI: 9.7, 17.3) dogs in the IN-P group coughed spontaneously for ≥1 day within 30 days of vaccination (P=0.37). The IN-BP and IN-BPA vaccines were 20.7 and 24.4% effective, respectively, in reducing coughing compared with a placebo vaccine. The strongest prognostic factor for coughing (regardless of vaccine group) was the number of days spent at the shelter, with each additional day increasing the risk of coughing by 3% (95% CI: 0.5, 6.3). The low incidence of coughing in the shelter during this study precluded observation of differences in vaccine effectiveness. No differences in vaccine-associated adverse events (coughing, sneezing, nasal or ocular discharge) were noted during the first 3 days post-administration or thereafter.
Keywords: Vaccine effectiveness, Kennel cough, Dogs, Animal shelter, Clinical trial, Infectious tracheobronchitis
1. Introduction
Infectious tracheobronchitis (kennel cough) is a clinical syndrome of dogs characterized by acute onset of a dry, hacking cough (often followed by retching or gagging on mucous secretions) (Appel and Percy, 1970, Bemis et al., 1977a, Shade and Goodnow, 1979, Azetaka and Konishi, 1988). Kennel cough can be due to a single pathogen or to a complex of bacteria and/or viruses (Appel and Percy, 1970, Bemis et al., 1977a, Appel, 1981). The primary bacterium associated with kennel cough is Bordetella bronchiseptica (Bemis et al., 1977a). Signs of infection due to B. bronchiseptica typically persist for 1–2 weeks, but organisms can be shed for 2–3 months following clinical recovery (Bemis et al., 1977a). Dogs can be resistant to re-infection for approximately 12 months following recovery (Bemis et al., 1977b). Mycoplasma spp., Pastuerella multocida, Pseudomonas aeruginosa, and Escherichia coli have been isolated from the respiratory tract of dogs and also might cause signs of kennel cough (Bemis et al., 1977b, Wagener et al., 1984). Primary viral pathogens implicated in kennel cough include canine-adenovirus type 2 and canine-parainfluenza virus (Appel and Percy, 1970, Azetaka and Konishi, 1988). Other viruses suspected of causing kennel cough include canine-adenovirus type 1, canine-herpesvirus, canine-distemper virus, and canine-reovirus types 1–3 (Dhein and Gorham, 1986, Appel and Binn, 1987). Transmission of pathogens associated with kennel cough is both by direct contact and indirectly by aerosolized droplets from coughing or sneezing dogs and by fomites (such as dishes, and the clothing and hands of dog handlers) (Ford and Vaden, 1990).
Dogs in kennels, shelters, and pet stores are at increased risk for kennel cough due to increased population density and the influx of susceptible animals and pathogens resulting from high population turnover, and (sometimes) inadequate ventilation (Appel and Percy, 1970, Bemis et al., 1977a, Azetaka and Konishi, 1988, Ford and Vaden, 1998). Signs of kennel cough often occur within 5–10 days (Appel, 1981) after admission to a shelter and morbidity can be as high as 50% among susceptible dogs; mortality is usually low (Appel and Binn, 1987). A major issue for shelters is that coughing dogs might not be adoptable and might require medical care. Furthermore, signs of kennel cough might not become apparent until a dog is adopted—causing concern and frustration among new owners.
Methods to prevent kennel cough include vaccination of dogs as soon as possible following admission to a shelter, quarantine of newly introduced dogs, isolation of sick dogs, and adequate sanitation and ventilation. Commercial vaccines are either killed or modified live for parenteral or intranasal (IN) administration. Ten to 14 days usually are required for partial immunity to develop following subcutaneous administration, and a second dose is required 2–4 weeks later. IN modified-live vaccines usually produce a rapid, localized (mucosal) protection (Shade and Goodnow, 1979, Bey et al., 1981, Glickman and Appel, 1981, Kontor et al., 1981) and protective immunity can develop within 4 days following a single dose (Bey et al., 1981). IN vaccination has been recommended for use during outbreaks or when exposure is imminent (such as prior to boarding, being placed in a shelter, or attending a dog show) (Appel and Binn, 1987). Shelters usually do not have adequate space to quarantine vaccinated dogs to allow protective immunity to develop or to isolate symptomatic dogs. Use of bleach solutions for cleaning cages and bedding, use of disposable gloves and dishware, diligent hand washing, and adequate ventilation/filtration to reduce the concentration of viral and bacterial particles in the environment also help to prevent the spread of kennel cough (Ford and Vaden, 1990).
