Highlights
► Canine parvovirus strains circulating in Brazil were detected and characterised. ► Stool samples were analysed by PCR and sequenced. ► Phylogenetic analysis of the variants circulating was performed. ► CPV-2c was the predominant subtype circulating in Brazil between years 2008 and 2010.
Keywords: Canine parvovirus, CPV-2, Detection, Characterisation, Phylogeny
Abstract
Detection and characterisation of the canine parvovirus (CPV-2) strains that are currently circulating are essential for the understanding of viral evolution and the development of measures to control its spread. In the present study, stool samples from 144 dogs were analysed by polymerase chain reaction (PCR) for CPV-2, and 29.2% (42/144) of them were positive. From the 42 positive strains, 71.4% (30) of the dogs had signs of haemorrhagic gastroenteritis. The sequencing of the 583 bp fragment of the VP2 gene from the positive strains identified 78.6% (33/42) of them as type 2c, 19% (8/42) as type 2b and 2.4% (1/42) as type 2a. A phylogenetic analysis of the variants circulating in the canine population of Brazil showed that they are very similar to those found in other countries and type 2c has become the predominant type circulating in Brazil.
1. Introduction
Treatment of gastrointestinal diseases comprises a large part of small animal medicine, in which typical clinical signs are vomiting and diarrhoea that can lead to death of the animal (Sellon, 2005). Among the main viruses that cause diarrhea are the canine parvovirus-2 (CPV-2), canine enteric coronavirus (CCoV), canine rotavirus (CRV) and canine distemper virus (CDV) (Hoskins, 1997, Tams, 2003). Since the late 1970s, the CPV-2 strain has been known to be a major etiologic agent of infectious gastroenteritis in young dogs (Appel et al., 1979, Morais and Costa, 2007). Thus, canine parvovirus, which occurs with high frequency and endures for long periods of time in the environment, has stood out among other diseases because it results in high rates of morbidity and mortality (Hoskins, 1997). CPV-2 was first isolated in 1978, and since then, it has given rise to new antigenic types that have spread in the dog population (Appel et al., 1979, Morais and Costa, 2007). Over time, the original CPV-2 has been replaced by its antigenic variants, CPV-2a and CPV-2b (Decaro et al., 2008). A strain with an amino acid change, (Asp) – 426 to (Glu) – 426, in an important antigenic site was recognised in Italy in 2000 and was named CPV-2c (Buonavoglia et al., 2001). Subsequently, this strain was identified in Vietnam (Nakamura et al., 2004) and Spain (Decaro et al., 2006). In South America, this new type was first reported in Uruguay (Pérez et al., 2007), followed by Brazil (Streck et al., 2009) and Argentina (Calderón et al., 2009). The identification and monitoring of the types of CPV-2 circulating in the canine population are important in the understanding of viral evolution and the development of measures to control its spread. The present study aimed to detect the presence and characterise the types of CPV-2 circulating in Brazil between the years 2008 and 2010.
2. Materials and methods
2.1. Samples
The analysis was performed on 144 samples of dog faeces collected in 20 districts of six Brazilian states from April 2008 to July 2010. These animals, which were aged between 1 month and 1 year, included animals with or without symptoms of haemorrhagic gastroenteritis (HGE), animals with or without a vaccination history and animals from different breeds and both genders.
2.2. DNA extraction
The stool samples were diluted to 20% (w/v) in phosphate buffered saline (PBS, pH 7.4). The solution was frozen and thawed three times and then centrifuged at 1500 × g for 10 min. DNA was extracted from the supernatant using a commercial kit based on guanidine isothiocyanate and silica (Biotechnology Simbios, Canoas-RS, Brazil) as described by Boom et al. (1990) and stored at −20 °C until use. A commercial vaccine was used as a positive control and distilled water was used as a negative control.
