Abstract
This study aimed to find novel information concerning pathogen detection and some probable coinfection factors in hand, foot, and mouth disease (HFMD). In this study, 1104 clinically diagnosed HFMD patients were included. Enterovirus 71 (EV71), coxsackievirus A16 (CA16), and 14 different respiratory pathogens were examined from nasopharyngeal swabs using polymerase chain reaction (PCR) or reverse transcriptase PCR (RT-PCR). To evaluate the immune activation in HFMD patients, 8 cytokines and IgM antibodies to EV71 and CA16 from mild and severe patients were detected. Our results indicated that the severity of HFMD may affect the pathogen detection. The lower positive rates of enterovirus and respiratory viruses in severe HFMD cases by RT-PCR were probably related to stronger immune response. Therefore, immunological tests such as ELISA are essential supplements to PCR or RT-PCR in order to increase pathogen diagnosis in HFMD, especially in severe cases.
Keywords: Hand, foot, and mouth disease; Enterovirus; Immune activation; Respiratory pathogen; Cytokine detection
1. Introduction
Hand, foot, and mouth disease (HFMD) is generally a febrile exanthematous disease mostly prevalent in children younger than 10 years of age. Manifestations are vesicles on the palmar and plantar surfaces of the hands and feet, and on the buccal mucosa, tongue, and buttocks. Although other types of enterovirus (EV), such as coxsackie virus A4–A7, B2–B5, and EV18 are related to HFMD, human EV71 and coxsackievirus A16 (CA16) are the main pathogens (Bruu, 2003). Severe cases with neurological and cardiopulmonary complications, such as aseptic meningitis, encephalitis, and poliomyelitis-like paralysis, are mainly caused by EV71 (Chong et al., 2003, McMinn et al., 2001, Wang et al., 1999, Weng et al., 2010). In addition, EV71 is associated with higher mortality rates.
The early diagnosis of HFMD relies on typical clinical manifestations, together with RT-PCR assays from nasopharyngeal swab, vesicular fluid, or feces, with a detection rate of various EV that ranges from 48.0% to 88.4% (Chen et al., 2009, Jiang et al., 2012, Singh et al., 2002, Yang et al., 2011). It has been reported that different types of sample, early or multiple sampling may affect the positive rate of RT-PCR (Singh et al., 2002). Immune detection of IgM antibody against EV71 or CA16 using serum samples, which is meaningful for early diagnosis, has been established (Hong et al., 2012, Xu et al., 2010). However, the performance of the methods had not been fully evaluated. Recent studies have indicated that some antiviral proinflammatory and inflammatory cytokines were increasing after EV infection, especially in severe HFMD cases, and have been associated with certain adverse outcomes (Chung et al., 2008, Khong et al., 2011, Lin et al., 2002). The strong or abnormal activation of the immune system in severe HFMD cases may affect the method choice concerning pathogen detection using either molecular methods or immune detection focus on IgM antibody.
On the other hand, it has been reported that various viruses, such as human adenovirus (HAdV), norovirus, and rotavirus, were related to fatal HFMD cases (Cardosa et al., 1999, Liu et al., 2012). A broader insight into other common community-acquired pathogens (CAP) such as respiratory viruses (RV), Chlamydophila pneumoniae (CP), and Mycoplasma pneumoniae (MP) coinfection is necessary for understanding the pathogenesis and better management of HFMD. Laboratory detection of respiratory pathogens using molecular diagnostic tests such as RT-PCR have been frequently used in febrile children with respiratory symptoms, whereas the method and detection rate still need additional evaluation, especially in the situation when there is a coinfection with EV.
In this study, EV71, CA16, and 14 respiratory pathogens were examined using nasopharyngeal swabs from individuals with HFMD using commercially available PCR or RT-PCR kits. In addition, eight proinflammatory and inflammatory cytokines and the IgM antibodies released in both EV71 and CA16 infection were detected using serum samples from selected mild and severe HFMD cases. Our study yielded novel information concerning the pathogen detection in HFMD cases.
2. Materials and methods
2.1. Clinical specimen and data collection
After obtaining approval from the Ethics Committee of Beijing Youan Hospital, Beijing, China and consent from the study participants, a total of 1,104 clinically diagnosed HFMD patients were included in the study. Participants attended Beijing Youan Hospital between June and October in 2010 comprised 233 severe cases and 871 mild cases, with the mean age at 2.26, and 60.4% being male. Of the 233 severe cases, the mean age was 1.91, and 67.8% were male. Of the 871 mild cases, the mean age was 2.24, and 58.4% were male. All the patients were given a clinical diagnosis of HFMD by senior physicians with special training, and in accordance with the HFMD clinical diagnosis guidelines published in 2009 by the Chinese Ministry of Health. According to the guidelines, the mild cases were characterized by fever or not, with herpetic stomatitis and a rash on the hands and feet. The cases with any neurological signs and cardiopulmonary complications, such as aseptic meningitis, encephalitis, and poliomyelitis-like paralysis, were defined as severe cases.
Nasopharyngeal swabs from all the 1104 patients were collected within 5 days of the onset of illness (each patient sampled only once). The specimens were immediately placed in virus transport media tubes (YOCON, Beijing, China), which were temporarily stored at 4 °C for analysis or at −80 °C within 24 hours until use. Nasopharyngeal swabs from 348 cases, with 115 randomly selected mild cases as the mild group and 233 severe cases as the severe group, were chosen for CAP detection. Simultaneously, 103 serum samples were collected from the 348 patients for the immunological assay, with 33 samples from mild cases and 70 from severe cases. Serum samples were collected at the same time as nasopharyngeal swabs and stored at −80 °C until use.
2.2. Total RNA extraction and reverse transcription
RNA was extracted using QIAamp Viral RNA Mini Kits (Qiagen, Hilden, Germany), according to the protocol provided. Reverse transcription was carried out using Revert Aid First Strand cDNA Synthesis Kits (Fermentas, Shenzhen, China) following the procedure outlined by the manufacturer.