There are several commercially available IN vaccines to prevent kennel cough—but only the effectiveness of a vaccine containing B. bronchiseptica and canine-parainfluenza virus antigens has been evaluated in a controlled field trial (Glickman and Appel, 1981). Recently, an IN vaccine containing canine-adenovirus in addition to B. bronchiseptica and canine-parainfluenza was licensed. In May 2000, a higher-than-expected incidence of coughing in dogs (approximately 50%) was noted at the Tippecanoe County Humane Society (TCHS) in Lafayette, Indiana. A placebo-controlled trial was initiated at the TCHS to compare the effectiveness of two commercial IN vaccines (containing B. bronchiseptica and canine-parainfluenza virus with or without canine-adenovirus) with a placebo IN vaccine and with each other, for the prevention of kennel cough. Secondary objectives were to determine prognostic factors for development of kennel cough and the frequency of vaccine-associated adverse events.
2. Material and methods
2.1. Study site and population
During 2000, 2830 dogs and 2932 cats were admitted to TCHS. Dogs were housed in three rooms with a total of 5200 square feet of housing and shared the same air space. Kennels were cleaned daily with pressure washes followed by application of a quaternary-ammonia solution that remained on surfaces for 10 min before rinsing and scraping to remove water. Between June 2000 and March 2001, 1973 dogs were admitted to the shelter. At the time of the study, there were a total of 20 staff members and three managers, with six staff members and one manager working on any given day. Staff members worked varied schedules. The shelter was open every day of the week.
Prior to the trial, TCHS personnel were administering a single subcutaneous vaccine (Duramune® Max 5 CVK/LCI-GP, Fort Dodge Laboratories, Fort Dodge, IA) containing modified-live canine-distemper virus, canine-adenovirus type 2, canine-parainfluenza virus, and canine-parvovirus, killed coronavirus, and leptospirosis only to those dogs that met the shelter’s criteria for adoptability (including temperament). This determination was made between 3 and 5 days following admission to the shelter, and the vaccination was given at that time. The shelter continued this practice for the duration of the IN vaccine trial.
2.2. Protocol
On entry to the shelter, all dogs were examined by shelter staff and their age, gender, spay/neuter status, breed, and prior vaccination history were recorded. Puppies <8 weeks of age, dogs with any signs of respiratory disease at the time of admission, dogs surrendered for euthanasia, or dogs judged to be unadoptable (based on temperament) at the time of admission were excluded from the trial. Dogs admitted to TCHS more than once during the study period were included in the vaccine trial on their first admission only. Eligible dogs were assigned to one of three vaccine groups on a rotating-day basis. The first day at the shelter, apparently healthy dogs received either an IN vaccination containing B. bronchiseptica and canine-parainfluenza virus (Intra-Trac® II, Schering-Plough Animal Health Corporation, Omaha, NE) (IN-BP), an IN vaccine containing B. bronchiseptica and canine parainfluenza virus plus canine adenovirus (Bronchi-Shield® III, Fort Dodge Laboratories, Fort Dodge, IA) (IN-BPA), or a placebo IN (IN-P) vaccine consisting of a sterile diluent labeled as an experimental vaccine. Active vaccines were packaged in the commercially available form. All dogs admitted on a given day received the same vaccine. Dogs were housed in one of the three rooms of the shelter based on availability of kennel space on the admission date; small dogs and puppies were housed in the smallest room containing the smallest kennels. Therefore, no attempt was made to segregate unvaccinated dogs or to group dogs by admission date (and thus by vaccine). As necessary to facilitate cleaning, dogs were moved from one kennel to another during their stay in the shelter. Dogs were observed daily for coughing, sneezing, or nasal or ocular discharge, by shelter staff during activities of cleaning, feeding, and placement of new dog admissions into neighboring kennels. When these signs were observed by staff members, managers confirmed the observations and noted the signs on a reporting form. The TCHS admission date, the type of vaccine administered (by manufacturer’s name), and the date of vaccine administration were recorded in each dog’s written and computer records; this was the only indication that the dog was part of the study and of the vaccine it had received.