2.3. Polymerase chain reaction (PCR)
We amplified 583 bp of the VP2 gene (position 4003–4585) using the protocol described by Buonavoglia et al. (2001). The PCR products were electrophoresed in 2% agarose gels, visualised under UV light and compared with a 100 bp molecular weight ladder (Fermentas, USA).
2.4. Sequencing of the amplification products
The amplification products were purified using GFX PCR DNA and gel band purification (Amersham Bioscience, USA) and sequenced by using the Abi-Prism 3100 Genetic Analyzer (Applied Biosystems, USA). The alignment and analysis were interpreted using the Clustal method with the BioEdit 7.0.0 software (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). The sequences were submitted to GenBank (http://www.ncbi.nlm.nih.gov) and the accession numbers are shown in Table 1 .
Table 1.
Characteristics of the CPV-2 positive dogs in different locations in Brazil, describing the main differences in some nucleotides and amino acids.
| Accession no. | Breed | Gender | Age | Vaccin. program | CPV-2 type | Position of the codon and amino acid |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 426 | 431 | 440 | 461 | 546 | 547 | 555 | 573 | ||||||
| FJ222821/VP2 | 56/00 | – | – | – | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| EF375479 Uruguay | ST | NA | NA | V | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| DQ340409 | NA | NA | NA | NA | 2b | GAT(Asp) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| DQ340434 | NA | NA | NA | NA | 2a | AAT(Asn) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| EU797726 | Mo | M | 5 m | NV | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAC(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| EU797727 | GS | F | 4 m | V | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAC(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| EU797728 | Mo | F | NA | NV | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAC(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| FJ236063 | GS | F | 3 m | V(I) | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAC(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| FJ236064 | P | F | 2 m | V(I) | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAC(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| FJ236065 | NA | NA | 2 m | NA | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAC(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| FJ236066 | NA | NA | 2 m | NA | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAC(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| FJ236067 | NA | NA | 6 m | NA | 2b | GAT(Asp) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| FJ236068 | NA | F | NA | NA | 2b | GAT(Asp) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796211 | D | M | 2 m | NV | 2b | GAT(Asp) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796210 | Mo | M | 12 m | NV | 2b | GAT(Asp) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796208 | GS | M | 2 m | NV | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAC(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796209 | GS | F | 2 m | NV | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAC(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| GQ395692 | R | M | 3 m | V | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| GQ452297 | Mo | F | 3 m | V(I) | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796207 | S | F | 2 m | V(I) | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | ACA(Thr) | GTA(Val) | TTT(Phe) |
| JF796206 | BI | M | 2 m | V(I) | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | ACA(Thr) | GTA(Val) | TTT(Phe) |
| JF796205 | A | M | 4 m | NV | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796204 | Mo | M | 5 m | NV | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796203 | Mo | M | 3 m | NV | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796202 | Mo | F | 3 m | NV | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796201 | R | M | 4 m | NV | 2c | GAA(Glu) | GTA(Val) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796200 | Mo | M | 4 m | NV | 2a | AAT(Asn) | CTA(Leu) | ACA(Thr) | CCG(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796199 | GS | M | 4 m | NV | 2b | GAT(Asp) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796198 | R | M | 6 m | NV | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796197 | Mo | M | 3 m | V(I) | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796196 | Mo | F | 4 m | V | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796195 | Mo | F | 5 m | NV | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796194 | LR | M | 4 m | V | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796193 | Mo | M | 4 m | V | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796192 | P | F | 2 m | V(I) | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796191 | GS | M | 2 m | V(I) | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796190 | D | M | 2 m | NV | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796189 | Mo | F | 2 m | NV | 2b | GAT(Asp) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796188 | Y | M | 4 m | V | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796187 | Mo | M | 10 m | NV | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796186 | P | M | 6 m | V(I) | 2b | GAT(Asp) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796185 | GR | M | 1 m | V(I) | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAC(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796184 | S | F | 3 m | V(I) | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796183 | Mo | M | 2 m | NV | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796182 | GS | M | 2 m | V(I) | 2c | GAA(Glu) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
| JF796181 | Mo | F | 4 m | NV | 2b | GAT(Asp) | CTA(Leu) | ACA(Thr) | CCA(Pro) | AAT(Asn) | CCA(Pro) | GTA(Val) | TAT(Tyr) |
F: female; M: male; m: month; Mo: Mongrel; GS: German Shepherd; GR: Golden Retriever; P: Pinscher; Y: Yorkshire; LR: Labrador Retriever; R: Rottweiler; A: Akita; D: Dachshund; BI: English Bulldog; Sch: Schnauzer; S: Shi-tzu; ST: Short Terrier; NA: not available; NV: not vaccinated; V(I): incomplete vaccination; V: vaccinated. In bold are the mutations found in the CPV-2 Brazilian sequences.