2.3. EV71, CA16 and human enterovirus universal (EVU) RT-PCR assays
EV71 and CA16 were analyzed using one-step RT-PCR Detection Kits (Da An Gene Co. Ltd., Guangzhou, China) in 1104 HFMD cases. Overall, 137 hospitalized cases negative for EV71 and CA16 were investigated for other EV infections using Human EVU Fluorescence RT-PCR Diagnostic Kits (Da An Gene Co. Ltd., Guangzhou, China). RT-PCR amplification was carried out using the M×3000P PCR instrument (Stratagene, La Jolla, CA, USA), according to the manufacturer’s instructions.
2.4. Multiplex RT-PCR detection for RV
In 348 swabs chosen for CAP detection, multiplex RT-PCR was performed using Seeplex RV12 ACE Detection Kits (Seegene, Hangzhou, China) for RV detection, following the manufacturer’s instructions. cDNA samples were used for the virus testing, and each cDNA sample was tested against 2 sets of primers: one set was tested for human HAdV; human metapneumovirus; human coronavirus (HCoV) 229E/NL63; and human parainfluenza virus (HPIV) types 1, 2, and 3, and another set was tested for the influenza type B virus, HCoV-OC43/HKU1, human rhinovirus (HRV A/B), human respiratory syncytial virus (HRSV) B/A, and influenza type A virus (FLU A). The RT-PCR products were analyzed using agarose gel (2%) electrophoresis and stained with ethidium bromide. Each RT-PCR product from a specific virus was designed to migrate at a unique position in the gel, which corresponded to 6 molecular weight markers, each enabling the identification of a specific virus.
2.5. Detection of CP and MP
In the 348 swabs chosen for CAP detection, the presence of CP and MP DNA was examined using Diagnostic Kits (Da An Gene Co. Ltd., Guangzhou, China) according to the manufacturer’s instructions.
2.6. Cytokine detection
Eight different cytokines — interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), interferon-γ (INF-γ), granulocyte-macrophage colony stimulating factor (GM-CSF), and tumor necrosis factor (TNF) — were detected from the 103 serum samples by the liquid chip method using Bio-Plex Human 8-plex cytokine examination kits (Bio-Rad, Hercules, CA, USA), following the instructions provided.
2.7. Detection of EV71-IgM and CA16-IgM using ELISA
EV71-IgM and CA16-IgM were detected in 103 serum using EV-71 IgM ELISA kits and CA16 IgM ELISA kits (Wantai, Beijing, China), following the instructions provided.
2.8. Statistical analysis
Data were entered into the database and analyzed using SPSS software version 16.0 (SPSS, Chicago, IL, USA). The chi-square test was used for qualitative variables, the t test was used for quantitative variables subordinate to the normal distribution and homogeneity of variance, and the Mann–Whitney–Wilcoxon test was used for quantitative variables that were not normally distributed or showed homogeneity of variance, the expression of most cytokines were compared by this method. P < 0.05 was considered to be statistically significant.
3. Results
3.1. Determination of human EV infection by RT-PCR
Of the 1104 HFMD cases, 611 (55.3%) were tested positive for EV71/CA16. Of these, 512 cases (46.4%) were EV71 positive, and 101 cases (9.1%) were CA16 positive (two were positive for both). Specifically, of the 871 mild cases, 510 (58.6%) were EV71/CA16-positive, of which 413 (81.0%) were EV71-positive and 99 (19.4%) were CA16 positive (two were co-infected). Of the 233 severe cases, 101 (43.3%) were identified as EV71/CA16 positive, of which 99 (98.0%) were EV71 positive and only 2 (2.0%) were CA16 positive. The EV71/CA16-positive rate in mild cases was much higher than in severe cases (P = 0.000). The EV71 infection in severe cases was much higher than in mild cases (P = 0.001) (Fig. 1 ).
Fig. 1.
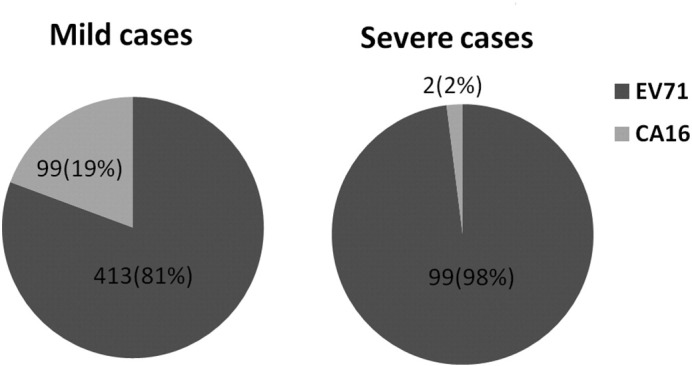
The proportion of EV71 and CA16 in severe and mild HFMD cases. EV71 indicates enterovirus 71; CA16, coxsackievirus A16. Among the severe cases positive for EV71/CA16, 99 (98.0%) cases were positive for EV71, and only 2 were positive for CA16. On the other hand, in the mild cases positive for EV71/CA16, 413 (81%) cases were positives for EV71, and 99 (19%) were positive for CA16. The result indicates that EV71 is the major pathogen in HFMD patients, especially in severe cases.
Of the 611 cases positive for EV71/CA16, 243 (39.8%) were infants aged ≤1 year, 160 (26.2%) were 2-year-olds, 80 (13.1%) were 3-year-olds, 64 (10.5%) were 4-year-olds, 31 (5.1%) were aged 5 years, and only 33 (5.4%) were older than 5 years (Fig. 2 ). Children younger than 5 years comprised the majority of study participants with a positive EV71/CA16 test result (94.9%).
Fig. 2.
