Abstract
Oncolytic virotherapy is a promising form of gene therapy for cancer, employing nature’s own agents to find and destroy malignant cells. The purpose of this review is to provide an introduction to this very topical field of research and to point out some of the current observations, insights and ideas circulating in the literature. We have strived to acknowledge as many different oncolytic viruses as possible to give a broader picture of targeting cancer using viruses. Some of the newest additions to the panel of oncolytic viruses include the avian adenovirus, foamy virus, myxoma virus, yaba-like disease virus, echovirus type 1, bovine herpesvirus 4, Saimiri virus, feline panleukopenia virus, Sendai virus and the non-human coronaviruses. Although promising, virotherapy still faces many obstacles that need to be addressed, including the emergence of virus-resistant tumor cells.
Abbreviations: CNS, central nervous system; ECM, extracellular matrix; HIV, human immunodeficiency virus; HSV, herpes simplex type 1 virus; IFN, interferon; MOI, multiplicity of infection; (Mo)MLV, (Moloney) murine leukemia virus; NDV, Newcastle disease virus; PKR, protein kinase R; PFU, plaque-forming unit; SFV, Semliki Forest virus; VSV, vesicular stomatitis virus; VV, Vaccinia virus; VLP, viral-like particle; TK, thymidine kinase; i.v., intravenous; i.t., intratumoral; i.p., intraperitoneal; i.c., intracranial; WT, wildtype; TAA, tumor associated antigen; FMG, fusogenic membrane glycoprotein
Keywords: Cancer gene therapy, Oncolytic, Replication-competent, Replication-deficient, Virotherapy, Virus-resistant
1. Introduction
The notion that viruses may be able to eradicate cancer has existed since the early 20th century [1], [2], [3]. Several viruses were tested both in experimental settings and in humans during the 1950s and 1960s. Among the first viruses to be used in a controlled fashion in clinical studies was a vaccine strain of rabies virus to treat 30 patients with melanomatosis, eight of whom showed tumor regression [4]. A few years later the oncolytic efficacy of adenovirus serotype type 4, which at that time was referred to as RI (respiratory infectious) virus, was tested in humans [5], as well as the flavivirus West Nile virus (strain Egypt 101) [3] and the paramyxoviruses mumps and Newcastle disease virus (NDV) [6], [7]. Also, many viruses were tested in animal models. For instance, bovine enterovirus showed efficient lysis of syngeneic tumors in immunocompetent mice [8]. Moreover, many case reports describe the use of several different viruses, including ones with previously undescribed pathogenesis. In one larger study, 10 human and one simian adenovirus serotypes were administered to 30 patients with cervical cancer resulting in necrosis and transient tumor regression in some patients [9]. In another report, six different viruses were administered in succession to a patient suffering from acute leukemia [10], and a third report summarizes the use of 13 different viruses or strains in a total of 57 patients with various types of advanced cancer, with no oncolytic effects observed using any of the viruses [11]. While these studies were groundbreaking and provided the first glimpse of therapeutic potential in humans, the efficacy of the virotherapy was unimpressive and many viruses elicited side-effects which ultimately ended the trials. As a result, the initial interest in viruses as anti-cancer agents declined during the following decades, as deduced from the number of publications from that time, and studies were mainly conducted using experimental models. As an example, a report from 1982 describes the testing of the oncolytic potential of 16 different viruses in 180-sarcoma and Erlich ascites tumors in mice [12]. Later in the 1990s, with the advent of modern biotechnology and the concept of gene therapy, interest in viruses as a treatment for cancer was rekindled, and today virotherapy is asserting itself as a formidable treatment option alongside surgery, chemo- and radiation therapy. Many new virotherapeutic candidates are emerging in what could be called the second coming of viruses.
2. Viruses and cancer – alternatives to oncolysis
In addition to direct tumor targeting several alternative strategies for cancer gene therapy have been developed, which are addressed only briefly in this review. Foremost among these is tumor vaccination or cancer immunotherapy, which aims at evoking tumor-specific immunity capable of eradicating established tumors as well as maintaining immunologic memory [13], [14], [15]. Tumor-specific immunity may be stimulated e.g. by the production of tumor associated antigens (TAAs) either within the tumor cells themselves or in other cells, or via opsonization of tumor cells by antibodies produced by viral vectors. Another approach involves the enhancement of tumor cell recognition by T cells or dendritic cells which have been transduced with viruses encoding immunostimulatory cytokines. Many viruses evoke a strong immune response and may thus function as adjuvants, and hence oncolysis can be considered a two-pronged strategy where cancer cells are destroyed on one hand by direct action of viral replication and on the other, they become targets for recognition by the immune system. During the 1950s this idea spurred the development of so called oncolysates which were used in several human trials to vaccinate against cancer [14], [15]. One of the most promising studies was led by Dr. William Cassel who treated patients with stage III malignant melanoma with NDV-oncolysates [16], [17]. The oncolysates were prepared ex vivo using either existing or isolated melanoma cell lines from the patients to be treated, and then injected subcutaneously into the thigh and forearm of the patients. The treatment regimen was gradually increased from weekly injections to 3 month intervals after 10 years of continued therapy. Of the 81 treated patients, 63% were tumor-free after 10 years [18] and 71% of these remained tumor-free for the following 10 years, totaling a 20-year survival of 44% (see Chapter 9 in Ref. [19]). Nine of the 15 patients who died between the 10 and 20 year follow-ups were tumor-free and died of other causes. While this trial was limited and included only historical controls, it is encouraging and warrants further study on a larger scale. In comparison, although survival is highly dependent on the substage of the disease (primarily determined by the number of regional lymph nodes involved), the overall 20-year survival in 2002 of stage III melanoma was reported to be 23% [20]. In fact, despite aggressive chemo- and radiation therapy, the overall survival rates of the most common forms of metastasizing cancer have not improved in the past decades, casting doubt on the true usefulness of the conventional forms of treatment and emphasizing the need for alternative therapies [21].
Oncolysates have also been used later in clinical trials with some degree of success [15], [22], [23], but results have been ambiguous even using the same virus (Chapter 5, Ref. [19]). Still, when measuring overall survival, viral oncolysates have proven better than conventional therapies, possibly because of the detrimental effects chemo- and radiation therapy have on the immune system (discussed in Chapter 4, Ref. [19]). The relationship between viruses, cancer and the immune system is complex and may reveal surprising aspects. For example, a growing amount of evidence shows that vaccination against common human pathogens, such as influenza, vaccinia and tuberculosis, reduces the risk of developing melanoma later in life [24], [25], [26]. While the molecular basis for this phenomenon is still unknown, cross-protection between viral epitopes and cancer has been proposed to play a role. For other types of cancer where a particular virus is the etiologic agent, e.g. papillomavirus in cervical cancer and hepatitis B virus in hepatocellular carcinoma, vaccines against these viruses have proven highly prophylactic, but only rarely therapeutic for established cancers [27], [28]. Moreover, the controlled clinical or laboratory experiments aside, hundreds of cases of spontaneous remission have been documented since the beginning of the 20th century [29]. Although the underlying mechanisms of these remissions remain unclear, in many of these cases remission phase has been preceded by fever, which has spurred the notion of fever therapy for cancer [30]. It is likely that the immune system has played a significant role in clearing the tumor mass, possibly augmented by common infections. Elevated body temperature may also be beneficial for virotherapy. For instance, normal cells become more refractory to adenoviruses when cultured at high temperatures, whereas cancer cells may display even enhanced permissiveness [31], [32]. As far as oncolytic bacteria are concerned, at least attenuated Salmonella typhimurium and Clostridium novyi are being used in clinical trials to target various types of cancer (see www.wiley.co.uk/genmed/clinical/ and www.clinicaltrials.gov/ct).
Finally, also tumor vasculature constitutes an ample target for viral transduction [33]. The goal is to achieve transduction of the endothelial cells of blood vessels in solid tumors in order to have them produce oncotoxic prodrugs or preventing tumor spread by restricting angiogenesis. In the coming chapters we will discuss the possible mechanisms of viral oncolysis and summarize some of the problems virotherapy is currently facing.
3. Molecular basis for the selective permissiveness of cancer cells to viruses
Why are cancer cells such generous hosts for viruses, being preferred over other types of cells? Many genetic and physiological features specific for malignant cells relating to particular gain-of-function or loss-of-function mutations explain this phenomenon. Cancer cells have undergone a mini-evolution, involving extensive point-mutations as well as larger chromosomal shifts and alterations, termed chromosomal instability, which provides them with selective growth advantages over normal cells [34]. However, while cancer cells may gain many growth-enhancing attributes, they may simultaneously lose critical components of the intracellular defense mechanisms and thus become fertile ground for the replication of many viruses. For instance, although many cancer cell types are resistant to apoptosis due to activation of Ras (gain-of-function), reovirus replicates better in these cells as the function of the double-stranded RNA-activated protein kinase R (PKR) is inhibited [35], [36]. Activation of Ras and the subsequent inhibition of PKR has also been thought to underlie the permissiveness of tumor cells to oncolytic herpes simplex viruses (HSV) which are deleted in the main neurovirulence gene γ34.5. Normally, the γ34.5 protein overcomes the restriction in replication posed by functional PKR, but in a recent report it was shown that even vectors deleted for γ34.5 can replicate in cancer cells in which PKR is functional [37]. In this case, defects in the PI 3-kinase pathway allow the γ34.5-deleted HSV genome to be translated.
Malignant cells may express higher levels of virus receptors compared to normal cells (gain-of-function), which makes them more susceptible to infection. For instance, many types of cancer cells overexpress ICAM-1 and DAF, the receptors for coxsackievirus A21 [38], [39]. Another enterovirus, echovirus type 1, gains preferential entry to ovarian cancer cells due to overexpression of the I domain of integrin α2β1 [40], and poliovirus infects cells expressing the CD155 receptor, abundant on many human cancer cell types [41], [42]. The alphavirus Sindbis virus gains preferential access to tumor cells as a result of overexpression of the 67 kDa laminin receptor [43]. However, for viruses which utilize receptors that are abundant also on normal cells the mechanisms that govern preferential replication in cancer cells are probably intracellular. For instance, despite that NDV or mumps virus use sialic acid as their receptor and alphaviruses use heparan sulphate or ICAM-1, all abundantly expressed also on many normal cells, these viruses show high cancer cell selectivity [44], [45], [46]. In these cases preference to cancer cell stems from the fact that many, if not most, tumor cells display an impaired anti-viral response due to failure of critical components of the interferon (IFN) response system (loss-of-function) [47], [48], [49], [50], [51]. In fact, one strategy to enhance the tumor cell specificity of oncolytic viruses is to mutate them to induce a more potent IFN response [52]. While this reduces the replication of such viruses in normal cells, cancer cells remain permissive to the viruses because they are unable to respond to IFN signaling.
Also, several cell type-specific promoters are available to target viruses to only those tumor cells in which the promoters are active, or alternatively, cancer cells may overexpress certain genes that make them susceptible to virally delivered prodrugs or growth inhibitors. For instance, squamous carcinoma cells overexpress survivin which makes it possible to use therapies specifically targeting this gene [53]. On the other hand, for many viruses the molecular basis for preferential infection of cancer cells is less clear, and some viruses may even display anti-cancer effects that do not involve the direct killing of the cells. As an example, while the parvoviral NS1 protein interferes with many cellular processes and can induce apoptosis particularly in transformed cells, parvoviruses may exhibit oncosuppressive activity (in addition to oncolysis) which prevents cellular transformation and tumorigenesis [54], [55]. Even more perplexing, pre-infection of Balb/c mice with mouse parvovirus led to enhanced graft rejection of syngeneic sarcoma cells despite that these cells were refractory to infection by the virus [56]. In another study, replication-defective human cytomegalovirus vectors were able to halt tumor growth in distant metastases in mice without direct infection of the tumor cells [57]. Both these mechanisms remain largely unresolved but may still be used in cancer therapy in the future.
Finally, some viruses have been engineered cancer cell specific. For example, some adenoviral vectors have been deleted for the E1B gene in order to restrict their replication to cells in which the function of the p53 protein is impaired, including many types of cancer cells. Whereas the E1B protein of wildtype (WT) adenovirus prevents p53 from inducing apoptosis in response to infection, E1B-deleted vectors can replicate productively only in cells lacking functional p53. This requirement is not absolute, however, and seems to be influenced by other factors, including cell type [58], [59]. As another example, herpes viruses lose the ability to infect non-dividing cells by deletion of the thymidine kinase gene, thereby forcing these viruses to prefer tumor cells over normal cells [60]. Viruses which have been mutated to be dependent on certain molecular defects in cancer cells are called conditionally replicating (i.e. restricted to replicate only in permissive cells). In the following chapter we introduce other types of viral vectors used in cancer targeting.
4. Classification of viral constructs
Frequently, in the literature terms such as amplicon, capsid-replacement vector, gutless vector, pseudovirion, replicon, single-cycle vector, virus based nanoparticle, viral-like particle (VLP) and virosome appear when delving into the world of viral vectors and gene therapy. These terms, attributed to the vectors in a manner habitual to the particular sub-field of research they originate in, imply that the constructs are unable to spread from cell to cell by means of autonomous replication. In other words, although replication of the viral genome may occur, such vectors cannot produce progeny virions. Hence the more descriptive terms replication-deficient or replication-defective. A distinction can be made between replication-deficient vectors that contain the genes necessary for replication (but not the viral particle), e.g. replicons or capsid-replacement vectors, and constructs that do not express any viral genes at all, e.g. amplicons, virus based nanoparticles, VLPs, gutless vectors and pseudovirions. However, there is some discrepancy in the use of these terms, as, for example, some use the term VLP to describe replicons [61], while others use the it for particles completely devoid of viral genomes [62].
The inability of replication-defective viruses to spread is caused by deletions in regions of the viral genome coding for the structural proteins. The mutations abrogate the production of the viral capsid and/or envelope proteins, and thus eliminate both the possibility and the risk of a spreading infection. Exceptions to this rule exist, however, as some replication-deficient vectors expressing fusogenic membrane glycoproteins (FMGs) may allow the vector to spread into neighboring cells in the absence of an extracellular step involving a virus particle. In such systems, the truncated viral genome is passively transferred into new cells via the formation of multinucleated syncytia as cells fuse together, seen for example in cell cultures infected with members of the paramyxoviridae family [63]. In addition to facilitating virus spread, syncytiosome formation may be directly toxic to the cells and syncytia are also more readily recognized by the immune system, increasing the tumor-killing capacity of oncolytic viruses even further or conferring killing capacity to otherwise apathogenic constructs [64]. Viral genomes may also piggyback on exocytosed vesicles or jump from cell to cell through tight junctions, as has been documented for a variety of different viruses and viral vectors [65], [66], [67]. Moreover, some vectors may not be based on any specific virus yet still be packaged into VLPs. For example, the so called plasmoviruses are plasmids in which the sole viral elements are the encapsidation signal and the genes for the virion itself (capsid/envelope). Plasmoviruses have been shown to drive the production of foreign inserts under the control of a heterologous promoter and to propagate the “infection” by production and budding of VLPs [68]. Whether deleted in the viral replicase or the structural genes, the cloning capacity of replication-deficient constructs is often impressive. However, due to the inability to produce progeny it is becoming clear that replication-deficient constructs lack in efficacy when compared to replication-competent counterparts [69].
In a generic sense, to achieve virally induced cell death either of the following mechanisms are required: (a) viral replication, which causes lysis due to accumulation of virions or because the cellular transcription/translation machinery is usurped, or (b) the expression or import of viral proteins which in themselves are cytotoxic or influence cellular functions that may lead to apoptosis. This means that viruses which are used to target cancer cells, i.e. oncotropic viruses, but do not kill them are not considered oncolytic. True oncolytic viruses, whether replication-deficient or replication-competent, meet the criteria above. Oncotropic viruses are taken up by human cells, but their genes are not expressed or the infection is abortive. Such viruses include canine parvovirus [62], baculovirus [70], [71] and canarypoxvirus [72], all of which are being developed into vectors for cancer targeting but none of which display intrinsic oncolytic capacity. Moreover, as many of the non-lytic viruses are highly immunogenic they may serve as adjuvants to boost anti-tumoral immune responses. For example, immunogenic viruses from the plant kingdom [73] and bacterial phages carrying TAAs [74] are being developed for tumor vaccination in humans.
Importantly, the virus particles of some virus families may by themselves induce cell death in the complete absence of replication. For instance, directly upon entry UV-inactivated avian reovirus induces apoptosis in several avian and mammalian cell lines [75]. Although the molecular basis for this phenomenon is unknown, the number of viral particles taken up and uncoated seems to play a crucial role. This in turn, puts constraints on drawing conclusions about viral oncolytic efficacy when very high MOIs are used – cell death in such cases could also result from high viral load. Also, many viral gene products are in themselves potent oncotoxic agents and may be used in conjunction with other viral vectors or as single therapeutics. For example, the 15 kDa protein apoptin encoded by the VP3 gene of the chicken anemia virus and the gene product of adenovirus E4 open reading frame 4 are both cytotoxic to cancer cells but not to normal cells [76]. Expression of apoptin by adenoviral or parvoviral vectors enhanced their oncolytic efficacy both in vitro and in vivo without increasing cytotoxicity towards non-transformed cells [77], [78].
5. If it can cause cancer, it can kill cancer
Members from an increasing number of virus families are being considered for cancer gene therapy (Table 1 ). As many human viruses have been implicated in carcinogenesis [79], the sudden “conversion” of an oncogenic virus into an oncolytic agent may seem implausible. Nevertheless, despite the continuous risk of insertional mutagenesis, leading to the unfortunate setbacks in a recent X-linked SCID gene therapy trial [80], retroviruses have not been excluded from being developed for virotherapy. In fact, significant effort is put into making these viruses safer, and recent breakthroughs with retroviral gene therapy indicate, perhaps somewhat counterintuitively, that under certain conditions genomic integration can be beneficial [81], [82]. A recent study has shown that while altering the expression of some genes in human T cells, retroviral integration did not affect T cell function or cell growth [83]. Another vehicle extensively used in cancer gene therapy, the vaccinia virus (VV), expresses the E3L protein which has been shown in some circumstances to be oncogenic [84]. Even more surprising, the polyomavirus SV40, which has been extensively used to transform different types of cells, has also recently proven efficient as a cancer targeting vector [85]. In this vector the Tag protein responsible for cellular transformation has been deleted, and although the recombinant SV40 vectors still integrate, tumorigenesis has not been observed [86]. Moreover, despite that the Epstein–Barr virus is associated with a number of different types of cancers and is therefore classified as a type 1 carcinogen, it has recently been developed into an expression vector for non-lytic tumor vaccination against B cell lymphomas [87]. The vector has been rendered safe (non-oncogenic and replication-deficient) by deleting two oncogenic genes and one gene critical for the lytic cycle of the virus. Another herpes virus with oncogenic properties, the human herpes simplex virus type 2, has recently been engineered for oncolytic cancer targeting by deleting the ribonucleotide reductase gene [88].
Table 1.
Viruses planned for or implemented in cancer gene therapy
| Genome | Family | Genus | Species/Strain/Vector |
|---|---|---|---|
| DNA | |||
| ds | Adenoviridae | Mastadenovirus | Ad serotype 5 and several derivatives in experimental settings [32], [59], [93], [94], [95], [96], [97], [102], [104], [110], [288] and in clinical trials [100], [289]. Conditionally replicating vectors based on canine adenovirus [111] |
| Aviadenovirus | Replication-deficient vector CELO [116] | ||
| Atadenovirus | The ovine adenovirus type 7 vectors OAdV623 and OAdV220 [114], [115] | ||
| Asfarviridae | n.f. | ||
| Herpesviridae | Simplexvirus | Several replication-competent vectors based on both natural and laboratory strains of human HSV-1 in experimental cancer targeting [60], [121], [124], [213] and in clinical trials [117], [119], [120]. Replication-competent vectors based on HSV-2 [88] | |
| Rhadinovirus | Replication-competent vectors based on bovine herpesvirus 4 [126] and saimiri virus [127], [128] | ||
| Varicellovirus | Replication-competent pseudorabies vectors [129] | ||
| Iridoviridae | n.f. | ||
| Papillomaviridae | n.f. | ||
| Polyomaviridae | Polyomavirus | Replication-deficient SV40 vector [85] | |
| Poxviridae | Orthopoxvirus | Replication-competent vectors based on vaccinia strains WR and Wyeth [132], [133], [134] | |
| Leporipoxvirus | Replication-competent vectors based on myxomavirus [139] | ||
| Yatapoxvirus | Replication-competent vectors based on yaba-like disease virus [138] | ||
| ds/ss | Hepadnaviridae | n.f. | |
| ss | Circoviridae | n.f. | |
| Parvoviridae | Parvovirus | Live autonomous rodent parvovirus H-1 [145]. Both live virus and replicons of minute virus of mice [131], [146]. Replicons of retargeted feline panleukopenia virus [147] | |
| Dependovirus | Replication-defective vectors based on several serotypes of adeno-associated virus [142], [143], [144] | ||
| RNA | |||
| ds | Birnaviridae | n.f. | |
| Reoviridae | Orthoreovirus | Live reovirus type 3 strain Dearing (T3D) in both pre-clinical experiments and in clinical trials [148], [149], [150] | |
| ss | − Arenaviridae | –⁎ | |
| Bornaviridae | n.f. | ||
| Bunyaviridae | –⁎ | ||
| Filoviridae | n.f. | ||
| Orthomyxoviridae | Influenza virus A | Replication-competent NS1 deleted influenza virus A [155] | |
| Paramyxoviridae | Avulavirus | Experimental therapy in several cancer models using live attenuated Newcastle disease virus strains such as 73-T, Ulster and Italien [69], [160]. Clinical trials with the live attenuated PV701 and MTH-68/H strains of Newcastle disease virus [2], [157], [159] | |
| Morbillivirus | Experimental cancer targeting with replication-competent vectors based on several measles virus strains, such as Jeryl Lynn, Moraten and Edmonston [46], [167], [168] Replication-competent measles vectors in clinical trials [169] | ||
| Respirovirus | Replication-deficient vector based on Sendai virus [63] | ||
| Rubulavirus | Live mumps virus in experimental settings [163] and in a clinical trial to treat ovarian cancer [290]. Non-oncolytic replication-competent vectors based on simian virus 5[164] | ||
| Rhabdoviridae | Vesiculovirus | Live attenuated vesicular stomatitis virus and recombinant derivatives [51], [52], [170], [171], [172], [174], [175], [176], [210] | |
| ss | + Arteriviridae | n.f. | |
| Astroviridae | n.f. | ||
| Caliciviridae | n.f. | ||
| Coronaviridae | Coronavirus | In vitro oncolytic activity of retargeted replication-competent vectors based on feline coronavirus and murine hepatitis virus [177], [178]. | |
| Flaviviridae | –⁎ | ||
| Nodaviridae | n.f. | ||
| Picornaviridae | Enterovirus | Live echovirus type 1 [40]. Live coxsackievirus A21 in experimental settings [38], [39] and in clinical trials [182]. Both a replicon vector [41] and replication-competent vectors of poliovirus type 1 [42], [179], also in a clinical trial (www.wiley.co.uk/genetherapy/clinical/). Live attenuated poliovirus [180] and bovine enterovirus [8], [183] in pre-clinical testing | |
| Unassigned | Live Seneca Valley virus SVV-001 in a clinical trial (www.neotropix.com) | ||
| Togaviridae | Alphavirus | Sindbis virus replicons [43], [186], [187] and live attenuated Sindbis virus [131], [188]. Replicons based on WT Semliki Forest virus [61], [189] and a replication-competent vector based on attenuated SFV [45] | |
| Retroviridae | Gammaretrovirus | Both replication-deficient and replication-competent MoMLV vectors [190], [191], [268], also used clinical trials [192], [193], [194] | |
| Lentivirus | Replication-deficient vectors based on HIV-1 [195], [276] | ||
| Spumavirus | Replication-competent vectors based on foamy virus [196] | ||
Listed are the virus families with a general vertebrate host range according to the 8th report of the International Committee on Taxonomy of Viruses (www.ncbi.nlm.nih.gov/ICTVdb/Ictv/index.htm) and those virus species, strains or vectors which currently are used or show promise of becoming tools for virotherapy. Although the list is by no means absolute it provides a glimpse of the extent to which the research field has expanded since the early days of gene therapy. Viruses used in the 1950s and 1960s [1] have generally not been listed unless they are still in use today. Importantly, this list shows only inherently oncolytic viruses and oncotropic vectors intended for direct tumor transduction – viral vectors which are used ex vivo, for transgene-mediated bystander killing or for tumor vaccination have been omitted. n.f. = data on the use of a member of this virus family for cancer targeting could not be found on PubMed using combinations of the virus family, species, strain or vector name and keywords such as cancer, oncolytic, virotherapy, tumor targeting and intratumoral. Underlined viruses are not oncolytic, only oncotropic.