2.5. Phylogenetic analysis
For the phylogenetic analysis, 28 CPV-2 sequences from 14 countries were retrieved from GenBank. Two Brazilian sequences (DQ340434 and DQ340409) (Pereira et al., 2000) and a sequence of feline panleukopenia virus (AB054225), representing the out group, were also included. The Bayesian inference (BI) of phylogeny was performed using MrBayes 3.1.2 (Huelsenbeck and Ronquist, 2001). The substitution model for BI analyses was found using MrMODELTEST (Nylander, 2002) with the AIC criterion. Four Markov chains, one cold and three heated, were run for 2 × 106 generations, trees were strained every 100 generations and those generated prior to stationary were discarded as “burn-in”. The posterior probability values for all clades in the final majority rule consensus tree are reported. The support values shown along each branch are Bayesian posterior probabilities (Nodal support values <50% not shown).
Samples were assigned to one CPV-2 type based in the presence of a deduced asparagine (2a), aspartic acid (2b) or a glutamic acid (2c) in the amino acid position 426 (Buonavoglia et al., 2000).
3. Results
Of the 144 stool samples analysed by PCR, 29.2% (42/144) were positive for CPV-2 (Table 1). Amplification of the partial VP2 gene displayed a single band with the expected 583 bp. Of the dogs examined, 38.8% had haemorrhagic gastroenteritis (HGE), but from the 42 positive samples, 71.4% displayed signs of HGE (Table 2 ). From Rio Grande do Sul, 111 samples were analysed, which were from the municipalities of Porto Alegre, Viamão, Cachoeirinha, Taquara, Canoas, Caxias do Sul, Passo Fundo, Bagé, Glorinha, Gravataí, Santana do Livramento, Lavras do Sul, Novo Hamburgo, Rio Pardo and São Francisco de Paula. The states of Santa Catarina (SC), Paraná (PR), São Paulo (SP), Rio de Janeiro (RJ) and Rondônia (RO) had a total of 33 samples that resulted in nine CPV-2-positive samples. Sequencing the amplification product from the 42 positive samples allowed us to identify nucleotide mutations, although the majority were silent (Table 1). It was possible to identify 78.6% (33/42) of the samples as type 2c, 19% (8/42) as type 2b and 2.4% (1/42) as type 2a. From the positive animals, 33.3% (14/42) were aged less than 2 months, 47.6% (20/42) had no vaccination history and the 6 animals with a complete immunisation schedule were positive for CPV-2c. Regarding breed, 11 mixed breeds, 6 German Shepherds and 3 Rottweilers were CPV-2c positive and their ages ranged from 2 to 10 months.
Table 2.
Results of the analysis by PCR for CPV-2 from stool samples of dogs with and without haemorrhagic gastroenteritis (HGE).
| Positive by PCR | Negative by PCR | Total | |
|---|---|---|---|
| Dogs with HGE | 30 | 26 | 56 |
| Dogs without HGE | 12 | 76 | 88 |
| Total | 42 | 102 | 144 |
In general, the BI and NJ trees (not shown here) were different because many possible topological resolutions were found, with weak support. Salient features of all trees included that (a) CPV-2 Brazilian variants can be considered to be cosmopolitan, with similar variants in South America, Europe, Africa and Asia and (b) no clear geographic structure was found showing that CPV-2 variants are widely dispersed along the studied area. The BI tree (Fig. 1 ) showed that the CPV-2 Brazilian variants have clustered into different groups.