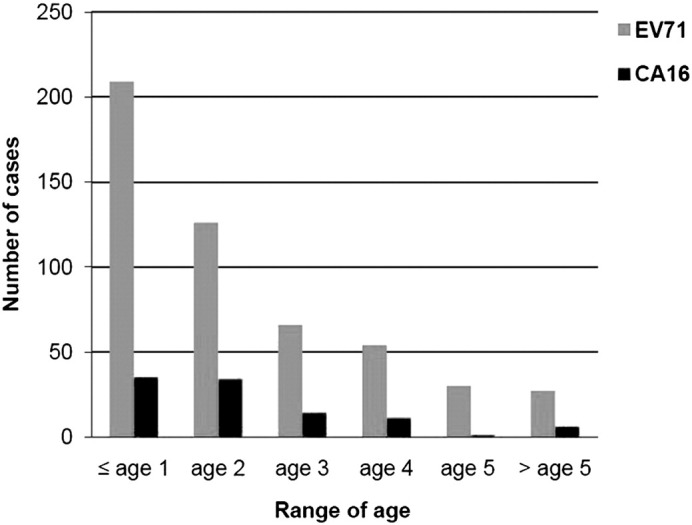
The age profile of 611 cases positive for EV71/CA16. The majority (94.9%) of the patients were children younger than 5 years.
In terms of the seasonal distribution of the 611 cases positive for EV71/CA16, 121 (19.8%) were infected in June, 309 (50.6%) in July, 107 (17.5%) in August, 53 (8.7%) in September, and 21 (3.4%) in October (Fig. 3 ). The peak incidence appeared to occur from June to August. Additionally, 14 (6.0%) cases were detected positive for EV other than EV71/CA16 in 233 severe HFMD cases.
Fig. 3.
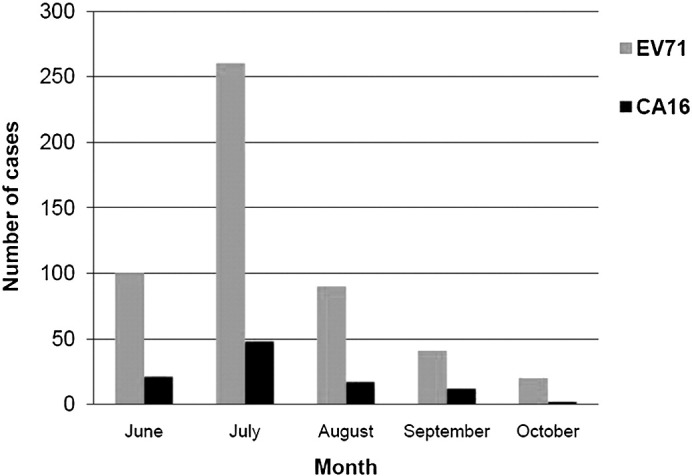
Monthly distribution of the cases positive for EV71/CA16. The majority of the patients positive for EV71/CA16 were during the period from June to August. The peak incidence occurred in July. Both EV71 and CA16 positive patients were in accord with this trend generally.
3.2. Respiratory pathogen detection
Among the 348 HFMD cases, 57 were positive for various respiratory pathogens, with 29 (12.4%) detected in the severe group, and 28 (24.3%) in the mild group. Detailed information is provided in Table 1 . The positive rate in the mild group was much higher than in the severe group (χ2 = 7.963, P = 0.005).
Table 1.
Common respiratory etiologies identified from children with HFMD.
| Group | RV (%) |
MP (%) | CP (%) | Total (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HRV A/B | FLU A | HPIV3 | HAdV | HCoV-229E/NL63 | HPIV 1 | HCoV-OC43/HKU | HRSV A | ||||
| Severe-group (n = 233) | 6 (2.6) | 4 (1.7) | 3(1.3) | 2 (0.9) | 3 (1.3) | 0 (0) | 0 (0) | 1 (0.4) | 13 (5.6) | 0 (0) | 29 (12.4) |
| Mild-group (n = 115) | 12 (10.4) | 2 (1.7) | 3 (2.6) | 2 (1.7) | 1 (0.8) | 1 (0.8) | 1 (0.8) | 0 (0) | 8 (7.0) | 0 (0) | 28 (24.3) |
| Total (n = 348) | 18 (5.2) | 6 (1.7) | 6 (1.7) | 4 (1.0) | 4 (1.1) | 1 (0.3) | 1 (0.3) | 1 (0.3) | 21 (6.0) | 0 (0) | 57 (16.4) |
The table shows the distribution of the positive respiratory pathogens in 348 HFMD patients. In total, eight types of RV and MP were detected positive. And totally 57 positive cases were found, and 4 cases were coinfected with multiple pathogens. The positive rate of CAP in the mild group is much higher than in the severe group.
Specifically, 36 (10.3%) participants were positive for RV, including 18 with HRV A/B, 6 with FLU A, 6 with HPIV3, 4 with HAdV, 4 with HCoV-229E/NL63, 1 with HPIV1, 1 with HCoV-OC43/HKU, and 1 with HRSVA. Four individuals were co-infected with multiple viruses. Sixteen (6.9%) out of 233 severe cases were positive for RV, as compared to 20 (17.4%) cases in the 115 mild group; the RV-positive rate in the mild group was much higher than in the severe group (χ2 = 9.195, P = 0.002). There was no significant difference in RV detection among age groups. However, the rate of RV-positive cases in September and October was much higher than in other months (χ2 = 5.702, P = 0.017).
In terms of CP and MP detection, 21 of the 348 cases were positive for MP, with 13 (5.6%) in severe group and 8 (7.0%) in mild group. No case was tested positive for CP in either group. MP-positive cases were mainly found in patients ≤1 year of age, with 10.0% being MP-positive, and the percentage was significantly higher than other age groups (χ2 = 9.557, P = 0.002). Although it was not statistically significant, a small peak of MP infection was observed in August. The age profile and seasonal distribution of the respiratory pathogens are shown in Fig. 4, Fig. 5 .