A multitude of different viruses have been used in studies to eradicate tumors in animal models but have not been developed further for the purpose of virotherapy. In one report up to 16 different viruses from the families Arenaviridae, Bunyaviridae, Flaviviridae, Reoviridae and Togaviridae families were tested for oncolytic efficacy in 180 sarcoma and Erlich ascites tumors in mice [12]. None of these viruses, with the exception of Sindbis virus, have to our knowledge been developed for virotherapy. Although in vitro studies in the 1950s revealed that the phlebovirus (family: Bunyaviridae) Rift Valley fever virus can lyse several tumor cell types, this virus has gained no further attention as a potential virotherapeutic [291]. Another bunyavirus, the Bunyamwera virus, was used in humans in the 1950s, but like the Rift Valley fever virus it has not been used for cancer targeting since [11]. As the West Nile virus (family: Flaviviridae) used concurrently to treat patients with advanced lymphomas sometimes gave rise to encephalitis, it has largely been dismissed as a candidate for cancer therapy [3], [11]. The same is true for the flaviviruses Ilheus and Dengue, as well as other viruses [11]. A third oncolytic flavivirus, the Russian Far East encephalitis virus never reached the clinics due to lethal encephalitis in animals [292].
Thus, even oncogenic viruses may serve as tools in cancer targeting if they have been deleted for the tumorigenic gene components. The versatility of a particular virus in cancer killing is not determined by the type of genome or whether the virus is enveloped or not, but rather by advantageous phenotypic characteristics such as tropism and apathogenicity in humans.
6. Oncolytic viruses
In this chapter, we will introduce the oncolytic viruses used in the field of cancer virotherapy as well as some viruses which display significant oncotropism (Table 1). Although many of these viruses may never make it to the clinics, they may contribute to virotherapy research in other ways, e.g. by functioning as models or providing insights into the mechanisms of oncolysis. For an extensive list of ongoing clinical trials, see reference [89]. We also warmly recommend the book Viral Therapy of Human Cancers [19] by Drs. Joseph Sinkovics and Joseph Horvath and other pioneers of the field as an encyclopedic source of information on oncolytic viruses, their history, mechanisms of action and application in clinical trials. A collection of excellent reviews can also be found in issue 52 of Oncogene, volume 24, November 2005.
6.1. Adenoviruses
Adenoviruses were isolated in 1953 from human adenoid tissue samples in culture undergoing “spontaneous” regression, and were dubbed adenoidal–pharyngeal–conjunctival viruses based on their capacity to induce disease symptoms in experimentally infected humans [90]. Since then adenoviruses have become the most widely used and most extensively studied viruses for gene delivery/therapy purposes. In China, oncolytic adenovirus mutants in combination with chemotherapy have been accepted as a standard treatment form for refractory nasopharyngeal cancer.
The immediate-early genes E1 are expressed promptly upon adenovirus entry into a cell. Two genes of this group in particular have been the targets of modification in order to create tumor-specific viruses: E1A and E1B (E1B55K). Normally the products of these genes act in concert to force the host cell to enter S phase, a prerequisite for the rest of the viral replication process. Deletion of E1A will render the virus susceptible to the anti-viral mechanisms of the retinoblastoma (Rb) protein, specifically by blocking the G1 to S transition. Deletion of E1B, on the other hand, allows p53 to induce apoptosis in infected cells, aborting replication and spread of the virus. Therefore, productive replication of adenoviral E1-deletion mutants can only take place in cells deficient in Rb and p53. Most gliomas fulfill these requirements and thus become ample targets for selective oncolysis by adenoviral E1-deletion mutants [91].
An example of an E1B-negative adenovirus (dl1520) is the Onyx-015 vector, which is frequently used in both experimental and clinical gene therapy [47], [58], [59], [89], [92], [93], [94], [95]. However, the specificity of E1B-deletion mutants to p53-negative cells is not absolute, as the these viruses still express the E1A protein which can override the block imposed by p53 in infected cells, eventually causing apoptosis even in normal cells. Also, the p53 status of the cell is not a reliable marker for permissiveness to E1B-deleted adenoviruses. In fact, the ability of such adenoviruses to replicate rather seems to be dependent on the nuclear RNA export machinery of the cell which is different in cancer cells compared to normal cells [32]. The lack of E1B can be compensated by drugs or hyperthermia, enhancing the replication of E1B-deleted adenoviruses. Elevated temperature does not predispose normal cells to the enhanced viral replication, but rather diminishes it [31], [32].
E1A-deletion mutants, such as Δ24 (dl922–947), have shown superior oncolytic efficacy compared to E1B mutants both in vitro and in vivo [96], [97]. To further enhance the oncolytic efficacy of E1A mutants they can be engineered to express functional p53, such as the commercial vector ADVEXIN™ (Introgen Therapeutics Inc.), which enhances late stage replication in p53-deficient tumor cells by inducing apoptosis and facilitating the release of new virions [98], [99]. This strategy is being implemented in several clinical trials [100]. However, since primary tumors often express low levels of the primary adenovirus receptor-the coxsackie-adenovirus receptor (CAR)-and since the oncolytic efficacy of adenoviruses directly correlates with the expression level of this receptor, adenoviruses may be limited in their tumor targeting potential [101], [102]. Therefore, several retargeting strategies have been developed. For example, the incorporation of tumor-targeting peptides into the fiber protein of adenoviruses has been shown to increases both the specificity and oncolytic efficacy in glioma cells [103], [104]. Similarly, placement of an RGD-motif into the fiber knob of a replication competent adenoviral vector dramatically enhanced its oncolytic efficacy in A549 human lung carcinoma xenografts in nude mice [105]. Moreover, a tremendous potential to retarget adenoviruses is provided by the possibility to replace the native fibers with the fibers from the more than 50 human adenovirus serotypes and several non-human adenoviruses identified so far. Human adenoviral vectors with fiber proteins from their canine or ovine counterparts show an expanded tropism in vivo, alleviating the strict dependence on the CAR receptor [106], [107], [108]. Exchanging the fiber protein from the human adenovirus type 5 to that from type 35 converts the tropism of adenoviral vectors to using CD46, often abundant on tumor cells compared to normal cells [109]. For an extensive review of adenoviruses, particularly their retargeting strategies, see [110].
The currently employed adenoviruses in clinical trials are all based on human adenovirus serotype 5, although they employ different modes of tumor selectivity. Several other adenoviral vectors have been developed. For example, although not specifically developed for human use, canine adenoviral vectors have been used in mouse models for cancer with promising results [111]. Further, vectors based on both porcine and bovine adenoviruses are available, showing efficient transduction of many human cell lines [112], [113]. The ovine adenovirus type 7 vectors OAdV623 and OAdV220 have been tested in animal models of cancer with good results [114], [115]. Finally, a recombinant vector based on the avian adenovirus chicken embryo lethal orphan (CELO) virus and expressing TK displayed (in combination with relevant prodrugs) modest oncolytic capacity in several human cancer cell lines in vitro and prolongation of animal survival in an immunocompetent mouse model for melanoma [116]. As with most non-human adenoviruses, CELO does not replicate productively in human cells and is therefore replication-deficient. It is possible to utilize these non-human adenoviruses in translational research and in the future they may be applied in clinical settings in humans.
6.2. Herpesviruses
Vectors based on herpes simplex virus (HSV)-1 are, together with the adenoviruses, furthest along in their development and testing for virotherapy. To date, a plethora of HSV vectors is available and the number of both preclinical studies and clinical trials is rapidly increasing [117], [118], [119]. The first replication-competent vector, dlsptk, for cancer therapy (malignant glioma) based on HSV-1 F strain was described in 1991 [60]. This vector was deleted in the thymidine kinase (TK) gene but was shown to be oncolytic to human glioma xenografts in nude mice. However, although TK-negative HSV vectors are attenuated in normal cells, the absence of this gene eliminates the possibility to use commercial anti-herpetic drugs to control the infection. Therefore, most HSV vectors in therapy use today are instead deleted for the main neurovirulence gene γ34.5, which severely restricts their ability to replicate in the adult central nervous system (CNS) and to form latency. Indeed, γ34.5 deletion-mutants have been the first HSV vehicles to enter clinical trials [120]. However, in order to avoid the generation of wildtype (WT) HSV, the so called second-generation vectors have been deleted in several genes. Moreover, as it has become clear that deletion of the γ34.5 gene also reduces replication efficiency, modifications restoring the efficiency have been introduced. For example, the third-generation vector G47Δ has been deleted for the ICP47 gene, and shows increased replication efficacy and oncolysis in animal models compared to the parental G207 vector [121]. Deletion of the ICP47 gene also reduces viral downregulation of MCH class I molecules and thus enhances vector immunogenicity. To date, five replication-competent HSV-1 vectors have entered clinical trials: (i) the first-generation vector 1716, a recombinant based on HSV-1 strain 17 and deleted for the γ34.5 gene, (ii) NV1020 (formerly R7020), a chimeric second-generation recombinant of HSV-1 and HSV-2 with deletions in the UL24 and UL56 genes and in one copy of the γ34.5 gene, (iii) the second-generation vector G207 deleted in the ICP6 gene (ribonucleotide reductase) and both copies of the γ34.5 gene, (iv) OncoVEX™ (GM-CSF) (BioVex, www.biovex.com), a recombinant vector deleted in the γ34.5 gene but engineered to express higher levels of US11 which compensates for the reduced replication efficiency, and (v) the HF10 vector, a multiple mutation recombinant HF strain of HSV-1 [117], [122]. Most of these vectors have proven safe, and phase II studies are ongoing.
In addition to the modified vectors described above, HSV variants with full replication efficiency have been generated by serial passage on human cancer cells in cell culture [123]. Also, as most recombinant HSV vectors are derived from the laboratory strains F or 17, they are less cytotoxic than clinical isolates due to culture adaptation. In this respect, a recombinant vector based on a clinical isolate of HSV-1 (strain JS1) recently showed enhanced oncolytic capacity compared to conventional HSV vectors, and due to ICP47 gene deletion it also elicited protective anti-tumoral immune responses [124]. Furthermore, the oncolytic capacity of many HSV vectors has been augmented by various inserts. An extensively explored strategy has been to produce cytokines and chemokines (e.g. IL-12 or GM-CSF) from the vectors to stimulate immune recognition and to establish tumor immunity [118]. Another strategy has been to express FMGs by HSV vectors, but it has even been possible to screen for recombinant HSV viruses with intrinsic membrane fusogenic properties by randomly mutating the (G207) viral backbone. Combining this built-in ability with the expression of foreign FMGs, a strategy used in the novel Synco-2D vector, has enhanced the oncolytic capacity even further [125].
Many vectors exist based on other members of the herpesviridae family. Notably, since also herpes simplex type 2 is a human pathogen, interest in this virus has resulted in its development into an oncolytic vector [88]. This construct, named FusOn-H2, was created by deletion of the ICP10 ribonucleotide reductase gene, corresponding to the ICP6 gene in HSV-1, which abrogates the tumorigenic potential of the vector and confers syncytia-forming properties. Indeed, when compared to the above mentioned Synco-2D vector and to Baco-1, another oncolytic vector based on HSV-1, FusOn-H2 demonstrated superior efficacy even after intravenous (i.v.) administration in a nude mouse model of human breast cancer. Making use of the natural tropism of Epstein–Barr virus (EBV), a recombinant non-oncolytic vector based on the prototype strain B95.8 was recently created for targeting of B-cells which are otherwise notoriously difficult to transduce [87]. This vector, expressing GM-CSF, was able to induce maturation of DCs in vitro and to induce specific T-cell activation, and is thus being considered for vaccination against B-cell lymphomas. Furthermore, it was recently shown that replication-competent bovine herpesvirus 4 was oncolytic for several cancer cell lines in culture and destroyed human A549 lung carcinoma xenografts in nude mice [126]. The observed apoptosis was shown to be dependent on the expression of viral genes. In addition to conventional HSV vectors, vehicles based on saimiri virus, the prototype of the γ-2 subfamily of herpes viruses, have shown promise of becoming new virotherapeutic agents. These viruses can persist non-lytically in T-cells, and when engineered to express TK, they have shown significant anti-tumoral efficacy in animal models of leukemia [127]. Saimiri virus, and also pseudorabies virus (genus varicellovirus) which is currently being developed to a cancer targeting tool, is oncolytic in some cancer cell lines but strictly non-lytic in others [128], [129].
Non-oncolytic amplicon vectors based on the roseoloviruses human herpesvirus 6A and 6B and 7 are being developed for tumor vaccination [130]. HHV-6 infects lymphocytes and macrophages via the CD46 receptor and HHV-7 shows strict CD4 T-cell tropism. Similar vectors based on the human cytomegalovirus have been created, and although the oncolytic capacity of the cytomegaloviruses is unknown, at least murine cytomegalovirus displays a remarkable tumor-controlling activity termed apoptotic crisis. This activity functions to induce cell death in distant lymphoma tumor nests without direct infection of the tumor cells [57]. The possibility to use this effect in anti-tumor therapy has been proposed. Finally, vectors based on equine herpesvirus 1 have recently been constructed, but these have not yet been tested for cancer targeting potential.
Taken together, the main advantage of herpesviral vectors is their capacity to carry large transgenes, exceeding 150 kb in some settings, while the main drawbacks include difficult cloning (even using new BAC-systems), safety issues such as neurotoxicity at high virus doses, the possible oncogenicity, and the risk of recombination with or activation of endogenous herpesviruses.
6.3. Polyomaviruses
Polyomaviruses are double-stranded DNA viruses with a relatively small (∼5.3 kb) circular genome. Recombinant polyomavirus vectors based on the SV40 virus have been around since the turn of the millennium, and although inherently non-lytic, Cordelier et al. [85] recently demonstrated significant tumor-specificity in vitro and growth inhibition in a mouse model of pancreatic cancer using tumor-specific promoters to drive the expression of an inhibitory protein from such a vector. In another study an SV40 vector was unable to transduce two human glioma cell lines [131]. The main advantages of polyomavirus vectors are their remarkable ability to escape immune recognition, i.e. they do not evoke a neutralizing antibody response, and durable transgene expression [86].
6.4. Poxviruses
Poxviruses are large, enveloped, dsDNA viruses with a complex genome harboring multiple immune-modulating genes. The most commonly used poxvirus in cancer targeting is vaccinia virus (VV). As the wildtype strain WR causes local tissue destruction (necrosom), the derived vectors have been attenuated by removal of thymidine kinase or other genes [132], [133]. Many VV vectors efficiently kill tumors in preclinical settings. As an example, the novel replication-competent vector JX-594 based on strain Wyeth showed impressive oncolysis of tumors and metastases in syngeneic models in both rats and rabbits upon i.v. administration [134]. However, although replication-competent vectors have been able to enhance the survival of both immunodeficient and immunocompetent mice carrying different types of tumors, significant infection of other organs has also been observed, indicating that VV vectors still need to be optimized [132].
Given the history of VV as the vaccine vector against smallpox, the most common anti-cancer strategy in clinical trials has been tumor vaccination or transfer of immunoregulatory genes [135], [136], [137]. Besides virus preparations such as PANVAC™ or TRICOM™, also non-oncolytic fowlpox virus (avipox genus) has been used in these trials. Cancer cell death in response to VV occurs both by apoptosis and other mechanisms, and a clear advantage of the vectors based on this virus is their well-established safety profile in humans. However, in order to avoid possible pre-existing host immunity also other members of the poxviridae family have been considered for cancer therapy. For instance, the yaba-like disease virus (YDV) has shown oncolytic potential both in vitro and in vivo [138]. Similarly, recombinant vectors based on myxomavirus, previously thought to infect only rabbits, were recently shown to efficiently kill several human glioma cell lines and primary glioma explants in vitro and to markedly prolong the survival of mice in two human glioma models [139]. Although these viruses replicated in vitro they were unable to spread to neighboring tumors in the other brain hemisphere. The oncolytic or oncotropic potential of other poxviruses, such as the Orf virus, have not been explored.
6.5. Parvoviruses
Parvoviruses are non-enveloped single-stranded DNA viruses (∼5 kb genome). The family includes the helper-dependent viruses (dependoviruses), including the adeno-associated viruses (AAV), which require molecular functions supplied in trans via co-infection with herpes- or adenoviruses, whereas the autonomous parvoviruses can replicate with the help of cellular factors. Of these, only the autonomous parvoviruses are oncolytic, although both display oncosuppressive effects. For example, parvoviruses can inhibit the transforming capacity of other oncogenic viruses [140], and have been shown to suppress the proliferation of some cancer cell lines by inducing cell cycle arrest and terminal differentiation [141]. The non-structural proteins of parvoviruses can cause epigenetic modifications in cancer cells and revert them to a benign phenotype [55]. Thus, anti-tumor efficacy by these viruses may involve both oncolysis and tumor suppression/reversion [54].
Vectors based on several members of the dependoviruses have been used for cancer targeting [142]. In a recent study the tumor-transduction efficacy of five different AAV strains was compared, and serotype 2 proved to be the most efficient killer of solid tumor cells [143]. In another study, replication-deficient vectors based on AAV type 8 expressing anti-angiogenic soluble VEGF receptor were able to significantly halt tumor progression in several rodent models of glioblastoma and were more efficient than vectors based on serotype 2 [144]. Dupressoir et al. [145] demonstrated the oncolytic effect of the autonomous rodent parvovirus H-1 in a mouse model of mammary cancer, both when given i.v. or when mixed with the tumor cells. The virus used did not cause tissue damage and its replication in non-transformed cells was considerably attenuated, providing an important safety aspect. Similar results were reported by Dupont et al. [146] who used recombinant minute virus of mice. The virus infected a panel of cancer cells much more efficiently than normal cells. Recently, Wollmann et al. [131] demonstrated variable permissiveness of human glioma cell lines to two strains of the minute virus of mice. Finally, parvovirus types unable to infect human cells can be used to target human cancer cells by capsid retargeting. For example, a modified feline panleukopenia virus is currently being tested for feasibility as a cancer targeting tool [147].
6.6. Reoviruses
Reoviruses are very common in the human respiratory and gastrointestinal tract (up to 100% seropositivity in the adult population, not associated with any disease). While attenuated in healthy tissue, reoviruses are inherently oncolytic and show high tumor-specificity upon remote administration. For example, marked inhibition of tumor growth and prolonged survival of immunocompetent mice was observed upon multiple i.v. injections in a model of lung-metastasizing mammary cancer [148]. In this study, the oncolytic efficacy was reduced in pre-immunized animals, indicating that pre-existing immunity poses an obstacle with this virus, but combining treatment with immunosuppression overcame the restriction. In another study, reovirus administered intracranially (i.c.) was able to eradicate breast cancer metastases in the CNS of nude mice and to prolong survival of immunocompetent rats with leptomeningeal breast cancer metastases when administered intrathecally [149]. The replication of these double stranded RNA viruses is inhibited in normal cells by the cellular anti-viral defense machinery, which is activated by recognition of the viral genome by the enzyme PKR. In many cancer cell types, however, reoviruses are able to replicate unhindered due to inactivation of PKR by the oncoprotein Ras. The main advantage of live reovirus is its well-established safety profile in man, and reovirus type 3 (Dearing strain) is currently being used in clinical trials (Reolysin™, Reosyn™, www.oncolyticsbiotech.com). For more information about reoviruses in cancer targeting, see [150].
6.7. Orthomyxoviruses
Influenza viruses were used for experimental cancer therapy already in the 1950s [151] and later as oncolysates in clinical trials [22]. However, influenza viruses and cancer may have a more complex relationship, as patients with different types of cancer are highly susceptible to infection with influenza virus or other respiratory viruses, particularly if under immunosuppression, yet such patients do not show increased incidence of tumor regression, nor has vaccination of cancer patients against influenza been reported to inhibit tumor progression [152], [153], [154]. In contrast, vaccination against influenza has been shown to provide protection against certain types of cancer later in life [24], [25], [26]. The molecular basis for this phenomenon remains unclear.
The NS1 protein of influenza virus inhibits the activity of PKR in target cells, which in turn permits viral replication. PKR is also inhibited by Ras in many tumor cell types, which thereby constitute preferential targets for infection by NS1-deleted influenza virus. In a recent study, Bergmann et al. [155] reported on the utilization of a recombinant NS1 deletion mutant of influenza virus A strain PR8 that efficiently lysed Ras-expressing cells, both in vitro and in a subcutaneous tumor model in SCID mice. As expected, normal cells were shown to be resistant, constituting an important safety aspect.
6.8. Paramyxoviruses
This vast group of viruses includes prototypic members such as measles virus, mumps virus and the Newcastle disease virus (NDV), all of which have been extensively used in cancer targeting. Although still being investigated, the specificity of these viruses to tumor cells likely stems from the inability of tumor cells to respond to type I interferons and to mount an anti-viral defense [49]. The history of NDV as an anti-tumor agent in humans began in 1965 with a clinical trial led by Dr’s Cassel and Garrett who used the live attenuated 73-T strain to treat a patient with cervical carcinoma [156]. Subsequently, NDV has been used both as oncolysate and live virus in several clinical trials. As an example of the latter, in one study a purified poultry vaccine dubbed MTH-68/H based on live attenuated NDV was administered i.v. to a total of 14 patients with grade IV glioblastoma multiforme since 1996 [157]. Of these patients, five died of the cancer, two died of other causes and the remaining seven were alive at the time of the publishing of the paper in 2004. Four of these patients who had been alive for 5–9 years had received i.v. virus as their sole form of treatment. While these anecdotal results await confirmation by other independent laboratories using proper controls, they strengthen the supposition that multiresistant brain tumors may be treated by peripheral administration of a tumor-homing, replication-competent oncolytic virus.
A Phase I/II clinical trial using i.v. administration of another live attenuated neurotropic NDV strain, NDV-HUJ, to treat glioblastoma and three phase I trials using the live NDV vector PV701 to treat patients with multiple types of cancers were recently completed [158], [159]. Despite that none of the 11 assessable patients in the NDV-HUJ trial or the altogether 113 patients in the three PV701 trials had durable responses, the viruses were well tolerated (less adverse events, including fever, in the NDV-HUJ trial than the PV701 trial), reaffirming the safety of NDV in humans. Moreover, in some cases tumor responses could with likelihood be ascribed to the virus treatment, which has warranted the continued development of these viruses for virotherapy. The NDV-HUJ virus is classified as being lentogenic, i.e. avirulent in poultry and not capable of producing infectious progeny in most tissues, whereas the PV701 strain and the other oncolytic NDV strains MTH-68/H and 73-T are considered mesogenic (moderately virulent in chickens). Over the years, the 73-T strain has also been used in humans in the form of oncolysates to target melanoma [16], [17], [18]. In a 20-year follow-up, the overall survival rate of 81 patients who had received the immunotherapy in addition to lymph node resection was 63% (see Chapter 9, Ref. [19]). The 73-T strain is perhaps the most potently oncolytic of the NDV strains studied to date [2], and has also re-emerged in experimental cancer treatment studies [69]. Additionally, a number of other strains, including Italien and the non-oncolytic lentogenic Ulster strain, have been used to target cancer cells both in vitro and in vivo with high specificity [160]. It has been reported that tumor vaccination using mixtures of non-oncolytic NDV and tumor cells may be more efficacious than using oncolysates [161]. Based on results from the numerous animal experiments and clinical trials conducted it seems NDV is an extremely safe oncolytic agent. For an excellent review of the oncolytic strains of Newcastle disease virus, see [2].
Mumps virus (genus rubulavirus) was also among the first paramyxoviruses to be tested in humans. Asada [6] reported partial or complete responses in 37 cases using the Urabe strain to treat 90 patients with different malignancies. Later this virus was used as oncolysate to treat a variety of cancers [23]. However, since the live Urabe vaccine strain causes CNS complications in up to 1% of vaccinated individuals, more attenuated strains have been developed, the newest not yet tested in cancer targeting [162]. One of the characterized mumps viruses, the live attenuated vaccine strain S79, shows promise as an oncolytic vehicle based on its selective infection of cancer cells in vitro and significant tumor inhibition in nude mice [163]. Another rubulavirus, simian virus 5, has been engineered for cancer targeting [164]. Although inherently non-cytolytic, this virus was shown to kill several cancer cell types in culture via production of HSV TK followed by treatment with ganciclovir or acyclovir.
