Infections of the lower respiratory tract, bronchitis and pneumonia, are very common in clinical practice. Clinical manifestation can be variable, ranging from acute to chronic and from mild to severe.3, 4, 27, 38, 39 Pneumonia is the sixth leading cause of death and the most common infectious disease cause of death in the United States. There are an estimated 4.5 million cases of community-acquired pneumonia annually, with at least 500,000 requiring hospitalization. Early empiric therapy is needed, because the mortality rate can be high, especially in the “high-risk” patient. Specific antibiotic recommendations are in flux, because of changing resistance patterns worldwide. Resistance is driven by an enormous overuse of antimicrobial agents, often in the setting of a non-bacterial infection or inflammation. Clearly a better approach to the specific diagnosis of lower respiratory infections is required to avoid overprescription of antimicrobials. Acute bronchitis needs to be carefully distinguished from chronic bronchitis. Hospitalization decisions have become more easily defined.3 Diagnostic and therapeutic strategies are determined by the clinical presentation, with special attention to the host's immunocompetency. Microbiological work-up may or may not be indicated. This article focuses primarily on diagnostic strategies in community-acquired lower respiratory tract infections.
BRONCHITIS
Bronchitis is an inflammatory condition of the tracheobronchial tree that commonly presents acutely or as an acute exacerbation of a chronic condition. Bronchitis peaks during winter months, when respiratory viruses are prevalent in the community. A recent stratification of bronchitis into four distinct categories has been suggested.20 The first category is acute tracheobronchitis usually of viral origin and not requiring antibiotic therapy; second is simple chronic bronchitis with forced expiratory volume in one second (FEV1) greater than 50% of normal, increased sputum volume, and purulence. Third is complicated chronic bronchitis with increased sputum volume and purulence plus at least one of the following: FEV1 greater than 50%, more than four exacerbations per year, advanced age and comorbid conditions like congestive heart failure (CHF). Categories 2 and 3 are usually caused by Haemophilus influenzae, Streptococcus pneumoniae, and/or Moraxella catarrhalis. Fourth is chronic bronchial infection similar to category 3 with continuous sputum production throughout the year, often associated with enteric gram-negative pathogens including Pseudomonas aeruginosa.
Acute Bronchitis
Acute bronchitis is associated with a generalized pulmonary inflammation infection, usually caused by viruses such as rhinovirus, corona virus, respiratory syncytial virus (RSV), influenza virus, and adenovirus.19, 21 Bordetella pertussis, Bordetella parapertussis, Mycoplasma pneumoniae, and Chlamydia pneumoniae are occasional causes of acute bronchitis. B. pertussis should be considered in any adult with a cough illness lasting more than 2 to 3 weeks.19 Acute bronchitis typically begins as an acute viral infection and manifests with cough and occasionally sputum production in the absence of tachycardia, tachypnea, significant fever (> 35° C), or an abnormal chest exam. Productive sputum is not predictive of viral, bacterial, or noninfectious causes of cough such as asthma, postnasal discharge, or gastroesophageal reflux disease.38, 39 Causative roles of S. pneumoniae and H. influenzae in acute bronchitis have not been demonstrated. Such roles are difficult to establish, because these organisms may colonize the normal respiratory tract.21, 38, 39 Acute bronchitis is a clinical diagnosis, and antimicrobial therapy in the uncomplicated host is not indicated. Bacterial cultures, sputum gram smears, and viral studies on respiratory secretions are not normally helpful and may be misleading.38, 39 A major exception is the adult patient with suspected pertussis in whom documentation of B. pertussis should be attempted. Culture, serology, and more sensitive and specific amplification methods (e.g., polymerase chain reaction [PCR]) are available in reference laboratories. Consensus recommendations for diagnosis of B. pertussis by PCR have been proposed and may become the “gold standard” for diagnosis of pertussis syndrome.12 Presently, however, there is no single, standardized PCR method available. Many laboratories also have the expertise and resources to provide culture and detection of organisms with direct fluorescent antibody (DFA) stains. DFA smears suffer inter-observer variability and lack sensitivity and specificity compared with culture. Calcium-alginate nasopharyngeal swabs or aspirates should be submitted in appropriate transport media. The swabs should never be transported dry. The choice of transport medium depends on the time required for delivery of the specimen to the laboratory. Individual laboratories should be contacted for appropriate transport media and instructions. B. pertussis becomes progressively more difficult to culture over the course of the infection. In adults in whom the course may be protracted, supplemental studies may increase the diagnostic yield. If available, the combination of serology and/or PCR in conjunction with culture may be warranted.22, 23
Chronic Bronchitis
Chronic bronchitis is defined by the continued presence of a productive cough over at least a 3-month period in each of 2 consecutive years.41
It is characterized by cough, shortness of breath, and secretion of excessive mucus caused by chronic, irreversible inflammatory changes in the tracheobronchial airways.41 Many environmental, infectious, and allergic factors contribute to chronic bronchitis. Ten percent to 25% of the adult population may be affected, and an estimated 12 million people in the United States suffer from the disease.41 Analysis of early morning sputum for presence of polymorphonuclear granulocytes (PMN), eosinophils, ciliated epithelial cells, and alveolar macrophage is helpful in the evaluation of chronic bronchitis. In contrast to normal patients, those with chronic bronchitis are commonly colonized (30% to 50%) in their lower airways with potentially pathogenic bacteria such as S. pneumoniae, nonencapsulated H. influenzae, and M. catarrhalis.
The role of bacteria in acute exacerbation of chronic bronchitis (AECB) has been debated, but there is evidence that their quantity in the lower respiratory tract may increase during AECB.29 This increase in bacterial numbers may stimulate the overproduction of mucus and contribute to the symptoms of AECB.41 Exacerbations are probably a result of a number of factors in addition to bacterial overgrowth. The role of comorbid conditions like underlying heart disease, chronic lung disease, or immunosuppression is important. Viruses, including influenza virus, parainfluenza virus, RSV, rhinovirus, and coronavirus, contribute to AECB.18 S. pneumoniae and H. influenzae are found in at least half of the patients with AECB, but they may colonize patients with and without AECB. Their isolation rate does not increase during symptomatic episodes. B. pertussis is recognized as a potential contributor to exacerbation. Anaerobes and M. pneumoniae are not commonly found in AECB, while C. pneumoniae is associated with a mild form of disease characterized by cough lasting for weeks. Other bacteria such as beta-hemolytic streptococci, staphylococci, as well as the gram-negative enteric bacilli, rarely contribute to exacerbations and are found in only 10% to 15% of sputum cultures from patients with AECB. Because most of these organisms are commonly present in respiratory secretions in patients with bronchitis, bacterial cultures of sputum are of little value, and the isolation of potential pathogens does not confirm their role in AECB. Smears may delineate the presence of a predominant organism, but microbiological work-up of respiratory secretions rarely contributes to therapeutic decisions. Thus, when required, initial therapy should be directed empirically and should normally cover pneumococci, Haemophilus, and Moraxella. In certain clinical settings (such as the patient with protracted cough lasting more than 3 weeks), B. pertussis should be considered.41 There will be patients who need more microbiologic evaluation than usual, so clinical judgment is needed. Compromised hosts such as patients with multiple myeloma, chronic lymphocytic leukemia, and patients on long-term corticosteroid therapy are in this category. Recurrent AECB and pneumonia should raise the specter of underlying immunoglobulin deficiency as in acquired hypogammaglobulinemia.