The primary outcome of interest was clinically apparent tracheobronchitis based on episodic spontaneous coughing of at least 1 day’s duration (Dhein and Gorham, 1986, Appel and Binn, 1987, Ford, 1995, Ford and Vaden, 1998). Dogs in the study were monitored for up to 30 days post-vaccination. If a dog was adopted or redeemed from TCHS prior to 30 days, the owner was given a written description of the study, along with a chart to record any clinical signs of tracheobronchitis (such as coughing, sneezing, and nasal and ocular discharge) and the duration of these signs. The vaccines the dogs had received were noted in the shelter record by manufacturer’s name, and owners received a copy of this record as part of the adoption paperwork. The owners of dogs adopted before 30 days post-vaccination were contacted by telephone 30–45 days following vaccination and asked about their dogs’ health. Any clinical signs that occurred during the 30 days following vaccination were recorded. Two primary outcomes were possible: no coughing or coughing (whether at TCHS or at home). Secondary outcomes included signs of upper-respiratory disease (such as coughing, sneezing, and nasal or ocular discharge) during the first 3 days following vaccination, and these were considered as potential vaccine-associated adverse events. Sneezing or nasal or ocular discharge occurring within 30 days post-vaccination also was recorded.
2.3. Microbiology
Before the start of the trial, nasal and pharyngeal swabs were obtained from three coughing dogs for viral isolation and bacterial culture. B. bronchiseptica was isolated from both nasal and pharyngeal swabs from one of these dogs. Toward the end of the trial, nasal and pharyngeal swabs from one of three unvaccinated, non-coughing dogs yielded canine-adenovirus. This testing involved a convenience sample of dogs, because funds were not available for regular sampling during the study.
2.4. Statistical analysis
All data were entered into a database (Microsoft® Access 97 SR-2, Microsoft Corporation, Redmond, WA). Kruskal–Wallis and χ 2-tests (SPSS for Windows® Release 9.0.0, SPSS, Chicago, IL) were used to compare baseline characteristics and frequency of clinical signs between the three vaccine groups. Cumulative incidence (Last, 2001) was calculated; this was defined as the proportion of dogs experiencing the onset of a sign of upper-respiratory disease during the study period. Relative risks (RR) with 95% confidence intervals (CI) were used to measure the strength of association between specific prognostic factors (vaccine type versus placebo, age, admission status (owned or stray), breed group (pure/single breed or mixed breed), gender, days spent at TCHS) and the likelihood of kennel cough. Prognostic factors for kennel cough were evaluated using logistic regression (odds ratios (OR)). The distributions of continuous variables were checked for log-linearity. Univariable logistic regression was used to determine OR, 95% CI of the OR, and significance values for each prognostic factor. Factors that changed ORs for other factors in logistic models by >10% were retained in multivariable models. Vaccine effectiveness was determined using the preventable fraction (Last, 2001): (incidence of coughing in the IN-P group−incidence of coughing in the IN-BP or IN-BPA group)/(the incidence of coughing in the IN-P group)×100. Dogs lost to follow-up during the first 2 days post-vaccination were excluded from the analysis of adverse events. Alpha was P<0.05, and all tests were two-sided because no assumptions were made as to the likely outcome.
3. Results
3.1. Study site and population
During the study period, 972 of the admitted 1973 dogs (49.3%) met the criteria for participation and were entered into the study. Information on ineligible dogs was not collected for this study; these would have included under-age puppies, dogs with obvious temperament problems (and which therefore could not have been vaccinated), dogs surrendered for euthanasia, dogs redeemed by their owners the same day as entry to the shelter and that had not yet been vaccinated, and dogs that were not vaccinated within 24 h. In a small number of cases, dogs within the study were admitted several times (repeated strays), and their number of admissions is reflected in the total. Except for management personnel, shelter staff turned over twice during this period. The study initially was planned to recruit 1500 dogs (with 500 per vaccine group)—but was halted when newly admitted dogs were housed in another location so that new surface coatings could be applied to the floors at TCHS. Rather than attempt to investigate the effectiveness of the vaccines at both sites, the study concluded when the last dog enrolled in the study was adopted.