Fig. 1.
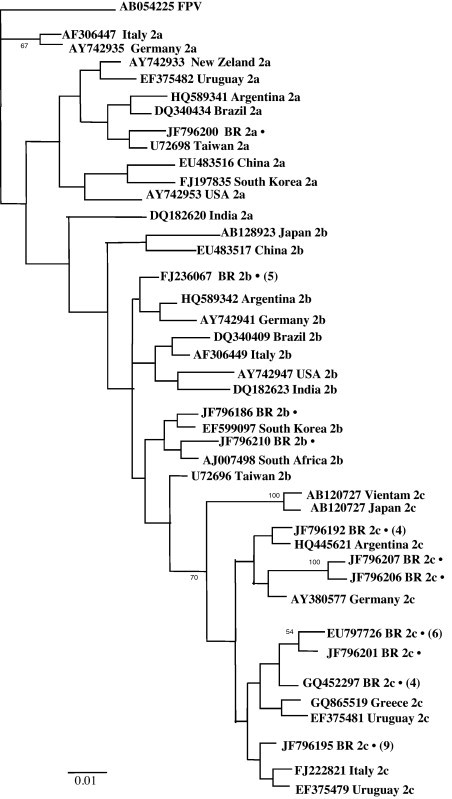
Nucleotide phylogenetic tree based on the VP2 gene of CPV-2 strains circulating in Brazil between the years 2008 and 2010 (●) with 28 other worldwide CPV-2 variants. Principal bootstrap values are indicated. The values in parentheses indicate the number of strains with identical sequences to that included in the tree. (5): FJ236068, JF796199, JF796181, JF796189, JF796211; (4): JF796190, JF796193, JF796203, JF796198; (6): EU797728, JF796209, FJ236065, EU797727, FJ236066, FJ236063; (4): JF796197, GQ395692, JF796196, JF796184; (9): JF796204, JF796182, JF796202, JF796191, JF796205, JF796183, JF796194, JF796188, JF796187.
4. Discussion
Since its emergence in the late 1970s, parvovirus has caused high rates of morbidity and mortality, but the severity was initially attributed to a lack of natural immunity in the canine population against the virus. Therefore, dog vaccination and natural exposure to infection should have improved protection; however, a high incidence of disease continues in animals aged between 6 weeks and 6 months (Morais and Costa, 2007). This trend was confirmed by data found in the present study, in which 95.2% of the positive animals were between 1 and 6 months of age. Puppies are more prone to the development of haemorrhagic gastroenteritis caused by CPV-2, although dogs of any age, gender or breed may be affected (McCandlish, 1999, Morais and Costa, 2007, Parrish, 1999). From the 42 positive animals detected in the present study, 12 displayed no signs of HGE, showing that the clinical outcome may depend on the degree of maternal immunity, virulence of the virus strain, infectious virus dose and the host's immune system (Homem et al., 1999, Sellon, 2005, Tams, 2003).
It should be noted that in Brazil, canine parvovirus vaccines consist of live attenuated virus (CPV-2 and CPV-2b) and which, although it is rare, may be detected in the faeces of dogs (Decaro et al., 2007). However, the sequencing analyses of the CPV-2b described in the current study demonstrated that a point mutation at residue position 570 (A–G), which is characteristic of the CPV-2b vaccine strain, was not observed in the present sequences, confirming that the CPV-2b strains detected were not related to vaccination. These data corroborate previous reports that have demonstrated the high stability of the virulence attenuation of the strains used in current vaccines (Decaro et al., 2006).