PNEUMONIA
General Principles
Pneumonia is the sixth most common infectious disease-related cause of death in the United States.11, 27 There are at least four million cases of pneumonia each year. This discussion concerns the more frequent community-acquired pneumonias (CAP), rather than the nosocomial pneumonias (NAP).
The clinical manifestations of CAP are rarely specific for a particular microbe. Initial manifestations may include cough, productive sputum, fever, and increasing respiratory distress. A preceding viral syndrome or underlying chronic lung condition may worsen over several days. In some hosts the onset is abrupt, with shaking chills, pleuritic chest pain, and obvious toxicity. Extremes of age often have atypical presentations, with the elderly presenting with increased mental confusion.13, 27 The physical examination helps to distinguish noninfectious diseases from infection, for example, CHF, signs of underlying malignancy, signs of thromboembolic phenomena, and hypertension with pulmonary edema; however, physical examination rarely provides insight into specific infective causes. Signs of consolidation on chest exam include dullness to percussion, increased tactile fremitus, increased clarity of whispered or spoken vocal sounds, and bronchial (tubular) breath sounds with a shortened inspiratory phase and a prolonged expiratory phase. Inspiratory crackles are usually heard. The presence of a heart murmur makes infective endocarditis a consideration. Mental status changes should raise immediate concerns about meningitis. Chest radiography usually defines pneumonia, but is rarely pathognomonic for a specific cause of pneumonia. The diagnosis of a specific etiology of pneumonia requires a positive culture from blood, pleural fluid, or direct lung puncture.
Microorganisms invade the lower respiratory tract primarily by way of aspiration from the oropharynx or inhalation of aerosols. Seeding of the lung by way of the bloodstream or by direct extension from a contiguous site is unusual, except in right-sided endovascular infections.44 The pathogens that classically cause CAP are changing in part because of the identification of pathogens not previously recognized such as Legionella species, Coxiella burnetii (Q fever), and C. pneumoniae. The prevalence of some pathogens is influenced by underlying diseases like acquired immunodeficiency syndrome (AIDS) (Pneumocystis carinii), by geographic location (coccidioidomycosis in the southwestern deserts, Q fever in rural Nova Scotia), and by socioeconomic factors (tuberculosis [TB]); however, a careful assessment of the patient's history, epidemiology, physical examination, and relevant preliminary laboratory information helps focus the differential diagnosis toward a specific etiology.11, 44 Although the etiologies are most commonly derived from the colonizing flora of the oropharynx, bacterial cultures of upper respiratory tract secretions do not reliably identify the etiology of lower respiratory tract infections.38, 39, 44
Indigenous And Colonizing Flora of the Upper Respiratory Tract
The oropharynx is colonized by a plethora of aerobic and anaerobic microorganisms. The flora are often altered by changes in the patient's immediate environment or health status. Colonization by potentially pathogenic organisms such as S. pneumoniae and H. influenzae can increase seasonally or from protracted exposure such as in parents with children attending day-care facilities. Gram-negative bacillary colonization increases rapidly under the influence of broad-spectrum antimicrobial use, chronic hospitalization, acute severe illness, and chronic illnesses such as diabetes mellitus and alcoholism. Healthy people have minimal sublaryngeal bacterial colonization. Chronic illness, especially pulmonary, and acute illness associated with aspiration or the need for intubation cause rapid colonization of the lower respiratory tract.44
Etiologies of Community-Acquired Pneumonia
The microbial causes of pneumonia may be divided into two groups: the commensal organisms that normally colonize the upper respiratory tract (primarily S. pneumoniae and H. influenzae) and the noncommensal organisms. The noncommensal organisms are best termed “professional pathogens,” because they rarely colonize without infecting. These pathogens include Mycobacterium tuberculosis (MTB), Legionella species, Pneumocystis carinii, some other fungi, and specific respiratory viruses such as influenza. These noncommensal organisms are often considered in certain hosts, and specialized, selective methods are required to identify them. Their isolation in patients with clinical pneumonia often establishes the specific cause of the pneumonia. The commensal organisms are much more common causes of pneumonia and require the clinician to ask if these organisms are truly causing the infection or merely colonizing respiratory surfaces.44
S. pneumoniae is the most common cause of CAP and is especially prevalent in patients requiring hospitalization.27, 38, 39, 44 Published incidence studies of CAP underestimate the true frequency of S. pneumoniae, because diagnosis of the specific etiology is rarely attempted.27 Specific etiology identification often requires detection in a normally sterile site such as the blood, pleural fluid, or lung. A Gram's stain of well-collected sputum showing many white blood cells (WBC), few squamous epithelial cells, and a predominance of gram-positive lancet-shaped diplococci with S. pneumoniae on culture is supportive of this diagnosis. Pneumococci still account for two thirds of the identified pathogens in hospitalized patients with CAP and in patients dying from CAP.14
Community-acquired pneumonias can be caused by other pyogenic bacteria such as H. influenzae, S. aureus, and enteric bacilli. The prevalence of these organisms rises in patients over the age of 60 years.27, 43 Legionella pneumophila and other Legionella species also cause CAP, and although rarely seen in healthy children and young adults, are more prevalent in patients under the age of 60 years. Their frequency varies geographically and may be negligible in certain parts of the United States.44 Like Legionella, M. pneumoniae, C. pneumoniae, and other atypical etiologies of CAP have a predilection for younger populations.27
In patients with underlying conditions such as chronic obstructive pulmonary disease (COPD), diabetes mellitus, and immunosuppression, additional bacterial etiologies must be considered. Legionella species and Nocardia species begin to play more prominent pathogenic roles. Rhodococcus equi, a gram-positive rod in the aerobic actinomycete group, can cause acute or chronic pneumonia in patients with more severe immunocompromising conditions, especially in patients with AIDS.