3.2. Kennel cough
There were no significant differences in baseline characteristics between the three vaccine groups (Table 1 ). Overall, 861 (88.6%) dogs did not cough, 53 (5.4%) dogs developed a cough at TCHS, and 58 (6.0%) dogs developed a cough at home following adoption. The number of dogs in each vaccine group that coughed within the 30 days post-vaccination was not significantly different (Table 2 ). It is interesting to us to note that the incidence of coughing was higher for dogs in their new homes than at TCHS for dogs in the active vaccine groups compared with dogs in the placebo group. During the first 3 days post-vaccination, the cumulative incidence of coughing was also not different among dogs in the three vaccine groups (Table 3 ). The cumulative incidence of coughing remained higher for dogs in the IN-BP group than the IN-BPA group, though not significantly so, for the remainder of the 30-day follow-up period (Fig. 1 ).
Table 1.
Baseline comparisons, length of stay, and length of follow-up for 972 dogs in an intranasal vaccine trial at a shelter in the USAa
| Variable | Vaccine group |
Test statistic | P | |||||||||||||
| IN-BP (n=308) |
IN-BPA (n=353) |
IN-P (n=311) |
||||||||||||||
| n | % | n | % | n | % | |||||||||||
| Gender | ||||||||||||||||
| Male | 159 | 51.6 | 185 | 52.4 | 154 | 49.5 | χ2=0.58 | 0.75 | ||||||||
| Female | 149 | 48.4 | 168 | 47.6 | 157 | 50.5 | ||||||||||
| Spay/neuter status | ||||||||||||||||
| Intact or unknown | 268 | 87.0 | 294 | 83.3 | 274 | 88.1 | χ2=3.57 | 0.17 | ||||||||
| Spayed or castrated | 40 | 13.0 | 59 | 16.7 | 37 | 11.9 | ||||||||||
| Breed | ||||||||||||||||
| Purebreed or only one breed given | 88 | 28.6 | 100 | 28.3 | 73 | 23.5 | χ2=2.66 | 0.26 | ||||||||
| Mixed breed | 220 | 71.4 | 253 | 71.7 | 238 | 76.5 | ||||||||||
| Admission status | ||||||||||||||||
| Owned (surrendered/abandoned) | 177 | 57.5 | 192 | 54.4 | 158 | 50.8 | χ2=2.77 | 0.25 | ||||||||
| Stray | 131 | 42.5 | 161 | 45.6 | 153 | 49.2 | ||||||||||
| Prior vaccine history | ||||||||||||||||
| Current or overdue | 12 | 3.9 | 19 | 5.4 | 8 | 2.6 | χ2=3.41 | 0.18 | ||||||||
| Never or unknown | 296 | 96.1 | 334 | 94.6 | 303 | 97.4 | ||||||||||
|
Median (minimum–maximum) |
|
|
|
|
|
|||||||||||
| IN-BP |
IN-BPA |
IN-P |
||||||||||||||
| Age (months) | 7.0 (2–120) | 9.0 (2–146) | 8.0 (2–156) | KW=2.66 | 0.26 | |||||||||||
| Days of stay at TCHS | 7.0 (1–30) | 7.0 (1–30) | 8.0 (1–30) | KW=3.21 | 0.20 | |||||||||||
| Days of follow-up | 10.0 (1–30) | 11.0 (1–30) | 10.0 (1–30) | KW=1.90 | 0.39 | |||||||||||
IN-P: intranasal placebo; IN-BP: intranasal vaccine with B. bronchiseptica and canine-parainfluenza virus; IN-BPA: intranasal vaccine with B. bronchiseptica, canine-parainfluenza virus, and canine-adenovirus. Test statistics: χ2; KW: Kruskal–Wallis.
Table 2.