There have been reports of regression of Hodgkin’s disease after a natural measles infection [165]. Inspired by this, measles virus has been used to target lymphoidic cancer cells by exploiting its natural tropism for CD150 or SLAM. Both the parental virus and a recombinant vector based on the Edmonston B strain (a vaccine strain that was originally converted less pathogenic to humans by passage in tissue culture) were able to inhibit the growth of human lymphoma xenografts in SCID mice by either i.t. or i.v. injection [166]. Later, Peng et al. [167] demonstrated the feasibility of using a recombinant measles vector to preferentially infect and destroy human epithelial ovarian cancer cells in vivo via CD46, the main receptor for the attenuated vaccine strains of measles virus. In this study the virus was able to significantly prolong the survival of the treated mice. Moreover, a recombinant vector based on the Edmonston virus and engineered to express carcinoembryonic antigen caused marked regression of i.c. U87 glioma xenografts in nude mice [168]. This vector has entered a phase I clinical trial to treat glioblastoma multiforme. Recently, the oncolytic efficacy of the culture-adapted Moraten vaccine strain and the live attenuated Jeryl Lynn strain of mumps virus were compared to the Edmonston vaccine strain both in vitro and in vivo [46]. Results revealed that while the replication kinetics of the viruses differed in cultured cells, all three showed similar oncolytic efficacy upon i.p. administration in nude mice harboring human ovarian cancer xenografts. These strains are included in approved commercial vaccines and therefore have undergone comprehensive testing in humans certifying their safety. Other measles vectors are being used in clinical studies to target ovarian cancer, myeloma and cutaneous T-cell lymphomas (www.wiley.co.uk/genmed/clinical; [169]).
Finally, a recent study describes the utilization of a vector based on Sendai virus (genus: respirovirus) to infect and kill several different cancer cell types in vitro as well as to eradicate human fibrosarcoma and human colorectal adenocarcinoma xenografts in nude mice [63]. An interesting property of this vector is that despite non-productive infection it can spread efficiently by cell-fusion mediated by the paramyxoviridae fusion glycoproteins. Altogether, many paramyxoviruses display remarkable potential as anti-cancer agents. In all cases mentioned above, oncolysis has required viral replication as UV-inactivated viruses have not mediated cell death.
6.9. Rhabdoviruses
The most extensively tested and so far the only rhabdovirus in cancer targeting is the vesicular stomatitis virus (VSV; genus: vesiculovirus), which is considerably attenuated in humans. Particularly since the development of recombinant VSV vectors by two independent groups in 1995 has interest in this virus as a potential virotherapeutic exploded [170]. In a recent study it was shown that i.v. administration of replication-competent VSV to immunocompetent rats with hepatocellular carcinoma significantly prolonged survival [171]. Ectopic expression of a fusogenic mutant F glycoprotein of Newcastle disease virus enhanced the oncolytic potency and extended animal survival compared to treatment with WT VSV [172]. In another study, replication-competent VSV engineered to express either IL-4 or HSV TK killed syngeneic breast carcinoma and melanoma in immunocompetent mice more efficiently than WT virus [173].
The tumor-specificity of VSV, as in many other RNA viruses, is largely determined by the inability of cancer cells to mount an effective anti-viral response due to defects in type I interferon signaling pathways [51]. However, recombinant VSV is still able to infect and kill normal fibroblasts and cause neurotoxicity at high doses, which may pose a problem in human use, and therefore prophylactic treatment with interferon to restrict the spread of VSV in normal cells has been considered [131], [174]. Another strategy has been to mutate the matrix (M) protein, which causes VSV to elicit a stronger IFN response and restricts its replication exclusively to cells with impaired IFN-signaling [52]. A recombinant VSV vector, VSVΔM51, with a single amino acid mutation in the M protein has recently been used to target experimental human glioma xenografts in nude mice and syngeneic breast cancer metastases in adult Balb/c mice [175], [176]. In these studies, the mutant virus was well tolerated when given i.v. and was able to cause marked prolongation of animal survival in both models. However, despite that the maximum tolerated dose of the mutant virus in the Balb/c mice was 100-fold higher compared to WT VSV, VSVΔM51 was lethal to nude mice when given i.c., showing that other mechanisms than susceptibility to type I interferons govern the neurotoxicity of recombinant VSV. Similarly to inactivated paramyxoviruses, dead rhabdovirus particles do not induce cell death. An excellent review of VSV as an oncolytic agent is provided in Ref. [170].
6.10. Coronaviruses
Coronaviruses, obtaining their name by their appearance under the electron microscope, are common respiratory pathogens of mammals and birds. Although human coronaviruses await conversion into cancer targeting vectors, non-human coronaviruses, including feline coronavirus and murine hepatitis coronavirus A59, display significant oncolytic activity in human cancer cells in vitro [177], [178]. The tropism of these viruses, however, has been changed, which is necessary to achieve infection as non-human coronaviruses do not normally infect human cells. Viral tropism is thus determined solely on the basis of expression of the proper viral receptors on the cell surface; once inside the cells non-human coronaviruses are capable of initiating productive replication. This is the complete opposite to the avipoxviruses which readily infect human cells but do not multiply in them.
6.11. Picornaviruses
Several members of these positive strand RNA viruses are being developed into tools for virotherapy. Poliovirus infects a wide variety of human cancer cell lines and primary explants [41]. By attenuation of a neurovirulent poliovirus strain by replacement of the viral internal ribosome entry site (IRES) with the corresponding sequence from the closely related human rhinovirus type 2, Gromeier et al. [179] were able to obtain a highly efficacious recombinant virus, PV1(PVS)-RIPO, which displayed significant tumor tropism and oncolytic potential in subcutaneous and i.c. human astrocytoma xenografts in nude mice. Whereas the subcutaneous tumors were efficiently eradicated upon a single i.v. administration, the brain tumor xenografts responded only to i.t. injection despite that the vector was neurotropic and replication-competent. In another study, this virus was able to cause complete tumor regression in a small number of athymic rats harboring i.c. human glioma xenografts upon intrathecal administration. In addition to the recombinant vectors, a live attenuated strain of poliovirus 1 has shown promise as an oncolytic agent [180]. Such neurotropic and attenuated viruses are good candidates for therapy of brain tumors. Indeed, the RIPO vector has entered a phase I clinical trial to treat malignant glioma.
Coxsackievirus was recognized as an anti-neoplastic agent in the 1950s and was tested for oncolytic efficacy in animal models [181]. Today, oncolytic vectors based on coxsackievirus A21 are available [38], [39], and phase I studies in targeting melanoma with this virus have been completed (results not yet published) [182]. Another oncolytic picornavirus tested in the 1950s, bovine enterovirus, has gained renewed attention as a potential virotherapeutic [8], [183]. This virus shows oncolytic potential towards a variety of human cancer cell types, including some of lymphoid origin. Moreover, echovirus type 1 was recently shown to harbor a strong tropism for human ovarian cancer cells [40]. In this study, tumor-free survival of SCID mice bearing multiple subcutaneous xenografts was observed for up to 15 weeks following a single i.t. injection of the virus into just one of the xenografts. Finally, live attenuated Seneca Valley virus SVV-001 (genus unassigned) has entered phase I studies to treat carcinomas of neuroendocrine origin (Neotropix, www.neotropix.com).
6.12. Togaviruses
During the 1950s when the first wave of interest in viruses as anti-cancer agents was peaking, also members of this family were considered. For example, an attenuated variant of the Trinidad donkey vaccine strain of Venezuelan Equine encephalitis virus (VEE), TC-80, caused an objective tumor response in four patients with moderately aggressive lymphomas [184]. Although recombinant VEE vectors have been available since the early 1990s and several studies in cancer vaccination using VEE replicons are ongoing, no reports on this virus in oncolysis have emerged since. Like many other RNA viruses, alphaviruses are highly sensitive to the anti-viral action of type I interferons and defects in the IFN-signaling enables more effective infection of cancer cells [185]. Tumor-specificity is also enhanced by the selective upregulation of alphaviral receptors, such as laminin, on cancer cells [43].
Two prototype members, Sindbis virus and Semliki Forest virus (SFV) both display prominent oncolytic potential. Sindbis virus replicons expressing reporter protein were shown to kill several cancer cell lines in vitro and to cause regression of several types of tumors in SCID mice upon repeated i.p. administration [186]. Expression of IL-12 from these vectors enhanced the anti-tumor efficacy, and in subsequent studies in immunocompetent mice the same vectors were able to reach disperse tumors upon systemic administration [43], [187]. Despite the oncolytic power of the replicons, however, the tumors eventually regrew, indicating that replicon-based therapy suffers from limited transduction regardless of repeated injections. In this respect, live Sindbis virus vectors have recently been introduced. A replication-competent vector based on the avirulent AR339 strain was shown to suppress tumor growth and prolong the survival of nude mice with both subcutaneous and metastasizing tumors, even following a single systemic injection [188]. In another study, a live Sindbis vector displayed superior efficacy and specificity to several other viruses [131].
Vectors based on SFV show similar properties to Sindbis virus. An SFV replicon was more efficient than a first generation adenoviral replicon in eradicating subcutaneous MC38 colon carcinoma tumors in immunocompetent mice [189]. Additionally, expression of IL-12 from an enhanced version of the replicon significantly improved the oncolytic capacity of the vector and it was possible to achieve oncolysis in pre-immunized animals, albeit at 20% lower efficacy compared to naïve mice. In contrast, in a study by Smyth et al. the oncolytic efficacy of SFV was clearly enhanced in pre-immunized mice compared to naïve animals, demonstrating that alphaviruses may also serve as an adjuvants in treatment of established tumors in vivo [61]. Similar to studies with Sindbis virus, repeated administrations of SFV did not reduce the efficacy of the treatment nor bring adverse effects. Although replication-competent SFV proved more efficacious than the corresponding replicon particles, tumors regrew once the treatment was stopped. Moreover, the replication-competent virus in this study was based on a moderately virulent strain of SFV and is thus less likely to be used in humans than an avirulent strain. Our own experiments showed that a replication-competent vector based on the avirulent A7(74) strain of SFV was able to destroy several cancer cell lines in vitro as well as human melanoma xenografts in SCID mice upon systemic administration [45]. Despite prominent homing to the tumors, the vectors retained their inherent neurotropism and established a persistent infection in the brains of the mice. Neurotropism may serve as an asset in targeting CNS tumors which are notoriously difficult to reach by surgery.
6.13. Retroviruses
Members of this virus family have been extensively used in different gene therapy applications. Perhaps the most frequently used retrovirus to target cancer is the Moloney murine leukemia virus (MoMLV). Due to the lack of active nuclear transport of the viral genome, all gammaretroviruses, including MoMLV, are unable to transduce non-dividing cells, which can be considered an important safety aspect. Complete transduction of human U87 glioma xenografts in nude mice was reported following a single i.c. administration of a replication-competent MoMLV vector [190]. Gliomas appearing at sites remote from the primary inoculation spot also stained positive for the viral envelope, suggesting that replication-competent retrovirus can be used to transduce tumors of the CNS with high efficiency. Furthermore, virus was not detected in any non-tumor tissue which demonstrates its specificity. In another study, replication-competent MoMLV vectors expressing HSV thymidine kinase (TK) were able to sensitize syngeneic glioblastoma cells in Lewis rats to ganciclovir and achieve up to 20% long-term (40 days) survivors [191]. Notably, this effect was not seen using replication-deficient vectors. Retroviruses have been used in clinical trials to treat cancer, but data from these studies suggest that the dissemination of the vectors in solid tumors needs to be improved in order to reach clinical efficacy [192], [193], [194].
Besides the gammaretroviruses, vectors based on lentiviruses have also been used to target cancer in pre-clinical experiments. For example, using equal amounts of infectious virus, VSV-G pseudotyped lentiviral vectors were shown to transduce a wide variety of tumor cells almost 10 times as efficiently as vectors based on adenovirus serotype 5 [195]. Like their retroviral counterparts, lentiviruses are not oncolytic. However, they do have the capacity to transduce both dividing and non-dividing cells, which may be advantageous under certain circumstances. In contrast to both gammaretroviruses and lentiviruses, the spumavirus foamy virus shows intrinsic oncolytic capacity. In a recent study, replication-competent vectors based on the prototype strain of this virus were able to control subcutaneous U87 tumors in nude mice for up to 25 weeks [196]. Although the vectors were observed to integrate in normal cells throughout the body of the infected mice, foamy virus vectors have in another study been shown to integrate in a pattern different from other retroviruses, with no apparent preference for active gene areas, and may thus be safer than these viruses in gene therapy applications [197].
7. The relevance of animal models in the study of oncolytic viruses
Despite a prominent increase in cost, anti-cancer drugs approved in 1995–2000 do not show increased efficacy in providing cures from cancer [198]. In fact, while many exciting breakthroughs have been achieved in the laboratories, survival rates for the most common types of cancer have barely budged since the 1950s [21]. Particularly in treatment of metastatic cancers have the conventional modalities (chemo- and radiation therapy and surgery) failed. Due to these reasons, complementary and alternative medicine is gaining support, and virotherapy is asserting itself as a potential treatment option alongside the conventional therapies. However, apart from special cases or anecdotal reports, successful translation into the clinics of virotherapy is also lacking.
A natural explanation for the discrepancy between results from animal studies and the clinics is the difference in host physiology, which may severely confound the assessment of the safety and efficacy of viruses in humans. For example, the pathways of complement activation are different in humans and in mice [199]. Another reason is that many human viruses do not replicate well in murine cells, and differences may even exist between rodent species. For example, experimental data suggests that VV, VSV and avirulent SFV replicate better in human than in mouse cells [8], [8], [45], [134], [175]. Andreansky et al. [200] reported that while glioma cells of both mouse and human origin were permissive for vectors based on HSV-1, glioma and other types of cancer cells from rats were not. Likewise, parvoviral strains show strict host-dependence, exemplified by the rat H-1 parvovirus which does not replicate in mouse cells, making analysis of tropism difficult in mouse models of human cancer. Unpublished observations by Cornelis et al. [54] reveal that the H-1 virus shows greater oncolytic efficacy against human cancer cells than does the prototypic minute virus of mouse (MVM) against the murine counterparts, supporting the development of the H-1 for human cancer therapy but making direct comparisons between these viral strains difficult based on studies in mouse models. Further, a common misconception is that SCID mice lack all capability to respond to viruses. In fact, despite being severely immunocompromised, SCID mice can mount an innate immune response including cytokines, complement, macrophages, NK cells and granulocytes. It was shown that neutrophils contribute significantly to the oncolytic efficacy of a recombinant attenuated measles virus vector expressing GM-CSF in SCID mice harboring human lymphoma (Raji cell) xenografts [201]. Also, the use of nude mice as a model for human cancer, although informative, has not correlated well with the therapeutic efficacy obtained in man [202]. The role of the subcutaneous xenograft model in immunodeficient mice (nude or SCID) should not be emphasized beyond its indicative value.
As a measure of safety, animal models may not always be reliable. Indeed, the fact that many oncolytic viruses employed today elicit pathology in animals has not prevented them from reaching the clinics. This may not be surprising, considering the extent of information available on the pathogenesis of some of the oncolytic viruses in humans. The vesicular stomatitis virus, a well known human pathogen which does not cause extrovert symptoms in man, gives rise to progressing neuropathology in adult mice with fatal outcome [203], [204]. Reovirus type 3 constitutes a similar contradiction, causing a fatal infection in immune-deficient mice called the black foot syndrome, but no disease in man. In addition, strain-dependent differences within virus families may muddle the assessment of pathogenicity in humans based on study of a single member of the family. For example, whereas the Indiana strain of VSV is lethal to many rodents, the New Jersey strain is considerably attenuated at least in adult hamsters [205]. The VSV strains currently used in virotherapy of cancer are more attenuated still, carrying several point mutations that enhance the interferon response towards these viruses and render them safe in humans [52].
In order to solve the problem of interspecies differences, new animal models are being developed. For instance, human poliovirus infection is restricted to cells expressing the CD155 receptor, and as rodents do not express this receptor CD155 transgenic mouse models have been developed to replace the neurovirulence tests in Cynomolgus monkeys [206]. Similarly, transgenic models have been developed to assess the pathogenicity and tropism of measles virus which normally does not infect mice [207]. The same is true for adenoviruses, which display strict host-dependence and rarely infect cells of another species, although expression of the early genes and cytotoxicity does occur. Several murine cancer cells have been shown to be permissive to human adenoviruses, facilitating the development of syngeneic immunocompetent tumor models [208].
It is likely that animal models will always function as the gateway to clinical trials and thus cannot be dismissed. Instead, they should be improved upon and utilized to maximal degree. As recurrence is the most common outcome of cancers with a poor prognosis, animal studies should be carried out to their full extent and not be arbitrarily stopped before regrowth is likely to appear. In all too many experimental studies is this crucial aspect ignored, despite that it would not be too difficult to follow the treatment out. Also, as metastasizing disease appears to be the real culprit, effort is being put into developing models for disseminated cancers and those already available will hopefully be used to a greater extent.
When used properly, animal models may provide invaluable clues about the interactions between viruses and tumors in a living host, and while not necessarily predictive in final therapeutic efficacy, in all cases has virotherapy translated in the same direction in animals as in humans (i.e. therapeutic in animals is therapeutic in humans). This is in contrast to for instance drugs developed for multiple sclerosis where the notorious anti-IFN-γ and anti-TNF-α treatments are ameliorating in the animal model EAE but worsen the human disease. Nevertheless, while the efficacy of virotherapy may be lacking, the principle is sound. As the plethora of candidate viruses for cancer therapy grows, so does the knowledge of their safety and efficacy in various hosts. It is important, however, to clearly distinguish which strain is being used and to not draw too broad conclusions about virus safety and efficacy in humans based on animal experiments.
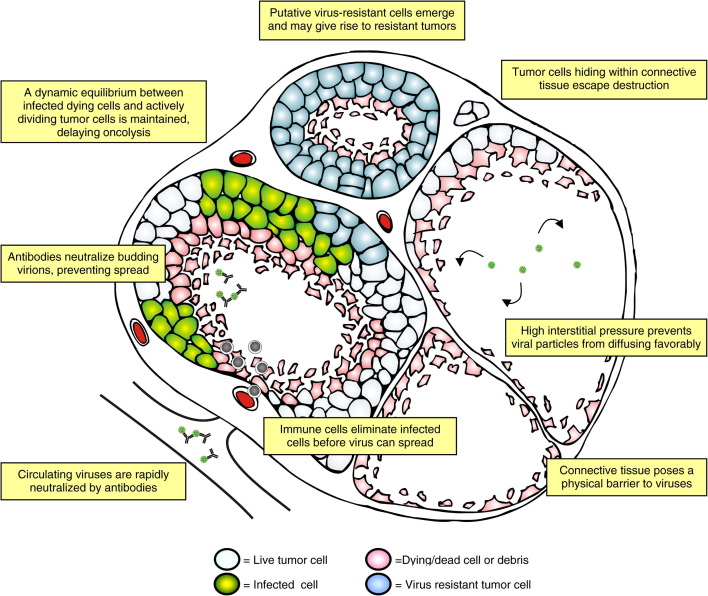
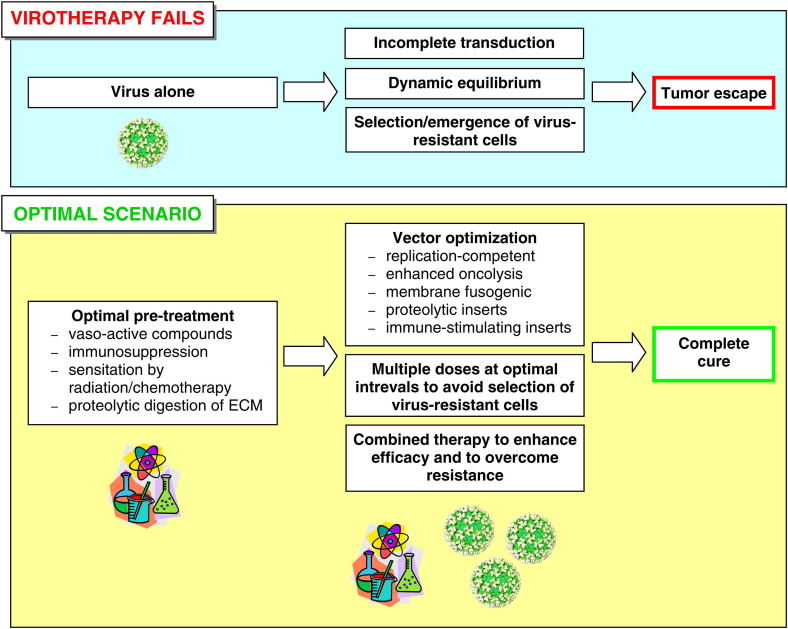
8. Obstacles viruses are facing
Unaided, the most common outcome of any virotherapy is the escape of tumor cells and subsequent regrowth of the tumor. In this chapter we discuss the reasons for this and delineate major hurdles that viruses face in cancer therapy. Some of these problems are illustrated in Fig. 1 .
Fig. 1.
Impediments to virotherapy. This schematic drawing represents a tumor mass, and highlighted are some of the known and putative problems oncolytic viruses are facing. Oncolysis is ongoing in the nodule to the left, whereas tumor destruction is almost complete in the nodules to the right. At the top, a new nodule has formed consisting of virus-resistant cells emerging under the selective pressure of oncolysis. Tumor cells may also avoid viral destruction by hiding within strands of connective tissue.
8.1. Incomplete transduction/dissemination
Irrespective of the virus used or whether it is replication-competent or not, the pattern of infection in many different types of tumors in animal models has upon histological examination been observed to be highly heterogenous and incomplete, even following direct i.t. administration [45], [61], [96], [139], [149], [166], [167], [171], [172], [186], [209], [210], [211], [212], [213], [214], [215]. Also, data from a growing number of clinical studies suggest that the dissemination of viruses in tumors in humans, such as gliomas, is incomplete and contributes to the failure of virotherapy [191], [192], [193], [194]. These observations have led researchers to study the interactions between viruses and tumor tissue and to scrutinize the fine morphology of the tumor mass.
Highly variable tumor transduction was observed in several tumor model systems using replication-deficient adenovirus vector [216]. While increasing virus load augmented delivery, transduction remained incomplete and plateaued off at a dose around 2.5 × 109 virus particles. The transduction efficacy was inversely proportional to tumor size; small tumors were transduced more efficiently than large ones [217]. In a study by Grote et al. [166], the oncolytic efficacy of live measles virus was better in small (<0.4 cm3) tumors than in large ones and also better with local delivery compared to systemic. Upon histological examination of extracted tumor nodules, heterogenous virus dissemination was observed, indicating physical barriers to virus spread. Similarly, Bilbao et al. [209] demonstrated limited access of adenoviral vectors to experimental hepatocellular carcinoma nodules in the livers of immunocompetent rats following both intra-arterial and intraportal delivery. In this study, the thickness of the extracellular matrix (ECM) surrounding tumor nodules was of critical importance, restricting access of the virus as the capsules grew in thickness (and the nodules in size). Also, a progressive increase in components of the basement membrane substantially reduced tumor permissiveness. Some of these barriers could be alleviated using vasoactive compounds, such as histamine or nitroglycerin, which revealed the tumor microvasculature to be largely responsive for accessibility into the tumors. However, despite increasing access from the blood, the vasoactive compounds did not bring complete transduction, and the permissiveness was highly dependent on tumor morphology, indicating that internal barriers still remained. In yet another study, despite optimized tumor targeting capacity of a modified adenoviral vector to CAR-independent tropism (CD46), very limited transduction of tumor metastases in the liver was observed following i.v. infusion and mainly into metastases and tumor nests with a visibly thinner extracellular matrix between endothelial cells and tumor cells compared to untransduced tumor foci [215]. This study was extended by Li et al. [214] who demonstrated using adenoviral vectors in immunodeficient mice carrying liver metastatic xenografts from a number of different human cancers, a relationship between the transducability of tumor cells and the availability of blood vessels adjacent to them. The authors showed that only those tumor cells that were in close proximity to the blood-vessels were transduced and that the ECM indeed posed a significant barrier to virus dissemination.