The mycobacterioses (infections caused by acid-fast-bacilli [AFB]) can be divided into two categories based on clinical and epidemiologic characteristics, and the causative organisms. Members of the M. tuberculosis complex, which includes MTB, cause tuberculosis, and isolation of MTB from clinical specimens is considered diagnostic. Mycobacterial species not included in the TB complex cause “nontuberculous mycobacterial infection” and are collectively known as the nontuberculous mycobacteria (NTM). These NTM infections normally present as chronic pneumonias in patients with underlying respiratory or immunological problems. Unlike MTB, NTM are ubiquitous in the environment and are not transmitted person-to-person. They may be found in water, dust, soil, and even domesticated pets. With the exception of Mycobacterium kansasii, the clinical significance of NTM in respiratory secretions is problematic, because their presence may represent transient contamination.51 Certain NTM such as those in the Mycobacterium avium complex (MAC) are increasing in frequency and involve younger people, many without apparent pre-existing lung disease.37 Women are infected with MAC more often than men and may have an indolent, progressive clinical presentation with cough, usually without weight loss or fever. Nodular, patchy, predominately lower lobe infiltrates that rarely cavitate may progress over months to years. The clinical laboratory will need to identify NTM isolates to species level. Therapeutic decisions will require that the clinician carefully consider the issue of colonization versus infection by looking at the patient and chest radiographs over time.51
Community-acquired fungal pneumonias have distinct geographic areas and should be considered if the patient resides or travels in these areas.45, 46 Immunocompromised hosts are at increased risk for certain respiratory fungi. Coccidioidomycosis afflicts patients residing in or traveling through the southwestern Sonoran desert states or in parts of Latin America. An estimated 100,000 new cases of C. immitis infection occur annually. Histoplasmosis and blastomycosis must be considered in patients who visit or live in the drainage systems of the Mississippi or Ohio Rivers. Histoplasma capsulatum is considered the most common fungal cause of respiratory infection, with an estimated 250,000 cases annually. Most cases of histoplasmosis and coccidioidomycosis are self-limited and go unrecognized. Cryptococcus neoformans is not geographically restricted and should be considered in immunocompromised hosts, especially when central nervous system involvement is apparent; isolated cryptococcal pneumonia is rare. Pneumocystis carinii, now considered a fungus, should also be included in the differential diagnosis in immunocompromised hosts, especially those on corticosteroids or with AIDS. Aspergilli, zygomycetes, and other molds are rare causes of CAP, unless neutropenia, diabetic ketoacidosis, or profound immunodeficiency are present.45, 46
Viruses, especially influenza A and B, play a significant role in CAP, although they are diagnosed in children under the age of 5 most frequently. Other significant viral causes of CAP include adenovirus, RSV, the parainfluenza viruses, and in some instances rhinoviruses.38, 39 In a recent Finnish study of 254 hospitalized pediatric patients with CAP, 62% of cases were caused by viruses, while 53% were caused by bacteria.25 The seasonal outbreak of influenza and RSV may promote adult pneumonia and act as a cofactor in worsening bacterial pneumonia in the elderly. Recently, influenza A infection has been closely linked to an outbreak of severe pneumococcal pneumonia in children.32 Additionally, the Hantavirus causing Hantavirus pulmonary syndrome (HPS) was first recognized in the United States in New Mexico in 1993. Hantaviruses are zoonotic, infecting wild rodents (e.g., deer mice) and are transmitted to humans through aerosolization of dried excrement. Although the first Hantavirus found in the Four Corners area of the southwest (named Sin Nombre virus) remains the most important, other Hantavirus cases have now been documented throughout the United States. HPS is rare but should be considered in healthy adults presenting with unexplained pulmonary edema or adult respiratory distress syndrome.35
DIAGNOSTIC METHODS
The evaluation of patients with suspected pneumonia begins with a comprehensive history and physical examination. Chest radiography confirms the presence of pulmonary inflammation, although in some instances infiltrates may be scant or missing because of dehydration, neutropenia, or a retrocardiac location.3, 4, 38 Chest radiography is rarely pathognomonic for a specific cause of pneumonia. Routine laboratory studies (complete blood count, comprehensive chemistry, urinalysis, pulse oxymetry) are not helpful in establishing a specific etiology of CAP, but can assist in stratifying patients according to the severity of their pneumonias and need for hospitalization.14, 15, 38
The usefulness of diagnostic testing in pneumonia remains in question. Guidelines from both the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) disagree on the extent of diagnostic testing needed in patients with CAP.1, 3 ATS guidelines recommend empiric therapy without routine microbiological work-up, whereas IDSA guidelines support work-up whenever possible and especially in patients requiring hospitalization. Microbiological diagnosis of CAP may fail in over 50% of patients. Increased success in microbiological diagnosis usually occurs in patients who produce sputum, have not been treated with antibiotics, and require hospitalization. A specific microbial diagnosis allows appropriate antimicrobial selection and detection of resistant organisms. Increasing worldwide microbial resistance may force more specific diagnosis of CAP. Likewise, the potential for less common but possibly more serious causes of CAP such as Legionella, TB, nocardiosis, pneumocystosis, and other local fungal etiologies needs to be considered. Clinical acumen is important, because blood and/or pleural fluid cultures are rarely positive, sputum Gram's stain and culture are difficult to interpret, and the clinical manifestations of pneumonia are rarely specific for a single pathogen. The clinician often can focus on the most likely etiology using the clinical history and a well-collected and examined Gram's stain of sputum, keeping in mind that “common things happen commonly.” Compromised hosts, nursing-home residents, and patients who received prior antimicrobial therapy may require broader antimicrobial coverage and a more accelerated, invasive diagnostic work-up.
Evaluation for Bacterial Infection
Laboratory diagnosis of the specific cause of CAP is indicated in the more ill patients, especially those requiring hospitalization.3, 4 Specific diagnosis is predicated on the availability of appropriate and adequate laboratory resources. Inadequate specimens or transport times commonly result in misidentification of the true cause of pneumonia. Specimens should be collected before patients receive antimicrobial therapy, because susceptible microorganisms can be rapidly inhibited once therapy is begun.
In addition to sputum evaluation, specimens may be collected from other sites to enhance the diagnosis (Table 1) . Two routine blood cultures should be collected. Blood cultures are rarely positive if therapy is initiated prior to collection of the blood.33 Pleural fluid collected by thoracentesis should be evaluated by culture and smear. Blood and pleural fluid cultures are often without growth; however, a positive culture is diagnostic of the specific cause of pneumonia.
Table 1.