Frequency of clinical signs of upper-respiratory disease within 30 days post-vaccination for 972 dogs by kennel cough vaccine group (in a humane shelter in the USA)a
| Variable | Vaccine group |
Test statistic, χ2 | P | |||||||||||||
| IN-BP (n=308) |
IN-BPA (n=353) |
IN-P (n=311) |
||||||||||||||
| n | % | n | % | n | % | |||||||||||
| Coughing | 33 | 10.7 | 36 | 10.2 | 42 | 13.5 | 2.01 | 0.37 | ||||||||
| Coughing location | ||||||||||||||||
| At TCHS | 13 | 4.2 | 15 | 4.2 | 25 | 8.0 | 6.11 | 0.19 | ||||||||
| At home | 20 | 6.5 | 21 | 5.9 | 17 | 5.5 | ||||||||||
| Sneezing | 41 | 13.3 | 40 | 11.3 | 49 | 15.8 | 2.80 | 0.25 | ||||||||
| Nasal discharge | 65 | 21.1 | 73 | 20.7 | 77 | 24.8 | 1.87 | 0.39 | ||||||||
| Ocular discharge | 28 | 9.1 | 33 | 9.3 | 34 | 10.9 | 0.71 | 0.70 | ||||||||
IN-P: intranasal placebo; IN-BP: intranasal vaccine with B. bronchiseptica and canine-parainfluenza virus; IN-BPA: intranasal vaccine with B. bronchiseptica, canine-parainfluenza virus, and canine-adenovirus. Test statistics, χ2.
Table 3.
Frequency of clinical signs of upper-respiratory disease within 3 days post-vaccination for 871 dogs by kennel cough vaccine group (in a shelter in the USA)a
| Variable | Vaccine groupb |
Test statistic, χ2 | P | |||||
| IN-BP (n=276) |
IN-BPA (n=310) |
IN-P (n=285) |
||||||
| n | % | n | % | n | % | |||
| Coughing | 1 | 0.4 | 2 | 0.6 | 1 | 0.4 | 0.36 | 0.83 |
| Sneezing | 3 | 1.1 | 1 | 0.3 | 2 | 0.7 | 1.25 | 0.54 |
| Nasal discharge | 14 | 5.1 | 23 | 7.4 | 18 | 6.3 | 1.36 | 0.51 |
| Ocular discharge | 7 | 2.5 | 13 | 4.2 | 9 | 3.2 | 1.29 | 0.53 |
IN-P: intranasal placebo; IN-BP: intranasal vaccine with B. bronchiseptica and canine-parainfluenza virus; IN-BPA: intranasal vaccine with B. bronchiseptica, canine-parainfluenza virus, and canine-adenovirus. Test statistics, χ2.
Dogs lost to follow-up in first 2 days were excluded from analysis.
Fig. 1.
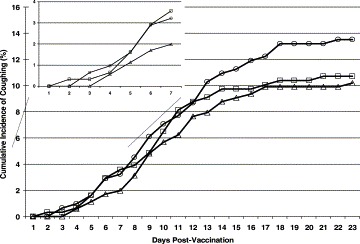
Cumulative incidence of coughing for 972 humane-shelter dogs by kennel-cough vaccine group and time from vaccination to onset of cough. Open circles: IN-placebo vaccine group; open squares: IN-BP vaccine group; open triangles: IN-BPA vaccine group.
The IN-BPA and IN-BP vaccines were 24.4% (95% CI: 22.5, 27.8) and 20.7% (95% CI: 17.9, 25.8) effective, respectively, in preventing coughing compared with the IN-P vaccine. During the period 4–30 days post-vaccination, the IN-BPA and IN-BP vaccines were 22.2% (95% CI: 20.4, 25.4) and 18.7% (95% CI: 16.4, 23.0) effective, respectively, in preventing coughing compared with the IN-P vaccine.
The length of follow-up for dogs in the three vaccine groups was not significantly different (Table 1). Based on univariable logistic regression, the only significant prognostic factor for coughing was length of stay at TCHS. Each additional day at TCHS increased the risk of coughing by 3% (RR=1.03, 95% CI: 1.01–1.06, P=0.02). No substantial (>10%) differences were observed between estimates of vaccine effectiveness regardless of whether or not they were adjusted for prognostic factors (vaccine type, age, admission status (owned or stray), breed group (pure/single breed or mixed breed), gender, or days spent at TCHS).