From these studies (and many others not listed here) several important conclusions can be drawn: first, viruses delivered via the vasculature must cross the blood vessel endothelium and any layer of matrix that may separate the endothelial cells from the tumor. Research has shown that viruses delivered i.v. are much less efficient in transducing tumor nodules that exceed a critical size, correlating with both the thickness and the stage of maturation of the capsule surrounding the nodules. This threshold may be different depending on the type and location of the tumor, both of which affect the degree of ECM deposition, but in general the tumor size will be a restricting factor for any macromolecular drug delivered via the circulation. Second, dissemination of virus within the tumor mass will be incomplete if only a single injection is given either intratumorally or at a remote location (whereby infection occurs via the blood). The degree of transduction will be dependent on virus dose and can be increased by multiple dosing or vaso-dilating compounds, but internal barriers within the tumor mass may still cause the infection to proceed unevenly. Third, host-derived connective tissue, including components of the basement membrane and the ECM, acts as a passive barrier to viral spread. Köpf–Maier and Kestenbach elegantly describe the kinetics of xenograft growth of 13 different human tumors in nude mice, showing that after an initial phase of cell death, mouse fibroblasts rapidly form strands of connective tissue into and around the tumors, providing substrate for the formation of blood-vessels, which in turn sustain the regrowing tumor tissue [218]. Normal host cells, including fibroblasts, are almost without exception refractory to the oncolytic viruses employed today, and the ECM itself may function as a decoy for virus binding, adsorbing the viral particles and thus preventing the infection of the tumor cells. It has also been shown that the ECM functions as a molecular sieve; whereas the movement of large particles (∼150 nm diameter) such as herpes viruses is hindered, smaller nanoparticles (∼20 nm) diffuse more easily through the matrix [219].
In addition to the physical barriers mentioned above, solid tumors contain physiologically different sub-compartments which may influence virus entry, diffusion and half-life. For example, in areas of necrosis or calcification, common to growing tumor masses in vivo, the prevailing microenvironment is characterized by hypoxia, acidosis and enhanced proteolytic activity, which may affect the virus particle itself or modify the cell surface of nearby live tumor cells in an unfavorable manner [220]. Moreover, the elevated interstitial pressure of solid tumors and pressure gradients within the tumors may repel viruses, preventing them from entering and spreading in the tumors. Internal pressure in solid tumors and nodules is demonstrably increased, constituting a clear obstacle for delivery and dissemination of other types of macromolecules [221], [222], [223], [224]. Finally, not only the size of the tumor mass, but also its bodily location influences the homing capacity of oncolytic viruses. Myers et al. [46] recently demonstrated residual tumor mass in most mice bearing human ovarian cancer xenografts treated i.p. with three different oncolytic viruses (two strains of measles virus and one mumps virus). Despite direct contact with the intraperitoneal cavity, the surviving tumors were located in the greater omentum which seemingly was not accessible by the injected viruses. In another study, although replication-competent poliovirus was able to find and eradicate subcutaneous tumors following a single i.v. injection, xenografts of the same cancer cells implanted in the brain were not cleared by peripheral administration and required i.c. virus injection to be affected [179].
8.2. Dynamic equilibrium
Many solid tumors contain a necrotic core which is palisaded by live tumor cells. Whereas the actively proliferating tumor cells are found outermost, virus antigen is frequently detected dispersedly at the interface between necrotic and viable tumor cells (see references in the previous chapter). This typical pattern of transduction as well as the fact that in many of the studies above both live virus and live tumor cells have been found, have spurred the notion of a dynamic equilibrium between infected dying cells and live tumor cells [166], [167]. According to this model, the respective growth kinetics of the virus and the tumor cells determine the outcome of the treatment. In the simplest scenario, tumors that grow as fast as or faster than the virus are not destroyed and therefore have a chance to regrow once the virus has been cleared. In immunodeficient mice, such a dynamic equilibrium could be maintained indefinitely [225], whereas in normal animals the immune system will probably eliminate the virus and tilt the equilibrium in favor of the tumors (unless also tumor-immunity is achieved).
Contributing to the unpredictable advancement of oncolysis, the so called cell contact effect alters the gene expression of cancer cells growing in three-dimensional tumor masses, such as spheroids, compared to monolayers, and directly influences the tumor response to various therapeutics [224]. Moreover, physiologically distinct subpopulations of cancer cells emerge within the tumor mass, termed microheterogeneity, which provides an additional impediment to any form of monotherapy [226]. For example, many oncolytic viruses show reduced replication in growth-arrested cells, common in hypoxic tumor regions. Finally, as viruses themselves are prone to adapt and mutate, the model of dynamic equilibrium is inadequate to describe the entire process of tumor oncolysis. Therefore, more elaborate mathematical models have been developed which take into account some of the confounding aspects that prevail during the interaction between viruses and cancers [227], [228]. Although still oversimplified, such models may prove valuable in studying and optimizing oncolytic virotherapies. They also show that in addition to growth kinetics, the degree of tumor permissiveness influences the outcome of the virotherapy.
8.3. Resistance to virus
A defining feature of a cancer cell is its ability to evade death. It does not respond to contact-mediated growth-inhibition or to apoptotic signals. Due to severe defects in the DNA repair machinery the genome of a cancer cell is highly malleable and undergoes several mutations during clonal expansion in vivo or passage in vitro [229]. The loss of other cellular functions, such as death-inducing pathways, also can make cancer cells particularly difficult to eradicate. Indeed, a common feature of the most severe malignancies is the development of resistance to both chemo- and radiation therapy, termed multiresistance. As cancer cells have the ability to adapt to almost any challenge, why would the concept of developing resistance to viruses be any different?
In our own studies we recently observed the emergence of completely virus-resistant melanoma cells during the course of virotherapy in SCID mice using replication-competent avirulent SFV [45]. The virus-resistant cells probably formed due to the selective pressure exerted by the virus or due to adaptive mutations in the tumor cells, which may have eliminated virus receptors or otherwise put the cells in a non-permissive state. Consistent with the concept of microheterogeneity, we could clearly show that the virus-resistant cells constituted only a portion of all cancer cells isolated from the residual tumors. They were likely to have contributed to tumor regrowth and ultimate failure of the therapy. Several other researchers have raised the possibility of virus-resistant cancer cells [69], [209], [212], [225], [230], [231], but despite promising tumor responses, e.g. to repeated dosing, none of these studies have analysed the emergence of such cells over the course of virotherapy by detailed scrutiny using both histochemistry and explant culturing. As an exception, in one study live tumor cells isolated from human lung carcinoma xenografts 21 days after treatment with oncolytic bovine herpesvirus 4 were still susceptible to the virus, which is in contrast to our findings [126]. However, it is likely that virus-resistant cells do not form equally fast with all viruses and that the isolation technique plays a role in finding these cells. Therefore, further studies are required.
Resistance of cancer cells to many different viruses may be promoted in vitro by multiple rounds of infection at low MOI, followed by passaging and re-infection of the surviving cells. This technique has been employed to generate tumor cells resistant to e.g. rat H-1 parvovirus [232], [233], coxsackie B virus [234], minute virus of mice [235], encephalomyocarditis virus [236] and adenovirus [231]. However, in many cases the so-called resistant cells have been persistently infected and resistant only to virally mediated cell death, in contrast to the cells obtained in our study which were resistant also to virus entry or replication. The distinction is important, as persistently infected cells may still be susceptible to cell death via cytotoxic gene products encoded by viral vectors. Recently Alain et al. [230] demonstrated using reovirus type 3 that persistently infected Raji cells (derived from Burkitt’s lymphoma) could be rendered virus-free by treatment with neutralizing antibody. Remarkably, despite remaining resistant to virus in vitro, these “cured” cells were efficiently killed by the virus in xenografts in SCID mice. This phenomenon was ascribed to the in vivo proteolytic tumor microenvironment which processed the virion into a form capable of infecting the resistant cells. The study by Alain et al. shows that virus-resistant cancer cells generated in vitro may be still be killed by oncolytic viruses in vivo. In contrast, however, the virus-resistant melanoma cells that emerged in vivo in our study remained resistant throughout the study (also in vitro), suggesting that such cells are distinct from virus-resistant cancer cells generated in vitro. Therefore, assessing the presence of virus-resistant cells in tumors that survive oncolysis in vivo may be more informative than to screen for virus-resistance in vitro.
Virus-resistant cancer cells generated in vitro to H-1 parvovirus have been found resistant also to TNF-α-mediated killing, demonstrating cross-protection due to overlapping molecular pathways of resistance [232], [233], [237]. These findings may have wider implications for combination therapy with oncolytic viruses and cytotoxic drugs. Interestingly, upon gaining resistance to virus, parvovirus-resistant cells lose their tumorigenic capacity, i.e. become revertants [55]. The tumorigenicity of the virus-resistant cell lines acquired in our own study [45] has not been established. It is possible that these cells have also become revertants but it is currently unknown whether the same is true for other viruses and whether tumor reversion occurs in vivo. Nevertheless, even if virus-resistant cells lose their tumorigenicity, they may still limit the success of virotherapy by posing a passive barrier to virus spread, tilting the dynamic equilibrium in favor of tumor growth. In this respect, it has been observed that infection of cancer cells with oncolytic herpes viruses induces apoptosis of neighboring cells, which may adsorb budding virions and prevent the infection from spreading [238]. Indeed, inhibiting apoptosis in cultures by chemical drugs considerably enhanced the oncolytic efficacy of the vectors. Thus, by inhibiting cell death, viral oncolysis could in fact be enhanced – a highly provocative idea which all the same may provide means to increase the success of virotherapy.
A recent case report describing the treatment of a 12-year-old boy with anaplastic astrocytoma (WHO grade III) with the live attenuated Newcastle disease virus MTH-68/H revealed significantly reduced virus replication in one of two (and in the final stages a third) different tumor masses [239]. The resistant mass was shown by histological examination post mortem to have arisen from the same original tumor. Thus, despite that the therapy virus may show efficient replication in a particular tumor mass in a patient, it may fail to do so in another of the same type in the same patient. The possible reasons for this striking phenomenon were discussed, but the spontaneous conversion of the tumor cells into a virus-resistant phenotype was not studied further. A similar case was reported in a study to treat glioblastoma multiforme using the lentogenic NDV-HUJ strain (given i.v.), where the tumor regrew in one patient after an initial complete response despite ongoing virotherapy [158]. The emergence of phenotypically indistinguishable but genetically different subclones of glioblastoma is not uncommon and may underlie the observed increased resistance to virus. The ongoing virotherapy may additionally have exerted selective pressure that provided growth advantage. In any case, it seems that tumor regrowth cannot be dismissed as mere incomplete transduction or poor toxicity – the underlying reasons may be related to intrinsic resistance to therapy (Chapter 5, Ref. [19]).
Resistance of cancer cells to various forms of challenge extends also to the realm of immunotherapy. For instance, following tumor vaccination using an influenza virus vector, Zheng et al. [240] observed tumor cells that were resistant to immune-mediated clearance due to downregulation of TAAs. In agreement with tumor microheterogeneity and the cell contact effect, such resistant tumors occurred only in mice with a heavy tumor burden at the start of the treatment, whereas animals with a smaller tumor burden remained tumor-free at the end of the study. Other types of tumor cells have also been observed to escape recognition by the immune system during ongoing immune surveillance [241], [242], [243]. Besides shedding TAAs, tumor cells may express immunosuppressive molecules or ignore external apoptotic signals [244]. In fact, not only are cancer cells resistant to immune-mediated apoptosis but can induce apoptosis in those immune cells that would potentially pose a threat, a phenomenon known as the tumor counter-attack [245].
Hypothetically, once a tumor has acquired the ability to resist both the immune system and any external onslaught, including chemo- and radiation therapy and oncolytic viruses, it has in practice become impervious to all forms of treatment. For simplicity, such a cell could be labeled omniresistant. In order to avoid this, successful treatment should include a combination of synergistic therapies. This puts even more pressure on the precise and scientifically backed planning of treatment regimens and to elucidate common molecular targets that enable facilitation between different forms of treatment. Ideally, the molecular mechanisms of cell death in virally infected tumor cells should be put into context with the mechanisms that prevail during chemotherapy, cytokine therapy or radiation therapy in order to reveal the optimal molecular and genetic targets for intervention and ultimately, to provide a tailored therapy to which the cancer cell is defenseless. Not surprisingly, combination therapy is nowadays considered the only veritable option using adenoviruses, and monotherapy employing other viruses may be just as limited.
8.4. Safety, side-effects and complications
Several clinical trials have been conducted using common human pathogens that are considered innocuous, e.g. influenza virus, coxsackievirus, measles, mumps and adenovirus. However, as cancer patients run an increased risk of opportunistic infections by such cold-producing viruses, sometimes with severe consequences, complications may arise also during virotherapy [152], [153], [154]. As all viruses are potentially immunogenic they may, particularly at high doses, elicit unwanted side-effects. For example, at the therapeutically efficacious doses of 1012 particles, adenoviruses administered i.v. elicit transient liver inflammation and low-grade disseminated intravascular coagulation which despite the good biosafety data accumulated to date is a problem [95], [246]. The unfortunate death in 1999 of a patient suffering from ornithine decarbamylase deficiency treated with high-dose adenovirus serves as a grim reminder of the perils involved in using viruses in humans. Although harmless during endemic infections, given directly into the blood these viruses bypass the body’s inherent defenses and may elicit a potentially fatal systemic response. Another setback for viral therapy of human diseases occurred in France where three children with severe X-linked immunodeficiency treated with retrovirally transduced T cells developed leukemia related to viral integration and activation of the LMO2 oncogene [80]. Other patients treated similarly before this trial have recently begun to show clonal dominance in their immune cell repertoire, owing to the integration of retroviral vectors [247]. This underpins the fact that despite their capacity to correct immune-related monogenetic disorders, integrating vectors come with serious risks that need to be controlled.
Pre-existing immunity may pose a problem for the efficacy of virotherapy (discussed in the next chapter), but in some cases it may also result in exacerbated toxicity. For example, intratumoral treatment of Balb/c mice harboring subcutaneous syngeneic breast cancer xenografts with an adenoviral vector led to significantly increased toxicity in pre-immunized mice (60% mortality) compared to naïve mice (15% mortality) at doses from 2 to 6 × 1011 particles, corresponding to the highest doses used in humans [248]. Pre-existing immunity prevented the transduction of peripheral organs but at the same time increased liver toxicity. In addition, therapy with high-dose virus may cause depletion of cytotoxic T cells or anti-viral interferons by a process known as immune exhaustion, compromising natural tumor immunity and reducing the efficacy of tumor vaccination, and rendering the host susceptible to further viral infection [249], [250]. This may have implications for virotherapy involving repeated dosing. Moreover, undesired side-effects may emerge during combination therapy where two (or more) treatment modalities may act synergistically to potentiate toxicity. For example, a portion of SCID mice harboring i.p. human ovarian cancer xenografts died when treated with gemcitabine and a retargeted Δ24 adenovirus, presumably due to liver or bone marrow toxicity [251]. The sensitization of normal cells to either the drug or the virus was offered as a plausible explanation for the occurrence. Finally, all oncolytic viruses may not be equally suitable for targeting tumors in sensitive locations, such as the brain, and the route of administration may also impact biosafety. For example, adenoviral vectors expressing HSV TK have been shown to cause CNS demyelination in mice upon i.c. inoculation [252]. In a study to target leptomeningeal metastases in rats and non-human primates, administration of adenovirus expressing TK into the cerebrospinal fluid followed by i.v. ganciclovir lead to viral meningitis, and a clinical trial was recently aborted as intrathecal retrovirus-TK therapy evoked severe toxicity in one patient [253].
Taken together, using live viruses in humans always comes with a certain risk. Some viruses may be more immunogenic or toxic than others, but it must be kept in mind that bypassing the innate defenses by administration of high doses of any biological agent, however innocuous, may have undesired consequences. During the past years, many virotherapy companies have emerged, some of which unfortunately do not publish their research in public forums. This puts even more pressure on health authorities to control the clinical trials, and with so many viruses being developed for virotherapy the burden is daunting. In order to facilitate the process and to provide an impartial assessment of the risks-benefit ratios, fastidious review of both the pre-clinical data and the proposed protocol by independent peers should become standard procedure.
8.5. Virus inactivation
As the majority of the human population is immune to several of the potential therapy viruses, pre-existing immunity may pose a problem. However, while virotherapy with some viruses seems to benefit from pre-existing immunity [61], the replication of at least HSV and adenovirus in immune hosts may be limited. Delman et al. [254] found that in contrast to high-dose treatment (107 PFU), i.v. low-dose (106 PFU) administration of either G207 or NV1020 failed to reduce the number of tumor nodules in pre-immunized mice with liver-metastasizing colorectal carcinoma. However, the oncolytic efficacy at the lower dose was not diminished if the viruses were given in close proximity to the tumor mass via intraportal injection. Similarly, in a mouse model of advanced peritoneal gastric cancer these vectors were unable to halt tumor growth when given i.v. instead of i.p. [255]. Thus, even replication-competent HSV vectors lose oncolytic efficacy when administered at sites remote from the tumor, and high doses of virus may be required to achieve efficacy in systemic virotherapy. Moreover, vectors based on HSV-1 and murine leukemia virus are prone to inactivation by complement [213], [256].
Adenoviral vectors are often rapidly neutralized by antibody [257], [258], [259] and may despite retargeting be trapped by the liver where they may cause significant toxicity, which in turn limits their use in systemic virotherapy [109], [211], [216]. Pre-existing immunity may also reduce the oncolytic capacity of reoviruses administered i.v., exemplified by a study to treat metastatic tumors [148]. In this study, immunosuppressive drugs and/or T-cell depletion significantly augmented virus spread in the tumors, prolonging survival and providing increased long-term cure. All in all, while the mechanisms of virus inactivation differ between host species and the results obtained using laboratory animals may not apply in humans [199], the problem of premature virus inactivation probably concerns most oncolytic viruses.
9. Strategies to enhance treatment efficacy
We outline here a few ideas that seem particularly promising in overcoming some of the severe limitations in cancer virotherapy.
9.1. Enhancing virus delivery
Recently, a number of strategies to enhance the delivery of drugs into solid tumors have been proposed [219], [220], [224]. For example, given the physical barriers to oncolytic viruses (presented in Chapter 8.1), pre-treatment with proteolytic enzymes, e.g. hyalorunidase or collagenase, could alter the ECM and thus facilitate virus penetration. Also, increasing the oxygenation of tumors to relieve hypoxia and to lower the interstitial pressure has been proposed to resensitize the tumors to radiation and facilitate drug delivery by normalizing the vasculature. This could be achieved by a number of strategies, including inhalation of hyperoxic gas, induction of local hyperthermia or blockade of vascular endothelial growth factor signaling. Vaso-active or vaso-normalizing compounds such as bevacizumab, bradikynin, low-dose paclitaxel or leukotrienes have also been used to increase the blood-tumor permeability. However, in one study even vaso-active drugs were not able to enhance adenoviral transduction of solid tumors exceeding a critical size [209]. This phenomenon has been corroborated later by a number of other observations and it has been proposed that the contribution of blood-vessel permeability to macromolecular penetration becomes dispensable in well-established fenestrated tumors where internal barriers are more likely to play the major role [224]. Also, while efficacious in increasing tumor permissiveness to viral vectors, vaso-active compounds may simultaneously increase the risk of tumor escape and metastasis (discussed in [209]). Nevertheless, it is becoming clear that pre-treatment of tumors with compounds that alter the microenvironment has a beneficial effect by resensitizing resistant tumors and by relieving some of the physical barriers to macromolecular delivery. Since tumor transduction by viruses in most cases is incomplete, particularly when the tumors have exceeded a certain size, pre-treatment to enhance virus delivery is likely to become a critical step in virotherapy.
9.2. Repeated dosing
With or without pre-treatment, the foremost strategy to enhance tumor transduction by viruses has been to use multiple administrations. For example, increasing treatment regimen to two i.t. doses decreased the variability in the transduction rate of a replication-deficient adenovirus vector [216]. Further, repeated i.v. injection of replication-competent VSV vector significantly prolonged animal survival compared to a single virus dose in a syngeneic immunocompetent breast cancer model, without increasing toxicity [175]. Five consecutive injections of an oncolytic herpes virus provided dramatically enhanced transduction in large (490–695 mm3) tumor xenografts compared to single injection of the same total dose, leading to complete responses in all the treated animals [260]. As many oncolytic viruses are based on common human pathogens, pre-existing immunity will rapidly clear the viruses and thus multiple dosing will probably be required for successful virotherapy. However, despite providing enhanced efficacy in many experimental settings, even this strategy may be limited due to internal barriers that remain in the tumors [95], [219]. Also, in the case of intracranial tumors, repeated i.t. injections may not be feasible. Therefore, other strategies, such as continuous delivery via catheter and convection-enhanced delivery have been applied to increase tumor transduction within the CNS. The latter strategy is being used in a prospective phase I/II study to treat glioblastoma multiforme using liposomally encapsulated SFV replicons [261].
9.3. Targeting/circumventing internal tumor barriers
In addition to pre-treating or co-injecting tumors with ECM-disrupting drugs or enzymes, viral vectors can be modified to express ECM-degrading proteins. For example, the oncolytic efficacy of an adenoviral vector engineered to express the proteolytic enzyme relaxin was significantly enhanced compared to parental virus in a number of tumor xenografts in nude mice [262]. Expression of relaxin reduced the internal collagen content of the tumors and clearly enhanced virus spread. Despite the obvious concern for increased metastasizing potential in a matrix-degrading environment, treatment with the relaxin-expressing vector in fact reduced the number of lung metastases in the B16BL6 spontaneous metastatic tumor model compared to the parental vector.
Members of several virus families, including the corona-, ortho- and paramyxoviruses, have the capacity to induce cell fusion via their FMGs. This leads to formation of multinucleated syncytia which provide the viruses with unsurpassed access to building blocks for new viruses and the possibility to spread the infection without an extracellular step. Despite that many membrane-fusogenic viruses have long been used to target cancer, the notion of using their fusogenic properties as separate anti-tumorigenic entities or in conjunction with other viruses has only recently been implemented in practice [263], [264]. While FMGs are already by themselves capable of killing cancer cells, they augment virotherapy even further by facilitating virus spread within the tumor. For example, expression of a fusogenic mutant F glycoprotein of Newcastle disease virus from a VSV vector enhanced its oncolytic potency and extended animal survival compared to WT virus [172]. Likewise, expression of the hyperfusogenic envelope glycoprotein of the gibbon ape leukemia virus from a replicon vector based on Sindbis virus brought significant enhancement of therapy efficacy in the U87 i.c. glioma model in nude mice [265]. Syncytia formation could circumvent some of the intrinsic barriers present inside the tumors, including the high interstitial pressure, as the oncolytic viruses are no longer dependent on particle budding and diffusion to infect neighboring cells.
9.4. Virus retargeting
A myriad of modified viruses have been developed in which the natural tropism has been altered in order to enhance the specificity to tumor cells or to alleviate innate restrictions to the tropism (e.g. to overcome the species-barrier). In addition, virus retargeting may function to increase safety by reducing infection of normal cells.
First, replication of DNA viruses and the expression of oncotoxic inserts can be confined to tumor cells by the use of tumor-specific promoters. For example, the progression elevated gene-3 (PEG-3) promoter or the human tyrosinase promoter with relevant enhancer elements significantly enhanced the tumor-specificity of adenoviral vectors in mouse models of human melanoma [266], [267]. Similarly, a number of tumor cell-specific promoters were able to alter the tropism of replication competent retroviral vectors [268].
Second, a commonly employed strategy to achieve tumor-specificity has been to modify or exchange the viral surface glycoproteins. In this respect, adenoviruses have undergone the most intensive research and a multitude of adenoviral vectors with altered tropism is available (reviewed in [110]). Retroviruses can be efficiently retargeted to specific cells by pseudotyping them with suitable surface glycoproteins (not just VSV-G) [269] and autonomous parvoviruses can be retargeted by modifying the capsid protein [147] Also, incorporation of tumor-specific peptides into several different gene delivery systems, including viruses, has been shown to significantly augment the targeting of many cancers [270]. For example, the tropism of measles virus has been made strictly cancer cell specific by expression of receptor-binding regions of single-chain antibodies on their surface, which also minimizes infection of non-tumor cells expressing the natural measles receptors CD64 or SLAM (CD150) [271]. However, peptide sequences incorporated into the surface glycoproteins of viruses may also function as tethers for tumor-specific antibodies. For example, lentiviral vectors pseudotyped with a modified E2 glycoprotein of Sindbis virus capable of accepting the Fc portion of IgG molecules displayed increased specificity to human melanoma cells in SCID mice when the viruses were incubated with specific antibodies prior to i.v. inoculation [272].