Fig. 4.
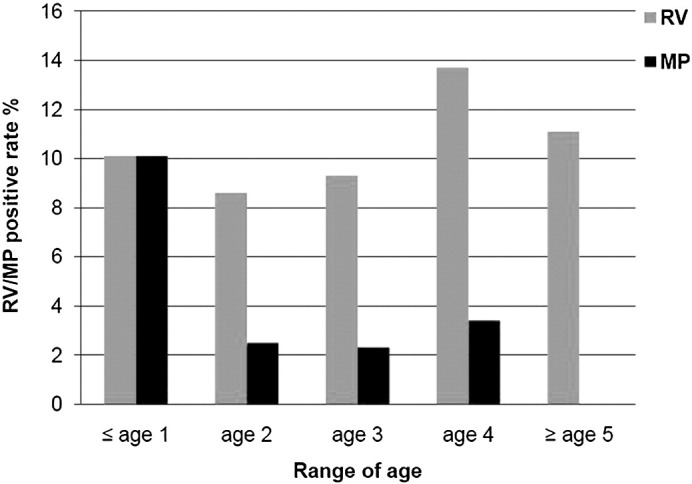
Age distribution of RV/MP in HFMD patients. Figure 4 shows the RV and MP positive rate in the HFMD patients among various age groups, no apparent difference of the RV detection rate among the 5 groups was observed. However, the MP positive rate (10%) in the patients ≤1 year of age was evidently higher than any other groups (χ2 = 9.557, P = 0.002). MP detection rarely occurred in the group ≥5 year of age.
Fig. 5.
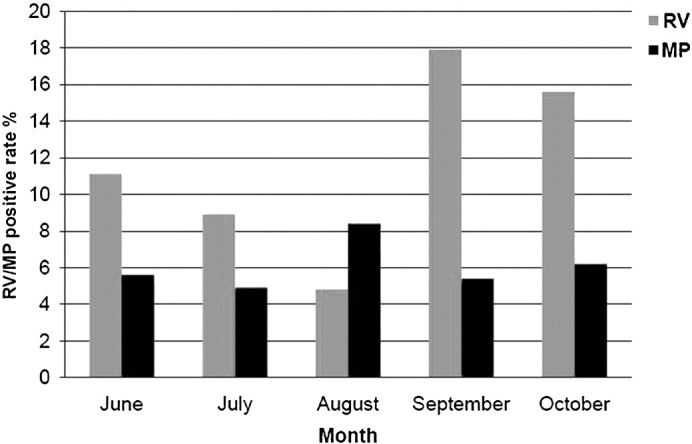
Monthly distribution of RV/MP infection in HFMD patients. RV indicates respiratory virus. Although no statistical difference of the MP positive incidence in different months was found, a small peak of MP infection was observed in August. RV infection was more prevalent in September (18%) and October (15%) than other months among the HFMD patients.
3.3. No interference between respiratory pathogens infection and the identification of EV
The 233 severe cases were subdivided into an EV-positive group of 118 cases and an EV-negative group of 115. In the EV-positive group, 16 cases were identified to have a respiratory pathogen infection, compared to 13 cases in the EV-negative group. There was no significant difference in the detection of respiratory pathogens between the two groups (χ2 = 0.272, P = 0.602). In more detail, eight RV-positive cases were found in the EV-positive group and 8 were in the EV-negative group. Correspondingly, 8 MP-positive cases were identified in the EV-positive group, compared to 5 in the EV-negative group. There was no significant difference in both RV (χ2 = 0.003, P = 0.957) and MP detection (χ2 = 0.654, P = 0.419) between the two groups (Table 2 ).
Table 2.
Distribution of the CAP positive cases in EV-positive and EV-negative groups.
| Group | RV | MP | CP | Total |
|---|---|---|---|---|
| EV-positive group (n = 118) | 8 | 8 | 0 | 16 |
| EV-negative group (n = 115) | 8 | 5 | 0 | 13 |
| P value | 0.957 | 0.419 | 0.602 |
CAP indicates community-acquired pathogens; P values indicate the difference in detection rate between mild and severe groups. No statistical difference of the CAP detection was found between EV-negative group and EV-positive group.
In the 233 severe cases, there were 204 CAP-negative cases and 29 CAP-positive. Of these, 87 of the 204 CAP-negative cases (42.6%) were positive for EV71/CA16 and 14 of the 29 CAP-positive cases (48.3%) were positive for EV71/CA16. There are no significant differences in the EV detection rate between the two groups statistically (χ2 = 0.328, P = 0.567).
3.4. Cytokine release in mild and severe HFMD cases
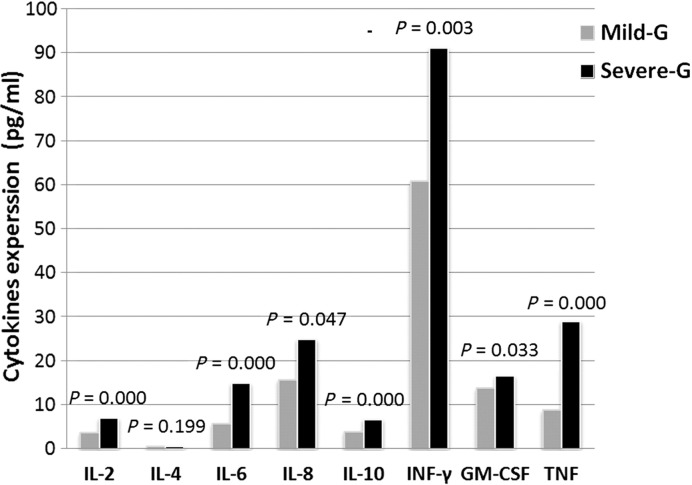
Eight different cytokines were detected in all the serum samples. These cytokines to some extent represent immune activation in HFMD. P values between the mild and severe groups were 0.000 (IL-2), 0.199 (IL-4), 0.000 (IL-6), 0.047 (IL-8), 0.000 (IL-10), 0.003 (INF-γ), 0.033 (GM-CSF), and 0.000 (TNF) (Fig. 6 ). Except for IL-4, the presence of cytokines was shown to be significantly higher in the severe group than in the mild group.