3.3. Vaccine-associated adverse events
Nasal discharge was the most common upper-respiratory sign observed during the first 3 days post-vaccination (Table 3). This remained the most frequently reported sign over the 30-day follow-up period (Table 2). As with coughing, there were also no differences between the groups with respect to the incidence within the first 3 days or over the 30-day follow-up period of nasal or ocular discharge, or of sneezing.
4. Discussion
The length of time a dog spent at TCHS was the only significant prognostic factor for coughing during this trial, with a 3% increased risk of coughing for each day spent at the shelter. This can be explained by continuous exposure of sheltered dogs to respiratory pathogens, by other dogs incubating pathogens, or by convalescent carrier dogs admitted to the shelter. The stress of being in a shelter might cause recrudescence of coughing among convalescent carriers. This finding is similar to that of an earlier intranasal vaccine study to prevent upper-respiratory disease in cats at a shelter (Edinboro et al., 1999). In that study, each additional day at the shelter increased a cat’s risk of respiratory disease by 5%.
In a large closed dog-breeding kennel (Glickman and Appel, 1981), an IN vaccine containing canine-adenovirus plus canine-parainfluenza virus and B. bronchiseptica was more effective than a placebo (efficacy: 71.2% in winter, 81.8% in summer) or an IN vaccine containing “only” canine-parainfluenza virus and B. bronchiseptica (efficacy: 45.3% in winter, 72.7% in summer) in reducing kennel cough. The lack of significant difference in effectiveness of the two IN vaccines in the present study might be from the small sample size. For example, for the difference seen here in the incidence of coughing between the vaccine groups and the placebo group to be significant at P<0.05 with 80% power, each vaccine group would have required at least 1500 dogs. Our original power calculation suggested that, given 500 dogs per group at α=0.05, this study would have had a power of approximately 85% to detect a 20% difference in the incidence of coughing between dogs in the IN-P group and each of the active vaccine groups or between these two vaccine groups. After the first month, however, the incidence of coughing was never above 20% in any group in this study.
Despite reported claims of adverse events associated with intranasal vaccination (Greene, 1998), we saw no differences between the vaccine groups and the placebo group in incidence of adverse events (defined as coughing, sneezing, or nasal or ocular discharge) in the first 3 days post-vaccination. The cumulative incidence of coughing was higher in the period >3 days post-vaccination for each group than it was in the first 3 days post-vaccination. Coughing that developed after the period of time when adverse events might be expected (>3 days) was probably not due to the vaccine administration. This suggests that adverse effects associated with the use of intranasal vaccines are minimal and do not contraindicate their use in shelters.
One advantage to use of IN vaccines in shelters is that protective immunity develops more rapidly than with parenteral vaccines and this might be sufficient to protect some newly admitted dogs. IN vaccines might also be more effective in protecting unvaccinated dogs by producing “herd immunity” in the shelter population, because vaccine antigens can be spread from dog to dog. Because the population in a shelter is dynamic, more effective vaccines are required to achieve herd immunity. Local humoral immunity in the upper-respiratory tract appears to be important in preventing infection with B. bronchiseptica (Bemis et al., 1977b) and canine-parainfluenza (Kontor et al., 1981). Thus, IN vaccines prevent infection and decrease signs of disease compared with parenteral vaccines that do not act locally to prevent pathogen entry.
Our findings suggest that even with effective IN vaccines, it is not possible to prevent some dogs from developing kennel cough in the shelter environment. IN vaccines containing appropriate antigens will be less effective if administered to incoming dogs that already are incubating viruses or bacteria that cause kennel cough. Ideally, B. bronchiseptica vaccine should be administered 5–7 days prior to exposure (Bemis, 1992, Ford, 1995). Also, agents that cause kennel cough but not present in commercially available IN vaccines might also be present in a shelter; these organisms include canine-herpesvirus and canine-distemper virus (Appel and Binn, 1987).