Third, in addition to modifying the viral surface, mutating other regions of the viral genome can increase the specificity and oncolytic efficacy of some viruses by altering viral replication kinetics in cancer cells compared to normal cells. For example, elimination of the SP-1 and SP-2 genes of vaccinia virus (strain WR) significantly reduced the cytotoxicity of the virus for two human and one mouse primary cell line while simultaneously increasing its toxicity for transformed or p53-negative counterparts [132]. Treatment with either IFN-α or IFN-γ or both at the same time failed to abolish replication of the virus in the transformed cells. Upon i.p. administration in mice, the modified vaccinia vector localized to subcutaneous tumors with greater specificity than its WT counterpart, while vector titers in peripheral organs were reduced compared to WT virus. Similarly, deletion of the TK gene from VV strain Wyeth significantly diminished the capacity of the virus to replicate in non-transformed non-proliferating cells, thus enhancing its tumor-specificity [134]. Introduction of mutations restoring replication efficiency to the conventional γ34.5 deleted HSV vectors has been shown to significantly enhance oncotoxicity without compromising virus attenuation [123].
Moreover, as cancer cells most often become hypoxic, targeting viruses to replicate exclusively in such cells has emerged a promising strategy to enhance the specificity of virotherapy. For example, placing the adenoviral E1A gene under the control of a hypoxia-inducible factor (HIF)-responsive element created a conditionally replicating virus, HYPR-Ad, that showed highly specific replication and spread in areas of low oxygen in U87 glioma xenografts in nude mice, with oncolytic efficacy close to that of replication-competent WT adenovirus [119]. Targeting hypoxic cells has the additional benefit of being a generic strategy which may apply to all solid cancers and thus is less likely to be dependent on specific molecular defects in the cancer cells, which may vary with cell type and be prone to mitigation by compensating mechanisms and overlapping pathways. An analogous strategy is to make use of the enhanced proteolytic microenvironment in tumors. This strategy has been implemented on measles virus which was rendered apathogenic in mice by engineering the activation of the fusogenic membrane glycoprotein F, necessary for infection, to be dependent on matrix metalloproteases (upregulated in tumors) rather than on furin [273]. The oncolytic efficacy of the virus was not diminished by the mutation.
Lastly, retargeting of viruses by coating them with tumor-specific antibodies is an additional option to facilitate at least the initial transduction of cancer cells. For instance, using a bi-specific antibody it was possible to target feline coronavirus to human epidermal growth factor expressing cancer cells [178]. However, in the case of replication-competent viruses, if the antibody is required for infection its presence in subsequent rounds of infection must be ensured, even within the tumor, and thus this strategy may be difficult to implement in practice. One possibility in this case is to have the vector express the required antibody.
9.5. Oncotoxic/oncosuppressive inserts
An obvious way of enhancing the cancer killing potency of an oncolytic virus is to engineer it produce a protein that is toxic to the cell, either directly or via sensitization to cell death. For example, expression of Fas-ligand, TRAIL or p53 by adenoviral vectors has been shown to enhance their oncolytic effect both in vitro and in vivo without increasing the toxicity to normal cells [274], [275]. In addition to being directly cytotoxic, anti-neoplastic proteins can function by suppressing tumor growth. For example, the parvovirus non-structural proteins are capable of causing tumor reversion. Also, the Vpr nuclear protein of HIV-1 causes cell cycle arrest in susceptible cancer cell types and has successfully been used in experimental immunocompetent settings to eradicate cancer [276]. Interestingly, no anti-tumor effect was observed in nude or SCID mice. Finally, oncotoxic inserts do not have to be proteins. Using short hairpin RNA expressed by a lentiviral vector to inhibit survivin mRNA gave good results in a nude mouse model of oral squamous cell carcinoma [53]. All in all, oncotoxic inserts may enhance the robustness of the virotherapy, particularly in situations where the cancer cells are moderately resistant to the backbone virus.
9.6. Evading/activating the immune system
Most viruses are recognized and eventually eliminated by the immune system. In human virotherapy this is a desirable trait, but can impede the efficacy of the treatment if occurring prematurely. It is not uncommon to achieve a better response in naïve immunocompetent animals than in animals with a defective immune system [201], [277]. This also means that immunosuppression, while allowing enhanced viral replication, may simultaneously diminish the anti-tumor immune responses and reduce the efficacy of the treatment [278]. Even so, for many viruses, including adeno-, herpes- and reovirus, transient immunosuppression has proven highly advantageous. For example, pre-immune IgM, which is present in the plasma of both rats and humans, has been shown to restrict the transduction capacity and spread of replication-competent HSV vectors administered intra-arterially [213]. Treatment with the B-cell immunosuppressive agent cyclophosphamide abolished this inhibition and allowed more efficient transduction of CNS tumors, increasing the survival time of the animals. In another study, treatment efficacy with reovirus of mice bearing multiple metastases of colorectal cancer was dramatically enhanced by pre-treating the animals with cyclosporine A [279].
In order to increase the safety of immunogenic viral vectors in humans, particularly as objective tumor responses often require large virus doses, strategies to desensitize the immune system have been developed. For example, priming the innate immune system with recombinant IFN-α prior to VSV administration reduced replication but significantly increased the threshold of virus toxicity in immunocompetent rats [174]. This strategy could potentially allow the use higher virus doses in humans than currently possible. A related strategy, the administration of low-dose virus before the actual therapeutic dose is currently being implemented in a number of clinical trials to minimize virally elicited immune-dependent toxicity [182]. However, adverse events may occur despite apparent tolerance to repeated virus dosing, as evidenced by a severe systemic inflammatory reaction that developed in a patient after the fourth dose of the dl1520 adenovirus via the hepatic artery in a clinical trial to treat liver-metastasizing gastrointestinal carcinoma [92]. Moreover, in addition to suppressing or desensitizing the immune system, viruses can be made less immunogenic. One strategy has been to mask viruses by polymer-coating. For adenoviruses, this method has enabled the simultaneous enhancement of targeting specificity and evasion of neutralizing antibody [257]. Another strategy involves the encapsulation of viruses in non-immunogenic liposomes, a technique currently employed in phase I/II studies to target glioblastoma multiforme with SFV replicons [261]. Also, simply by choosing an appropriate producer cell line, the half-life in the blood of certain viruses can be dramatically improved. For MLV, complement-mediated inactivation in human serum was reduced severalfold depending on the cell type used [256].
In immunocompetent hosts, viral oncolysis is always a complex process involving the immune system in addition to the virus and the tumor. As viral oncolysis and tumor vaccination are overlapping concepts, the integration of immune-stimulating modalities into oncolytic therapy, most often cytokines and chemokines, is gaining support. For example, expression of IFN-γ from a conditionally replicating adenoviral vector enhanced the oncolytic efficacy compared to the backbone virus, both in a human xenograft and a syngeneic mouse model for liver cancer [277]. Importantly, expression of IFN-γ led to the eradication of also large (>1 cm3) xenografts not achieved with the parental virus, which is encouraging when considering that large tumors are often refractory to oncolytic viruses (Chapter 8.1). In another study, expression of human GM-CSF from a targeted VV vector JX-594 in rabbits with metastasizing liver cancer proved highly efficacious (hGM-CSF is biologically active in rabbits, in contrast to mice) [134]. Further, expression of IL-12 from SFV replicons has increased their efficacy in vivo [189]. However, particularly in conjunction with immune-modulating treatment viruses may display unexpected behavior as viral replication is dependent on immune responses.
9.7. Using viruses in conjunction with other forms of treatment
In this last chapter we will briefly discuss some future aspects of oncolytic virotherapy. Data accumulated so far suggest that the oncolytic capacity of viruses, although impressive, is impeded at many levels and may require assistance in order to reach full efficacy. Combination strategies are emerging the only truly effective way of achieving full cure in humans (Fig. 2 ). Also, in order to avoid the generation of the aforementioned omniresistant cancer cells, tumors have to be subjected to a barrage of different treatment forms targeting both synergistic and non-overlapping molecular pathways.
Fig. 2.
Two hypothetical outcomes of virotherapy. In the upper panel, due to the highlighted problems the therapy will ultimately fail using virus infection alone. Several strategies have therefore been proposed to enhance treatment efficacy in order to achieve complete eradication of the cancer (lower panel). Finding a combination resulting in optimal treatment regimen will probably become increasingly important in the future.
In this respect, using viruses to sensitize cancer cells to other forms of treatment, or vice versa, has proven highly promising. For instance, in one report the beneficial effects of virotherapy were enhanced in combination with radiation therapy, and the authors speculated that possible resistance to virus may be overcome by introducing multiple treatment modalities [59]. In another study, adenoviral vectors engineered to silence the cell cycle checkpoint kinase 1 gene through antisense RNA were shown to sensitize otherwise resistant cancer cells to cisplatin in an animal model of liver cancer [280]. Moreover, one of the most prominently upregulated genes in multiresistant cancer cells compared to non-resistant counterparts is the gene for Y-box protein (YB)-1 [281]. Several strategies are therefore being developed to target YB-1 in order to resensitize these cells to conventional forms of therapy (i.e. chemo- and radiation therapy). In one study, treatment of prostate cancer cells with a modified conditionally replicating adenovirus, Xvir03, led to enhanced nuclear localization of YB-1, which resulted both in enhanced virus replication and increased sensitivity of the cells to chemotherapy [282]. The authors of this study termed the notion of using one form of therapy to sensitize cancer cells to another through a common molecular target mutually synergistic therapy. As another example, adenoviral vectors engineered to express a dominant-negative NF-κB inhibitor, IκBM, rendered HeLa cells susceptible to TNF-α mediated apoptosis both in vitro and in vivo, facilitating the release of virions and spread of the virus [283]. Finally, in clinical trials of squamous cell cancer of the head and neck, the conditionally replicating Onyx-015 vector was able to achieve only a ∼15% tumor response on its own, whereas combining virotherapy with chemotherapy increased the efficacy to a level where 19 of 30 patients had an at least 50% reduction in tumor size and 8 patients were cleared of the cancer [93], [94], [95].
In addition to combining virotherapy with drugs or radiation, synergy can also be achieved by using two or more viruses either in conjunction or succession. For instance, when used together, recombinant HSV was found to enhance the release of AAV in pancreatic cancer cells both in vitro and in vivo [284]. Also, since it is known that echovirus infection leads to the upregulation of ICAM-1-the cellular receptor for coxsackievirus A21-in infected ovarian cancer cells, it has been proposed that the combined administration of these viruses could lead to enhanced oncolysis [40]. Several clinical trials using combinations of two or more different vaccinia vectors in conjunction with chemotherapy have been started [136]. Moreover, one virus can be used to drive the production of another. Particularly the large dsDNA viruses, such as the herpes-, adeno- and poxviruses, are capable of accommodating very large inserts, including other viruses. For instance, hybrid HSV vectors have been used to drive the production of retroviral amplicons in target cells, enabling significantly enhanced tumor targeting as the transduced tumor cells become producer cells for the amplicons [285]. Parvoviral replicons have been integrated into adenoviruses, and despite that the parvoviral NS1 protein initially prevented vector production, hybrid vectors were obtained in producer cells that expressed anti-sense RNA against NS1 [286]. Furthermore, adenoviral vectors were recently engineered to produce SFV replicons via a tissue-specific promoter [287]. Here the natural tropism of adenoviruses was used to target the vector to liver tumors in rats, while tumor-cell specificity was ensured using the α-fetoprotein promoter and robust gene expression and cytotoxicity by SFV. This is an example of combining three strategies in one system to enhance both specificity and toxicity, while at the same time preserving safety. For extensive reviews covering combination therapy strategies, see [119], [246].
10. Conclusions
Despite the growing number of oncolytic viruses and despite the avid improvements made to several of these, tumor regrowth remains the most difficult challenge of virotherapy. One reason for this is the inadequacy of animal models to mimic cancer in humans, a problem which could be addressed by using more sophisticated models and by taking maximum advantage of the ones already available. The mechanisms of tumor survival should be elucidated in order to avoid the generation of cancer cells resistant to both conventional treatment and virotherapy. As results from both preclinical and clinical virotherapy studies accumulate, it is becoming increasingly clear which forms of therapy offer the best chances of success. In addition to oncolytic virus(es), the optimal treatment regimen in most cases probably includes a combination of radiation, chemo- or immunotherapy. However, while apparently effective, the combination of two or more treatment modalities in one host may introduce additional variables into an already complex equation, possibly with dire consequences. One prominent concern is the use of replication-competent viruses in patients with compromised immunity, e.g. after radiation therapy. Another is the difficulty to estimate the extent of the immune response to rapid oncolysis, particularly at high virus doses. Therefore, detailed knowledge on immunological mechanisms and greater understanding of the molecular interactions between viruses and other forms of treatment is needed.
Contributor Information
Markus J.V. Vähä-Koskela, Email: makoskel@abo.fi.
Jari E. Heikkilä, Email: jaheikki@abo.fi.
Ari E. Hinkkanen, Email: ahinkkan@abo.fi.
References
- 1.Sinkovics J., Horvath J. New developments in the virus therapy of cancer: a historical review. Intervirology. 1993;36:193–214. doi: 10.1159/000150339. [DOI] [PubMed] [Google Scholar]
- 2.Sinkovics J.G., Horvath J.C. Newcastle disease virus (NDV): brief history of its oncolytic strains. J. Clin. Virol. 2000;16:1–15. doi: 10.1016/s1386-6532(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 3.Southam C.M., Moore A.E. Clinical studies of viruses as antineoplastic agents with particular reference to Egypt 101 virus. Cancer. 1952;5:1025–1034. doi: 10.1002/1097-0142(195209)5:5<1025::aid-cncr2820050518>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Pack G.T. Note on the experimental use of rabies vaccine for melanomatosis. AMA Arch. Derm. Syphilol. 1950;62:694–695. doi: 10.1001/archderm.1950.01530180083015. [DOI] [PubMed] [Google Scholar]
- 5.Southam C.M., Hilleman M.R., Werner J.H. Pathogenicity and oncolytic capacity of RI virus strain RI-67 in man. J. Lab. Clin. Med. 1956;47:573–582. [PubMed] [Google Scholar]
- 6.Asada T. Treatment of human cancer with mumps virus. Cancer. 1974;34:1907–1928. doi: 10.1002/1097-0142(197412)34:6<1907::aid-cncr2820340609>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Okuno Y., Asada T., Yamanishi K., Otsuka T., Takahashi M., Tanioka T., Aoyama H., Fukui O., Matsumoto K., Uemura F., Wada A. Studies on the use of mumps virus for treatment of human cancer. Biken J. 1978;21:37–49. [PubMed] [Google Scholar]
- 8.Taylor M.W., Cordell B., Souhrada M., Prather S. Viruses as an aid to cancer therapy: regression of solid and ascites tumors in rodents after treatment with bovine enterovirus. Proc. Natl. Acad. Sci. USA. 1971;68:836–840. doi: 10.1073/pnas.68.4.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith R.R., Huebner R.J., Rowe W.P., Schatten W.E., Thomas L.B. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9:1211–1218. doi: 10.1002/1097-0142(195611/12)9:6<1211::aid-cncr2820090624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Wheelock E.F., Dingle J.H. Observations on the repeated administration of viruses to a patient with acute leukemia. A preliminary report. N. Engl. J. Med. 1964;271:645–651. doi: 10.1056/NEJM196409242711302. [DOI] [PubMed] [Google Scholar]
- 11.Newman W., Southam C.M. Virus treatment in advanced cancer; a pathological study of fifty-seven cases. Cancer. 1954;7:106–118. doi: 10.1002/1097-0142(195401)7:1<106::aid-cncr2820070112>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Mettler N.E., Clarke D.H., Casals J. Virus inoculation in mice bearing Ehrlich ascitic tumors: antigen production and tumor regression. Infect. Immun. 1982;37:23–27. doi: 10.1128/iai.37.1.23-27.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuster M., Nechansky A., Kircheis R. Cancer immunotherapy. Biotechnol. J. 2006;1:138–147. doi: 10.1002/biot.200500044. [DOI] [PubMed] [Google Scholar]
- 14.Sinkovics J.G., Horvath J.C. Vaccination against human cancers (review) Int. J. Oncol. 2000;16:81–96. doi: 10.3892/ijo.16.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Sinkovics J.G., Horvath J.C. Evidence accumulating in support of cancer vaccines combined with chemotherapy: a pragmatic review of past and present efforts. Int. J. Oncol. 2006;29:765–777. [PubMed] [Google Scholar]
- 16.Cassel W.A., Murray D.R., Torbin A.H., Olkowski Z.L., Moore M.E. Viral oncolysate in the management of malignant melanoma. I. Preparation of the oncolysate and measurement of immunologic responses. Cancer. 1977;40:672–679. doi: 10.1002/1097-0142(197708)40:2<672::aid-cncr2820400213>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Murray D.R., Cassel W.A., Torbin A.H., Olkowski Z.L., Moore M.E. Viral oncolysate in the management of malignant melanoma. II. Clinical studies. Cancer. 1977;40:680–686. doi: 10.1002/1097-0142(197708)40:2<680::aid-cncr2820400214>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Cassel W.A., Murray D.R. A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med. Oncol. Tumor Pharmacother. 1992;9:169–171. doi: 10.1007/BF02987752. [DOI] [PubMed] [Google Scholar]
- 19.Sinkovics J.G., Horvath J.C. Marcel Dekker; Boca Raton, FL: 2005. Viral therapy of human cancers. [Google Scholar]
- 20.White R.R., Stanley W.E., Johnson J.L., Tyler D.S., Seigler H.F. Long-term survival in 2505 patients with melanoma with regional lymph node metastasis. Ann. Surg. 2002;235:879–887. doi: 10.1097/00000658-200206000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leaf C. Why we’re losing the war on cancer [and how to win it] Fortune. 2004:76–92. [PubMed] [Google Scholar]
- 22.Freedman R.S., Edwards C.L., Bowen J.M., Lotzova E., Katz R., Lewis E., Atkinson N., Carsetti R. Viral oncolysates in patients with advanced ovarian cancer. Gynecol. Oncol. 1988;29:337–347. doi: 10.1016/0090-8258(88)90233-8. [DOI] [PubMed] [Google Scholar]
- 23.Neagoe G., Stoian M. Methods of active immunotherapy and viral oncolysis in some forms of cancer. Med. Interne. 1986;24:125–142. [PubMed] [Google Scholar]
- 24.Kolmel K.F., Grange J.M., Krone B., Mastrangelo G., Rossi C.R., Henz B.M., Seebacher C., Botev I.N., Niin M., Lambert D., Shafir R., Kokoschka E.M., Kleeberg U.R., Gefeller O., Pfahlberg A. Prior immunisation of patients with malignant melanoma with vaccinia or BCG is associated with better survival. An European Organization for Research and Treatment of Cancer cohort study on 542 patients. Eur. J. Cancer. 2005;41:118–125. doi: 10.1016/j.ejca.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Krone B., Kolmel K.F., Grange J.M., Mastrangelo G., Henz B.M., Botev I.N., Niin M., Seebacher C., Lambert D., Shafir R., Kokoschka E.M., Kleeberg U.R., Gefeller O., Pfahlberg A. Impact of vaccinations and infectious diseases on the risk of melanoma–evaluation of an EORTC case-control study. Eur. J. Cancer. 2003;39:2372–2378. doi: 10.1016/s0959-8049(03)00625-7. [DOI] [PubMed] [Google Scholar]
- 26.Mastrangelo G., Rossi C.R., Pfahlberg A., Marzia V., Barba A., Baldo M., Fadda E., Milan G., Kolmel K.F. Is there a relationship between influenza vaccinations and risk of melanoma? A population-based case-control study. Eur. J. Epidemiol. 2000;16:777–782. doi: 10.1023/a:1007658503740. [DOI] [PubMed] [Google Scholar]
- 27.Hilleman M.R. Critical overview and outlook: pathogenesis, prevention, and treatment of hepatitis and hepatocarcinoma caused by hepatitis B virus. Vaccine. 2003;21:4626–4649. doi: 10.1016/s0264-410x(03)00529-2. [DOI] [PubMed] [Google Scholar]
- 28.Mahdavi A., Monk B.J. Vaccines against human papillomavirus and cervical cancer: promises and challenges. Oncologist. 2005;10:528–538. doi: 10.1634/theoncologist.10-7-528. [DOI] [PubMed] [Google Scholar]
- 29.Challis G.B., Stam H.J. The spontaneous regression of cancer. A review of cases from 1900 to 1987. Acta Oncol. 1990;29:545–550. doi: 10.3109/02841869009090048. [DOI] [PubMed] [Google Scholar]
