Abstract
Ebola virus (EBOV), a member of Filoviridae virus family under the genus Ebolavirus, has emerged as a dangerous and potential threat to human health globally. It causes a severe and deadly hemorrhagic fever in humans and other mammals, called Ebola Virus Disease (EVD). In recent outbreaks of EVD, there has been loss of large numbers of individual’s life. Therefore, EBOV has attracted researchers and increased interests in developing new models for virus evolution, and therapies. The EBOV interacts with the immune system of the host which led to understand how the virus functions and effects immune system behaviour. This article presents an exhaustive review on Ebola research which includes EVD illness, symptoms, transmission patterns, patho-physiology conditions, development of antiviral agents and vaccines, resilient health system, dynamics and mathematical model of EBOV, challenges and prospects for future studies.
Keywords: Ebola virus, Ebola hemorrhagic fever, Ebola virus disease, Resilient healthcare system
1. Introduction
Ebola virus (EBOV) has emerged as a potential threat to human health worldwide, which cause a critical and serious ailment, often deadly if not cured properly. It is a member of “Filoviridae” virus family, under the genus “Ebolavirus”, having five species: Zaire Ebola, Tai Forest Ebola, Sudan Ebola, Bundibugyo Ebola, and Reston Ebola Virus [1]. Out of these species, former four cause illness in humans, whereas the Reston Ebola Virus causes illness in primates. Ebola virus causes an illness called as hemorrhagic fever [2], especially in humans and other mammals, named as “Ebola Virus Disease” (EVD), and therefore EVD is also known as “Ebola Hemorrhagic Fever” (EHF). Ebola viruses are native to a number of African countries. The disease was firstly recognized in 1976 in two concurrent outbreaks: one of them in Nzara, an state in South Sudan, and another one in village Yambuku, previously known as Democratic Republic of Congo, adjacent to ‘Ebola River’, and hence the name Ebola Virus Disease [3].
1.1. EBOV and the immune system
EBOV is an enveloped virus having a 19 kb single-stranded, negative-sense RNA genome which encodes 7 proteins [4]. The core of the virus is composed of the RNA genome, called nucleoprotein (NP), that covers the genomic RNA and nucleocapsid viral protein 30 (VP30) which is covered by a lipid envelope having projected surface composed of a glycoprotein (GP). The surface glycoprotein is a multimer having roles in cell attachment, fusion and cell entry, helps in immune evasion and pathogenesis of disease [5]. The diagrammatic representation of Ebola genome is shown in Fig. 1 .
Fig. 1.
Diagrammatic representation of Ebola genome (7 identified genes).
Zaire Ebola Virus triggers the immune response and then punctures the vascular system. When the virus enters the body, it infects various types of immune cells by initially targeting monocytes and macrophages, and cause the release of proinflammatory cytokines and chemokines [98]. In the previous studies it was shown that host Toll-like receptor 4 (TLR4) is a sensor for EBOV glycoprotein (GP) on virus-like particles (VLPs) and that resultant TLR4 signaling pathways lead to the production of proinflammatory cytokines and suppressor of cytokine signaling 1 (SOCS1) in a human monocytic cell line and in HEK293-TLR4/MD2 cells stably expressing the TLR4/MD2 complex. EBOV GP interacts with TLR4, and on VLPs it was able to stimulate expression of NF-κB in a TLR4-dependent manner. It was also found that budding of EBOV VLPs was more pronounced in TLR4-stimulated cells than in unstimulated control cells. These findings identify the host innate immune protein TLR4 as a sensor for Ebola virus GP and play an important role in the immune-pathogenesis.
The immune system forms a complex network of cells communicating to each other with the help of soluble mediators such as cytokines. Immune system has both innate and adaptive immunities to protect the body against pathogens. Nucleotides from RNA viruses are recognized by retinoic acid inducible gene I (RIG-I)-like helicases (RLHs) and Toll-like receptors (TLRs). It triggers signaling cascades that induce anti-viral mediators such as type I interferons (IFNs) and pro-inflammatory cytokines.
In many viral infections, the early action of cytokines produced by infected cells and dendritic cells is sufficient to eliminate the pathogen [6]. In some cases, innate defenses are not able to handle viral infection, and second-line of defenses is mobilized to ensure host survival. The adaptive defense consists of antibodies and lymphocytes, often called the humoral response and the cell mediated responses which are essential for destruction of viruses [6]. Innate and adaptive responses usually work together to eliminate the viruses, but in some cases both these immune responses are not able to eradicate the viruses which led to diseases. Filoviridae family of viruses is one of the examples which are not destroyed by the immune system.
Many studies shown that the induction of an innate immune response lead to infection or stimulation of macrophages/monocytes and DCs with Ebola virus or VLPs, respectively. For example, incubation of Ebola virus VP40 + GP VLPs with DCs led to the induction of interleukin-6 (IL-6), IL-8, NF-κB and ERK1/2. Ebola virus VP40 + GP VLPs, but not VP40 VLPs, induced cytokine and SOCS1 expression in a TLR4/MD2 dependent manner both in a human monocytic cell line (THP-1 cells) and in 293T cells expressing a functional TLR4/MD2 receptor. Stimulation of TLR4 by Ebola virus envelope GP results in an innate host response, induction of SOCS1 protein, and potential enhancement of virus egress. The detailed coverage of various genes involved in immune response can be found in [5], [6], [7], [46].
There were several EVD outbreaks in the past years, but the most severe and the largest was the recent widespread outbreak in West Africa (2013–2016), having 28,616 cases and 11,310 deaths, and estimated case fatality rate of 71% [8], [9]. Fig. 2 presents country and year-wise outbreaks, number of cases registered, and number of deaths..
Fig. 2.
Country and year-wise EVD outbreaks statistics (1976 – 2017) showing year of outbreaks, number of cases registered (numerator) and number of deaths (denominator). Data sources: http://www.who.int/mediacentre/factsheets/fs103/en/, https://www.cdc.gov/vhf/ebola/outbreaks/history/summaries.html.
1.2. Clinical features and complications
Signs and symptoms of EVD start between 2–21 days after contacting the virus, and the most common symptoms are fever, sore throat, muscular pain and headache [10] (Fig. 3 ). With high concentration of virus, vomiting, diarrhoea, and rashes are usually occurred which decrease the function of liver and kidneys. It also causes bleeding both internally and externally which lead to high risk of death and killing 90% of infected people due to low blood pressure from body fluids [11]. The severity of symptoms of EVD compared with ZikV [12], Dengue, Chikungunya and West Nile are presented in Table 1 .
Fig. 3.
Most common signs and symptoms of EVD after contacting the virus.
Table 1.
Comparison of severity of symptoms: EBOV to ZikV, Dengue, Chikungunya and West Nile.
| Symptoms | EBOV | ZikV | Dengue | Chikungunya | West Nile |
|---|---|---|---|---|---|
| Conjunctivitis | ✓✓✓✓ | ✓✓✓ | ✓✓ | ✓ | ✓✓ |
| Diarrhoea | ✓✓✓✓ | ✓ | ✓✓ | ✓ | ✓ |
| Dysphagia | ✓✓✓✓ | ✓ | ✓ | ✓ | ✓ |
| Hiccups | ✓✓✓✓ | ✓ | ✓ | ✓ | ✓ |
| Nausea | ✓✓✓✓ | ✓✓ | ✓ | ✓ | ✓ |
| Rashes | ✓✓✓✓ | ✓✓✓✓ | ✓✓ | ✓✓ | ✓✓ |
| Chest pain | ✓✓✓ | ✓ | ✓ | ✓ | ✓ |
| Headache | ✓✓✓ | ✓✓ | ✓✓✓ | ✓✓ | ✓ |
| Loss of appetite | ✓✓✓ | ✓ | ✓ | ✓ | ✓ |
| Muscle Pain | ✓✓✓ | ✓✓ | ✓✓✓ | ✓✓ | ✓✓ |
| Weakness | ✓✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ |
| Vomiting | ✓✓✓ | ✓ | ✓✓ | ✓ | ✓ |
| Abdominal pain | ✓✓ | ✓ | ✓ | ✓ | ✓ |
| Bleeding | ✓✓ | ✓ | ✓ | ✓ | ✓ |
| Fatigue | ✓✓ | ✓ | ✓✓ | ✓✓✓ | ✓ |
| Joint Pain | ✓✓ | ✓✓ | ✓ | ✓✓✓✓ | ✓ |
| Fever | ✓ | ✓ | ✓✓✓✓ | ✓✓✓✓ | ✓✓ |
Ebola virus is responsible for haemorrhagic fever leading to various complications such as malaise, fever, vascular permeability, decreased plasma volume, coagulation abnormalities, and varying degrees of hemorrhage [13]. It is also notified that the incubation period of filoviruses is 2–21days after infection, with identifying symptoms such as chills, fever, myalgia and malaise, which is followed by lethargy, nausea, vomiting, abdominal pain, anorexia, diarrhea, coughing, headache, hypotension, maculopapular rash and mucosal bleeding, in the gastrointestinal and genitourinary tracts. With the pertaining of these diseases, specially as a result of hypotensive shock and multi-organ failure (including hepatic damage and renal failure), death usually occurs in 6–16 days after the onset of symptoms [14].
In a statistical study of EVD cases [15], the amount of survived and died individuals are determined by various parameters such as age, sex and symptoms as shown as charts in Fig. 4 . It can be inferred from Fig. 4 that mortality rate of EVD patients are higher in males than females. Similarly, the bleeding events from faeces show a high mortality rate during admission as well as hospitalization.
Fig. 4.
Pie Charts showing epidemiology of EBOV based on different parameters (such as age, sex and symptoms) (Source of data: [15]).
Several people have survived from EVD during the previous major outbreaks in Sierra leone (2014-16). However, many complications of EVD and its factors persist and responsible for death [16].Various signs and symptoms of EBOV infection has been associated with many complications [16], [17], given in Table 2 .
Table 2.