RECOMMENDED DIAGNOSTIC APPROACHES TO BACTERIAL COMMUNITY-ACQUIRED PNEUMONIA
| Etiology | Diagnostic Approaches and Comments* |
|---|---|
| Usual pyogenic bacterial causes† | Routine blood cultures X 2 (before therapy). |
| Gram's stain of sputum for adequacy screen and etiology; routine culture (if screen adequate). | |
| Clinical use of urine antigen forStreptococcus pneumoniae not yet confirmed. | |
| Legionella species | Legionella culture requested (BCYE agars). |
| DFA‡ not recommended on respiratory secretions. | |
| Sputum may be clear; adequacy criteria not followed. | |
| Urine antigen study for Legionella pneumophila serogroup 1 may be helpful in geographic areas in which it is prevalent or when sputum is not available. | |
| Serologies not helpful in diagnosis of acute disease. | |
| Mycoplasma | Not routinely tested, because culture and serology not clinically helpful. Paired (acute/convalescent) sera needed in adults; single serum for immunoglobulin M/immunoglobulin G (IgM/IgG) may be useful in children. |
| Cold agglutinins not helpful. | |
| Chlamydia pneumoniae | Routine diagnosis not recommended. |
| Mycobacteria (acid-fast bacilli) | Order mycobacterial acid-fast bacilli cultures and stains. |
| Adequacy criteria not followed. | |
| Auramine/rhodamine fluorescent stains most useful for direct observation in smears. | |
| Decontamination and concentration of sputum necessary. | |
| Routine susceptibility studies required routinely only on Mycobacterium tuberculosis and rapid-growing acid-fast bacilli. | |
| Amplification methods for direct detection in sputum available, but sensitivity best in smear-positive specimens. | |
| Other, less common bacteria§ | Call laboratory for availability and instructions for specific pathogens. |
Although amplification methods using polymerase chain reaction are available, they are not standardized, are difficult to interpret, and are not at this time recommended for routine evaluation of lower respiratory secretion in the diagnosis of community-acquired pneumonia.
S. pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and enteric gram-negative rods most common.
DFA: direct fluorescent antigen detection in specimens.
e.g., Coxiella burnetti (Q fever), Chlamydia psittaci (psittacosis), Francisella tularensis (tularemia), Yersinia pestis (plague).
Studies detecting urinary antigens of pneumococci and Legionella are available, but their clinical usefulness is either still in question (Pneumococcus) or depends on geographic prevalence of the specific serogroups detected. Sensitivities of the pneumococcal antigen urinary test, although surpassing those of Gram's stain, may vary from 75% to 95%. The legionella urinary antigen studies include only L. pneumophila group 1, and sensitivities range from 70% to 90%.24 Its clinical usefulness may depend on the geographic prevalence of serogroup 1. Larger studies indicating substantiated benefits of urine antigen studies on the initial therapy of CAP are not yet universally available.38
A productive sputum should be carefully acquired for analysis. The patient must be taught appropriate collection techniques. Secretions from the lower respiratory tract (expectorated sputum, deep cough) rather than a collection of oropharyngeal saliva (spit) are essential. Induced sputum does not increase recovery of bacteria, mycobacteria, or fungi, but may help in the diagnosis of pneumocystosis in the immunocompromised host.16 Induced sputum may be submitted for mycobacterial and fungal studies if expectorated sputum is not available. Swabs of nasopharyngeal secretions and 24-hour collections of sputum are not useful.
Appropriate storage and transport must be available to assure specimen integrity. Specimens should be processed within 2 hours or refrigerated for up to 24 hours.28 Viability of pathogenic bacteria with potential overgrowth with commensal organisms is always an issue, so specimens should be processed as rapidly as possible.
Purulent areas of sputum should be screened microscopically (Gram's stain, methylene blue wet mount) at low magnification for adequacy. Several criteria comparing presence of squamous epithelial cells (SEC) with polymorphonuclear cells (PMN) and predominant morphologies of bacteria have been evaluated and promoted.30, 49 The presence of increased numbers of SECs (12 to 25/100 × magnification field) and a paucity of PMNs (<25) represents a specimen that is inadequate for routine bacterial culture and should be rejected (Figs. 1 and 2) . Collection of additional expectorated sputum specimens for routine culture after rejection of an initial specimen is not useful if a patient has begun therapy. A well-prepared Gram's stain of purulent sputum demonstrating a predominant bacterial morphology associated with WBCs is useful in guiding pathogen-oriented therapy (Figs. 3 and 4) .42 Sputum Gram's stains that demonstrate many WBCs but no organisms suggest a “stealth organism,” such as a virus, mycoplasma, C. pneumoniae, Legionella species, or M. tuberculosis. In the absence of many WBCs and a predominant bacterial morphology, sputum microscopy is not useful. The Gram's stain can be viewed as a road map to the sputum culture. If what grows in the culture is not associated with WBCs on the smear, the culture is suspect.
Figure 1.
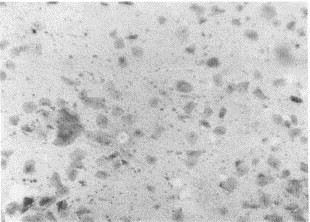
Gram's stain of respiratory secretions showing overabundance of buccal epithelial cells and few white blood cells. Such specimens are considered saliva and should not be evaluated further for usual bacterial etiologies of community-acquired pneumonia (original low-power magnification × 120).
Figure 2.
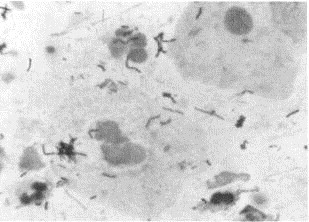
Gram's stain of saliva showing contamination with multiple organisms made up of normal oral flora (original high-power magnification × 1200).
Figure 3.
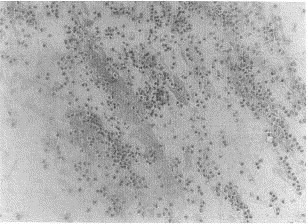
Gram's stain of sputum with predominance of polymorphonuclear cells (PMNs) and few epithelial cells. Cultures may be set up on such specimens (original low-power magnification × 120).
Figure 4.

Gram's stain of sputum showing a predominant organism associated with the PMNs and compatible with Streptococcus pneumoniae (original high-power magnification × 1200).
Acceptable sputum specimens should be inoculated onto agar media that allow growth of the most common bacterial pathogens and help provide early differentiation of species. A usual battery of media includes sheep blood agar, chocolate agar, and McConkey agar plates. Other media and incubation characteristics may be indicated by the result of the Gram's stain. Slower growing bacteria such as Nocardia require longer incubation times. Direct communication with the laboratory is essential whenever dealing with a compromised host or the potential for less common pathogens requiring special media or lengthy incubation. Anaerobic cultures are not recommended on expectorated or aspirated sputum. These should be set up only on specimens collected by biopsy or protected bronchoscopic brushing.