Seven of the 15 (47%) cats at a small shelter and 22 of the 47 (47%) cats in a municipal shelter were reported to be culture-positive for B. bronchiseptica during periods of feline upper-respiratory infection and canine kennel cough (Foley et al., 2002). At times when no kennel cough was reported, 3 of the 30 (10%) cats at the small shelter and 0 of the 48 cats at the municipal shelter had positive cultures for B. bronchiseptica (Foley et al., 2002). Thus, kennel cough prevention in dogs should also consider the potential for cross-species spread of pathogens and the possibility that cats can serve as reservoirs of B. bronchiseptica for dogs.
This study had several limitations. The dogs were not randomly assigned to the three vaccine groups and the shelter staff was not formally blinded to the vaccines given. However, there were no significant differences in host or environmental characteristics at baseline among dogs in the three vaccine groups (Table 1). Although viral and bacterial cultures were performed at the start and near the conclusion of the trial, the pattern of pathogens in the shelter was not monitored throughout the trial. Some upper-respiratory signs of disease might, therefore, have been caused by pathogens for which antigens were not present in the vaccine. The finding of B. bronchiseptica before the study started confirmed that this organism was present, and at least in part responsible for the rate of coughing reported to us. The finding of canine-adenovirus months later suggests that at least in this shelter at this time, this pathogen might have contributed to clinical disease and argues in favor of trivalent vaccine use. Staff members were not blinded to the vaccines given the dogs. Because this study was not funded and used commercially packaged vaccines (each of which had a distinct appearance and delivery system), the simplest procedure was rotating-day assignment. Owners could discover the name of the manufacturer of the vaccines given to their dogs within the adoption paperwork. It is possible that the lack of blinding contributed to the small differences seen in this study. For example, if there had been an expectation that the experimental vaccine would be effective, then the increased coughing incidence in the IN-P group might actually have been under-reported—leading to decreased effectiveness for the active vaccines. On the other hand, potential misclassification of coughing might have resulted in either under- or over-reporting of coughing in the other vaccine groups, again making it difficult to discern true incidence (Rothman and Greenland, 1998). Ideally, vaccines would have been packaged identically with no distinguishing characteristics and with a coding scheme for their identification. In this small municipal shelter, with a limited number of staff members and without funding, the practical solution was the field trial we conducted.
5. Conclusion
Under conditions of continuous exposure to unvaccinated dogs and a low cumulative incidence of coughing, our study showed no difference in the incidence of coughing among dogs receiving intranasal vaccines containing at least canine-parainfluenza and B. bronchiseptica. The significant prognostic factor for development of coughing was the length of time dogs remained in the shelter. No differences in adverse events associated with intranasal administration of vaccines were observed.
Acknowledgements
We thank Andrea Ragard, LVT, AAS, BS, for contacting owners and Ms. Max Champion (former Executive Director) and her staff (including Ms. Mary Sandberg and Ms. Stephanie Schmidt) at the Tippecanoe County Humane Society for their assistance. A Kenneth Scott Fellowship in Epidemiology and Animal Welfare provided financial support for Dr. Edinboro. Fort Dodge Laboratories and Schering-Plough Animal Health Corporation donated the commercially-packaged vaccines used in this study.
References
- Appel M.J. Canine infectious tracheobronchitis (kennel cough): a status report. Comp. Cont. Ed. 1981;3:70–79. [PubMed] [Google Scholar]
- Appel, M., Binn, L.N., 1987. Canine infectious tracheobronchitis. Short review: kennel cough. In: Appel, M.J. (Ed.), Virus Infections of Carnivores. Elsevier, Amsterdam, pp. 201–211.