Persistence of signs, symptoms, and diagnoses in EVD Survivors of in the Follow-up Program of Médecins Sans Frontières in Freetown, Sierra leone extracted from [16]. Data are presented as number as well as in percent within parenthesis. Abbreviations: ETC, Ebola treatment center; GERD, gastroesophageal reflux disease; STI, sexually transmitted infection.
| Signs, symptoms and diagnoses | All cases, N = 166 | Adults age ≥ 16 yrs, n = 128 | Children age ≤ 15 yrs n = 38 | Days from ETC discharge to first follow-up visit |
|||
|---|---|---|---|---|---|---|---|
| 0–30days, n = 62 | 31–60 days, n = 45 | 61–90 days, n = 21 | ≥91 days, n = 38 | ||||
| Arthralgia | 129(77.7) | 109(85.1%) | 20(52.6%) | 48(77.4%) | 34(75.5%) | 18(85.7%) | 29(76.3%) |
| Fatigue | 116(69.8) | 94(73.4%) | 22(57.8%) | 51(82.3%) | 22(48.9%) | 16(76.1%) | 27(71.0%) |
| Abdominal Pain | 90(54.2%) | 72(56.2%) | 18(47.3%) | 40(64.5%) | 21(51.1%) | 14(66.7%) | 12(34.2%) |
| Headache | 87(52.4%) | 66(51.5%) | 21(55.3%) | 36(58.0%) | 25(55.6%) | 11(52.4%) | 15(39.5%) |
| Anemia | 83(50%) | 55(42.9%) | 28(73.7%) | 36(58.0%) | 22 (48.8%) | 11(52.3%) | 14(36.8%) |
| Skin rash / infection/itching | 81(48.8%) | 54(41.2%) | 27(71.0%) | 38(61.2%) | 21(46.7%) | 9(42.8%) | 13(34.2%) |
| Back pain | 54(32.5%) | 46(35.9%) | 8(21.0%) | 25(40.3%) | 14(31.1%) | 8(38.1%) | 7(18.4%) |
| Alopecia (diffuse) | 53(31.9%) | 43(33.5%) | 10(26.3%) | 23(37.1%) | 8(17.8%) | 10(47.6%) | 12(31.6%) |
| Respiratory tract infection + otitis | 45(27.1%) | 29(22.6%) | 16(42.1%) | 17(27.4%) | 18(40.0%) | 3(14.3%) | 7(18.4%) |
| Anorexia | 43(25.9%) | 36(28.1%) | 7(18.4%) | 21(33.8%) | 11(24.4%) | 5(23.8%) | 6(15.8%) |
| Genital/urinary tract infection/STI | 38(22.8%) | 36(28.1%) | 2(5.3%) | 22(35.5%) | 5(11.1%) | 3(14.3%) | 8(21.0%) |
| Insomnia | 30(18.0%) | 29(22.6%) | 1(2.6%) | 10(16.1%) | 7(15.6%) | 4(19.0%) | 9(23.7%) |
| Gastritis/ulcer/GERD | 27(16.2%) | 26(20.3%) | 1(2.6%) | 11(17.7%) | 7(15.5%) | 4(19.0%) | 5(13.2%) |
| Malaria | 23(13.8%) | 14(10.9%) | 9(23.7%) | 10(16.1%) | 3(6.7%) | 2(9.5%) | 8(21.0%) |
| Moderate acute malnutrition | 19(11.4%) | 7(5.4%) | 12(31.6%) | 10(16.1%) | 6(13.3%) | 1(4.7%) | 2(5.2%) |
| Cardiopathy/valvulopathy/tachycardia | 19(11.4%) | 17(13.2%) | 2(5.3%) | 7(11.3%) | 6(13.3%) | 2(9.5%) | 4(10.5%) |
| Arterial hypertension | 12(7.2%) | 12(9.3%) | 0 (0%) | 4(6.4%) | 4(8.4%) | 1(4.7%) | 3(7.8%) |
| Diarrhea/gastroenteritis | 9(5.4%) | 7(5.4%) | 2(5.3%) | 5(8.0%) | 2(4.4%) | 0 (0 %) | 2(5.3%) |
| Any ocular complication | 94(56.6%) | 74(57.8%) | 20(52.6%) | 41(66.1%) | 22(48.8%) | 11(52.3%) | 20(52.6%) |
| Uveitis | 58(34.9%) | 50(39.0%) | 8(32.1%) | 26(41.9%) | 10(22.2%) | 8(38.0%) | 14(36.8%) |
The most common and frequent complications were Neurological problems (brain as well as spinal cord problems), Ocular complications (eye difficulties), and Musculoskeletal problems (including body’s muscles problems) [18].
1.3. EBOV transmissions
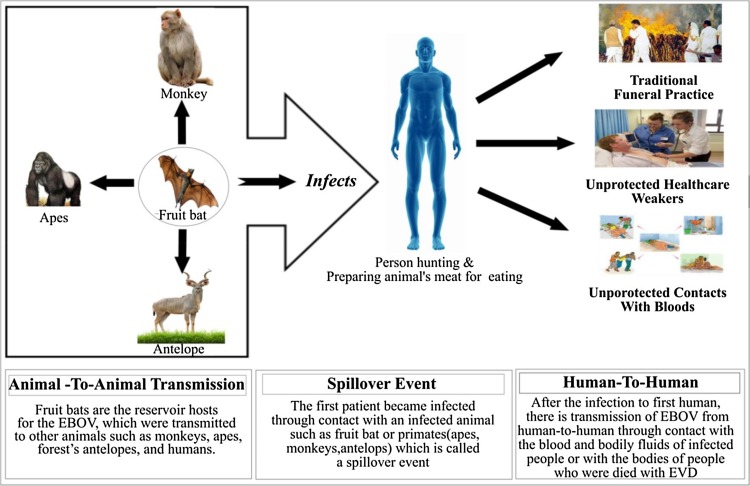
EVD is a zoonotic disease which involves animals and humans [19]. However, there is no evidence of the natural reservoir host. The scientists investigated that the fruit bats are the carrier hosts of the EBOV and usually contain the virus particles. The viruses which were carried by bats are transmitted to animals, such as monkeys, apes, forest’s antelopes, and also to humans, who came in contact with the bodily fluids like blood and secretions of such infected organisms. These transmissions are possible between animal-to-animal, animal-to-human, and human-to-human (Fig. 5 ).
Fig. 5.
EBOV transmission from fruit-bat to animals, animals-to-humans, and humans-to-humans.
In addition to infected skin, EVD also transmits through bodily fluids such as blood and secretions of infected people, and with some kind of objects such as beds and clothes spoiled with such type of fluids [19]. The EVD patients also cause illness to many health-care workers during care and treatment. Transmission of EBOV has also been reported when people attend traditional funeral practices which involve direct contact with the body of the deceased people (Fig. 5).
1.4. EVD epidemics and persistence of virus after recovery
After the transmission of Ebola virus to different part of the West Africa such as Guinea, Liberia, and Sierra Leone, epidemic of Ebola virus disease had resulted in a total of 28,646 cases and 11,323 deaths. In addition, Ebola cases were also exported to several other African and European countries including United States, with further transmission to healthcare workers across several countries [13]. The large number of Ebola survivors has the frequency of persistent symptoms and the possibility of virus persistence in sanctuary sites, leading to delayed transmission. EBOV may also persist in immune-privileged sites and semen of survivors which lead to re-ignition of EVD outbreak. As EBOV RNA can remain detectable in immune-privileged tissues for prolonged periods of time after clearance from the blood, suggesting that it may persists during convalescence and thereafter [97]. Eliminating persistent EBOV is important to ensure full recovery of survivors and decrease the risk of outbreak re-ignition caused by EBOV spread from healthy survivors to new contact [20].
Due to persistence of EVD in West Africa, most of the survivors have many residues of infection of disease and complications, such as arthritis and vision-threatening uveitis [97]. Many of the symptoms such as fatigue, sleep disturbance, blurred vision, retro-orbital pain, hearing loss, difficulty swallowing, and muscle weakness were reported after 21 months of follow-up. This lead to delayed clearance of viruses in selected and other body compartments, semen of the men, pregnant women, fetuses, products of conception, and breast milk [21].
2. Dynamics of the mechanism of EBOV
There is a very interesting relationship between the ecosystem and the human population, where ecosystem is like a natural home for humans. Wildlife plays an important source of infectious diseases for the human race. There are myriads of serious, harmful zoonotic infections that have been caused by bats (vector), for example, EBOV, Marburg, SARS-corona virus, Nipah, Hendra, multiple types of rabies etc. There is an intrinsic relationship between the ecosystem health and the human health which, unfortunately, has not been understood well [22], [23].
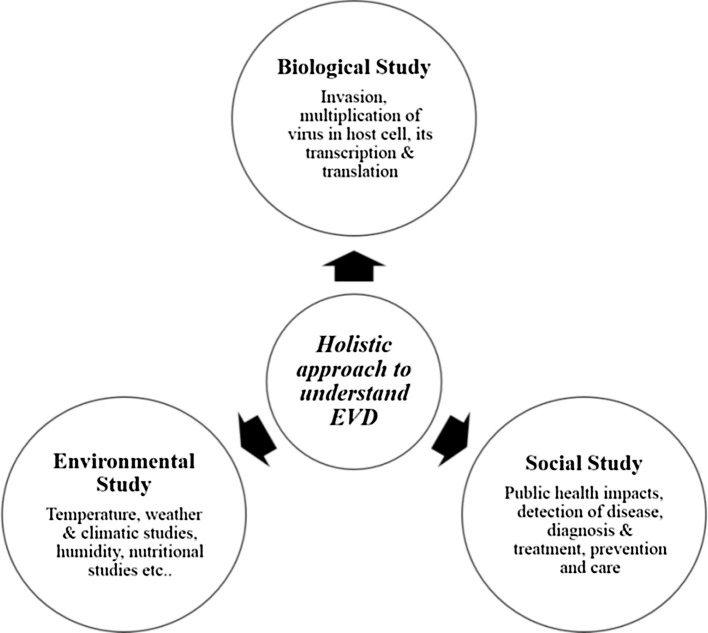
Our ecosystem is not just composed of the goody-goody beings, but also some of the most threatening pathogens, such as RNA viruses. These viruses are very harmful as they have the potential to adapt to new hosts and environments [24]. Henceforth, we have a challenge to protect the wildlife and their habitats, by that safeguarding vital ecosystem structures and functions having local as well as broader consequences for human wellbeing [25].There are high public health risks, disease threats which are because of RNA viruses associated with fruit bats. The spill-over of infections occurs from bats to humans, but the compendium of dynamics involved depends on the extent of spill-over that has been recognized and understood and revolves around three important factors: Biological Factors, Environmental Factors, and Social Factors.
Zoonosis related health viruses also focus less studied social and environmental queries, like why are bats the only vectors for EBOV? How do people interact with bats, both directly and indirectly? What are the environmental factors that force viral pathogen dynamics and spill-over between many species of bats? To what extent bats cause infectious diseases in human being? How do the above mentioned factors influenced in the spread of the diseases? Does the bats ancestry, its life history, and feeding habits play a role in deciding whether it is capable of becoming a vector or not? Are bats the only ones which act as a cave for many deadly viruses, or are there more vectors? To answer some of these questions, biological factors have been studied in detail, but there is a need to study the social and environmental factors. For this, it is mandatory to take up a holistic approach of study [25].