Only organisms associated with WBCs as noted in the direct Gram's stained smear of the specimen should be evaluated (Fig. 4). Susceptibility studies are indicated when pathogens noted on Gram's stain are isolated and known to have increased resistance to antimicrobial agents of choice. For in vitro antimicrobial studies and laboratory reporting formats, the standards and recommendations of the National Committee for Clinical Laboratory Standards (NCCLS) should be followed.31
Legionella
Presence of fever, cough, pulmonary infiltrates, and one or more symptoms of mental confusion, headache, abdominal pain, elevated liver enzymes, and bradycardia should heighten suspicion for legionellosis.34 Legionella culture should be specifically requested, because special processing and selective and nonselective agar media (buffered charcoal yeast extract supplemented with α-ketoglutarate) capable of growing legionella are necessary. Culture of respiratory secretions remains the “gold standard” for identifying Legionella species, because this is the only method available for detecting legionella other than L. pneumophila. Secretions, however, are not always purulent and may be clear and watery. Hence cultures should be set up without screening for adequacy.34 Very ill or immunocompromised patients may require bronchoalveolar lavage (BAL), lung biopsy, and/or pleural fluid collection. Specimens for legionella culture may be refrigerated for 48 hours or transported frozen if longer delays are anticipated.34 Pretreatment with acid is recommended for sputum from patients with cystic fibrosis to decrease contamination. Cultures require incubation in ambient air for 1 week before being considered negative.
DFA techniques for detection of legionella in respiratory secretions lack adequate sensitivity and specificity and should not be performed on sputum. An enzyme immunoassay (EIA) is available for detection of L. pneumophila serogroup 1 antigen in urine.34 The test may be useful in areas of high prevalence of serogroup 1 organisms, but would miss the other 62 serovariants of legionella. Recently, a kit for detecting a broad group of legionella became available, but its performance characteristics have yet to be determined.34
Serologic studies for diagnosis of legionellosis should not be performed, because antibodies to the organisms develop slowly (>3 weeks), and many people living in endemic areas may have elevated titers.34 Because of the intracellular nature of legionella, results of in vitro susceptibility studies frequently do not correlate to clinical response and are not normally indicated or available.
Mycoplasma
Culture and serologic methods for diagnosis of mycoplasma infection are too insensitive and slow to be useful. They cannot be recommended in routine CAP or other respiratory tract infections at this time.50 Cold agglutinin studies are neither sensitive nor specific enough to warrant their use as diagnostic tools. Testing for immunoglobulin M (IgM) antibody by IFA or enzyme-linked immunosorbent assay (ELISA) may be useful for diagnosis of mycoplasma in pediatric patients, if specimens are collected within 7 to 10 days of onset of symptoms; however, IgM antibody may be lacking in adults.50
Evaluation of Mycobacterial Infection
The introduction of more rapid methods for the laboratory isolation, identification, and susceptibility testing of mycobacteria has significantly improved the ability to diagnose infections caused by these agents.47 The average time to recovery of acid-fast organisms in culture has decreased from 21 to 9 days. Culture and identification of MTB and M. avium complex (MAC) is now possible within 10 to 12 days of specimen set-up. Susceptibility studies of MTB against the primary antituberculosis agents isoniazid, rifampin, streptomycin, and ethambutol should be performed on all initial isolates and require only an additional five days using newer technology. Radiometric broth techniques for isolation and susceptibility testing and molecular probes for identification of MTB, MAC, and M. kansasii in cultures enable these changes.47 The introduction of high-pressure liquid chromatographic (HPLC) methods to identify the mycobacteria by evaluation of the characteristic presence of long-carbon chain fatty acids (mycolic acids or mycolates) in the cell wall has allowed for the rapid and accurate identification of the NTM, as well as the MTB.
Notwithstanding the newer methods, three sputum samples collected on three separate days are still necessary for adequate sampling of respiratory secretions, and the sputum still requires decontamination and concentration prior to inoculation of specialized media.47 The auramine/rhodamine fluorescent stain remains the microscopic specimen screening method of choice. In hard to diagnose cases, it may be helpful to perform bronchoscopy followed immediately by several expectorated sputum specimens, because the latter increases the yield.47
Recently, molecular amplification methods have become available for the diagnosis of MTB in sputum specimens. These methods have high sensitivity and specificity when smears show AFB (sensitivity and specificity >95%) but poorer predictive values when AFB are not seen on the smear. Their clinical usefulness depends on the clinician suspecting TB. In all cases, a specific isolate is needed for susceptibility testing.
The introduction of a fluorescent column for evaluation of mycolates by HPLC has heightened that method's sensitivity and has allowed for its potential use on respiratory specimens. Its sensitivity is greatest when testing smear-positive specimens. HPLC analysis has the added value of ascertaining the specific species of the organism present in the specimen, rather than just screening for MTB.
Routine antimicrobial susceptibility testing of MTB is mandatory, but testing of the NTM is discouraged and often can provide erroneous information for therapy. Routine susceptibility testing of the NTM is only recommended for rapidly growing mycobacteria such as M. fortuitum, M. chelonei, and M. abscessus.51
Evaluation of Fungal Infection
The diagnosis of fungal infections usually includes histology and culture, although serologic studies are critical when coccidioidomycosis is suspected (Table 2) . Culture on fungal media and characterization of an isolate's phenotypic morphology or biochemical reactions are still necessary for diagnoses of many mycoses.45, 46
Table 2.
RECOMMENDED DIAGNOSTIC APPROACHES TO COMMUNITY-ACQUIRED PNEUMONIA CAUSED BY FUNGI
| Etiology | Diagnostic Approaches and Comments |
|---|---|
| Blastomyces dermatitidis | Endemic to Mississippi/Ohio River valleys. |
| Sputum, BAL for fungal stains and culture. | |
| No serologies. | |
| Coccidioides immitis | Endemic to southwest United States and parts of Latin America. |
| Sputum, bronchoalveolar lavage (BAL) for fungal stains and culture. | |
| Serologies (immunoglobulin M/immunoglobulin G [IgM/IgG] by EIA or immunodiffusion); complement fixation titers (IgG). | |
| Cryptococcus neoformans | Worldwide; immunocompromised patient at risk. |
| Consider possible dissemination (especially to cerebrospinal fluid [CSF]) if isolated; CSF, tissue for fungal stains and culture. Serologies not useful, but antigen studies useful in disseminated disease. | |
| Histoplasma capsulatum | Endemic to Mississippi/Ohio River valleys. |
| Sputum, BAL for lung infection; in suspected dissemination tissue, blood, bone marrow, urine for fungal stains and culture. | |
| Antigen studies on urine, serum, CSF, and BAL may be helpful, but false-positive reactions may occur. Thus, serum should be submitted simultaneously with urine. | |
| Serologies (immunodiffusion and complement fixation) useful. | |
| Skin testing is contraindicated. | |
| Pneumocystis carinii | Worldwide; usually associated with immunocompromised patient. |
| Induced sputum, BAL for direct fluorescent antigen studies. | |
| No culture or serologies. | |
| Other fungi | Aspergillus, zygomycetes, dematiaceous molds on rare occasions may be associated with immunocompromised patients. Call laboratory for information/instructions. |
Stains or molecular probes that increase the capability to detect organisms (fluorescent stains) directly in clinical specimens or allow rapid identification (genetic probes) of isolates from culture have been introduced. The calcofluor white stain is used for the direct visualization of fungi in specimens. This stain incorporates a fluorescent dye that binds chitin in the fungal cell wall, thereby increasing the sensitivity of direct detection. Unfortunately, the stain is not organism specific, and experienced personnel are required to read the smears accurately, because artifacts may occur. Sensitive fluorescent monoclonal antibodies specific for the direct visualization of P. carinii are commercially available and have become the diagnostic method of choice. P. carinii does not grow on culture media.