German Shepherds and Rottweilers were among the breeds of dogs that were CPV positive. These two breeds, as well as other medium and large breeds, are more susceptible and can develop a more severe clinical picture when infected by CPV (Morais and Costa, 2007, Sellon, 2005). Interestingly, the six animals that had received a complete vaccination protocol were infected by the variant CPV-2c and displayed HGE, which resulted in death for one of them. These data corroborate previous findings in the United States and Uruguay (Kapil et al., 2007, Pérez et al., 2007), although it had been shown that vaccination would protect dogs exposed to CPV-2c (Spibey et al., 2008). However, in field situations, the vaccination protocol and maternal antibody titers are variable and may influence the appropriate response to the vaccine (Sellon, 2005).
The Brazilian CPV-2a strain contained the amino acid valine at position 555 of VP2, which has been described by Pérez et al. (2007). In the 1980s, CPV-2a strains from many countries displayed the amino acid valine in this position (Cavalli et al., 2008, Decaro et al., 2006). The Ile555Val change can be considered to be a reversion to the original CPV-2 (Martella et al., 2006, Pereira et al., 2007). Comparison of the CPV-2c strains from Europe and Uruguay with those found in Brazil revealed silent mutations in residue 461 (AG) and 546 (CT) from eleven strains and amino acid mutations in two strains (Pro547Thr and Tyr573Phe). The Brazilian strains did not show the mutation at position 440 (TA) that had been previously described (Calderón et al., 2011, Decaro et al., 2009, Hong et al., 2007, Kapil et al., 2007). The CPV-2c was the main subtype detected in the Brazilian samples, as previously found in the United States (Hong et al., 2007), Uruguay (Pérez et al., 2007) and Argentina (Calderón et al., 2009). The detection of CPV-2c in Europe, Asia and the Americas is an indication of the worldwide spread of this new variant in the canine population (Decaro et al., 2006, Decaro et al., 2007). Martella et al. (2005) indicated an increasing replacement of subtypes CPV-2a and CPV-2b by CPV-2c that, from 2000 until 2004, increased from 17% to 60% of the samples in Italy. Pérez et al. (2007) suggested that the rapid spread of subtype 2c was likely associated with an important nucleotide substitution and might lead to the elimination of the other subtypes of CPV-2 over time. In other countries, such as India (Raj et al., 2010), Belgium and Italy (Decaro et al., 2009), the predominance of the type 2a strains has been verified. The CPV-2b has a more homogeneous distribution and has been detected in different Brazilian regions and in other European countries, such as France and the UK (Decaro et al., 2009, Pereira et al., 2000).
Phylogenetic analysis of the variants circulating in the canine population of some regions of Brazil has shown that they are very similar to those found in other countries and do not indicate a common geographical origin and are thus termed cosmopolitan. In contrast, mutations were detected in two amino acids (547 and 573) from sequences with the accession numbers JF796207 and JF796206, which were not reported in any other country.
The results of the present study demonstrate that 2c is the main CPV type that circulated in Brazil between the years 2008 and 2010, affecting puppies either with or without a complete vaccination protocol. Most of the positive samples, as identified by PCR, were from dogs with signs of haemorrhagic gastroenteritis. Phylogenetic analysis of the variants circulating in the canine population of Brazil shows that they are very similar to those from other countries in the Americas.
Conflict of interest statement
No conflicts of interest exist regarding the publication of this paper.
Acknowledgements
We are thankful to Simbios Biotecnologia Ltda. for kindly supplying the DNA extraction kits. We thank the veterinarians who collected samples of dog faeces and the graduate and post graduate students of the Laboratório de Virologia for their collaboration in this work. Financial support was provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and Propesq/UFRGS.