- 30.Hobohm U. Fever therapy revisited. Br. J. Cancer. 2005;92:421–425. doi: 10.1038/sj.bjc.6602386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorne S.H., Brooks G., Lee Y.L., Au T., Eng L.F., Reid T. Effects of febrile temperature on adenoviral infection and replication: implications for viral therapy of cancer. J. Virol. 2005;79:581–591. doi: 10.1128/JVI.79.1.581-591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Shea C.C., Soria C., Bagus B., McCormick F. Heat shock phenocopies E1B-55K late functions and selectively sensitizes refractory tumor cells to ONYX-015 oncolytic viral therapy. Cancer Cell. 2005;8:61–74. doi: 10.1016/j.ccr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 33.De P.M., Venneri M.A., Naldini L. In vivo targeting of tumor endothelial cells by systemic delivery of lentiviral vectors. Hum. Gene Ther. 2003;14:1193–1206. doi: 10.1089/104303403322168028. [DOI] [PubMed] [Google Scholar]
- 34.Cahill D.P., Kinzler K.W., Vogelstein B., Lengauer C. Genetic instability and darwinian selection in tumours. Trends Cell Biol. 1999;9:M57–M60. [PubMed] [Google Scholar]
- 35.Coffey M.C., Strong J.E., Forsyth P.A., Lee P.W. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 36.Mundschau L.J., Faller D.V. Endogenous inhibitors of the dsRNA-dependent eIF-2 alpha protein kinase PKR in normal and ras-transformed cells. Biochimie. 1994;76:792–800. doi: 10.1016/0300-9084(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 37.Sarinella F., Calistri A., Sette P., Palu G., Parolin C. Oncolysis of pancreatic tumour cells by a gamma34.5-deleted HSV-1 does not rely upon Ras-activation, but on the PI 3-kinase pathway. Gene Ther. 2006;13:1080–1087. doi: 10.1038/sj.gt.3302770. [DOI] [PubMed] [Google Scholar]
- 38.Au G.G., Lindberg A.M., Barry R.D., Shafren D.R. Oncolysis of vascular malignant human melanoma tumors by Coxsackievirus A21. Int. J. Oncol. 2005;26:1471–1476. doi: 10.3892/ijo.26.6.1471. [DOI] [PubMed] [Google Scholar]
- 39.Shafren D.R., Au G.G., Nguyen T., Newcombe N.G., Haley E.S., Beagley L., Johansson E.S., Hersey P., Barry R.D. Systemic therapy of malignant human melanoma tumors by a common cold-producing enterovirus, coxsackievirus A21. Clin. Cancer Res. 2004;10:53–60. doi: 10.1158/1078-0432.ccr-0690-3. [DOI] [PubMed] [Google Scholar]
- 40.Shafren D.R., Sylvester D., Johansson E.S., Campbell I.G., Barry R.D. Oncolysis of human ovarian cancers by echovirus type 1. Int. J. Cancer. 2005;115:320–328. doi: 10.1002/ijc.20866. [DOI] [PubMed] [Google Scholar]
- 41.Ansardi D.C., Porter D.C., Jackson C.A., Gillespie G.Y., Morrow C.D. RNA replicons derived from poliovirus are directly oncolytic for human tumor cells of diverse origins. Cancer Res. 2001;61:8470–8479. [PubMed] [Google Scholar]
- 42.Ochiai H., Campbell S.A., Archer G.E., Chewning T.A., Dragunsky E., Ivanov A., Gromeier M., Sampson J.H. Targeted therapy for glioblastoma multiforme neoplastic meningitis with intrathecal delivery of an oncolytic recombinant poliovirus. Clin. Cancer Res. 2006;12:1349–1354. doi: 10.1158/1078-0432.CCR-05-1595. [DOI] [PubMed] [Google Scholar]
- 43.Tseng J.C., Hurtado A., Yee H., Levin B., Boivin C., Benet M., Blank S.V., Pellicer A., Meruelo D. Using sindbis viral vectors for specific detection and suppression of advanced ovarian cancer in animal models. Cancer Res. 2004;64:6684–6692. doi: 10.1158/0008-5472.CAN-04-1924. [DOI] [PubMed] [Google Scholar]
- 44.Reichard K.W., Lorence R.M., Cascino C.J., Peeples M.E., Walter R.J., Fernando M.B., Reyes H.M., Greager J.A. Newcastle disease virus selectively kills human tumor cells. J. Surg. Res. 1992;52:448–453. doi: 10.1016/0022-4804(92)90310-v. [DOI] [PubMed] [Google Scholar]
- 45.Vähä-Koskela M.J., Kallio J.P., Jansson L.C., Heikkilä J.E., Zakhartchenko V.A., Kallajoki M.A., Kähäri V.M., Hinkkanen A.E. Oncolytic capacity of attenuated replicative semliki forest virus in human melanoma xenografts in severe combined immunodeficient mice. Cancer Res. 2006;66:7185–7194. doi: 10.1158/0008-5472.CAN-05-2214. [DOI] [PubMed] [Google Scholar]
- 46.Myers R., Greiner S., Harvey M., Soeffker D., Frenzke M., Abraham K., Shaw A., Rozenblatt S., Federspiel M.J., Russell S.J., Peng K.W. Oncolytic activities of approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene Ther. 2005;12:593–599. doi: 10.1038/sj.cgt.7700823. [DOI] [PubMed] [Google Scholar]
- 47.Kirn D., Martuza R.L., Zwiebel J. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat. Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- 48.Chawla-Sarkar M., Leaman D.W., Jacobs B.S., Tuthill R.J., Chatterjee-Kishore M., Stark G.R., Borden E.C. Resistance to interferons in melanoma cells does not correlate with the expression or activation of signal transducer and activator of transcription 1 (Stat1) J. Interferon Cytokine Res. 2002;22:603–613. doi: 10.1089/10799900252982089. [DOI] [PubMed] [Google Scholar]
- 49.Krishnamurthy S., Takimoto T., Scroggs R.A., Portner A. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J. Virol. 2006;80:5145–5155. doi: 10.1128/JVI.02618-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong L.H., Krauer K.G., Hatzinisiriou I., Estcourt M.J., Hersey P., Tam N.D., Edmondson S., Devenish R.J., Ralph S.J. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3gamma. J. Biol. Chem. 1997;272:28779–28785. doi: 10.1074/jbc.272.45.28779. [DOI] [PubMed] [Google Scholar]
- 51.Stojdl D.F., Lichty B., Knowles S., Marius R., Atkins H., Sonenberg N., Bell J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 52.Stojdl D.F., Lichty B.D., tenOever B.R., Paterson J.M., Power A.T., Knowles S., Marius R., Reynard J., Poliquin L., Atkins H., Brown E.G., Durbin R.K., Durbin J.E., Hiscott J., Bell J.C. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 53.Jiang G., Li J., Zeng Z., Xian L. Lentivirus-mediated gene therapy by suppressing survivin in BALB/c nude mice bearing oral squamous cell carcinoma. Cancer Biol. Ther. 2006;5:435–440. doi: 10.4161/cbt.5.4.2542. [DOI] [PubMed] [Google Scholar]
- 54.Cornelis J.J., Lang S.I., Stroh-Dege A.Y., Balboni G., Dinsart C., Rommelaere J. Cancer gene therapy through autonomous parvovirus-mediated gene transfer. Curr. Gene Ther. 2004;4:249–261. doi: 10.2174/1566523043346228. [DOI] [PubMed] [Google Scholar]
- 55.Iseki H., Shimizukawa R., Sugiyama F., Kunita S., Iwama A., Onodera M., Nakauchi H., Yagami K. Parvovirus nonstructural proteins induce an epigenetic modification through histone acetylation in host genes and revert tumor malignancy to benignancy. J. Virol. 2005;79:8886–8893. doi: 10.1128/JVI.79.14.8886-8893.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McKisic M.D., Paturzo F.X., Smith A.L. Mouse parvovirus infection potentiates rejection of tumor allografts and modulates T cell effector functions. Transplantation. 1996;61:292–299. doi: 10.1097/00007890-199601270-00022. [DOI] [PubMed] [Google Scholar]
- 57.Erlach K.C., Bohm V., Seckert C.K., Reddehase M.J., Podlech J. Lymphoma cell apoptosis in the liver induced by distant murine cytomegalovirus infection. J. Virol. 2006;80:4801–4819. doi: 10.1128/JVI.80.10.4801-4819.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geoerger B., Grill J., Opolon P., Morizet J., Aubert G., Terrier-Lacombe M.J., Bressac De-Paillerets B., Barrois M., Feunteun J., Kirn D.H., Vassal G. Oncolytic activity of the E1B-55 kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xenografts. Cancer Res. 2002;62:764–772. [PubMed] [Google Scholar]
- 59.Rogulski K.R., Freytag S.O., Zhang K., Gilbert J.D., Paielli D.L., Kim J.H., Heise C.C., Kirn D.H. In vivo antitumor activity of ONYX-015 is influenced by p53 status and is augmented by radiotherapy. Cancer Res. 2000;60:1193–1196. [PubMed] [Google Scholar]
- 60.Martuza R.L., Malick A., Markert J.M., Ruffner K.L., Coen D.M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 61.Smyth J.W., Fleeton M.N., Sheahan B.J., Atkins G.J. Treatment of rapidly growing K-BALB and CT26 mouse tumours using Semliki Forest virus and its derived vector. Gene Ther. 2005;12:147–159. doi: 10.1038/sj.gt.3302390. [DOI] [PubMed] [Google Scholar]
- 62.Singh P., Destito G., Schneemann A., Manchester M. Canine parvovirus-like particles, a novel nanomaterial for tumor targeting. J. Nanobiotechnol. 2006;4:2. doi: 10.1186/1477-3155-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kinoh H., Inoue M., Washizawa K., Yamamoto T., Fujikawa S., Tokusumi Y., Iida A., Nagai Y., Hasegawa M. Generation of a recombinant Sendai virus that is selectively activated and lyses human tumor cells expressing matrix metalloproteinases. Gene Ther. 2004;11:1137–1145. doi: 10.1038/sj.gt.3302272. [DOI] [PubMed] [Google Scholar]
- 64.Bateman A., Bullough F., Murphy S., Emiliusen L., Lavillette D., Cosset F.L., Cattaneo R., Russell S.J., Vile R.G. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res. 2000;60:1492–1497. [PubMed] [Google Scholar]
- 65.Dingwell K.S., Johnson D.C. The herpes simplex virus gE–gI complex facilitates cell-to-cell spread and binds to components of cell junctions. J. Virol. 1998;72:8933–8942. doi: 10.1128/jvi.72.11.8933-8942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Zeijst B.A., Noyes B.E., Mirault M.E., Parker B., Osterhaus A.D., Swyryd E.A., Bleumink N., Horzinek M.C., Stark G.R. Persistent infection of some standard cell lines by lymphocytic choriomeningitis virus: transmission of infection by an intracellular agent. J. Virol. 1983;48:249–261. doi: 10.1128/jvi.48.1.249-261.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rolls M.M., Webster P., Balba N.H., Rose J.K. Novel infectious particles generated by expression of the vesicular stomatitis virus glycoprotein from a self-replicating RNA. Cell. 1994;79:497–506. doi: 10.1016/0092-8674(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 68.Noguiez-Hellin P., Meur M.R., Salzmann J.L., Klatzmann D. Plasmoviruses: nonviral/viral vectors for gene therapy. Proc. Natl. Acad. Sci. USA. 1996;93:4175–4180. doi: 10.1073/pnas.93.9.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phuangsab A., Lorence R.M., Reichard K.W., Peeples M.E., Walter R.J. Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration. Cancer Lett. 2001;172:27–36. doi: 10.1016/s0304-3835(01)00617-6. [DOI] [PubMed] [Google Scholar]
- 70.Wang C.Y., Li F., Yang Y., Guo H.Y., Wu C.X., Wang S. Recombinant baculovirus containing the diphtheria toxin A gene for malignant glioma therapy. Cancer Res. 2006;66:5798–5806. doi: 10.1158/0008-5472.CAN-05-4514. [DOI] [PubMed] [Google Scholar]
- 71.Mäkelä A.R., Matilainen H., White D.J., Ruoslahti E., Oker-Blom C. Enhanced baculovirus-mediated transduction of human cancer cells by tumor-homing peptides. J. Virol. 2006;80:6603–6611. doi: 10.1128/JVI.00528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Griffith T.S., Kawakita M., Tian J., Ritchey J., Tartaglia J., Sehgal I., Thompson T.C., Zhao W., Ratliff T.L. Inhibition of murine prostate tumor growth and activation of immunoregulatory cells with recombinant canarypox viruses. J. Natl. Cancer Inst. 2001;93:998–1007. doi: 10.1093/jnci/93.13.998. [DOI] [PubMed] [Google Scholar]
- 73.Pokorna D., Cerovska N., Smahel M., Moravec T., Ludvikova V., Mackova J., Synkova H., Duskova M., Hozak P., Veleminsky J. DNA vaccines based on chimeric potyvirus-like particles carrying HPV16 E7 peptide (aa 44–60) Oncol. Rep. 2005;14:1045–1053. [PubMed] [Google Scholar]
- 74.Eriksson F., Culp W.D., Massey R., Egevad L., Garland D., Persson M.A., Pisa P. Tumor specific phage particles promote tumor regression in a mouse melanoma model. Cancer Immunol. Immunother. 2006 doi: 10.1007/s00262-006-0227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Labrada L., Bodelon G., Vinuela J., Benavente J. Avian reoviruses cause apoptosis in cultured cells: viral uncoating, but not viral gene expression, is required for apoptosis induction. J. Virol. 2002;76:7932–7941. doi: 10.1128/JVI.76.16.7932-7941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maddika S., Mendoza F.J., Hauff K., Zamzow C.R., Paranjothy T., Los M. Cancer-selective therapy of the future: apoptin and its mechanism of action. Cancer Biol. Ther. 2006;5:10–19. doi: 10.4161/cbt.5.1.2400. [DOI] [PubMed] [Google Scholar]
- 77.Olijslagers S., Dege A.Y., Dinsart C., Voorhoeve M., Rommelaere J., Noteborn M.H., Cornelis J.J. Potentiation of a recombinant oncolytic parvovirus by expression of Apoptin. Cancer Gene Ther. 2001;8:958–965. doi: 10.1038/sj.cgt.7700392. [DOI] [PubMed] [Google Scholar]
- 78.Pietersen A.M., van der Eb M.M., Rademaker H.J., van den Wollenberg D.J., Rabelink M.J., Kuppen P.J., van Dierendonck J.H., van O.H., Masman D., Van de Velde C.J., Van der Eb A.J., Hoeben R.C., Noteborn M.H. Specific tumor-cell killing with adenovirus vectors containing the apoptin gene. Gene Ther. 1999;6:882–892. doi: 10.1038/sj.gt.3300876. [DOI] [PubMed] [Google Scholar]
- 79.Butel J.S. Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis. 2000;21:405–426. doi: 10.1093/carcin/21.3.405. [DOI] [PubMed] [Google Scholar]
- 80.Hacein-Bey-Abina S., von K.C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E., Sorensen R., Forster A., Fraser P., Cohen J.I., de Saint B.G., Alexander I., Wintergerst U., Frebourg T., Aurias A., Stoppa-Lyonnet D., Romana S., Radford-Weiss I., Gross F., Valensi F., Delabesse E., Macintyre E., Sigaux F., Soulier J., Leiva L.E., Wissler M., Prinz C., Rabbitts T.H., Le D.F., Fischer A., Cavazzana-Calvo M. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 81.Sinn P.L., Sauter S.L., McCray P.B., Jr. Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors – design, biosafety, and production. Gene Ther. 2005;12:1089–1098. doi: 10.1038/sj.gt.3302570. [DOI] [PubMed] [Google Scholar]
- 82.Ott M.G., Schmidt M., Schwarzwaelder K., Stein S., Siler U., Koehl U., Glimm H., Kuhlcke K., Schilz A., Kunkel H., Naundorf S., Brinkmann A., Deichmann A., Fischer M., Ball C., Pilz I., Dunbar C., Du Y., Jenkins N.A., Copeland N.G., Luthi U., Hassan M., Thrasher A.J., Hoelzer D., von K.C., Seger R., Grez M. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 83.Recchia A., Bonini C., Magnani Z., Urbinati F., Sartori D., Muraro S., Tagliafico E., Bondanza A., Stanghellini M.T., Bernardi M., Pescarollo A., Ciceri F., Bordignon C., Mavilio F. Retroviral vector integration deregulates gene expression but has no consequence on the biology and function of transplanted T cells. Proc. Natl. Acad. Sci. USA. 2006;103:1457–1462. doi: 10.1073/pnas.0507496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garcia M.A., Guerra S., Gil J., Jimenez V., Esteban M. Anti-apoptotic and oncogenic properties of the dsRNA-binding protein of vaccinia virus, E3L. Oncogene. 2002;21:8379–8387. doi: 10.1038/sj.onc.1206036. [DOI] [PubMed] [Google Scholar]
- 85.Cordelier P., Bienvenu C., Lulka H., Marrache F., Bouisson M., Openheim A., Strayer D.S., Vaysse N., Pradayrol L., Buscail L. Replication-deficient rSV40 mediate pancreatic gene transfer and long-term inhibition of tumor growth. Cancer Gene Ther. 2006 doi: 10.1038/sj.cgt.7700987. [DOI] [PubMed] [Google Scholar]
- 86.Strayer D.S., Cordelier P., Kondo R., Liu B., Matskevich A.A., McKee H.J., Nichols C.N., Mitchell C.B., Geverd D.A., White M.K., Strayer M.S. What they are, how they work and why they do what they do? The story of SV40-derived gene therapy vectors and what they have to offer. Curr. Gene Ther. 2005;5:151–165. doi: 10.2174/1566523053544281. [DOI] [PubMed] [Google Scholar]
- 87.Hellebrand E., Mautner J., Reisbach G., Nimmerjahn F., Hallek M., Mocikat R., Hammerschmidt W. Epstein–Barr virus vector-mediated gene transfer into human B cells: potential for antitumor vaccination. Gene Ther. 2006;13:150–162. doi: 10.1038/sj.gt.3302602. [DOI] [PubMed] [Google Scholar]
- 88.Fu X., Tao L., Cai R., Prigge J., Zhang X. A mutant type 2 herpes simplex virus deleted for the protein kinase domain of the ICP10 gene is a potent oncolytic virus. Mol. Ther. 2006;13:882–890. doi: 10.1016/j.ymthe.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 89.Aghi M., Martuza R.L. Oncolytic viral therapies – the clinical experience. Oncogene. 2005;24:7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 90.Rowe W.P., Huebner R.J., Gilmore L.K., Parrott R.H., Ward T.G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 1953;84:570–573. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- 91.Jiang H., McCormick F., Lang F.F., Gomez-Manzano C., Fueyo J. Oncolytic adenoviruses as antiglioma agents. Expert. Rev. Anticancer Ther. 2006;6:697–708. doi: 10.1586/14737140.6.5.697. [DOI] [PubMed] [Google Scholar]
- 92.Reid T., Galanis E., Abbruzzese J., Sze D., Wein L.M., Andrews J., Randlev B., Heise C., Uprichard M., Hatfield M., Rome L., Rubin J., Kirn D. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62:6070–6079. [PubMed] [Google Scholar]
- 93.Ganly I., Kirn D., Eckhardt G., Rodriguez G.I., Soutar D.S., Otto R., Robertson A.G., Park O., Gulley M.L., Heise C., Von Hoff D.D., Kaye S.B. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin. Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 94.Khuri F.R., Nemunaitis J., Ganly I., Arseneau J., Tannock I.F., Romel L., Gore M., Ironside J., MacDougall R.H., Heise C., Randlev B., Gillenwater A.M., Bruso P., Kaye S.B., Hong W.K., Kirn D.H. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat. Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 95.Nemunaitis J., Khuri F., Ganly I., Arseneau J., Posner M., Vokes E., Kuhn J., McCarty T., Landers S., Blackburn A., Romel L., Randlev B., Kaye S., Kirn D. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J. Clin. Oncol. 2001;19:289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 96.Heise C., Hermiston T., Johnson L., Brooks G., Sampson-Johannes A., Williams A., Hawkins L., Kirn D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 97.Fueyo J., Gomez-Manzano C., Alemany R., Lee P.S., McDonnell T.J., Mitlianga P., Shi Y.X., Levin V.A., Yung W.K., Kyritsis A.P. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 98.van Beusechem V., van den Doel P.B., Grill J., Pinedo H.M., Gerritsen W.R. Conditionally replicative adenovirus expressing p53 exhibits enhanced oncolytic potency. Cancer Res. 2002;62:6165–6171. [PubMed] [Google Scholar]
- 99.Merritt J.A., Roth J.A., Logothetis C.J. Clinical evaluation of adenoviral-mediated p53 gene transfer: review of INGN 201 studies. Semin. Oncol. 2001;28:105–114. doi: 10.1016/s0093-7754(01)90288-x. [DOI] [PubMed] [Google Scholar]
- 100.Vecil G.G., Lang F.F. Clinical trials of adenoviruses in brain tumors: a review of Ad-p53 and oncolytic adenoviruses. J. Neurooncol. 2003;65:237–246. doi: 10.1023/b:neon.0000003653.45635.32. [DOI] [PubMed] [Google Scholar]
- 101.Kim M., Zinn K.R., Barnett B.G., Sumerel L.A., Krasnykh V., Curiel D.T., Douglas J.T. The therapeutic efficacy of adenoviral vectors for cancer gene therapy is limited by a low level of primary adenovirus receptors on tumour cells. Eur. J. Cancer. 2002;38:1917–1926. doi: 10.1016/s0959-8049(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 102.Douglas J.T., Kim M., Sumerel L.A., Carey D.E., Curiel D.T. Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res. 2001;61:813–817. [PubMed] [Google Scholar]
- 103.Shinoura N., Yoshida Y., Tsunoda R., Ohashi M., Zhang W., Asai A., Kirino T., Hamada H. Highly augmented cytopathic effect of a fiber-mutant E1B-defective adenovirus for gene therapy of gliomas. Cancer Res. 1999;59:3411–3416. [PubMed] [Google Scholar]
- 104.Yoshida Y., Sadata A., Zhang W., Saito K., Shinoura N., Hamada H. Generation of fiber-mutant recombinant adenoviruses for gene therapy of malignant glioma. Hum. Gene Ther. 1998;9:2503–2515. doi: 10.1089/hum.1998.9.17-2503. [DOI] [PubMed] [Google Scholar]
- 105.Suzuki K., Fueyo J., Krasnykh V., Reynolds P.N., Curiel D.T., Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin. Cancer Res. 2001;7:120–126. [PubMed] [Google Scholar]
- 106.Glasgow J.N., Kremer E.J., Hemminki A., Siegal G.P., Douglas J.T., Curiel D.T. An adenovirus vector with a chimeric fiber derived from canine adenovirus type 2 displays novel tropism. Virology. 2004;324:103–116. doi: 10.1016/j.virol.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 107.Nakayama M., Both G.W., Banizs B., Tsuruta Y., Yamamoto S., Kawakami Y., Douglas J.T., Tani K., Curiel D.T., Glasgow J.N. An adenovirus serotype 5 vector with fibers derived from ovine atadenovirus demonstrates CAR-independent tropism and unique biodistribution in mice. Virology. 2006;350:103–115. doi: 10.1016/j.virol.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 108.Stoff-Khalili M.A., Rivera A.A., Glasgow J.N., Le L.P., Stoff A., Everts M., Tsuruta Y., Kawakami Y., Bauerschmitz G.J., Mathis J.M., Pereboeva L., Seigal G.P., Dall P., Curiel D.T. A human adenoviral vector with a chimeric fiber from canine adenovirus type 1 results in novel expanded tropism for cancer gene therapy. Gene Ther. 2005;12:1696–1706. doi: 10.1038/sj.gt.3302588. [DOI] [PubMed] [Google Scholar]
- 109.Bernt K.M., Ni S., Gaggar A., Li Z.Y., Shayakhmetov D.M., Lieber A. The effect of sequestration by nontarget tissues on anti-tumor efficacy of systemically applied, conditionally replicating adenovirus vectors. Mol. Ther. 2003;8:746–755. doi: 10.1016/j.ymthe.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 110.Mathis J.M., Stoff-Khalili M.A., Curiel D.T. Oncolytic adenoviruses – selective retargeting to tumor cells. Oncogene. 2005;24:7775–7791. doi: 10.1038/sj.onc.1209044. [DOI] [PubMed] [Google Scholar]
- 111.Hemminki A., Kanerva A., Kremer E.J., Bauerschmitz G.J., Smith B.F., Liu B., Wang M., Desmond R.A., Keriel A., Barnett B., Baker H.J., Siegal G.P., Curiel D.T. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol. Ther. 2003;7:163–173. doi: 10.1016/s1525-0016(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 112.Bangari D.S., Shukla S., Mittal S.K. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem. Biophys. Res. Commun. 2005;327:960–966. doi: 10.1016/j.bbrc.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 113.Löser P., Hüser A., Hillgenberg M., Kümin D., Both G.W., Hofmann C. Advances in the development of non-human viral DNA-vectors for gene delivery. Curr. Gene Ther. 2002;2:161–171. doi: 10.2174/1566523024605555. [DOI] [PubMed] [Google Scholar]
- 114.Voeks D., Martiniello-Wilks R., Madden V., Smith K., Bennetts E., Both G.W., Russell P.J. Gene therapy for prostate cancer delivered by ovine adenovirus and mediated by purine nucleoside phosphorylase and fludarabine in mouse models. Gene Ther. 2002;9:759–768. doi: 10.1038/sj.gt.3301698. [DOI] [PubMed] [Google Scholar]
- 115.Wang X.Y., Martiniello-Wilks R., Shaw J.M., Ho T., Coulston N., Cooke-Yarborough C., Molloy P.L., Cameron F., Moghaddam M., Lockett T.J., Webster L.K., Smith I.K., Both G.W., Russell P.J. Preclinical evaluation of a prostate-targeted gene-directed enzyme prodrug therapy delivered by ovine atadenovirus. Gene Ther. 2004;11:1559–1567. doi: 10.1038/sj.gt.3302308. [DOI] [PubMed] [Google Scholar]