Bastomyces dermatitidis can be demonstrated readily by direct microscopic examination or culture of respiratory specimens. BAL specimens are diagnostic in over 90% of cases. In disseminated disease, the fungus may be recovered from bronchoscopy specimens, skin lesions, cerebrospinal fluid (CSF), and blood.45, 46
The diagnosis of coccidioidomycosis is established by histology, culture, and/or serology. Microscopic evaluation of sputum specimens rarely demonstrates spherules. The sensitivity of microscopy increases with specimens from BAL, pleural fluid, or lung biopsy. Mycelial forms rather than spherules may be present in specimens collected from cavitary lesions.45, 46
Coccidioides immitis is easy to culture and almost always recoverable within 1 week. The presence of spherules on direct microscopy is diagnostic of C. immitis. When spherules are not seen in clinical material, an isolate's identity should be confirmed. C. immitis is best identified using a species-specific genetic probe (GenProbe, San Diego).
Coccidioidomycosis is easily diagnosed by serologic means. Skin tests are no longer available. EIAs for detection of fungus-specific antibodies correlating to the IgM and IgG classes are now available. Detection of an IgM class of antibody requires confirmation by an immunodiffusion (ID) or tube precipitin (TP) method. The complement fixation (CF) test for the quantitative determination of IgG antibody is useful for diagnosis and prognosis. CF titers above 1:32 suggest dissemination; however, there is no standard procedure for CF testing, and values obtained by different laboratories may differ.45, 46
The diagnosis of histoplasmosis is predicated on the progression of disease. In nondisseminated, self-limited infection, pulmonary specimens rarely reveal the fungus.45, 46 Thoracotomy or lung biopsy may be necessary in very ill patients to adequately document Histoplasma. Direct microscopic visualization of yeast cells has poor sensitivity in both pulmonary disease (<10%) and disseminated infection (60%). With dissemination, however, recovery of the organism is possible from blood, bone marrow, skin lesions, or respiratory secretions in over 65% to 90% of cases. Bone marrow provides the highest yield.
Histoplasma requires from 5 days to 4 weeks for recovery by culture. A species-specific genetic probe is available for rapid confirmation of the isolate's identity. The identity may also be confirmed by conversion of the mycelial phase of growth to the yeast phase at 35°C.
An EIA is available for rapid diagnosis of histoplasmosis. It detects a histoplasma-specific glycoprotein antigen in BAL fluid, serum, urine, or CSF. The test's sensitivity is greatest with disseminated disease and is higher in urine (92%) than serum (82%); in less acute disease the sensitivity of EIA drops. Although the specificity of EIA is normally high (>90%), false-positive serum results can occur. To avoid erroneous interpretation, serum and urine specimens should be submitted for concurrent evaluations. Antigenemia without antigenuria is rare, and such a finding should raise suspicion of a false-positive result.52 Antigen EIA studies are available only from the Histoplasmosis Reference Laboratory at Indiana University (1001 W. Tenth St., Indianapolis, IN 46202; 1-800-HIS-TODG).52
Although antibodies are produced slowly, serologies may be useful.52 The combination of immunodiffusion and CF tests has a sensitivity approaching 100%, except in the immunocompromised patient who may mount an inadequate response. Skin testing with histoplasmin is of no diagnostic value and can produce antibodies that interfere with serologic diagnosis.
Cryptococcus neoformans is normally contracted through the respiratory tract, but is uncommonly found in the lung. Dissemination involves skin, bone marrow, and the central nervous system. Finding cryptococci in the lung should spur a careful evaluation for infection in other sites, especially the central nervous system (CNS).45
Cryptococcus neoformans can be easily isolated on a variety of media, including routine sheep blood agar plates and fungal media. It will not grow on selective fungal media containing cycloheximide. Cryptococcus will grow in routine bacterial blood cultures, but often requires staining or subculture for recognition of its presence in the blood culture medium. It is inadequately stained with the Gram's stain and requires fungal stains or India ink preparations for visualization.
Commercial latex agglutination or enzyme-linked immunoassay systems are also available for rapid detection of cryptococcal polysaccharide antigen in CSF and serum during infection. These tests have high sensitivity (95%) and specificity (98%) in disseminated cryptococcosis. Serum antigen detection, however, is not useful when infection is limited to the respiratory tract, because sensitivity drops to less than 50%.45, 46, 52
Pneumocystis carinii causes pneumonia in immunosuppressed patients. The monoclonal fluorescent antibody stain directly on sputum has become the diagnostic gold standard. Depending on experience, cytospin centrifuged smear preparations of induced sputum have a yield for P. carinii of 50% to 90%, whereas transbronchial biopsy and/or BAL have yields of over 90%.5, 45, 46 Expectorated sputum provides poor yields.
Aspergillosis and zygomycosis (often incorrectly referred to as mucormycosis) are seen almost exclusively in compromised hosts including diabetics in ketoacidosis. Candida species almost never cause frank CAP, even in the immunocompromised patient.44, 45
Susceptibility testing of fungi is covered in a separate article in this series.
Evaluation of Viral Infection
Viral pneumonias may be diagnosed by tissue cell line cultures and viral antigen detection methods directly on respiratory secretions.48 Improved tissue culture techniques such as shell vial cultures, with early detection of viral replication by fluorescent, EIA, or molecular techniques have decreased the time for respiratory virus recovery (to <48 hours). From a clinical perspective, direct fluorescent antibody or EIA methods for detecting the presence of virus directly in cytocentrifuged specimens are most useful. These direct testing methods are particularly useful for viruses like RSV that lose viability when transported. Specimens most likely to yield positive viral results include nasopharyngeal (NP) aspirates, followed by NP swabs, and then throat swabs submitted in appropriate viral transport media.
The recent introduction of anti-influenza A and B therapies has led to a proliferation of commercial products capable of rapidly detecting both viruses in clinical specimens.8, 48 Unfortunately, their usefulness is hindered by a lack of sensitivity and clinical benefit studies. NP aspirates improve the sensitivity compared with swabs of the nasopharynx or throat. Influenza therapies are time-dependent and useful only if administered within the first 48 hours of illness. The majority of patients with influenza do not present to their physicians within 48 hours, so any therapeutic intervention based on these rapid tests would be too late for the therapies.