2.1. Need of the hour: the holistic approach
An integrated study of the three factors: Biological, Social, Environmental is mandatory to understand and explain the actual mechanism of zoonotic emergence. Fig. 6 depicts and describes the holistic approach to understand zoonotic disease like EVD. We need to understand the virus evolution, transmission and its spill-over to humans using mathematical and ecological models. The dynamics of individual spill-over episodes are based around the notion of the pyramid (the triangle that has been shown in Fig. 7 ) of pathogen emergence. It is suggested that humans are confronted by animal derived pathogens, however, only a small fraction of people (having weak immunity) get affected. Spill-over dynamics are liable to a range of local impact and practice, both social and environmental influences on viral pathogen dynamics in the manner that interaction with susceptible sympatric species [25]. These models give us a skeletal framework for studying the dynamics of infections in bat reservoir populations [26].
Fig. 6.
Holistic approach to understanding zoonotic disease like EVD.
Fig. 7.
A schematic diagram showing dynamics of individual spill-over (from EBOV to population P) in the local system, and how it is connected to three-factors Biological, Social, and Environmental domains.
2.2. Role of systems biology in elucidating the framework
Using advanced computational and analytical techniques, we can capture and develop a framework that adds the dynamic relationship between the virus (EBOV), bats, animals (candidate hosts) and humans (target) in a local system of three factors explained in Fig. 7. Systems Biology can be used for modeling the events, and deriving the answers to all those questions which are still unanswered. Systematic Modeling and Interaction Modeling can be used extensively to model biological phenomenon. With the help of computational and analytics approaches, we can unlock many new theories related to EVD transmission, and mechanism of EBOV invasions. We can even develop better vaccines and drugs for the diseases [27]. Infectious diseases are growing rapidly as the pathogens are evolving themselves against our therapeutics. There has been an urgent need for improved remedies against pathogens. Systems Biology based studies have proved to be icing on the cake! Systems Biology based analysis have been worked out recently in order to capture how the host genes are associated with the viral replication that tend to occupy space in the human protein interactome [28], [29]. A comprehensive systems-level analysis of the virus-host interactome is very important for understanding the roles of host factors with the end goal of discovering new druggable antiviral targets ([30]; Chasman, et al., 2014).
Many antiviral drug discovery approaches have produced appreciable achievements but there are no medications available in order to fight it. Systems biology based antiviral drug studies have tremendously helped in the identification of new antiviral candidates. Cheng et al. [31] have proposed an integrated approach to determine new antiviral candidates for the existing drugs by adding drug-gene patterns into the virus-host interactome. Using systems biology analysis they have determined three molecule drugs namely, Ajmaline, Piroxicam and Azlocillin, as the new drug candidates for anti-Ebola virus treatment. Along with this, they have also proposed the mechanism showing how these drugs can block the Ebola virus and henceforth, inhibit the infection.
In addition, software tools are also developed to identify EVD patients, along with the risk of EVD suffering in the future. Ebinformatics Software [32], known to be Ebola Fuzzy Informatics system, is developed in order to diagnose, prescribe appropriate medications to EVD patients. Based on the user evaluation result, this software has proven to be a valuable addition to the operational and research enterprise to fight against Ebola [32]. This multifaceted yet single platform has been achieved by gathering information such as factors that cause Ebola-like signs and symptoms, and a system was developed based on facts for the prognosis and diagnosis of the disease. This system relates the conditions and answer whether one is suffering from EVD or not.
2.3. Need to correlate the three factors
These are the biological, social, and environmental factors that are responsible for the disease spread. Such factors, as mentioned earlier, are not understood well for most of the zoonotic diseases. Thus, there is a need for some sort of empirical modeling techniques which can elucidate and derive a correlation of disease spill-over and their forces at various levels [27], [33]. Spatially categorical ecosystem data, including wildlife densities, livestock densities, human population densities, climate and socio-economic variables are mostly accessible for such analyses [27], [33].
In a nutshell, there is an urgent need for a framework for the holistic study of diseases caused by bats as the vector. Interdisciplinary studies play a vital role in mining the zoonotic diseases. The collaboration between Life Sciences, Mathematics, Systems Biology, Computational and Analytical approaches is a must for studying the disease dynamics. This will also help us to understand how to cope up with such apex diseases, and preventing them from spreading further.
3. Clinical strategies and challenges in the treatment
The most recent and the biggest epidemic implicating more than 28,000 cases in West Africa was triggered by a variant strain of EBOV with an approximate global death of ∼40% [34]. We lacked the certified therapeutics to treat EVD during 2014–15 outbreaks which demolished West African countries [35].
As diagnostic techniques for EBOV, Enzyme-linked immune-sorbent assay (ELISA) and real-time Reverse Transcription Polymerase Chain Reaction (rRT-PCR) are the mostly used. Antigen or antibody (IgM/IgG) is ascertained by ELISA and rRT-PCR through which presence of Ebola virus-specific genes are identified after virus isolation. However, antibody-capture ELISA method is restricted to be applied during early diagnosis due to non-appearance of antibodies within 1–2 week(s) of illness [36]. The developmental stages of therapeutics and drugs against EBOV have been very slow as most of the procedures related to the research and development has always been tricky and a big challenge, plus, it requires bio-safety level-4 laboratories.
Though a number of vaccines and anti-viral drugs have been developed against EBOV which provided satisfying results only for the first phase of clinical trials, but phase II, III and IV studies didn’t pass the trial lately. This failure highlights the fact that we still need better equipment, resources and technology to develop an optimized treatment for preventing and curing EVD. International organizations are establishing treatment centers which must be prepared to enlist patients for clinical trials, and have appropriate structures for the management of ethical and medico-legal requirements [37], [38].
Other challenges to conduct clinical trials are the need to train personnel to enter the “red zone” of Ebola Treatment Centres, thus, avoiding from risk exposure to Ebola, seeking consent from serious EVD patients for complex studies, and managing and expediting ethical review process between various governmental and non-governmental healthcare/research organizations [39]. The following section mentions known therapeutics which comprises of vaccines, small molecule based therapies, antibody based therapies, and also showcases how far we have reached in developing latest vaccines and drugs to eliminate EBOV [40].
3.1. Symptomatic treatment against Ebola
Symptomatic treatment refers to the immediate and urgent treatment that is given when a person gets infected. This treatment is completely dependent on clinical requirements, viz. oral/intravenous therapy, analgesics, antipyretics, anti-diarrheal medications, antipsychotic drugs etc.., as per the patient’s signs and symptoms [41], [90]. Table 3 summarizes detailed information about the medications that are used for the symptomatic treatment along with the side effects that may arise.
Table 3.
Summarized table about the symptomatic treatment for initial symptoms developed by patients who get infected with Ebola, along with the description of medications and side effects. The table has been derived from Department of Health, Republic of South Africa (Clinical Management of patients with Ebola virus disease (2015), (URL:http://www.nicd.ac.za/assets/files/clin_mx_feb15.pdf) and Ebola Clinical Care Guidelines organized by the Public Health Agency of Canada (URL www.ammi.ca/media/69846/Ebola%20Clinical%20Care%20Guidelines%202%20Sep%202014.pdf).
| Symptoms observed in the patients | Symptomatic treatment | Description of the treatment | Side-effects that may occur |
|---|---|---|---|
| Headache, Fever, Chills, Body Ache | Acetaminophen (Paracetamol) | Acetoaminophen is a p-aminophenol derivative with analgesic and antipyretic properties. The antipyretic property is because of inhibition of prostaglandin synthesis and release in the CNS. | (i) May cause skin, eyes, and respiratory irritations. (ii) It may be harmful, if swallowed. |
| Pain (Joints, Chest, Bones, Abdomen) | Morphine, Fentanil (fentanyl) | Morphine is an opiate alkaloid extracted from the Poppy plant (Papaver somniferum). It binds and activates specific opiate receptors that are involved in controlling most of the functions of brain. Fentanil is a synthetic, lipophilic phenyl piperidine opoid agonist anesthetic and analgesic property. | (i) Morphine, if taken in excess may cause drowsiness and dizziness. (ii) Fentanil if swallowed, may be fatal, cause breathing difficulties, skin allergies, damages fertility and harm to breast fed children. |
| Diarrhoea | Imodium (Loperamide) | Loperamide is a synthetic agent chemically related to opiates with anti-diarrheal properties. | Toxic if swallowed. |
| Vomiting / Nausea | Metaclopramide, Ondansetron; some physicians may also recommend NG tube (Nasogastric Intubation) | Metaclopramide is a substituted benzamide and a derivative of para-aminobenzoic acid (PABA), with gastroprokinetic and anti-emetic effects. Ondansetron is a competitive serotonin type 3 receptor antagonist viz. used to treat nausea and vomiting. | (i) Metaclopramide may have reproductive toxicity. (ii) Ondansetron generally causes headaches, constipation and dizziness, and skin irritations. |
| Psychotic Episodes | Diazepam, Haloperidol | Diazepam is a benzodiazepine derivative with sedative, hypnotic and anticonvulsant properties. Haloperidol is a phenyl piperdinyl-butyrophenone that is used to treat schizophrenia and other psychoses. |
|
| Hiccups | Chlorpromazine may also be treated by using NG tube. | It is basically an anti-psychotic drug but has many other properties, it is used as an anti-emetic drug and it also used in the treatment of intractable hiccups. | Acute toxicity and causes skin problems. |
3.2. Therapeutics involved in treatment
Vaccinations are known to play a pivot role in reducing risks of many diseases, including EVD. Since the rise of biotechnology, pharmaceuticals have also used the antibodies (IgG/IgM) and other small molecules (lipids) to reduce the risk of Ebola, summarized details is given in Table 4 .Vaccination alone is no “cure-all remedy”. We need to have a better management system which abstains itself from all the bigotry and favoritism.
Table 4.