- 116.Shashkova E.V., Cherenova L.V., Kazansky D.B., Doronin K. Avian adenovirus vector CELO-TK displays anticancer activity in human cancer cells and suppresses established murine melanoma tumors. Cancer Gene Ther. 2005;12:617–626. doi: 10.1038/sj.cgt.7700822. [DOI] [PubMed] [Google Scholar]
- 117.Varghese S., Rabkin S.D. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9:967–978. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 118.Epstein A.L., Marconi P., Argnani R., Manservigi R. HSV-1-derived recombinant and amplicon vectors for gene transfer and gene therapy. Curr. Gene Ther. 2005;5:445–458. doi: 10.2174/156652305774329285. [DOI] [PubMed] [Google Scholar]
- 119.Post D.E., Fulci G., Chiocca E.A., Van Meir E.G. Replicative oncolytic herpes simplex viruses in combination cancer therapies. Curr. Gene Ther. 2004;4:41–51. doi: 10.2174/1566523044577988. [DOI] [PubMed] [Google Scholar]
- 120.Markert J.M., Medlock M.D., Rabkin S.D., Gillespie G.Y., Todo T., Hunter W.D., Palmer C.A., Feigenbaum F., Tornatore C., Tufaro F., Martuza R.L. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 121.Todo T., Martuza R.L., Rabkin S.D., Johnson P.A. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc. Natl. Acad. Sci. USA. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakao A., Kimata H., Imai T., Kikumori T., Teshigahara O., Nagasaka T., Goshima F., Nishiyama Y. Intratumoral injection of herpes simplex virus HF10 in recurrent breast cancer. Ann. Oncol. 2004;15:988–989. doi: 10.1093/annonc/mdh225. [DOI] [PubMed] [Google Scholar]
- 123.Taneja S., MacGregor J., Markus S., Ha S., Mohr I. Enhanced antitumor efficacy of a herpes simplex virus mutant isolated by genetic selection in cancer cells. Proc. Natl. Acad. Sci. USA. 2001;98:8804–8808. doi: 10.1073/pnas.161011798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu B.L., Robinson M., Han Z.Q., Branston R.H., English C., Reay P., McGrath Y., Thomas S.K., Thornton M., Bullock P., Love C.A., Coffin R.S. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 125.Nakamori M., Fu X., Meng F., Jin A., Tao L., Bast R.C., Jr., Zhang X. Effective therapy of metastatic ovarian cancer with an oncolytic herpes simplex virus incorporating two membrane fusion mechanisms. Clin. Cancer Res. 2003;9:2727–2733. [PubMed] [Google Scholar]
- 126.Gillet L., Dewals B., Farnir F., de L.L., Vanderplasschen A. Bovine herpesvirus 4 induces apoptosis of human carcinoma cell lines in vitro and in vivo. Cancer Res. 2005;65:9463–9472. doi: 10.1158/0008-5472.CAN-05-1076. [DOI] [PubMed] [Google Scholar]
- 127.Griffiths R.A., Boyne J.R., Whitehouse A. Herpesvirus saimiri-based gene delivery vectors. Curr. Gene Ther. 2006;6:1–15. doi: 10.2174/156652306775515529. [DOI] [PubMed] [Google Scholar]
- 128.Stevenson A.J., Giles M.S., Hall K.T., Goodwin D.J., Calderwood M.A., Markham A.F., Whitehouse A. Specific oncolytic activity of herpesvirus saimiri in pancreatic cancer cells. Br. J. Cancer. 2000;83:329–332. doi: 10.1054/bjoc.2000.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Boldogköi Z., Nógrádi A. Gene and cancer therapy – pseudorabies virus: a novel research and therapeutic tool? Curr. Gene Ther. 2003;3:155–182. doi: 10.2174/1566523034578393. [DOI] [PubMed] [Google Scholar]
- 130.Frenkel N., Borenstein R. Characterization of the lymphotropic Amplicons-6 and Tamplicon-7 vectors derived from HHV-6 and HHV-7. Curr. Gene Ther. 2006;6:399–420. doi: 10.2174/156652306777592036. [DOI] [PubMed] [Google Scholar]
- 131.Wollmann G., Tattersall P., van den Pol A.N. Targeting human glioblastoma cells: comparison of nine viruses with oncolytic potential. J. Virol. 2005;79:6005–6022. doi: 10.1128/JVI.79.10.6005-6022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guo Z.S., Naik A., O’Malley M.E., Popovic P., Demarco R., Hu Y., Yin X., Yang S., Zeh H.J., Moss B., Lotze M.T., Bartlett D.L. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res. 2005;65:9991–9998. doi: 10.1158/0008-5472.CAN-05-1630. [DOI] [PubMed] [Google Scholar]
- 133.McCart J.A., Ward J.M., Lee J., Hu Y., Alexander H.R., Libutti S.K., Moss B., Bartlett D.L. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
- 134.Kim J.H., Oh J.Y., Park B.H., Lee D.E., Kim J.S., Park H.E., Roh M.S., Je J.E., Yoon J.H., Thorne S.H., Kirn D., Hwang T.H. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 135.Mastrangelo M.J., Lattime E.C. Virotherapy clinical trials for regional disease: in situ immune modulation using recombinant poxvirus vectors. Cancer Gene Ther. 2002;9:1013–1021. doi: 10.1038/sj.cgt.7700538. [DOI] [PubMed] [Google Scholar]
- 136.Arlen P.M., Pazdur M., Skarupa L., Rauckhorst M., Gulley J.L. A randomized phase II study of docetaxel alone or in combination with PANVAC-V (vaccinia) and PANVAC-F (fowlpox) in patients with metastatic breast cancer (NCI 05-C-0229) Clin. Breast Cancer. 2006;7:176–179. doi: 10.3816/CBC.2006.n.032. [DOI] [PubMed] [Google Scholar]
- 137.Petrulio C.A., Kaufman H.L. Development of the PANVAC-VF vaccine for pancreatic cancer. Expert. Rev. Vaccines. 2006;5:9–19. doi: 10.1586/14760584.5.1.9. [DOI] [PubMed] [Google Scholar]
- 138.Hu Y., Lee J., McCart J.A., Xu H., Moss B., Alexander H.R., Bartlett D.L. Yaba-like disease virus: an alternative replicating poxvirus vector for cancer gene therapy. J. Virol. 2001;75:10300–10308. doi: 10.1128/JVI.75.21.10300-10308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lun X., Yang W., Alain T., Shi Z.Q., Muzik H., Barrett J.W., McFadden G., Bell J., Hamilton M.G., Senger D.L., Forsyth P.A. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005;65:9982–9990. doi: 10.1158/0008-5472.CAN-05-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mousset S., Rommelaere J. Minute virus of mice inhibits cell transformation by simian virus 40. Nature. 1982;300:537–539. doi: 10.1038/300537a0. [DOI] [PubMed] [Google Scholar]
- 141.Bantel-Schaal U. Growth properties of a human melanoma cell line are altered by adeno-associated parvovirus type 2. Int. J. Cancer. 1995;60:269–274. doi: 10.1002/ijc.2910600223. [DOI] [PubMed] [Google Scholar]
- 142.Li C., Bowles D.E., van D.T., Samulski R.J. Adeno-associated virus vectors: potential applications for cancer gene therapy. Cancer Gene Ther. 2005;12:913–925. doi: 10.1038/sj.cgt.7700876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hacker U.T., Wingenfeld L., Kofler D.M., Schuhmann N.K., Lutz S., Herold T., King S.B., Gerner F.M., Perabo L., Rabinowitz J., McCarty D.M., Samulski R.J., Hallek M., Buning H. Adeno-associated virus serotypes 1 to 5 mediated tumor cell directed gene transfer and improvement of transduction efficiency. J. Gene Med. 2005;7:1429–1438. doi: 10.1002/jgm.782. [DOI] [PubMed] [Google Scholar]
- 144.Harding T.C., Lalani A.S., Roberts B.N., Yendluri S., Luan B., Koprivnikar K.E., Gonzalez-Edick M., Huan-Tu G., Musterer R., VanRoey M.J., Ozawa T., LeCouter R.A., Deen D., Dickinson P.J., Jooss K. AAV serotype 8-mediated gene delivery of a soluble VEGF receptor to the CNS for the treatment of glioblastoma. Mol. Ther. 2006;13:956–966. doi: 10.1016/j.ymthe.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 145.Dupressoir T., Vanacker J.M., Cornelis J.J., Duponchel N., Rommelaere J. Inhibition by parvovirus H-1 of the formation of tumors in nude mice and colonies in vitro by transformed human mammary epithelial cells. Cancer Res. 1989;49:3203–3208. [PubMed] [Google Scholar]
- 146.Dupont F., Avalosse B., Karim A., Mine N., Bosseler M., Maron A., Van den Broeke A.V., Ghanem G.E., Burny A., Zeicher M. Tumor-selective gene transduction and cell killing with an oncotropic autonomous parvovirus-based vector. Gene Ther. 2000;7:790–796. doi: 10.1038/sj.gt.3301161. [DOI] [PubMed] [Google Scholar]
- 147.Maxwell I.H., Chapman J.T., Scherrer L.C., Spitzer A.L., Leptihn S., Maxwell F., Corsini J.A. Expansion of tropism of a feline parvovirus to target a human tumor cell line by display of an alpha(v) integrin binding peptide on the capsid. Gene Ther. 2001;8:324–331. doi: 10.1038/sj.gt.3301399. [DOI] [PubMed] [Google Scholar]
- 148.Hirasawa K., Nishikawa S.G., Norman K.L., Coffey M.C., Thompson B.G., Yoon C.S., Waisman D.M., Lee P.W. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63:348–353. [PubMed] [Google Scholar]
- 149.Yang W.Q., Senger D.L., Lun X.Q., Muzik H., Shi Z.Q., Dyck R.H., Norman K., Brasher P.M., Rewcastle N.B., George D., Stewart D., Lee P.W., Forsyth P.A. Reovirus as an experimental therapeutic for brain and leptomeningeal metastases from breast cancer. Gene Ther. 2004;11:1579–1589. doi: 10.1038/sj.gt.3302319. [DOI] [PubMed] [Google Scholar]
- 150.Norman K.L., Lee P.W. Not all viruses are bad guys: the case for reovirus in cancer therapy. Drug Discov. Today. 2005;10:847–855. doi: 10.1016/S1359-6446(05)03483-5. [DOI] [PubMed] [Google Scholar]
- 151.Wagner R.R. Influenza virus infection of transplanted tumors. I. Multiplication of a neurotropic strain and its effect on solid neoplasms. Cancer Res. 1954;14:377–385. [PubMed] [Google Scholar]
- 152.Chisholm J., Howe K., Taj M., Zambon M. Influenza immunisation in children with solid tumours. Eur. J. Cancer. 2005;41:2280–2287. doi: 10.1016/j.ejca.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 153.Cooksley C.D., Avritscher E.B., Bekele B.N., Rolston K.V., Geraci J.M., Elting L.S. Epidemiology and outcomes of serious influenza-related infections in the cancer population. Cancer. 2005;104:618–628. doi: 10.1002/cncr.21203. [DOI] [PubMed] [Google Scholar]
- 154.Hicks K.L., Chemaly R.F., Kontoyiannis D.P. Common community respiratory viruses in patients with cancer: more than just “common colds”. Cancer. 2003;97:2576–2587. doi: 10.1002/cncr.11353. [DOI] [PubMed] [Google Scholar]
- 155.Bergmann M., Romirer I., Sachet M., Fleischhacker R., Garcia-Sastre A., Palese P., Wolff K., Pehamberger H., Jakesz R., Muster T. A genetically engineered influenza A virus with ras-dependent oncolytic properties. Cancer Res. 2001;61:8188–8193. [PubMed] [Google Scholar]
- 156.Cassel W.A., Garrett R.E. Newcastle disease virus as an antineoplastic agent. Cancer. 1965;18:863–868. doi: 10.1002/1097-0142(196507)18:7<863::aid-cncr2820180714>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 157.Csatary L.K., Gosztonyi G., Szeberenyi J., Fabian Z., Liszka V., Bodey B., Csatary C.M. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J. Neurooncol. 2004;67:83–93. doi: 10.1023/b:neon.0000021735.85511.05. [DOI] [PubMed] [Google Scholar]
- 158.Freeman A.I., Zakay-Rones Z., Gomori J.M., Linetsky E., Rasooly L., Greenbaum E., Rozenman-Yair S., Panet A., Libson E., Irving C.S., Galun E., Siegal T. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 2006;13:221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 159.Lorence R.M., Pecora A.L., Major P.P., Hotte S.J., Laurie S.A., Roberts M.S., Groene W.S., Bamat M.K. Overview of phase I studies of intravenous administration of PV701, an oncolytic virus. Curr. Opin. Mol. Ther. 2003;5:618–624. [PubMed] [Google Scholar]
- 160.Schirrmacher V., Griesbach A., Ahlert T. Antitumor effects of Newcastle Disease Virus in vivo: local versus systemic effects. Int. J. Oncol. 2001;18:945–952. doi: 10.3892/ijo.18.5.945. [DOI] [PubMed] [Google Scholar]
- 161.Ahlert T., Sauerbrei W., Bastert G., Ruhland S., Bartik B., Simiantonaki N., Schumacher J., Hacker B., Schumacher M., Schirrmacher V. Tumor-cell number and viability as quality and efficacy parameters of autologous virus-modified cancer vaccines in patients with breast or ovarian cancer. J. Clin. Oncol. 1997;15:1354–1366. doi: 10.1200/JCO.1997.15.4.1354. [DOI] [PubMed] [Google Scholar]
- 162.Saika S., Kidokoro M., Kubonoya H., Ito K., Ohkawa T., Aoki A., Nagata N., Suzuki K. Development and biological properties of a new live attenuated mumps vaccine. Comp. Immunol. Microbiol. Infect. Dis. 2006;29:89–99. doi: 10.1016/j.cimid.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 163.Yan Y.F., Chen X., Zhu Y., Wu J.G., Dong C.Y. Selective cytolysis of tumor cells by mumps virus S79. Intervirology. 2005;48:292–296. doi: 10.1159/000085097. [DOI] [PubMed] [Google Scholar]
- 164.Parks G.D., Young V.A., Koumenis C., Wansley E.K., Layer J.L., Cooke K.M. Controlled cell killing by a recombinant nonsegmented negative-strand RNA virus. Virology. 2002;293:192–203. doi: 10.1006/viro.2001.1298. [DOI] [PubMed] [Google Scholar]
- 165.Taqi A.M., Abdurrahman M.B., Yakubu A.M., Fleming A.F. Regression of Hodgkin’s disease after measles. Lancet. 1981;1:1112. doi: 10.1016/s0140-6736(81)92286-8. [DOI] [PubMed] [Google Scholar]
- 166.Grote D., Russell S.J., Cornu T.I., Cattaneo R., Vile R., Poland G.A., Fielding A.K. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97:3746–3754. doi: 10.1182/blood.v97.12.3746. [DOI] [PubMed] [Google Scholar]
- 167.Peng K.W., TenEyck C.J., Galanis E., Kalli K.R., Hartmann L.C., Russell S.J. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- 168.Phuong L.K., Allen C., Peng K.W., Giannini C., Greiner S., TenEyck C.J., Mishra P.K., Macura S.I., Russell S.J., Galanis E.C. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–2469. [PubMed] [Google Scholar]
- 169.Heinzerling L., Kunzi V., Oberholzer P.A., Kundig T., Naim H., Dummer R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood. 2005;106:2287–2294. doi: 10.1182/blood-2004-11-4558. [DOI] [PubMed] [Google Scholar]
- 170.Barber G.N. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- 171.Shinozaki K., Ebert O., Kournioti C., Tai Y.S., Woo S.L. Oncolysis of multifocal hepatocellular carcinoma in the rat liver by hepatic artery infusion of vesicular stomatitis virus. Mol. Ther. 2004;9:368–376. doi: 10.1016/j.ymthe.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 172.Ebert O., Shinozaki K., Kournioti C., Park M.S., Garcia-Sastre A., Woo S.L. Syncytia induction enhances the oncolytic potential of vesicular stomatitis virus in virotherapy for cancer. Cancer Res. 2004;64:3265–3270. doi: 10.1158/0008-5472.can-03-3753. [DOI] [PubMed] [Google Scholar]
- 173.Fernandez M., Porosnicu M., Markovic D., Barber G.N. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J. Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shinozaki K., Ebert O., Suriawinata A., Thung S.N., Woo S.L. Prophylactic alpha interferon treatment increases the therapeutic index of oncolytic vesicular stomatitis virus virotherapy for advanced hepatocellular carcinoma in immune-competent rats. J. Virol. 2005;79:13705–13713. doi: 10.1128/JVI.79.21.13705-13713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ebert O., Harbaran S., Shinozaki K., Woo S.L. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Ther. 2005;12:350–358. doi: 10.1038/sj.cgt.7700794. [DOI] [PubMed] [Google Scholar]
- 176.Lun X., Senger D.L., Alain T., Oprea A., Parato K., Stojdl D., Lichty B., Power A., Johnston R.N., Hamilton M., Parney I., Bell J.C., Forsyth P.A. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(deltaM51)) on multifocal and invasive gliomas. J. Natl. Cancer Inst. 2006;98:1546–1557. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- 177.Verheije M.H., Wurdinger T., van Beusechem V., de Haan C.A., Gerritsen W.R., Rottier P.J. Redirecting coronavirus to a nonnative receptor through a virus-encoded targeting adapter. J. Virol. 2006;80:1250–1260. doi: 10.1128/JVI.80.3.1250-1260.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wurdinger T., Verheije M.H., Raaben M., Bosch B.J., de Haan C.A., van Beusechem V., Rottier P.J., Gerritsen W.R. Targeting non-human coronaviruses to human cancer cells using a bispecific single-chain antibody. Gene Ther. 2005;12:1394–1404. doi: 10.1038/sj.gt.3302535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gromeier M., Lachmann S., Rosenfeld M.R., Gutin P.H., Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc. Natl. Acad. Sci. USA. 2000;97:6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Toyoda H., Ido M., Hayashi T., Gabazza E.C., Suzuki K., Kisenge R.R., Kang J., Hori H., Komada Y. Experimental treatment of human neuroblastoma using live-attenuated poliovirus. Int. J. Oncol. 2004;24:49–58. [PubMed] [Google Scholar]
- 181.Suskind R.G., Huebner R.J., Rowe W.P., Love R. Viral agents oncolytic for human tumors in heterologous host; oncolytic effect of Coxsackie B viruses. Proc. Soc. Exp. Biol. Med. 1957;94:309–318. doi: 10.3181/00379727-94-22931. [DOI] [PubMed] [Google Scholar]
- 182.Parato K.A., Senger D., Forsyth P.A., Bell J.C. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 183.Smyth M., Symonds A., Brazinova S., Martin J. Bovine enterovirus as an oncolytic virus: foetal calf serum facilitates its infection of human cells. Int. J. Mol. Med. 2002;10:49–53. doi: 10.3892/ijmm.10.1.49. [DOI] [PubMed] [Google Scholar]
- 184.Tigertt W.D., Crosby W.H., Berge T.O., Howie D.L., Kress S., Dangerfield H.G., Bass J.W., Frank W. The virus of Venezuelan equine encephalomyelitis as an antineoplastic agent in man. Cancer. 1962;15:628–632. doi: 10.1002/1097-0142(196205/06)15:3<628::aid-cncr2820150326>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 185.Nilsen T.W., Wood D.L., Baglioni C. Virus-specific effects of interferon in embryonal carcinoma cells. Nature. 1980;286:178–180. doi: 10.1038/286178a0. [DOI] [PubMed] [Google Scholar]
- 186.Tseng J.C., Levin B., Hirano T., Yee H., Pampeno C., Meruelo D. In vivo antitumor activity of Sindbis viral vectors. J. Natl. Cancer Inst. 2002;94:1790–1802. doi: 10.1093/jnci/94.23.1790. [DOI] [PubMed] [Google Scholar]
- 187.Tseng J.C., Levin B., Hurtado A., Yee H., Perez de Castro I., Jimenez M., Shamamian P., Jin R., Novick R.P., Pellicer A., Meruelo D. Systemic tumor targeting and killing by Sindbis viral vectors. Nat. Biotechnol. 2004;22:70–77. doi: 10.1038/nbt917. [DOI] [PubMed] [Google Scholar]
- 188.Unno Y., Shino Y., Kondo F., Igarashi N., Wang G., Shimura R., Yamaguchi T., Asano T., Saisho H., Sekiya S., Shirasawa H. Oncolytic viral therapy for cervical and ovarian cancer cells by Sindbis virus AR339 strain. Clin. Cancer Res. 2005;11:4553–4560. doi: 10.1158/1078-0432.CCR-04-2610. [DOI] [PubMed] [Google Scholar]
- 189.Rodriguez-Madoz J.R., Prieto J., Smerdou C. Semliki forest virus vectors engineered to express higher IL-12 levels induce efficient elimination of murine colon adenocarcinomas. Mol. Ther. 2005;12:153–163. doi: 10.1016/j.ymthe.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 190.Tai C.K., Wang W.J., Chen T.C., Kasahara N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol. Ther. 2005;12:842–851. doi: 10.1016/j.ymthe.2005.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Solly S.K., Trajcevski S., Frisen C., Holzer G.W., Nelson E., Clerc B., bordo-Adesida E., Castro M., Lowenstein P., Klatzmann D. Replicative retroviral vectors for cancer gene therapy. Cancer Gene Ther. 2003;10:30–39. doi: 10.1038/sj.cgt.7700521. [DOI] [PubMed] [Google Scholar]
- 192.Ram Z., Culver K.W., Oshiro E.M., Viola J.J., DeVroom H.L., Otto E., Long Z., Chiang Y., McGarrity G.J., Muul L.M., Katz D., Blaese R.M., Oldfield E.H. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat. Med. 1997;3:1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 193.Pulkkanen K.J., Ylä-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol. Ther. 2005;12:585–598. doi: 10.1016/j.ymthe.2005.07.357. [DOI] [PubMed] [Google Scholar]
- 194.Colombo F., Barzon L., Franchin E., Pacenti M., Pinna V., Danieli D., Zanusso M., Palu G. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: biological and clinical results. Cancer Gene Ther. 2005;12:835–848. doi: 10.1038/sj.cgt.7700851. [DOI] [PubMed] [Google Scholar]
- 195.Pellinen R., Hakkarainen T., Wahlfors T., Tulimaki K., Ketola A., Tenhunen A., Salonen T., Wahlfors J. Cancer cells as targets for lentivirus-mediated gene transfer and gene therapy. Int. J. Oncol. 2004;25:1753–1762. [PubMed] [Google Scholar]
- 196.Heinkelein M., Hoffmann U., Lucke M., Imrich H., Muller J.G., Meixensberger J., Westphahl M., Kretschmer A., Rethwilm A. Experimental therapy of allogeneic solid tumors induced in athymic mice with suicide gene-transducing replication-competent foamy virus vectors. Cancer Gene Ther. 2005;12:947–953. doi: 10.1038/sj.cgt.7700855. [DOI] [PubMed] [Google Scholar]
- 197.Trobridge G.D., Miller D.G., Jacobs M.A., Allen J.M., Kiem H.P., Kaul R., Russell D.W. Foamy virus vector integration sites in normal human cells. Proc. Natl. Acad. Sci. USA. 2006;103:1498–1503. doi: 10.1073/pnas.0510046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Garattini S., Bertele V. Efficacy, safety, and cost of new anticancer drugs. BMJ. 2002;325:269–271. doi: 10.1136/bmj.325.7358.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wakimoto H., Ikeda K., Abe T., Ichikawa T., Hochberg F.H., Ezekowitz R.A., Pasternack M.S., Chiocca E.A. The complement response against an oncolytic virus is species-specific in its activation pathways. Mol. Ther. 2002;5:275–282. doi: 10.1006/mthe.2002.0547. [DOI] [PubMed] [Google Scholar]
- 200.Andreansky S.S., He B., Gillespie G.Y., Soroceanu L., Markert J., Chou J., Roizman B., Whitley R.J. The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors. Proc. Natl. Acad. Sci. USA. 1996;93:11313–11318. doi: 10.1073/pnas.93.21.11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Grote D., Cattaneo R., Fielding A.K. Neutrophils contribute to the measles virus-induced antitumor effect: enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 2003;63:6463–6468. [PubMed] [Google Scholar]
- 202.Kelland L.R. Of mice and men: values and liabilities of the athymic nude mouse model in anticancer drug development. Eur. J. Cancer. 2004;40:827–836. doi: 10.1016/j.ejca.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 203.Huneycutt B.S., Bi Z., Aoki C.J., Reiss C.S. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J. Virol. 1993;67:6698–6706. doi: 10.1128/jvi.67.11.6698-6706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Thomsen A.R., Nansen A., Andersen C., Johansen J., Marker O., Christensen J.P. Cooperation of B cells and T cells is required for survival of mice infected with vesicular stomatitis virus. Int. Immunol. 1997;9:1757–1766. doi: 10.1093/intimm/9.11.1757. [DOI] [PubMed] [Google Scholar]
- 205.Fultz P.N., Holland J.J. Differing responses of hamsters to infection by vesicular stomatitis virus Indiana and New Jersey serotypes. Virus Res. 1985;3:129–140. doi: 10.1016/0168-1702(85)90003-6. [DOI] [PubMed] [Google Scholar]
- 206.Dragunsky E., Taffs R., Chernokhvostova Y., Nomura T., Hioki K., Gardner D., Norwood L., Levenbook I. A poliovirus-susceptible transgenic mouse model as a possible replacement for the monkey neurovirulence test of oral poliovirus vaccine. Biologicals. 1996;24:77–86. doi: 10.1006/biol.1996.0010. [DOI] [PubMed] [Google Scholar]