- Appel M.J., Percy D.H. SV-5-like parainfluenza virus in dogs. J. Am. Vet. Med. Assoc. 1970;156:1778–1781. [PubMed] [Google Scholar]
- Azetaka M., Konishi S. Kennel cough complex: confirmation and analysis of the outbreak in Japan. Jpn. J. Vet. Sci. 1988;50:851–858. doi: 10.1292/jvms1939.50.851. [DOI] [PubMed] [Google Scholar]
- Bemis D.A. Bordetella and mycoplasma respiratory infections in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 1992;22:1173–1186. doi: 10.1016/s0195-5616(92)50308-4. [DOI] [PubMed] [Google Scholar]
- Bemis D.A., Carmichael L.E., Appel M.J. Naturally occurring respiratory disease in a kennel caused by Bordetella bronchiseptica. Cornell Vet. 1977;67:282–293. [PubMed] [Google Scholar]
- Bemis D.A., Greisen H.A., Appel M.J. Pathogenesis of canine bordetellosis. J. Infect. Dis. 1977;135:753–762. doi: 10.1093/infdis/135.5.753. [DOI] [PubMed] [Google Scholar]
- Bey R.F., Shade F.J., Goodnow R.A., Johnson R.C. Intranasal vaccination of dogs with live avirulent Bordetella bronchiseptica: correlation with serum agglutination titer and the formation of secretory IgA with protection against experimentally induced infectious tracheobronchitis. Am. J. Vet. Res. 1981;42:1130–1132. [PubMed] [Google Scholar]
- Dhein, C.R., Gorham, J.R., 1986. Canine respiratory infections. In: Scott, F.W. (Ed.), Infectious Diseases. Churchill Livingstone, New York, pp. 177–205.
- Edinboro, C.H., Janowitz, L.K., Guptill-Yoran, L., Glickman, L.T., 1999. A clinical trial of intranasal and subcutaneous vaccines to prevent upper respiratory infection in cats at an animal shelter. Feline Pract. 27, 7–11, 13.
- Foley J.E., Rand C., Bannasch M.J., Norris C.R., Milan J. Molecular epidemiology of feline bordetellosis in two animal shelters in California, USA. Prev. Vet. Med. 2002;54:141–156. doi: 10.1016/s0167-5877(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Ford, R.B., 1995. Infectious tracheobronchitis. In: Bonagura, J.D. (Ed.), Kirk’s Current Veterinary Therapy, vol. XII. Saunders, Philadelphia, pp. 905–908.
- Ford, R.B., Vaden, S.L., 1990. Canine infectious tracheobronchitis. In: Greene, C.E. (Ed.), Infectious Diseases of the Dog and Cat. Saunders, Philadelphia, pp. 259–265.
- Ford, R.B., Vaden, S.L., 1998. Canine infectious tracheobronchitis. In: Greene, C.E. (Ed.), Infectious Diseases of the Dog and Cat. Saunders, Philadelphia, pp. 33–38.
- Glickman L., Appel M. Intranasal vaccine trial for canine infectious tracheobronchitis (kennel cough) Lab. Anim. Sci. 1981;31:397–399. [PubMed] [Google Scholar]
- Greene, C.E., 1998. Immunoprophylaxis and immunotherapy. In: Greene, C.E. (Ed.), Infectious Diseases of the Dog and Cat. Saunders, Philadelphia, pp. 717–750.
- Kontor E.J., Wegrzyn R.J., Goodnow R.A. Canine infectious tracheobronchitis: effects of an intranasal live canine parainfluenza-Bordetella bronchiseptica vaccine on viral shedding and clinical tracheobronchitis (kennel cough) Am. J. Vet. Res. 1981;42:1694–1698. [PubMed] [Google Scholar]
- Last, J.M. (Ed.), 2001. A Dictionary of Epidemiology. Oxford University Press, New York.
- Rothman, K.J., Greenland, S., 1998. Precision and validity in epidemiologic studies. In: Rothman, K.J., Greenland, S. (Eds.), Modern Epidemiology. Lippincott-Raven, Philadelphia, pp. 115–134.
- Shade F.J., Goodnow R.A. Intranasal immunization of dogs against Bordetella bronchiseptica-induced tracheobronchitis (kennel cough) with modified live-Bordetella bronchiseptica vaccine. Am. J. Vet. Res. 1979;40:1241–1243. [PubMed] [Google Scholar]
- Wagener J.S., Sobonya R., Minnich L., Taussig L.M. Role of canine parainfluenza virus and Bordetella bronchiseptica in kennel cough. Am. J. Vet. Res. 1984;45:1862–1866. [PubMed] [Google Scholar]