Summarized therapies used for EVD.
| Product | Company | Description | Intake | Clinical trial stages of evaluation |
|---|---|---|---|---|
| Favipiravir (T-705, Avigan) | Toyama Chemical, Fujifilm Group (Tokyo, Japan) | 6-Fluoro-3-hydroxy-2-pyranizecarboxamide licensed anti-influenza drug, viral RNA Pol(L) inhibitor. | Oral | In Phase III but drug not tested on EVD patient. |
| ZMapp | MappBio (San Diego, USA) | Cocktail of 3 humanized chimeric neutralizing antibodies c13c6, 2G4,4G7, targets viral GP. | Intravenous Injection | Currently in Phase I & II. Drug tested on EVD patients. |
| Convalescent Plasma | N/A | ABO-compatible plasma from convalescent donors | Intravenous Injection | Not confirmed, but studies reveal that out of 8 patients only 1 died. |
| JK-05 | Sihuan Pharmaceutical(Beijing, China) | Inhibitor of the viral RNA pol. | Intravenous Injection | Drug not tested, but it is studied for army personnel for emergency use only. |
| BCX4430 | BioCryst Pharmaceuticals(North Carolina) | Broad spectrum antiviral effective against RNA viruses. | N/A | In Phase I but drug not tested. |
| TKM-Ebola(TKM) | Arbutus BioPharma(Canada) | siRNA lipid nanoparticle product used to target mRNAs of viral RNA pol (L) and viral protein 35(VP35). | Intravenous Injection | In Phase II, but drug not tested. |
| Brincidofovir(CMX001) | Chimerix (Durham, USA) | 3-Hexadecycloxy-1-propanol(HDP) lipid associate of acyclic nucleoside phosphonatecidofovir EBOV, but mode of action in not known. | Oral | Withdrawn from Phase II, butdrug not tested. |
| Anti-Ebola hyperimmune globulin | N/A | Prepared from plasma of donors having high antibodies against specific antigen. | Intravenous Injection | Animal studies completed. |
| GS-5734 | Gilead (Foster City, U.S.A.) | Monophosphoramidate prodrug which acts as an inhibitor for RNA pol in viruses. | Intravenous Injection | Phase II passed. |
| AVI-6002 | Sarepta Therapeutics (Cambridge, MA) | Combination of 2 phosphorodiamidatemorpholino antisense oligomers [PMOs] with positive charges on tabbed subunits (PMOplus™). Interferes with RNA signaling involved in protein synthesis. | Intravenous Injection | Passed In Phase I, but drug not tested. |
| rGP nanoparticle (Novavax) | Gaithersburg (Maryland, USA) | Needs 2 injections. | Intravenous Injection | In Phase II. |
| ChAd-EBOV, Ad5-EBOV, cAd3-EBOV (GSK), Ad26 and MVA-EBOV | Merck, gsk, J&J, CanSinotech. | Long term immunity. | Intramuscular Injection | Recruitment is unknown, but passed Phase I. |
| FGI-103 | Investigational New Drug (IND, U.S.A.) | Antiviral drug, mechanism of action not known. | Intraperitoneal Injection (Mice) | In Phase III. |
| U18666A(3β)-3-[2-(Diethylamino)ethoxy]androst-5-en-17-one hydrochloride) | N/A | A cell permeable drug which inhibits cholesterol trafficking, cholesterol transport from late endosomes/lysosomes to the endoplasmic reticulum (ER), but not cholesterol transport to bilipid layered membrane. | Oral | In Phase I. |
| Clomiphene | Approved by AHFS (U.S.A.) | Comprises of 2 isomers: enclomiphene (trans-clomiphene) and zuclomiphene (cis-clomiphene). Enclomiphene is an estradiol receptor antagonist while the latter being an estradiol receptor agonist. | Intraperitoneal Injection (Mice) | Between Phase II and III. |
| Toremiphene | Approved by AHFS (U.S.A.) | It is an oral Selective Estrogen Receptor Modulator (SERM), opposes the actions of estrogenin the body. | Intraperitoneal Injection (Mice) | In Phase III. |
| FX06 | Fibrex Medical Inc. (Austria) | Naturally occurring peptide derived from the neo-N-terminus of fibrin Bbeta(15-42). It prohibits leukocyte migration over the gap junctions of endothelial cells. FX06 has authenticated safety in acute and subchronic toxicological studies and has entered clinical development lately. | Fluid Intake | In Phase II. |
| Chloroquine (7-chloro-N-[5-(diethylamino)pentan-2-yl]quinolin-4-amine) | Bayer AG (Germany) | Chloroquine emerged as optimum in vitro EBOV inhibitors. | Oral | Phase IV. |
| Statins | Sankyo (Japan) | Lipid-lowering drug which shows anti-inflammatory, immune modulatory & antiviral characteristics. | Oral | In Phase II. |
| Fingolimod (FTY720) | Novartis (Switzerland) | Advantageous agent for avoidance of transplant rejection & autoimmune diseases; provide to reduce inflammation and fatality in mice. | Oral | Phase IV complete. |
| Ribavirin (Rebetol) | Fulford Limited (India), and Lupin Laboratories Limited (Pinnacle) | Interferes with capping of viral mRNA and not recommended because of severe adverse effects. | Oral (Capsule) | In Phase IV. |
| Lamivudine | Glaxosmith Keine (gsk), USA | A nucleoside analogue that interfere gene replication; no survival benefit demonstrated in EVD. | Oral | In Phase II. |
| Amiodarone | Approved by FDA | N/A | Oral | In Phase III. |
Drug discovery and development is a very expensive process in both the terms time and money. Once such medications are ready to be circulated, these medications are not easily available to the low economic countries. Therefore, it is an utmost responsibility of the pharmaceutical industries and the governments to join their hands and to make such medications available at cheap costs. After all, a human being is a resource, and a life saved is more important than to make heaps of stocks of money. The current drugs that are being used to treat Ebola affected patients are so costly, for example: Favipiravir (T-705) 25mg costs around $280, whereas, the same medicine but of a higher dosage, about 100mg costs around $780. Such therapeutic treatments and medications are very costly, which actually can be one of the reasons why we still lag behind in completely eliminating Ebola roots from the world. Epidemiologic practices, like toss-back analyses to establish and lazaretto people debunked to Ebola, are momentous to curb the spill-over. Such control practices are desired to train people on the ground in even the most lonesome locale [87]. Given that nosocomial transmission has contributed heavily to past Ebola epidemics [42]. It is also indispensible to coalesce vaccination with nosocomial contact precautions and quarantining [43], [44].
Several problems were faced by the scientists while evaluating the drugs mentioned in Table 4. Before testing any drug, patients need to be isolated and there should be no contact between the patients and the rest of the people, which can cause further contamination [98]. The blood samples are taken and rapidly sent to the pathological laboratories for testing. In the fresh phase of the disease, fluid replacement therapy hysterically escalates the probability of endurability. Ribavirin, the only acknowledged antiviral viz. competent against certain VHF pathogens such as Lassa fever, is not active against Ebola viruses, which is unfortunate as it has already passed the 3rd phase of clinical trials. ZMapp, on the other hand, is a combination of monoclonal antibodies used to treat Ebola patients. Its role in treating EVD is still not certified as enough data proving it to be efficient is yet not published. It works well when tested on non-primates as it was capable enough to revert back the EVD. Favipiravir is the most commonly used and tested drug on humans. When tested on mice, it prevented its death, but its activity in non-human models still need to be validated. BCX4430 is another drug extensively studied and worked on, and has proved to be effective against Marburg Viruses in non-human primate models and Ebola model of mice. Ebola outbreak of Kikwit in 1995 was treated with patients being treated to Convalescent Plasma treatment, which at that time proved to be better as fewer people died and many survived. These days, with latest techniques and technology, this treatment is not being used much [45].
Other drugs such as TKM, AVI6002, Amiodarone, Lamivudine, Statins, Clomiphene, U18666-A, and Novavax are still under developed (Phase-I and Phase-II of clinical trials). These drugs have been proven to be effective against mice but there is no trace of their efficacy in humans. Both developed and developing therapeutics are under clinical trials and have some Achilles heels, but most of them have passed phase II trials. It means that they are capable enough of treating Ebola patients, but at the same time it needs to be suitable enough and efficient enough to work on the human body. These drugs pass the test on animal, non-primates human models, but usually fail to respond testing on human because of its complex mechanism. Furthermore, EBOV attacks the innate and the adaptive immunity, thereby creating chaos which restricts these drugs in the human body.
3.3. Mode of vaccine delivery
Vaccines can be delivered using two main routes: Cytomegalovirus (CMV) based vaccination, and Intranasal Route. The CMV-based vaccination has been studied in rodents [46], [47]. A single dosage of CMV expressing a CD8T cell epitope from nucleoprotein of EBOV persuaded durable CD8 + T cell immunity up to 33 weeks. No EVD was detected in vaccinated mice. These mice did, nevertheless, confirm loss of weight, implying protection [39]. The intranasal route was suggested as an effective vaccine delivery route [48]. This approach has the potential to escalate compliance, especially in populations dubious of Western therapeutics and medications [39].
3.4. Candidate vaccines
There are several vaccines which are developed, but Live Attenuated Vaccines are aforethought and examined as an aboriginal, a competitor, full of potency, and asylum were tested using nonhuman anthropoids as primary animal figurines. Despite, the results did not ascertain any cogent vaccine candidates even in commensuration of animal model [92]. Nevertheless, Ebola vaccine design and development studies have been going on for the last several decades, but unfortunately, the pace was not so rapid or rewarding. As a result, the development of vaccines related to EBOV has several limitations related to safety and personal expertise [36]. Some approaches were based on DNA, called DNA Vaccine, and were initiated during 2000s. Such approaches were tested and found to be successful. In DNA vaccine, infused nucleoprotein (NP) or glycoprotein (GP) of EBOV acts as an antigen to activate host immune responses and infused antigen-delivering DNA boosts the immunization [49]. Recently, a trial using Vesicular Stomatitis Virus (VSV) platform has been devised as bivalent vaccine against EBOV showing systemic immune responses safe-guarding animals after injection [50]. This non-segmented, negative stranded RNA virus is likewise celebrated to be a superlative competitor for recombinant DNA vaccine belvedere across several filoviruses as the virtue of the virus is an animal pathogen that does not coax any kind of serious manifestations in humans [50], [51].VSV based candidate vaccines (VSV-GP) have been successful in mice and have proved to give complete protection and also, have shown to be effective in the post exposure treatment regime. Since VSV-based vaccines are replication competent recombinant viruses, their use have been suspected in humans [88]. VSV based vaccines are an exclusive feature that is very precious in laboratory accidents, outbreak and bio-weapon exposure situations [52]. A review on phase-I trials of EBOV vaccines can be in found in Lambe et al. [53].
3.5. Antibodies based therapy
Human survivors of EVD are liable to early accumulate long standing neutralizing antibodies (NAbs) which may bind to EBOV structural enveloped glycoprotein (GP) [54]. Identifying such NAbs and their mechanism of activity is important for the development of novel immunotherapy and vaccines against EBOV. The broadness of aegis of such NAbs is wavering and still enduring study, which includes use of Convalescent Blood Products (Plasma) and ZMapp. Convalescent Plasma therapy is an immunotherapy, whereas ZMapp is a monoclonal antibody which degrades the EBOV transmembrane glycoprotein, helping the virus to attach, fuse and enter the host organism [55].