- 207.Schneider-Schaulies J., Meulen V., Schneider-Schaulies S. Measles infection of the central nervous system. J. Neurovirol. 2003;9:247–252. doi: 10.1080/13550280390193993. [DOI] [PubMed] [Google Scholar]
- 208.Hallden G., Hill R., Wang Y., Anand A., Liu T.C., Lemoine N.R., Francis J., Hawkins L., Kirn D. Novel immunocompetent murine tumor models for the assessment of replication-competent oncolytic adenovirus efficacy. Mol. Ther. 2003;8:412–424. doi: 10.1016/s1525-0016(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 209.Bilbao R., Bustos M., Alzuguren P., Pajares M.J., Drozdzik M., Qian C., Prieto J. A blood-tumor barrier limits gene transfer to experimental liver cancer: the effect of vasoactive compounds. Gene Ther. 2000;7:1824–1832. doi: 10.1038/sj.gt.3301312. [DOI] [PubMed] [Google Scholar]
- 210.Ebert O., Shinozaki K., Huang T.G., Savontaus M.J., Garcia-Sastre A., Woo S.L. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 2003;63:3605–3611. [PubMed] [Google Scholar]
- 211.Fechner H., Haack A., Wang H., Wang X., Eizema K., Pauschinger M., Schoemaker R., Veghel R., Houtsmuller A., Schultheiss H.P., Lamers J., Poller W. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- 212.Harrison D., Sauthoff H., Heitner S., Jagirdar J., Rom W.N., Hay J.G. Wild-type adenovirus decreases tumor xenograft growth, but despite viral persistence complete tumor responses are rarely achieved–deletion of the viral E1b-19-kD gene increases the viral oncolytic effect. Hum. Gene Ther. 2001;12:1323–1332. doi: 10.1089/104303401750270977. [DOI] [PubMed] [Google Scholar]
- 213.Ikeda K., Wakimoto H., Ichikawa T., Jhung S., Hochberg F.H., Louis D.N., Chiocca E.A. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J. Virol. 2000;74:4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li Z.Y., Ni S., Yang X., Kiviat N., Lieber A. Xenograft models for liver metastasis: relationship between tumor morphology and adenovirus vector transduction. Mol. Ther. 2004;9:650–657. doi: 10.1016/j.ymthe.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 215.Shayakhmetov D.M., Li Z.Y., Ni S., Lieber A. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res. 2002;62:1063–1068. [PubMed] [Google Scholar]
- 216.Djeha A.H., Thomson T.A., Leung H., Searle P.F., Young L.S., Kerr D.J., Harris P.A., Mountain A., Wrighton C.J. Combined adenovirus-mediated nitroreductase gene delivery and CB1954 treatment: a well-tolerated therapy for established solid tumors. Mol. Ther. 2001;3:233–240. doi: 10.1006/mthe.2000.0250. [DOI] [PubMed] [Google Scholar]
- 217.Cordaro T.A., de Visser K.E., Tirion F.H., Graus Y.M., Haanen J.B., Kioussis D., Kruisbeek A.M. Tumor size at the time of adoptive transfer determines whether tumor rejection occurs. Eur. J. Immunol. 2000;30:1297–1307. doi: 10.1002/(SICI)1521-4141(200005)30:5<1297::AID-IMMU1297>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 218.Köpf-Maier P., Kestenbach U. The interaction between host-supplied connective tissue and xenografted human tumor cells. Anticancer Res. 1990;10:161–171. [PubMed] [Google Scholar]
- 219.McKee T.D., Grandi P., Mok W., Alexandrakis G., Insin N., Zimmer J.P., Bawendi M.G., Boucher Y., Breakefield X.O., Jain R.K. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 220.Cairns R., Papandreou I., Denko N. Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol. Cancer Res. 2006;4:61–70. doi: 10.1158/1541-7786.MCR-06-0002. [DOI] [PubMed] [Google Scholar]
- 221.Jain R.K. Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 2001;46:149–168. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 222.Tanaka T., Yamanaka N., Oriyama T., Furukawa K., Okamoto E. Factors regulating tumor pressure in hepatocellular carcinoma and implications for tumor spread. Hepatology. 1997;26:283–287. doi: 10.1002/hep.510260205. [DOI] [PubMed] [Google Scholar]
- 223.Stohrer M., Boucher Y., Stangassinger M., Jain R.K. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60:4251–4255. [PubMed] [Google Scholar]
- 224.Minchinton A.I., Tannock I.F. Drug penetration in solid tumours. Nat. Rev. Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 225.Kanerva A., Zinn K.R., Peng K.W., Ranki T., Kangasniemi L., Chaudhuri T.R., Desmond R.A., Wang M., Takayama K., Hakkarainen T., Alfthan H., Stenman U.H., Curiel D.T., Hemminki A. Noninvasive dual modality in vivo monitoring of the persistence and potency of a tumor targeted conditionally replicating adenovirus. Gene Ther. 2005;12:87–94. doi: 10.1038/sj.gt.3302387. [DOI] [PubMed] [Google Scholar]
- 226.Gonzalez-Garcia I., Sole R.V., Costa J. Metapopulation dynamics and spatial heterogeneity in cancer. Proc. Natl. Acad. Sci. USA. 2002;99:13085–13089. doi: 10.1073/pnas.202139299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Karev G.P., Novozhilov A.S., Koonin E.V. Mathematical modeling of tumor therapy with oncolytic viruses: effects of parametric heterogeneity on cell dynamics. Biol. Direct. 2006;1:30. doi: 10.1186/1745-6150-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Novozhilov A.S., Berezovskaya F.S., Koonin E.V., Karev G.P. Mathematical modeling of tumor therapy with oncolytic viruses: regimes with complete tumor elimination within the framework of deterministic models. Biol. Direct. 2006;1:6. doi: 10.1186/1745-6150-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Carey T.E., Van Dyke D.L., Worsham M.J. Nonrandom chromosome aberrations and clonal populations in head and neck cancer. Anticancer Res. 1993;13:2561–2567. [PubMed] [Google Scholar]
- 230.Alain T., Kim M., Johnston R.N., Urbanski S., Kossakowska A.E., Forsyth P.A., Lee P.W. The oncolytic effect in vivo of reovirus on tumour cells that have survived reovirus cell killing in vitro. Br. J. Cancer. 2006;95:1020–1027. doi: 10.1038/sj.bjc.6603363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kim Y.T., Ganly I., Brown R., Stuart D. Acquired resistance to cytolysis of the E1B-attenuated adenovirus, dl1520, in ovarian tumour cell lines. Cancer Gene Ther. 2003;10:589–590. doi: 10.1038/sj.cgt.7700607. [DOI] [PubMed] [Google Scholar]
- 232.Telerman A., Tuynder M., Dupressoir T., Robaye B., Sigaux F., Shaulian E., Oren M., Rommelaere J., Amson R. A model for tumor suppression using H-1 parvovirus. Proc. Natl. Acad. Sci. USA. 1993;90:8702–8706. doi: 10.1073/pnas.90.18.8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lopez-Guerrero J.A., Rayet B., Tuynder M., Rommelaere J., Dinsart C. Constitutive activation of U937 promonocytic cell clones selected for their resistance to parvovirus H-1 infection. Blood. 1997;89:1642–1653. [PubMed] [Google Scholar]
- 234.Cao Y., Walen K.H., Schnurr D. Coxsackievirus B-3 selection of virus resistant Buffalo green monkey kidney cells and chromosome analysis of parental and resistant cells. Arch. Virol. 1988;101:209–219. doi: 10.1007/BF01311002. [DOI] [PubMed] [Google Scholar]
- 235.Ron D., Tal J. Spontaneous curing of a minute virus of mice carrier state by selection of cells with an intracellular block of viral replication. J. Virol. 1986;58:26–30. doi: 10.1128/jvi.58.1.26-30.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Taber R., Alexander V., Wald N., Jr. The selection of virus-resistant Chinese hamster ovary cells. Cell. 1976;8:529–533. doi: 10.1016/0092-8674(76)90221-x. [DOI] [PubMed] [Google Scholar]
- 237.Rayet B., Lopez-Guerrero J.A., Rommelaere J., Dinsart C. Induction of programmed cell death by parvovirus H-1 in U937 cells: connection with the tumor necrosis factor alpha signalling pathway. J. Virol. 1998;72:8893–8903. doi: 10.1128/jvi.72.11.8893-8903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Stanziale S.F., Petrowsky H., Adusumilli P.S., Ben-Porat L., Gonen M., Fong Y. Infection with oncolytic herpes simplex virus-1 induces apoptosis in neighboring human cancer cells: a potential target to increase anticancer activity. Clin. Cancer Res. 2004;10:3225–3232. doi: 10.1158/1078-0432.ccr-1083-3. [DOI] [PubMed] [Google Scholar]
- 239.Wagner S., Csatary C.M., Gosztonyi G., Koch H.C., Hartmann C., Peters O., Hernaiz-Driever P., Theallier-Janko A., Zintl F., Langler A., Wolff J.E., Csatary L.K. Combined treatment of pediatric high-grade glioma with the oncolytic viral strain MTH-68/H and oral valproic acid. APMIS. 2006;114:731–743. doi: 10.1111/j.1600-0463.2006.apm_516.x. [DOI] [PubMed] [Google Scholar]
- 240.Zheng H., Palese P., Garcia-Sastre A. Antitumor properties of influenza virus vectors. Cancer Res. 2000;60:6972–6976. [PubMed] [Google Scholar]
- 241.Uyttenhove C., Maryanski J., Boon T. Escape of mouse mastocytoma P815 after nearly complete rejection is due to antigen-loss variants rather than immunosuppression. J. Exp. Med. 1983;157:1040–1052. doi: 10.1084/jem.157.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Uyttenhove C., Pilotte L., Theate I., Stroobant V., Colau D., Parmentier N., Boon T., Van den Eynde B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 243.Ward P.L., Koeppen H.K., Hurteau T., Rowley D.A., Schreiber H. Major histocompatibility complex class I and unique antigen expression by murine tumors that escaped from CD8+ T-cell-dependent surveillance. Cancer Res. 1990;50:3851–3858. [PubMed] [Google Scholar]
- 244.Campoli M., Ferrone S., Zea A.H., Rodriguez P.C., Ochoa A.C. Mechanisms of tumor evasion. Cancer Treat. Res. 2005;123:61–88. doi: 10.1007/0-387-27545-2_3. [DOI] [PubMed] [Google Scholar]
- 245.Igney F.H., Krammer P.H. Immune escape of tumors: apoptosis resistance and tumor counterattack. J. Leukoc. Biol. 2002;71:907–920. [PubMed] [Google Scholar]
- 246.Gomez-Manzano C., Yung W.K., Alemany R., Fueyo J. Genetically modified adenoviruses against gliomas: from bench to bedside. Neurology. 2004;63:418–426. doi: 10.1212/01.wnl.0000133302.15022.7f. [DOI] [PubMed] [Google Scholar]
- 247.Nienhuis A.W., Dunbar C.E., Sorrentino B.P. Genotoxicity of retroviral integration in hematopoietic cells. Mol. Ther. 2006;13:1031–1049. doi: 10.1016/j.ymthe.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 248.Vlachaki M.T., Hernandez-Garcia A., Ittmann M., Chhikara M., Aguilar L.K., Zhu X., Teh B.S., Butler E.B., Woo S., Thompson T.C., Barrera-Saldana H., guilar-Cordova E. Impact of preimmunization on adenoviral vector expression and toxicity in a subcutaneous mouse cancer model. Mol. Ther. 2002;6:342–348. doi: 10.1006/mthe.2002.0669. [DOI] [PubMed] [Google Scholar]
- 249.Krebs P., Scandella E., Odermatt B., Ludewig B. Rapid functional exhaustion and deletion of CTL following immunization with recombinant adenovirus. J. Immunol. 2005;174:4559–4566. doi: 10.4049/jimmunol.174.8.4559. [DOI] [PubMed] [Google Scholar]
- 250.Alsharifi M., Regner M., Blanden R., Lobigs M., Lee E., Koskinen A., Mullbacher A. Exhaustion of type I interferon response following an acute viral infection. J. Immunol. 2006;177:3235–3241. doi: 10.4049/jimmunol.177.5.3235. [DOI] [PubMed] [Google Scholar]
- 251.Raki M., Kanerva A., Ristimaki A., Desmond R.A., Chen D.T., Ranki T., Sarkioja M., Kangasniemi L., Hemminki A. Combination of gemcitabine and Ad5/3-Delta24, a tropism modified conditionally replicating adenovirus, for the treatment of ovarian cancer. Gene Ther. 2005;12:1198–1205. doi: 10.1038/sj.gt.3302517. [DOI] [PubMed] [Google Scholar]
- 252.Dewey R.A., Morrissey G., Cowsill C.M., Stone D., Bolognani F., Dodd N.J., Southgate T.D., Klatzmann D., Lassmann H., Castro M.G., Lowenstein P.R. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat. Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 253.Driesse M.J., Vincent A.J., Sillevis Smitt P.A., Kros J.M., Hoogerbrugge P.M., Avezaat C.J., Valerio D., Bout A. Intracerebral injection of adenovirus harboring the HSVtk gene combined with ganciclovir administration: toxicity study in nonhuman primates. Gene Ther. 1998;5:1122–1129. doi: 10.1038/sj.gt.3300695. [DOI] [PubMed] [Google Scholar]
- 254.Delman K.A., Bennett J.J., Zager J.S., Burt B.M., McAuliffe P.F., Petrowsky H., Kooby D.A., Hawkins W.G., Horsburgh B.C., Johnson P., Fong Y. Effects of preexisting immunity on the response to herpes simplex-based oncolytic viral therapy. Hum. Gene Ther. 2000;11:2465–2472. doi: 10.1089/10430340050207957. [DOI] [PubMed] [Google Scholar]
- 255.Bennett J.J., Delman K.A., Burt B.M., Mariotti A., Malhotra S., Zager J., Petrowsky H., Mastorides S., Federoff H., Fong Y. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Ther. 2002;9:935–945. doi: 10.1038/sj.cgt.7700510. [DOI] [PubMed] [Google Scholar]
- 256.Pensiero M.N., Wysocki C.A., Nader K., Kikuchi G.E. Development of amphotropic murine retrovirus vectors resistant to inactivation by human serum. Hum. Gene Ther. 1996;7:1095–1101. doi: 10.1089/hum.1996.7.9-1095. [DOI] [PubMed] [Google Scholar]
- 257.Fisher K.D., Stallwood Y., Green N.K., Ulbrich K., Mautner V., Seymour L.W. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8:341–348. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- 258.Green N.K., Seymour L.W. Adenoviral vectors: systemic delivery and tumor targeting. Cancer Gene Ther. 2002;9:1036–1042. doi: 10.1038/sj.cgt.7700541. [DOI] [PubMed] [Google Scholar]
- 259.Massari I., Donnini A., Argentati K., Straino S., Mangoni A., Gaetano C., Viticchi C., Capogrossi M., Provinciali M. Age-dependent effects of repeated immunization with a first generation adenovirus vector on the immune response and transgene expression in young and old rats. Exp. Gerontol. 2002;37:823–831. doi: 10.1016/s0531-5565(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 260.Currier M.A., Adams L.C., Mahller Y.Y., Cripe T.P. Widespread intratumoral virus distribution with fractionated injection enables local control of large human rhabdomyosarcoma xenografts by oncolytic herpes simplex viruses. Cancer Gene Ther. 2005;12:407–416. doi: 10.1038/sj.cgt.7700799. [DOI] [PubMed] [Google Scholar]
- 261.Ren H., Boulikas T., Lundstrom K., Soling A., Warnke P.C., Rainov N.G. Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication-incompetent Semliki forest virus vector carrying the human interleukin-12 gene–a phase I/II clinical protocol. J. Neurooncol. 2003;64:147–154. doi: 10.1007/BF02700029. [DOI] [PubMed] [Google Scholar]
- 262.Kim J.H., Lee Y.S., Kim H., Huang J.H., Yoon A.R., Yun C.O. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J. Natl. Cancer Inst. 2006;98:1482–1493. doi: 10.1093/jnci/djj397. [DOI] [PubMed] [Google Scholar]
- 263.Bateman A., Bullough F., Murphy S., Emiliusen L., Lavillette D., Cosset F.L., Cattaneo R., Russell S.J., Vile R.G. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res. 2000;60:1492–1497. [PubMed] [Google Scholar]
- 264.Li H., Haviv Y.S., Derdeyn C.A., Lam J., Coolidge C., Hunter E., Curiel D.T., Blackwell J.L. Human immunodeficiency virus type 1-mediated syncytium formation is compatible with adenovirus replication and facilitates efficient dispersion of viral gene products and de novo-synthesized virus particles. Hum. Gene Ther. 2001;12:2155–2165. doi: 10.1089/10430340152710504. [DOI] [PubMed] [Google Scholar]
- 265.Zhang J., Frolov I., Russell S.J. Gene therapy for malignant glioma using Sindbis vectors expressing a fusogenic membrane glycoprotein. J. Gene Med. 2004;6:1082–1091. doi: 10.1002/jgm.605. [DOI] [PubMed] [Google Scholar]
- 266.Lillehammer T., Tveito S., Engesaeter B.O., Fodstad O., Maelandsmo G.M., Engebraaten O. Melanoma-specific expression in first-generation adenoviral vectors in vitro and in vivo – use of the human tyrosinase promoter with human enhancers. Cancer Gene Ther. 2005;12:864–872. doi: 10.1038/sj.cgt.7700852. [DOI] [PubMed] [Google Scholar]
- 267.Sarkar D., Su Z.Z., Vozhilla N., Park E.S., Randolph A., Valerie K., Fisher P.B. Targeted virus replication plus immunotherapy eradicates primary and distant pancreatic tumors in nude mice. Cancer Res. 2005;65:9056–9063. doi: 10.1158/0008-5472.CAN-05-1261. [DOI] [PubMed] [Google Scholar]
- 268.Metzl C., Mischek D., Salmons B., Gunzburg W.H., Renner M., Portsmouth D. Tissue- and tumor-specific targeting of murine leukemia virus-based replication-competent retroviral vectors. J. Virol. 2006;80:7070–7078. doi: 10.1128/JVI.00020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Steffens S., Tebbets J., Kramm C.M., Lindemann D., Flake A., Sena-Esteves M. Transduction of human glial and neuronal tumor cells with different lentivirus vector pseudotypes. J. Neurooncol. 2004;70:281–288. doi: 10.1007/s11060-004-6046-8. [DOI] [PubMed] [Google Scholar]
- 270.Parker A.L., Fisher K.D., Oupicky D., Read M.L., Nicklin S.A., Baker A.H., Seymour L.W. Enhanced gene transfer activity of peptide-targeted gene-delivery vectors. J. Drug Target. 2005;13:39–51. doi: 10.1080/10611860400020449. [DOI] [PubMed] [Google Scholar]
- 271.Nakamura T., Peng K.W., Harvey M., Greiner S., Lorimer I.A., James C.D., Russell S.J. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- 272.Morizono K., Xie Y., Ringpis G.E., Johnson M., Nassanian H., Lee B., Wu L., Chen I.S. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat. Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 273.Springfeld C., von Messling V., Frenzke M., Ungerechts G., Buchholz C.J., Cattaneo R. Oncolytic efficacy and enhanced safety of measles virus activated by tumor-secreted matrix metalloproteinases. Cancer Res. 2006;66:7694–7700. doi: 10.1158/0008-5472.CAN-06-0538. [DOI] [PubMed] [Google Scholar]
- 274.Shinoura N., Yoshida Y., Asai A., Kirino T., Hamada H. Adenovirus-mediated transfer of p53 and Fas ligand drastically enhances apoptosis in gliomas. Cancer Gene Ther. 2000;7:732–738. doi: 10.1038/sj.cgt.7700160. [DOI] [PubMed] [Google Scholar]
- 275.Zhao L., Dong A., Gu J., Liu Z., Zhang Y., Zhang W., Wang Y., He L., Qian C., Qian Q., Liu X. The antitumor activity of TRAIL and IL-24 with replicating oncolytic adenovirus in colorectal cancer. Cancer Gene Ther. 2006;13:1011–1022. doi: 10.1038/sj.cgt.7700969. [DOI] [PubMed] [Google Scholar]
- 276.Pang S., Kang M.K., Kung S., Yu D., Lee A., Poon B., Chen I.S., Lindemann B., Park N.H. Anticancer effect of a lentiviral vector capable of expressing HIV-1 Vpr. Clin. Cancer Res. 2001;7:3567–3573. [PubMed] [Google Scholar]
- 277.Su C., Peng L., Sham J., Wang X., Zhang Q., Chua D., Liu C., Cui Z., Xue H., Wu H., Yang Q., Zhang B., Liu X., Wu M., Qian Q. Immune gene-viral therapy with triplex efficacy mediated by oncolytic adenovirus carrying an interferon-gamma gene yields efficient antitumor activity in immunodeficient and immunocompetent mice. Mol. Ther. 2006;13:918–927. doi: 10.1016/j.ymthe.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 278.Lou E. Oncolytic viral therapy and immunotherapy of malignant brain tumors: two potential new approaches of translational research. Ann. Med. 2004;36:2–8. doi: 10.1080/07853890310016315. [DOI] [PubMed] [Google Scholar]
- 279.Smakman N., van der Bilt J.D., van den Wollenberg D.J., Hoeben R.C., Borel R., Kranenburg I.O. Immunosuppression promotes reovirus therapy of colorectal liver metastases. Cancer Gene Ther. 2006;13:815–818. doi: 10.1038/sj.cgt.7700949. [DOI] [PubMed] [Google Scholar]
- 280.Gao Q., Huang X., Tang D., Cao Y., Chen G., Lu Y., Zhuang L., Wang S., Xu G., Zhou J., Ma D. Influence of chk1 and plk1 silencing on radiation- or cisplatin-induced cytotoxicity in human malignant cells. Apoptosis. 2006;11:1789–1800. doi: 10.1007/s10495-006-9421-4. [DOI] [PubMed] [Google Scholar]
- 281.Levenson V.V., Davidovich I.A., Roninson I.B. Pleiotropic resistance to DNA-interactive drugs is associated with increased expression of genes involved in DNA replication, repair, and stress response. Cancer Res. 2000;60:5027–5030. [PubMed] [Google Scholar]
- 282.Mantwill K., Kohler-Vargas N., Bernshausen A., Bieler A., Lage H., Kaszubiak A., Surowiak P., Dravits T., Treiber U., Hartung R., Gansbacher B., Holm P.S. Inhibition of the multidrug-resistant phenotype by targeting YB-1 with a conditionally oncolytic adenovirus: implications for combinatorial treatment regimen with chemotherapeutic agents. Cancer Res. 2006;66:7195–7202. doi: 10.1158/0008-5472.CAN-05-2339. [DOI] [PubMed] [Google Scholar]
- 283.Mi J., Li Z.Y., Ni S., Steinwaerder D., Lieber A. Induced apoptosis supports spread of adenovirus vectors in tumors. Hum. Gene Ther. 2001;12:1343–1352. doi: 10.1089/104303401750270995. [DOI] [PubMed] [Google Scholar]
- 284.Kasuya H., Mizuno M., Yoshida J., Nishiyama Y., Nomoto S., Nakao A. Combined effects of adeno-associated virus vector and a herpes simplex virus mutant as neoplastic therapy. J. Surg. Oncol. 2000;74:214–218. doi: 10.1002/1096-9098(200007)74:3<214::aid-jso12>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 285.Hampl J.A., Camp S.M., Mydlarz W.K., Hampl M., Ichikawa T., Chiocca E.A., Louis D.N., Sena-Esteves M., Breakefield X.O. Potentiated gene delivery to tumors using herpes simplex virus/Epstein–Barr virus/RV tribrid amplicon vectors. Hum. Gene Ther. 2003;14:611–626. doi: 10.1089/104303403321618137. [DOI] [PubMed] [Google Scholar]
- 286.Raykov Z., Legrand V., Homann H.E., Rommelaere J. Transient suppression of transgene expression by means of antisense oligonucleotides: a method for the production of toxin-transducing recombinant viruses. Gene Ther. 2002;9:358–362. doi: 10.1038/sj.gt.3301660. [DOI] [PubMed] [Google Scholar]
- 287.Guan M., Rodriguez-Madoz J.R., Alzuguren P., Gomar C., Kramer M.G., Kochanek S., Prieto J., Smerdou C., Qian C. Increased efficacy and safety in the treatment of experimental liver cancer with a novel adenovirus-alphavirus hybrid vector. Cancer Res. 2006;66:1620–1629. doi: 10.1158/0008-5472.CAN-05-0877. [DOI] [PubMed] [Google Scholar]
- 288.Abou El Hassan M.A., van der Meulen-Muileman I., Abbas S., Kruyt F.A. Conditionally replicating adenoviruses kill tumor cells via a basic apoptotic machinery-independent mechanism that resembles necrosis-like programmed cell death. J. Virol. 2004;78:12243–12251. doi: 10.1128/JVI.78.22.12243-12251.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Herman J.R., Adler H.L., guilar-Cordova E., Rojas-Martinez A., Woo S., Timme T.L., Wheeler T.M., Thompson T.C., Scardino P.T. In situ gene therapy for adenocarcinoma of the prostate: a phase I clinical trial. Hum. Gene Ther. 1999;10:1239–1249. doi: 10.1089/10430349950018229. [DOI] [PubMed] [Google Scholar]
- 290.Shimizu Y., Hasumi K., Okudaira Y., Yamanishi K., Takahashi M. Immunotherapy of advanced gynecologic cancer patients utilizing mumps virus. Cancer Detect. Prev. 1988;12:487–495. [PubMed] [Google Scholar]
- 291.Takemori N., Nakano M., Hemmi M., Ikeda H., Yanagida S., Kitaoka M. Destruction of tumour cells by Rift Valley fever virus. Nature. 1954;174:698–700. doi: 10.1038/174698b0. [DOI] [PubMed] [Google Scholar]
- 292.Moore A.E. Inhibition of growth of five transplantable mouse tumors by the virus of Russian Far East encephalitis. Cancer. 1951;4:375–382. doi: 10.1002/1097-0142(195103)4:2<375::aid-cncr2820040227>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]