Fig. 6.
Inflammatory cytokines expression in mild and severe HFMD cases. Eight different cytokines were detected, and 7 cytokines were much higher in severe group than in mild group. As those cytokines to some extent represent the strength of immune response in HFMD, this result suggested the immune response in severe cases was enhanced.
3.5. EV71/CA16-IgM detection using ELISA method
There were 84 (81.6%) cases detected positive for EV71/CA16-IgM by ELISA in all serum samples. The results in different HFMD cases were discordant from the RT-PCR method. Of the 70 severe cases, 62 (88.6%) were positive for EV71/CA6-IgM, and 22 (66.7%) of 33 mild cases were positive for EV71/CA6-IgM; the positive rate of EV71/CA16-IgM in severe cases was much higher than in mild cases (χ2 = 7.153, P = 0.007).
4. Discussion
The co-circulation of EV71 and CA16 in HFMD patients has frequently been described (Li et al., 2005, Zhu et al., 2010). Numerous EV71-associated HFMD outbreaks with high morbidity and mortality have occurred in a range of different countries and regions (Lee et al., 2010, Wu et al., 2010, Zhang et al., 2009). Overwhelming virus replication combined with the induction of massive inflammatory cytokines have been proposed to be responsible for EV71 pathogenesis (Khong et al., 2011). Their co-infection with other respiratory pathogens may be another factor that contributes to disease progression (Cardosa et al., 1999).
Rapid identification of the causative agents for HFMD, together with the pathogens responsible for co-infection, can help clinical management by focusing attention on the possible complications. It is also important with regards to predicting the severity of the outbreak and initiating appropriate public health interventions. The nucleotide-based RT-PCR methods have been developed and are now used in many clinical laboratories (de Crom et al., 2011, Guney et al., 2003, Watkins-Riedel et al., 2002). Effective and accurate virological diagnosis depends on the correct sampling time and use of appropriate clinical specimens, as well as their transportation to laboratory under optimal conditions. This requires close cooperation between laboratory experts and clinicians. However, there should be other factors that can affect the detection by RT-PCR.
In the present study, we detected EV71, CA16, and 14 other CAP pathogens using molecular-based methods with pharyngeal swabs and immune assays of serum samples taken from patients with HFMD. After analyzing the HFMD swabs using RT-PCR, EV71 and CA16 were still the leading causes of the disease, with the majority of positive cases being infected by EV71. In addition, there were 6.0% severe cases being positive for EV other than EV71/CA16. As a result, non-EV71 and non-CA16 EV infection should not be ignored in clinical practice.
Among the severe cases that were positive for EV71/CA16, the proportion of patients identified positive for EV71 was particularly high at 98.1%. An interesting finding in our study was that the positive rate for EV71/CA16 in mild cases was much higher than that in severe cases. An earlier report, which examined pharyngeal, stool and vesicular liquid samples from patients using the RT-PCR method, showed a EV71/CA16-positive rate of 85.4% in mild cases and 28.6% in severe cases (Wang et al., 2008). This result, together with ours, suggested that EV detection might be associated with the severity of HFMD.
Among those EV71/CA16 positive cases, more than 60% were younger than 2 years, and more than 90% were under the age of 5 years, which is consistent with earlier reports (Liu et al., 2011, Zhang et al., 2011). The peak of infection occurred between June and August, and a trend for peaks in the summer and fall can also be found in other reports (Baek et al., 2011). Age profile and seasonal distribution could be factors that affect the infection of EV71 and CA16.
Of the 348 HFMD cases, 57 (16.4%) were tested positive for respiratory pathogens. The RV identification rate was much higher in the mild group than in the severe group, which was similar to our findings with EV71/CA16. The result confirmed that CAP infection or CAP carrier did exist in some HFMD cases. However, it did not suggest that CAP enhanced the severity of HFMD as generally expected, whereas the severity of HFMD influenced the detection of CAP, especially RV. A study had suggested HAdv infection was a reason for the death of severe HFMD patients (Cardosa et al., 1999). However, there was no statistical difference in HAdv detection between the severe and mild groups in this study (P = 0.602). The only case that died from encephalitis in the severe group was detected with HRV A/B. RV infection was more prevalent in September and October than other months, and such trend can be found in other studies (Kim et al., 1996). Therefore, even though the incidence of HFMD drops to a low level during autumn and winter, co-infection of patients with HFMD with RV should not be ignored.
As for the incidence of MP and CP, no significant difference was found between the mild and severe groups, which suggests that the severity of HFMD does not affect MP and CP detection. However, MP detection was much more common (10.0%) in patients aged less than 1 year compared with other age groups. The incidence of both MP and CP was lower than in some other reports (Defilippi et al., 2008, Kese et al., 2002, Mc et al., 2009); this may be due to the fact that we did not focus on children with acute respiratory infection (Defilippi et al., 2008) or on patients in winter and spring when there are more likely to be epidemics of CP or MP (Kese et al., 2002, Mc et al., 2007, Mc et al., 2009). Furthermore, it may be that the pharyngeal swabs special for virus preservation decreased the MP/CP detection rate compared with sputum samples used in other studies, and the single sampling may also decreased detection rate. Some other studies reported that children younger than 3 years, which are the majority in our study, are rarely infected by MP or CP (Almasri et al., 2011, Kamata et al., 2011).
With regards to CAP detection, no statistical difference was found between the EV-positive group and EV-negative group. This result indicates that the presence of EV71/CA16 does not affect the detection of CAP. Correspondingly, in terms of EV71/CA16 detection, no apparent difference was found between the CAP-positive group and the CAP-negative group, suggesting that CAP also does not influence EV71/CA16 detection.