3.6. Under-sized molecules and drugs
There are numerous known and accepted abettors and pin-pointed compounds that show paradoxical-EBOV activity. For instance, benzodiazepine derivative which forestalls EBOV entry into the host cells [56]. We have matured drugs against EBOV, which have proved to be competent, like Favipiravir. Favipiravir performs antiviral activities against negative stranded RNA viruses, and has been administered in European Centres in the last EVD outbreak. Molecules such as Phosphorodiamidate Morpholino Oligomers (PMOs), and small interfering RNAs (siRNAs) are found useful in abbreviating carnage when supervised to NHPs up to 1 h after disclosure [57], [58]. AVI 6002 is a unification of positively charged PMOs devised to point mRNA sequences of VP24 and VP35 in EBOV. It is presently going through phase-I clinical trials for EBOV post-exposure treatment [39]. Brincidofovir (CMX001) is a prodrug of cidofovir and is an emphatic anti-DNA antiviral antidote by impeding viral replication, discouraging viral DNA polymerases. Currently, it is going through Phase-III clinical trials for its application against adenovirus and cytomegalovirus. Hence, Brincidofovir are used in crunch positions in subjects vitiated with EBOV [59]. BCX4430, another antiviral, restrains viral RNA polymerase activity circumlocutory through non-obligate RNA chain termination, exhibiting adequacy in pre-exposure EBOV treatment in vitro in small animal model. It showed protection from antagonist EBOV after taking BD oral and IM administration for 9days [60]. However, combination of ZMapp and high dosage of Favipiravir have proven to yield better results than the rest.
4. Resistance or Resilience: what is better for combating Ebola?
In ecology, resistance refers to the characteristic feature of populations (communities), which remain unchanged when subjected to any turbulent episode, both natural and artificial (man-made). On the other hand, resilience is defined as the potential of the populations (communities) to respond to the disturbances by resisting the damage caused and by rapid recovery. If we correlate the two terms, we may answer how communities have coped EVD. Resilience in general means “Elasticity”, i.e., transforming our medical health systems into resilient ones in order to become multifaceted, giving care and support to the patients to overcome the past and prepare them for a better future [61], [62]. Healthcare systems can be termed as resilient only when they have the zeal and springiness in their mode of action towards such epidemic disease spills [63]. They must also provide patients with everyday benefits and optimistic results for living an exceptionally beautiful future. This joy of double benefit and an upgraded performance in all times is what is known as “the resilience dividend” [64].
Resistance in disease spill-over is rarely observed. Resilience, on the other hand, proves to be a better option. The Ebola outbreak in the past has shown us a mirror that we lag behind when it comes to a resilient human healthcare system. Constructing resilience is ambience-dependent and verbose which needs progressive appraisal of healthcare systems, strengths and weaknesses, contributions in susceptible and sensitive peripherals of the system ahead of any cataclysmic episodes, support during such emergencies, and an analysis and audit of the conduct of work after the outbreak. Resilience is not a passive or stagnant construct, for instance, the accelerated measures of improvement from a disaster is a fundamental and an essential part of accomplishment [64].
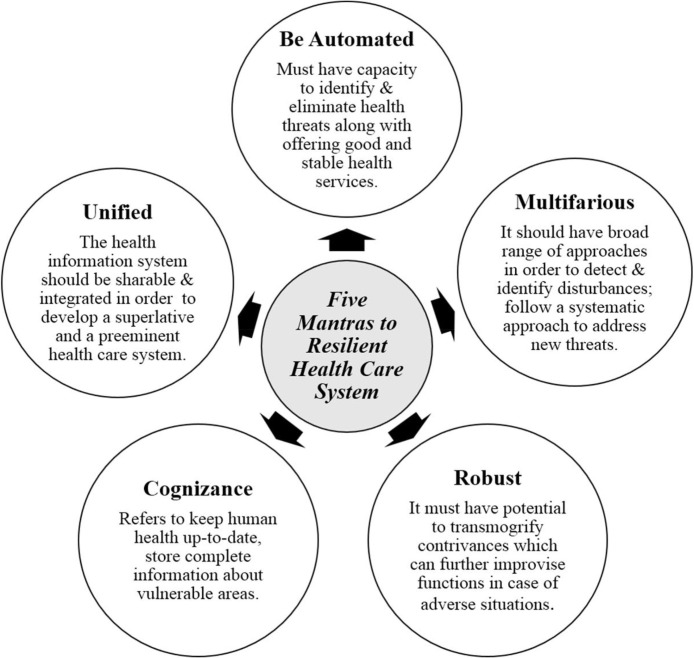
Resilient Heath System (RHS) is a comprehensive and a universal civic congenial approach which requires concerted and a corporate acknowledgement from all over the world. Capitalizing for this antiphon can materialize from conventional subdued donor sources, or from international sources where countries contribute a hefty amount [65]. Diversified fields such as- ecology, engineering, complex adaptive systems, psychology, and civil health have created resilience scheme [66], [67]. Here we propose five mantras to a RHS, composed of five rules that must be followed by every medical healthcare system, as depicted and described in Fig. 8 .
Fig. 8.
The 5 mantras (features) of a Resilient Health System.
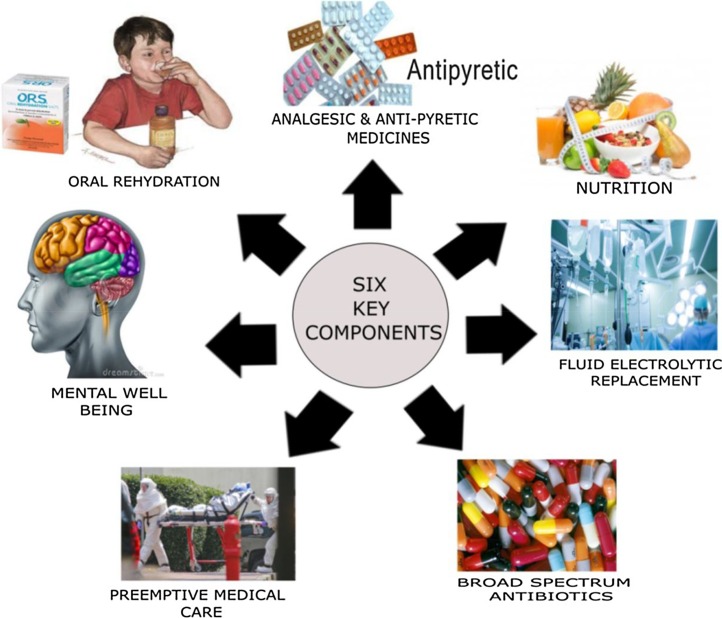
Along with the therapies mentioned in previous section, EVD patient needs a special yet specified care. Clinical researches can be integrated into standard care towards the evaluation of EBOV specific therapies [68]. Optimized Standard Of Care (oSOC) is focused on the standardized routine of care, which involves 6 steps [68], depicted in Fig. 9 , which is self-explanatory.
Fig. 9.
Optimized Standard of Care routine involving six steps .
5. Ebola mathematical model with control
Optimal control theory has been applied to study the dynamic mechanism of EBOV, which can be used to reduce its susceptibility in the population. The mathematical model of EBOV was created by Bonyah and collaborators [69], which provides EBOV control strategy. In this mode, two treatment controls are applied to the infected and late stage infected (super) human population. For this, Pontryagin’s Maximum Principle [70] is employed to characterize optimally control, which is then solved numerically. It is observed that time optimal control exist in the EBOV model. The activation of each control showed a positive reduction of infection. The overall effect of activation of all the controls simultaneously reduced the effort required for the reduction of the infection quickly [69].
In Bonyah et al. [69], mathematical model of EBOV disease include prevention and treatments measures as optimal control. By exploring and using Pontryagin’s maximum principle, condition for optimal control to minimize disease in a finite time can be derived and analyzed. The model system of Ebola has six NLDEs (Non-Linear Ordinary Differential Equations), which have been given by [70] and Bonyah et al. [69], as follows:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
Given all S(0), E(0), I 1(0), I 2(0), F(0), and R(0) > = 0, where the model partitions total populations, denoted by epidemiological sub population of susceptible (S), those exposed to Ebola virus (E), those individuals with the first stage of infection (I 1), those individuals with the second stage of infection(I 2), and the fraction of the population who recovered are denoted by (R). The fraction of population who died, and/or being processed for burial is represented as F. Coefficients β1, β2 and βf are the rates at which the susceptible become infected by an infectious individual in the first, second, and burial stage per unit of time, respectively. An exposed individual moves to the first stage of infectious class at a rate of α. The average length of first stage of illness is denoted by γ1 −1 and the average length of second stage of illness is γ2 −1. The average time from death till one is buried is denoted by γf −1. Average duration of Ebola treatment unit bed occupancy after recovery is γr −1. δ1 denotes the fraction of infected who progress to the second stage of infection and δ2 is the fraction who subsequently progress to death. The natural per capita mortality of EVD rate is denoted by μ. Fig. 10 presents a schematic diagram as compartment of Ebola Model, where population of susceptible (S) may be exposed to EBOV (E), which can further go to stage 1 (I 1) and may either recover (R) if treated properly, or moved to stage 2 (I 2). From stage 2 (I 2), patient may either be recovered (R), or died (F).
Fig. 10.
Schematic diagram showing unit compartments of Ebola Model, where population of susceptible (S) may be exposed to EBOV (E), which can further go to stage 1 (I1). From I1, it may either recover (R) if treated properly, or moved to stage 2 (I2). From stage 2 (I2), patient may either be recovered (R), or died (F) (Source: [69]).
The control functions u 1(t), u 2(t), and u 3(t) are bounded Lebesques integrable functions. The objective is to reduce the number of infected individuals with the EBOV, while at the same time keeping the cost of treatment very low [69].The optimal control strategy applied to this model shows that proper medication on the right time basis will ensure that EVD can be cured [69].