SUMMARY
This article has focused on the evaluation of outpatients with lower respiratory illness. In large part, the need for microbiological work-up is host-dependent. Healthy patients usually do well, and laboratory data are often unnecessary. The abnormal host requires a different approach and, in general, the more compromised the host, the more aggressive the laboratory evaluation. A renal transplant patient with respiratory symptoms often follows the dictum that “common things happen commonly;” however, the clinician needs that extra level of assurance in this case. Some transplant patients may have respiratory illness caused by strongyloidiasis. Cystic fibrosis is another example of the need for a more comprehensive laboratory evaluation. Specialized selective media and additional susceptibility studies may be needed to evaluate isolates associated with exacerbation of symptoms in these patients. The clinical laboratory should be forewarned of any materials coming from invasive diagnostic techniques, so they can prepare and offer useful advice regarding specimens, transport, and follow-up. Microbiological laboratories are often most knowledgeable regarding what type of testing is appropriate. Direct communication with the laboratory is essential to assure the best patient care.
Footnotes
Address reprint requests to:Michael A. Saubolle, PhD Department of Clinical Pathology Good Samaritan Regional Medical Center 1111 E. McDowell Rd Phoenix, AZ 85006 e-mail: mail45622@pop.net
References
- 1.American Thoracic Society Guidelines for the initial management of adults with community-acquired pneumonia: Diagnosis, assessment of severity, and initial antimicrobial therapy. Am Rev Respir Dis. 1993;148:1418–1426. doi: 10.1164/ajrccm/148.5.1418. [DOI] [PubMed] [Google Scholar]
- 2.Barrett-Connor E. The nonvalue of sputum culture in the diagnosis of pneumococcal pneumonia. Am Rev Respir Dis. 1970;103:845–848. doi: 10.1164/arrd.1971.103.6.845. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett J.G., Breiman R.F., Mandell L.A. Community-acquired pneumonia in adults: Guidelines for management. Clin Infect Dis. 1998;26:811–838. doi: 10.1086/513953. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett J.G., Mundy L.M. Current concepts: Community-acquired pneumonia. N Engl J Med. 1995;333:1618–1624. doi: 10.1056/NEJM199512143332408. [DOI] [PubMed] [Google Scholar]
- 5.Baughman R.P., Conrado C.E. Diagnosis of lower respiratory tract infections: What we have and what would be nice. Chest. 1998;113:219S–223S. doi: 10.1378/chest.113.3_supplement.219s. [DOI] [PubMed] [Google Scholar]
- 6.Berk S.L. Justifying use of blood cultures when diagnosing community-acquired pneumonia. Chest. 1995;108:891–892. doi: 10.1378/chest.108.4.891. [DOI] [PubMed] [Google Scholar]
- 7.Boermer D.F., Zwadick P. The value of the sputum Gram's stain in community-acquired pneumonia. JAMA. 1982;247:642–645. [PubMed] [Google Scholar]
- 8.Boivin G., Hardy I., Kress A. Evaluation of a rapid optical immunoassay for influenza viruses (FLU OIA Test) in comparison with cell culture and reverse transcriptase-PCR. J Clin Microbiol. 2001;39:730–732. doi: 10.1128/JCM.39.2.730-732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalasani N.P., Valdecanas M.A.L., Gopal A.K. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined risks. Chest. 1995;108:932–936. doi: 10.1378/chest.108.4.932. [DOI] [PubMed] [Google Scholar]
- 10.Dismukes W.E. Chronic pneumonia. In: Mandell G.L., Bennett J.E., Dolin R., editors. Principles and Practice of Infectious Diseases. ed 5. Churchill Livingstone; New York: 2000. pp. 755–767. [Google Scholar]
- 11.Donowitz G.R., Mandell G.L. Acute pneumonia. In: Mandell G.L., Bennett J.E., Dolin R., editors. Principles and Practice of Infectious Diseases. ed 5. Churchill Livingstone; New York: 2000. pp. 717–743. [Google Scholar]
- 12.Farrell D.J., McKeon M., Daggard G. Rapid-cycle PCR method to detect Bordetella pertussis that fulfills all consensus recommendations for use of PCR in diagnosis of pertussis. J Clin Microbiol. 2000;38:4499–4502. doi: 10.1128/jcm.38.12.4499-4502.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.File T.M., Jr, Tan J.S., Plouffe J.F. Community-acquired pneumonia: What's needed for accurate diagnosis. Postgrad Med. 1996;99:107–195. [PubMed] [Google Scholar]
- 14.Fine M.J., Smith M.A., Carson C.A. Prognosis and outcomes of patients with community-acquired pneumonia: A meta-analysis. JAMA. 1996;275:134–141. [PubMed] [Google Scholar]
- 15.Fine M.J., Stone R.A., Singer D.E. Processes and outcomes of care for patients with community-acquired pneumonia: Results from the pneumonia patient outcomes research team (PORT) cohort study. Arch Intern Med. 1999;159:970–980. doi: 10.1001/archinte.159.9.970. [DOI] [PubMed] [Google Scholar]
- 16.Fishman J.A., Roth R.S., Zanzot E. Use of induced sputum specimens for microbiological diagnosis of infections due to organisms other than Pneumocystis carinii. J Clin Microbiol. 1994;32:131–134. doi: 10.1128/jcm.32.1.131-134.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gleckman R., DeVita J., Hibert D. Sputum gram stain assessment in community-acquired bacteremic pneumonia. J Clin Microbiol. 1988;26:846–849. doi: 10.1128/jcm.26.5.846-849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glezen W.P., Greenberg S.B., Atmar R.L. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 19.Gonzales R., Sande M.A. Uncomplicated acute bronchitis. Ann Intern Med. 2000;133:981–991. doi: 10.7326/0003-4819-133-12-200012190-00014. [DOI] [PubMed] [Google Scholar]
- 20.Grossman R.F. Guidelines for the treatment of acute exacerbations of chronic bronchitis. Chest. 1997;112:310S–313S. doi: 10.1378/chest.112.6_supplement.310s. [DOI] [PubMed] [Google Scholar]
- 21.Gwaltney J., Jr . Acute bronchitis. In: Mandell G.L., Bennett J.E., Dolin R., editors. Principles and Practice of Infectious Diseases. ed 5. Churchill Livingstone; New York: 2000. pp. 703–706. [Google Scholar]