Identification rates of both RV and EV in the mild group were much higher than in the severe group by RT-PCR. Therefore, a hypothesis was proposed that this difference was a result of stronger activation of acute immune reaction in the severe patients. Earlier studies have indicated that increased proinflammatory and inflammatory cytokines such as TNF-α and IL-6 secretion was detected after EV or respiratory pathogen infection (Cello et al., 1996, Chung et al., 2008, Härkönen et al., 2003, Khong et al., 2011, Lin et al., 2002). Studies have suggested that the enhanced expression of IL-1, IL-6, IL-10, IL-13, IFN-γ and TNF is associated with life-threatening complications in severe HFMD cases (Lin et al., 2002, Lin et al., 2003, Wang et al., 2003). Some cytokines, including IL-6, IL-10, IFN-γ, transforming growth factor, and GM-CSF, have been reported to have specific or non-specific antiviral effects (Ank et al., 2006, Brooks et al., 2006, Hong et al., 2012, Huang et al., 2011). A recent study indicated that EV71 infection increased the expression of IFN-γ-inducible protein 10 (IP-10). High level of IP-10 was found in the plasma of severe HFMD patients with encephalitis, with functions to enhance viral clearance, increase IFN-γ expression and boost the infiltration of CD8 T cells (Shen et al., 2013). Therefore, such a strong immunoreaction not only plays an important role in the pathogenesis of severe HFMD but also might affect the choice of the detection method. In this study, seven cytokines were much higher in severe cases than in mild cases. This finding supports our hypothesis and also indicates that detecting the levels of these cytokines may be utilized as an auxiliary diagnostic method to evaluate disease progression.
The detection rate of serum IgM to EV71/CA16 in severe cases was much higher than in mild cases, which could be an evidence of enhanced immune response in severe HFMD cases. In this situation, stronger immune activation in severe HFMD is coupled with the generation of specific antibodies and elimination of specific pathogens. Therefore, detection rate of EV an RV by RT-PCR was decreased. There was no difference in MP and CP detection between the severe group and mild group, suggesting that the elimination mechanism of CP/MP infection should differ from that of RV and EV. That is, the immune response which takes place in EV/RV infection is ineffective with CP/MP.
Whereas virus isolation is still the “golden standard” for the HFMD diagnosis, it detects active replicative viruses relative to RT-PCR. Some relevant studies indicated the EV71 detection rate by virus culture is about 20% or even lower in the RT-PCR positive cases (Singh et al., 2002). Practical RT-PCR methods have been used in worldwide (de Crom et al., 2011, Song et al., 2011, Watkins-Riedel et al., 2002). However, enhanced immunoreaction in severe HFMD may eliminate EV and RV quickly from the nasopharynx, thus decreasing the detection rate by RT-PCR. Alternatively, studies indicated that the positive rates of IgM antibody for CA16 and EV71 could get more than 90% at day 7 after onset (Xu et al., 2010, Xu et al., 2011). The rapid generation of such antibodies was confirmed again in present study and, on the other hand, clarified the characteristics of immune response to EV. In view of this, RT-PCR detection combined with serum immunoassays, such as ELISA and cytokine profile detection, could be used for the early diagnosis of EV infection, especially in cases of severe HFMD.
In conclusion, our results illustrate that the severity of HFMD influences pathogen detection in HFMD. Furthermore, lower positive rates of EV and RV in clinically severe cases are possibly due to a stronger immune response. Therefore, immunological tests such as ELISA and cytokine detection could be essential supplements to RT-PCR in order to increase pathogen diagnosis in HFMD patients, especially in severe clinical cases.
Acknowledgments
This study was supported by grants from the Ministry of Science and Technology of China ("11th five-year" project) (2009ZX10004-110), the Capital Medical Development Scientific Research Fund (2009–1057), National Natural Science Foundation of China (30973388) and the High-level Health Professional Disciplines of Beijing Health System, China (2011–2014). The founders had no role in study design, data collection and analysis, interpretation of data, decision to publish, or preparation of the manuscript.
Footnotes
The current “Instructions to Authors” have been read, and we declare to comply with the instructions and stated conditions. All authors and acknowledged parties agreed to the submitted version, and agreed to our inclusion. The material is original and it has been neither published elsewhere nor submitted for publication simultaneously. If accepted, the paper will not be published elsewhere.