6. Challenges ahead
Ebola has been proven to be the most dangerous and fatal disease to mankind since its first outbreak in 1976. The disease transmission is known to be uncontrolled, and it is probable for its spill over to the unaffected areas of the world. The Centre for Disease Control and Prevention (CDC) is engaged extensively with allies to help stop the epidemic at its source in Africa. Every month, thousands of people from affected areas travel to different part of the world including other parts of Africa, United States, Europe, and Asia. As long as Ebola is spreading in these regions, clinicians are required to be vigilant to the likelihood of EVD, assess travel history, and immediately separate and test ill travelers who have travelled toEVD affected regions in the past 3 weeks, and have manifestations persistent with EVD [71]. The CDC has circulated guidelines about identifying, separating, diagnosing, and treating patients (http://www.cdc.gov/vhf/ebola/hcp/infection-prevention-and-control-recommendations.html). Some of the major challenges in Ebola research are as follows:
6.1. Directions for management of EVD
A large number of healthcare workers need to be trained to manage severe and fatal EVD. It may also be planned to treatment post-EVD complications, including social and psychological support. Although, several governmental and non-governmental agencies have been established to control the activities related to any outbreak, and establishment of supportive health-care system, but still basic challenge is that survivors need some specialised services. Therefore, mental health, eye care, and professionals need to be trained, and clinics need to be established [21].
6.2. Setting sight on targeting the virulent pathogen-contingencies and challenges
Remedial treatment for viral contagion can be universally assorted as agents that intrude the virus and its replication cycle without any deviation, or as abettors that aid and entrench host immune deterrence. According to the golden rule, there are bountiful targets and multifarious plans for both the above mentioned categories. This exuberant of quiescent targets could result in numerous remedial approaches. In conjunction with antisense targeting of the viral genome, inhibition of the replicase or polymerase activity by small-molecule inhibitors, including other specific molecular targets are important for the formation of a replication-competent complex [72].The modernistic advancement of reverse genetics and filovirus reporter-based mini-genomes [73], conjointly, Green Fluorescent Protein (GFP)expressing EBOV [74], is likely to promote the apperception of inhibitors of filovirus replication.
6.3. Ancillary therapeutics for Ebola
Filovirus infections are correlated with many pathological circumstances, inclusive of promulgated intravascular coagulation, that has given results from up-regulation of tissue factor on the surface of leukocytes [75]. Limited accomplishment contra to EBOV bugs in rhesus monkeys applying recombinant nematode anticoagulant protein C2 has lately been proclaimed [76]. Despite the fact, the study is promising, the service of anticoagulant therapy in humans which needs supplementary studies distinctly in consolidation with clear-cut antiviral remedies [77].
6.4. Aggrandizing elemental immunity
The ambition of a few antiviral abettors is to tip antithesis of the immune response facing innate immunity and avows definite immune consent mechanisms, viz. adaptive immunity, to ratify [7]. At the crisis of copious native (elemental), immune responses are interferons, a family of molecules that can precisely elicits antiviral responses. Though, the services of interferons as broad-spectrum antivirals antiquate finite both by the ephemerality and lethality of their effects. This has threatened attention about the expectancies for a wide array of other newly characterized cytokines that also encourage innate immunity. Furthermore, other conveniences for drug interference have emerged in targeting viral pathogens. The recognition of proteins originated by vaccination and influenza virus which serve as interferon antagonists [78], [79] was ensued by presentation that Ebola viruses [80] and Marburg viruses [81] also produce interferon antagonists.
6.5. Is Ebola the new bio-weapon?
Bioterrorism and Bio-weapons are the new tinge of today’s demagogic society. The discombobulating caused by the incendiary communities can be anything, of any form. The spill-over of virulent bugs put a perpetual impendence to the global health. Enough heed is paid to the emanation of comparatively unfamiliar or unknown bugs, viruses etc, e.g. EBOV. Most epidemics materialize due to external and topographical factors. Occasionally, notwithstanding, hominid prying with nature impacts lead to the spill-over of the diseases. A few zoonoses plunge to human host because the rainforest habitat of former animal hosts is reduced. The success of bio-terroristic effort is measured by societal disruption and panic, and not by abrupt number of casualties [82]. Among three classes of bioterrorism agents defined by CDC, category A includes the highest-prerogative abettor which can be easily transmitted from person-to-person causing secondary and tertiary cases. It causes high fatality and high impact on public health, public panic and social disruption. Filoviridae have been placed in category A, and EBOV also causes lethalness, may be misused as an ultimate destructive agent for mankind.
6.6. Lesson learn from past outbreaks
Modern lessons learnt from Ebola outbreaks in the past should be standardized. The previous experiences of malfunctioning and failure have to be remembered and accordingly new practices have to be implemented [83]. The basic lessons that have been learnt seeing the past outbreaks are as follows [84]:
Disease detection and disease response systems must be invigorated.
International funding must be present beforehand. International stocks of protective apparatus and supplies must be controlled and its uses must be available.
The public health society must identify the positive potential of the international media in organizing public ideas and resources for infectious disease control, and they must entertain their needs without backing off from the primary mission.
It becomes an utmost responsibility of WHO to educate people round the globe about any new outbreaks of diseases by utilizing electronic communication systems.
Challenges that still lie ahead in eliminating EBOV
There is an urgent need to accelerate the rate of drug and vaccine development so as to prevent the spread of the disease, and to eliminate it. Ethical issues have been raised for usage of experimental treatments and vaccines which are in very limited supply. However, a vaccine which is safe and effective would further protect in outbreak situations [71].
Candidate vaccines should be evaluated and updated on a regular basis.
Novel technologies must be quickly adopted, as they are very likely to yield faster and better results. These technologies and analytical equipment must be inexpensive so that any small laboratory can also work with them.
A holistic approach should be used in order to understand the mechanism and treat the atrocious EVD.
In order to understand the mechanism and the dynamics of the virus, Systems Biology, Computational, Analytical, Mathematical fields of studies must be amalgamated, which eventually would lead to the formation of framework, describing the intrinsic details of Ebola.
There is a need to strengthen our health and security agendas, to fight with the virulent pathogens.
More and more consultants, nurses, are needed who are willing to treat the Ebola patients. There should be better hospital infrastructures, and clinics, which are fully fuelled with the laboratories and technologies.
Ebola outbreak also comes with a chance to scale up mental healthcare sorely needed within the affected countries. It is very important to provide heed to the mental health being [85].
7. Conclusion
Ebola Virus Disease (EVD) is a potentially threatening illness to human health globally, caused by Ebola Virus (EBOV) of the virus family Filoviridae. EBOV is an enveloped virus composed of 18.8–19 kb single-stranded, negative sense RNA genome that encodes 7 proteins and has surface glycoprotein presenting roles in cell attachment, fusion and cell entry involved in immune evasion and pathogenesis of disease. Transmission of the virus is generally zoonosis which involves animals and humans. As it was identified that fruit bats are the reservoir hosts of the Ebola Virus and therefore they carried this virus and transmits to other animals and further to humans. The spill-over of infections basically occurs from bats to humans but the dynamical mechanism of EBOV is understood by 3 factors, namely, Biological Factors, Environmental Factors and Social Factors, and therefore, the infectious interaction between bats and the ecosystem is responsible for the spill-over dynamics of deadly diseases either directly (bats -> humans) or indirectly (bats -> animals -> humans). So, to understand the framework for the holistic study of disease dynamics mechanism of EBOV, various approaches such as Life Sciences, Mathematics, Systems Biology, Computational, and Analytical methods need to be applied.
The Ebola outbreak in the past has shown us a mirror that we dawdle behind when it comes to a resilient human healthcare system. Constructing resilience is ambience-dependent and bombastic which needs progressive appraisal of healthcare systems, strengths and weaknesses, contributions in susceptible and sensitive components of the system ahead of any cataclysmic episodes. Resilient Heath System (RHS) is a global and a universal civic congenial approach which requires concerted and a corporate acknowledgement from all over the world.
There is a big challenge in the treatment of EVD as there is no approved therapeutics so far. But some of the diagnostic methods such as ELISA and rRT-PCR are generally used. In addition, a number of vaccines and antiviral drugs are under the development against EVD by using animal models such as mice, guinea pigs, and NHPs to test the safety and efficacy of the vaccines or drugs, which gives results for only phase I clinical trials. Phase II, III, and IV trials didn’t give better results due to lack of equipment, resources and technology. Various small molecules and antiviral drugs, including as Favipiravir, siRNAs, PMOs, AVI6002, Brincidofovir, BCX4430, and ZMapp, have been developed. Among these, the most promising and active antiviral drugs are ZMapp and Favipiravir. Hence, to get a victory against Ebola, we still require powerful drugs and vaccines. In addition to therapeutics and medications, optimized care using resilient health system is very important.
Optimal control theory was used to develop dynamic mathematical model of EVD which can be used to reduce the sensitivity in the population. The Centre for Disease Control and Prevention is engrossed broadly with allies to help stop the epidemic at its source in Africa. Thousands of people from affected areas travel to different part of the world. As long as Ebola is growing, practitioners are required to be very alert to the probability of EVD, check travel history, and immediately separate and test ill travelers who have travelled to EVD affected regions in the past 3 weeks [71]. However, it is a challenging task. Regardless of the fact, the service of anticoagulant therapy in humans needs additional studies distinctly in confining with clear-cut antiviral remedies. Though, the services of interferons as broad-spectrum antivirals archaize finite both by the brevity and fatality of their effects. This has jeopardized attention about the expectancies for a wide array of other newly characterized cytokines that also inspires innate immunity. To elevate the predictability, it becomes mandatory to identify infected persons as soon as possible, which requires latest diagnostic techniques and treatment facilities. A single nonchalant activity can be disastrous. By following precautionary measures, spread of the disease can be restricted to a large extent. Challenges in combating Ebola are not only limited to resources and knowledge, but also public and personal hygiene that must be adopted in the society.
Disclosures
The authors declare that there is any conflict of interest in the publication of this manuscript.
Acknowledgements
The corresponding author acknowledges senior colleagues of his University for necessary discussions and suggestions on the initial version of the manuscript. Authors acknowledge the anonymous reviewers for their valuable comments and suggestions.