References
- Appel M.J.G., Cooper B.J., Greisen H., Scott F.W., Carmichael L.E. Canine viral enteritis. I. Status report on corona and parvo-like viral enteritides. Cornell Vet. 1979;69:123–133. [PubMed] [Google Scholar]
- Boom R., Sol C.J.A., Salimans M.M.M., Jansen C.L., Wertheim-Van Dillen P.M.E., Van Der Noordaa J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonavoglia D., Cavalli A., Pratelli A., Martella V., Greco G., Tempesta M., Buonavoglia C. Antigenic analysis of canine parvovirus strains isolated in Italy. New Microbiol. 2000;23:93–96. [PubMed] [Google Scholar]
- Buonavoglia C., Martella V., Pratelli A., Tempesta M., Cavalli A., Buonavoglia D., Bozzo G., Elia G., Decaro N., Carmichael L. Evidence for evolution of canine parvovirus type 2 in Italy. J. Gen. Virol. 2001;82:3021–3025. doi: 10.1099/0022-1317-82-12-3021. [DOI] [PubMed] [Google Scholar]
- Calderón M.G., Mattion N., Bucafusco D., Fogel F., Remorini P., La Torre J. Molecular characterization of canine parvovirus strains in Argentina: detection of the pathogenic variant CPV2c in vaccinated dogs. J. Virol. Methods. 2009;159:141–145. doi: 10.1016/j.jviromet.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Calderón M.G., Romanutti C., D’Antuono A., Keller L., Mattion N., Torre J.L. Evolution of canine parvovirus in Argentina between years 2003 and 2010: CPV 2c has become the predominant variant affecting the domestic dog population. Virus Res. 2011;157:106–110. doi: 10.1016/j.virusres.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli A., Martella V., Desario C., Camero M., Bellacicco A.L., Palo P., Decaro N., Elia G., Buonavoglia C. Evaluation of the antigenic relationships among canine parvovirus type 2 variants. Clin. Vaccine Immunol. 2008;15:534–539. doi: 10.1128/CVI.00444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Martella V., Desario C., Bellacicco A.L., Camero M., Manna L., D’Aloja D., Buonavoglia C. First detection of canine parvovirus type 2c in pups with hemorrhagic enteritis in Spain. J. Vet. Med. 2006;53:468–472. doi: 10.1111/j.1439-0450.2006.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Desario C., Elia G., Campolo M., Lorusso A., Mari V., Martella V., Buonavoglia C. Occurrence of severe gastroenteritis in pups after canine parvovirus vaccine administration: a clinical and laboratory diagnostic dilemma. Vaccine. 2007;25:1161–1166. doi: 10.1016/j.vaccine.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Desario C., Elia G., Martella V., Mari V., Lavazz A., Nardi M., Buonavoglia C. Evidence for immunization failure in vaccinated adult dogs infected with canine parvovirus type 2c. New Microbiol. 2008;31:125–130. [PubMed] [Google Scholar]
- Decaro N., Desario C., Parisi A., Martella V., Lorusso A., Miccolupo A., Mari V., Colaianni M.L., Cavalli A., Di Trani L., Buonavoglia C. Genetic analysis of canine parvovirus type 2c. Virology. 2009;385:5–10. doi: 10.1016/j.virol.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Homem V.S.F., Mendes Y.G., Linhares A.C. Gastroenterite canina-Agentes virais nas fezes de cães diarréicos e não diarréicos. Arq. Bras. Med. Vet. Zootec. 1999;51:531–536. [Google Scholar]
- Hong C., Decaro N., Desario C., Tanner P., Pardo M.C., Sanchez S., Buonavoglia C., Saliki J.T. Occurrence of canine parvovirus type 2c in the United States. J. Vet. Diagn. Invest. 2007;19:535–539. doi: 10.1177/104063870701900512. [DOI] [PubMed] [Google Scholar]