- 22.Halperin S.A. Interpretation of pertussis serologic tests. Pediatr Infect Dis J. 1991;10:791–792. [PubMed] [Google Scholar]
- 23.Hoppe J.E. Bordetella. In: Murray P.R., Baron E.J., Phaller M.A., editors. Manual of Clinical Microbiology. ed 7. American Society for Microbiology Press; Washington, D.C.: 1999. pp. 614–624. [Google Scholar]
- 24.Hindiyeh M., Carroll K.C. Laboratory diagnosis of atypical pneumonia. Semin Respir Infect. 2000;15:101–113. doi: 10.1053/srin.2000.9592. [DOI] [PubMed] [Google Scholar]
- 25.Juven T., Mertsola J., Wais M. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Lentino J.R., Lucks D.A. Nonvalue of sputum culture in management of lower respiratory tract infections. J Clin Microbiol. 1987;25:758–762. doi: 10.1128/jcm.25.5.758-762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marrie T.J. Community-acquired pneumonia in the elderly. Clin Infect Dis. 2000;31:1066–1078. doi: 10.1086/318124. [DOI] [PubMed] [Google Scholar]
- 28.Miller J.M., Holmes H.T. Specimen collection, transport, and storage. In: Murray P.R., Baron E.J., Phaller M.A., editors. Manual of Clinical Microbiology. ed 7. American Society for Microbiology Press; Washington, D.C.: 1999. pp. 33–61. [Google Scholar]
- 29.Monso E., Ruiz J., Rosell A. Bacterial infection in chronic obstructive pulmonary disease: A study of stable and exacerbated outpatients using protected specimen brush. Am J Respir Crit Care Med. 1995;152:1316–1320. doi: 10.1164/ajrccm.152.4.7551388. [DOI] [PubMed] [Google Scholar]
- 30.Murray P.R., Washington J.A. Microscopic and bacteriologic analysis of expectorated sputum. Mayo Clin Proc. 1975;50:339–344. [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards . National Committee for Clinical Laboratory Standards; Wayne, Pa.: 2000. Performance standards for antimicrobial susceptibility testing: Eleventh informational supplement. NCCLS document M100-S11. [Google Scholar]
- 32.O'Brien K.L., Walters M.I., Sellman J. Severe pneumococcal pneumonia in previously healthy children: The role of preceding influenza infection. Clin Infect Dis. 2000;30:784–789. doi: 10.1086/313772. [DOI] [PubMed] [Google Scholar]
- 33.Ostergaard L., Andersen P.L. Etiology of community-acquired pneumonia. Evaluation by transtracheal aspiration, blood culture, or serology. Chest. 1993;104:1400–1407. doi: 10.1378/chest.104.5.1400. [DOI] [PubMed] [Google Scholar]
- 34.Pasculle W. Update on legionella. Clinical Microbiology Newsletter. 2000;22:97–101. [Google Scholar]
- 35.Peters C.J. California encephalitis, Hantavirus pulmonary syndrome, and Bunyavirid hemorrhagic fevers. In: Mandell G.L., Bennett J.E., Dolin R., editors. Principles and Practice of Infectious Diseases. ed 5. Churchill Livingstone; New York: 2000. pp. 1849–1855. [Google Scholar]
- 36.Plouffe J.F., McNally C., File T.M., Jr Value of noninvasive studies in community-acquired pneumonia. Infect Dis Clin North Am. 1998;12:689–699. doi: 10.1016/s0891-5520(05)70205-1. [DOI] [PubMed] [Google Scholar]
- 37.Prince D.S., Peterson D.D., Steiner R.M. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321:863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 38.Reimer L.G. Community-acquired bacterial pneumonias. Semin Respir Infect. 2000;15:95–100. doi: 10.1053/srin.2000.9591. [DOI] [PubMed] [Google Scholar]
- 39.Reimer L.G., Carroll K.C. Role of the microbiology laboratory in the diagnosis of lower respiratory tract infections. Clin Infect Dis. 1998;26:742–748. doi: 10.1086/514583. [DOI] [PubMed] [Google Scholar]
- 40.Rein M.F., Gwaltney J.M., Jr, O'Brien W.M. Accuracy of Gram's stain in identifying pneumococci in sputum. JAMA. 1978;239:2671–2673. doi: 10.1001/jama.239.25.2671. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds H.Y. Chronic bronchitis and acute infectious exacerbations. In: Mandell G.L., Bennett J.E., Dolin R., editors. Principles and Practice of Infectious Diseases. ed 5. Churchill Livingstone; New York: 2000. pp. 706–710. [Google Scholar]
- 42.Roson B., Carratala J., Verdaguer R. Prospective study of the usefulness of sputum Gram's stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin Infect Dis. 2000;31:869–874. doi: 10.1086/318151. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz M., Ewig S., Marcos M.A. Etiology of community-acquired pneumonias: Impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160:397–405. doi: 10.1164/ajrccm.160.2.9808045. [DOI] [PubMed] [Google Scholar]
- 44.Saubolle M.A. Lower respiratory tract specimens. In: Tilton R.C., Balows A., Hohnadel D.C., editors. Clinical Laboratory Medicine. ed 1. Mosby Year Book; St. Louis: 1992. pp. 591–603. [Google Scholar]
- 45.Saubolle M.A. Fungal pneumonias. Semin Respir Infect. 2000;15:162–177. doi: 10.1053/srin.2000.9598. [DOI] [PubMed] [Google Scholar]
- 46.Saubolle M.A. Mycology and the clinical laboratory in the diagnosis of respiratory mycoses. In: Sarosi G.A., Davies S.F., editors. Fungal diseases of the lung. ed 3. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 1–16. [Google Scholar]
- 47.Saubolle MA: Aerobic actinomycetes. In McClatchey KD (ed): Clinical laboratory medicine, ed 2. Philadelphia, Lippincott Williams & Wilkins, in press
- 48.Storch G.A. Diagnostic virology. Clin Infect Dis. 2000;31:739–751. doi: 10.1086/314015. [DOI] [PubMed] [Google Scholar]
- 49.Valenstein P.N. Semiquantitation of bacteria in sputum Gram's stains. J Clin Microbiol. 1988;26:1791–1794. doi: 10.1128/jcm.26.9.1791-1794.1988. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Waites K.B., Bebear C.M., Robertson J.A. American Society for Microbiology; Washington D.C.: 2000. Cumitech 34, laboratory diagnosis of mycoplasmal infections; pp. 1–30. [Google Scholar]
- 51.Wallace R.J., Jr, Glassroth J., Griffith D.E. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med. 1997;156:S1–S25. doi: 10.1164/ajrccm.156.2.atsstatement. [DOI] [PubMed] [Google Scholar]
- 52.Wheat L.J. Serologic diagnosis of fungal disease. In: Sarosi G.A., Davies S.F., editors. Fungal diseases of the lung. ed 3. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 17–29. [Google Scholar]