References
- Almasri M., Diza E., Papa A., Eboriadou M., Souliou1 E. Mycoplasma pneumoniae respiratory tract infections among Greek children. Hippokratia. 2011;15:147–152. [PMC free article] [PubMed] [Google Scholar]
- Ank N., West H., Bartholdy C., Eriksson K., Thomsen A.R., Paludan S.R. Lambda interferon (IFN-λ), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek K.A., Yeo S.G., Lee B.H., Park K.S., Song J.H., Yu J.S. Epidemics of enterovirus infection in Chungnam Korea, 2008 and 2009. Virol J. 2011;8:297. doi: 10.1186/1743-422X-8-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D.G., Trifilo M.J., Edelmann K.H., Teyton L., McGavern D.B., Oldstone M.B.A. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruu A.L. Enteroviruses: polioviruses, coxsackieviruses, echoviruses and newer enteroviruses. In: Haaheim L.R., Pattison J.R., Whitley R.J., editors. A practical guide to clinical virology, 2nd ed. John Wiley & Sons, Ltd; 2003. pp. 44–45. [Google Scholar]
- Cardosa M.J., Krishnan S., Tio P.H., Perera D., Wong S.C. Isolation of subgenus B adenovirus during a fatal outbreak of enterovirus 71-associated hand, foot, and mouth disease in Sibu, Sarawak. Lancet. 1999;354:987–991. doi: 10.1016/S0140-6736(98)11032-2. [DOI] [PubMed] [Google Scholar]
- Cello J., Strannegård Ö., Svennerholm B. A study of the cellular immune response to enteroviruses in humans: identification of cross-reactive T cell epitopes on the structural proteins of enteroviruses. J Gen Virol. 1996;77:2097–2108. doi: 10.1099/0022-1317-77-9-2097. [DOI] [PubMed] [Google Scholar]
- Chen H., Ma J.T., Ma X.M. Analysis of etiology detection result of HFMD cases in Ningxia in 2008. Ningxia Med J. 2009;33:515–516. [Google Scholar]
- Chong C.Y., Chan K.P., Shah V.A., Ng W.Y.M., Lau G., Teo T.S. Hand, foot and mouth disease in Singapore: a comparison of fatal and non-fatal cases. Acta Paediatr. 2003;92:1163–1169. [PubMed] [Google Scholar]
- Chung Y.C., Ho M.S., Wu J.C., Chen W.J., Huang J.H., Chou S.T. Immunization with virus-like particles of enterovirus 71 elicits potent immune responses and protects mice against lethal challenge. Vaccine. 2008;26:1855–1862. doi: 10.1016/j.vaccine.2008.01.058. [DOI] [PubMed] [Google Scholar]
- de Crom S.C.M., Obihara C.C., van Loon A.M., ArgilagosAlvarez A.A., Peeters M.F., van Furth A.M. Detection of enterovirus RNA in cerebrospinal fluid: comparison of two molecular assays. J Virol Methods. 2011;179:104–107. doi: 10.1016/j.jviromet.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Defilippi A., Silvestri M., Tacchella A., Giacchino R., Melioli G., Di Marco E. Epidemiology and clinical features of Mycoplasma pneumoniae infection in children. Respir Med. 2008;102:1762–1768. doi: 10.1016/j.rmed.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Guney C., Ozkaya E., Yapar M., Gumus I., Kubar A., Doganci L. Laboratory diagnosis of enteroviral infections of the central nervous system by using a nested RT-polymerase chain reaction (PCR) assay. Diagn Microbiol Infect Dis. 2003;47:557–562. doi: 10.1016/s0732-8893(03)00148-2. [DOI] [PubMed] [Google Scholar]
- Härkönen T., Paananen A., Lankinen H., Hovi T., Vaarala O., Roivainen M. Enterovirus infection may induce humoral immune response reacting with islet cell autoantigens in humans. J Med Virol. 2003;69:426–440. doi: 10.1002/jmv.10306. [DOI] [PubMed] [Google Scholar]
- Hong M.-H., Chou Y.-C., Wu Y.-C., Tsai K.-N., C-p Hu., Jeng K.-S. Transforming growth factor-β1 suppresses hepatitis B virus replication by the reduction of hepatocyte nuclear factor-4α expression. PLoS One. 2012;7:e30360. doi: 10.1371/journal.pone.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F.-F., Barnes P.F., Feng Y., Donis R., Chroneos Z.C., Idell S. GM-CSF in the lung protects against lethal influenza infection. Am J Respir Crit Care Med. 2011;184:259–268. doi: 10.1164/rccm.201012-2036OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Zhang J., You X., Dong C., Cheng X., Dai X. Diagnosis of hand, foot, and mouth disease caused by EV71 and other enteroviruses by a one-step, single tube, duplex RT-PCR. J Med Virol. 2012;84:1803–1808. doi: 10.1002/jmv.23391. [DOI] [PubMed] [Google Scholar]
- Kamata A., Obinata K., Niizuma T., Kinoshita K., Shimizu T. The validity of the criteria for primary infection of Chlamydophila pneumoniae in children by measuring ELISA IgM antibodies. J Infect Chemother. 2011;17:1–5. doi: 10.1007/s10156-011-0327-x. [DOI] [PubMed] [Google Scholar]
- Kese D., Cizman M., Marin J., AvsicZupanc T. Chlamydia pneumoniae infections in patients with community-acquired pneumonia in Slovenia. Scand J Infect Dis. 2002;34:172–176. doi: 10.1080/00365540110077399. [DOI] [PubMed] [Google Scholar]
- Khong W.X., Foo D.G.W., Trasti S.L., Tan E.L., Alonso S. Sustained high levels of interleukin-6 contribute to the pathogenesis of enterovirus 71 in a neonate mouse model. J Virol. 2011;85:3067–3076. doi: 10.1128/JVI.01779-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P.E., Musher D.M., Glezen W.P., Barradas M.C.R., Nahm W.K., Wright C.E. Association of invasive pneumococcal disease with season, atmospheric conditions, Air pollution, and the isolation of respiratory viruses. Clin Infect Dis. 1996;22:100–106. doi: 10.1093/clinids/22.1.100. [DOI] [PubMed] [Google Scholar]
- Lee M.S., Lin T.Y., Chiang P.S., Li W.C., Luo S.T., Tsao K.C. An investigation of epidemic enterovirus 71 infection in Taiwan, 2008: clinical, virologic, and serologic features. Pediatr Infect Dis J. 2010;29:1030–1034. doi: 10.1097/INF.0b013e3181e52945. 1010.1097/INF.1030b1013e3181e52945. [DOI] [PubMed] [Google Scholar]