References
- 1.Rajak H., Jain D.K., Singh A., Sharma A.K., Dixit A. Ebola virus disease: past, present and future. Asia. Pac. J. Trop. Biomed. 2015;5(5):337–343. [Google Scholar]
- 2.Feldmann H., Geisbert T.W. Ebola haemorrhagic fever. Lancet. 2011;377(9768):849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldmann H., Slenczka W., Klenk H.D. Imported Virus Infections. Springer; Vienna: 1996. Emerging and reemerging of filoviruses; pp. 77–100. [DOI] [PubMed] [Google Scholar]
- 4.Sridhar S. Clinical development of Ebola vaccines. Ther. Adv. Vacc. 2015;3(5–6):125–138. doi: 10.1177/2051013615611017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan N., Yang Z.Y., Nabel G.J. Ebola virus pathogenesis: implications for vaccines and therapies. J. Virol. 2003;77(18):9733–9737. doi: 10.1128/JVI.77.18.9733-9737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A. Ebola virus is altered innate and adaptive immune response signaling pathways: implications for novel therapeutic approaches. Infect. Disord.- Drug. Targets. 2016;16(2):79–94. doi: 10.2174/1871526516666160108114644. [DOI] [PubMed] [Google Scholar]
- 7.Mohamadzadeh M., Luftig R. Dendritic cells: in the forefront of immunopathogenesis and vaccine development–A review. J. Immune Based Therapies Vaccines. 2004;2(1):1. doi: 10.1186/1476-8518-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . World Health Organization; Geneva: 2014. Sierra Leone: Helping the Ebola Survivors Turn the Page.http://www.who.int/features/2014/post-ebola-syndrome/en (on July 10, 2017) Retrieved from: [Google Scholar]
- 9.Centers for Disease Control and Prevention . 2014. 2014 Ebola Outbreak in West Africa—case Counts.https://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.htmlon (July 21, 2017) Retrieved from. [Google Scholar]
- 10.Bwaka M.A., Bonnet M.J., Calain P., Colebunders R., De Roo A., Guimard Y., Mapanda B.B. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J. Infect. Dis. 1999;179(Supplement_1):S1–S7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]
- 11.Groseth A., Feldmann H., Strong J.E. The ecology of Ebola virus. Trends Microbiol. 2007;15(9):408–416. doi: 10.1016/j.tim.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Alam A., Imam N., Ali S., Malik M.Z., Ishrat R. Recent trends in ZikV research: A step away from cure. Biomed. Pharmacother. 2017;91:1152–1159. doi: 10.1016/j.biopha.2017.05.045. [DOI] [PubMed] [Google Scholar]
- 13.Caviness K., Kuhn J.H., Palacios G. Ebola virus persistence as a new focus in clinical research. Curr. Opin. Virol. 2017;23:43–48. doi: 10.1016/j.coviro.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Baseler L., Chertow D.S., Johnson K.M., Feldmann H., Morens D.M. The pathogenesis of ebola virus disease. Annu. Rev. Pathol.: Mech. Dis. 2017;12:387–418. doi: 10.1146/annurev-pathol-052016-100506. [DOI] [PubMed] [Google Scholar]
- 15.Haaskjold Y.L., Bolkan H.A., Krogh K.Ø., Jongopi J., Lundeby K.M., Mellesmo S., Fuentes L.M.Z. Clinical features of and risk factors for fatal Ebola virus disease, Moyamba District, Sierra Leone, December 2014–February 2015. Emerg. Infect. Dis. 2016;22(9):1537. doi: 10.3201/eid2209.151621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiffany A., Vetter P., Mattia J., Dayer J.A., Bartsch M., Kasztura M., Ciglenecki I. Ebola virus disease complications as experienced by survivors in sierra Leone. Clin. Infect. Dis. 2016;62(11):1360–1366. doi: 10.1093/cid/ciw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark D.V., Kibuuka H., Millard M., Wakabi S., Lukwago L., Taylor A., Olinger G.G. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. The Lancet Infectious Diseases. 2015;15(8):905–912. doi: 10.1016/S1473-3099(15)70152-0. [DOI] [PubMed] [Google Scholar]
- 18.Wappes J. 2016. Studies on Ebola Survivors Show Range of Complications. Center For Infectious Disease Research And Policy (CIDRAP) News. Feb 25. [Google Scholar]
- 19.Rewar S., Mirdha D. Transmission of Ebola virus disease: an overview. Ann. Global Health. 2014;80(6):444–451. doi: 10.1016/j.aogh.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Lo T.Q., Marston B.J., Dahl B.A., De Cock K.M. Ebola: anatomy of an epidemic. Annu. Rev. Med. 2017;68:359–370. doi: 10.1146/annurev-med-052915-015604. [DOI] [PubMed] [Google Scholar]
- 21.Vetter P., Kaiser L., Schibler M., Ciglenecki I., Bausch D.G. Sequelae of Ebola virus disease: the emergency within the emergency. Lancet Infect. Dis. 2016;16(6):e82–e91. doi: 10.1016/S1473-3099(16)00077-3. [DOI] [PubMed] [Google Scholar]
- 22.Sachs J.D., et al. Biodiversity conservation and the millennium development goals. Science. 2009;325:1502–1503. doi: 10.1126/science.1175035. [DOI] [PubMed] [Google Scholar]
- 23.Naeem S. Ecology: Gini in the bottle. Nature. 2009;458:579–580. doi: 10.1038/458579a. [DOI] [PubMed] [Google Scholar]
- 24.Grenfell B.T., Pybus O.G., Gog J.R., Wood J.L., Daly J.M., Mumford J.A., Holmes E.C. Unifying the epidemiological and evolutionary dynamics of pathogens. Science. 2004;303(5656):327–332. doi: 10.1126/science.1090727. [DOI] [PubMed] [Google Scholar]
- 25.Wood J.L., Leach M., Waldman L., MacGregor H., Fooks A.R., Jones K.E., Peel A.J. A framework for the study of zoonotic disease emergence and its drivers: spillover of bat pathogens as a case study. Phil. Trans. R. Soc. B. 2012;367(1604):2881–2892. doi: 10.1098/rstb.2012.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Restif O., Hayman D.T., Pulliam J.R., Plowright R.K., George D.B., Luis A.D., Wood J.L. Model‐guided fieldwork: practical guidelines for multidisciplinary research on wildlife ecological and epidemiological dynamics. Ecol. Lett. 2012;15(10):1083–1094. doi: 10.1111/j.1461-0248.2012.01836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keesing F., Belden L.K., Daszak P., Dobson A., Harvell C.D., Holt R.D., Myers S.S. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468(7324):647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strauss S. Biotech drugs too little, too late for Ebola outbreak. Nat. Bioechnol. 2014;32:849–850. doi: 10.1038/nbt0914-849a. [DOI] [PubMed] [Google Scholar]
- 29.Qiu X., Wong G., et al. Reversion of advanced Ebola virus disease in non human primates with Zmapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan S.L., Ganji G., et al. Systems biology and the host response to viral infection. Nat. Biotechnol. 2007;25:1383–1389. doi: 10.1038/nbt1207-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng F., Murray J.L., Zhao J., Sheng J., Zhao Z., Rubin D.H. Systems biology-based investigation of cellular antiviral drug targets identified by gene-trap insertional mutagenesis. PLoS Comput. Biol. 2016;12(9):e1005074. doi: 10.1371/journal.pcbi.1005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oluwagbemi O., Oluwagbemi F., Abimbola O. Ebinformatics: Ebola fuzzy informatics systems on the diagnosis, prediction and recommendation of appropriate treatments for Ebola virus disease (EVD) Inf. Med. Unlocked. 2016;2:12–37. [Google Scholar]
- 33.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baize S., Pannetier D., Oestereich L., Rieger T., Koivogui L., Magassouba N.F., Tiffany A. Emergence of Zaire Ebola virus disease in Guinea. New Engl. J. Med. 2014;371(15):1418–1425. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 35.Bradfute S.B. The early clinical development of Ebola virus treatments. Expert Opin. Investig. Drugs. 2017;26(1):1–4. doi: 10.1080/13543784.2017.1260545. [DOI] [PubMed] [Google Scholar]
- 36.Choi W.Y., Hong K.J., Hong J.E., Lee W.J. Progress of vaccine and drug development for Ebola preparedness. Clin. Exp. Vacc. Res. 2015;4(1):11–16. doi: 10.7774/cevr.2015.4.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammadi D. First trials for Ebola treatments announced. Lancet. 2014;384(9957):1833. doi: 10.1016/s0140-6736(14)62043-2. [DOI] [PubMed] [Google Scholar]
- 38.Cooper B.S., Boni M.F., Pan-ngum W., Day N.P., Horby P.W., Olliaro P., Whitehead J. Evaluating clinical trial designs for investigational treatments of ebola virus disease. PLoS Med. 2015;12(4):e1001815. doi: 10.1371/journal.pmed.1001815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trad M.A., Naughton W., Yeung A., Mazlin L., O’sullivan M., Gilroy N., Stuart R.L. Ebola virus disease: an update on current prevention and management strategies. J. Clin. Virol. 2017;86:5–13. doi: 10.1016/j.jcv.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Burnett J.C., Henchal E.A., Schmaljohn A.L., Bavari S. The evolving field of biodefence: therapeutic developments and diagnostics. Nat. Rev. Drug Discov. 2005;4(4):281. doi: 10.1038/nrd1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olszanecki R., Gawlik G. Pharmacotherapy of Ebola hemorrhagic fever: a brief review of current status and future perspectives. Folia Med. Cracov. 2014;3:67–77. [PubMed] [Google Scholar]
- 42.Khan A.S., Tshioko F.K., Heymann D.L., Le Guenno B., Nabeth P., Kerstiëns B., Rollin P.E. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. J. Infect. Dis. 1999;179(Supplement_1):S76–S86. doi: 10.1086/514306. [DOI] [PubMed] [Google Scholar]
- 43.Halperin S.A., Arribas J.R., Rupp R., Andrews C.P., Chu L., Das R., Helmond F.A. Six-month safety data of recombinant vesicular stomatitis virus–zaire ebola virus envelope glycoprotein vaccine in a phase 3 double-blind, placebo-controlled randomized study in healthy adults. J. Infect. Dis. 2017;215(12):1789–1798. doi: 10.1093/infdis/jix189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henao-Restrepo A.M., Camacho A., Longini I.M., Watson C.H., Edmunds W.J., Egger M., Draguez B. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) The Lancet. 2017;389(10068):505–518. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goeijenbier M., et al. Ebola virus disease: a review on epidemiology, symptoms, treatment and pathogenesis. J. Med. 2014;72(9):442–448. [PubMed] [Google Scholar]