- Hoskins J.D. Update on canine parvoviral enteritis. Vet. Med. 1997;92:694–709. [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Kapil S., Cooper E., Lamm C., Murray B., Rezabek G., Johnston L., III, Campbell G., Johnson B. Canine parvovirus types 2c and 2b circulating in North American dogs: 2006 and 2007. J. Clin. Microbiol. 2007;45:4044–4047. doi: 10.1128/JCM.01300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V., Cavalli A., Decaro N., Elia G., Desario C., Campolo M., Bozzo G., Tarsitano E., Buonavoglia C. Immunogenicity of an intranasally administered modified live canine parvovirus type 2b vaccine in pups with maternally derived antibodies. Clin. Diagn. Lab. Immunol. 2005;10(12):1243–1245. doi: 10.1128/CDLI.12.10.1243-1245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V., Decaro N., Buonavoglia C. Evolution of CPV-2 and implication for antigenic/genetic characterization. Virus Genes. 2006;33:11–13. doi: 10.1007/s11262-005-0034-8. [DOI] [PubMed] [Google Scholar]
- McCandlish I.A.P. Infections specific canine. In: Dunn J.K., editor. Textbook of Small Animal Medicine. W.B. Saunders Company; London: 1999. pp. 915–920. [Google Scholar]
- Morais M.P., Costa P.R. Parvoviridae. In: Flores E.F., editor. Virologia Veterinária. Ed. da UFSM; Santa Maria: 2007. pp. 388–392. [Google Scholar]
- Nakamura M., Tohya Y., Miyazawa T., Mochizuki M., Phung H.T., Nguyen N.P., Huynh L.M., Nguyen L.T., Nguyen P.N., Nguyen P.V., Nguyen N.P., Akashi H. A novel antigenic variant of canine parvovirus from a Vietnamese dog. Arch. Virol. 2004;149:2261–2269. doi: 10.1007/s00705-004-0367-y. [DOI] [PubMed] [Google Scholar]
- Nylander, J.A.A., 2002. MrModeltest v.1.0b. Program distributed by the author. http://www.ebc.uu.se/systzoo/staff/nylander.html.
- Parrish C.R. Host range relationships and the evolution of canine parvovirus. Vet. Microbiol. 1999;69:29–40. doi: 10.1016/s0378-1135(99)00084-x. [DOI] [PubMed] [Google Scholar]
- Pereira C.A.D., Monezi T.A., Mehnert U., D’Angelo M., Durigon E.L. Molecular characterization of canine parvovirus in Brazil by polymerase chain reaction assay. Vet. Microbiol. 2000;75:127–133. doi: 10.1016/s0378-1135(00)00214-5. [DOI] [PubMed] [Google Scholar]
- Pereira C.A., Leal E.S., Durigon E.L. Selective regimen shift and demographic growth increase associated with the emergence of high-fitness variants of canine parvovirus. Infect. Genet. Evol. 2007;7:399–409. doi: 10.1016/j.meegid.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Pérez R., Francia L., Romero V., Maya L. First detection of canine parvovirus type 2c in South America. Vet. Microbiol. 2007;124:147–152. doi: 10.1016/j.vetmic.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Raj J.M., Mukhopadhyay H.K., Thanislass J., Antony P.X., Pillai R.M. Isolation, molecular, characterization and phylogenetic analysis of canine parvovirus. Infect. Genet. Evol. 2010;10:1237–1241. doi: 10.1016/j.meegid.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Sellon R.K. Canine virus disease. In: Ettinger S.J., Feldman E.C., editors. Veterinary Internal Medicine. sixth ed. Elsevier Saunders; St. Louis, Missouri: 2005. pp. 646–652. [Google Scholar]
- Spibey N., Greenwood N.M., Sutton D., Chalmers W.S.K., Tarpey I. Canine parvovirus type 2 vaccine protects against virulent challenge with type 2c virus. Vet. Microbiol. 2008;128:48–55. doi: 10.1016/j.vetmic.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Streck A.F., Souza C.K., Gonçalves K.R., Zang L., Pinto L.D., Canal C.W. First detection of canine parvovirus type 2c in Brazil. Braz. J. Microbiol. 2009;40:465–469. doi: 10.1590/S1517-83822009000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tams T.R. second ed. Saunders Company; St. Louis: 2003. Handbook of Small Animal Gastroenterology. [Google Scholar]