- Li L.L., He Y.Q., Yang H., Zhu J.P., Xu X.Y., Dong J. Genetic characteristics of human enterovirus 71 and coxsackievirus A16 circulating from 1999 to 2004 in Shenzhen, People's Republic of China. J Clin Microbiol. 2005;43:3835–3839. doi: 10.1128/JCM.43.8.3835-3839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.-Y., Hsia S.-H., Huang Y.-C., Wu C.-T., Chang L.-Y. Proinflammatory cytokine reactions in enterovirus 71 infections of the central nervous system. Clin Infect Dis. 2003;36:269–274. doi: 10.1086/345905. [DOI] [PubMed] [Google Scholar]
- Lin T.Y., Chang L.Y., Huang Y.C., Hsu K.H., Chiu C.H., Yang K.D. Different proinflammatory reactions in fatal and non-fatal enterovirus 71 infections: implications for early recognition and therapy. Acta Paediatr. 2002;91:632–635. doi: 10.1080/080352502760069016. [DOI] [PubMed] [Google Scholar]
- Liu L.-J., Xu H.-M., Li X.-J., Wang J., Wang X.-J., Ding S.-J. Co-detection in the pathogenesis of severe hand-foot-mouth disease. Arch Virol. 2012;157:2219–2222. doi: 10.1007/s00705-012-1396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.Y., Liu W.Y., Luo J., Liu Y.L., Zhu Y., Berman H. Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010. PLoS One. 2011;6:e252. doi: 10.1371/journal.pone.0025287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc H., Tm L., Ct H. Chlamydia pneumoniae respiratory infections in Taiwan. Microbiol Immunol. 2007;51:539–542. doi: 10.1111/j.1348-0421.2007.tb03942.x. [DOI] [PubMed] [Google Scholar]
- Mc X., Hh M., Qq O., Aw L., Gl R., Xy W. Epidemiological and clinical analysis of Mycoplasma pneumoniae infection in children with acute respiratory tract infection. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29:2082–2083. [PubMed] [Google Scholar]
- McMinn P., Stratov I., Nagarajan L., Davis S. Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot, and mouth disease in Western Australia. Clin Infect Dis. 2001;32:236–242. doi: 10.1086/318454. [DOI] [PubMed] [Google Scholar]
- Shen F.-H., Tsai C.-C., Wang L.-C., Chang K.-C., Tung Y.-Y., Su I.-J. Enterovirus 71 infection increases expression of interferon-gamma-inducible protein 10 which protects mice by reducing viral burden in multiple tissues. J Gen Virol. 2013 doi: 10.1099/vir.0.046383-0. [DOI] [PubMed] [Google Scholar]
- Singh S., Chow V.T., Phoon M.C., Chan K.P., Poh C.L. Direct detection of enterovirus 71 (EV71) in clinical specimens from a hand, foot, and mouth disease outbreak in Singapore by reverse transcription-PCR with universal enterovirus and EV71-specific primers. J Clin Microbiol. 2002;40:2823–2827. doi: 10.1128/JCM.40.8.2823-2827.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L.Q., Sun S.P., Li B., Pan Y., Li W.L., Zhang K. External quality assessment for enterovirus 71 and coxsackievirus A16 detection by reverse transcription-PCR using armored RNA as a virus surrogate. J Clin Microbiol. 2011;49:3591–3595. doi: 10.1128/JCM.00686-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.J., Wang L., Tian X.P., Yu H.L., Yu P.B. Clinical and epidemiological characteristics of 5676 cases of hand·foot-mouth disease. Chinese J Dis Control Prev. 2008;12:567–569. [Google Scholar]
- Wang S.-M., Lei H.-Y., Huang K.-J., Wu J.-M., Wang J.-R., Yu C.-K. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J Infect Dis. 2003;188:564–570. doi: 10.1086/376998. [DOI] [PubMed] [Google Scholar]
- Wang S.M., Liu C.C., Tseng H.W., Wang J.R., Huang C.C., Chen Y.J. Clinical spectrum of enterovirus 71 infection in children in Southern Taiwan, with an emphasis on neurological complications. Clin Infect Dis. 1999;29:184–190. doi: 10.1086/520149. [DOI] [PubMed] [Google Scholar]
- Watkins-Riedel T., Woegerbauer M., Hollemann D., Hufnagl P. Rapid diagnosis of enterovirus infections by real-time PCR on the LightCycler using the TaqMan format. Diagn Microbiol Infect Dis. 2002;42:99–105. doi: 10.1016/s0732-8893(01)00330-3. [DOI] [PubMed] [Google Scholar]
- Weng K.F., Chen L.L., Huang P.N., Shih S.R. Neural pathogenesis of enterovirus 71 infection. Microbes Infect. 2010;12:505–510. doi: 10.1016/j.micinf.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Wu Y., Yeo A., Phoon M.C., Tan E.L., Poh C.L., Quak S.H. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:e1076–e1081. doi: 10.1016/j.ijid.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Xu F., He D., He S., Wu B., Guan L., Niu J. Development of an IgM-capture ELISA for coxsackievirus A16 infection. J Virol Methods. 2011;171:107–110. doi: 10.1016/j.jviromet.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Xu F., Yan Q., Wang H., Niu J., Li L., Zhu F. Performance of detecting IgM antibodies against enterovirus 71 for early diagnosis. PLoS One. 2010;5:e11388. doi: 10.1371/journal.pone.0011388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Zhang T., Hu Y.F., Wang X.F., Du J., Li Y.F. Survey of enterovirus infections from hand, foot and mouth disease outbreak in china, 2009. Virol J. 2011;8:508. doi: 10.1186/1743-422X-8-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Sun J.L., Chang Z.R., Zhang W.D., Wang Z.J., Feng Z.J. Characterization of hand, foot, and mouth disease in China between 2008 and 2009. Biomed Environ Sci. 2011;24:214–221. doi: 10.3967/0895-3988.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Tan X.J., Wang H.Y., Yan D.M., Zhu S.L., Wang D.Y. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J Clin Virol. 2009;44:262–267. doi: 10.1016/j.jcv.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Zhu S.L., Guo X.B., Wang J.T., Wang D.Y., Yan D.M. Retrospective seroepidemiology indicated that human enterovirus 71 and coxsackievirus A16 circulated wildly in central and southern China before large-scale outbreaks from 2008. Virol J. 2010;7:300. doi: 10.1186/1743-422X-7-300. [DOI] [PMC free article] [PubMed] [Google Scholar]