- 46.Tsuda Y., Hoenen T., Banadyga L., Weisend C., Ricklefs S.M., Porcella S.F., Ebihara H. An improved reverse genetics system to overcome cell-type–dependent ebola virus genome plasticity. J. Infect. Dis. 2015;212(suppl_2):S129–S137. doi: 10.1093/infdis/jiu681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuda Y., Parkins C.J., Caposio P., Feldmann F., Botto S., Ball S., Jarvis M.A. A cytomegalovirus-based vaccine provides long-lasting protection against lethal Ebola virus challenge after a single dose. Vaccine. 2015;33(19):2261–2266. doi: 10.1016/j.vaccine.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jonsson-Schmunk K., Croyle M.A. 2015. A Long-Lasting, Single-Dose Nasal Vaccine for Ebola: a Practical Armament for an Outbreak With Significant Global Impact. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanderzanden L., Bray M., Fuller D., Roberts T., Custer D., Spik K., Schmaljohn C. DNA vaccines expressing either the GP or NP genes of ebola virus protect mice from lethal challenge. Virology. 1998;246(1):134–144. doi: 10.1006/viro.1998.9176. [DOI] [PubMed] [Google Scholar]
- 50.Warfield K.L., Goetzmann J.E., Biggins J.E., Kasda M.B., Unfer R.C., Vu H., Walsh P.D. Vaccinating captive chimpanzees to save wild chimpanzees. Proc. Natl. Acad. Sci. 2014;111(24):8873–8876. doi: 10.1073/pnas.1316902111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geisbert T.W., Feldmann H. Recombinant vesicular stomatitis virus–based vaccines against Ebola and Marburg virus infections. J. Infect. Dis. 2011;204(suppl_3):S1075–S1081. doi: 10.1093/infdis/jir349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson J.S., Dekker J.D., et al. Recent advances in Ebolavirus vaccine development. Hum. Vaccin. 2010;6(6):439–449. doi: 10.4161/hv.6.6.11097. [DOI] [PubMed] [Google Scholar]
- 53.Lambe T., Bowyer G., Ewer K.J. A review of phase I trials of Ebola virus vaccines: what can we learn from the race to develop novel vaccines? Phil. Trans. R. Soc. B. 2017;372(1721) doi: 10.1098/rstb.2016.0295. 20160295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shedlock D.J., Bailey M.A., Popernack P.M., Cunningham J.M., Burton D.R., Sullivan N.J. Antibody-mediated neutralization of Ebola virus can occur by two distinct mechanisms. Virology. 2010;401(2):228–235. doi: 10.1016/j.virol.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin M.J., et al. Ebola virus infection: overview and update on prevention and treatment. Infect. Dis. Ther. 2015;4(4):365–390. doi: 10.1007/s40121-015-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basu A., Li B., Mills D.M., Panchal R.G., Cardinale S.C., Butler M.M., Moir D.T. Identification of a small-molecule entry inhibitor for filoviruses. J. Virol. 2011;85(7):3106–3119. doi: 10.1128/JVI.01456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geisbert T.W., Lee A.C., Robbins M., Geisbert J.B., Honko A.N., Sood V., Hensley L.E. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. The Lancet. 2010;375(9729):1896–1905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warren T.K., Warfield K.L., Wells J., Swenson D.L., Donner K.S., Van Tongeren S.A., Nichols D.K. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat. Med. 2010;16(9):991–994. doi: 10.1038/nm.2202. [DOI] [PubMed] [Google Scholar]
- 59.Florescu D.F., Keck M.A. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev. Anti Infect. Ther. 2014;12(10):1171–1178. doi: 10.1586/14787210.2014.948847. [DOI] [PubMed] [Google Scholar]
- 60.Warren T.K., Wells J., Panchal R.G., Stuthman K.S., Garza N.L., Van Tongeren S.A., Honnold S. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508(7496):402. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kieny M.P., Evans D.B., Schmets G., Kadandale S. Health-system resilience: reflections on the Ebola crisis in western Africa. Bull. World Health Organ. 2014;92(12):850. doi: 10.2471/BLT.14.149278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kruk M.E., Myers M., Varpilah S.T., Dahn B.T. What is a resilient health system? Lessons from Ebola. The Lancet. 2015;385(9980):1910–1912. doi: 10.1016/S0140-6736(15)60755-3. [DOI] [PubMed] [Google Scholar]
- 63.Masten A.S. Ordinary magic: resilience processes in development. Am. Psychol. 2001;56(3):227. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- 64.Rodin J. 2014. The Resilience Dividend: Being Strong in a World Where Things Go Wrong. Public Affairs. [Google Scholar]
- 65.Gostin L.O. Ebola: towards an international health systems fund. Lancet. 2014;384:e49–e51. doi: 10.1016/S0140-6736(14)61345-3. [DOI] [PubMed] [Google Scholar]
- 66.Dalziell E.P., McManus S.T. 2004. Resilience, Vulnerability, and Adaptive Capacity: Implications for System Performance. [Google Scholar]
- 67.Allenby B., Fink J. Toward inherently secure and resilient societies. Science. 2005;309(5737):1034–1036. doi: 10.1126/science.1111534. [DOI] [PubMed] [Google Scholar]
- 68.Duraffour S., Malvy D., Sissoko D. How to treat ebola virus infections? A lesson from the field. Curr. Opin. Virol. 2017;24:9–15. doi: 10.1016/j.coviro.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Bonyah E., Badu K., Asiedu-Addo S.K. Optimal control application to an Ebola model. Asia. Pac. J. Trop. Biomed. 2016;6(4):283–289. doi: 10.1016/j.apjtb.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pontryagin L.S. CRC Press; 1987. Mathematical Theory of Optimal Processes. [Google Scholar]
- 71.Frieden T.R., Damon I., Bell B.P., Kenyon T., Nichol S. Ebola 2014—new challenges, new global response and responsibility. New Engl. J. Med. 2014;371(13):1177–1180. doi: 10.1056/NEJMp1409903. [DOI] [PubMed] [Google Scholar]
- 72.Goodchild J. Oligonucleotide, antibody and peptide therapeutics--from design to the clinic. Curr. Opin. Mol. Ther. 2004;6(2):119. [PubMed] [Google Scholar]
- 73.Mühlberger E., Weik M., Volchkov V.E., Klenk H.D., Becker S. Comparison of the transcription and replication strategies of Marburg virus and Ebola virus by using artificial replication systems. J. Virol. 1999;73(3):2333–2342. doi: 10.1128/jvi.73.3.2333-2342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Towner J.S., Paragas J., Dover J.E., Gupta M., Goldsmith C.S., Huggins J.W., Nichol S.T. Generation of eGFP expressing recombinant Zaire ebolavirus for analysis of early pathogenesis events and high-throughput antiviral drug screening. Virology. 2005;332(1):20–27. doi: 10.1016/j.virol.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 75.Geisbert T.W., Hensley L.E., Jahrling P.B., Larsen T., Geisbert J.B., Paragas J., Vlasuk G.P. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003;362(9400):1953–1958. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 76.Geisbert T.W., Young H.A., Jahrling P.B., Davis K.J., Kagan E., Hensley L.E. Mechanisms underlying coagulation abnormalities in Ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J. Infect. Dis. 2003;188(11):1618–1629. doi: 10.1086/379724. [DOI] [PubMed] [Google Scholar]
- 77.Yen J.Y., Garamszegi S., Geisbert J.B., Rubins K.H., Geisbert T.W., Honko A., Hensley L.E. Therapeutics of Ebola hemorrhagic fever: whole-genome transcriptional analysis of successful disease mitigation. J. Infect. Dis. 2011;204(suppl_3):S1043–S1052. doi: 10.1093/infdis/jir345. [DOI] [PubMed] [Google Scholar]
- 78.Chang H.W., Watson J.C., Jacobs B.L. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. 1992;89(11):4825–4829. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palese P., Muster T., Zheng H., O'Neill R., Garcia-Sastre A. Learning from our foes: a novel vaccine concept for influenza virus. Arch. Virol. Suppl. 1999:131–138. doi: 10.1007/978-3-7091-6425-9_9. [DOI] [PubMed] [Google Scholar]
- 80.Basler C.F., Wang X., Mühlberger E., Volchkov V., Paragas J., Klenk H.D., Palese P. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl. Acad. Sci. 2000;97(22):12289–12294. doi: 10.1073/pnas.220398297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bosio C.M., Aman M.J., Grogan C., Hogan R., Ruthel G., Negley D., Schmaljohn A. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J. Infect. Dis. 2003;188(11):1630–1638. doi: 10.1086/379199. [DOI] [PubMed] [Google Scholar]
- 82.Jansen H.J., Breeveld F.J., Stijnis C., Grobusch M.P. Biological warfare, bioterrorism, and biocrime. Clin. Microbiol. Infect. 2014;20(6):488–496. doi: 10.1111/1469-0691.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gates B. The next epidemic- lessons from Ebola. The New. Engl. J. Med. 2015;372:1381–1384. doi: 10.1056/NEJMp1502918. [DOI] [PubMed] [Google Scholar]
- 84.Heymann D., Barakamfitiye D., et al. Ebola hemorrhagic fever: lessons from Kikwit, Democratic Republic of Congo. J. Infect. Dis. 1999;179:S283–S286. doi: 10.1086/514287. [DOI] [PubMed] [Google Scholar]
- 85.Cheung E.Y. An outbreak of fear, rumours and stigma: psychosocial support for the Ebola virus disease outbreak in West Africa. Intervention. 2015;13(1):70–76. [Google Scholar]
- 87.Gire S.K., Goba A., Andersen K.G., Sealfon R.S., Park D.J., Kanneh L., Wohl S. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science. 2014;345(6202):1369–1372. doi: 10.1126/science.1259657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ilinykh P.A., Lubaki N.M., Widen S.G., Renn L.A., Theisen T.C., Rabin R.L., Bukreyev A. Different temporal effects of Ebola virus VP35 and VP24 proteins on global gene expression in human dendritic cells. J. Virol. 2015;89(15):7567–7583. doi: 10.1128/JVI.00924-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lakshmi V.D., et al. Ebola virus- symptomatology and intervention. Int. J. Bus. Admin. Res. Rev. 2013;1(8):144–148. [Google Scholar]
- 92.Okumura A., Rasmussen A.L., Halfmann P., Feldmann F., Yoshimura A., Feldmann H., Katze M.G. Suppressor of cytokine signaling 3 is an inducible host factor that regulates virus egress during Ebola virus infection. J. Virol. 2015;89(20):10399–10406. doi: 10.1128/JVI.01736-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Varkey J.B., Shantha J.G., Crozier I., Kraft C.S., Lyon G.M., Mehta A.K., Ströher U. Persistence of Ebola virus in ocular fluid during convalescence. New Engl. J. Med. 2015;372(25):2423–2427. doi: 10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wahl-Jensen V., Kurz S., Feldmann F., Buehler L.K., Kindrachuk J., DeFilippis V., Feldmann H. Ebola virion attachment and entry into human macrophages profoundly effects early cellular gene expression. PLoS Negl. Trop. Dis. 2011;5(10):e1359. doi: 10.1371/journal.pntd.0001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haque A., Hober D., Blondiaux J. Addressing therapeutic options for ebola virus infection in current and future outbreaks. Antimicrob. Agents Chemother. 2015;59(10):5892–5902. doi: 10.1128/AAC.01105-15. [DOI] [PMC free article] [PubMed] [Google Scholar]